Abstract
IMPORTANCE
Substance use disorders (SUDs) are among the most common sequelae of childhood maltreatment, yet the independent contributions of SUDs and childhood maltreatment to neurobiological changes and the effect of the latter on relapse risk (a critical variable in addiction treatment) are relatively unknown.
OBJECTIVES
To identify structural neural characteristics independently associated with childhood maltreatment (CM; a common type of childhood adversity), comparing a sample with SUD with a demographically comparable control sample, and to examine the relationship between CM-related structural brain changes and subsequent relapse.
DESIGN, SETTING, AND PARTICIPANTS
Structural magnetic resonance imaging study comparing 79 treatment-engaged participants with SUD in acute remission in inpatient treatment at a community mental health center vs 98 healthy control participants at an outpatient research center at an academic medical center. Both groups included individuals with a range of CM experiences. Participants with SUD were followed up prospectively for 90 days to assess relapse and relapse severity.
INTERVENTION
Standard 12-step, recovery-based, inpatient addiction treatment for all participants with SUD.
MAIN OUTCOMES AND MEASURES
Gray matter volume (GMV), subsequent substance use relapse, days to relapse, and severity of relapse.
RESULTS
Controlling for SUD and psychiatric comorbidity, CM (dichotomously classified) was uniquely associated with lower GMV across all participants in the left hippocampus (cornu ammonis 1-3, dentate gyrus), parahippocampus (presubiculum, parasubiculum, prosubiculum, subiculum, and entorhinal cortex), and anterior fusiform gyrus (corrected P < .05; uncorrected P = .001). Among the sample with SUD, CM prospectively predicted a shorter relapse to use of any drug (P = .048), while CM-related GMV reductions predicted severity of substance use relapse (P = .04).
CONCLUSIONS AND RELEVANCE
Findings indicate that CM was related to decreased GMV in limbic regions, which in turn predicted increased risk of relapse in SUD. These results suggest that CM may significantly affect the course of SUD treatment outcomes and that SUD treatment planning may benefit from identifying and addressing CM.
Childhood adversity plays a role in approximately 30% of all mental illness worldwide.1 Estimates suggest that approximately 40% to 50% of those who experienced childhood maltreatment (CM), herein defined as physical, sexual, and emotional abuse as well as physical and emotional neglect, will develop substance abuse problems.2 Those who experience CM are at least twice as likely as those without CM to initiate illicit substance use and 2 to 3 times more likely to do so in early adolescence.2 Childhood maltreatment is associated with increased severity of substance use disorders (SUDs) and may also increase the number or frequency of relapse.3,4 While neurobiological alterations have been associated with both CM5-7 and SUD8,9 separately, dissociating the contributions of CM from SUD has been difficult. Unique contributions of CM and SUD, their possible additive or interactive effects on neurobiological function, and the impact on course and severity of psychopathology remain largely unknown.
Human research assessing neurobiological effects of early adversity has focused on regions of interest (ROIs) such as the hippocampus and amygdala5,7 with a priori hypotheses based on preclinical literature. While validating earlier findings, this approach does not allow investigation of the effects of CM on the whole brain, especially regions known to be affected by CM (eg, orbitofrontal cortex, nucleus accumbens, striatum, prefrontal cortex).6,10 Research showing adversity-related neurobiological sequelae in multiple brain regions raises the important question of whether these effects are specific to childhood adversity or instead are linked to other types of insults and environmental deprivation, including substance use and psychiatric comorbidity associated with such adversity. Further, evidence suggests that neurobiological alterations related to substance use may be significant in the course of SUD and associated rates of relapse,11 but to our knowledge such studies have not examined the potential role of CM in the neurobiological alterations associated with SUD.
To examine the neurobiological specificity of childhood adversity, we examined the specific effects of CM vs SUD on brain morphology. The methods included whole-brain voxel-based morphometry (VBM)12 in a sample of 98 healthy control individuals from the community with no history of substance abuse or dependence and 79 treatment-engaged individuals with SUD who were abstinent for 4 to 5 weeks and met criteria for alcohol, cocaine, and/or cannabis use disorders. Changes in whole-brain morphometry were examined to assess the independent and interactive relationships of CM and SUD. After identifying significant brain regions associated with CM by whole-brain VBM, we also examined the specific effects of CM and SUD on estimates of gray matter volume (GMV) in significant regions. Finally, we prospectively examined the ability of CM as well as CM-related GMV changes to predict substance use relapse and relapse severity during a 90-day postdischarge follow-up period among the subsample of inpatient-treated, recovering participants with SUD.
Methods
Participants
Individuals aged 25 to 50 years were selected for this study from a recruited sample of 255 participants to limit possible effects of substance abuse on neurodevelopmental processes.13 A total of 98 healthy controls (HCs) with no history of SUD from the community and 79 participants who met DSM-IV criteria for SUD limited to alcohol, cocaine, and/or cannabis use disorders participated. All HCs were recruited from advertisements in local newspapers, advertisements on websites, and flyers in the community. Treatment-seeking participants with SUD were recruited using the same processes as well as via addiction treatment centers in the greater New Haven, Connecticut, area. The SUD and HC groups were group matched on relevant demographic variables.
All participants with SUD had been abstinent for approximately 4 to 5 weeks and were engaged in standard inpatient treatment at the time of the scan. Weekly substance use was verified by a combination of self-report, breathalyzer, and urine toxicology prior to inpatient admission. Individuals with SUD specific to opioids, sedatives, methamphetamines, and any other drugs (except nicotine) were excluded. Those with primary Axis I diagnoses (other than SUD, as described earlier), those receiving psychoactive medications, and those with any history of psychosis were excluded. Individuals with secondary lifetime and/or current major depressive disorder or anxiety disorders were not excluded given high comorbidity with SUD.14 The HC group comprised 98 comparison participants who were free of Axis I psychiatric diagnoses (other than lifetime history of major depressive disorder or any anxiety disorders), confirmed by the Structured Clinical Interview for DSM-IV Axis I Disorders,15 and had no lifetime or current history of SUD (including alcohol).
Across both groups, potential participants were excluded for any history of serious head trauma (defined by a blow or knock to the head with accompanying loss of consciousness ≥30 minutes) or neurological conditions. All participants underwent medical examination to ensure good physical health; individuals taking prescription medications and those requiring acute medical attention were excluded. All participants gave both written and verbal informed consent. The study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Procedures
Participants completed the Structured Clinical Interview for DSM-IV Axis I Disorders,15 Childhood Trauma Questionnaire (CTQ),16 and other measures as described in previous analyses of GMV with subgroups of participants from the current sample, including assessment of cumulative adversity in the HCs, individuals with alcohol dependence and relapse, and sex differences in cocaine dependence.17-19 Standardized cutoffs recommended by the CTQ manual20 and used in prior studies21 were used to classify individuals into a group with CM if they exceeded cutoff scores on any 1 or more of the CTQ subscales (cutoff scores: emotional abuse, 13; physical abuse, 10; sexual abuse, 8; emotional neglect, 15; physical neglect, 10). Categorical groups for CM were used to ensure a focus on moderate to severe CM.
Participants with SUD completed a magnetic resonance imaging (MRI) scan between weeks 4 and 5 of abstinence. Participants with SUD were discharged from inpatient treatment soon after and returned for repeated face-to-face follow-up interviews at 14, 30, and 90 days after discharge, at which time relapse outcomes were evaluated using urine and breathalyzer samples and the Form 90 substance use calendar.22 The HCs completed all assessments over 2 or 3 appointments and the MRI scan was conducted in a separate, subsequent session. Breathalyzer and urine toxicology screens were conducted for HCs at each appointment.
MRI Data Acquisition
Participants were scanned on a 3-T scanner (TIM Trio; Siemens AG). Data for each participant consisted of a single sagittally acquired high-resolution T1-weighted magnetization-prepared rapid-acquisition gradient-echo scan (176 slices; 1-mm3 isotropic voxels; field of view, 256 × 256 mm; acquisition matrix, 256 × 256; repetition time, 2530 milliseconds; echo time, 3.66 milliseconds; flip angle, 7°).
Segmentation and Registration
Image segmentation and registration were performed in Statistical Parametric Mapping version 8 software (SPM8; Wellcome Trust Centre for Neuroimaging, University College London) using the VBM toolbox (VBM8; Department of Psychiatry, University of Jena). Resulting segmentations were validated visually23,24 to approximate the gold standard (ie, manual segmentation25,26); segmentation demonstrated appropriate face validity in all images. Rigid-body aligned segmentations for each participant were registered to an average template generated by diffeomorphic anatomical registration through exponentiated Lie algebra (DARTEL)27 using gray matter, white matter, and cerebrospinal fluid segmentations. The DARTEL-registered data were then affine transformed to Montreal Neurological Institute space, modulated, and smoothed with a 6-mm Gaussian filter. The default parameter settings were used for all steps of the DARTEL registration. Final outputs were smoothed, modulated gray matter segments (1.5-mm3 voxels).
Data Analysis
The GMV was regressed on CM group (dummy coded), SUD group (dummy coded), and any psychiatric history (dummy coded as present or absent) using a multiple regression model implemented in SPM8. Covariates controlled for participant age, sex, and total intracranial volume (TICV) in both cases,28 with the additional inclusion of the age-by-SUD interaction term. The TICV was calculated by voxelwise summation. While substance use duration has been shown to be a relevant variable to GMV changes in addiction,29 preliminary analysis suggested that it was not a significant covariate in our model; it was therefore excluded.
Whole-Brain Analysis
Whole-brain statistical analysis was conducted using topological false discovery rate (FDR) correction for multiple comparisons.30 Topological FDR ensures that the familywise error rate is equivalent to P < .05 for cluster-level inference. Data were height thresholded at P < .005, and subsequently extent thresholded for contiguously activated voxels, based on the SPM-generated FDR cluster threshold.30
Anatomical Localization
Anatomical regions within significant clusters were initially identified using the Automated Anatomical Labeling atlas.31 To acquire localized specificity of volumetric differences specific to subfields of medial temporal lobe structures, significant clusters were mapped to probabilistic cytoarchitectonic maps32 using the SPM Anatomy toolbox version 2.0.33,34
Volume Estimation
We extracted ROIs using the MarsBaR toolbox (http://marsbar.sourceforge.net/). Individual volumes were estimated for each cluster of interest with a modified version of the voxelwise summation used to estimate TICV.
Relapse Estimation
Relapse was assessed by face-to-face interview on days 14, 30, and 90 of the postdischarge period, with urine and breathalyzer samples at each interview and, where necessary, collateral informant report (ie, family members or significant others; for additional details, see the articles by Rando et al18 and Seo et al35).
The Kaplan-Meier (survival) estimator36 was used to examine time to relapse to any drug (ie, alcohol, cocaine, or cannabis) as a function of primary dependence group, duration of drug use, CM group, and whole-brain identified ROIs (for both SUD and CM). The Kaplan-Meier estimator was used to examine significant differences in relapse rates between groups because of its greater sensitivity to survival differences over early and short assessment periods.37
In addition to examining relapse rate, severity of relapse was examined. This variable was defined as days of use (in the 90-day follow-up period) of an individual’s most commonly used drug. Backward stepwise regressions examined whether any of the whole-brain identified GMV clusters were able to predict severity of relapse. As relapse is relatively easy to identify (dichotomous outcome), while quantity of use is more difficult to self-report,38 severity of relapse was examined only in individuals for whom there was additional information (ie, urine and breathalyzer data, secondary report, official records).
Results
Demographic Characteristics
Demographic, substance use, and individual characteristics are provided in Table 1 and Table 2. The HC and SUD groups significantly differed on age, education, and psychiatric history. Age and psychiatric history were included as covariates in the main model. Separate analyses included educational history as an additional covariate. Further analyses indicated that while psychiatric comorbidity was higher in the SUD group than in the HC group, psychiatric comorbidity was actually better accounted for by CM than SUD (eAppendix in Supplement).
Table 1.
Demographic Characteristics of Sample Subgroups
| No. (%) |
||||||
|---|---|---|---|---|---|---|
| HCs |
SUD |
|||||
| Characteristic | No CM (n = 73) |
CM (n = 25) |
No CM (n = 35) |
CM (n = 44) |
P Value | Group Differencesa |
| Male | 46 (63.0) | 14 (56.0) | 27 (77.1) | 26 (59.1) | .28 | NA |
|
| ||||||
| Age, mean (SD), y | 34.1 (1.6) | 33.4 (7.9) | 38.3 (7.2) | 37.9 (6.1) | .003 | 1 < 3, 4 |
|
| ||||||
| Education, mean (SD), y | 15.5 (2.4) | 15.2 (1.9) | 13.1 (2.1) | 12.4 (1.6) | <.001 | 1, 2 > 3, 4 |
|
| ||||||
| Minority race | 23 (31.5) | 12 (48.0) | 15 (42.9) | 19 (43.2) | .37 | NA |
|
| ||||||
| African American | 15 (20.5) | 8 (32.0) | 14 (40.0) | 19 (43.2) | ||
|
| ||||||
| Hispanic or Latino | 4 (5.5) | 2 (8.0) | 1 (2.9) | 0 | ||
|
| ||||||
| Other | 4 (5.5) | 2 (8.0) | 0 | 0 | ||
|
| ||||||
| Childhood Trauma Questionnaire score, mean (SD) |
31.6 (5.7) | 55.4 (16.7) | 32.5 (5.9) | 59.6 (15.4) | <.001 | 1, 3 < 2, 4 |
|
| ||||||
| Any psychiatric historyb | 4 (5.5) | 6 (24.0) | 3 (8.6) | 22 (50.0) | <.001 | 1, 3 < 2 < 4 |
|
| ||||||
| Depression | ||||||
|
| ||||||
| Lifetime | 4 (5.5) | 4 (16.0) | 1 (2.9) | 10 (22.7) | .008 | 1, 3 < 2, 4 |
|
| ||||||
| Current | 0 | 1 (4.0) | 0 | 1 (2.3) | .31 | NA |
|
| ||||||
| PTSD | ||||||
|
| ||||||
| Lifetime | 0 | 3 (12.0) | 1 (2.9) | 13 (29.5) | <.001 | 1, 3 < 2, 4 |
|
| ||||||
| Current | 0 | 2 (8.0) | 1 (2.9) | 10 (22.7) | <.001 | 1, 3 < 2, 4 |
|
| ||||||
| Anxiety disorder, excluding PTSD |
||||||
|
| ||||||
| Lifetime | 0 | 0 | 1 (2.9) | 8 (18.2) | <.001 | 1, 3 < 2 < 4 |
|
| ||||||
| Current | 0 | 0 | 1 (2.9) | 7 (15.9) | <.001 | 1, 3 < 2 < 4 |
Abbreviations: CM, childhood maltreatment; HCs, healthy controls; NA, not applicable; PTSD, posttraumatic stress disorder; SUD, substance use disorder.
Groups are indicated by numbers to facilitate comparison: 1 indicates HCs with no CM; 2, HCs with CM; 3, participants with SUD and no CM; and 4, participants with SUD and CM. Group differences represent significant post hoc differences at P < .05, controlled for multiple comparisons.
Psychiatric comorbidity is higher in participants with SUD than HCs, but there is no significant difference in comorbidity between HCs with CM and participants who have SUD with CM.
Table 2.
Substance Use Statistics for Individuals With Substance Use Disordera
| Variable | Value |
|---|---|
| Primary substance of abuse, No. (%) | |
|
| |
| Alcohol | 45 (57.0) |
|
| |
| Cocaine | 34 (43.0) |
|
| |
| Lifetime diagnosis, No. (%) | |
|
| |
| Alcohol use disorder | 69 (95.8) |
|
| |
| Cocaine use disorder | 57 (79.2) |
|
| |
| Cannabis use disorder | 56 (77.8) |
|
| |
| Lifetime alcohol, cocaine, and/or cannabis use, No. (%) | |
|
| |
| Substance of dependence only | 7 (9.7) |
|
| |
| Substance of dependence + 1 other substance | 20 (27.8) |
|
| |
| Substance of dependence + 2 other substances | 45 (62.5) |
|
| |
| Duration of substance abuse, mean (SD), y | |
|
| |
| Alcohol | 17.3 (8.1) |
|
| |
| Cocaine | 9.5 (6.8) |
|
| |
| Cannabis | 11.1 (7.4) |
Data were available for all 79 individuals with substance use disorder for primary substance of abuse but were available for only 72 individuals with substance use disorder for all other variables.
Whole-Brain GMV Differences
Controlling for age, sex, TICV, substance dependence, psychiatric history (ie, depression, anxiety disorder, and posttraumatic stress disorder), and age-by-SUD interaction, VBM results indicated that CM was associated with smaller GMV in a cluster that encompassed the left hippocampus as well as the parahippocampal and fusiform gyri (corrected P < .05; uncorrected P = .001; 1087 voxels) (Figure 1 and eTable 1 in Supplement). Close examination of the CM-related cluster revealed that 40.8% (443 voxels) of the cluster overlapped the parahippocampal gyrus, 38.4% (417 voxels) overlapped the fusiform gyrus, and 10.9% (118 voxels) overlapped the hippocampus. Childhood maltreatment was not associated with increased volume in any region. Probabilistic cytoarchitectural mapping using the SPM Anatomy toolbox version 2.033 revealed significant GMV reductions associated with CM in subregions of the parahippocampus (including presubiculum, parasubiculum, prosubiculum, subiculum, and entorhinal cortex), hippocampus (including cornu ammonis regions 1-3 and dentate gyrus), amygdala, and cerebellum (Table 3).
Figure 1. Gray Matter Volume Differences Related to Childhood Maltreatment.
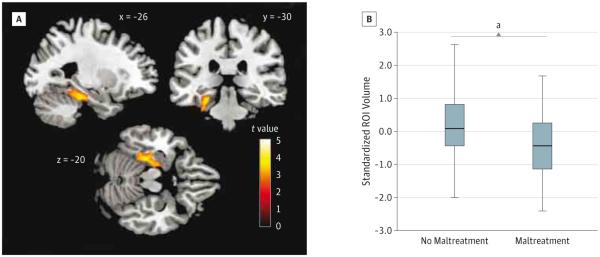
Using whole-brain voxel-based morphometry, childhood maltreatment was associated with lower mean gray matter volume, after controlling for substance use disorder, psychiatric history, age, sex, total intracranial volume, and substance dependence by age, in the left medial temporal lobe (1087 voxels; maximum: x = −20, y = −16, z = −26). A, The gray matter volume cluster predominantly includes regions of the hippocampus and parahippocampal and fusiform gyri. Statistical parametric maps have been height thresholded at P < .005 (t ≥ 2.61) and cluster thresholded using topological false discovery rate to set the overall error rate to P < .05. B, Box-and-whisker plot of estimated, standardized region-of-interest (ROI) cluster volumes by group (no trauma vs trauma). The shaded boxes indicate the 75th (top) and 25th (bottom) percentiles; horizontal lines in the middle of the boxes, the median (50th percentile); and whiskers, the minimum and maximum volumes.
aA t test revealed significant differences in the estimated volumes for the ROI between the 2 groups at P < .001.
Table 3.
Regional Localization of Significant Cluster Difference Related to Childhood Maltreatment Based on Probabilistic Cytoarchitectural Mappinga
| Anatomical Region | Voxels, No. | % of Cluster | % of Anatomical Region |
|---|---|---|---|
| Amygdala | |||
|
| |||
| Superficial nucleus | 3.6 | 0.3 | 1.9 |
|
| |||
| Basolateral nucleus | 0.9 | 0.1 | 0.2 |
|
| |||
| Cerebellum | |||
|
| |||
| Lobules I-IV | 3.6 | 0.3 | 0.2 |
|
| |||
| Lobule V | 75.3 | 6.9 | 3.1 |
|
| |||
| Lobule VI | 3.3 | 0.3 | 0.1 |
|
| |||
| Entorhinal cortex | 24.8 | 2.3 | 3.9 |
|
| |||
| Hippocampal-amygdala transitional area | 18.2 | 1.7 | 47.3 |
|
| |||
| Hippocampus | |||
|
| |||
| CA1 | 207.1 | 19.1 | 28.9 |
|
| |||
| CA2 | 5.4 | 0.5 | 4.0 |
|
| |||
| CA3 | 58.6 | 5.4 | 44.4 |
|
| |||
| Dentate gyrus | 7.8 | 0.7 | 2.4 |
|
| |||
| Parasubiculum | 26.0 | 2.4 | 57.2 |
|
| |||
| Presubiculum | 50.2 | 4.6 | 22.0 |
|
| |||
| Prosubiculum | 103.4 | 9.5 | 41.0 |
|
| |||
| Subiculum | 85.5 | 7.9 | 29.4 |
Abbreviation: CA, cornu ammonis.
Only regions that can be adequately assigned to specific anatomical regions are represented (ie, 62.0% of the total cluster). Notably, the probabilistic maps did not include aspects of the anterior fusiform gyrus.
The SUD status (a single group contrast of SUD vs no SUD) predicted decreased GMV across 4 clusters primarily in the following regions: (1) bilateral thalamus, (2) left midcingulate gyrus and bilateral supplementary motor area, (3) bilateral posterior cingulate gyrus and right cuneus and precuneus, and (4) right fusiform gyrus (Figure 2 and eTable 2 in Supplement). Whole-brain GMV interaction analysis (CM by SUD) resulted in no significant clusters. We examined SUD- and CM-related ROIs for all subgroups as well (eFigure 1 in Supplement); there were only main effects of CM and SUD.
Figure 2. Gray Matter Volume Differences Related to Substance Use Disorders.
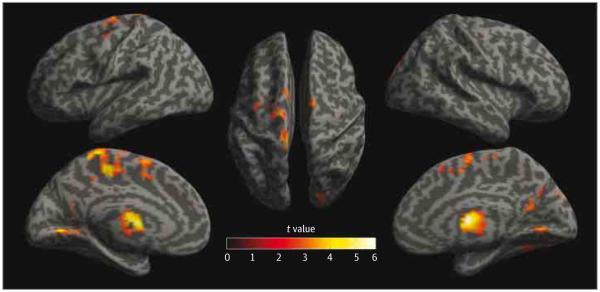
Using whole-brain voxel-based morphometry, substance use disorder was associated with lower mean gray matter volume, after controlling for childhood maltreatment, psychiatric history, age, sex, total intracranial volume, and substance use disorders by age, across 4 clusters. Gray matter volume differences are depicted as statistical parametric maps on rendered surfaces in the bilateral thalamus (1337 voxels; maximum: x = 6, y = −4, z = 10), left midcingulate gyrus and bilateral supplementary motor area (3899 voxels; maximum: x = −6, y = −31, z = 48), bilateral posterior cingulate gyrus and right cuneus and precuneus (2166 voxels; maximum: x = −5, y = −61, z = 7), and right fusiform gyrus (1157 voxels; maximum: x = 35, y = −73, z = −15). Statistical parametric maps have been height thresholded at P < .005 (t ≥ 2.61) and cluster thresholded using topological false discovery rate to set the overall error rate to P < .05.
Psychiatric history (psychiatric history vs no history) yielded no clusters that exceeded the FDR-corrected threshold. Post hoc analyses of the CM-identified ROI cluster by any psychiatric diagnosis and posttraumatic stress disorder alone revealed no significant differences by affective disorder history (eAppendix in Supplement). As previous analysis in the cocaine-dependent subsample showed sex differences in GMV,19 we also examined whether there was an interaction between CM and sex on GMV. No clusters exceeded an FDR-corrected threshold at P < .05. Analyses including education as an additional covariate indicated no effect on CM-related GMV findings but changes to SUD-related GMV findings (eAppendix and eFigure 2 in Supplement). Finally, secondary analyses examining continuous CTQ scores (with age, sex, and TICV as covariates) indicated significant GMV decreases in the bilateral medial temporal lobe (eAppendix, eFigure 3, and eTable 3 in Supplement).
Trauma-Related Effects on Prospective SUD Relapse Risk and Severity
Seventy-two of the 79 participants with SUD (91.1%) were successfully followed up during the 90-day period subsequent to treatment. Neither duration of primary substance use nor educational attainment were significant predictors of relapse and were therefore omitted from subsequent analyses. There was no difference in likelihood of relapse as a function of CM status ( = 1.14; P = .29). However, those with CM exhibited a significantly shorter time to any drug relapse than those with no CM (mean [SD], 27.60 [5.07] vs 42.03 [6.08] days, respectively; generalized Wilcoxon χ2 = 3.90; P = .048). Figure 3A shows survival function.
Figure 3. Relapse Rate and Severity by Trauma Group and Childhood Maltreatment–Related Gray Matter Volume Differences.
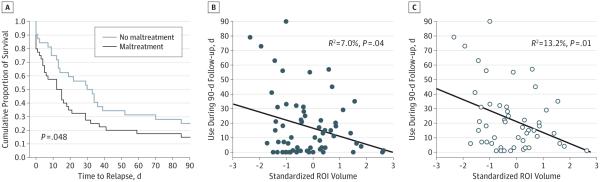
Among the subset of the substance-dependent sample for whom additional data were available (n = 72 [91.1%]), relapse time course was examined as a function of childhood maltreatment (A), and severity of relapse (defined as maximum number of days of drug use [alcohol, cocaine, and cannabis] during the 90-day follow-up) was examined as a function of childhood maltreatment–predicted gray matter volume differences in a whole-brain identified region of interest (ROI) (B and C). A, Significantly faster rate of drug relapse for participants with childhood maltreatment compared with those without childhood maltreatment (P value reflects the generalized Wilcoxon χ2 test). B, Correlation between ROI gray matter volume and severity of relapse in the entire subsample (n = 72). C, Relationship between ROI gray matter volume and severity of relapse among those individuals for whom severity of relapse was greater than 0 days (n = 52 [72.2% of subsample]).
None of the 4 SUD-identified or CM-identified clusters predicted time to relapse. However, the CM-related GMV estimate was significantly predictive of substance use severity after initial relapse. A backward stepwise regression indicated that GMV for the CM-related cluster was a significant predictor of days of use during the follow-up period (r = −0.265; F = 4.52; P = .04; R2 = 0.070). Decreasing GMV of the trauma-related cluster predicted increasing severity of use. Examining only those who relapsed (50 of 63 participants [79.4%]), the relationship was even stronger (r = −0.363; P = .01; R2 = 0.132) (Figure 3B and C).
Discussion
Using whole-brain VBM analyses, lower GMV in the left medial temporal lobe or limbic region was uniquely associated with CM. Probabilistic cytoarchitectural mapping revealed that the CM ROI included subfields of the hippocampal complex that are critically involved in neurogenesis (eg, cornu ammonis 3 and dentate gyrus7) and regulation of the hypothalamic-pituitary-adrenal axis (eg, subiculum39) and more generally contribute to heightened risk for anxious temperament.40 Notably, the regions identified overlap with a number of brain regions shown to be affected by early life stress in preclinical studies.41,42 Further, while CM itself was predictive of time to relapse, GMV differences in the CM-related limbic ROI (but not any of the ROIs identified in relation to SUD) were predictive of severity of relapse (measured in days of drug use during a 90-day follow-up period). The present findings represent the first documentation of the specific associations of CM-related limbic GMV alterations on the clinical course of SUD (alcohol, cocaine, and cannabis use disorders), demonstrating its prospective association to relapse severity in a defined episode of abstinence and early recovery. While our study focused on one of the most common sequelae of CM (ie, SUD43) and the relapsing nature of SUD, the impairments of this specific limbic region may generalize to other psychiatric conditions as well.44,45
Our findings are especially relevant for the neurobiological basis of CM and its role in addictive behaviors. Childhood adversity, including CM, is a major contributing factor to the development of addictive behaviors,43 although there are numerous methodological limitations in this area of research, not the least of which is the fact that SUDs are commonly related to neurobiological changes on their own.8,9,46 Interestingly, in this study, those GMV changes statistically associated with SUD were distinct from CM, and only 1 of the SUD-related clusters (located in the posterior cingulate, cuneus, and precuneus) replicated our previous analyses of cocaine-dependent19 and alcohol-dependent18 samples. Further, this study failed to show a significant interaction between CM and SUD on GMV. The findings also indicated that the CM-specific ROI was predictive of severity of relapse, while SUD-related ROIs were not. Thus, CM and SUD may have unique, albeit possibly additive, neurobiological effects. As previous research on the neurobiology of SUDs has often failed to consider CM, it may be the case that previous identifications of GMV deficits in SUD may actually relate to CM. Regular assessment of CM in addiction treatment settings may help clinicians identify those at greater risk for relapse, potentially permitting individually tailored treatments to address CM-related pathophysiology. As the current results used the CTQ clinical cutoff thresholds, they support use of these clinical cutoffs in the clinical setting to identify individuals with SUD who have CM-related pathophysiology for CM-related specialized services. Additionally, CM-related neurobiological changes in SUD reported herein may inform the development of new therapies to improve outcomes in individuals with both SUD and CM.
The neuroanatomical alterations related to CM may also have important functional implications. The present data suggest that CM is primarily associated with the structural integrity of the hippocampal complex. Broadly speaking, deficits in the hippocampal complex may contribute to altered learning mechanisms,47 especially in the context of reward-based learning.48 These deficits may create inflexibility with regard to rule-based learning47 and the emotion-memory interface,49 which interact with temperamental alterations40 to create predispositions for anxiety, depression, and addictive behaviors. There may be unique contributions of altered hippocampal complex structure and function to anxiety,50 depression,51 and/or addictions11; however, it seems more likely that these mechanisms are involved in an interactive, dynamic way to create shared vulnerability that may revolve around the processing of and response to stress.41
Childhood maltreatment in this study was retrospectively self-reported. Despite the inherent limitations of retrospective self-report, numerous studies have established the reliability and validity of retrospective self-report of childhood adversity and CM.52-57 Additionally, an impressive body of literature has established a link between retrospective report of early childhood deficits and psychiatric and physical illness.55,58 We also cross-sectionally assessed CM; thus, our results cannot speak to the timing and duration of CM. Further, the CTQ assesses only childhood abuse and neglect. Because there are important links between childhood adversity and a host of neurobiological and medical sequelae,55 future studies should examine a broader context of childhood adversity (eAppendix in Supplement).59
As our MRI collection was cross-sectional, all findings related to GMV are subject to questions about directionality in terms of relationships between variables. Our SUD sample was limited to individuals with alcohol, cocaine, and/or cannabis use disorders; hence, the findings may not generalize to individuals with opioid, methamphetamine, sedative, and any other SUDs. Our HC and SUD samples also differed on age, education, and psychiatric comorbidity, which were statistically accounted for in the analyses and did not appear to significantly affect the main findings pertaining to CM or CM-related risk for relapse.
Conclusions
Our findings indicate that CM is associated with altered structural integrity of the hippocampal complex. Such neuroanatomical specificity on the hippocampal complex provides an important translational link between the preclinical literature on early life stress and clinical research related to CM in at-risk and SUD samples. More importantly, we present the first evidence, to our knowledge, directly linking CM-related neurobiological effects to relapse risk and severity in SUD. These findings suggest that routine and thorough assessment of CM during adulthood and especially among individuals seeking treatment for substance abuse may be highly relevant in treatment planning for those with comorbid CM and SUD.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by grants P50-DA016556 (Dr Sinha), R01-AA13892 (Dr Sinha), and RL1-AA017539 (Dr Potenza) from the National Institutes of Health, Roadmap Fund for Medical Research grants UL1-DE019586 (Dr Sinha), PL1-DA024859 (Dr Sinha), and UL1-RR024139 (Yale Clinical and Translational Science Award) from the National Institutes of Health, and the State of Connecticut Department of Mental Health and Addiction Services.
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Audio Interview at jamapsychiatry.com
Supplemental content at jamapsychiatry.com
Author Contributions: Dr Sinha had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Rando, Tuit, Sinha.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Van Dam, Rando, Sinha.
Critical revision of the manuscript for important intellectual content: Van Dam, Potenza, Tuit, Sinha.
Statistical analysis: Van Dam, Rando, Sinha.
Obtained funding: Potenza, Sinha.
Administrative, technical, or material support: Rando, Tuit, Sinha.
Study supervision: Tuit, Sinha.
Conflict of Interest Disclosures: Dr Potenza has consulted for and advised Boehringer Ingelheim; has consulted for and has financial interests in Somaxon; has received research support from the National Institutes of Health, Veterans Affairs, Mohegan Sun Casino, National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, Forest Laboratories, Psyadon, Ortho-McNeil, Oy-Control/Biotie, and GlaxoSmithKline; has participated in surveys, mailings, or telephone consultations related to drug addiction, impulse control disorders, or other health topics; has consulted for law offices and the federal public defender’s office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has given academic lectures in grand rounds, continuing medical education events, and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. Dr Sinha is on the scientific advisory board for Embera NeuroTherapeutics.
Disclaimer: The contents of the article are the sole responsibility of the authors and do not necessarily represent the official views of any of the funding agencies.
REFERENCES
- 1.Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry. 2010;197(5):378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the Adverse Childhood Experiences Study. Pediatrics. 2003;111(3):564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- 3.Westermeyer J, Wahmanholm K, Thuras P. Effects of childhood physical abuse on course and severity of substance abuse. Am J Addict. 2001;10(2):101–110. doi: 10.1080/105504901750227769. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher JA, Coffey SF, Stasiewicz PR. Symptom severity, alcohol craving, and age of trauma onset in childhood and adolescent trauma survivors with comorbid alcohol dependence and posttraumatic stress disorder. Am J Addict. 2006;15(6):422–425. doi: 10.1080/10550490600996355. [DOI] [PubMed] [Google Scholar]
- 5.Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Hanson JL, Chung MK, Avants BB, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A. 2012;109(9):563–572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335(6068):601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 9.Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 11.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 12.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage. 2000;11(6):805–821. doi: 10.1006/nimg.2000.0582. pt 1. [DOI] [PubMed] [Google Scholar]
- 13.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10(4):372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 14.Martins SS, Gorelick DA. Conditional substance abuse and dependence by diagnosis of mood or anxiety disorder or schizophrenia in the US population. Drug Alcohol Depend. 2011;119(1-2):28–36. doi: 10.1016/j.drugalcdep.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- 16.Bernstein D, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-report Questionnaire and Manual. Psychological Corp; San Antonio, TX: 1998. [Google Scholar]
- 17.Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72(1):57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rando K, Hong KI, Bhagwagar Z, et al. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry. 2011;168(2):183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rando K, Tuit K, Hannestad J, Guarnaccia J, Sinha R. Sex differences in decreased limbic and cortical grey matter volume in cocaine dependence: a voxel-based morphometric study. Addict Biol. 2013;18(1):147–160. doi: 10.1111/adb.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Heim C, Nater UM, Maloney E, Boneva R, Jones JF, Reeves WC. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch Gen Psychiatry. 2009;66(1):72–80. doi: 10.1001/archgenpsychiatry.2008.508. [DOI] [PubMed] [Google Scholar]
- 22.Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- 23.Cerasa A, Messina D, Nicoletti G, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. AJNR Am J Neuroradiol. 2009;30(6):1240–1243. doi: 10.3174/ajnr.A1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies RR, Scahill VL, Graham A, Williams GB, Graham KS, Hodges JR. Development of an MRI rating scale for multiple brain regions: comparison with volumetrics and with voxel-based morphometry. Neuroradiology. 2009;51(8):491–503. doi: 10.1007/s00234-009-0521-z. [DOI] [PubMed] [Google Scholar]
- 25.Bouix S, Martin-Fernandez M, Ungar L, et al. On evaluating brain tissue classifiers without a ground truth. Neuroimage. 2007;36(4):1207–1224. doi: 10.1016/j.neuroimage.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Boer R, Vrooman HA, Ikram MA, et al. Accuracy and reproducibility study of automatic MRI brain tissue segmentation methods. Neuroimage. 2010;51(3):1047–1056. doi: 10.1016/j.neuroimage.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Barnes J, Ridgway GR, Bartlett J, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53(4):1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated grey matter changes with prolonged addiction and extended abstinence in cocaine users. PLoS One. 2013;8(3):e59645. doi: 10.1371/journal.pone.0059645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44(1):62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 32.Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210(5-6):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 33.Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 34.Eickhoff SB, Paus T, Caspers S, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 35.Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70(7):727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 37.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient, II: analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison L, Hughes A. The Validity of Self-reported Drug Use: Improving the Accuracy of Survey Estimates. National Institute on Drug Abuse; Rockville, MD: 1997. [PubMed] [Google Scholar]
- 39.O’Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat. 2005;207(3):271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oler JA, Fox AS, Shelton SE, et al. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466(7308):864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 42.McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(suppl):38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green JG, McLaughlin KA, Berglund PA, et al. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 48.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 50.Leonardo ED, Hen R. Anxiety as a developmental disorder. Neuropsychopharmacology. 2008;33(1):134–140. doi: 10.1038/sj.npp.1301569. [DOI] [PubMed] [Google Scholar]
- 51.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 52.Dill DL, Chu JA, Grob MC, Eisen SV. The reliability of abuse history reports: a comparison of two inquiry formats. Compr Psychiatry. 1991;32(2):166–169. doi: 10.1016/0010-440x(91)90009-2. [DOI] [PubMed] [Google Scholar]
- 53.Brewin CR, Andrews B, Gotlib IH. Psychopathology and early experience: a reappraisal of retrospective reports. Psychol Bull. 1993;113(1):82–98. doi: 10.1037/0033-2909.113.1.82. [DOI] [PubMed] [Google Scholar]
- 54.Bifulco A, Brown GW, Lillie A, Jarvis J. Memories of childhood neglect and abuse: corroboration in a series of sisters. J Child Psychol Psychiatry. 1997;38(3):365–374. doi: 10.1111/j.1469-7610.1997.tb01520.x. [DOI] [PubMed] [Google Scholar]
- 55.Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 57.Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry. 2004;45(2):260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 58.Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci U S A. 2010;107(19):8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strand VC, Sarmiento TL, Pasquale LE. Assessment and screening tools for trauma in children and adolescents: a review. Trauma Violence Abuse. 2005;6(1):55–78. doi: 10.1177/1524838004272559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


