Abstract
The development of an on-animal separation-based sensor that can be employed for monitoring drug metabolism in a freely roaming sheep is described. The system consists of microdialysis sampling coupled directly to microchip electrophoresis with electrochemical detection (MD-ME-EC). Separations were accomplished using an all-glass chip with integrated platinum working and reference electrodes. Discrete samples from the microdialysis flow were introduced into the electrophoresis chip using a flow-gated injection approach. Electrochemical detection was accomplished in-channel using a two-electrode isolated potentiostat. Nitrite was separated by microchip electrophoresis using reverse polarity and a run buffer consisting of 50 mM phosphate at pH 7.4. The entire system was under telemetry control. The system was first tested with rats to monitor the production of nitrite following introduction of nitroglycerin into the subdermal tissue using a linear probe. The data acquired using the on-line MD-ME-EC system was compared to that obtained off-line analysis by liquid chromatography with electrochemical detection (LC-EC), using a second microdialysis probe implanted parallel to the first probe in the same animal. The MD-ME-EC device was then used on-animal to monitor the subdermal metabolism of nitroglycerin in sheep. The ultimate goal is to use this device to simultaneously monitor drug metabolism and behavior in a freely roaming animal.
Keywords: microchip electrophoresis, microdialysis, electrochemical detection, nitrite, sheep, nitroglycerin, skin
INTRODUCTION
Drugs are known to influence both physiology and behavior. Pharmaceutical compounds for treatment of behavioral disorders including ADHD, drug addiction, and depression are continuously under development.1–3 However, some pharmaceuticals have been shown to produce undesirable behavioral effects such as those observed with many of the pharmaceuticals used to treat insomnia.4 Therefore, methods that can be employed for the simultaneous monitoring of drug metabolism and disposition along with neurotransmitter release and behavior are important for drug development. In vivo voltammetry and analyte-specific biosensors have been used extensively to monitor the release of neurotransmitters as well as changes in the concentrations of other endogenous molecules, such as glucose, in freely moving animals.5, 6 These techniques can provide information regarding the relative concentrations of specific substances in the brain, blood, or other tissues with temporal resolution ranging from milliseconds to days. However, the primary disadvantage of these approaches is that they are generally restricted to a single analyte and are therefore not useful for monitoring drug metabolism.
Microdialysis sampling (MD) is a technique that has been used extensively to monitor the metabolism and disposition of drugs in a variety of tissues, as well as for monitoring neurotransmitter release in the brain.7–10 The primary advantage of microdialysis over in vivo voltammetry and biosensors is that it is a generic sampling method that can be used to collect low molecular weight compounds from the extracellular fluid of interest, which can then be analyzed by almost any analytical method. Therefore, it is possible to customize the analytical method for the specific analyte(s) of interest. If a separation-based analytical method is employed, the result is a “separation-based sensor” that makes it possible to monitor several analytes concurrently. Depending on the volume requirements of the analytical method, it is also possible to analyze the same sample using multiple analytical methods.
Commercially available systems, such as the Raturn® (BASi, West Lafayette, IN), allow simultaneous microdialysis sampling and behavior measurements in rats and mice. If multiple microdialysis probes are used, it is possible to simultaneously monitor blood, brain, and tissue concentrations of the drug and endogenous molecules in a single animal. An excellent example of this is the report by Huff and Davies in which they used multiple probes to monitor the transport of Ritalin across the blood-brain barrier along with brain dopamine concentrations.11 In that study, they were also able to correlate blood and brain drug concentrations with the activity of the rat using a motion detector attached to the Raturn®. However, although the rat was freely moving in these studies, it was still connected to the MD system using a liquid swivel and was therefore not freely roaming.
A system that permits a rat to be freely roaming during microdialysis sampling was reported by Cooper et al.12 In this approach, an osmotic pump implanted under the skin of the rat was used to deliver the perfusate through the probe at a flow rate of approximately 200 nL/min. On-rat collection was accomplished using a plastic vial that was attached to the outlet of the probe. With this system it was possible to collect neurochemical data with the rat outside of the Raturn® bowl. However, the collection vial had to be manually removed from the rat to obtain a sample for analysis. This limited the temporal resolution that could be obtained with this approach.
Capillary electrophoresis (CE) has been shown to be an ideal analytical method for the analysis of microdialysis samples due to its small sample volume requirements and fast separation times.13, 14 The pL-to-nL volume requirements of CE make it possible to monitor biochemical events using microdialysis with good temporal resolution.15, 16 The on-line coupling of microdialysis to capillary electrophoresis was first reported in 1994; it provided the added advantage of eliminating manual manipulation of submicroliter samples, leading to better injection precision and the ability to perform near real-time monitoring.17 The use of capillary electrophoresis also made it possible to independently choose the separation mode and the detector that was best suited for the analytes of interest. These separation-based sensor sensors have been used for a variety of applications, including the investigation of drug metabolism and neurotransmitter release13–15, 18–20.
The goal of the present work was to develop an on-animal separation-based sensor that could be used for the simultaneous monitoring of drug metabolism and behavior in freely roaming animals. Microchip electrophoresis (ME) is a miniaturized version of CE that has shown considerable promise for on-animal measurements. Chips are small and planar, making them easier to integrate into a portable analysis system. Also, the chips used for ME generally require lower total voltages for the separation than those used for CE. This makes it possible to use miniaturized high voltage power supplies and detectors as part of the system.
Several approaches have been reported for coupling microdialysis to electrophoretic separations on chip.13, 21 The first paper employed a flow-gated interface to inject picoliter volumes from the microdialysate perfusate into the separation channel.22 More recently, pneumatic injection valves23–25 and segmented flow26 have been employed for this purpose. The most common detection scheme for MD-ME includes on-chip derivatization with a fluorophore and laser-induced fluorescence detection.24, 26–30
Electrochemical detection (EC) has been less frequently explored for MD-CE or MD-ME. In 1999, Zhou et al. described an online MD-CE-EC system to monitor the transdermal delivery and metabolism of nicotine.18 The Martin group was the first to report the coupling of microdialysis to microchip electrophoresis with electrochemical detection using a PDMS based pneumatic actuated valving system.25 They used MD-ME-EC to monitor catecholamine release from cultured PC 12 cells with a carbon-based working electrode.
Recently, we reported the development of an all-glass chip containing an embedded platinum working electrode for MD-ME-EC.31 This chip employs flow-gated injection, which relies on precise timing of the application of potentials to different reservoirs. This injection mode is easier to miniaturize and control remotely than approaches that employ pneumatic valves or droplets to introduce the sample into the chip and is, therefore, more suitable for on-animal experiments. Electrochemical detection has the additional advantage for on-animal applications in that the electrodes and potentiostat can easily be miniaturized.
Microdialysis studies have been performed in sheep to study maternal behavior, breeding and drug metabolism.32–37 Sheep have also been used as an animal model for social behavior.38, 39 In this paper, a miniaturized separation-based sensor using microdialysis coupled to microchip electrophoresis with electrochemical detection for on-animal sensing is described. The system consists of an on-line interface to couple microdialysis to microchip electrophoresis, high voltage power supplies, and an electrically isolated potentiostat. The instrument is controlled and data are collected remotely using a Bluetooth® interface and a laptop PC. The system was demonstrated with sheep as a model subject by monitoring nitrite production during subdermal delivery of nitroglycerin. The complete system, which is about the size of a lunchbox, was mounted on the back of the sheep. This system opens up the possibility of simultaneous and continuous monitoring of drug metabolism and behavior in freely roaming animals.
EXPERIMENTAL SECTION
Materials and Reagents
Calcium chloride, disodium phosphate heptahydrate, magnesium chloride, monosodium phosphate monohydrate, sodium chloride, sodium nitrite, tetrabutylammonium hydroxide (TBAOH), boric acid, and potassium chloride were purchased from Sigma-Aldrich (St. Louis, MO). Acetone, hydrochloric acid, hydrofluoric acid, hydrogen peroxide, isopropyl alcohol, methanol, nitric acid, and sulfuric acid were purchased from Fisher Scientific (Pittsburgh, PA). DEA-NONate was purchased from Cayman Chemical (Ann Arbor, MI). Ultrapure water was generated using a Millipore Synthesis (18 mOhm) A10 system (Millipore, Kansas City, MO). Chrome and AZ1518 photoresist-coated soda-lime glass blanks were obtained from Nanofilm (West Lake Village, CA). AZ® 300 MIF was purchased from Capitol Scientific, Inc. (Austin, TX). Chrome etchant CR-7S was purchased from Cyantek Corp. (Fremont, CA). Platinum (Pt) and titanium (Ti) sputtering targets were purchased from the Kurt J. Lesker Company (Jefferson Hills, PA). Colloidal silver (Ag) was purchased from Ted Pella, Inc. (Redding, CA). Nitroglycerin injectable USP (5 mg/mL) was obtained from American Regent, Inc. (Shirley, NY). Lidocaine HCl 2% USP injectable was purchased from Bimeda (Oakbrook Terrace, IL)
Microchip Fabrication
The all-glass microfluidic devices with platinum working and reference electrodes embedded into the glass substrate were fabricated in-house at the Adams Institute cleanroom facilities as described previously.31 Briefly, the microfluidic channels and integrated electrode designs were drawn using AutoCad software (Autodesk, San Rafael, CA) to produce a negative tone mask transparency that was printed by Infinite Graphics Inc. (Minneapolis, MN). The chrome and AZ1518 photoresist-coated soda-lime glass blanks (Nanofilm, Westlake Village, CA) were photolithographically patterned using an i-line UV flood source (ABM Inc., Scotts Valley, CA) with an exposure of 86 mJ/cm3 and developed in an MIF 300 developer for 30 s (Integrated Micro Materials, Argyle, TX). Once the AZ® 1518 photoresist was developed and dried, the exposed chrome was etched using chrome etchant (CR-7S, KMG Chemicals, Houston, TX.).
In the top microfluidic channel plate, fluid wells and electrode access holes were drilled using a 1.55 mm diamond drill bit (TrueBite, Inc., Vestal, NY) mounted in a Dremel drill press. The channels in the plate were then etched at a rate of 5 µm/min in a 20:14:66 solution (49% hydrofluoric acid: concentrated nitric acid: water) to a depth of 15 microns. Etch depths were verified using an Alpha-Step 200 stylus profilometer (KLA-Tencor, Milpitas, CA). The patterned and etched glass blanks were then cut into individual chips using a tungsten carbide cutting wheel, resulting in four chips per plate. Following a thorough water rinse, the etched plates were placed in an acetone bath to dissolve the remaining AZ 1518 photoresist. The remaining chromium was then removed from each piece using chrome etchant.
The bottom electrode plate was fabricated by etching the glass plate in 10:1 buffered oxide etchant (VWR International, Rador, PA) with an etch rate of 0.35 µm min−1 to produce a trench approximately 500 nm deep for deposition of the platinum electrodes. The working electrode width was patterned to 15 µm with the length of the electrode being defined by the microfluidic channel width. The Pt reference electrode was 300 microns wide. The glass plate was placed into the vacuum chamber of an Axxis DC Magnatron sputtering system (Kurt J. Lesker Co., Jefferson Hills, PA) and a 40-nm adhesion layer of titanium was deposited, followed by a 460-nm layer of platinum. The excess metal was lifted off in an acetone bath. The remaining chromium layer was removed from each piece using CR-75 etchant.
All devices used in this study were bonded using the calcium-assisted bonding step previously reported by Allen et al.40 Briefly, during the calcium-assisted bonding step, the substrate surfaces were washed with a 5% Alconox™ solution using a fiber wipe to gently scrub each plate with the solution. The substrates were then washed again three times in a 5% Alconox™/5% calcium acetate solution. The plates were rinsed with water while the electrodes and the microfluidic channels were roughly aligned by hand. The plates were then aligned under a microscope so that the edge of the working electrode was positioned no more than five microns into the end of the separation channel. The assembled chip was clamped together using binder clips to ensure that the electrode alignment was not disturbed during processing. The chip was then placed in a low temperature oven (Lindberg/Blue-M, SPX Thermal Product Solutions, Riverside, MI) at 65°C for 1 h, after which time the temperature was increased to 110°C for a minimum of 2 h. The assembled chip was inspected for proper electrode alignment and for areas of nonspecific binding identified by Newton rings. In the case of electrode misalignment or the formation of Newton rings, the chip were easily taken apart and reassembled by repeating the procedure above until all requirements were met. After assembling a chip in this manner, it was placed in a programmable muffle furnace (750 Series, Fisher Scientific) for a full thermal bonding at 630°C for 3 hours.
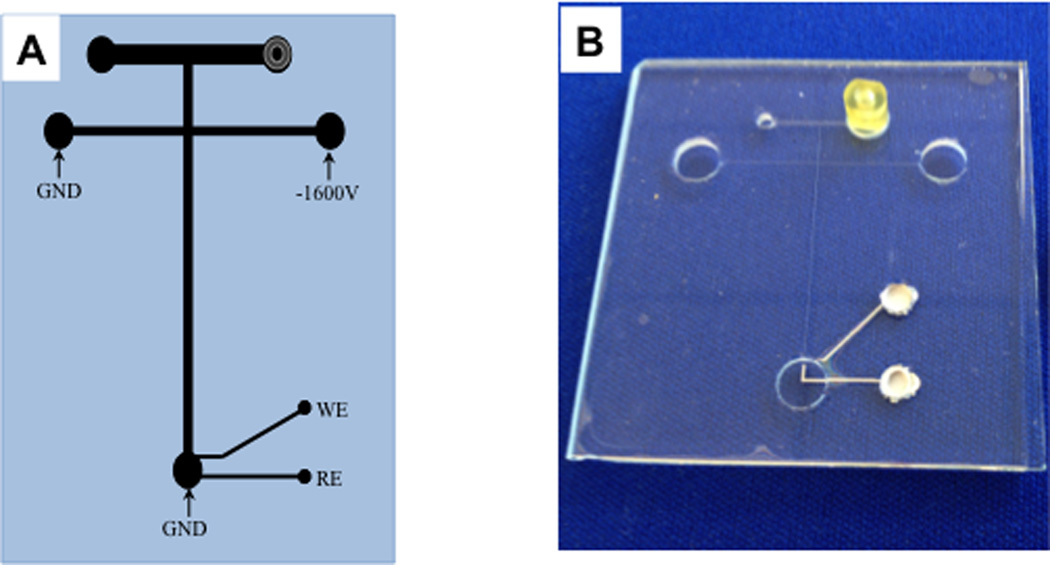
After a batch of microchips was fabricated, each chip was prepared for use. Using a two-part adhesive (Labsmith, Livermore, CA), Labsmith port connectors were bonded over one of the access holes to the microdialysis sampling channel. Colloidal silver (Ted Pella, Inc.) was then used to fill the electrode access holes, making a solid contact between the potentiostat and the platinum working and reference electrodes. (Figure 1)
Figure 1.
Chip used for MD-ME-EC. A. Schematic of the “double-T” microchip design with electrochemical detection used in these studies. The separation channel was 2.5 cm long and the side arm were 1.3 cm long. Channels were 15 µm deep × 40 µm wide. The microdialysis flow-through channel was 1.5 cm long, 15 µm deep, and 500 µm wide. A 15µm platinum working electrode and 300 µm platinum reference electrode were employed. B. Photo of the microfluidic device.
Instrumental Set-up
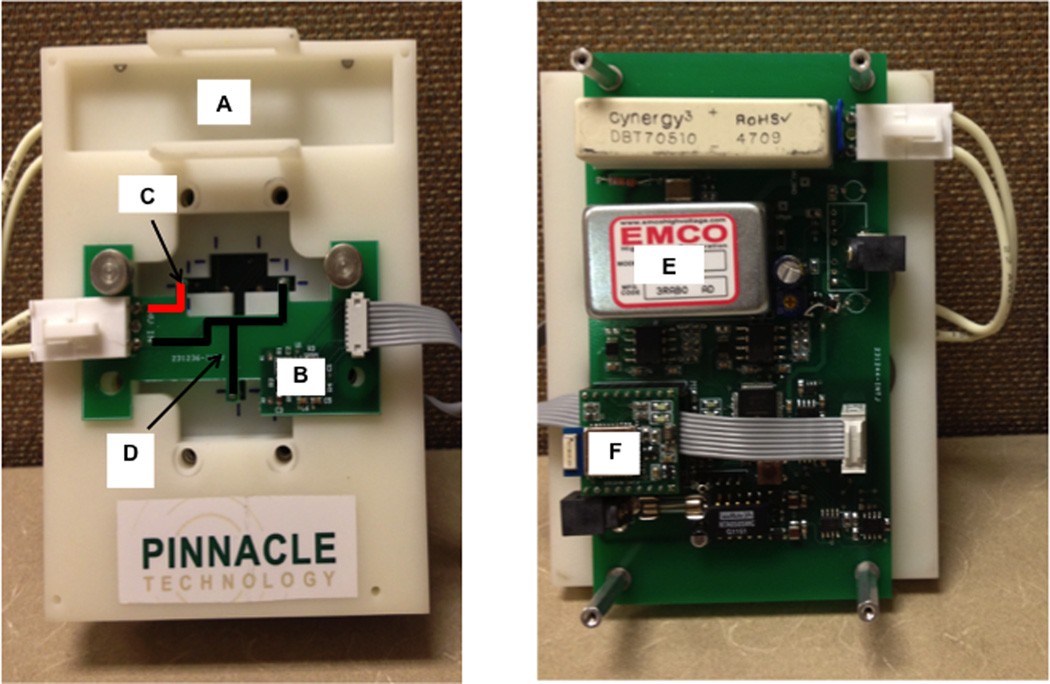
The hardware for the portable analysis system is shown n Figure 2 and consisted of an EMCO high voltage power supply (Model CA20N-5, EMCO High Voltage Corporation, Sutter Creek, CA), two external adjustable voltage (3–19 V) Tekkeon laptop batteries (Model MP3450, Tekkeon Inc., Irvine, CA), and an electrically isolated potentiostat set at a data sampling rate of 30 Hz. Data collection and device control were accomplished using integrated telemetry via Bluetooth® module Mitsumi WML-C46AHR (Mitsumi, Tokyo, Japan). All data were collected and analyzed using a modified version of the Sirenia® software suite (Pinnacle Technology, Lawrence, KS).
Figure 2.
Basic components of the MD-ME-EC system hardware. (A) holder for chip and microdialysis pump that also serves as a mount for all major electrical components, (B) electrically isolated potentiostat, (C) high voltage lead highlighted in red, (D) ground connectors highlighted in black, (E) high voltage power supply, and (F) Bluetooth® module.
The top plate of an epoxy-based photopolymer resin holder (Harvest Technologies, Belton, TX) secured the microfluidic device to the platform, which held the primary control board and high voltage power supply (Figure 2A). A smaller board with a built-in Pinnacle isolated potentiostat (Figure 2B) and high voltage leads and ground leads (Figures 2C and 2D) was secured onto the top plate and placed into contact with the microfluidic device. The isolated potentiostat was telemetry controlled via Bluetooth® (Figure 2F), with an applied voltage range between 0.1 and 1.2 volts. The potentiostat was coupled to the microfluidic device with spring-loaded gold contact pins (MILL-MAX, Oyster Bay, NY) connecting the potentiostat to the contact pads fabricated into the working and reference electrodes. The electrochemical detection cell was a two-electrode system, consisting of a 15 micron-wide platinum working electrode placed in in-channel configuration with a 300 µm embedded platinum reference electrode. The working electrode potential was set at +1.05 V versus the platinum counter (pseudoreference) electrode unless otherwise indicated.
Three leads were built into the smaller board. One lead was used to apply the voltage generated by the EMCO power supply (potential range between −200 and −1800 volts) and the other two leads were connected to ground. Platinum wire was used to make electric contact between the built-in leads and the microfluidic device. The high voltage lead was placed into the buffer reservoir and held at a potential of −1600 V for reverse polarity experiments. The ground leads were placed in the buffer waste and the sample waste.
Sampling, Buffer, and Injection Parameters
All sampling for analysis using the microfluidic device occurred by either direct infusion or microdialysis sampling. For direct infusion, one end of a 15 cm 1/32 × 0.005 PEEK tubing was coupled to the chip using a 1/32 LabSmith port connector. The other was directly attached to the CMA 107 syringe pump (CMA, N. Chelmsford, MA) using tubing connectors. Microdialysis sampling was accomplished using a 30 kDa 1 cm BASi loop microdialysis probe (BASi). Each side of the 1 cm semipermeable PAN membrane was attached to 16 cm of fluorinated ethylene propylene (FEP) tubing, which was cut to the desired length. The FEP tubing from the probe was connected to the CMA 107 syringe pump. To couple the probe to the chip, approximately 5 cm of the 16 cm of the FEP tubing was removed and replaced with an equivalent amount of PEEK tubing that was connected using 1/32 LabSmith connectors and fittings (LabSmith).
Before each experiment, a conditioning procedure was performed as described previously.31 The chip was then secured in the Pinnacle board chip holder (Pinnacle Technology) shown in Figure 2. In these experiments, the syringe pump was set at a flow rate of 1 µL/min, and sample introduction and gating was performed as previously described.22, 29, 31 The potential applied across the separation channel was set to −1600 V in order to establish a gate and overcome the hydrodynamic pressure induced by the pump. The high voltage was floated for 1 s to achieve an injection time of 1 s.
Several different perfusate compositions were employed during the course of this study. The ruggedness experiments that were conducted early in the experimental process employed a perfusate and a separation buffer consisting of 10 mM borate buffer containing 2 mM TTAB. A perfusate and separation buffer consisting of 50 mM phosphate buffer at pH 7.4 was used for the reproducibility experiments as well as for monitoring the production of nitrite from DEA-NONOate, as shown in the supporting information. In all other experiments, a perfusate of 50 mM phosphate buffer with 119 mM NaCl at pH 7.4 was used. For all animal experiments a buffer consisting of 50 mM phosphate pH 7.4 was employed for ME with a separation voltage of −1600 V. The working electrode was set at +1.05V vs. the Pt reference electrode unless otherwise indicated.
On-line MD-ME-EC Rat Studies
Male Sprague-Dawley rats weighing 300–350 g were used for these experiments. All experiments were performed in accordance with regulations of the Institutional Animal Care and Use Committee (IACUC) at the University of Kansas, which operates with accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Animals were acclimated for 7 days prior to the experiment, and all experiments were performed during the following week.
The rat was induced into a light plane of anesthesia using an isoflurane chamber before being given a ketamine-xylazine-acepromazine cocktail (KXA) via intraperitoneal injection. The animal was prepared for surgery by shaving and cleaning a three-inch by one-inch area on the left dorsal side of the midline between the anterior and posterior of the animal. Two 1-cm BASi loop microdialysis probes were implanted subcutaneously parallel to each other approximately 1.5 cm apart. One probe was used for direct analysis using the MD-ME-EC system and the second probe was used for sample collection for off-line analysis on a conventional LC-EC system. A nose cone was used to maintain anesthesia by inhalation of isoflurane using a vaporizer. The nitroglycerin solutions used for these and other animal studies were prepared from a 5 mg/mL stock solution of the drug formulated in an ethanol (30%) and polyethylene glycol (30%) solution (American Regent, Inc.).
A 1-h baseline microdialysis perfusion was performed using a solution consisting of 50 mM phosphate buffer with 119 mM NaCl at pH 7.4 pumped through the probe at a flow rate of 1 µL/min. After baseline monitoring, the subcutaneous delivery of nitroglycerin commenced, immediately followed by a 1–2 hour sampling period. This was accomplished by switching syringes from the separation buffer to a solution of 50 mM phosphate buffer and 119 mM NaCl containing 4.8 mg/mL of nitroglycerin. To estimate the concentration of nitrite being produced in the in vivo studies, the on-line ME-EC system was calibrated off-line using direct perfusion before the animal experiments began, using standard solutions of nitrite ranging from 2.5 to 25 µM prepared in 50 mM phosphate buffer with 119 mM NaCl at pH 7.4. Samples for analysis by LC-EC were collected off-line in 10 mL aliquots from a separate probe implanted parallel to that for the on-line system.
In vivo Analysis in Sheep
All animal protocols involving sheep were approved by the Purdue Animal Care and Use Committee and were conducted at the Purdue University College of Veterinary Medicine using donated female domesticated sheep. The sheep were secured in a small calf cart for ease of probe implantation and in order to properly secure a “double back” harness (Ruffwear Inc., Bend, OR) and mount the complete analysis system on the animal. The 1-cm BASi loop microdialysis probe implant site was prepared by shaving and cleaning an area on the left dorsal side of the sheep before the harness and system were secured to the animal. Once the system was secured on the animal, the implantation site was cleansed and a local anesthetic of Lidocaine HCl 2% USP injection (Bimeda) was administered. Two half-inch incisions were made into the skin approximately 3 cm apart, and two separate microdialysis probes were implanted parallel to one another through the incision points. As described above for the rat experiments, one probe was used for on-line analysis and the other for sample collection for off-line analysis by LC-EC. A 1-h baseline perfusion was performed first using 50 mM phosphate with 119 mM NaCl at pH 7.4 at a flow rate of 1 µL/min. Following baseline monitoring, the perfusate was changed to a solution containing 4.8 mg/mL of intravenous nitroglycerin solution for 2 h. The perfusion of nitroglycerin was started by switching syringes to a solution of 50 mM phosphate buffer and 119 mM NaCl with 4.8 mg/mL of nitroglycerin. Dialysate samples were collected from both probes simultaneously. Samples from the first probe were were either analyzed immediately on-line by MD-ME-EC while those from the parallel probe were collected off-line every 10 min in a centrifuge tube, immediately placed on dry ice and then stored in a −80 °C freezer for later analysis by LC-EC.
Off-line Analysis of Nitrite using Liquid Chromatography/Electrochemistry
Liquid chromatographic analysis of the off-line samples was accomplished using a Shimadzu® LC-20AD pump operating at a flow rate of 0.4 mL/min−1. A Phenomenex® Synergi Hydro-RP 4 µm 80Å 2 × 150 mm C-18 column was employed, and the mobile phase consisted of 1 mM TBAOH and 15 mM H2SO4 at pH 4.0 for the separation of nitrite. A 2 µL Rheodyne® 7725i injection valve was used (IDEX Health & Science, Oak Harbor, WA). Detection was accomplished using a BASi amperometric detector Model LC-4C (BASi) with a glassy carbon electrode at an applied potential of +1.025 V vs. a Ag/AgCl reference electrode (BASi). A calibration curve from 2.5 to 50 µM was obtained prior to analysis for quantitation.
RESULTS AND DISCUSSION
Device Development and Performance
The microfluidic devices used in these studies were constructed from soda lime glass using conventional photolithographic procedures.31 Soda lime glass is softer than the more commonly used borosilicate or quartz glass substrates. It is easily etched and can be bonded at lower temperatures with shorter bonding times. Because soda lime is easier to etch than quartz, the relatively shallow depths of the electrode patterns (500 nm) were simpler to fabricate with this substrate. Bonding at lower temperatures also allowed the electrode to go through the thermal bonding process without damaging the electrode material. Figure 1 shows the overall chip design, including the dimensions and electrode placement. The two-electrode electrochemical detection cell consisted of a 15-micron-wide platinum working electrode placed in the in-channel configuration with a 300-micron embedded platinum reference electrode.
The chip contained a double-T interface that has been has been previously described for coupling microdialysis sampling to the microchip electrophoresis system.22 The chips used in these studies employed flow-gated injection to introduce sample into the separation channel. This type of injection procedure was adapted from Lin et al.,41 and has been used with other chip designs previously described by our group.22, 28, 29, 31 To briefly explain, a syringe pump was used to deliver the dialysate at 1 µL/min through the 500 µm-wide flow channel at the top of the device. To prevent the sample from prematurely entering the separation channel, a gate was established by applying a voltage at the buffer reservoir to the buffer and sample waste reservoirs. In these experiments, a voltage of −1600 V was used, which generated sufficient EOF to overcome the hydrodynamic force being applied by the syringe pump. Injection of the microdialysis sample into the separation channel was accomplished by floating the high voltage for 1 s. This temporarily eliminated the EOF, which allowed the hydrodynamic pressure to become the dominant force inside the microfluidic device and introduced a discrete plug of sample into the separation channel. The gate was then reestablished by reapplying the high voltage at the buffer reservoir. The injection time of 1s defined the sample plug size that was introduced into the separation channel.
The overall reproducibility of the MD-ME-EC chip and the gated injection process was evaluated by running the system in the direct infusion mode continuously for 24-h. The migration times and peak heights for a 1 mM concentration of nitrite were monitored over the 24-h period with a sample plug injected every 2 min, resulting in 720 consecutive injections and giving a RSD for the peak height of approximately 9.6 % for the entire period. More details are provided in S1.
Experiments were also performed to evaluate the overall ruggedness of the chip when placed on a moving cart or a shaker (S1). In addition, the device was tested in vitro for monitoring nitrite production using DEA-NONOate (S2). Lastly, the perfusate and run buffer composition used in these experiments were optimized for biocompatibility and maximum detector response (S3). It was found that stacking of nitrite occurred with a run buffer of 50 mM phosphate pH 7.4 when a perfusate solution containing 119 mM NaCl was used, leading to lower LODs. Therefore a 50 mM phosphate run buffer was employed as the separation buffer for all MD-ME-EC animal experiments. Details regarding the experimental design and results of the above experiments are provided in the supplemental information along with a video of the device on a freely roaming sheep.
Although the within chip reproducibility was good, because these chips were fabricated individually, there was considerable chip-to-chip variability with regard to the current response obtained for equivalent concentrations of nitrite and other electroactive compounds. We believe this is due to slight differences in electrode alignment between chips. Therefore, for quantitative experiments, each chip was calibrated prior to use using standards. In the future, these chips could be produced by a commercial laboratory with better precision with regard to fabrication and, hopefully, this variation would be less of an issue.
In vivo Studies Off-animal with Rats
Nitroglycerin is a prodrug that undergoes complex metabolic biotransformation, predominantly in the smooth muscle intracellular space. The exact mechanism by which vasodilation occurs due to nitroglycerin administration is still largely unknown.42, 43 It is believed that vasodilation is caused by nitric oxide (NO), which is generated from nitroglycerin in the presence of glutathione-S-transferase and other enzymes.43–45 Nitric oxide is converted to nitrite in the presence of oxygen in vivo, and is often used as an indicator of NO production.46 Intravenous nitroglycerin is readily available in a 5 mg/mL solution.
For the in vivo microdialysis experiments, two separate loop microdialysis probes were implanted subcutaneously into the epidermal tissue. One probe was coupled directly to the MD-ME-EC system, and the other was used to collect samples for later analysis by LC-EC. This method allowed direct comparison of nitrite production in the animals without having to compensate for errors produced by animal-to-animal variability. Baseline collection was performed for 1 h before the drug was administered to stimulate nitrite production for the following 2 h.
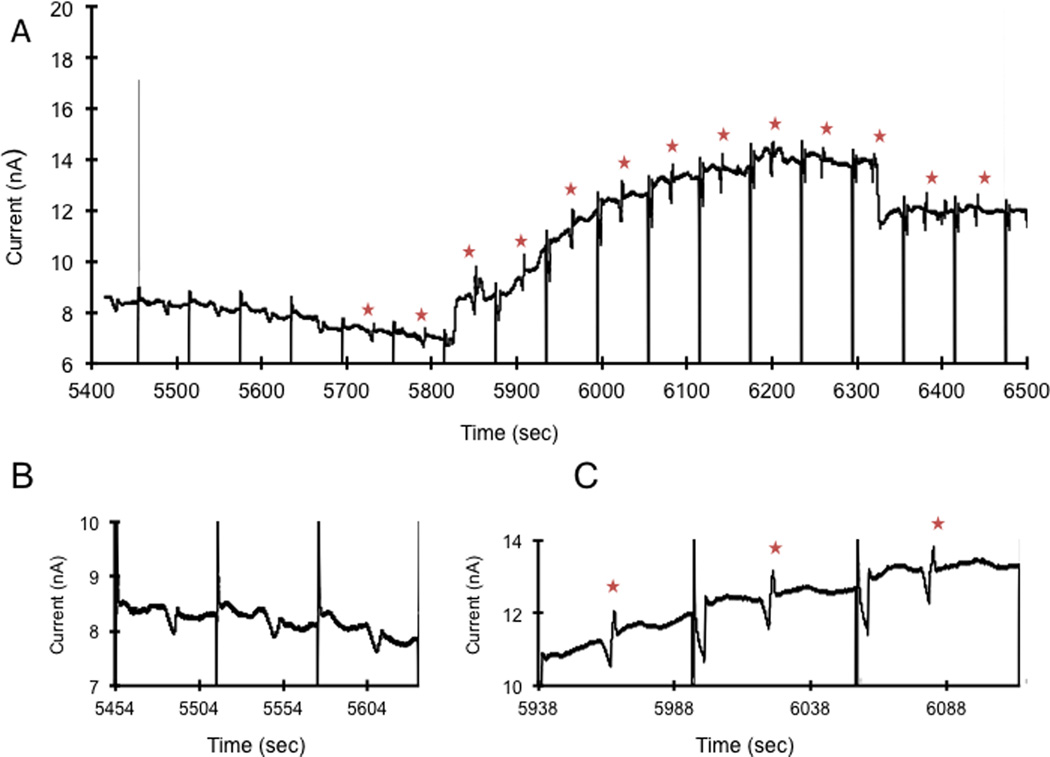
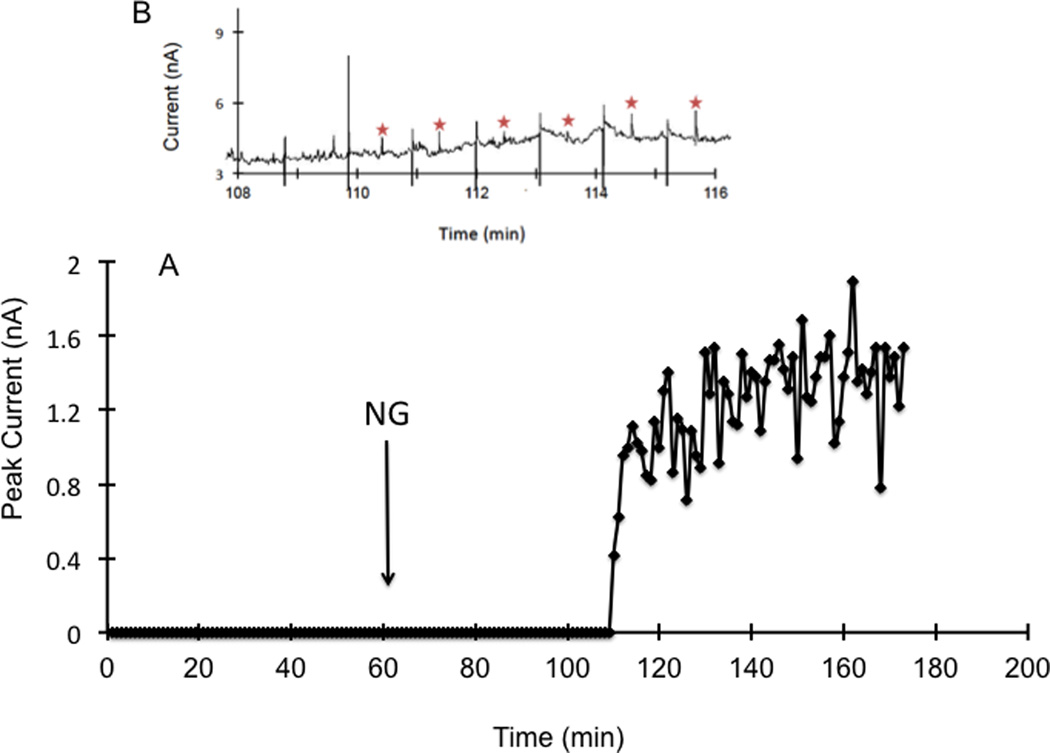
To ensure that the system would work for on-animal in vivo measurements, the system was first evaluated off-animal with rats. Using LC-EC, the relative recovery of the microdialysis probe for nitrite was determined to be 78%. First a calibration curve for the MD-ME-EC system was made by direct infusion of different concentrations of nitrite from the syringe pump. The calibration curve was constructed using five concentrations ranging from 2.5 to 50 µM, giving an R2 value of 0.98. During the first hour a perfusate of 50 mM phosphate buffer with 119 mM NaCl was used. After 1 h, the composition of the perfusate was changed to 4.8 mg/mL nitroglycerin with 50 mM phosphate buffer and 119 mM NaCl. The raw data obtained using the MD-ME-EC system is shown in Figure 3. No nitrite was detected in the baseline samples (Figures 3A 3B). Nitrite began to appear in the electropherograms approximately 30 minutes after changing the perfusate to nitroglycerin (Figures 3A and 3C).
Figure 3.
Monitoring the production of nitrite in the rat following subcutaneous perfusion of nitroglycerin through a loop microdialysis probe with MD-ME-EC.. Working electrode set at +1.05 V vs. Pt pseudo reference/counter electrode. The separation buffer consisted of 50 mM sodium phosphate pH 7.4. Separation voltage was −1600 V with a 1 second injection. Flow rate for microdialysis experiment was 1 µL/min. For the first 60 min, the perfusate consisted of 50 mM phosphate buffer with 119 mM NaCl. After 1 h, the perfusate was changed to 4.8 mg/mL nitroglycerin dissolved in 50 mM phosphate buffer and 119 mM NaCl, indicated by NG on the graph. (A) Continuous electropherograms obtained between 90 and 108 minutes (5400–6500 sec) using the on-line system. Nitrate peaks are marked with a star. Injection spikes were attenuated in the figure. (B) Representative electropherograms obtained between 90–93 minutes before nitrite was detected (C) Representative on-line electropherograms obtained between 98–102 minutes showing nitrite production due to nitroglycerin metabolism.
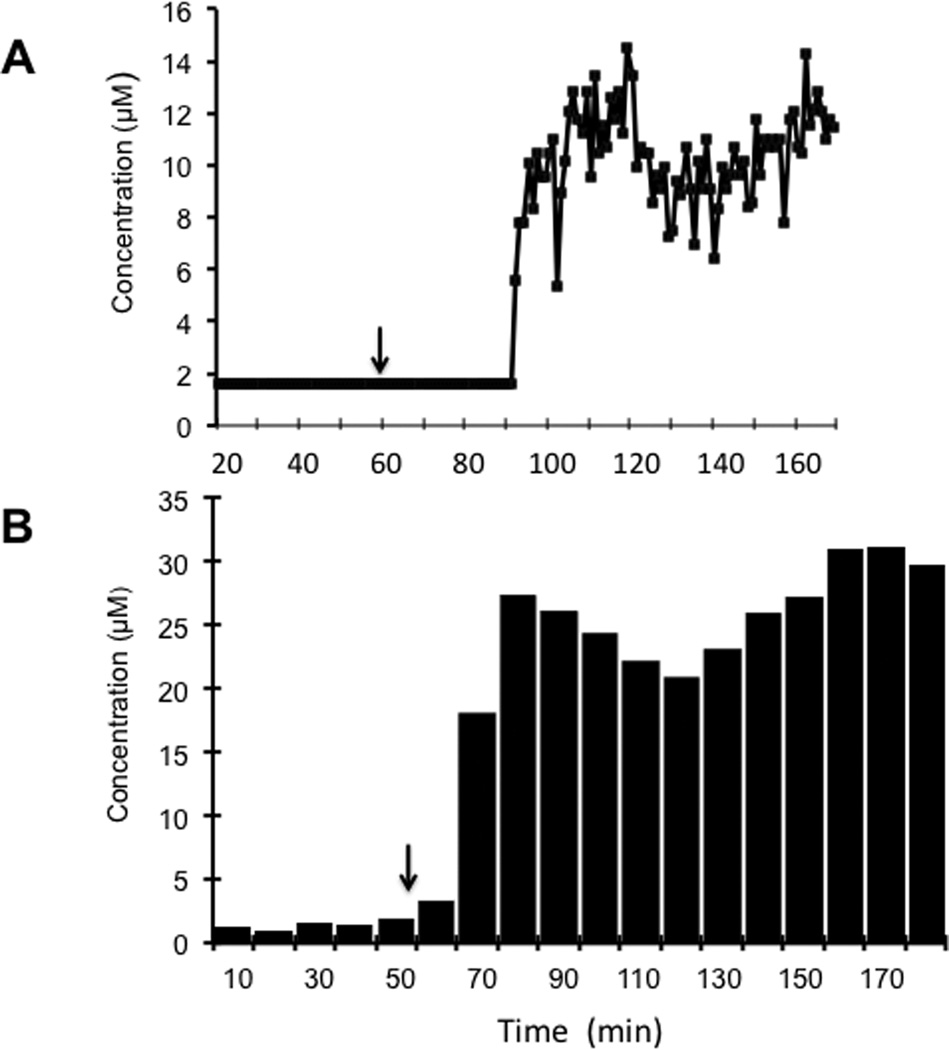
Plots of obtained for nitrite concentration versus time using the on-line system (Figure 4a) and off-line analysis using LC-EC (Figure 4b) for the same rat are shown in Figure 4. Since the off-line analysis gives the average concentration of nitrite over the 10-min sampling period, these data are depicted as a bar graph. In contrast, the on-line system gives a single point measurement every minute, so these data are represented as points on the graph. It can be seen in Figure 4 that there is an increase in lag time for the on-line system compared to off-line analysis due to the additional tubing length required to couple the microdialysis probe implanted in the rat to the microfluidic device.
Figure 4.
Comparison of data obtained by MD-ME-EC and LC-EC for the nitroglycerin perfusion experiment shown in Figure 3. Two separate probes were implanted subcutaneously in the rat and perfused with the nitroglycerin solution. (A) Nitrite concentration versus time plot for data obtained using the MD-ME-EC system. A calibration curve was generated before the experiment produced using direct infusion of standard nitrite solutions. (B) Histogram of concentrations of nitrite obtained with off-line sample collection and analysis by LC-EC. Each box corresponds to the concentration obtained for a 10 minute (10 microliter) sample. The difference in lag time for the MD-ME-EC system is due to length of additional tubing required for tubing connectors. The experimental conditions are identical to those in Figure 3.
On-animal Experiments Using Sheep
Sheep are the ideal model animal for the study of the miniaturized MD-ME-EC system (Figure 5) because of their size, general docile character, and the fact that they have been used previously for behavioral studies and pharmacokinetic studies using microdialysis.32–34, 36, 37 It is also a species that recovers well from general anesthesia and surgery. In these experiments, the complete on-line MD-ME-EC system was attached to a harness that fastened the system to the sheep. The working system, including all electronics and the microdialysis pump, was fixed within the box attached to the harness. Two laptop batteries used to run the system were easily secured underneath the box to straps sewn into the harness. Figure 5 depicts a complete and running telemetry system with data being collected via Bluetooth®. Duplicate experiments matching those completed on the rat model were performed on the sheep. The only noticeable difference in the experimental design for the sheep animal model was that the sheep was awake and not anesthetized during the course of the experiment. Although the sheep were not completely freely roaming throughout these experiments, later the device was operated while the sheep were freely roaming and in contact with other sheep.
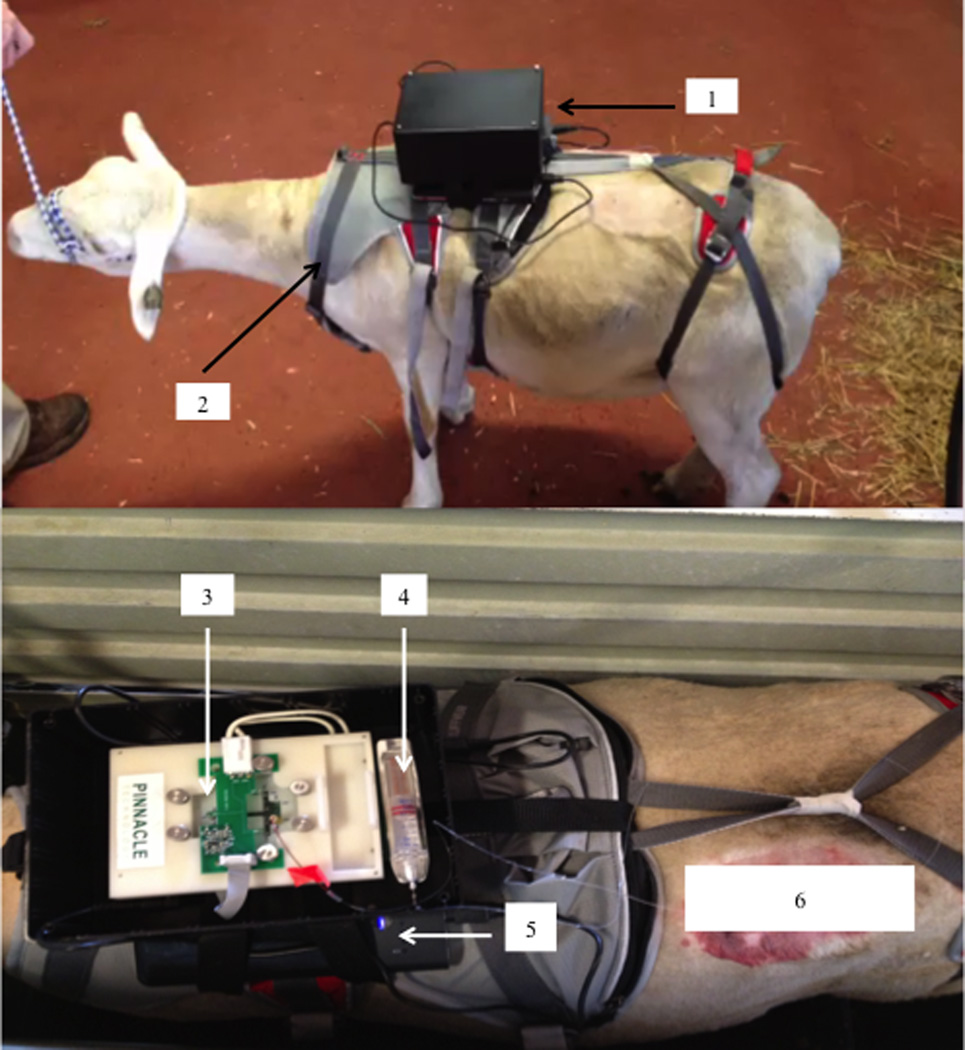
Figure 5.
Miniaturized MD-ME-EC system placed on a sheep: (1) complete system mounted on animal; (2) harness that secures system to the animal; (3) sensor components secured within the box and attached to the harness; (4) syringe pump secured to the chip holder; (5) laptop batteries; and (6) microdialysis probe implant site. This figure depicts a fully functioning and operational telemetry system with data being collected via Bluetooth®.
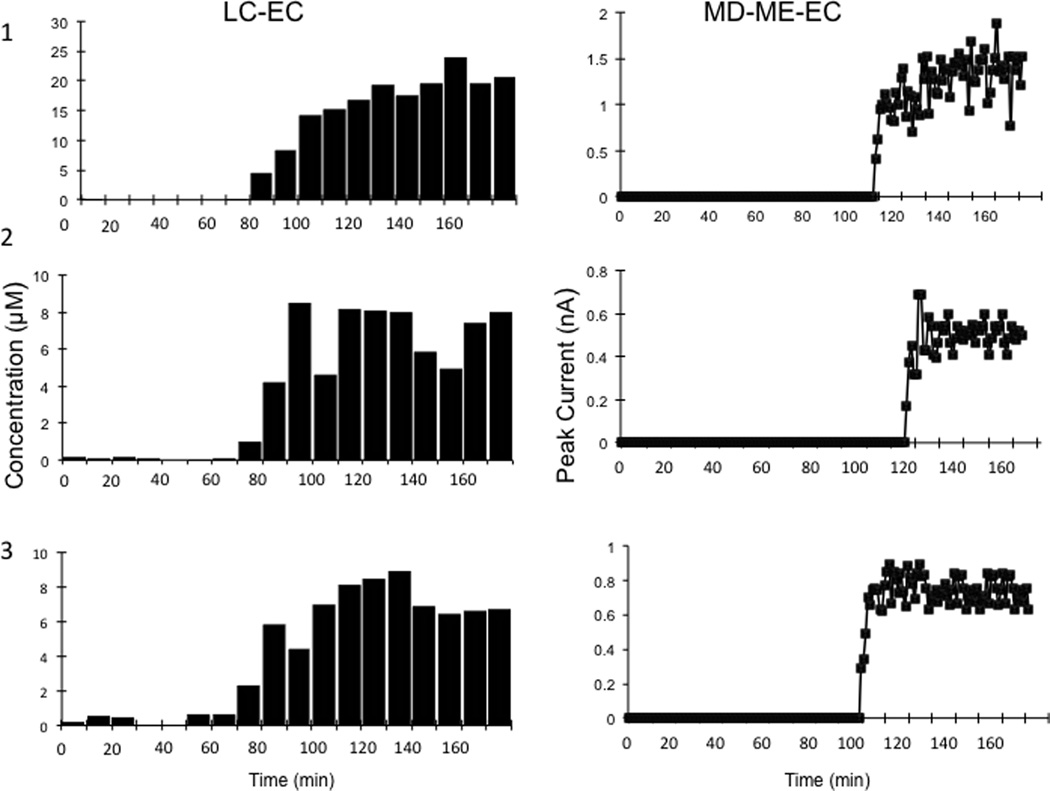
Figure 6A shows the peak height for nitrite for each injection made during the experiment using MD-ME-EC. Figure 6B shows a representative area of the electropherogram from multiple injections, with the nitrite peak indicated. As with the rat studies, the results obtained on-animal using the MD-ME-EC system were compared to those obtained using another probe on the animal and off-line analysis (Figure 7). Using LC-EC, nitrite concentrations were measured starting at the 10-min mark of the baseline perfusion. After nitroglycerin perfusion had started at the 60-min mark, an increase in nitrite levels was seen, with a peak concentration intensity at approximately 100 min. After the peak nitrite response was observed, a slight decrease occurred for the remainder of the experiment.
Figure 6.
Time course of production of nitrite in sheep due to the subcutaneous perfusion of nitroglycerin through the microdialysis probe. (A) Peak height is shown as a function of time for nitrite during the experiment. The first 60 min is from a perfusion of 50 mM phosphate buffer with 119 mM NaCl. After 1 h, 4.8 mg/mL nitroglycerin with 50 mM phosphate buffer and 119 mM NaCl was perfused through the microdialysis probe. (B) Representative data from multiple injections during the peak concentrations with a star indicating the nitrite peak. Operating conditions are identical to those in Figure 3.
Figure 7.
Comparison of the production of nitrite in three sheep due to the subcutaneous perfusion of nitroglycerin through the microdialysis probe. Two separate probes with identical perfusion of nitroglycerin were used. Left (LC-EC) column: Dialysates were collected every 10 min from a microdialysis probe with a flow of 1 µL/min and analyzed off-line by LC-EC. Samples. Right (MD-ME-EC) column: Data obtained in near real-time using the MD-ME-EC system.
The identity of nitrite was confirmed off-line by spiking collected sample with a nitrite standard and testing it using LC-EC. The maximum concentration of nitrite produced by the infusion was estimated to be approximately 17 µM by LC-EC, taking recovery into account. A lag time of 50 min prior to the detection of nitrite was observed with the MD-ME-EC system. This lag time is a combination of the system delay due to the connecting tubing between the probe and the on-line system, as well as the time required for enzymatic conversion of nitroglycerin to detectable levels of nitrite. For the off-line collection and analysis by LCEC, the lag time was less than the sampling time (10 minutes) and a change in nitrite concentration was observed immediately.
The comparison of the results obtained by MD-ME-EC with off-line analysis by LC-EC is shown in Figure 7. The MD-ME-EC system exhibited better temporal resolution than off-line measurements with LCEC system. The microdialysate was introduced directly into the chip and analyzed in near real-time while the system was tethered to the back of the animal, and all data were wirelessly streamed to a PC interface for immediate interpretation. The system was able to collect and analyze data from in vivo monitoring every 60 seconds with immediately interpretable data. In comparison, for the LC-EC system, 10 µL samples were collected via microdialysis and stored for later analysis. At a flow rate of 1 µL min−1, this produced one sample for analysis every 10 min. The data shown in Figure 7 for the LC-EC system are therefore an average concentration for the entire 10-min sampling time, whereas the MD-ME-EC system produced a data point every 60 seconds.
The device as shown in Figure 5 was tested on an untethered and unrestrained freely roaming sheep (video in supplemental information). During these evaluations the sheep was allowed to interact in a pen with two other sheep while communication with the device was being maintained via Bluetooth®. The functionality of the device was monitored for a short time in which the animal was freely roaming. Communication was maintained with the device, and the system could be manipulated via telemetry while the animal was unrestrained. However, only the system functionality and the ability to maintain communication via telemetry were tested during this time due to concerns for welfare of the prototype device.
The studies described in this paper were primarily intended to test the performance of the device for on-animal sampling, and the experiments generally lasted 2–3 h. For behavioral studies lasting longer than 24 h, the effect of tissue inflammation on probe recovery will need to be taken into consideration. In future studies, the incorporation of an internal standard in the dialysate (retrodialysis) will be employed to monitor changes in probe recovery over time.
CONCLUSIONS
The development of a miniaturized on-animal separation-based sensor using microdialysis sampling and electrochemical detection on a microfluidic platform is described. The complete system utilized a miniaturized high voltage power supply and potentiostat. The microfluidic microchip was integrated into the device, and the whole system was controlled wirelessly. In these studies, the overall functionality of the device was tested in an effort to demonstrate its capability as an on-animal separation-based sensor. The ultimate goal is to apply this methodology to monitor neurotransmitters in freely roaming sheep and correlate concentration with behavior.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge financial support from the National Institutes of Health (NINDS NS42929 and NINDS 64644). All microfluidic devices used in this research were manufactured in the Ralph N. Adams Institute COBRE Core Microfabrication Facility, which is supported by the National Institutes of Health grant NIGMS 5P20GM103638. We would also like to acknowledge Dr. Matt Hulvey, Dr. Pradyot Nandi, Hans Harmon, Rachel Saylor, Dr. Dulan Gunasekara, Dr. Anne Regel, and Ryan Grigsby for technical assistance and helpful discussions throughout the project. The authors also thank Crystal Haggen and Pam Lachcik at Purdue University, who contributed greatly with their time and expertise in the sheep experiments. Lastly, we thank Nancy Harmony for her editorial assistance in preparation of the manuscript.
REFERENCES
- 1.Heal DJ, Smith SL, Gosden J, Nutt DJ. Journal of Psychopharmacology. 2013;27:479–496. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidbreder C. Naunyn-Schmiedeberg's Archives of Pharmacology. 2013;386:167–176. doi: 10.1007/s00210-012-0803-6. [DOI] [PubMed] [Google Scholar]
- 3.Uppal A, Singh A, Gahtori P, Ghosh SK, Ahmad MZ. Current Pharmaceutical Design. 2010;16:4243–4253. doi: 10.2174/138161210794519110. [DOI] [PubMed] [Google Scholar]
- 4.Gunja N. Journal of Medical Toxicology. 2013;9:155–162. doi: 10.1007/s13181-013-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson GS, Johnson MA. Chemical Reviews. 2008;108:2462–2481. doi: 10.1021/cr068082i. [DOI] [PubMed] [Google Scholar]
- 6.Robinson DL, Hermans A, Seipel AT, Wightman RM. Chemical Reviews. 2008;108:2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhnline Sloan CD, Nandi P, Linz TH, Aldrich JV, Audus KL, Lunte SM. Annual Review of Analytical Chemistry. 2012;5:505–531. doi: 10.1146/annurev-anchem-062011-143002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nandi P, Kuhnline CD, Lunte SM. In: Applications of Microdialysis in Pharmaceutical Science. Tsai T-H, editor. Vol. 1. Singapore: John Wiley & Sons, Inc.; 2011. pp. 39–92. [Google Scholar]
- 9.Kennedy RT. Current Opinion in Chemical Biology. 2013;17:860–867. doi: 10.1016/j.cbpa.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westerink BHC, Cremers TIFH. Handbook of Microdialysis : Methods, Applications, and Perspectives. 1st edn. Amsterdam: Elsevier/Academic Press; 2007. [Google Scholar]
- 11.Huff JK, Davies MI. Journal of Pharmacuetical and Biomedical Analysis. 2002;29:767–777. doi: 10.1016/s0731-7085(02)00196-6. [DOI] [PubMed] [Google Scholar]
- 12.Cooper JD, Heppert KE, Davies MI, Lunte SM. Journal of Neuroscience Methods. 2007;160:269–275. doi: 10.1016/j.jneumeth.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nandi P, Lunte SM. Analytica Chimica Acta. 2009;651:1–14. doi: 10.1016/j.aca.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz KN, Kennedy RT. Annual Review of Analytical Chemistry. 2008;1:627–661. doi: 10.1146/annurev.anchem.1.031207.113047. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Slaney T, Mabrouk O, Kennedy RT. Journal of Neuroscience. Methods. 2010;190:39–48. doi: 10.1016/j.jneumeth.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaul S, Faiman MD, Lunte CE. Analytical. Methods. 2011;3:1514–1520. [Google Scholar]
- 17.Hogan BL, Lunte SM, Stobaugh JF, Lunte CE. Analytical Chemistry. 1994;66:596–602. doi: 10.1021/ac00077a004. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Heckert DM, Zuo H, Lunte CE, Lunte SM. Analytica Chim. Acta. 1999;379:307–317. [Google Scholar]
- 19.Hogerton AL, Bowser MT. Analytical Chemistryz. 2013;85:9070–9077. doi: 10.1021/ac401631k. [DOI] [PubMed] [Google Scholar]
- 20.Lada MW, Kennedy RT. Analytical. Chemistry. 1996;68:2790–2797. doi: 10.1021/ac960178x. [DOI] [PubMed] [Google Scholar]
- 21.Saylor RA, Lunte SM. Journal of Chromatography A. 2015 doi: 10.1016/j.chroma.2014.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huynh BH, Fogarty BA, Martin RS, Lunte SM. Analytical Chemistry. 2004;76:6440–6447. doi: 10.1021/ac049365i. [DOI] [PubMed] [Google Scholar]
- 23.Li MW, Huynh BH, Hulvey MK, Lunte SM, Martin RS. Analytical Chemistry. 2006;78:1042–1051. doi: 10.1021/ac051592c. [DOI] [PubMed] [Google Scholar]
- 24.Mecker LC, Martin RS. Journal of Association for Laboratory Automation. 2007;12:296–302. [Google Scholar]
- 25.Mecker LC, Martin RS. Analytical Chemistry. 2008;80:9257–9264. doi: 10.1021/ac801614r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Roman GT, Perry ML, Kennedy RT. Analytical Chemistry. 2009;81:9072–9078. doi: 10.1021/ac901731v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandlin ZD, Shou M, Shackman JG, Kennedy RT. Analytical Chemistry. 2005;77:7702–7708. doi: 10.1021/ac051044z. [DOI] [PubMed] [Google Scholar]
- 28.Nandi P, Scott DE, Desai D, Lunte SM. Electrophoresis. 2013;34:895–902. doi: 10.1002/elps.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huynh BH, Fogarty BA, Nandi P, Lunte SM. Journal of Pharmaceutical and Biomedical Analysis. 2006;42:529–534. doi: 10.1016/j.jpba.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Nandi P, Desai DP, Lunte SM. Electrophoresis. 2010;31:1414–1422. doi: 10.1002/elps.200900612. [DOI] [PubMed] [Google Scholar]
- 31.Scott DE, Grigsby RJ, Lunte SM. Chemphyschem. 2013;14:2288–2294. doi: 10.1002/cphc.201300449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendrick KM. Techniques Behavioral Neural Science. 1991;7:327–348. [Google Scholar]
- 33.Westerink BHC. Behavioral Brain Research. 1995;70:103–124. doi: 10.1016/0166-4328(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 34.Au-Yeung SC, Riggs KW, Gruber N, Rurak DW. Drug metabolism and disposition: the biological fate of chemicals. 2007;35:1285–1291. doi: 10.1124/dmd.106.013995. [DOI] [PubMed] [Google Scholar]
- 35.Cook CJ. Journal of Neuroscience Methods. 1997;72:161–166. doi: 10.1016/s0165-0270(96)02175-9. [DOI] [PubMed] [Google Scholar]
- 36.Bengtsson J, Ederoth P, Ley D, Hansson S, Amer-Waahlin I, Hellstroem-Westas L, Marsal K, Nordstroem CH, Hammarlund-Udenaes M. British Jounral of Pharmacology. 2009;157:1085–1096. doi: 10.1111/j.1476-5381.2009.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Costa APC, Guevara-Guzman RG, Ohkura S, Goode JA, Kendrick KM. Journal of Neuroendocrinology. 1996;8:163–177. doi: 10.1046/j.1365-2826.1996.04411.x. [DOI] [PubMed] [Google Scholar]
- 38.Kendrick KM, da Costa AP, Leigh AE, Hinton MR, Peirce JW. Nature. 2001;414:165–166. doi: 10.1038/35102669. [DOI] [PubMed] [Google Scholar]
- 39.Lim MM, Young LJ. Hormones and Behavior. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 40.Allen PB, Chiu DT. Analytical Chemistry. 2008;80:7153–7157. doi: 10.1021/ac801059h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Y-H, Lee G-B, Li C-W, Huang G-R, Chen S-H. Journal of Chromatography A. 2001;937:115–125. doi: 10.1016/s0021-9673(01)01326-7. [DOI] [PubMed] [Google Scholar]
- 42.Page NA, Fung H-L. Journal of Pharmaceutical Science. 2013;102:3070–3081. doi: 10.1002/jps.23550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Münzel T, Steven S, Daiber A. Vascular Pharmacology. 2014;63:105–113. doi: 10.1016/j.vph.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Higo N, Hinz RS, Lau DTW, Benet LZ, Guy RH. Pharmaceutical Research. 1992;9:187–190. doi: 10.1023/a:1018925004345. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z, Zhang J, Stamler JS. Proceedings of the National Academy of Sciences. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ignarro LJ. Angewante Chemie. 1999;38:1882–1892. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1882::AID-ANIE1882>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.