Summary
A growing body of evidence suggests that resting cortisol levels are elevated in patients with schizophrenia and closely tied to symptom severity. However, there is limited research on the biological stress system during the ultra high-risk (UHR) period immediately preceding the onset of psychosis, and cortisol has not been examined in relation to individual characteristics such as self-concept or potential stressors such as putative familial environment in this critical population. In the present study, salivary cortisol samples were collected on 37 UHR and 42 matched control adolescents, and these individuals were assessed with clinical interviews as well as a measure of self-concept. For a subsection of the sample (23 UHR and 20 control adolescents), a participating relative/caretaker was also assessed with an expressed emotion interview designed to gauge psychosocial environment. Consistent with previous studies, UHR participants exhibited elevated resting cortisol levels when compared with controls. In addition, UHR adolescents exhibited increased negative self-concept and their relatives/caretakers endorsed significantly fewer initial positive statements about the participant. Interestingly, a strong trend in the UHR group suggests that higher cortisol levels are associated with higher rates of critical statements from relatives/caretakers. Furthermore, elevated cortisol levels in the participants were associated with increased negative self-concept as well as fewer initial positive comments from relatives/caretakers. Results suggest that hypothalamic-pituitary-adrenal axis (HPA) dysfunction is closely associated with both individual and environmental-level characteristics. Taken together, these findings support a neural diathesis-stress model of psychosis and future studies, designed to examine causal relationships, stand to inform both our understanding of pathogenic processes in the high-risk period as well as early intervention efforts.
Keywords: Cortisol, HPA Axis, Prodrome, High-Risk, Schizophrenia, Expressed Emotion
1. Introduction
Accumulating evidence suggests that hypothalamic-pituitary-adrenal (HPA) axis dysfunction can have detrimental effects on mental health (Goodyer et al., 2001) and that adolescence is a period that is characterized by both increased sensitivity as well as significant changes in this integral system (Walker et al., 2008). A neural diathesis-stress model posits that an early biological vulnerability to the HPA system later interacts in the adolescent period with both individual factors and environmental stressors as well as normative and pathological neuroendocrine development, eventually leading to the onset of psychotic disorders such as schizophrenia (Walker et al., 2008). Consistent with this research, elevated stress hormones have been observed in individuals with chronic psychosis (Corcoran et al., 2003). An emerging body of evidence supports this theory and suggests that resting cortisol levels are elevated in the psychosis-risk period as well (Mittal and Walker, 2011; Walker et al., 2013; Karanikas and Garyfallos, 2014). However, while individual characteristics, such as self-concept (Fowler et al., 2006; Smith et al., 2006; Taylor et al., 2013), and psychosocial stressors, such as family environment (Barrowclough and Hooley, 2003), have been reliably linked to psychosis outcome, to our knowledge, there have been no empirical studies examining relationships between these factors and markers of HPA dysfunction. Understanding the association between neuroendocrine dysfunction, self-concept, and environmental stressors in the period immediately prior to the onset of psychotic disorders is integral, as the prodrome is a viable period of intervention in which considerable neuroendocrine development is still underway (Sowell et al., 1999; Dahl, 2004; Spear, 2004).
As noted, studies have observed that cortisol levels are elevated in patients with schizophrenia when compared to healthy controls (Belvederi Murri et al., 2012) and are closely associated with positive symptoms (Franzen, 1971; Walder et al., 2000; Collip et al., 2011). Recent investigations are also now observing similar elevations in psychosis risk (Walker et al., 2010; Walker et al., 2013; Karanikas and Garyfallos, 2014); however, the relationship is nuanced, and group differences are not always observed (Cullen et al., 2014; Day et al., 2014). The root of stress hormone elevations in UHR individuals remains unclear as HPA dysregulation in this population has been linked to anxiety (Corcoran et al., 2012; Karanikas and Garyfallos, 2014), suspiciousness (Corcoran et al., 2012), stressful life events (Labad et al., 2014), and stress intolerance (Pruessner et al., 2013; Karanikas and Garyfallos, 2014). Alternatively, several studies have reported that abnormal stress hormone levels are closely tied to the illness progression in the UHR period and are ultimately predictive of transition to psychosis (Walker et al., 2010; Walker et al., 2013).
Self-concept formation (i.e. the development of beliefs about the self) occurs during childhood and adolescence (Cole et al., 2001), and cognitive theorist have posited that the development of negative beliefs about the self can result in maladaptive responses and mental states (Beck, 1979; Garety et al., 2001). Interestingly, negative beliefs about the self have been linked to cortisol activity in healthy individuals (Kirschbaum et al., 1995) and have consistently been exhibited by individuals with chronic psychosis (Fowler et al., 2006; Smith et al., 2006; Taylor et al., 2013). Given this finding, researchers have theorized that dysfunctional self-concept, particularly negative self-beliefs, plays a role in the development of psychosis symptoms (Garety et al., 2001; Smith et al., 2006). Although the literature is limited, available studies examining this phenomenon in UHR adolescents have produced inconsistent results leading to a lack of understanding of how poor self-concept relates to illness development. While some investigations observed that UHR individuals endorse more negative self-beliefs than healthy controls (Perivoliotis et al., 2009; Stowkowy and Addington, 2012; Saleem et al., 2014), other studies have not observed a relationship with symptomatology (Saleem et al., 2014).
In normative samples, family environment during adolescence has been tied to endocrine function (Flinn and England, 1995; Luecken et al., 2009) as well as aspects of self-concept, such as self-esteem (Schmidt and Padilla, 2003). Within the context of psychosis, family environment is most commonly assessed through measuring expressed emotion (EE), which is defined as the attitudes and feelings a relative or caretaker expresses about their mentally ill family member (Barrowclough and Hooley, 2003). More specifically, high levels of criticism and hostility in the family environment are linked to increased risk in relapse in schizophrenia (Bachmann et al., 2002). There is a small but accumulating body of literature examining the relationship of EE and symptoms in UHR adolescents, and the findings generally show that greater levels of criticism and emotional over-involvement are associated with higher positive and negative symptoms (O'Brien et al., 2006; McFarlane and Cook, 2007; Schlosser et al., 2010; Dominguez-Martinez et al., 2014). However, to date, there have been no studies comparing EE in a UHR sample to healthy controls and it remains unclear as to how this social risk factor is linked to endocrine activity or self-concept.
In the present study, including a total of 79 adolescents (37 UHR and 42 matched healthy participations), we evaluated resting cortisol levels as well as links with self-concept, family environment, and symptomatology. We made the following predictions based on the available research. First we predicted that the present sample of UHR participants would exhibit elevated cortisol levels when compared to matched healthy controls and that this would be associated with increased symptomatology (i.e. higher positive and negative symptoms). We also predicted that the UHR group would exhibit elevated negative self-concept compared to healthy controls, and that increased negative self-concept would be associated with elevated cortisol levels in turn. Because there is not yet a strong guiding literature to inform predictions, the relationships between cortisol levels and positive self-concept, as well as the relationship between both categories of self-concept and symptoms, were examined in exploratory analyses. In an analysis of family environment, assessed in a subset of 43 participants (23 UHR and 20 control adolescents), we predicted that the family environment of UHR individuals would be characterized by lower levels of positive initial statements and warmth, and elevated criticism and emotional over-involvement compared to the family environment of control participants. Finally, we predicted that elevated dysfunctional expressed emotion variables would be linked with elevated cortisol and increased negative self-concept. An exploratory analysis of family environment's relationship with symptoms and positive self-concept was also conducted.
2. Methods
2.1 Participants
Participants were recruited at the Adolescent Development and Preventative Treatment (ADAPT) research program (see Table 1 for demographic characteristics of this sample). Adolescent UHR and control participants (mean age=18.59) were recruited by Craigslist, e-mail postings, newspaper ads, bus ads, and community professional referrals. Exclusion criteria included history of head injury, the presence of a neurological disorder, and lifetime substance dependence. The presence of an Axis I psychotic disorder (e.g. schizophrenia, schizoaffective disorder, schizophreniform) was an exclusion criterion for UHR participants. Other comorbid Axis I disorders were not exclusion criteria for UHR participants. Rates of current comorbid Axis I disorders in the UHR participants included 13(35%) affective disorders, 4(11%) PTSD, 14(38%) other anxiety disorders, and 4(11%) ADHD. Comorbid Axis I disorders are typical of UHR individuals and the present rates are comparable to other studies (Fusar-Poli et al., 2014). UHR individuals met criteria for a prodromal syndrome including: (a) recent onset or escalation of moderate levels of attenuated positive symptoms (a score of 3-5), and/or (b) a decline in global functioning over the last 12 months accompanying the presence of schizotypal personality disorder (SPD), and/or (c) a decline in global functioning over the last 12 months accompanying the presence of a first-degree relative with a psychotic disorder such as schizophrenia (Miller et al., 1999). Meeting for an Axis I disorder or the presence of a psychotic disorder in a first-degree relative were exclusionary criteria for controls.
Table 1.
Participant Demographics, Symptoms, Resting Cortisol, Self-Concept
| Group | Healthy Control | Ultra High-Risk | p-value |
|---|---|---|---|
| Gender | |||
| Males | 22(52.4) | 24(64.9) | |
| Female | 20(47.6) | 13(35.1) | p = .27 |
| Total | 42 | 37 | |
| Age | |||
| Mean Years(SD) | 18.38(2.43) | 18.84(1.69) | p = .17 |
| Parent Education | |||
| Mean Years(SD) | 14.73(4.01) | 15.10(3.12) | p = .34 |
| Cortisol | |||
| AUCg Mean(SD) | 34(.19) | .44(.27) | p = .04 |
| BCSS | |||
| Negative-Self Mean(SD) | 1.70(2.02) | 5.31(5.15) | p = .001 |
| Positive-Self Mean(SD) | 16.39(5.15) | 12.64(5.91) | p = .001 |
| SIPS Symptoms | |||
| Positive Mean(SD) | .71(1.54) | 12.38(4.83) | p = .001 |
| Negative Mean(SD) | .36(1.10) | 3.57(4.92) | p = .001 |
Note: The Units of cortisol are listed in μg/dl. AUCg refers to the total area under the curve with respect to ground; Scores on the Brief Core Schema Scale (BCSS) range from 0-24; Positive and negative symptoms reflect total sums from domains from the Structured Interview for Prodromal Syndromes (SIPS).
The protocol and informed consent procedures were approved by the University Institutional Review Board. In both groups, participants’ family members were invited to participate in the study; however, parent participation and availability was not an inclusion requirement, and as a result, a subsection participated in this portion of the study (54%). However, there were no significant differences in demographic, cortisol, self-concept, or symptom variables between the individuals whose relative/caretaker did or did not participate in the study.
2.2 Clinical interviews
The Structured Interview for Prodromal Syndromes (SIPS; Miller et al., 1999) was administered to diagnose a prodromal syndrome. As noted, UHR participants in the present study met criteria for a prodromal or high-risk syndrome. The SIPS gauges several distinct categories of prodromal symptom domains including positive and negative dimensions. A sum score for each category is used as an indicator of the respective dimensions of symptomatology. The Structured Clinical Interview for Axis-I DSM-IV Disorders (SCID-IV; First et al., 1995) was also administered to rule out formal psychosis in UHR participants and an Axis I disorder in the control group (noted exclusionary criterion). This measure has been demonstrated to have excellent interrater reliability in adolescent populations (Martin et al., 2000) and has been used in several previous studies focusing on adolescent populations with schizophrenia spectrum disorders (Howes et al., 2009). Training of interviewers (who were advanced doctoral students) was conducted over a 2-month period, and interrater reliabilities exceed the minimum study criterion of Kappa ≥ .80.
2.3 Self-Concept
The Brief Core Schema Scale (BCSS; Fowler et al., 2006) was used to assess self-concept. The BCSS gauges several distinct categories of self-concept including two categories that evaluate beliefs about the self: negative-self and positive-self. These are two separate categories and while unlikely, it is possible to have a high or low score on both at the same time. This measure was chosen due to its wide use in schizophrenia and schizophrenia risk studies (Smith et al., 2006; Stowkowy and Addington, 2012; Taylor et al., 2013; Saleem et al., 2014). This scale has been demonstrated to be a valid and appropriate scale to use in adolescent and UHR populations (Addington and Tran, 2009) and has strong internal consistency and concurrent and discriminate validity (Fowler et al., 2006; Addington and Tran, 2009).
2.4 Family Environment
The Five Minute Speech Sample (FMSS; Magana et al., 1986) was employed to assess family environment. The FMSS elicits a response from the participant's key relative/caretaker and was designed to identify attitudes and feelings about the participant as well as perceptions of the quality of their relationship. Audio-recorded speech samples, masked regarding group, were rated by the developer of the FMSS-EE coding system (Magana et al., 1986). These ratings were then recoded into dichotomous categories of interest including: positive initial statement (i.e. “I love my daughter very much.”), emotional over-involvement (i.e. “I would do absolutely anything to make sure my daughter is always safe.”), criticism (i.e. “It feels like we have always had issues with my son.”), and warmth (i.e. “My son is a very kind person.”). The initial statement category was included as a variable of interest because it has been found to be reliable and valid (Daley et al., 2003), and research suggests that the quality of a caretaker's initial statement about their family member may be a useful tool to distinguish risk groups (Kershner et al., 1996). Emotional over-involvement, criticism, and warmth were included in the study because they are classic EE variables. The categories were coded as present or not present where present means the caregiver expressed that type of statement at lest once during the speech sample. The FMSS has been found to be a reliable and stable measure and matches well with the traditional method for assessing EE, the Camberwell Family Interview (CFI; Magana et al., 1986; Malla et al., 1991; Moore and Kuipers, 1999), and has good predictive validity (Leeb et al., 1991; Marom et al., 2005).
2.5 Cortisol
The participants were assessed in the morning and gave three saliva samples over the course of 2 hours (every 60 minutes). Based on our prior studies, saliva was collected utilizing a passive-drool method (Mittal et al., 2007; Mittal and Walker, 2011). The participants were not exposed to a stressor; the cortisol level indexed represents the participant's cortisol secretion in the context of the novelty of the assessment. Subjects and their parent/guardian were provided with written and verbal dietary instructions to observe the evening before and the morning of sampling. Instructions allowed a light breakfast but instructed participants to refrain from caffeine, alcohol, dairy products, and nonprescription medications, as well as brushing teeth within 30 min prior to sampling. Subjects were questioned to confirm their compliance with the instructions and it was not necessary to exclude any participants on this basis. A small portion of the sample (6, 7/5%) was taking neuroleptic medication and there was no difference in cortisol levels between those UHR participants on or off medication [t(35)=-.86, p=.42]. The cortisol samples were stored in a -20C freezer until ready for assay. The Salimetrics High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics, LLC, College Park, PA) was used and following gold standard procedures, samples were subjected to duplicate analyses and the average of all points was calculated to yield the resting cortisol variable.
2.6 Statistical approach
Independent t-tests and chi-square tests were employed to examine differences between groups in respective continuous and categorical demographic variables. Independent t-tests were also used to compare group differences on hormone, self-concept, and symptom variables while chi-share tests were employed to examine group differences in the dichotomous (Y/N) EE variables. Based on an equation provided by Pruessner et al. (2003), mean cortisol values for each of the three sample points were used to calculate the total area under the curve with respect to ground (AUCg). This method was utilized to examine cortisol levels as the measure takes into account both sensitivity (the difference between the single measurements from each other) and intensity (the distance of these measures from ground) (Pruessner et al., 2003). Bivariate correlations were used to examine the relationship between cortisol and both negative/positive self-concept and positive/negative symptoms, and point-biserial correlations were employed to examine the association between cortisol and the dichotomous EE variables (i.e., initial positive statement, criticism, over-involvement, warmth). Because the variables of interest vary across subjects in both groups in a continuous fashion (e.g., there are varying levels of cortisol, symptoms, self concept, and familial EE among UHR and healthy control individuals), the present study examined associations across the entire sample. Further, to determine if any associations were specific to the clinical group, the analyses were repeated in the UHR group alone. Exploratory analyses will also be presented and a Bonferroni correction will be applied to these correlations. It is then noted whether any significant findings from exploratory analyses passed the threshold of this correction.
3. Results
As noted, a total of 79 (37 UHR and 42 control) adolescents participated in a study of cortisol and self-concept and of this group, a total of 43 (23 UHR and 20 control) had family members/caretakers participate to provide EE data. There were no group differences in the number of first-degree relatives and caretakers between groups [t(41)=-.91, p=.18], (UHR: 78% first degree relatives, 22% partner/significant other; Control: 90% first degree relatives, 10% partner/significant other). Within the UHR adolescents with EE data, 3(13%) had a first-degree relative with a psychotic disorder and 7(30%) had a first-degree relative with an affective or anxiety disorder. It is important to note that none of the family members who participated in the EE interview had a psychosis diagnosis. There were no significant differences in demographic characteristics such as age [t(77)=.97, p=.33], parental education [t(76)=.45, p=.65], or sex [χ2(1)=1.26, p=.27] between the UHR and control groups. This also held for the UHR and control groups in the subsample with EE data. As expected, the UHR group showed significantly more positive [t(77)=14.07, p=.001] and negative [t(77)=3.81, p=.001] symptoms when compared with healthy controls (see Table 1). One-sample Kolmogorov-Smirnov tests revealed that distributions for target continuous variables were normally distributed, meeting assumptions for parametric statistics. As noted, the EE variables were examined with non-parametric statistics.
3.1 Group Differences in Cortisol and Self-Concept
Consistent with our prediction, the UHR group showed significantly elevated resting cortisol levels when compared with matched healthy control adolescents, [t(77)=1.85, p=.04]. With respect to self-concept, the UHR individuals reported significantly more negative self-concept than controls, [t(76)=3.96, p=.001]. In contrast, healthy control adolescent reported significant elevation on an orthogonal scale of positive self-concept, [t(76)=-2.98, p=.001] (see Table 1).
3.2 Associations Between Cortisol, Self-Concept, and Symptom Variables
Correlational analyses indicated that elevated cortisol in the entire sample was moderately associated with increased negative self-concept scores (see Table 2). It is also interesting to note that there was a trend (p=.08) to suggest that lower cortisol levels were associated with increased positive beliefs about the self from the independent positive self-concept scale. While there was a weak trend (p=.10) to suggest that elevated cortisol was associated with higher positive symptoms, the relationship with negative symptoms did not approach significance. Several other interesting significant associations emerged from exploratory analyses. Specifically, it is noteworthy that elevated negative self-concept scores were strongly associated with higher positive and negative symptom levels, while higher positive self-concept scores were moderately linked to lower symptoms in both domains (did not pass Bonferroni correction).
Table 2.
Whole Sample Associations Between Resting Cortisol, Self-Concept, and Symptoms
| Domain | Cortisol AUCg | Negative Self | Positive Self | Positive Symp. | Negative Symp. |
|---|---|---|---|---|---|
| Cortisol AUCg | 1 | ||||
| Negative Self | .36** | 1 | |||
| Positive Self | −.16† | −.56** | 1 | ||
| Positive Symptoms | .15† | .48** | −.28** | 1 | |
| Negative Symptoms | −.03 | .52** | −.33** | .53** | 1 |
Note:
p<0.01
p≤ 0.10 (trend)
Bivariate correlations were used to examine the relationship between Cortisol AUCg, BCSS negative/positive self-concept, and SIPS positive/negative symptoms.
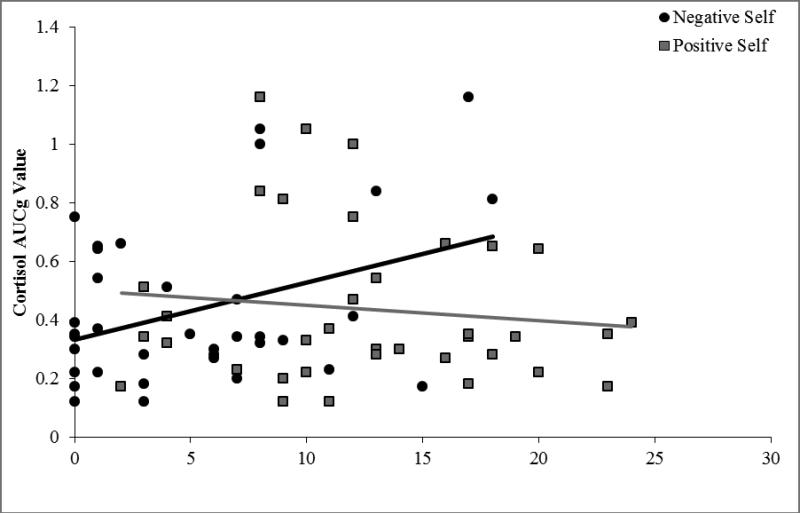
Correlational analyses in the UHR group alone also indicated a moderate association between elevated cortisol and increased negative self-concept (r=.37, p=.01). There was not a significant association between cortisol levels and positive self-concept (r=-.12, p=.25), nor positive (r=-.11, p=.26), and negative symptom domains (r=-.18, p=.14). Additionally, elevated negative self-concepts scores in the UHR group continued to be moderately associated with higher negative symptom levels (r=.44, p=.004), and higher positive self-concept scores were moderately linked to lower negative symptom levels (r=-.33, p=.03) in the exploratory analyses. There was not a significant association between negative (r=.20, p=.12) or positive (r=.03, p=.43) self-concept scores and positive symptom levels. Correlations between self-concept and symptom level did not pass Bonferroni correction. Figure 1 illustrates correlations between cortisol and both negative and positive self-concept.
Figure 1.
Associations Between Resting Cortisol and Self-Concept in UHR Individuals
Note: Bivariate correlations were used to examine the relationship between cortisol AUCg and BCSS negative/positive self-concept; n = 37 UHR adolescents.
3.3 Group Differences in Expressed Emotion
Consistent with predictions, caretakers of UHR individuals (22%) expressed a significantly lower frequency of positive initial statements about their respective participants when compared to the caretakers of healthy individuals (45%), [χ2(1)=2.63, p=.05]. However, the caretakers in both groups did not differ on critical statements (UHR=78.3%, control=80.0%) or emotional over-involvement statements (UHR=61.0%, control=60.0%) in the speech sample. Finally, while the overall frequency of low warmth speech samples (i.e., did not endorse a warm statement during the speech sample) was quite low (4.7% of the sample), both cases were provided by caretakers of UHR individuals, and as a result there was a moderate trend to suggest group differences, [χ2(1)=1.82, p=.09], (UHR=8.7%, control= 0.0%). Table 3 presents the group comparisons for EE variables.
Table 3.
Expressed Emotion Group Comparisons
| Group | Healthy Control | Ultra High-Risk | χ 2 | p-value |
|---|---|---|---|---|
| Initial Statement Positive | ||||
| Endorsed Frequency(%) | 9(45%) | 5(22%) | 2.64 | p = .05 |
| Not Endorsed Frequency(%) | 11(55%) | 18(78%) | ||
| Criticism | ||||
| Endorsed Frequency(%) | 16(80%) | 18(78%) | 0.02 | p = .44 |
| Not Endorsed Frequency(%) | 4(20%) | 5(22%) | ||
| Emotional Over-Involvement | ||||
| Endorsed Frequency(%) | 12(60%) | 14(61%) | 0.003 | p = .48 |
| Not Endorsed Frequency(%) | 8(40%) | 9(39%) | ||
| Warmth | ||||
| Endorsed Frequency(%) | 20(100%) | 21(91%) | 1.82 | p = .09 |
| Not Endorsed Frequency(%) | 0(0%) | 2(9%) |
Note: Chi-share tests were employed to examine group differences in the dichotomous (Y/N) EE variables from the Five Minute Speech Sample (FMSS).
3.4 Associations with Expressed Emotion Variables
Point-biserial correlations in the entire sample revealed that participants whose caretakers provided fewer positive initial statements also showed heightened levels of resting cortisol (r=-.34, p=.01). There was also a trend (r=.22, p=.08) to suggest that more critical statements were correlated with elevated resting-cortisol levels. However, it is interesting that both over-involvement and warmth variables were not associated with levels of cortisol. With regard to self-concept, it appears that both fewer initial positive statements (r=-.30, p=.02) and elevated critical statements (r=.27, p=.04) were moderately associated with negative self-concept. It is also not surprising that warm statements were moderately associated with positive self-concept scores (r=.30, p=.03). However, given the high frequency of this behavior (all but two cases in this sample reported warm statements), this last result should be interpreted with caution. In the exploratory analysis, the relationships between critical statements and positive symptoms was not significant, but there were weak trends to suggest that fewer positive statements (r=-.18, p=.13) and elevated over-involvement (r=.17, p=.14) statements were associated with increased positive symptoms. Increased warm statements were moderately associated with lower positive symptoms (r=-.26, p=.04). We also observed that fewer initial positive statements were associated with increased negative symptomatology (r=-.30, p=.03). While there were not associations with criticism and over-involvement, there was a weak trend to suggest that a lack of warm caretaker statements were associated with increased negative symptoms (r=-.20, p=.10). The correlations in the exploratory analysis of family environment with self-concept and symptoms did not pass Bonferroni corrections.
Point-biserial correlations in the UHR group alone should be interpreted with caution due to the small sample size and lack of power. The analysis revealed a moderate correlation at the trend level for participants whose caretakers provided more critical statements and heightened levels of resting cortisol (r=-.32, p=.07). There was no longer an associated between the number of positive initial statements and cortisol levels (r=-.26, p=.12) and both over-involvement and warmth variables continued to not be associated with cortisol levels (r=-.19, p=.20, r=.13, p=.28). Increased negative self-concept in the UHR sample continued to be moderately associated with elevated critical statements (r=.35, p=.05) but not with the initial positive (r=-.23, p=.15), over-involvement (r=.18, p=.20), and warm (r=-.02, p=.46) statements. Positive self-concept scores continued to only be associated with warm statements at the moderate level (r=.36, p=.05). While there were no significant relationships between positive statements, over-involvement, and warmth with positive symptoms, a new moderate correlation at the trend level emerged between critical statements and positive symptoms (r=.33, p=.06). The relationship between fewer initial positive statements and increased negative symptomatology in the UHR group fell to trend level (r=-.28, p=.10). The correlations in the exploratory analysis of family environment with self-concept and symptoms in the UHR group did not pass Bonferroni corrections.
4. Discussion
The present investigation provides a unique perspective of the adolescent high-risk period by examining UHR individuals and matched healthy controls at the endocrine (cortisol), individual (self-concept) and environmental (EE) level. This approach is effective at providing a novel understanding of the relationship between important factors which have until now been largely studied alone, and primarily in chronic populations (i.e., schizophrenia patients). Understanding these relationships is an important first step, which future longitudinal studies can build upon to evaluate causation. Taken together, we observed several noteworthy findings that are largely consistent with a neural diathesis-stress conception. Specifically, observations of HPA dysregulation in the UHR group replicate the results from several previous studies in distinct samples of high-risk individuals (Walker et al., 2010; Walker et al., 2013; Day et al., 2014; Karanikas and Garyfallos, 2014). In addition, findings that suggest that UHR adolescents report elevated negative self-concept improve our understanding of what may be an important risk factor and viable target for psychosocial intervention. While EE data was only available for a subset of the sample and results should be interpreted with caution, we observed significant differences in specific EE behaviors between the relatives/care-takers of the UHR participants and healthy controls, which were largely consistent with what is observed in the families of patients with schizophrenia (Bachmann et al., 2002). It is particularly noteworthy that some family behaviors such as criticism and emotional over-involvement were found to be associated with increased cortisol, negative self-concept, and symptom levels, whereas other behaviors such as positive and warm statements appear to be linked with the opposite effect. This is the first study to evaluate these relationships in a psychosis risk group and the results could have important ramifications for multi-family group interventions, which have been designed to reduce EE in the families of those with serious mental illness (Miklowitz et al., 2000). Finally, while it is not possible to determine causality with the present design, the close associations seen between the hormone, individual-level, and environmental factors support a neural diathesis-stress conception, and can serve as the framework for future large-scale longitudinal research in this vital area.
Studies have shown that the HPA axis is dysregulated in patients with schizophrenia (Belvederi Murri et al., 2012) and elevated cortisol levels are closely associated with positive symptoms such as unusual thoughts, suspiciousness, and perceptual anomalies (Franzen, 1971; Walder et al., 2000; Collip et al., 2011). As noted, present observations of HPA dysregulation are also consistent with previous studies showing that at-risk individuals have higher cortisol levels when compared to healthy controls (Mittal et al. 2007, Walker et al. 2010, Walker et al. 2013). One particular study that strongly supports this theory found that cortisol at baseline predicted eventual conversion to a psychotic disorder (in a 2 year period) (Walker et al., 2010). This speaks to how abnormalities in endocrine function appear in part to be driving the pathogenesis disorder.
However, some studies have found that HPA dysregulation is not related to symptoms in UHR individuals and suggest that there might be a more complicated relationship between HPA dysregulation and symptoms (Corcoran et al., 2012; Sugranyes et al., 2012; Cullen et al., 2014; Day et al., 2014). In particular anxiety (Corcoran et al., 2012; Karanikas and Garyfallos, 2014), suspiciousness (Corcoran et al., 2012), stressful life events (Labad et al., 2014), and stress intolerance (Pruessner et al., 2013; Karanikas and Garyfallos, 2014) have been found to be more closely related to cortisol levels in UHR individuals than symptoms. Given these divergent results, future research that systematically examines these relationships (with larger samples and specialized instruments) is needed to better understand HPA dysregulation in UHR individuals as the findings will be critical in further understanding the diathesis-stress model. While resting cortisol has been studied in a variety of ways in the UHR period, the field still has a poor understanding of behavioral correlates, particularly at the individual and environmental levels explored in the present study.
Negative self-concept has recently become an area of interest as a potential risk factor for developing psychosis, yet this is the first time this domain has been examined within the context of other biological and social risk factors in the UHR population. The current findings replicate previous observations that high-risk adolescents exhibit significantly higher negative self-concept when compared to healthy controls (Perivoliotis et al., 2009; Stowkowy and Addington, 2012; Saleem et al., 2014), which supports the theory that negative self-concept is present prior to the development of psychotic disorders such as schizophrenia. It is also important to consider that studies in healthy populations have found that negative self-concept is associated with heightened cortisol levels (Kirschbaum et al., 1995). Consistent with this research, present findings that higher negative self-concept was moderately associated with elevated cortisol suggest negative self-concept could be a risk factor that interacts with the underlying biological vulnerability of HPA dysfunction. In addition, greater negative self-concept was also strongly associated with higher positive and negative symptoms. While this finding is consistent with previous studies (Fowler et al., 2006; Smith et al., 2006; Taylor et al., 2013), it is important to note that the relationship is also complex, and not all investigations have detected an association (Saleem et al., 2014). In contrast, the healthy control adolescents in our study exhibited significantly higher positive self-concept when compared to the UHR group and across the sample there was a trend to suggest that lower cortisol levels were associated increased positive self-concept. Consistent with the direction of this finding, we also observed that higher positive self-concept scores were moderately linked to lower positive and negative symptoms. These findings have not been reported in previous studies, and future longitudinal studies should further examine this relationship as it may yield important new treatment targets.
Family environment and its impact on mental health has been the focus of significant previous research and the evidence suggests that it has a particularly strong impact on symptom severity for patients with schizophrenia (Barrowclough and Hooley, 2003). In healthy individuals, family environment during adolescence has been previously linked to endocrine function (Flinn and England, 1995) and the formation of self-concept (Schmidt and Padilla, 2003); however, this is the first exploratory examination of these relationships in a high-risk sample. It has been well established that family members of patients with schizophrenia have higher rates of criticism and emotional over-involvement and that these rates are highly related to symptom severity (Bachmann et al., 2002). While the present results should be interpreted with caution due to limited power and correlational design, it is surprising that there was a low rate of reported criticism and emotional over-involvement in the sample. One possibility is that this behavior emerges after the onset of schizophrenia, and reflects covariance between the patient's acute symptomatology and related behavioral dysfunction and the family's subsequent behaviors (Nuechterlein et al., 1992). The relationship is certainly complex and as a result, the few studies assessing EE in family members of UHR adolescents have provided mixed results. Some studies report that UHR adolescents living in critical family environments show significantly worse positive symptoms relative to those at-risk individuals in warm environments and warmth and optimal family involvement jointly predicts improved functioning (O'Brien et al., 2006; McFarlane and Cook, 2007; Schlosser et al., 2010). However, Meneghelli et al. (2011) did not find any links between severity of illness and EE in a sample of UHR adolescents. These divergent results suggest that criticism and emotional over-involvement might not be the best measures of EE in the UHR sample. The present finding that caretaker's initial statements were more negative and neutral in UHR group and this lack of positive statements had the strongest relationship to symptom severity supports previous observations and suggests that initial statement valance might be a more accurate measure of EE in UHR samples (Kershner et al., 1996; Daley et al., 2003). This pattern of statements was also associated with heightened levels of resting cortisol and negative self-concept. While the link with negative comments is highly consistent with theory (Barrowclough and Hooley, 2003), the finding related to disengagement and lower positive statements is open to interpretation.
As noted, a neural diathesis-stress model posits that an early biological vulnerability of the HPA system later interacts in the adolescent period with both individual factors (e.g., resiliency, maladaptive coping style) and environmental stressors as well as normative and pathological neuroendocrine development, eventually leading to the onset of psychotic disorders such as schizophrenia (Walker et al., 2008). The present findings support this model and provide a better understanding of the specific individual factors (self-concept) and environmental stress (family member's/caretaker's attitudes towards the participant) that are associated with HPA dysfunction. Future longitudinal studies are needed to better understand the causal relationship between these factors and to help the field understand how psychosocial variables are tied to the neuroendocrine abnormalities in part driving the disorder. These results are highly encouraging given that these factors have been successful targets of treatment and sensitive to change (Miklowitz et al., 2000; Lower et al., 2014), and suggest that self-concept and family environment should be targeted in early preventative treatments.
4.1 Study Limitations
The present study held several important innovations, and current results are consistent with a broader literature on biological, individual, and psychosocial risk factors for developing psychosis. The current approach has several strengths including acquiring data from multiple sources (i.e., biological, self-report, family member/caretaker), screening for formal psychosis, and conservative assessment of cortisol (i.e., duplicate analysis, collecting several samples to rule-out artifacts due to the novel environment, controlling for diet/exercise/contraceptives). However, there were also several limitations that we have highlighted.
Notable limitations of the present study include the correlational design and lack of power to examine the impact of other possible risk factors. Though group differences and correlations were observed it is impossible to determine causality and future studies with larger samples should be conducted before any definitive conclusions are made. Future studies examining causality should aim to determine if the putative family environment factors are contributing to a negative self-concept and cortisol levels directly, or if the self-concept domain moderates this relationship. Further, the high frequency of trend results may be a consequence of the lack of power and there is related potential for Type I error. Additionally, accumulating studies have found that there are a variety of potential contributors to HPA dysregulation in psychosis (Hunter et al., 2011; Corcoran et al., 2012; Pariante et al., 2012; Cullen et al., 2014; Karanikas and Garyfallos, 2014; Labad et al., 2014). Future studies should systematically examine the impact of important factors including comorbid mental illness, and trauma on cortisol levels in UHR individuals and use specific specialized scales. Trauma and early adversity should be examined in particular due to its observed relationship to both cortisol levels (Hunter et al., 2011) and negative sense of self (Addington et al., 2013).
Another limitation of the present study relates to the small number of participants with EE data. Specifically, only a subset of participants had family member/caretakers participate and there may be a possible sampling bias between those whose families chose to participate and those whose families did not. Although the present study did not find any differences between the whole sample and the subgroup of participants with EE data, it is possible that 3rd variable factors relating to a families willingness to participate may have affected the conclusions and overall generalizability of the present findings. Additionally, the sample size did not allow differences in type of caregiver to be examined (i.e. mother vs. father, parent with mental illness vs. not). Further, it is well known that parental psychopathology has genetic and environmental impacts on childhood risk for psychopathology (Tsuang, 2000; Tienari et al., 2004; McLaughlin et al., 2012) and it will be important for future studies to explore implications for family environment based on family history of psychiatric disorder. Additionally, it is important to note that some studies have found that the FMSS under-rates the occurrence of high EE (i.e. critical and over involvement statements) relative to the CFI (Halford, 1992). Therefore, it is possible that the present findings represent a conservative view of EE behaviors in these families. Future studies should consider incorporating additional self-report and continuous measures of EE to more accurately assess for family environment. Taken together, we believe that the results from this innovative study are an important first-step for what will hopefully be an area of research with expanded samples, specialized measures, and large scale studies.
Highlights.
Cortisol, self-concept, and family environment in UHR adolescents were analyzed.
The UHR group exhibited abnormal cortisol levels and elevated negative self-concept.
UHR relatives and caretakers reported fewer initial positive statements.
Elevated cortisol was associated with negative self–concept and family environment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Stowkowy J, Cadenhead KS, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Cannon TD. Early traumatic experiences in those at clinical high risk for psychosis. Early Interv. Psychiatr. 2013;7:300–305. doi: 10.1111/eip.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Tran L. Using the brief core schema scales with individuals at clinical high risk of psychosis. Behav. Cogn. Psychother. 2009;37:227–231. doi: 10.1017/S1352465809005116. [DOI] [PubMed] [Google Scholar]
- Bachmann S, Bottmer C, Jacob S, Kronmuller KT, Backenstrass M, Mundt C, Renneberg B, Fiedler P, Schroder J. Expressed emotion in relatives of first-episode and chronic patients with schizophrenia and major depressive disorder-a comparison. Psychiatr. Res. 2002;112:239–250. doi: 10.1016/s0165-1781(02)00226-3. [DOI] [PubMed] [Google Scholar]
- Barrowclough C, Hooley JM. Attributions and expressed emotion: a review. Clin. Pychol. Rev. 2003;23:849–880. doi: 10.1016/s0272-7358(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive Therapy and the Emotional Disoders. Penguin Group Penguin Books; New York, New York: 1979. [Google Scholar]
- Belvederi Murri M, Pariante CM, Dazzan P, Hepgul N, Papadopoulos AS, Zunszain P, Di Forti M, Murray RM, Mondelli V. Hypothalamic-pituitary-adrenal axis and clinical symptoms in first-episode psychosis. Psychoneuroendocrinol. 2012;37:629–644. doi: 10.1016/j.psyneuen.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Cole DA, Maxwell SE, Martin JM, Peeke LG, Seroczynski AD, Tram JM, Hoffman KB, Ruiz MD, Jacquez F, Maschman T. The development of multiple domains of child and adolescent self-concept: a cohort sequential longitudinal design. Child Dev. 2001;72:1723–1746. doi: 10.1111/1467-8624.00375. [DOI] [PubMed] [Google Scholar]
- Collip D, Nicolson NA, Lardinois M, Lataster T, van Os J, Myin-Germeys I. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol. Med. 2011;41:2305–2315. doi: 10.1017/S0033291711000602. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophr. Bull. 2003;29:671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- Corcoran CM, Smith C, McLaughlin D, Auther A, Malaspina D, Cornblatt B. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr. Res. 2012;135:170–174. doi: 10.1016/j.schres.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen AE, Fisher HL, Roberts RE, Pariante CM, Laurens KR. Daily stressors and negative life events in children at elevated risk of developing schizophrenia. Br. J. Psychiatr.: J. Ment. Sci. 2014;204:354–360. doi: 10.1192/bjp.bp.113.127001. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann. N. Y. Acad. of Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Daley D, Sonuga-Barke EJ, Thompson M. Assessing expressed emotion in mothers of preschool AD/HD children: psychometric properties of a modified speech sample. Br. J. Clin. Psych. / Br. Psych. Soc. 2003;42:53–67. doi: 10.1348/014466503762842011. [DOI] [PubMed] [Google Scholar]
- Day FL, Valmaggia LR, Mondelli V, Papadopoulos A, Papadopoulos I, Pariante CM, McGuire P. Blunted cortisol awakening response in people at ultra high risk of developing psychosis. Schizophr. Res. 2014;158:25–31. doi: 10.1016/j.schres.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Dominguez-Martinez T, Medina-Pradas C, Kwapil TR, Barrantes-Vidal N. Relatives illness attributions mediate the association of expressed emotion with early psychosis symptoms and functioning. Psychiatr. Res. 2014;218:48–53. doi: 10.1016/j.psychres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I) Patient Edition American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- Flinn MV, England BG. Childhood Stress and Family Environment Current Anthropology. 1995;36:854–866. [Google Scholar]
- Fowler D, Freeman D, Smith B, Kuipers E, Bebbington P, Bashforth H, Coker S, Hodgekins J, Gracie A, Dunn G, Garety P. The Brief Core Schema Scales (BCSS): psychometric properties and associations with paranoia and grandiosity in non-clinical and psychosis samples. Psych. Med. 2006;36:749–759. doi: 10.1017/S0033291706007355. [DOI] [PubMed] [Google Scholar]
- Franzen G. Serum cortisol in chronic schizophrenia. A study of the siurnal rhythm in relation to psychiatric mental status. J. Psychosom. Res. 1971;15:367–373. doi: 10.1016/0022-3999(71)90050-x. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr. Bull. 2014;40:120–131. doi: 10.1093/schbul/sbs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psych. Med. 2001;31:189–195. doi: 10.1017/s0033291701003312. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. B. J. Psychiatr.: J. Ment. Sci. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- Halford WK. Assessment of family interaction with a schizophrenic member, Schizophrenia. Springer; 1992. pp. 254–274. [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. Elevated Striatal Dopamine Function Linked to Prodromal Signs of Schizophrenia. Arch. Gen. Psychiatr. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Hunter AL, Minnis H, Wilson P. Altered stress responses in children exposed to early adversity: a systematic review of salivary cortisol studies. Stress. 2011;14:614–626. doi: 10.3109/10253890.2011.577848. [DOI] [PubMed] [Google Scholar]
- Karanikas E, Garyfallos G. The role of cortisol in At Risk for Psychosis mental state and psychopathological correlates: a systematic review. Psychiatr. Clin. Neurosci. 2014 doi: 10.1111/pcn.12259. DOI http://dx.doi.org/10.1111/pcn.12259. [DOI] [PubMed]
- Kershner JG, Cohen NJ, Coyne JC. Expressed emotion in families of clinically referred and nonreferred children: Toward a further understanding of the expressed emotion index. J. Fam. Psych. 1996;10:97–106. [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom. Med. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Labad J, Stojanovic-Perez A, Montalvo I, Sole M, Cabezas A, Ortega L, Moreno I, Vilella E, Martorell L, Reynolds RM, Gutierrez-Zotes A. Stress biomarkers as predictors of transition to psychosis in at-risk mental states: Roles for cortisol, prolactin and albumin. J. Psychiatr. Res. 2014;60C:163–169. doi: 10.1016/j.jpsychires.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Leeb B, Hahlweg K, Goldstein MJ, Feinstein E, Mueller U, Dose M, Magana-Amato A. Cross-national reliability, concurrent validity, and stability of a brief method for assessing expressed emotion. Psychiatr. Res. 1991;39:25–31. doi: 10.1016/0165-1781(91)90005-a. [DOI] [PubMed] [Google Scholar]
- Lower R, Wilson J, Medin E, Corlett E, Turner R, Wheeler K, Fowler D. Evaluating an early intervention in psychosis service for ‘high-risk’ adolescents: symptomatic and social recovery outcomes. Early Interv. Psychiatr. 2014 doi: 10.1111/eip.12139. DOI http://dx.doi.org/10.1111/eip.12139. [DOI] [PubMed]
- Luecken LJ, Kraft A, Hagan MJ. Negative relationships in the family-of-originpredict attenuated cortisol in emerging adults. Horm. Behav. 2009;55:412–417. doi: 10.1016/j.yhbeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magana AB, Goldstein MJ, Karno M, Miklowitz DJ, Jenkins J, Falloon IRH. A Brief Method for Assessing Expressed Emotion in Relatives of Psychiatric-Patients. Psychiatr. Res. 1986;17:203–212. doi: 10.1016/0165-1781(86)90049-1. [DOI] [PubMed] [Google Scholar]
- Malla AK, Kazarian SS, Barnes S, Cole JD. Validation of the five minute speech sample in measuring expressed emotion. Can. J. Psychiatr. 1991;36:297–299. doi: 10.1177/070674379103600411. [DOI] [PubMed] [Google Scholar]
- Marom S, Munitz H, Jones PB, Weizman A, Hermesh H. Expressed emotion: relevance to rehospitalization in schizophrenia over 7 years. Schizophr. Bull. 2005;31:751–758. doi: 10.1093/schbul/sbi016. [DOI] [PubMed] [Google Scholar]
- Martin CS, Pollock NK, Bukstein OG, Lynch KG. Inter-rater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug Alcohol Depen. 2000;59:173–176. doi: 10.1016/s0376-8716(99)00119-2. [DOI] [PubMed] [Google Scholar]
- McFarlane WR, Cook WL. Family expressed emotion prior to onset of psychosis. Fam. Process. 2007;46:185–197. doi: 10.1111/j.1545-5300.2007.00203.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Gadermann AM, Hwang I, Sampson NA, Al-Hamzawi A, Andrade LH, Angermeyer MC, Benjet C, Bromet EJ, Bruffaerts R, Caldas-de-Almeida JM, de Girolamo G, de Graaf R, Florescu S, Gureje O, Haro JM, Hinkov HR, Horiguchi I, Hu C, Karam AN, Kovess-Masfety V, Lee S, Murphy SD, Nizamie SH, Posada-Villa J, Williams DR, Kessler RC. Parent psychopathology and offspring mental disorders: results from the WHO World Mental Health Surveys. Br. J. Psychiatr.: J. Ment. Sci. 2012;200:290–299. doi: 10.1192/bjp.bp.111.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghelli A, Alpi A, Pafumi N, Patelli G, Preti A, Cocchi A. Expressed emotion in first-episode schizophrenia and in ultra high-risk patients: results from the Programma2000 (Milan, Italy). Psychiatr. Res. 2011;189:331–338. doi: 10.1016/j.psychres.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Simoneau TL, George EL, Richards JA, Kalbag A, Sachs-Ericsson N, Suddath R. Family-focused treatment of bipolar disorder: 1-year effects of a psychoeducational program in conjunction with pharmacotherapy. Biol. Psychiatr. 2000;48:582–592. doi: 10.1016/s0006-3223(00)00931-8. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr. Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: Minor physical anomalies, movement abnormalities, and salivary cortisol. Biol. Psychiatr. 2007;61:1179–1186. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Walker EF. Minor physical anomalies and vulnerability in prodromal youth. Schizophr. Res. 2011;129:116–121. doi: 10.1016/j.schres.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E, Kuipers E. The measurement of expressed emotion in relationships between staff and service users: the use of short speech samples. Br. J. Clin. Psychol. /Br. Psychol. Soc. 1999;38(Pt 4):345–356. doi: 10.1348/014466599162953. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, Yee CM, Mintz J. Developmental Processes in Schizophrenic Disorders: longitudinal studies of vulnerability and stress. Schizophr. Bull. 1992;18:387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- O'Brien MP, Gordon JL, Bearden CE, Lopez SR, Kopelowicz A, Cannon TD. Positive family environment predicts improvement in symptoms and social functioning among adolescents at imminent risk for onset of psychosis. Schizophr. Res. 2006;81:269–275. doi: 10.1016/j.schres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Alhaj HA, Arulnathan VE, Gallagher P, Hanson A, Massey E, McAllister-Williams RH. Central glucocorticoid receptor-mediated effects of the antidepressant, citalopram, in humans: a study using EEG and cognitive testing. Psychoneuroendocrinol. 2012;37:618–628. doi: 10.1016/j.psyneuen.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Perivoliotis D, Morrison AP, Grant PM, French P, Beck AT. Negative performance beliefs and negative symptoms in individuals at ultra-high risk of psychosis: a preliminary study. Psychopathol. 2009;42:375–379. doi: 10.1159/000236909. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinol. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Bechard-Evans L, Boekestyn L, Iyer SN, Pruessner JC, Malla AK. Attenuated cortisol response to acute psychosocial stress in individuals at ultra-high risk for psychosis. Schizophr. Res. 2013;146:79–86. doi: 10.1016/j.schres.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Saleem MM, Stowkowy J, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Addington J. Perceived discrimination in those at clinical high risk for psychosis. Early Interv. Psychiatr. 2014;8:77–81. doi: 10.1111/eip.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser DA, Zinberg JL, Loewy RL, Casey-Cannon S, O'Brien MP, Bearden CE, Vinogradov S, Cannon TD. Predicting the longitudinal effects of the family environment on prodromal symptoms and functioning in patients at-risk for psychosis. Schizophr. Res. 2010;118:69–75. doi: 10.1016/j.schres.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JA, Padilla B. Self-Esteem and Family Challenge: An Investigation of Their Effects on Achiev. J. Youth Adolesc. 2003;32:37–46. [Google Scholar]
- Smith B, Fowler DG, Freeman D, Bebbington P, Bashforth H, Garety P, Dunn G, Kuipers E. Emotion and psychosis: links between depression, self-esteem, negative schematic beliefs and delusions and hallucinations. Schizophr. Res. 2006;86:181–188. doi: 10.1016/j.schres.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Ann. N. Y. Acad. Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Stowkowy J, Addington J. Maladaptive schemas as a mediator between social defeat and positive symptoms in young people at clinical high risk for psychosis. Early Interv. Psychiatr. 2012;6:87–90. doi: 10.1111/j.1751-7893.2011.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugranyes G, Thompson JL, Corcoran CM. HPA-axis function, symptoms, and medication exposure in youths at clinical high risk for psychosis. J. Psychiatr. Res. 2012;46:1389–1393. doi: 10.1016/j.jpsychires.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HE, Stewart SL, Dunn G, Parker S, Fowler D, Morrison AP. Core Schemas across the Continuum of Psychosis: A Comparison of Clinical and Non-Clinical Groups. Behav. Cogn. Psychother. 2013;42:1–13. doi: 10.1017/S1352465813000593. [DOI] [PubMed] [Google Scholar]
- Tienari P, Wynne LC, Sorri A, Lahti I, Laksy K, Moring J, Naarala M, Nieminen P, Wahlberg KE. Genotype-environment interaction in schizophrenia-spectrum disorder. Long-term follow-up study of Finnish adoptees. Br. J. Psychiatr.: J. Ment. Sci. 2004;184:216–222. doi: 10.1192/bjp.184.3.216. [DOI] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: genes and environment. Biol. Psychiatr. 2000;47:210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Walker EF, Lewine RJ. Cognitive functioning, cortisol release, and symptom severity in patients with schizophrenia. Biol. Psychiatr. 2000;48:1121–1132. doi: 10.1016/s0006-3223(00)01052-0. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Ann. Rev. Clin. Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J. Abnorm. Psychol. 2010;119:401–408. doi: 10.1037/a0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, Heinssen R, Mathalon DH, Perkins DO, Seidman LJ, Tsuang MT, Cannon TD, McGlashan TH, Woods SW. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol. Psychiatr. 2013;74:410–417. doi: 10.1016/j.biopsych.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]