Abstract
Oral contraceptive (OC) use is associated with reduced ovarian cancer risk; however, associations with other contraceptive methods, such as intrauterine device (IUD) and tubal ligation (TL), are less clear. Women in China differ from western women in regard to mechanisms and duration of use of contraception. This study was undertaken to evaluate associations between contraceptive methods and ovarian cancer risk using data from the prospective Shanghai Women’s Health Study. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated by Cox proportional hazards regression. A total of 174 epithelial ovarian cancer cases were found to occur among 70,259 women that were followed for a total of 888,258 person years. The majority of women had ever used any contraception (77.0%), including IUD use (55.6%), OC use (20.4%), TL (14.7%), and contraceptive shots (2.6%). Ever use of any contraception was associated with a non-significant reduction in ovarian cancer risk (HR: 0.86, 95% CI: 0.60–1.24). Longer duration of IUD use was associated with lower ovarian cancer risk (P-value for trend=0.04). Compared to never users, women with durations of IUD use longer than the median (20 years) were 38% less likely to develop ovarian cancer (HR: 0.62, 95% CI: 0.40–0.97). Based on the high prevalence and long duration of IUD use among Chinese women, we estimate a preventive fraction of 9.3%, corresponding to approximately 16 ovarian cancer cases. High prevalence of long-term IUD use may therefore contribute to the low incidence of ovarian cancer observed in China.
Keywords: contraceptive, ovarian cancer risk, prospective cohort study
Introduction
Ovarian cancer is the sixth most common cancer and the seventh cause of cancer deaths among women worldwide.1 Due to the absence of specific symptoms and the lack of effective screening and early diagnostic techniques, ovarian cancer remains a highly deadly malignancy.2 Despite the lethality of ovarian cancer, the etiology of this disease remains poorly understood. Two hypotheses have been proposed: incessant ovulation3,4 and gonadotropin5,6 stimulation. Repeated ovulation and gonadotropin hormones both stimulate the ovarian surface epithelium, and may contribute to the development of ovarian cancer. However, a recent paradigm shift has resulted from a growing body of evidence that indicates that the majority of ovarian cancers, the serous papillary subtype, actually arise from the distal fallopian tube, and involve the ovary only secondarily.7–10 Regardless of the origins of ovarian cancer, oral contraceptive (OC) use is known to reduce ovarian cancer risk.11,12 Associations with other contraceptive methods, such as intrauterine device (IUD) and tubal ligation (TL), are less clear. The majority of studies conducted to date on contraceptive measures and ovarian cancer risk have used case-control designs;13,14 fewer cohort studies have been conducted.15,16 Further, most study populations have been comprised predominantly of Caucasian women.
The prevalence of contraception use in China ranks among the highest in the world.17 More than 269 million (88.95%) married women of reproductive age used contraception in China in 2009.18 Modes of contraceptive use are also unique to China: long-term contraceptive use, including IUD, TL and male sterilization, have been very common, with prevalence rates of approximately 80%, which have been stable since the 1980s.18 As patterns of contraception use differ between women in China and in Western countries, where use is dominated by short-term contraceptive methods,17 differences in associations with ovarian cancer risk may also exist. Therefore, we examined ovarian risk in relation to different contraceptive methods, including IUD, OC, TL and contraceptive shots, among Chinese women participating in a large-scale prospective cohort study.
Materials and Methods
Study Population
The Shanghai Women’s Health Study (SWHS) is an ongoing, population-based, prospective cohort study. Detailed descriptions of the SWHS have been previously described.19 Briefly, at baseline from March 1997 to May 2000, adult Chinese women aged 40 to 70 years were recruited from seven urban communities of Shanghai, China. Of women recruited, 74,941 (92%) completed a detailed baseline survey. Follow-up was administered by biennial in-person interviews and periodic linkage to the population-based Shanghai Cancer and Vital Statistics Registries. Incident cancer cases were verified by home visits and medical chart review. For analysis, ovarian cancer cases were defined as women for whom ovarian cancer was the first cancer diagnosis (ICD-9 code 183); only invasive malignancies were included (ICD-O-2 5th-digit behavior code 3). Women who had a previous diagnosis of any cancer (N = 1,598), an unverified diagnosis of cancer (N = 69), a prior oophorectomy (N = 3,117), unknown menopausal status (N=15), and those who died of cancer without a specific cancer diagnosis (N = 138), or were lost to follow-up (N = 5) were excluded; a total of 70,259 women were included in our analysis. This study was approved by the Institutional Review Boards of all relevant institutions in both the People’s Republic of China and the United States (US). All participants provided informed written consent.
Statistical Analysis
Cox proportional hazards regression was used to derive hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between ovarian cancer risk and contraceptive methods. For regression models, participant age was used as the time scale, with entry at age at recruitment, and exit at age of cancer diagnosis or else censored at the date of death or last contact (truncated at December 31, 2011) for non-cases. Validity of proportional hazards assumptions was examined by visual inspection of log (-log of the estimated survivor functions) versus log (time) plots as well as formal tests of interactions with time. Contraceptive methods evaluated in this study included IUD, OC, TL and contraceptive shots; ever use was compared to never use. Durations of exposure time were calculated as the interval between start and end of use, except for TL and IUD where removal had not occurred by baseline interview. For TL, exposure durations ended at age at menopause for postmenopausal women and at age 53 (mean age at menopause plus one standard deviation) for premenopausal women. For IUD durations where removal had not occurred prior to interview, duration stopped at age at interview plus two years for postmenopausal women, or age 53 for women premenopausal at interview. Had exposure time been defined as the duration to disease diagnosis for cases and the duration to the end of follow-up for controls, differential misclassification could have resulted in biased estimates of associations with contraception durations. Durations of use were dichotomized at the median values among controls; IUD use was further categorized by quartiles among controls. Cut-points were rounded to the nearest whole numbers. In regression models, factors evaluated as potential confounders included age at recruitment, education, years of ovulation, irregular ovulatory cycles (yes/no), first-degree family history of cancer (yes/no), body mass index (BMI), regular physical activity within 5 years (yes/no) and other contraceptive methods (never/ever). Total years of ovulation was calculated by subtracting age at menarche from either age at exit time (if premenopausal) or age at menopause (if postmenopausal), and then subtracting the total time spent pregnant, using oral contraceptives, and 55% of the time spent breastfeeding.20 In addition, analyses stratified by potential effect measure modifiers were conducted; interactions were evaluated with the likelihood ratio test. Histologic subtype was our a priori potential effect modifier; differences by parity were also examined based on our evaluation of the data. Histologic subtype was determined by ICD-O morphology code, and dichotomized as either serous or non-serous; cases with unknown histologic subtype (N=48) were excluded from these stratified analyses. The preventive fraction (PF) of ovarian cancer cases was calculated as (1-incidence in whole population/ incidence in unexposed population). All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) and used a two-sided probability with a significance level of 0.05.
Results
As shown in Table 1, 174 epithelial ovarian cancer cases occurred among 70,259 women after a total follow-up time of 888,258 person years (mean follow-up per person: 12.6 years, standard deviation (SD): 2.3). Ovarian cancer cases were diagnosed after a mean follow-up time of 6.5 years; these cases had a mean survival time of 4.2 years (data not shown). Distributions of demographic and selected ovarian cancer risk factors were found to differ between cases and non-cases; compared to non-cases, ovarian cancer cases were less likely to have a history of irregular ovulatory cycles (P=0.04), and more likely to have a later age at menopause (P=0.03) and longer duration of total years of ovulation (P=0.03).
Table 1.
Demographic and selected ovarian cancer risk factors among participants; the Shanghai Women’s Health Study
| Characteristics | Cases* | Non-Cases* | P** |
|---|---|---|---|
| Participants (N) | 174 | 70085 | – |
| Total person-years | 1132.4 | 887126.0 | – |
| Age at recruitment | 52.6±8.7 | 52.5±9.1 | 0.90 |
| Education | 0.83 | ||
| Elementary School or below | 40 (23.0%) | 15074 (21.5%) | |
| Junior high shool | 59 (33.9%) | 26179 (37.4%) | |
| High School | 51 (29.3%) | 19504 (27.8%) | |
| College or above | 24 (13.8%) | 9315 (13.3%) | |
| Ever married | 173 (99.43%) | 69480 (99.12%) | 1.00 |
| Age at menarche | 14.9±1.7 | 14.9±1.7 | 0.63 |
| Ever had irregular ovulatory cycles | 5 (2.9%) | 4688 (6.7%) | 0.04 |
| History of infertility | 3 (1.7%) | 362 (0.5%) | 0.06 |
| Ever pregnant | 167 (96.0%) | 68297 (97.5%) | 0.22 |
| Ever had a live birth | 167 (96.0%) | 67873 (96.8%) | 0.51 |
| Ever had an abortion | 89 (51.2%) | 40973 (58.5%) | 0.05 |
| Ever had a miscarriage | 27 (15.5%) | 8347 (11.9%) | 0.14 |
| Ever had salpingocyesis or ectopic pregnancies | 3 (1.7%) | 702 (1.0%) | 0.25 |
| Number of pregnancies | 2.8±1.5 | 2.8±1.5 | 0.89 |
| Number of live births | 1.8±1.3 | 1.8±1.2 | 0.97 |
| Premenopausal | 87 (50.0%) | 36653 (52.3%) | 0.54 |
| Age at menopause1 | 50.1±3.4 | 49.2±3.7 | 0.03 |
| Total weeks of pregnancies | 77.7±50.4 | 78.2±49.8 | 0.88 |
| Total months of breastfeeding | 16.0±20.0 | 15.6±18.4 | 0.78 |
| Total years of ovulation | 29.5±4.8 | 28.7±5.0 | 0.03 |
| Ever used hormone replacement therapy (HRT) | 3 (1.7%) | 1154 (1.7%) | 0.77 |
| First-degree family history of cancer | 50 (28.7%) | 18443 (26.3%) | 0.47 |
| Family history of breast cancer or ovarian cancer | 4 (2.3%) | 1473 (2.1%) | 0.79 |
| Exercised regularly in past 5 years | 60 (34.5%) | 24372 (34.8%) | 0.94 |
| Ever smoked regularly | 8 (4.6%) | 1951 (2.8%) | 0.16 |
| Ever drank regularly | 5 (2.9%) | 1585 (2.3%) | 0.60 |
| Ever drank tea regularly | 53 (30.5%) | 20946 (29.9%) | 0.87 |
| BMI | 24.4±3.8 | 24.0±3.4 | 0.15 |
Among women with natural menopause
n (%) for categorical variables or mean ± sd for continuous variables
P-values derived from χ2 or exact tests for categorical variables or t-tests for continuous variables
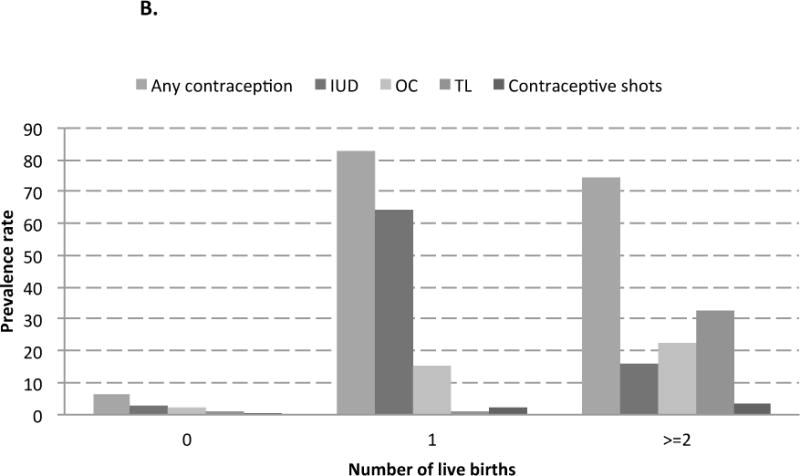
The majority of women in the SWHS had ever used any contraception (77.0%), including IUD use (55.6%), OC use (20.4%), TL (14.7%), and contraceptive shots use (2.6%). Modes of contraceptive use were found to differ by age and parity among women in the SWHS (Figure 1). Younger women (40–49 years old at recruitment) or uniparous women had the highest prevalence of contraceptive use; IUD was the most frequent contraceptive method ever used (prevalence rates of 68.4% and 64.3%, respectively). Among middle age women (50–59 years old at recruitment), oral contraceptives were the most frequent contraceptive method ever used (31.2% prevalence). Among older women (60–70 years old at recruitment) and multiparous women, TL was the most frequent contraceptive measure (prevalence rates of 37.7% and 32.8%, respectively).
Figure 1.
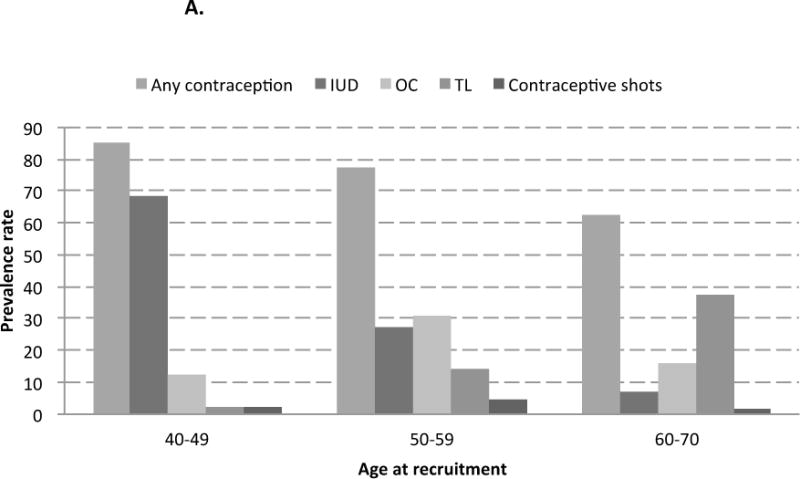
A. Contraceptive methods ever used, by age at recruitment, the Shanghai Women’s Health Study
B. Contraceptive methods ever used, by number of live births, the Shanghai Women’s Health Study
Associations between contraceptive use and ovarian cancer risk among all women are shown in Table 2. Ever use of any contraception was associated with a non-significant reduction in ovarian cancer risk (HR: 0.86, 95% CI: 0.60–1.24). Durations of use, dichotomized at the median among controls, were also compared to never users, except for contraceptive shots which had a very low prevalence rate and resulted in unstable estimates. Durations of contraception use longer than the median (20 years) also had a non-significant reduction in ovarian cancer risk (HR: 0.84, 95% CI: 0.50–1.42). A significant decreasing trend in ovarian cancer risk was found for women with increasing durations of IUD use (P-value for trend=0.04). Compared to never users, women with durations of IUD use longer than the median (20 years) were 38% less likely to develop ovarian cancer (HR: 0.62, 95% CI: 0.40–0.97). Women with durations of OC use longer than the median (2 years) also tended to have a decreased ovarian cancer risk (HR: 0.72, 95% CI: 0.38–1.36), although this association was not significant.
Table 2.
Association of contraceptive methods with ovarian cancer risk; the Shanghai Women’s Health Study
| Total | Cases | Non-Cases | HR1 | P | HR2 | P | |
|---|---|---|---|---|---|---|---|
| Ever Use | |||||||
| IUD | |||||||
| Never | 31205 (44.4%) | 83 (47.7%) | 31122 (44.4%) | 1 (reference) | – | 1 (reference) | – |
| Ever | 39054 (55.6%) | 91 (52.3%) | 38963 (55.6%) | 0.82 (0.57–1.16) | 0.26 | 0.79 (0.55–1.13) | 0.19 |
| OC | |||||||
| Never | 55911 (79.6%) | 137 (78.7%) | 55774 (79.6%) | 1 (reference) | – | 1 (reference) | – |
| Ever | 14348 (20.4%) | 37 (21.3%) | 14311 (20.4%) | 0.95 (0.66–1.37) | 0.78 | 1.02 (0.69–1.51) | 0.91 |
| TL | |||||||
| Never | 59947 (85.3%) | 148 (85.1%) | 59799 (85.3%) | 1 (reference) | – | 1 (reference) | – |
| Ever | 10311 (14.7%) | 26 (14.9%) | 10285 (14.7%) | 1.06 (0.67–1.68) | 0.80 | 0.96 (0.60–1.56) | 0.88 |
| Contraceptive Shots | |||||||
| Never | 68439 (97.4%) | 168 (96.6%) | 68271 (97.4%) | 1 (reference) | – | 1 (reference) | – |
| Ever | 1819 (2.6%) | 6 (3.5%) | 1813 (2.6%) | 1.24 (0.55–2.81) | 0.60 | 1.33 (0.58–3.04) | 0.51 |
| Any Contraception | |||||||
| Never | 16146 (23.0%) | 44 (25.3%) | 16102 (23.0%) | 1 (reference) | – | 1 (reference) | – |
| Ever | 54113 (77.0%) | 130 (74.7%) | 53983 (77.0%) | 0.86 (0.61–1.23) | 0.41 | 0.86 (0.60–1.24) | 0.42 |
| Years of Use* | |||||||
| IUD* | |||||||
| Never Used | 31205 (44.4%) | 83 (47.7%) | 31122 (44.4%) | 1 (reference) | – | 1 (reference) | – |
| <20 | 17857 (25.4%) | 51 (29.3%) | 17806 (25.4%) | 0.95 (0.65–1.39) | 0.79 | 0.93 (0.63–1.37) | 0.70 |
| ≥20 | 21197 (30.2%) | 40 (23.0%) | 21157 (30.2%) | 0.65 (0.42–1.02) | 0.06 | 0.62 (0.40–0.97) | 0.04 |
| P-value for trend | 0.07 | 0.04 | |||||
| OC | |||||||
| Never Used | 55911 (79.6%) | 137 (78.7%) | 55774 (79.6%) | 1 (reference) | – | 1 (reference) | – |
| <2 | 7135 (10.2%) | 24 (13.8%) | 7111 (10.2%) | 1.24 (0.80–1.92) | 0.34 | 1.24 (0.79–1.94) | 0.34 |
| ≥2 | 7208 (10.3%) | 13 (7.5%) | 7195 (10.3%) | 0.66 (0.37–1.17) | 0.16 | 0.72 (0.38–1.36) | 0.31 |
| P-value for trend | 0.36 | 0.65 | |||||
| TL** | |||||||
| Never Used | 59948 (85.3%) | 148 (85.1%) | 59800 (85.3%) | 1 (reference) | – | 1 (reference) | – |
| <20 | 5184 (7.4%) | 10 (5.8%) | 5174 (7.4%) | 0.83 (0.42–1.63) | 0.59 | 0.82 (0.41–1.64) | 0.57 |
| ≥20 | 5127 (7.3%) | 16 (9.2%) | 5111 (7.3%) | 1.27 (0.74–2.18) | 0.39 | 1.08 (0.61–1.91) | 0.79 |
| P-value for trend | 0.53 | 0.92 | |||||
| Any Contraception | |||||||
| Never Used | 16146 (23.0%) | 44 (25.3%) | 16102 (23.0%) | 1 (reference) | – | 1 (reference) | – |
| <20 | 23967 (34.1%) | 66 (37.9%) | 23901 (34.1%) | 0.97 (0.66–1.42) | 0.86 | 1.12 (0.67–1.87) | 0.67 |
| ≥20 | 30140 (42.9%) | 64 (36.7%) | 30076 (42.9%) | 0.76 (0.51–1.14) | 0.18 | 0.84 (0.50–1.42) | 0.52 |
| P-value for trend | 0.16 | 0.11 | |||||
Adjusted for age at recruitment
Adjusted for age at recruitment, education, years of ovulation, irregular ovulatory cycles (yes/no), first-degree family history of cancer regular exercise in past 5 years (yes/no), and other contraceptive methods (never/ever)
Years of use if IUD removed, or until age at interview plus two years for post-menopausal women or to age 53 (mean plus one standard deviation) for pre-menopausal women with IUDs present at interview
Years of use until menopause for post-menopausal women, or to age 53 (mean plus one standard deviation) for women pre-menopausal at interview
Given our high prevalence of IUD use, a more thorough exploration of the association between years of IUD use and ovarian cancer risk was conducted (Table 3). Associations among IUD users also indicated that women with longer durations of IUD use had lower risk of ovarian cancer (P-value for trend=0.01). Compared to women with less than 12 years of use, women with more than 24 years of IUD use were 44% less likely to develop ovarian cancer (HR: 0.56, 95% CI: 0.33–0.93). IUD removal status among IUD users at interview was also evaluated; a significant risk reduction was only evident for women whose IUD was present (HR: 0.55, 95% CI: 0.35–0.88). This finding was unaltered when non-users were included as the reference group; ovarian cancer risk only reduced among women with IUDs not removed by baseline interview (data not shown).
Table 3.
Duration and Cessation of IUD Use and Ovarian Cancer Risk, among users, the Shanghai Women’s Health Study
| Total | Cases | Non-Cases | HR1 | P | HR2 | P | |
|---|---|---|---|---|---|---|---|
| Among IUD Users | |||||||
| Years of Use* | |||||||
| <12 years | 9235 (23.7%) | 31 (34.1%) | 9204 (23.6%) | 1 (reference) | – | 1 (reference) | – |
| 12≤ to <20 years | 8622 (22.1%) | 20 (22.0%) | 8602 (22.1%) | 0.71 (0.43–1.15) | 0.16 | 0.67 (0.41–1.10) | 0.11 |
| 20≤ to <24 years | 9811 (25.1%) | 20 (22.0%) | 9791 (25.1%) | 0.65 (0.39–1.07) | 0.09 | 0.61 (0.37–1.02) | 0.06 |
| ≥24 years | 11386 (29.2%) | 20 (22.0%) | 11366 (29.2%) | 0.59 (0.35–0.98) | 0.04 | 0.56 (0.33–0.93) | 0.02 |
| P-value for trend | 0.02 | 0.01 | |||||
| IUD Removal | |||||||
| Removed by Interview | 16023 (41.0%) | 48 (52.8%) | 15975 (41.0%) | 1 (reference) | – | 1 (reference) | – |
| Present at Interview | 23031 (59.0%) | 43 (47.3%) | 22988 (59.0%) | 0.58 (0.37–0.91) | 0.02 | 0.55 (0.35–0.88) | 0.01 |
Adjusted for age at recruitment
Adjusted for age at recruitment, education, years of ovulation, irregular ovulatory cycles (yes/no), first-degree family history of cancer (yes/no), BMI, exercised regularly in past 5 years (yes/no), and other contraceptive methods (never/ever)
Years of use if IUD removed, or until age at interview plus two years for post-menopausal women or to age 53 (mean plus one standard deviation) for pre-menopausal women with IUDs present at interview
To examine if these associations differed by histologic subtype, stratified analyses were conducted for serous and non-serous ovarian cancers; cases with unspecified histologic subtype were excluded. The only significant interaction by histologic subtype was for contraceptive shots, where estimates were imprecise due to a low prevalence of the exposure. Additional analyses were also conducted to examine effect modification by parity, however, our power was lacking, due to the small sample size of uniparous cases who had ever had TL (N=1). Sensitivity analyses were conducted to evaluate the robustness of our findings by excluding women who had a history of infertility (N=372), had never been pregnant (N=1,810) or who had reasons for cessation of menstruation other than naturally occurring menopause (N=2,102); results from these analyses yielded very similar associations as those found among all women (data not shown). In addition, analyses excluding women with IUDs not removed by baseline interview (N=23,021) were conducted; the protective association between ovarian cancer risk and longer duration of IUD use was attenuated (HR: 0.80, 95% CI: 0.48–1.31). Notably, these findings were markedly different as the median duration of IUD use decreased from 20 to 10 years, and 43 cases were excluded from analysis. Finally, validity of the proportional hazards assumptions was evaluated; only whether or not IUD was removed was found to significantly interact with time (P-value=0.01).
Discussion
To the best of our knowledge, this is the first prospective cohort study examining the associations between contraceptive use and ovarian cancer risk among Asian women. We found that longer duration of IUD use was associated with lower risk of ovarian cancer in our study population of 70,259 women followed for a mean of 12.6 years, and that this result did not substantially differ by histologic subtype. Based on a preventative fraction of 9.3%, which was estimated as (1-incidence of ovarian cancer in all participants (174/70259) / incidence in women without long-term IUD (no longer than the median) use (134/49062)), we estimated that approximately 16 (preventative fraction (9.3%) times the total number of cases (174)) ovarian cancer cases were prevented by long-term IUD use in the Shanghai Women’s Health Study. Notably, modes of contraceptive use were found to differ by age and parity among women in the SWHS. This is in general agreement with the changes in family planning that occurred in China over the last forty years,18,21 and indicates that findings from studies on contraceptive measures or reproductive factors and women’s health outcomes among Chinese study populations should consider possible resulting effects.
IUD use is the most common contraceptive method in China. The prevalence rate of IUD use among married women of reproductive age in China reached 48.2% in 2010,18 and Chinese women comprise nearly two-thirds of the IUD users worldwide.22 Further, IUD use among Chinese women has been shown to be long; a cross-sectional survey found that more than 50% of postmenopausal IUD users had durations of use of more than 20 years.23 The underlying biologic mechanisms of IUD on ovarian cancer risk remain unclear, but a lowered ovarian cancer risk related to IUD use has been reported by several case-control studies conducted among Asian women.24–27 While our analysis for ever versus never IUD use did not reach statistical significance, we did find that longer duration of IUD use was associated with lower ovarian cancer risk. This finding is inconsistent with a prior report from the SWHS that found no association between IUD use and ovarian cancer risk.28 However, the earlier analysis was based on 94 ovarian cancer cases with 7.5 years of follow-up, and dichotomized duration of use at 14 years. With nearly double the cases and follow-up time, the current report has considerably greater power to detect an association, should it exist, and found an association among women with more than 20 years of use. The current finding is also inconsistent with results from the US Nurses’ Health Study (NHS), which reported that ever use of IUD was associated with an elevated ovarian cancer risk (RR = 1.76, 95% CI: 1.08, 2.85).16 However, no information on the duration of IUD use was included in this report, and the prevalence of IUD use in this US study population was very low (2.9%). It is also possible that the relation between IUD use and ovarian cancer risk differs by the type of IUD, as the mechanism of how IUD prevents pregnancy varies by IUD type.29 Most IUD use in our study population was from 1975 to 1990; about 90% of IUD use during this period in China was the stainless steel ring (SSR) IUD.30 Most IUD use in the Nurses’ Health Study was in the 1970s–1980s,16 when the malleable polyethylene plastic Dalkon Shield IUD was the most common model in the US.31 The Dalkon Shield IUD was later found to cause numerous negative health effects including bleeding, pelvic inflammatory disease and infertility due to its flawed design, which included a multifilament string.31 Given that inflammation may contribute to ovarian cancer tumorigenesis and progression,32 the elevated risk observed in the US Nurses’ Health Study may be due to the high prevalence of Dalkon Shield use among IUD users, and the high inflammatory rate caused by it.33 It is worth noting that after the SSR was proved to have a high failure rate of preventing pregnancy, copper-bearing IUDs replaced SSRs and became the dominant IUD used in China since the 1990s.34 Thus, the association of IUD use with ovarian cancer risk in the current study may not translate to the long-term effects of IUDs that are currently used in China. Finally, in the current study, a reduced risk of ovarian cancer was found only among women without IUD removal by baseline. This may be due to the difference in mean duration of use between women with IUDs removed and present at interview (10 vs. 25 years; p<0.01). Thus, differences in the duration of IUD use may also contribute to differences in associations with ovarian cancer risk across different study populations. Further research on the underlying biologic mechanisms between use of different IUD types and ovarian cancer risk is needed.
OC use is a well-known protective factor for ovarian cancer.11 Biologic mechanisms underlying this association include the reduction in number of lifetime ovulations and the elimination of the midcycle surge of luteinizing hormone during use.12 OC use reduces ovarian cancer risk in a duration-dependent manner: the benefit is more pronounced among women with long-term use,16,35 the effect lasts for 20–30 years after cessation of use,36 after which attenuation of the benefit occurs.16 In the current study, we did not find a significant association between ever OC use or long duration of OC use and ovarian cancer risk. This is in agreement with other case-control studies conducted among Chinese or Asian women.26,27,37,38 The prevalence rate of OC use in China has remained relatively low, as compared to the rest of the world.18 Only 20.4% participants in our study had ever used OC, and only 24.5% of the users had more than 5 years duration of use; this resulted in a very low prevalence of long-term OC users (5.0%) in our study population. Further, given that OC use usually occurs during early reproductive years,16 our older study population (mean age at recruitment: 52.5 years) resulted in a long duration since cessation of use (mean duration since last use: 31.9 years). Because of the low prevalence rate of long-term OC use and long duration since cessation of OC use in our study population, the protective effect from OC may be too small to detect or else already attenuated by the time of recruitment, thus prohibiting us from exploring the association between OC and ovarian cancer risk in this study population.
TL was a dominant contraceptive method in China in the 1980s (35% prevalence rate), but its prevalence has gradually declined (26.6% in 2010). Moreover, the prevalence of TL varies by geographical region; the overall prevalence is lower in urban areas than rural areas (15.2% versus 38.0%), and lower in eastern regions than central or western regions (10.9% versus 19.5% or 49.4%, respectively).18 The prevalence of TL in our study population, women from Shanghai, a large eastern city of China, was 14.7%. A number of studies have reported that TL decreases the risk of developing ovarian cancer, including case-control studies conducted among Asian women.26,27,37 A recent pooled analysis of data from 13 studies from the Ovarian Cancer Association Consortium also found a significant protective effects for TL; this was strongest for the invasive endometrioid histologic subtype.39 Proposed biological mechanisms for the reduction in risk include limiting the ascension of inflammatory agents or retrograde menstruation.40 In the current study, we did not find a significant association between either TL or time since TL and ovarian cancer risk.
The primary limitation of our study is the relatively small number of ovarian cancer cases that occurred in our study population. This is because China has a low incidence of ovarian cancer (3.8/100,000, compared to 6.3/100,000 worldwide in 2008).41 With only 174 ovarian cancer cases, our analyses stratified by histologic subtype were limited to dichotomizing into serous and non-serous, and excluding unknown and unspecified cancer types. Another limitation is that information on contraception use was only collected at baseline interview; updated information on IUD removal will be solicited in a future SWHS questionnaire. Additional limitations include the low prevalence of contraceptive shots and TL, long-term OC use, and the long duration of time since cessation of OC use; this prohibited us from fully exploring these associations. Finally, condom use was not included in our original questionnaire, nor was it included in the analyses of any contraception use. Given that condom use has not been reported to be associated with ovarian cancer risk, and that the prevalence of condom use among Chinese women was previously found to be low (2.35% in 1980),18 we believe that condom use would not likely alter our results on the association between “ever-use of any contraception” and ovarian cancer risk. Strengths of this study include a large-scale population based prospective study design, and that contraceptive information was collected before ovarian cancer occurred, avoiding the issue of differential recall bias. Further, the high prevalence and long-term use of IUD among Chinese women provided a unique opportunity to examine these associations.
In summary, we found a strong reduction in ovarian cancer risk among women with long-term IUD use in a prospective cohort study of Chinese women. This association occurred only among women with IUDs present at baseline interview. The high prevalence of long-term IUD use and the associated strong protective effect may contribute to the low incidence of ovarian cancer observed in China. Notably, these findings may not be generalizable to other populations, as the prevalence of IUD use and types of IUD commonly in use differ by geographic location and time. An additional implication of this research is that very large study populations, with very long durations of follow-up are needed in order to fully characterize the effects of contraceptive measures and ovarian cancer risk; research on the underlying biologic mechanisms of how different types of IUD influence ovarian cancer risk and whether these associations differ by ovarian cancer subtypes is also recommended.
Women in China have a high prevalence of long-term contraception use that includes intrauterine device (IUD) use and tubal ligation (TL). Results from a large prospective study of Chinese women indicate that women with durations of IUD use longer than the median (20 years) had significantly lower ovarian cancer risk. High prevalence of long-term IUD use may contribute to the low incidence of ovarian cancer in China.
Acknowledgments
The authors thank investigators and staff members of the research teams and study participants for their contributions and support for the study.
Funding: This study was supported by a grant from the US NIH (R37 CA070867, PI. W. Zheng). Zhezhou Huang is supported by the Vanderbilt-Shanghai Chronic Disease Research Training Program grant from the Fogarty International Center (D43 TW008313, PI. X.O. Shu). Dr. Beeghly-Fadiel is supported in part by a grant from the National Institutes of Health, National Institute of Child Health and Human Development (5 K12 HD 043483-05; PI: K. Hartmann).
Footnotes
Conflict of interest: None declared.
References
- 1.Boyle P, Levin B. World Cancer Report 2008 [Internet] Lyon: International Agency for Research on Cancer (IARC); 2008. pp. 412–417. Available from: http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/wcr_2008.pdf. [Google Scholar]
- 2.Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecol Oncol. 2013;129:258–64. doi: 10.1016/j.ygyno.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casagrande JT, Louie EW, Pike MC, Roy S, Ross RK, Henderson BE. “Incessant ovulation” and ovarian cancer. Lancet. 1979;2:170–3. doi: 10.1016/s0140-6736(79)91435-1. [DOI] [PubMed] [Google Scholar]
- 4.Fathalla MF. Incessant ovulation–a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 5.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71:717–21. [PubMed] [Google Scholar]
- 6.Mohle J, Whittemore A, Pike M, Darby S. Gonadotrophins and ovarian cancer risk. J Natl Cancer Inst. 1985;75:178–80. [PubMed] [Google Scholar]
- 7.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 8.Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch Int J Pathol. 2012;460:237–49. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- 9.Erickson BK, Conner MG, Landen CN., Jr The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013 doi: 10.1016/j.ajog.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nik NN, Vang R, Shih I-M, Kurman RJ. Origin and Pathogenesis of Pelvic (Ovarian, Tubal, and Primary Peritoneal) Serous Carcinoma. Annu Rev Pathol. 2013 doi: 10.1146/annurev-pathol-020712-163949. [DOI] [PubMed] [Google Scholar]
- 11.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol Clifton NJ. 2009;472:413–37. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]
- 12.Ness RB, Grisso JA, Vergona R, Klapper J, Morgan M, Wheeler JE. Oral Contraceptives, Other Methods of Contraception, and Risk Reduction for Ovarian Cancer. Epidemiology. 2001;12:307–12. doi: 10.1097/00001648-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 13.La Vecchia C. Oral contraceptives and ovarian cancer: an update, 1998–2004. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP. 2006;15:117–24. doi: 10.1097/01.cej.0000179274.24200.9d. [DOI] [PubMed] [Google Scholar]
- 14.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–14. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 15.Kumle M, Weiderpass E, Braaten T, Adami H-O, Lund E, Norwegian-Swedish Women’s Lifestyle and Health Cohort Study Risk for invasive and borderline epithelial ovarian neoplasias following use of hormonal contraceptives: the Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Br J Cancer. 2004;90:1386–91. doi: 10.1038/sj.bjc.6601715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166:894–901. doi: 10.1093/aje/kwm157. [DOI] [PubMed] [Google Scholar]
- 17.World Contraceptive Use 2011 [Internet] United Nations; 2011. Available from: http://www.un.org/esa/population/publications/contraceptive2011/contraceptive2011.htm. [Google Scholar]
- 18.Wang C. Trends in contraceptive use and determinants of choice in China: 1980–2010. Contraception. 2012;85:570–9. doi: 10.1016/j.contraception.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Zheng W, Chow W-H, Yang G, Jin F, Rothman N, Blair A, Li H-L, Wen W, Ji B-T, Li Q, Shu X-O, Gao Y-T. The Shanghai Women’s Health Study: Rationale, Study Design, and Baseline Characteristics. Am J Epidemiol. 2005;162:1123–31. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 20.Risch HA, Weiss NS, Lyon JL, Daling JR, Liff JM. Events of reproductive life and the incidence of epithelial ovarian cancer. Am J Epidemiol. 1983;117:128–39. doi: 10.1093/oxfordjournals.aje.a113523. [DOI] [PubMed] [Google Scholar]
- 21.Wang C. History of the Chinese Family Planning program: 1970–2010. Contraception. 2012;85:563–9. doi: 10.1016/j.contraception.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhan G, Ling L, Ying L, Li Y, Jin W, Li W. Regional economic levels and adverse events linked to intrauterine devices. J Evid-Based Med. 2011;4:8–14. doi: 10.1111/j.1756-5391.2011.01115.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang R-F, Liu X-Q, Chen J-P, Zhang Y-E, Chai L-P, Miao M-H, Yuan W, Liang H. Removal rate and service time of intrauterine devices among 2054 peri- or postmenopausal women. Reprod Contracept. 2014;34:41–5. [Google Scholar]
- 24.Miao Q, Kong B-H. A case-control study on etiology of epithelial ovarian cancer in Shandong Province. Ai Zheng Aizheng Chin J Cancer. 2006;25:871–5. [PubMed] [Google Scholar]
- 25.Zhang G, Jiang S, Zhang F. Influence factors in etiology of epithelial ovarian cancer. Zhonghua Fu Chan Ke Za Zhi. 1996;31:357–60. [PubMed] [Google Scholar]
- 26.Shu XO, Brinton LA, Gao YT, Yuan JM. Population-based case-control study of ovarian cancer in Shanghai. Cancer Res. 1989;49:3670–4. [PubMed] [Google Scholar]
- 27.Le D-C, Kubo T, Fujino Y, Sokal DC, Vach TH, Pham T-M, Matsuda S. Reproductive factors in relation to ovarian cancer: a case-control study in Northern Vietnam. Contraception. 2012;86:494–9. doi: 10.1016/j.contraception.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Dorjgochoo T, Shu X-O, Li H-L, Qian H-Z, Yang G, Cai H, Gao Y-T, Zheng W. Use of oral contraceptives, intrauterine devices and tubal sterilization and cancer risk in a large prospective study, from 1996 to 2006. Int J Cancer J Int Cancer. 2009;124:2442–9. doi: 10.1002/ijc.24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mechanism of action, safety and efficacy of intrauterine devices. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1987;753:1–91. [PubMed] [Google Scholar]
- 30.Kaufman J. The Cost of IUD Failure in China. Stud Fam Plann. 1993;24:194–6. [PubMed] [Google Scholar]
- 31.Sonfield A. Popularity Disparity: Attitudes About the IUD in Europe and the United States. Guttmacher Policy Rev. 2007;10:19–24. [Google Scholar]
- 32.Macciò A, Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012;58:133–47. doi: 10.1016/j.cyto.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Lee NC, Rubin GL, Ory HW, Burkman RT. Type of intrauterine device and the risk of pelvic inflammatory disease. Obstet Gynecol. 1983;62:1–6. [PubMed] [Google Scholar]
- 34.Bilian X. Chinese experience with intrauterine devices. Contraception. 2007;75:S31–S34. doi: 10.1016/j.contraception.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Bosetti C, Negri E, Trichopoulos D, Franceschi S, Beral V, Tzonou A, Parazzini F, Greggi S, La Vecchia C. Long-term effects of oral contraceptives on ovarian cancer risk. Int J Cancer J Int Cancer. 2002;102:262–5. doi: 10.1002/ijc.10696. [DOI] [PubMed] [Google Scholar]
- 36.Siskind V, Green A, Bain C, Purdie D. Beyond Ovulation: Oral Contraceptives and Epithelial Ovarian Cancer. Epidemiology. 2000;11:106–10. doi: 10.1097/00001648-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Wu PC, Lang JH, Ge WJ, Hartge P, Brinton LA. Risk factors for epithelial ovarian cancer in Beijing, China. Int J Epidemiol. 1992;21:23–9. doi: 10.1093/ije/21.1.23. [DOI] [PubMed] [Google Scholar]
- 38.Pasalich M, Su D, Binns CW, Lee AH. Reproductive factors for ovarian cancer in southern Chinese women. J Gynecol Oncol. 2013;24:135–40. doi: 10.3802/jgo.2013.24.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sieh W, Salvador S, McGuire V, Weber RP, Terry KL, Rossing MA, Risch H, Wu AH, Webb PM, Moysich K, Doherty JA, Felberg A, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013;42:579–89. doi: 10.1093/ije/dyt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cibula D, Widschwendter M, Májek O, Dusek L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update. 2011;17:55–67. doi: 10.1093/humupd/dmq030. [DOI] [PubMed] [Google Scholar]
- 41.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]


