Abstract
Bipolar Disorder (BD) is a unique disorder that transcends domains of function since the same patient can exhibit depression or mania, states with polar opposite mood symptoms. During depression, people feel helplessness, reduced energy, and risk aversion, while with mania behaviors include grandiosity, increased energy, less sleep, and risk preference. The neural mechanism(s) underlying each state are gaining clarity, with catecholaminergic disruption seen during mania, and cholinergic dysfunction during depression. The fact that the same patient cycles/switches between these states is the defining characteristic of BD however. Of greater importance therefore, is the mechanism(s) underlying cycling from one state - and its associated neural changes - to another, considered the ‘holy grail’ of BD research. Herein, we review studies investigating triggers that induce switching to these states. By identifying such triggers, researchers can study neural mechanisms underlying each state and importantly how such mechanistic changes can occur in the same subject. Current animal models of this switch are also discussed, from submissive- and dominant-behaviors to kindling effects. Focus however, is placed on how seasonal changes can induce manic and depressive states in BD sufferers. Importantly, changing photoperiod lengths can induce local switches in neurotransmitter expression in normal animals, from increased catecholaminergic expression during periods of high activity, to increased somatostatin and corticotrophin releasing factor during periods of low activity. Identifying susceptibilities to this switch would enable the development of targeted animal models. From animal models, targeted treatments could be developed and tested that would minimize the likelihood of switching.
1. Introduction
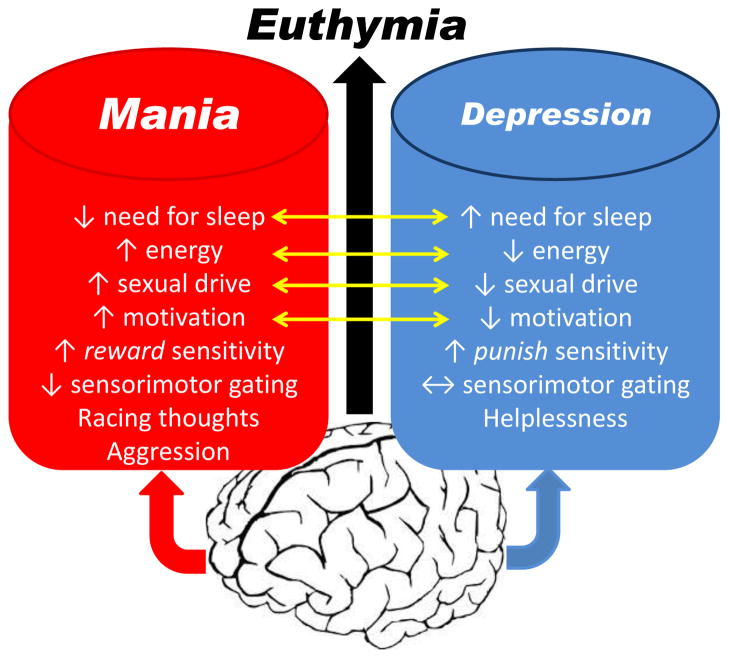
Bipolar disorder (BD) is a life-long neuropsychiatric disorder affecting approximately 2–5% of the world’s population (Akiskal et al., 2000; Judd et al., 2003; Merikangas et al., 2007). BD sufferers have a markedly increased suicide mortality rate (Osby et al., 2001) where one in three patients attempt suicide (Novick et al., 2010). The lifetime cost from treatment and lost productivity from people suffering from BD amounts to approximately $24 billion (Begley et al., 2001). BD is the sixth leading cause of disability among physical and psychological disorders worldwide (Murray and Lopez, 1996). BD is so-called because patients exhibit states of mania or depression, states that have polar opposite symptoms. Periods between extreme states where behavior is not as extreme (relatively normal) are referred to as a euthymic states. During a state of mania, people exhibit symptoms of euphoria, aggression, irritation, increased physical activity, racing thoughts, high reward-seeking behavior, and reduced need for sleep. In contrast, during a state of depression, people exhibit symptoms of reduced sense of self, helplessness, reduced energy, punish sensitivity, and increased sleep (Fig. 1). Importantly, BD is a unique disorder that transcends domains of function because the same patient can exhibit depression or mania. Hence, the fact that sufferers cycle/switch between states is the preeminent defining characteristic of BD (Goodwin and Jamison, 1990).
Figure 1. Symptoms of the extreme states of bipolar disorder, mania and depression.
Bipolar disorder is a unique disorder that transcends domains of function because the same patient can exhibit depression or mania at different points in their life. Periods when they exhibit symptoms of neither state are referred to as euthymia. Some symptoms are on a scale (yellow arrows) whereby the symptoms of each state are on the opposite ends of the spectrum. Some symptoms do not link with each other, where deficits seen in a manic state are normal during depression (e.g., sensorimotor gating as measured by prepulse inhibition). Importantly, identifying triggers that change the neural circuitry of the brain toward mania or depressive symptoms is a vital step toward delineating the key circuitry that allows for a hypersensitivity to such triggers.
BD therefore, is an extremely complex neuropsychiatric condition. Beyond switching between mood states, subgroups of BD also exist. In BD type I, sufferers exhibit aspects of mania and depression, in type II patients exhibit aspects of hypomania and depression (Belmaker, 2004). Other subgroups of BD exist, including sufferers that exhibit rapid cycling between states (Fountoulakis et al., 2013; Zupancic, 2011), which may not be stable (Carvalho et al., 2014), or some sufferers that exhibit mixed states (McIntyre et al., 2012; Ouanes et al., 2014; Price and Marzani-Nissen, 2012). In addition, juvenile-onset of BD is being documented (Luckenbaugh et al., 2009), adding another aspect of sufferers of BD. Importantly, all sufferers exhibit mania and depressive symptoms in varying degrees. In addition to these subgroups, the differing neurobiological substrates that likely underlie depressed vs. manic states (Brady et al., 2014; Janowsky et al., 1983; Savitz et al., 2014; van Enkhuizen et al., 2014b) is the likely reason that the only approved treatments for BD have been discovered serendipitously or first for other disorders (Gould and Einat, 2007; Young et al., 2011). To develop treatments targeted for BD, the mechanism(s) underlying swithcing between states - described as the ‘holy grail of BD research’ (Blumberg, 2012) - needs to be discovered.
In order to test hypotheses on potential mechanism(s) underlying such switching, animals that are manipulated by such mechanisms would be extremely beneficial. In combination with such manipulations, testing the behavioral output with relevance to cognitive and behavioral states using tests relevant to those affected in mania and depression will prove vital (Young et al, 2011; (Geyer et al., 2012; Keeler and Robbins, 2011; Malkesman et al., 2009; Markou et al., 2008; van Enkhuizen et al., 2014b; Young and Geyer, 2014; Young et al., 2011). There is a dearth of animal manipulations targeting the etiologies of BD (Malkesman et al., 2009; Young et al., 2011), and what manipulations exist, few utilize cross-species tests relevant to mania or depression (van Enkhuizen et al., 2014b; Young et al., 2011). In fact, the vast majority of current models only attempt to model the mania aspect of BD (Einat, 2007; Gessa et al., 1995; Gould and Einat, 2007; Kara and Einat, 2013; Machado-Vieira et al., 2004; Malkesman et al., 2009; van Enkhuizen et al., 2014b; Young et al., 2007). Those studies that attempt to recreate depression do not do so as a form of depression relevant to bipolar disorder (Abelaira et al., 2013; Belzung, 2014; Berton et al., 2012; Carboni, 2013; Kreiner et al., 2013; O’Leary and Cryan, 2013; Pryce and Klaus, 2013). Even fewer studies attempt to model the switching between states of BD.
This review will bring into focus; 1) current models of cycling in BD; 2) the extant literature detailing causes of switching between states in BD; 3) how switching between states can also switch neurotransmitter mechanisms, underlying the varied behavior between states; and 4) how future models of switching between states will have to be tested in order to determine their relevance to that state.
2. Current attempts at modeling switching between states
Some attempts have been made to create animal models relevant to cycling in BD. These models range from in vitro to in vivo studies. Naturally, in vivo studies offer the opportunity to study the behavioral effects of the manipulation, especially behaviors with relevant to mania or depression depending on the time of manipulation.
In (1967), Price proposed an evolutionary dominance hierarchy theory for developing mental illness. Briefly, this theory proposed that the maintenance of a hierarchy is essential for a social groups well-being, and since changes in the hierarchy are inevitable, certain behavioral characteristics during these changes make such transitions smoother. For example, the transition of defeated alpha male to a lower rank would be smoother if they exhibited low energy, disinterest, and reduced sexual drive - behaviors associated with depression. Based on this premise, Malatynska and colleagues have used dominant-submissive behaviors of rats as models of mania and depression [respectively; (Malatynska and Knapp, 2005)]. Similarities of submissive behaivor to depression include increased defensive behaviors, weight loss, sleep disturbances (Price et al., 1994). Dominant and submissive behaviors can be induced when resources are made scarce [e.g., social competition model; (Cumming et al., 2014; Pucilowski et al., 1990; Taylor and Moore, 1975)]. Interestingly, such dominant and submissive behaviors are heritable (Nesher et al., 2013). Importantly for modeling switching in BD, antidepressants can attenuate submissive behaviors (Koolhaas et al., 1990; Mitchell and Redfern, 1992). Treatments for anxiety can also attenuate submissive behavior however (Nesher et al., 2013; Piret et al., 1991), which are not used as treatments for depression in BD. Furthermore, lithium did not affect such submissive behavior (Nesher et al., 2013), despite being a treatment depression in BD. Additional support for this model comes however, from evidence that dominant behaviors are sensitive to reversal due to anti-manic treatments such as lithium and valproate (Malatynska et al., 2002; Nesher et al., 2013). The use of single doses as opposed to chronic treatment is in contrast with their efficacy in human treatment studies however (Young et al., 2011). When investigating potentially novel treatments, clonidine (an alpha2 adrenergic antagonist) reversed the dominance of rats (Malatynska et al., 2002). Clonidine has since received some support as a potential treatment for mania (Chou, 1991; Hardy et al., 1986; Jouvent et al., 1988; Maguire and Singh, 1987; Zubenko et al., 1984), although it - nor any mechanism of its type - has not been approved for treating mania. Additional concerns of this model include evidence that anxiogenic treatments attenuate dominant behavior of rats in social competition settings (Joly and Sanger, 1992), which are not used as treatments for mania. Another concern is that amphetamine attenuates the dominant behavior of rats (Malatynska and Kostowski, 1984; Miczek and Gold, 1983), yet can induce a manic state in patients with BD (see section 3.1.). Additionally, dominant males become submissive when they encounter a more dominant male (Willner et al., 1995), which hardly equates to a treatment for mania. Most importantly for this review, the behavior of these animals were rarely tested beyond their submissive or dominant behaviors, where neurocognitive and mood-related testing would be useful.. Furthermore, the animals used in these behaviors are consistently submissive or dominant, not switching between the two states. Hence, the suitability of this dominant/submissive behavior as a model of switching relevant to BD is likely limited.
Another model of switching in BD resulted in the kindling hypothesis. Kindling refers to a progressive decline in the strength of electrical current required to elicit seizure activity. Although a strong current is needed initially, the electrical threshold required diminishes over time until a point of ‘spontaneous epilepsy’ develops. It was hypothesized that this kindling process could be elicited by introducing repetitive stimuli or psychomotor stimulant presentation (Post et al., 1982), although of some concern is that the mechanisms underlying each may be distinct (Weiss and Post, 1987). Post and Weiss (1989) suggested that since repeated treatment with cocaine can induce mania-like episodes, animals receiving different dosing regimens of cocaine can produce differing effects from motoric hyperactivity to convulsions. Moreover, anticonvulsant treatments such as carbamazepine are effective at inhibiting this ‘kindling’. In part from this work, Post (1992) postulated the kindling hypothesis of mood disorders, whereby major psychological stressors play a greater role in the initial - as opposed to later - episodes of a mood disorder. While the initial hypothesis was to cover both unipolar depression and BD, most work has focused on evidence from unipolar depression (Monroe and Harkness, 2005). The utility of this model as a hypothesis for BD was reviewed recently, whereby only 1 in 3 retrospective human studies have provided support for such a hypothesis (Bender and Alloy, 2011). Numerous animal studies related to this kindling hypothesis have been conducted.
Antelman and colleagues (1998) investigated the effects of different numbers of cocaine treatment on oscillatory changes of dopamine efflux in response to amphetamine from rat slices from the nucleus accumbens and striatum. They also investigated the ability of lithium to block any observed effects. It was observed that a single treatment with cocaine reduced dopamine efflux, two treatments (1 week apart) had no effect, while 3 treatments also reduced dopamine efflux (2 weeks apart). When co-treated with lithium without cocaine, or 1 or 3 treatments, an increase in dopamine efflux was observed. Similar findings were observed for efflux from the striatum, although lithium did not increase efflux alone. A hypoalagesic response to shocks were seen when some multiple treatments of cocaine were given (1, 2, and 4 pretreatments) that was not reversed by lithium treatment. The authors claim that this effect is appropriate for evaluating the switching between states that occurs in BD (Antelman et al., 1998). It remains unclear what relevance a hypoalagesic effect has to behaviors seen in BD sufferers however. Evaluating the behavioral outcomes of these models using BD-relevant tests for mania or depression would prove more useful. Furthermore, it is clear that repeated cocaine treatment in humans does not result in a cycling state consistent with BD and so these effects may simply relate to the pharmacological effect of cocaine/amphetamine/lithium treatment. Ultimately, this model may be more relevant to seizures since anticonvulsants such as topiramate, gabapetin, and tiagabine are effective in this model but not for treating BD mania or depression (Weiss, 2004). Such studies have also been related to the modeling of disease progression in epilepsy, pain syndrome, and post-traumatic stress disorder (Grillon et al., 1996; McFarlane et al., 2002; Post, 2002). Hence, these studies ultimately support the assertion of Bender and Alloy (2011) that the kindling hypothesis is not fully supported for switching in BD and that other hypotheses, such as the social rhythm disruption theory - that led to social rhythm therapies (SRT; see section 3.2.) - may fit better.
Testing manipulations in whole animals would allow their behavioral testing in cross-species tasks that are relevant to mania and depression (Fig. 1). Such studies will be vital to testing hypotheses underlying switching in BD. Ideally, these manipulations should be taken from etiological studies in BD patients identified as inducing a transition into another state.
3. Key studies investigating cycling/switching into other states in BD
There have been numerous studies/reports on triggers that can induce switches of BD sufferers into other affective states (e.g., mania or depression). Although there are many methodological issues surrounding the definition of what constitutes a switch, (Salvadore et al, 2010), attempts have been made to review mechanisms that underlie switching to hypomania and mania (Chen et al., 2010; Salvadore et al., 2010) as well as switches to depression (Blumberg, 2012; Salvadore et al., 2010).
In a prospective 10 year study, it was observed that gender, a positive family history of BD, nor age were positive predictors of switching (Maj et al., 2002). This study focused on previous hospitalizations on switching, indicating that a higher number of hospitalizations was associated with switching. In another study, a mixed manic episode was more likely to predict a switch to a depressed state compared to exhibitions of mania symptoms alone (Zarate et al., 2001). While of importance for current treatment of sufferers, this clinical information may not prove useful for identifying mechanisms or specific triggers that could identify mechanisms. Other, more specific triggers, have been identified however.
3.1. Pharmacological triggers of switching between states in BD
One area of clinical importance that can also be informative for understanding mechanisms and model testing is that of anti-depressant treatment-induced switching to a hypomanic or manic state in BD. Chronic treatment with tricyclic antidepressants have consistently been linked with producing a switch at rates ranging from 9–69% (Bunney et al., 1970; Lewis and Winokur, 1982; Peet, 1994). Tricyclic antidepressants largely act to block both serotonin and norepinephrine re-uptake from the synaptic cleft, although there are a variety of effects at other receptors, notably in the serotonergic system. These combined inhibitors induce mania/hypomania at higher rates than from selective serotonin reuptake inhibitors (Bottlender et al., 2001; Peet, 1994). Treatment with bupropion - a combined dopamine/norepinephrine reuptake inhibitor - also induces switches in rates up to 19% (Sachs et al., 2007), although many studies focused on its use as an adjunctive therapy to mood stabilizing treatment (Salvadore et al., 2010). Ultimately, it appears that elevating norepinephrine or dopamine levels via blockade of their reuptake site likely induce switches from a depressed to a hypomanic or manic state, even during mood stabilizing co-treatment.
Direct treatment resulting in elevated dopamine/norepinephrine levels also induce mania-like states. For some time it was clear that treatment with the dopamine precursor L-dopa induced a hypomanic state of mood (Murphy et al., 1971). This treatment effect is consistent with observations that treating Parkinson’s disease patients with L-dopa can induce mania-relevant behavior such as gambling and risk-taking (Claassen et al., 2011; Djamshidian et al., 2011; Raja and Bentivoglio, 2012). Indirect dopamine/norepinephrine stimulation by \the combined reuptake inhibitor amphetamine induces manic/hypomanic episodes in patients and mania-like behaviors in healthy subjects (Asghar et al., 2003; Corp et al., 2014; Jacobs and Silverstone, 1986). Conformation of the importance of reuptake sites to this mood-elevating effect of amphetamine comes from studies demonstrating that polymorphisms of these transporter sites can alter the effects of amphetamine. (Dlugos et al., 2007; Lott et al., 2005). Interestingly however, it is not simply elevation of levels of these neurotransmitters, because reducing levels of tyrosine hydroxylase (TH; the precursor to dopamine/norepinephrine) via α-methyl-p-tyrosine (AMPT) treatment also induced hypomanic mood elevation (Anand et al., 1999). Although it is unclear whether AMPT would reduce mania behaviors in people undergoing a manic episode, this study makes it clear that it is the homeostatic control of catecholamines that are important for switching to mania/hypomania (Anand et al., 1999; van Enkhuizen et al., 2014b).
Manic episodes can be induced in BD patients using treatments beyond pharmacological-induced neurotransmitter manipulation. In particular, manipulation of the hypothalamic-pituitary-adrenal (HPA) axis can induce switching of mood states in 30–80% of cases in a dose-related fashion (Janowsky et al., 1983; Lewis and Smith, 1983; Phelan, 1989; Wada et al., 2000), especially when associated with a family history (Brown et al, 2002). Specifically, prednisone treatment can induce manic episodes in BD sufferers (Wada et al., 2000), while steroidal-based treatments for asthma can also induce a switch to a manic state (Phelan, 1989). Furthermore, HPA hyperactivity has been reported in rapid switching from mania to depression in case studies (Bunney et al., 1965; Fries et al., 2014). Hence, altered HPA function, or hypersensitivity to glucocorticoids, may represent a viable trigger related to switching between mood states.
Little is known about pharmacological treatments that can induce switching to a depressive state. One treatment used to improve memory in Alzheimer’s disease is physostigmine, an acetylcholinesterase inhibitor (inhibits the breakdown of acetylcholine, hence indirectly elevates acetylcholine). Physostigmine treatment can reduce mania symptoms in BD patients (Janowsky et al., 1972). In healthy subjects, and euthymic patients with BD, physostigmine can also induce a state of depression (Risch et al., 1981; Shopsin et al., 1974). More recently, this work has been followed-up with observations that elevated acetylcholine levels are observed in patients with BD and unipolar depression (Hannestad et al., 2013) as well as altered cholinergic receptor levels in BD sufferers (Saricicek et al., 2012). Hence, elevating acetylcholine levels likely induces a depressed state in BD patients.
Other neurotransmitter systems have been investigated that may underlie switching in patients with BD. Neither lamotrigine nor riluzole, which inhibit glutamate release, do not increase switching in depressed BD patients (Calabrese et al., 1999; McIntyre et al., 2013; Zarate et al., 2005). Other novel treatments for depression - including BD depression - includes intravenous treatment with NMDA receptor antagonists such as ketamine (Lally et al., 2014; McGirr et al., 2014; Villasenor et al., 2014). No reports of ketamine-induced switching of BD depressed patients to mania have been reported. Hence, glutamatergic links to switching in BD appear unlikely.
3.2. Environmental factors that induce switching between states in BD
Other environmental factors of switching are important to take into consideration beyond pharmacological treatments however. Disturbed sleep patterns have been observed at the time of the switch to mania in euthymic unmedicated patients with BD, including increases in catecholamine release (Goodwin et al., 2007). Full sleep deprivation can induce a manic episode in up to 30% of BD patients (Colombo et al., 1999; Leibenluft et al., 1996; Wright, 1993), and mania-like behavior in healthy subjects (Aydin et al., 2013; McKenna and Eyler, 2012; van Enkhuizen et al., 2013a). Sleep deprivation-induced changes in mood supports the social rhythm disruption (SRD) (social zeitgeber) theory of affective disorders (Grandin et al., 2006; Malkoff-Schwartz et al., 2000). This SRD theory posits that a disruption in social/biological rhythm can induce changes in mood in vulnerable individuals. Such disruption can take the form of stressful live events stressors (Ellicott et al., 1990; Hirschfeld and Cross, 1982; Post, 1992), but can also include positive events (Bender and Alloy, 2011). Further support includes that the sleep-wake cycle and biological rhythms are altered in BD (Gonzalez, 2014; Robillard et al., 2013) and likely play a role in the pathophysiology of BD (Kripke et al., 2009; McCarthy et al., 2012; McCarthy and Welsh, 2012; McClung, 2007). Hence, BD sufferers may have a susceptibility to SRD that underlies shifting moods. Although the mechanism(s) underlying this theory have yet to be determined, it has resulted in efficacious treatment development called social rhythm therapy (SRT) designed to maintain a stable lifestyle (Bouwkamp et al., 2013; Frank et al., 2008; Harvey, 2011; Hlastala et al., 2010; Sachs, 2008). Hence, beyond pharmacological challenges, changes in the environment of the sufferer can induce switches in mood.
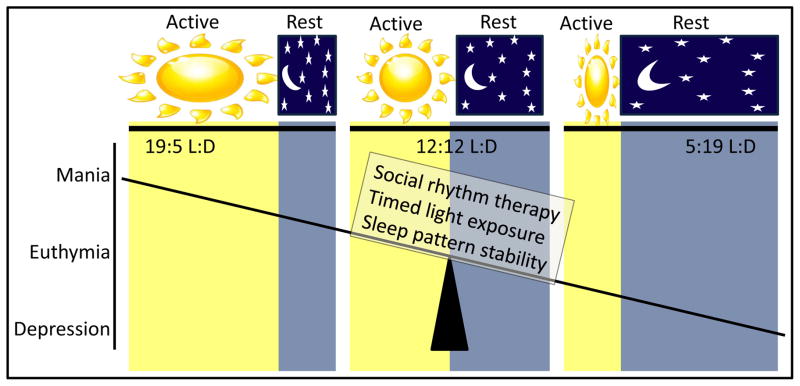
One of the greatest environmental triggers of changes in mood has been the changing of seasons. Seasonal variations effecting mood in BD have been recognized for over 2000 years when Hippocrates described the correlation between seasonal changes and the occurrence of mania in BD. This premise has been supported by numerous studies reporting more mania episodes during the spring and summer (Barbini et al., 1995; Lee et al., 2007; Mulder et al., 1990; Parker and Walter, 1982; Sayer et al., 1991; Takei et al., 1992; Volpe and Del Porto, 2006), although this has not always been seen ((Silverstone et al., 1995; Whitney et al., 1999). Changing seasons do not only affect sufferers of BD, but also people experiencing depression. Seasonal affective disorder is now a widely recognized condition wherein which people entering periods of seasons of short sunlight experience depressive symptoms (Jacobsen et al., 1987; Knapen et al., 2014; Rosenthal et al., 1984) and has been reviewed extensively (Gupta et al., 2013; Niemegeers et al., 2013). Light therapy (exposure to ultraviolet light at set times of the day) is used to treat seasonal affective disorder (Golden et al., 2006; Niemegeers et al., 2013) and is also used as an antidepressant in patients with BD (Postolache and Oren, 2005; Wu et al., 2009). Shortening day-lengths may represent one of the more consistent environmental triggers inducing a switch to depression in people with BD. Hence, it appears likely that changing seasons can exert extreme effects on mood in those susceptible to such effects (Fig. 2). As reviewed by Wang and Chen (2013), the majority of studies (23/29) provide supporting evidence for seasonal mania (manic episodes occurring in spring and summer). They highlight that the heterogeneity of BD may reflect why some sufferers do not show such seasonality, hence seasonal-variation induced switching in BD might be a sub-population of sufferers. In fact, this sub-population may exhibit with a more severe form of the disorder seen in males and females, although rapid cycling is more likely seen in females (Geoffroy et al., 2013). Such seasonality may be associated with BD type II patients, particularly in subjects that evidence with late evening preference and irregular bed-rise times (Baek et al., 2014). It was recommended that future studies aimed at understanding seasonal variation in BD require stringent research criterion (Murray et al., 2013). Considering the advent of modern electric lighting and its widespread use, such studies should also utilize light level readers. Importantly however, seasonality in BD is associated with a family history of BD (Brambilla et al., 2012) and appeared heritable in a twin study (Jang et al., 1997). Hence, it is likely there is a strong genetic component to seasonal changes-induced switches in mood.
Figure 2. Photoperiod length-induced changes of mood.
As sufferers of bipolar disorder enter spring/summer, where longer light-induced active periods are present, they are more likely to exhibit mania symptoms. During shorter light-induced inactive periods however, bipolar sufferers are more likely to exhibit depressive symptoms. Maintaining stability of photoperiod supports a euthymic, non-clinical state, consistent with psychosocial social rhythm therapy, timed light exposure, and sleep pattern stabilization treatments.
In any investigation on the putative mechanism(s) underlying BD it is important to keep in mind that BD is a genetic-based disorder. Heritability studies of BD estimate a 60 – 80% heritability supporting this genetic basis for sufferers. Numerous GWAS studies have been conducted in BD (Baum et al., 2008; Greenwood et al., 2012; Greenwood et al., 2006b; Lee et al., 2013; Sklar et al., 2008; Smith et al., 2009), but few observed statistical significant findings required for such studies. Such lack of effect may come from the heterogeneity of BD groups where studying subsets of patients may provide greater information. A GWAS study on patients self-identifying as having seasonal patterns of mania vs. non-seasonal sufferers discovered an association for rs41350144 which lies within the intron of NF1A on 1p31 (Lee et al., 2013). Importantly, genes contributing to the control of circadian rhythm are also implicated in BD. Haplotype polymorphisms of the circadian genes Period3 and ARNTL have been associated with BD, as have circadian locomotor output cycles kaput (CLOCK) and GSK3β (McClung, 2013; Nievergelt et al., 2006). At the core molecular level, the mammalian circadian circuit involves the CLOCK, ARNTL (also known as BMAL1) and NPAS2 transcription factors activating the transcription of cryptochrome (CRY1 and CRY2), period (PER1, PER2, and PER3) and other clock-controlled genes (McClung, 2013; Zhang and Kay, 2010). Although not specifically associated with circadian rhythm, the Val66Met genetic polymorphism of the brain-derived neurotrophic factor (BDNF) has specifically been associated with rapid cycling in bipolar disorder (Green et al., 2006; Liu et al., 2008; Muller et al., 2006). Subsequently, elevated plasma levels of BDNF were observed across all states of bipolar disorder (Munkholm et al., 2014). Since BDNF is a major regulator of synaptic transmission and plasticity, investigating its role in the switch between states in BD will prove important.
3.2.1. Origin of seasonal variation-induced changes in mood?
Modern day humans (Homo sapiens) originated in the Awash Valley of Ethiopia, a location close to equator where people were subject to a consistent 12:12 light dark cycle [12 h of daylight followed by 12 h of light; (White et al., 2003)]. Out of Africa migration of the human population led to genetic modulation of the circadian clock systems. This process also lead to risk variants associated with neuropsychiatric disorders, providing candidate genes for photoperiod-related environmental interactions (Forni et al., 2014). Such pressures may not have acted alone however. As these modern day humans entered the northern hemisphere, they were likely to encounter our predecessors Homo neanderthalensis. We know now that Neanderthals did not simply die out, but interbred with modern humans because modern humans originating from outside of Africa carry 1–4% of Neanderthal genes (Green et al., 2010). Support that Neanderthal genes may have contributed to susceptibility genes for BD comes from studies demonstrating a lower incidence of BD among those of African descent (Goodwin and Jamison, 1990; Neighbors et al., 2003; Perron et al., 2010; Weber et al., 2010). Considering Neanderthals spent 100,000s of years in the northern hemisphere, extreme responses to seasonal variation could have arisen leading to the disorder we now know as BD (Sherman, 2012). Seasonal changes require the body to exert homeostatic control, whereby decreased day lengths perceived through the eye and processed by the SCN of the hypothalamus can induce a reduced metabolic rate, slower movement, greater need for sleep, reduced responsiveness to sex, etc. These behaviors are all associated with depression. When the days start to lengthen, the same mechanism should induce increased metabolic rate, more energy, a greater desire to forage and find mates (goal-directed activities), behaviors associated with mania/hypomania. Therefore, it remains possible that during such a time-period evolutionary pressures could introduce random mutations producing aberrant responses to such changes in day-length, representing poor homestatic control. This evolutionary origin of bipolar disorder theory was detailed by Sherman (2012). Links to the mechanism(s) of how altered seasons, or photoperiod lengths, could induce such vast changes in behavior have however - up until recently - remained elusive.
3.2.2. Photoperiod-length-dependent neurotransmitter switching
Seasonal variation can induce changes in mood in those with a susceptibility to such effects. The mechanism of these effects, especially how the same person can exhibit such varied behaviors with differing underlying mechanisms, has been elusive. Conventional wisdom thought that the adult brain was hard-wired with specific neurons releasing set neurotransmitter levels. Increasingly however, the plasticity of the adult brain has been demonstrated, including in response to varied photoperiods as occur in seasonal variation. The first observation of photoperiod-dependent neurotransmitter respecification following changes in physiological levels of environmental illumination came from a developmental study in Xenopus laevis (Dulcis and Spitzer, 2008). The authors found that light can dynamically regulate the number of dopaminergic neurons in the developing hypothalamus and by innervating the melanotrope cells, it control skin pigmentation in tadpoles. An increase in the number of neurons expressing the dopaminergic phenotype occurred following two-hour exposure of tadpoles to a white background or to constant light. The newly expressing neurons were not new added cells via proliferation or migration. The additional neurons came from recruitment of neuropeptide Y (NPY)-expressing neurons that were already integrated in the neuronal network controlling skin pigmentation. Recruited NPY neurons responded to a 2-hour hyperactivation of the retinohypothalamic projection by co-expressing dopamine as an additional neurotransmitter. Ablation experiments aimed at compromising endogenous dopaminergic cells in the same network, showed that the recruitment of such reserve pool neurons could restore the camouflage behavior almost completely when natively dopaminergic neurons were ablated.
The exuberant plasticity of the young nervous system is often present in a more restrained form in the adult nervous system. Activity-dependent modulation of axonal sprouting, dendritic remodeling and synaptic strengths is robust in the developing nervous system and more modest but still present in the mature nervous system (Kozorovitskiy et al., 2005). Transmitter respecification however, does not appear to require either morphological remodeling or new network formation (involving changes in axon pathfinding, dendritic remodeling and synaptic strength), and is compatible with the limited remodeling ability of the mature CNS.
The follow-up questions that remained to be addressed was whether this novel form of neuroplasticity could be elicited in the nervous system of higher vertebrates, including mammals, and in the mature nervous system. There were few examples in the literature that provided insights on the possibility that neurons could switch to a different neurotransmitter in the mammalian system following activity/photoperiod manipulation. Neurons of the cervical spinal cord in cats display this type of recruitment for the cholinergic phenotype during development (Chakrabarty and Martin, 2010; Chakrabarty et al., 2009). The authors of these studies found that extensive circuit activation of the corticospinal tract affects the total number of ChAT-expressing neurons during a two-week period. In this case, newly cholinergic neurons were recruited from a reserve pool of spinal neurons that received the descending cortical input. Pharmacological manipulations aimed at blocking activity during this critical period abolished refinement of ChAT neurons in the spinal cord that displayed an immature neurotransmitter expression pattern.
As far as neurotransmitter switching in the mature nervous system, striatal TH-positive neurons increase in number in mouse and macaque models of Parkinson’s disease (Aumann et al., 2008; Tande et al., 2006). Following MPTP- and 6-OHDA-treatments, lesions of the dopaminergic substantia nigra pars compacta were observed to partially recover by inducing phenotypic respecification of neurons intrinsic to the mouse striatum. Interestingly, newly TH-immunoreactive neurons in the macaque striatum expressed both DAT and glutamic acid decarboxylase, suggesting that they were recruited from a population of GABAergic interneurons (Tande et al., 2006).
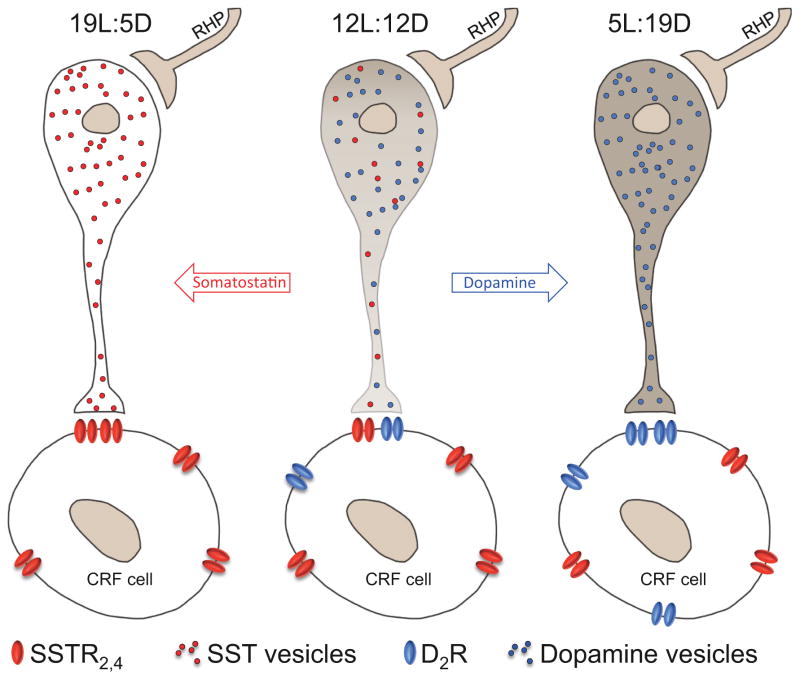
Despite the studies summarized above, the first clear demonstration that neurotransmitter plasticity can be induced in the mature nervous system and can affect behavior came from a study in adult rats following exposure to altered photoperiods (Dulcis et al., 2013). In this study, adult rats were kept in either long-day low-activity inducing (19 h of light and 5 h of dark) or short-day high-activity inducing (5 h of light and 19 h of dark) cycles. After one week, neurotransmitters released by hypothalamic neurons switched from dopamine to somatostatin phenotype during long-day cycles (Fig. 3). The opposite occurred after 1-week exposure to short-days (switch from somatostatin to dopamine). Hence, the altered photoperiod diverging from a balanced day-night cycle (12 h light and 12 h dark a day) induced individual neurons in the hypothalamus to change expression of their neurotransmitters. In the same study, authors were able to show that neurotransmitter switching was coupled to dopamine- and somatostatin- receptor matching in the post-synaptic targets, the corticotrophin releasing factor (CRF)-releasing cells. The appearance of more dopaminergic interneurons presynaptically was paralleled by an increased expression of dopamine type-2 receptors (D2Rs) postsynaptically (Fig. 3). Long-day photoperiods, lead to an increase of CRF titer in the cerebral spinal fluid (CSF) collected in the third ventricle following photoperiod manipulation. It is well known how changes in CRF concentration in the CSF modulate the level of circulating corticosteroids, which play a role in stress and depression. Dopamine is a neurotransmitter that plays a role in cognition (Arnsten et al., 1994; Vijayraghavan et al., 2007) motivation (Matsumoto and Hikosaka, 2009; Young, 2009; Young and Geyer, 2010), reward (Schultz, 2002), and decision-making (Floresco et al., 2006; Floresco et al., 2009; St Onge and Floresco, 2009; van Enkhuizen et al., 2014a). To investigate whether photoperiod-induced changes in dopamine expression had any consequences on animal behavior, the rats were tested in the elevated plus maze (EPM) and forced swim test (FST) as indicators of anxiety/risk-preference and despair-like behaviors respectively. Relative to controls, rats exposed to short-day cycles spent more time exploring the open arms of the EPM and spent less time immobile in the FST. In contrast, long-day exposure produced the opposite effects: rats explored the open arms less and exhibited increased immobility time. Interestingly, CRF expression can induce elevations in hippocampal acetylcholine (Desvignes et al., 2003). Such an increase is consistent with physostigmine-induced increases in immobility in the FST in mice that is hippocampal-mediated (Mineur et al., 2013). Further links between acetylcholine levels and behaivoral despair are reports that acetylcholinesterase levels in the frontal cortex of mice negatively correlated with immobility in the FST (Livneh et al., 2010). Interestingly, this effect can also be seen in diurnal rodent species, whereby long-darkness photoperiod lengths induced depression-relevant behaviors in sand rats (Ashkenazy-Frolinger et al., 2010). Hence, there is evidence that 7-day exposure of rodents to short-activity photoperiod lengths can have a cascade effect which could result in elevated acetylcholine levels leading to depression-relevant behaviors.
Figure 3. Schematic model of photoperiod-induced neurotransmitter switching and receptor matching as occurs in nocturnal rats.
Activation of the retino-hypothalamic projection (RHP) during a short-activity (19L:5D) photoperiod shifts transmitter expression in PaVN neurons to somatostatin (SST) while a long-activity (5L:19D) photoperiod shifts transmitter expression to dopamine. Dopamine type-2 receptor (D2R) expression on corticotrophin releasing factor (CRF) cells changes in parallel with changes in dopamine expression while SST type-2,4 receptor (SSTR2,4) expression remains constant.
The identity of the neurotransmitters expressed in a population of mature neurons has been thought to be fixed and immutable. In vivo observation of changes in transmitter specification in the adult rat brain linked to changes in behavior will substantially advance our scientific knowledge and suggest the value of determining whether there are comparable changes in the striatum, cortex, and hippocampus. The switch between TH to somatostatin and CRF production in adult rats dependent upon photoperiod length provides mechanistic support that the same adult human could exhibit extreme opposite behaviors when exposed to similar seasonal-induced changes in photoperiod length. The heritability of BD would promote studies identifying a genetic polymorphism that induces a susceptibility to such photoperiod-induced neurotransmitter switching.
3.3. Summary of putative mechanism(s) underlying switching between states of BD
It is clear from this review of the literature that the mechanism(s) underlying cycling/switching between states in BD will not be easily discovered. Switches to manic episodes appear to be driven by elevated catecholamines (dopamine and norepinephrine, or their precursor TH), or at least poor catecholaminergic homeostasis. Disruption in daily rhythm, or entry into high-activity photoperiod lengths (spring/summer) can also induce switches into a manic episode. It is interesting to note that entry into high-activity photoperiod lengths (spring/summer) elevate TH levels. Hence, there is distinct overlap between the stimuli that can induce a manic state. The triggers that switch sufferers to a depressed state appear less clear, with elevated acetylcholine levels, stress, and entry into low-activity photoperiod lengths (fall/winter) among the few certainties. Again, there is overlap between mechanisms underlying switching to this state, whereby entry into low-activity photoperiods can indirectly increase stress-related neurotransmitter and acetylcholine levels (Table 1). Most importantly, the neurotransmitter switching that occurs in response to changing photoperiod lengths provide insight into how the same adult brain can exhibit polar opposite behaviors representing distinct neural mechanisms at different time points. An important question remains however, as to what genetic polymorphism(s) make sufferers susceptible to extreme variation to these environments.
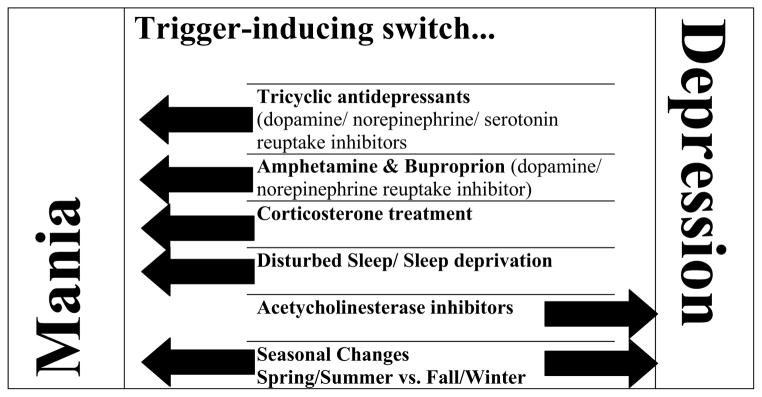
Table 1. Summary of triggers that induce switching to each state of bipolar disorder.
Clear from the table is that altering catecholaminergic homestatic control induces mania, as does sleep deprivation, and long-activity photoperiod lengths. While antidepressants and sleep deprivation can treat depression, patients can switch to a manic episode. There are few specific triggers that induce depression, but elevating acetylcholine levels and entering periods of low photoperiod lengths are two known triggers. Interestingly, changing photoperiod lengths to high-activity can imbalance catecholaminergic control, while low-activity lengths can elevate acetylcholine levels. Hence, this photoperiod changing hypersensitivity mechanism may have originally underlain switching between states and the other triggers simply alter that system. Any model organism relevant to switching/cycling in bipolar disorder should exhibit a hypersensitivity to these some if not all of these triggers.
|
4. Future directions
It remains unclear as to whether there are specific behaviors associated with the triggers mentioned above that induce switching in BD. The literature almost exclusively measures mood using clinician-interviewed rating scales of mood, which are difficult to quantify and test across species since interpretative leaps must be made for animals (Geyer et al., 2012; Young et al., 2011; Young et al., 2013; Young et al., 2009). While measuring mood state is important, equally important would be to quantify behavioral changes using clinical neuroscience techniques that are likely more closely tied to altered brain function at the circuit level, as detailed by NIMH-sponsored initiative such as CNTRICS and RDoC (Barch and Carter, 2008; Carter et al., 2011; Cuthbert and Insel, 2010; Morris and Cuthbert, 2012). Collectively, these findings would provide a framework into which experimental assessment of these neural mechanistic changes and their behavioral effects (in humans and animals) can be investigated. Numerous laboratory-based cross-species tests of cognitive state exist however (Young and Geyer, 2014) and could be used to quantify changes in behavior of patients during switches from one mood state to another. Hence, one of the most important strategies for identifying mechanisms underlying such switching will be to utilize these cross-species relevant behavioral tests during pharmacological treatments, or changing seasons. Utilizing cross-species relevant behaviors, would enable similar tests to be conducted on model organisms thought to reflect the altered neurocircuitry seen in BD patients, consistent with the approach by the RDoC initiative (Cuthbert and Insel, 2010; Morris and Cuthbert, 2012). Considering the cognitive performance of patients with BD is linked to their functional outcome (Green, 2006), measuring such performance across mood states will be vital for treatment development. Current treatments can deleteriously affect cognitive performance. For example, lithium significantly impairs cognitive performance in a variety of domains (Amado et al., 2005; Stip et al., 2000). Thus, by using clinical neuroscience tools, targets could also be identified that relate to cognitive dysfunction, enabling targeted treatments for this long-overlooked domain to be developed. Longitudinal studies on treatment- and light exposure-induced switching of behavior should also be conducted using such tests. Behaviors to focus on will be those that can be conducted repeatedly and that likely differentiate depending on when in a manic or depressed state. A key example of such laboratory-based techniques differentiating behavior in the two states is the hypersensitivity of patients in a manic or depressed to reward (Cassidy et al., 1998) or punishment (Adida et al., 2011; Must et al., 2013) respectively. Tasks such as the Iowa Gambling Task which differentially measures responses to reward and punishment (Bechara et al., 1994) would be useful since it is possible to test the risk-preference of mice (van Enkhuizen et al., 2014a) and rats (Rivalan et al., 2009; Rivalan et al., 2013) in such a task. Another example includes sensorimotor gating measured using the prepulse inhibition (PPI) of startle. PPI is impaired in patients with BD mania (Perry et al., 2001), an effect replicated in severely hyperdopaminergic mice (Powell et al., 2008), but these deficits are not observed in depressed subjects (Kohl et al., 2013) or mice with elevated acetylcholine (Clark et al., 2005). Hence, future studies using cross-species assessment tools in BD sufferers exposed to switch-inducing triggers can be conducted.
In addition to the assessment of patient and model organisms in cross-species relevant tasks, a key future direction will be to identify what manipulation(s) would provide the optimal model organism. As was described above, rs41350144 which lies within the intron of NF1A on 1p31 may be a susceptibility gene for seasonality in BD (Lee et al., 2013). Other genes linked to psychiatric conditions have been recreated and inserted into mice, such as the disrupted in schizophrenia 1 (DISC1)/boymaw transfusion gene (Ji et al., 2014) that has been related to schizophrenia and BD. Furthermore, a key element of catecholaminergic homeostasis in the prefrontal cortex is the catecho-methyl-transferase (COMT) gene (Allen et al., 1997; Risbrough et al., 2014; Tunbridge et al., 2012). COMT clears dopamine in the prefrontal cortex and the allelic variants of COMT result in faster (val/val) or slower (met/met) dopamine clearance (Allen et al., 1997). These allelic variants have been recreated in mice and have similar functional consequences (Risbrough et al., 2014). Another genetic polymorphism identified as relevant to rapid cycling in BD is the BDNF Val66Met polymorphism (Green et al., 2006; Liu et al., 2008; Muller et al., 2006). Given that this polymorphism has also been recreated in mice (Bath et al., 2012; Chen et al., 2006), testing the effects of pharmacological treatments or seasonal variation known to induce switching in any of these mouse lines on cross-species relevant behaviors could provide insight as to their importance for cycling in BD.
The literature review highlighted that altered catecholaminergic homeostasis likely plays a role in switching between mood states in BD. One key mechanism of such homeostasis in addition to COMT is the re-uptake of dopamine, and to a lesser extent norepinephrine, by the DAT. Several studies link altered DAT functioning in patients with BD. For example, polymorphisms in the DAT gene have been linked with BD (Greenwood et al., 2006a; Pinsonneault et al., 2011), which likely lower functional DAT levels (Horschitz et al., 2005). In support of this possibility, reduced striatal DAT levels are seen using positron emission tomography study in unmedicated BD patients (Anand et al., 2011) and from their postmortem tissue (Rao et al., 2012). Hence, mice with reduced functioning of the DAT may recreate the impaired catecholaminergic homeostasis seen in patients and mediate switching between states. Mice with extremely reduced DAT function (10% compared to wildtype controls) exhibit numerous behavioral abnormalities that are consistent with BD mania patients, including: 1) Hyper-exploration and straighter movement seen in the Behavioral Pattern Monitor (Perry et al., 2009; Young et al., 2010a; b); 2) that is treated with chronic valproate administration (van Enkhuizen et al., 2012); 3) which degrades with repeated testing, similar to SRT treatment (Young et al., 2010b); 4) high-reward preference increased risk-preference as measured by the Iowa Gambling Task (van Enkhuizen et al., 2014a). The 10% of DAT levels seen in these mice are much lower than unmedicated BD patients however [approximately 80% (Anand et al., 2011)]. Interestingly, rats placed in high-activity photoperiod lengths (that induce EPM-related risk-preferring behavior) have lower DAT levels compared to those kept in a balanced photoperiod (Dulcis et al., 2013). Hence, it is possible that mice with low, but not extremely low DAT levels, may better recreate the state seen in BD sufferers, whereby stimuli resulting in a switch to a mania-like state would further lower their DAT levels.
Other manipulations that would be useful to investigate whether they contribute to switching are manipulations of genes that underlie circadian rhythm. Naturally, any animal with an altered circadian rhythm may elicit altered behavioral responses to changing photoperiod length in a manner consistent with that of BD. Genetic mutants such as ClockΔ19 mice have a deletion of exon 19 in the CLOCK gene and exhibit BD-relevant behaviors such as lower immobility in the FST, sweet sucrose solution and cocaine preference, and have lower reward thresholds identified using intra-cranial self-stimulation (McClung et al., 2005; Roybal et al., 2007), and impaired PPI (van Enkhuizen et al., 2013b). Some of these behaviors are attenuated by lithium treatment (Roybal et al., 2007). These ClockΔ19 mice do not however exhibit increased specific exploration or straight line movements (van Enkhuizen et al., 2013b) seen in BD mania patients (Minassian et al., 2011; Perry et al., 2009). Moreover, this mutant arose from a random deletion not a targeted recreation of changes seen in patients with BD. Importantly, the effect of altered photoperiod lengths in these mice have yet to be established, nor have they been conducted in other circadian gene relevant BMAL1 and NPAS2 mutant mice.
Finally, while it is clear that there has been a strong seasonal variation driving switches in mood states in BD, this effect may have been reduced over time with the increased use of artificial lighting. In fact, people’s internal circadian clock do not entrain to solar time unless they are exposed to a minimum of 7 days of natural sunlight during wilderness camping (Wright et al., 2013). The increased availability of low level lighting from smartphones, tablets, and readers likely reduces solar entrainment even further. Importantly, while 7 days were required for human solar entrainment, the same number of days were required for entraining rats to altered photoperiod length-induced neurotransmitter switching (Dulcis et al., 2013). Combined, these premises support the possibility that mood switching in BD could have derived from natural sunlight variation in seasons, and that modern electrics may confound these effects. Irrespective of modern lighting-induced limitation in seasonal switching, this hypothesis would mean that the mechanisms underlying switching in BD remain and can be investigated. This mechanism could also be recreated in animals exposed to altered photoperiod lengths. Furthermore, studies identifying a susceptibility gene that engenders hypersensitivity to seasonality switching could also be conducted (e.g., NF1, BDNF, CLOCK, DAT etc). Importantly, using photoperiod chambers and the ability to conduct cross-species relevant testing of mania- and depression-relevant behaviors in mice, such seasonality-induced switching studies can be conducted. The key remains to identify a manipulation that results in a hypersensitivity to such changes, and the triggers outlined above.
Acknowledgments
We thank Drs. Geyer, Minassian, Perry, Spitzer for their support. These studies were supported by NIH grants R01-MH042228, R01-MH071916, a Kavli grant KIBM-2012-008, as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelaira HM, Reus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Rev Bras Psiquiatr. 2013;35(Suppl 2):S112–120. doi: 10.1590/1516-4446-2013-1098. [DOI] [PubMed] [Google Scholar]
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, Mazzola-Pomietto P, Jeanningros R, Goodwin GM, Azorin JM, Courtet P. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–365. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Bourgeois ML, Angst J, Post R, Moller H, Hirschfeld R. Reevaluating the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. J Affect Disord. 2000;59(Suppl 1):S5–S30. doi: 10.1016/s0165-0327(00)00203-2. [DOI] [PubMed] [Google Scholar]
- Allen E, Pettigrew A, Frank D, Thompson S, Myers C, Yamashita T, Blumer JL. Alterations in dopamine clearance and catechol-O-methyltransferase activity by dopamine infusions in children. Crit Care Med. 1997;25:181–189. doi: 10.1097/00003246-199701000-00032. [DOI] [PubMed] [Google Scholar]
- Amado I, Galinowski A, Daban C, Ramdane-Cherif Z, Poirier E, Bourdel MC, Poirier MF, Krebs MO. Effects of lithium on saccadic eye movements in healthy subjects in a ten-day double-blind placebo-controlled cross-over pilot study. Pharmacopsychiatry. 2005;38:321–325. doi: 10.1055/s-2005-916188. [DOI] [PubMed] [Google Scholar]
- Anand A, Barkay G, Dzemidzic M, Albrecht D, Karne H, Zheng QH, Hutchins GD, Normandin MD, Yoder KK. Striatal dopamine transporter availability in unmedicated bipolar disorder. Bipolar Disorder. 2011;13:406–413. doi: 10.1111/j.1399-5618.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- Anand A, Darnell A, Miller HL, Berman RM, Cappiello A, Oren DA, Woods SW, Charney DS. Effect of catecholamine depletion on lithium-induced long-term remission of bipolar disorder. Biol Psychiatry. 1999;45:972–978. doi: 10.1016/s0006-3223(98)00293-5. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR, Kucinski BJ, Fowler H, Gershon S, Edwards DJ, Austin MC, Stiller R, Kiss S, Kocan D. The effects of lithium on a potential cycling model of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:495–510. doi: 10.1016/s0278-5846(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Asghar SJ, Tanay VA, Baker GB, Greenshaw A, Silverstone PH. Relationship of plasma amphetamine levels to physiological, subjective, cognitive and biochemical measures in healthy volunteers. Hum Psychopharmacol. 2003;18:291–299. doi: 10.1002/hup.480. [DOI] [PubMed] [Google Scholar]
- Ashkenazy-Frolinger T, Kronfeld-Schor N, Juetten J, Einat H. It is darkness and not light: Depression-like behaviors of diurnal unstriped Nile grass rats maintained under a short photoperiod schedule. J Neurosci Methods. 2010;186:165–170. doi: 10.1016/j.jneumeth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Aumann TD, Gantois I, Egan K, Vais A, Tomas D, Drago J, Horne MK. SK channel function regulates the dopamine phenotype of neurons in the substantia nigra pars compacta. Exp Neurol. 2008;213:419–430. doi: 10.1016/j.expneurol.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Aydin A, Selvi Y, Besiroglu L, Boysan M, Atli A, Ozdemir O, Kilic S, Balaharoglu R. Mood and metabolic consequences of sleep deprivation as a potential endophenotype’ in bipolar disorder. J Affect Disord. 2013;150:284–294. doi: 10.1016/j.jad.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Baek JH, Kim JS, Kim MJ, Ryu S, Lee K, Ha K, Hong KS. Lifetime Characteristics of Evening-Preference and Irregular Bed-Rise Time Are Associated With Lifetime Seasonal Variation of Mood and Behavior: Comparison Between Individuals With Bipolar Disorder and Healthy Controls. Behav Sleep Med. 2014:1–14. doi: 10.1080/15402002.2014.974179. [DOI] [PubMed] [Google Scholar]
- Barbini B, Di Molfetta D, Gasperini M, Manfredonia M, Smeraldi E. Seasonal concordance of recurrence in mood disorder patients. Eur Psychiatry. 1995;10:171–174. doi: 10.1016/0767-399X(96)80060-5. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS. Measurement issues in the use of cognitive neuroscience tasks in drug development for impaired cognition in schizophrenia: a report of the second consensus building conference of the CNTRICS initiative. Schizophr Bull. 2008;34:613–618. doi: 10.1093/schbul/sbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Jing DQ, Dincheva I, Neeb CC, Pattwell SS, Chao MV, Lee FS, Ninan I. BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology. 2012;37:1297–1304. doi: 10.1038/npp.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, Schulze TG, Cichon S, Rietschel M, Nothen MM, Georgi A, Schumacher J, Schwarz M, Abou Jamra R, Hofels S, Propping P, Satagopan J, Detera-Wadleigh SD, Hardy J, McMahon FJ. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Begley CE, Annegers JF, Swann AC, Lewis C, Coan S, Schnapp WB, Bryant-Comstock L. The lifetime cost of bipolar disorder in the US: an estimate for new cases in 1998. Pharmacoeconomics. 2001;19:483–495. doi: 10.2165/00019053-200119050-00004. [DOI] [PubMed] [Google Scholar]
- Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- Belzung C. Innovative drugs to treat depression: did animal models fail to be predictive or did clinical trials fail to detect effects? Neuropsychopharmacology. 2014;39:1041–1051. doi: 10.1038/npp.2013.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RE, Alloy LB. Life stress and kindling in bipolar disorder: review of the evidence and integration with emerging biopsychosocial theories. Clin Psychol Rev. 2011;31:383–398. doi: 10.1016/j.cpr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Hahn CG, Thase ME. Are we getting closer to valid translational models for major depression? Science. 2012;338:75–79. doi: 10.1126/science.1222940. [DOI] [PubMed] [Google Scholar]
- Blumberg HP. Euthymia, depression, and mania: what do we know about the switch? Biol Psychiatry. 2012;71:570–571. doi: 10.1016/j.biopsych.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottlender R, Rudolf D, Strauss A, Moller HJ. Mood-stabilisers reduce the risk of developing antidepressant-induced maniform states in acute treatment of bipolar I depressed patients. J Affect Disord. 2001;63:79–83. doi: 10.1016/s0165-0327(00)00172-5. [DOI] [PubMed] [Google Scholar]
- Bouwkamp CG, de Kruiff ME, van Troost TM, Snippe D, Blom MJ, de Winter RF, Judith Haffmans PM. Interpersonal and social rhythm group therapy for patients with bipolar disorder. Int J Group Psychother. 2013;63:97–115. doi: 10.1521/ijgp.2013.63.1.97. [DOI] [PubMed] [Google Scholar]
- Brady R, Jr, Ongur D, Keshavan M. Neurobiology of mood-state shifts in bipolar disorder: a selective review and a hypothesis. Harv Rev Psychiatry. 2014;22:23–30. doi: 10.1097/HRP.0000000000000004. [DOI] [PubMed] [Google Scholar]
- Brambilla C, Gavinelli C, Delmonte D, Fulgosi MC, Barbini B, Colombo C, Smeraldi E. Seasonality and sleep: a clinical study on euthymic mood disorder patients. Depress Res Treat. 2012;2012:978962. doi: 10.1155/2012/978962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney WE, Jr, Hartmann EL, Mason JW. Study of a Patient with 48-Hour Manic-Depressive Cycles. Ii. Strong Positive Correlation between Endocrine Factors and Manic Defense Patterns. Arch Gen Psychiatry. 1965;12:619–625. doi: 10.1001/archpsyc.1965.01720360091015. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Jr, Murphy DL, Goodwin FK, Borge GF. The switch process from depression to mania: relationship to drugs which alter brain amines. Lancet. 1970;1:1022–1027. doi: 10.1016/s0140-6736(70)91151-7. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60:79–88. doi: 10.4088/jcp.v60n0203. [DOI] [PubMed] [Google Scholar]
- Carboni L. Peripheral biomarkers in animal models of major depressive disorder. Dis Markers. 2013;35:33–41. doi: 10.1155/2013/284543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Barch DM, Bullmore E, Breiling J, Buchanan RW, Butler P, Cohen JD, Geyer M, Gollub R, Green MF, Jaeger J, Krystal JH, Moore H, Nuechterlein K, Robbins T, Silverstein S, Smith EE, Strauss M, Wykes T. Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia II: developing imaging biomarkers to enhance treatment development for schizophrenia and related disorders. Biol Psychiatry. 2011;70:7–12. doi: 10.1016/j.biopsych.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Dimellis D, Gonda X, Vieta E, McLntyre RS, Fountoulakis KN. Rapid cycling in bipolar disorder: a systematic review. J Clin Psychiatry. 2014;75:e578–586. doi: 10.4088/JCP.13r08905. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Forest K, Murry E, Carroll BJ. A factor analysis of the signs and symptoms of mania. Arch Gen Psychiatry. 1998;55:27–32. doi: 10.1001/archpsyc.55.1.27. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S, Martin JH. Postnatal development of a segmental switch enables corticospinal tract transmission to spinal forelimb motor circuits. J Neurosci. 2010;30:2277–2288. doi: 10.1523/JNEUROSCI.5286-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S, Shulman B, Martin JH. Activity-dependent codevelopment of the corticospinal system and target interneurons in the cervical spinal cord. J Neurosci. 2009;29:8816–8827. doi: 10.1523/JNEUROSCI.0735-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Henter ID, Manji HK. Translational research in bipolar disorder: emerging insights from genetically based models. Mol Psychiatry. 2010;15:883–895. doi: 10.1038/mp.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JC. Recent advances in treatment of acute mania. J Clin Psychopharmacol. 1991;11:3–21. [PubMed] [Google Scholar]
- Claassen DO, van den Wildenberg WP, Ridderinkhof KR, Jessup CK, Harrison MB, Wooten GF, Wylie SA. The risky business of dopamine agonists in Parkinson disease and impulse control disorders. Behav Neurosci. 2011;125:492–500. doi: 10.1037/a0023795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MG, Sun W, Myers TM, Bansal R, Doctor BP, Saxena A. Effects of physostigmine and human butyrylcholinesterase on acoustic startle reflex and prepulse inhibition in C57BL/6J mice. Pharmacol Biochem Behav. 2005;81:497–505. doi: 10.1016/j.pbb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Colombo C, Benedetti F, Barbini B, Campori E, Smeraldi E. Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Res. 1999;86:267–270. doi: 10.1016/s0165-1781(99)00036-0. [DOI] [PubMed] [Google Scholar]
- Corp SA, Gitlin MJ, Altshuler LL. A review of the use of stimulants and stimulant alternatives in treating bipolar depression and major depressive disorder. J Clin Psychiatry. 2014;75:1010–1018. doi: 10.4088/JCP.13r08851. [DOI] [PubMed] [Google Scholar]
- Cumming MJ, Thompson MA, McCormick CM. Adolescent social instability stress increases aggression in a food competition task in adult male Long-Evans rats. Dev Psychobiol. 2014;56:1575–1588. doi: 10.1002/dev.21252. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvignes C, Rouquier L, Souilhac J, Mons G, Rodier D, Soubrie P, Steinberg R. Control by tachykinin NK(2) receptors of CRF(1) receptor-mediated activation of hippocampal acetylcholine release in the rat and guinea-pig. Neuropeptides. 2003;37:89–97. doi: 10.1016/s0143-4179(03)00019-2. [DOI] [PubMed] [Google Scholar]
- Djamshidian A, Cardoso F, Grosset D, Bowden-Jones H, Lees AJ. Pathological gambling in Parkinson’s disease--a review of the literature. Mov Disord. 2011;26:1976–1984. doi: 10.1002/mds.23821. [DOI] [PubMed] [Google Scholar]
- Dlugos A, Freitag C, Hohoff C, McDonald J, Cook EH, Deckert J, de Wit H. Norepinephrine transporter gene variation modulates acute response to D-amphetamine. Biol Psychiatry. 2007;61:1296–1305. doi: 10.1016/j.biopsych.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340:449–453. doi: 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- Dulcis D, Spitzer NC. Illumination controls differentiation of dopamine neurons regulating behaviour. Nature. 2008;456:195–201. doi: 10.1038/nature07569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H. Establishment of a battery of simple models for facets of bipolar disorder: a practical approach to achieve increased validity, better screening and possible insights into endophenotypes of disease. Behav Genet. 2007;37:244–255. doi: 10.1007/s10519-006-9093-4. [DOI] [PubMed] [Google Scholar]
- Ellicott A, Hammen C, Gitlin M, Brown G, Jamison K. Life events and the course of bipolar disorder. Am J Psychiatry. 1990;147:1194–1198. doi: 10.1176/ajp.147.9.1194. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Forni D, Cagliani R, Tresoldi C, Pozzoli U, De Gioia L, Filippi G, Riva S, Menozzi G, Colleoni M, Biasin M, Lo Caputo S, Mazzotta F, Comi GP, Bresolin N, Clerici M, Sironi M. An evolutionary analysis of antigen processing and presentation across different timescales reveals pervasive selection. PLoS Genet. 2014;10:e1004189. doi: 10.1371/journal.pgen.1004189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Kontis D, Gonda X, Yatham LN. A systematic review of the evidence on the treatment of rapid cycling bipolar disorder. Bipolar Disord. 2013;15:115–137. doi: 10.1111/bdi.12045. [DOI] [PubMed] [Google Scholar]
- Frank E, Soreca I, Swartz HA, Fagiolini AM, Mallinger AG, Thase ME, Grochocinski VJ, Houck PR, Kupfer DJ. The role of interpersonal and social rhythm therapy in improving occupational functioning in patients with bipolar I disorder. Am J Psychiatry. 2008;165:1559–1565. doi: 10.1176/appi.ajp.2008.07121953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries GR, Vasconcelos-Moreno MP, Gubert C, Santos BT, Sartori J, Eisele B, Ferrari P, Fijtman A, Ruegg J, Gassen NC, Kapczinski F, Rein T, Kauer-Sant’Anna M. Hypothalamic-pituitary-adrenal axis dysfunction and illness progression in bipolar disorder. Int J Neuropsychopharmacol. 2014:18. doi: 10.1093/ijnp/pyu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy PA, Bellivier F, Scott J, Boudebesse C, Lajnef M, Gard S, Kahn JP, Azorin JM, Henry C, Leboyer M, Etain B. Bipolar disorder with seasonal pattern: clinical characteristics and gender influences. Chronobiol Int. 2013;30:1101–1107. doi: 10.3109/07420528.2013.800091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Pani L, Fadda P, Fratta W. Sleep deprivation in the rat: an animal model of mania. Eur Neuropsychopharmacol. 1995;5(Suppl):89–93. doi: 10.1016/0924-977x(95)00023-i. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Olivier B, Joels M, Kahn RS. From antipsychotic to anti-schizophrenia drugs: role of animal models. Trends Pharmacol Sci. 2012;33:515–521. doi: 10.1016/j.tips.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden AM, Dalgleish T, Spinks H. Dysfunctional attitudes in seasonal affective disorder. Behav Res Ther. 2006;44:1159–1164. doi: 10.1016/j.brat.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Gonzalez R. The relationship between bipolar disorder and biological rhythms. J Clin Psychiatry. 2014;75:e323–331. doi: 10.4088/JCP.13r08507. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic Depressive Illness. Oxford University Press; New York: 1990. [Google Scholar]
- Goodwin FK, Jamison KR, Ghaemi SN. Manic-depressive illness: bipolar disorders and recurrent depression. Oxford University Press; New York: 2007. [Google Scholar]
- Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: a critical need for improvement. Neurosci Biobehav Rev. 2007;31:825–831. doi: 10.1016/j.neubiorev.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clin Psychol Rev. 2006;26:679–694. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Green EK, Raybould R, Macgregor S, Hyde S, Young AH, O’Donovan MC, Owen MJ, Kirov G, Jones L, Jones I, Craddock N. Genetic variation of brain-derived neurotrophic factor (BDNF) in bipolar disorder: case-control study of over 3000 individuals from the UK. Br J Psychiatry. 2006;188:21–25. doi: 10.1192/bjp.bp.105.009969. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai W, Fritz MH, Hansen NF, Durand EY, Malaspinas AS, Jensen JD, Marques-Bonet T, Alkan C, Prufer K, Meyer M, Burbano HA, Good JM, Schultz R, Aximu-Petri A, Butthof A, Hober B, Hoffner B, Siegemund M, Weihmann A, Nusbaum C, Lander ES, Russ C, Novod N, Affourtit J, Egholm M, Verna C, Rudan P, Brajkovic D, Kucan Z, Gusic I, Doronichev VB, Golovanova LV, Lalueza-Fox C, de la Rasilla M, Fortea J, Rosas A, Schmitz RW, Johnson PL, Eichler EE, Falush D, Birney E, Mullikin JC, Slatkin M, Nielsen R, Kelso J, Lachmann M, Reich D, Paabo S. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Akiskal HS, Akiskal KK, Kelsoe JR. Genome-wide association study of temperament in bipolar disorder reveals significant associations with three novel Loci. Biol Psychiatry. 2012;72:303–310. doi: 10.1016/j.biopsych.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol Psychiatry. 2006a;11:125–133. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol Psychiatry. 2006b;11:125–133. 115. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry. 1996;1:278–297. [PubMed] [Google Scholar]
- Gupta A, Sharma PK, Garg VK, Singh AK, Mondal SC. Role of serotonin in seasonal affective disorder. Eur Rev Med Pharmacol Sci. 2013;17:49–55. [PubMed] [Google Scholar]
- Hannestad JO, Cosgrove KP, DellaGioia NF, Perkins E, Bois F, Bhagwagar Z, Seibyl JP, McClure-Begley TD, Picciotto MR, Esterlis I. Changes in the cholinergic system between bipolar depression and euthymia as measured with [123I]5IA single photon emission computed tomography. Biol Psychiatry. 2013;74:768–776. doi: 10.1016/j.biopsych.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MC, Lecrubier Y, Widlocher D. Efficacy of clonidine in 24 patients with acute mania. Am J Psychiatry. 1986;143:1450–1453. doi: 10.1176/ajp.143.11.1450. [DOI] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian functioning: critical mechanisms in the mood disorders? Annu Rev Clin Psychol. 2011;7:297–319. doi: 10.1146/annurev-clinpsy-032210-104550. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Cross CK. Epidemiology of affective disorders. Arch Gen Psychiatry. 1982;39:35–46. doi: 10.1001/archpsyc.1982.04290010013003. [DOI] [PubMed] [Google Scholar]
- Hlastala SA, Kotler JS, McClellan JM, McCauley EA. Interpersonal and social rhythm therapy for adolescents with bipolar disorder: treatment development and results from an open trial. Depress Anxiety. 2010;27:457–464. doi: 10.1002/da.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Lau T, Rietschel M, Schloss P. A dopamine transporter mutation associated with bipolar affective disorder causes inhibition of transporter cell surface expression. Mol Psychiatry. 2005;10:1104–1109. doi: 10.1038/sj.mp.4001730. [DOI] [PubMed] [Google Scholar]
- Jacobs D, Silverstone T. Dextroamphetamine-induced arousal in human subjects as a model for mania. Psychol Med. 1986;16:323–329. doi: 10.1017/s0033291700009132. [DOI] [PubMed] [Google Scholar]
- Jacobsen FM, Wehr TA, Skwerer RA, Sack DA, Rosenthal NE. Morning versus midday phototherapy of seasonal affective disorder. Am J Psychiatry. 1987;144:1301–1305. doi: 10.1176/ajp.144.10.1301. [DOI] [PubMed] [Google Scholar]
- Jang KL, Lam RW, Livesley WJ, Vernon PA. Gender differences in the heritability of seasonal mood change. Psychiatry Res. 1997;70:145–154. doi: 10.1016/s0165-1781(97)00030-9. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Risch SC, Huey LY, Judd LL, Rausch JL. Hypothalamic-pituitary-adrenal regulation, neurotransmitters and affective disorders. Peptides. 1983;4:775–784. doi: 10.1016/0196-9781(83)90035-9. [DOI] [PubMed] [Google Scholar]
- Ji B, Higa KK, Kim M, Zhou L, Young JW, Geyer MA, Zhou X. Inhibition of protein translation by the DISC1-Boymaw fusion gene from a Scottish family with major psychiatric disorders. Hum Mol Genet. 2014;23:5683–5705. doi: 10.1093/hmg/ddu285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly D, Sanger DJ. Social competition in dominant rats can be attenuated by anxiogenic drugs. Behav Pharmacol. 1992;3:83–88. doi: 10.1097/00008877-199203010-00012. [DOI] [PubMed] [Google Scholar]
- Jouvent R, Lecrubier Y, Hardy MC, Widlocher D. Clonidine and neuroleptic-resistant mania. Br J Psychiatry. 1988;152:293–294. [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Akiskal HS, Maser J, Coryell W, Solomon D, Endicott J, Keller M. Long-term symptomatic status of bipolar I vs. bipolar II disorders. Int J Neuropsychopharmacol. 2003;6:127–137. doi: 10.1017/S1461145703003341. [DOI] [PubMed] [Google Scholar]
- Kara NZ, Einat H. Rodent models for mania: practical approaches. Cell Tissue Res. 2013;354:191–201. doi: 10.1007/s00441-013-1594-x. [DOI] [PubMed] [Google Scholar]
- Keeler JF, Robbins TW. Translating cognition from animals to humans. Biochem Pharmacol. 2011;81:1356–1366. doi: 10.1016/j.bcp.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Knapen SE, van de Werken M, Gordijn MC, Meesters Y. The duration of light treatment and therapy outcome in seasonal affective disorder. J Affect Disord. 2014;166:343–346. doi: 10.1016/j.jad.2014.05.034. [DOI] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkotter J, Kuhn J. Prepulse inhibition in psychiatric disorders--apart from schizophrenia. J Psychiatr Res. 2013;47:445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Hermann PM, Kemperman C, Bohus B, Van den Hoofdakker RH, Beersma DGM. Single social defeat in male rats induces a gradual but long lasting behavioral change: a model of depression? Neuroscience Research Communications. 1990;7:36–42. [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci U S A. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner G, Chmielarz P, Roman A, Nalepa I. Gender differences in genetic mouse models evaluated for depressive-like and antidepressant behavior. Pharmacol Rep. 2013;65:1580–1590. doi: 10.1016/s1734-1140(13)71519-6. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Tsai SY, Lin HC. Seasonal variations in bipolar disorder admissions and the association with climate: a population-based study. J Affect Disord. 2007;97:61–69. doi: 10.1016/j.jad.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Woo HG, Greenwood TA, Kripke DF, Kelsoe JR. A genome-wide association study of seasonal pattern mania identifies NF1A as a possible susceptibility gene for bipolar disorder. J Affect Disord. 2013;145:200–207. doi: 10.1016/j.jad.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Albert PS, Rosenthal NE, Wehr TA. Relationship between sleep and mood in patients with rapid-cycling bipolar disorder. Psychiatry Res. 1996;63:161–168. doi: 10.1016/0165-1781(96)02854-5. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Smith RE. Steroid-induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5:319–332. doi: 10.1016/0165-0327(83)90022-8. [DOI] [PubMed] [Google Scholar]
- Lewis JL, Winokur G. The induction of mania. A natural history study with controls. Arch Gen Psychiatry. 1982;39:303–306. doi: 10.1001/archpsyc.1982.04290030041007. [DOI] [PubMed] [Google Scholar]
- Liu L, Foroud T, Xuei X, Berrettini W, Byerley W, Coryell W, El-Mallakh R, Gershon ES, Kelsoe JR, Lawson WB, MacKinnon DF, McInnis M, McMahon FJ, Murphy DL, Rice J, Scheftner W, Zandi PP, Lohoff FW, Niculescu AB, Meyer ET, Edenberg HJ, Nurnberger JI., Jr Evidence of association between brain-derived neurotrophic factor gene and bipolar disorder. Psychiatr Genet. 2008;18:267–274. doi: 10.1097/YPG.0b013e3283060f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh U, Dori A, Katzav A, Kofman O. Strain and regional dependence of alternate splicing of acetylcholinesterase in the murine brain following stress or treatment with diisopropylfluorophosphate. Behav Brain Res. 2010;210:107–115. doi: 10.1016/j.bbr.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Lott DC, Kim SJ, Cook EH, Jr, de Wit H. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacology. 2005;30:602–609. doi: 10.1038/sj.npp.1300637. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Findling RL, Leverich GS, Pizzarello SM, Post RM. Earliest symptoms discriminating juvenile-onset bipolar illness from ADHD. Bipolar Disord. 2009;11:441–451. doi: 10.1111/j.1399-5618.2009.00684.x. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Kapczinski F, Soares JC. Perspectives for the development of animal models of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:209–224. doi: 10.1016/j.pnpbp.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Maguire J, Singh AN. Clonidine. An effective anti-manic agent? Br J Psychiatry. 1987;150:863–864. doi: 10.1192/bjp.150.6.863. [DOI] [PubMed] [Google Scholar]
- Maj M, Pirozzo R, Magliano L, Bartoli L. The prognostic significance of “switching” in patients with bipolar disorder: a 10-year prospective follow-up study. American Journal of Psychiatry. 2002;159:1711. doi: 10.1176/appi.ajp.159.10.1711. [DOI] [PubMed] [Google Scholar]
- Malatynska E, Goldenberg R, Shuck L, Haque A, Zamecki P, Crites G, Schindler N, Knapp RJ. Reduction of submissive behavior in rats: a test for antidepressant drug activity. Pharmacology. 2002;64:8–17. doi: 10.1159/000056145. [DOI] [PubMed] [Google Scholar]
- Malatynska E, Knapp RJ. Dominant-submissive behavior as models of mania and depression. Neurosci Biobehav Rev. 2005;29:715–737. doi: 10.1016/j.neubiorev.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Malatynska E, Kostowski W. The effect of antidepressant drugs on dominance behavior in rats competing for food. Pol J Pharmacol Pharm. 1984;36:531–540. [PubMed] [Google Scholar]
- Malkesman O, Austin DR, Chen G, Manji HK. Reverse translational strategies for developing animal models of bipolar disorder. Dis Model Mech. 2009;2:238–245. doi: 10.1242/dmm.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, Sherrill JT, Houck PR, Kupfer DJ. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychol Med. 2000;30:1005–1016. doi: 10.1017/s0033291799002706. [DOI] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing Obstacles in Neuroscience Drug Discovery: The Future Path for Animal Models. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Nievergelt CM, Kelsoe JR, Welsh DK. A survey of genomic studies supports association of circadian clock genes with bipolar disorder spectrum illnesses and lithium response. PLoS One. 2012;7:e32091. doi: 10.1371/journal.pone.0032091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. J Biol Rhythms. 2012;27:339–352. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- McClung CA. Clock genes and bipolar disorder: implications for therapy. Pharmacogenomics. 2007;8:1097–1100. doi: 10.2217/14622416.8.9.1097. [DOI] [PubMed] [Google Scholar]
- McClung CA. How might circadian rhythms control mood? Let me count the ways. Biol Psychiatry. 2013;74:242–249. doi: 10.1016/j.biopsych.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane AC, Yehuda R, Clark CR. Biologic models of traumatic memories and post-traumatic stress disorder. The role of neural networks. Psychiatr Clin North Am. 2002;25:253–270. v. doi: 10.1016/s0193-953x(01)00008-9. [DOI] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2014:1–12. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Cha DS, Kim RD, Mansur RB. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013;18(Suppl 1):4–20. doi: 10.1017/S1092852913000746. quiz 21. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Rosenbluth M, Ramasubbu R, Bond DJ, Taylor VH, Beaulieu S, Schaffer A. Managing medical and psychiatric comorbidity in individuals with major depressive disorder and bipolar disorder. Ann Clin Psychiatry. 2012;24:163–169. [PubMed] [Google Scholar]
- McKenna BS, Eyler LT. Overlapping prefrontal systems involved in cognitive and emotional processing in euthymic bipolar disorder and following sleep deprivation: a review of functional neuroimaging studies. Clin Psychol Rev. 2012;32:650–663. doi: 10.1016/j.cpr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Gold LH. Ethological analysis of amphetamine action on social behavior in squirrel monkeys (Saimiri sciureus) Prog Clin Biol Res. 1983;131:137–155. [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. PLoS One. 2011;6:e24185. doi: 10.1371/journal.pone.0024185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PJ, Redfern PH. Acute and chronic antidepressant drug treatments induce opposite effects in the social behaviour of rats. J Psychopharmacol. 1992;6:241–257. doi: 10.1177/026988119200600218. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder RT, Cosgriff JP, Smith AM, Joyce PR. Seasonality of mania in New Zealand. Aust N Z J Psychiatry. 1990;24:187–190. doi: 10.3109/00048679009077681. [DOI] [PubMed] [Google Scholar]
- Muller DJ, de Luca V, Sicard T, King N, Strauss J, Kennedy JL. Brain-derived neurotrophic factor (BDNF) gene and rapid-cycling bipolar disorder: family-based association study. Br J Psychiatry. 2006;189:317–323. doi: 10.1192/bjp.bp.105.010587. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Pedersen BK, Kessing LV, Vinberg M. Elevated levels of plasma brain derived neurotrophic factor in rapid cycling bipolar disorder patients. Psychoneuroendocrinology. 2014;47:199–211. doi: 10.1016/j.psyneuen.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Brodie HK, Goodwin FK, Bunney WE., Jr Regular induction of hypomania by L-dopa in “bipolar” manic-depressive patients. Nature. 1971;229:135–136. doi: 10.1038/229135a0. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- Murray G, Lam RW, Sharma V. Do the symptoms of bipolar disorder really show seasonal variation? Bipolar Disord. 2013;15:808–810. doi: 10.1111/bdi.12111. [DOI] [PubMed] [Google Scholar]
- Must A, Horvath S, Nemeth VL, Janka Z. The Iowa Gambling Task in depression - what have we learned about sub-optimal decision-making strategies? Frontiers in psychology. 2013;4:732. doi: 10.3389/fpsyg.2013.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors HW, Trierweiler SJ, Ford BC, Muroff JR. Racial differences in DSM diagnosis using a semi-structured instrument: the importance of clinical judgment in the diagnosis of African Americans. J Health Soc Behav. 2003;44:237–256. [PubMed] [Google Scholar]
- Nesher E, Gross M, Lisson S, Tikhonov T, Yadid G, Pinhasov A. Differential responses to distinct psychotropic agents of selectively bred dominant and submissive animals. Behav Brain Res. 2013;236:225–235. doi: 10.1016/j.bbr.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Niemegeers P, Dumont GJ, Patteet L, Neels H, Sabbe BG. Bupropion for the treatment of seasonal affective disorder. Expert Opin Drug Metab Toxicol. 2013;9:1229–1240. doi: 10.1517/17425255.2013.804062. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, McElroy SL, Keck PE, Jr, Schork NJ, Kelsoe JR. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick DM, Swartz HA, Frank E. Suicide attempts in bipolar I and bipolar II disorder: a review and meta-analysis of the evidence. Bipolar disorders. 2010;12:1–9. doi: 10.1111/j.1399-5618.2009.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary OF, Cryan JF. Towards translational rodent models of depression. Cell Tissue Res. 2013;354:141–153. doi: 10.1007/s00441-013-1587-9. [DOI] [PubMed] [Google Scholar]
- Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- Ouanes S, Chennoufi L, Cheour M. An update on the treatment of mixed bipolar states: what is new in 2013? J Affect Disord. 2014;158:53–55. doi: 10.1016/j.jad.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Parker G, Walter S. Seasonal variation in depressive disorders and suicidal deaths in New South Wales. Br J Psychiatry. 1982;140:626–632. doi: 10.1192/bjp.140.6.626. [DOI] [PubMed] [Google Scholar]
- Peet M. Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry. 1994;164:549–550. doi: 10.1192/bjp.164.4.549. [DOI] [PubMed] [Google Scholar]
- Perron BE, Fries LE, Kilbourne AM, Vaughn MG, Bauer MS. Racial/Ethnic group differences in bipolar symptomatology in a community sample of persons with bipolar I disorder. J Nerv Ment Dis. 2010;198:16–21. doi: 10.1097/NMD.0b013e3181c818c5. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry. 2001;50:418–424. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan MC. Beclomethasone mania. Br J Psychiatry. 1989;155:871–872. doi: 10.1192/bjp.155.6.871. [DOI] [PubMed] [Google Scholar]
- Pinsonneault JK, Han DD, Burdick KE, Kataki M, Bertolino A, Malhotra AK, Gu HH, Sadee W. Dopamine transporter gene variant affecting expression in human brain is associated with bipolar disorder. Neuropsychopharmacology. 2011;36:1644–1655. doi: 10.1038/npp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piret B, Depaulis A, Vergnes M. Opposite effects of agonist and inverse agonist ligands of benzodiazepine receptor on self-defensive and submissive postures in the rat. Psychopharmacology (Berl) 1991;103:56–61. doi: 10.1007/BF02244074. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Post RM. Do the epilepsies, pain syndromes, and affective disorders share common kindling-like mechanisms? Epilepsy Res. 2002;50:203–219. doi: 10.1016/s0920-1211(02)00081-5. [DOI] [PubMed] [Google Scholar]
- Post RM, Susan R, Weiss B. Sensitization, kindling, and carbamazepine: an update on their implications for the course of affective illness. Pharmacopsychiatry. 1992;25:41–43. doi: 10.1055/s-2007-1014386. [DOI] [PubMed] [Google Scholar]
- Post RM, Uhde TW, Putnam FW, Ballenger JC, Berrettini WH. Kindling and carbamazepine in affective illness. J Nerv Ment Dis. 1982;170:717–731. doi: 10.1097/00005053-198212000-00002. [DOI] [PubMed] [Google Scholar]
- Post RM, Weiss SR. Sensitization, kindling, and anticonvulsants in mania. J Clin Psychiatry. 1989;50(Suppl):23–30. discussion 45–27. [PubMed] [Google Scholar]
- Postolache TT, Oren DA. Circadian phase shifting, alerting, and antidepressant effects of bright light treatment. Clin Sports Med. 2005;24:381–413. xii. doi: 10.1016/j.csm.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Powell SB, Young JW, Ong JC, Caron MG, Geyer MA. Atypical antipsychotics clozapine and quetiapine attenuate prepulse inhibition deficits in dopamine transporter knockout mice. Behav Pharmacol. 2008;19:562–565. doi: 10.1097/FBP.0b013e32830dc110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Marzani-Nissen GR. Bipolar disorders: a review. Am Fam Physician. 2012;85:483–493. [PubMed] [Google Scholar]
- Price J, Sloman L, Gardner R, Jr, Gilbert P, Rohde P. The social competition hypothesis of depression. Br J Psychiatry. 1994;164:309–315. doi: 10.1192/bjp.164.3.309. [DOI] [PubMed] [Google Scholar]
- Price JS. Hypothesis: the dominance hierarchy and the evolution of mental illness. Lancet. 1967;2:243–246. [Google Scholar]
- Pryce CR, Klaus F. Translating the evidence for gene association with depression into mouse models of depression-relevant behaviour: current limitations and future potential. Neurosci Biobehav Rev. 2013;37:1380–1402. doi: 10.1016/j.neubiorev.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Pucilowski O, Trzaskowska E, Jankowska E, Kostowski W, Kupryszewski G. Effects of intra-amygdaloid TRH injections on motor activity and dominant-submissive behavior in rats competing for water. Acta Physiol Pol. 1990;41:71–77. [PubMed] [Google Scholar]
- Raja M, Bentivoglio AR. Impulsive and compulsive behaviors during dopamine replacement treatment in Parkinson’s Disease and other disorders. Curr Drug Saf. 2012;7:63–75. doi: 10.2174/157488612800492726. [DOI] [PubMed] [Google Scholar]
- Rao JS, Kellom M, Reese EA, Rapoport SI, Kim HW. Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. J Affect Disord. 2012;136:63–71. doi: 10.1016/j.jad.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Risbrough V, Ji B, Hauger R, Zhou X. Generation and characterization of humanized mice carrying COMT158 Met/Val alleles. Neuropsychopharmacology. 2014;39:1823–1832. doi: 10.1038/npp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch SC, Cohen RM, Janowsky DS, Kalin NH, Sitaram N, Gillin JC, Murphy DL. Physostigmine induction of depressive symptomatology in normal human subjects. Psychiatry Res. 1981;4:89–94. doi: 10.1016/0165-1781(81)90012-3. [DOI] [PubMed] [Google Scholar]
- Rivalan M, Ahmed SH, Dellu-Hagedorn F. Risk-prone individuals prefer the wrong options on a rat version of the Iowa Gambling Task. Biol Psychiatry. 2009;66:743–749. doi: 10.1016/j.biopsych.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Rivalan M, Valton V, Series P, Marchand AR, Dellu-Hagedorn F. Elucidating poor decision-making in a rat gambling task. PLoS One. 2013;8:e82052. doi: 10.1371/journal.pone.0082052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard R, Naismith SL, Rogers NL, Scott EM, Ip TK, Hermens DF, Hickie IB. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: comparison of unipolar and bipolar phenotypes. Eur Psychiatry. 2013;28:412–416. doi: 10.1016/j.eurpsy.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Mueller PS, Newsome DA, Wehr TA. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs GS. Psychosocial interventions as adjunctive therapy for bipolar disorder. J Psychiatr Pract. 2008;14(Suppl 2):39–44. doi: 10.1097/01.pra.0000320125.99423.b7. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, Friedman ES, Bowden CL, Fossey MD, Ostacher MJ, Ketter TA, Patel J, Hauser P, Rapport D, Martinez JM, Allen MH, Miklowitz DJ, Otto MW, Dennehy EB, Thase ME. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356:1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Quiroz JA, Machado-Vieira R, Henter ID, Manji HK, Zarate CA., Jr The neurobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010;71:1488–1501. doi: 10.4088/JCP.09r05259gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, Chen JI, Cosgrove KP, Kerestes R, Ghose S, Tamminga CA, Pittman B, Bois F, Tamagnan G, Seibyl J, Picciotto MR, Staley JK, Bhagwagar Z. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:851–859. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Price JL, Drevets WC. Neuropathological and neuromorphometric abnormalities in bipolar disorder: view from the medial prefrontal cortical network. Neurosci Biobehav Rev. 2014;42:132–147. doi: 10.1016/j.neubiorev.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Sayer HK, Marshall S, Mellsop GW. Mania and seasonality in the southern hemisphere. J Affect Disord. 1991;23:151–156. doi: 10.1016/0165-0327(91)90027-p. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Sherman JA. Evolutionary origin of bipolar disorder-revised: EOBD-R. Med Hypotheses. 2012;78:113–122. doi: 10.1016/j.mehy.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Shopsin B, Janowsky D, Davis J, Gershon S. Proceedings: Rebound phenomenon in manic patients following physostigmine: towards an understanding of aminergic mechanisms underlying affective disorders. Psychopharmacol Bull. 1974;10:50–51. [PubMed] [Google Scholar]
- Silverstone T, Romans S, Hunt N, McPherson H. Is there a seasonal pattern of relapse in bipolar affective disorders? A dual northern and southern hemisphere cohort study. Br J Psychiatry. 1995;167:58–60. doi: 10.1192/bjp.167.1.58. [DOI] [PubMed] [Google Scholar]
- Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, Nimgaonkar VL, McQueen MB, Faraone SV, Kirby A, de Bakker PI, Ogdie MN, Thase ME, Sachs GS, Todd-Brown K, Gabriel SB, Sougnez C, Gates C, Blumenstiel B, Defelice M, Ardlie KG, Franklin J, Muir WJ, McGhee KA, MacIntyre DJ, McLean A, VanBeck M, McQuillin A, Bass NJ, Robinson M, Lawrence J, Anjorin A, Curtis D, Scolnick EM, Daly MJ, Blackwood DH, Gurling HM, Purcell SM. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W, Byerley W, Coryell W, Craig D, Edenberg HJ, Eskin E, Foroud T, Gershon E, Greenwood TA, Hipolito M, Koller DL, Lawson WB, Liu C, Lohoff F, McInnis MG, McMahon FJ, Mirel DB, Murray SS, Nievergelt C, Nurnberger J, Nwulia EA, Paschall J, Potash JB, Rice J, Schulze TG, Scheftner W, Panganiban C, Zaitlen N, Zandi PP, Zollner S, Schork NJ, Kelsoe JR. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Stip E, Dufresne J, Lussier I, Yatham L. A double-blind, placebo-controlled study of the effects of lithium on cognition in healthy subjects: mild and selective effects on learning. J Affect Disord. 2000;60:147–157. doi: 10.1016/s0165-0327(99)00178-0. [DOI] [PubMed] [Google Scholar]
- Takei N, O’Callaghan E, Sham P, Glover G, Tamura A, Murray R. Seasonality of admissions in the psychoses: effect of diagnosis, sex, and age at onset. Br J Psychiatry. 1992;161:506–511. doi: 10.1192/bjp.161.4.506. [DOI] [PubMed] [Google Scholar]
- Tande D, Hoglinger G, Debeir T, Freundlieb N, Hirsch EC, Francois C. New striatal dopamine neurons in MPTP-treated macaques result from a phenotypic shift and not neurogenesis. Brain. 2006;129:1194–1200. doi: 10.1093/brain/awl041. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Moore S. Social position and competition in laboratory rats. J Comp Physiol Psychol. 1975;88:424–430. doi: 10.1037/h0076224. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Huber A, Farrell SM, Stumpenhorst K, Harrison PJ, Walton ME. The role of catechol-O-methyltransferase in reward processing and addiction. CNS Neurol Disord Drug Targets. 2012;11:306–323. doi: 10.2174/187152712800672409. [DOI] [PubMed] [Google Scholar]
- van Enkhuizen J, Acheson D, Risbrough V, Drummond S, Geyer MA, Young JW. Sleep deprivation impairs performance in the 5-choice continuous performance test: Similarities between humans and mice. Behav Brain Res. 2013a;261C:40–48. doi: 10.1016/j.bbr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Geyer MA, Kooistra K, Young JW. Chronic valproate attenuates some, but not all, facets of mania-like behaviour in mice. Int J Neuropsychopharmacol. 2012:1–11. doi: 10.1017/S1461145712001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Henry BL, Minassian A, Perry W, Milienne-Petiot M, Higa KK, Geyer MA, Young JW. Reduced dopamine transporter functioning induces high-reward risk-preference consistent with bipolar disorder. Neuropsychopharmacology. 2014a;39:3112–3122. doi: 10.1038/npp.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Janowsky DS, Olivier B, Minassian A, Perry W, Young JW, Geyer MA. The catecholaminergic-cholinergic balance hypothesis of bipolar disorder revisited. Eur J Pharmacol. 2014b doi: 10.1016/j.ejphar.2014.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Minassian A, Young JW. Further evidence for ClockDelta19 mice as a model for bipolar disorder mania using cross-species tests of exploration and sensorimotor gating. Behav Brain Res. 2013b doi: 10.1016/j.bbr.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Villasenor A, Ramamoorthy A, Silva dos Santos M, Lorenzo MP, Laje G, Zarate C, Jr, Barbas C, Wainer IW. A pilot study of plasma metabolomic patterns from patients treated with ketamine for bipolar depression: evidence for a response-related difference in mitochondrial networks. Br J Pharmacol. 2014;171:2230–2242. doi: 10.1111/bph.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe FM, Del Porto JA. Seasonality of admissions for mania in a psychiatric hospital of Belo Horizonte, Brazil. J Affect Disord. 2006;94:243–248. doi: 10.1016/j.jad.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Wada K, Yamada N, Suzuki H, Lee Y, Kuroda S. Recurrent cases of corticosteroid-induced mood disorder: clinical characteristics and treatment. J Clin Psychiatry. 2000;61:261–267. doi: 10.4088/jcp.v61n0404. [DOI] [PubMed] [Google Scholar]
- Wang B, Chen D. Evidence for seasonal mania: a review. J Psychiatr Pract. 2013;19:301–308. doi: 10.1097/01.pra.0000432600.32384.c5. [DOI] [PubMed] [Google Scholar]
- Weber NS, Cowan DN, Bedno SA, Niebuhr DW. Descriptive epidemiology of bipolar I disorder among United States military personnel. Mil Med. 2010;175:247–251. doi: 10.7205/milmed-d-09-00106. [DOI] [PubMed] [Google Scholar]
- Weiss RD. Treating patients with bipolar disorder and substance dependence: lessons learned. J Subst Abuse Treat. 2004;27:307–312. doi: 10.1016/j.jsat.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Weiss SR, Post RM. Carbamazepine and carbamazepine-10,11-epoxide inhibit amygdala-kindled seizures in the rat but do not block their development. Clin Neuropharmacol. 1987;10:272–279. doi: 10.1097/00002826-198706000-00008. [DOI] [PubMed] [Google Scholar]
- White TD, Asfaw B, DeGusta D, Gilbert H, Richards GD, Suwa G, Howell FC. Pleistocene Homo sapiens from Middle Awash, Ethiopia. Nature. 2003;423:742–747. doi: 10.1038/nature01669. [DOI] [PubMed] [Google Scholar]
- Whitney DK, Sharma V, Kueneman K. Seasonality of manic depressive illness in Canada. J Affect Disord. 1999;55:99–105. doi: 10.1016/s0165-0327(98)00197-9. [DOI] [PubMed] [Google Scholar]
- Willner P, D’Aquila PS, Coventry T, Brain P. Loss of social status: preliminary evaluation of a novel animal model of depression. J Psychopharmacol. 1995;9:207–213. doi: 10.1177/026988119500900302. [DOI] [PubMed] [Google Scholar]
- Wright JB. Mania following sleep deprivation. Br J Psychiatry. 1993;163:679–680. doi: 10.1192/bjp.163.5.679. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Kelsoe JR, Schachat C, Bunney BG, DeModena A, Golshan S, Gillin JC, Potkin SG, Bunney WE. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66:298–301. doi: 10.1016/j.biopsych.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Young J, Geyer M. Developing treatments for cognitive deficits in schizophrenia: The challenge of translation. J Psychopharmacol. 2014 doi: 10.1177/0269881114555252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW. Dopamine D1 and D2 receptor family contributions to modafinil-induced wakefulness. J Neurosci. 2009;29:2663–2665. doi: 10.1523/JNEUROSCI.5843-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Action of Modafinil-Increased Motivation Via the Dopamine Transporter Inhibition and D1 Receptors? Biol Psychiatry. 2010;67:784–787. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010a;208:443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacology Biochemistry and Behavior. 2010b;96:7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol. 2011;164:1263–1284. doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Jentsch JD, Bussey TJ, Wallace TL, Hutcheson DM. Consideration of species differences in developing novel molecules as cognition enhancers. Neurosci Biobehav Rev. 2013;37:2181–2193. doi: 10.1016/j.neubiorev.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neurosci Biobehav Rev. 2007;31:882–896. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005;57:430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Tohen M, Fletcher K. Cycling into depression from a first episode of mania: a case-comparison study. Am J Psychiatry. 2001;158:1524–1526. doi: 10.1176/appi.ajp.158.9.1524. [DOI] [PubMed] [Google Scholar]
- Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Cohen BM, Lipinski JF, Jr, Jonas JM. Clonidine in the treatment of mania and mixed bipolar disorder. Am J Psychiatry. 1984;141:1617–1618. doi: 10.1176/ajp.141.12.1617. [DOI] [PubMed] [Google Scholar]
- Zupancic ML. Role of atypical antipsychotics in rapid cycling bipolar disorder: a review of the literature. Ann Clin Psychiatry. 2011;23:141–149. [PubMed] [Google Scholar]