Abstract
Intervertebral disc (IVD) degeneration and associated low back pain (LBP) remains a major burden to our society without a significant improvement in treatment strategies or patient’s quality of life. While the recent cell-transplantation studies for treatment of degenerative disc disease showed promising results, to better gauge the success and functional outcomes of these therapies, it is crucial to understand if transplanted cells give rise to healthy nucleus pulposus (NP) tissue. NP cell phenotype is unique and is defined by expression of a characteristic set of markers that reflect their specialized physiology and function. This review summarizes phenotypic markers that mirror unique physiology and function of NP cells and their progenitors and should be considered to measure outcomes of cell-based therapies to treat disc degeneration.
BACKGROUND
Degeneration of the intervertebral discs (IVDs) and associated back pain remain prevalent and costly conditions today, despite ongoing research and recent clinical advancements. It is estimated that two out of three adults will suffer from back pain at some time during their lifetime, and much of this pain is directly attributable to disc disease [1, 2]. In a recent 20-year study, low back pain (LBP) was ranked the highest in number of years lived with disability; neck pain ranked fourth. In addition, LBP was fourth highest in disability-adjusted life-year ranking – a measure of missed “healthy” years of life [3]. Costs from the disease continue to increase, with spine-related expenditures in the United States estimated at $85.9 billion for 2005. Unfortunately, from 1997 to 2005, a Medical Expenditure Panel Survey found no significant improvement in several parameters surveyed, including self-assessed health status, functional disability, work limitations, and social functioning [4]. Moreover, one of the most common surgeries to relieve back pain stemming from degenerative discs – fusion – has been shown to negatively affect mechanics of surrounding discs [5]. It is clear from these statistics that there is a need for novel, effective strategies for the treatment of IVD degeneration.
An early characteristic of disc degeneration is the loss of cell number in the inner, gelatinous nucleus pulpous (NP) of the disc [6, 7]. The NP of the healthy adult disc is cell-sparse and proteoglycan-rich, affording the tissue its high water content and, thus, mechanical function of distributing loads applied to the spine [8]. Resident NP cells are responsible for maintenance of this critical extracellular matrix through production of proteoglycans, mainly aggrecan, and collagens [9, 10]. Since the activity of NP cells underlies function of the disc, and their capacity to support the tissue declines with degeneration, one logical approach to alleviating the effects of disc degeneration is to regenerate or replace these resident cells.
Several studies have investigated cell-based therapy for treating disc degeneration. Use of endogenous disc progenitor cells or transplantation of mature disc cells or mesenchymal stem cells (MSCs) has been extensively explored. Direct transplantation of NP cells or chondrocytes, from both autologous and allogenic sources, has been proven to decrease degenerative phenotype in animal models [11-14]. Identification of an endogenous progenitor population within the disc has further expanded the possibility of cell-based therapy [15]. Finally, MSCs derived from bone marrow or other tissues have been extensively studied as a potential source of regeneration for the NP, and have shown promising results [16]. While these studies provide hope that stem cell therapy can be used to maintain disc health, several obstacles still remain. One critical component of a regenerative therapy is that the new tissue is able to replace or support function of diseased tissue. To recapitulate the healthy NP cell phenotype, it must first be clearly defined [17]. Thus, this review focuses on the key phenotypic characteristics of NP cells that must be mirrored in a successful cell replacement therapy, and provides a broad overview of stem/progenitor cell based therapy for disc degeneration.
IDENTIFICATION OF DISC PROGENITOR CELLS
Although the turnover of disc cells is generally thought to be slow, minor regenerative processes have been observed in the IVD, especially in the outer regions of annulus fibrosus (AF) [18, 19] and possibly in the inner AF and the NP [20]. The idea of promoting disc cell self-renewal is supported by several studies showing evidence of stem cells or progenitor cells within the disc. From degenerative human disc tissue, Risbud et al. [21] identified cells that express CD105, CD166, CD63, CD49a, CD90, CD73, p75 low affinity nerve growth factor receptor, and CD133/1, all of which are characteristically expressed on BM-MSCs. These cells also showed osteogenic, adipogenic, and chondrogenic differentiation capacity when cultured in appropriate media [21]. Similarly, Brisby et al. [22] reported expression of OCT3/4, CD105, CD90, STRO-1, and NOTCH1 genes and proteins in degenerative human disc tissues. According to a study by Blanco et al., when compared with the BM-MSCs, NP-derived MSCs had comparable morphology, immunophenotype, and differentiation capacity, with the exception that NP-derived MSCs could not differentiate into adipocytes [23]. Furthermore, Feng et al. [24] isolated AF cells from non-degenerative human discs and found that these cells express MSC markers including CD29, CD49e, CD51, CD73, CD90, CD105, CD166, CD184, and STRO-1, and the neuronal stem cell markers nestin and neuron-specific enolase. The authors also demonstrated that these AF cells had the ability to differentiate into adipocytes, osteoblasts, chondrocytes, neurons, and endothelial cells [24]. In accordance with these studies, Henriksson et al. [25] found in rabbit discs that both NP and AF contained 5-bromo-2’-deoxyuridine (BrdU) positive cells, indicating slow but ongoing cell proliferation. In the region of the AF border to ligament zone and the perichondrium region, a high number of BrdU positive cells were found at early time points, which substantially decreased at later time points, similar to the pattern of a stem cell niche. In a more recent study, Henriksson et al. [26] confirmed the presence of BrdU positive cells in the previously reported stem cell niche near the epiphyseal plate. Interestingly, BrdU positive cells found in this region in the 3-month-old rabbit were significantly reduced at 9-months. Rather, in the older rabbits BrdU positive cells were found in a new niche in the mature AF [26].
Studies by Sakai et al. [27] and Yasen et al. [28] further confirmed the existence of a pool of endogenous progenitors within the disc. These two studies also emphasize the change in the progenitor pool with age and degeneration. Age-dependent reduction in the Tie2+ NP progenitor cell population in human and mouse [27] as well as reduction of progenitor cell marker expression in rabbit IVD [28] suggest there may be some difficulties in utilizing endogenous progenitors for disc repair simply due to reduced availability of the progenitor cells in aged discs (Figure 1). Still, results from these studies confirm the presence of endogenous progenitor/stem cells in a niche, and suggest that these cells can be used to replace old and damaged cells to replenish the disc structure.
Figure 1.
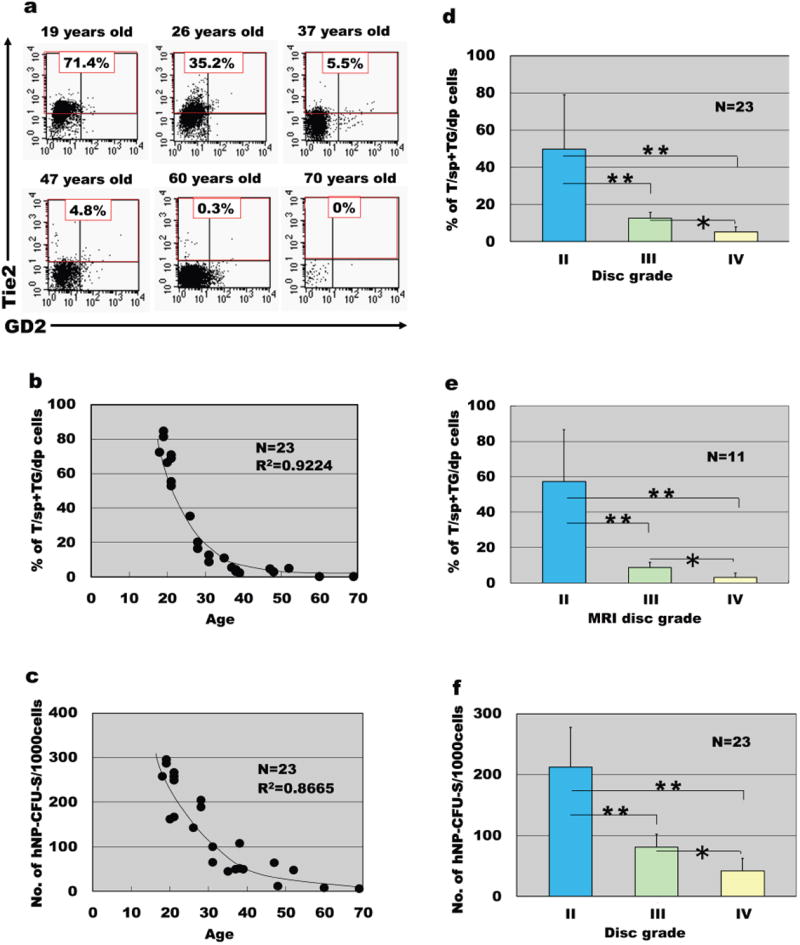
Decrease of Tie2+ NP cells with ageing and degeneration. Human NP cells donated by patients were studied together with their clinical profiles. Cells were freshly dispersed and only cells detected by flow cytometry within the live and the PI-negative gate were analysed. (a) Representative flow cytometry data of Tie2 and GD2 cell positivity in different age groups. (b) The frequency of Tie2+ cells (T/sp and TG/dp hNP cells) began to decrease before 20 years of age and correlated negatively with age (n=23, R2=0.9224). (c) The frequency of hNP-CFU-S generation also decreased with age (n=23, R2=0.8665). (d) The frequency of Tie2+ cells (T/sp and TG/dp hNP cells) decreased in relation to the extent of disc degeneration graded by morphology and (e) with disc degeneration graded by diagnostic magnetic resonance imaging (n=11). (f) The frequency of hNP-CFU-S generation decreased in relation to the extent of disc degeneration graded by morphology (n=23). *P<0.05, **P<0.01 (ANOVA with Mann–Whitney U-test). Data are represented as mean±s.d. Adapted from Ref. 27.
PROGENITOR CELL MARKERS AND NOTCH SIGNALING IN IVD
Studies that have identified progenitor cells within the IVD have often used classical MSC markers recommended by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cell Therapy: CD90+, CD73+, CD105+, CD45-, CD34-, CD14-, CD11b-, CD79α-/CD19-, and HLA-DR- [29]. Additional markers expressed by the disc progenitor cells or used to determine localization of these cells in the disc include CD24, CD29, CD44, CD49a/e/f, CD51, CD56, CD63, CD73, CD133/1, CD166, CD184, OCT3/4, Notch receptors and ligands, C-KIT, KI67, STRO-1, Tie2, and GD2 [21-24, 27, 28]. Two additional criteria proposed by the International Society for Cellular Therapy for defining human MSC are 1) adherent property to plastic in standard culture condition, and 2) ability to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro [29]. The latter of the two are especially critical in determining the pluripotency of the MSCs, and therefore important in identifying progenitor disc cells.
The Notch signaling pathway is essential for progenitor cell activity, proliferation, and fate determination in various tissues, and thus, its related molecules are utilized as stem/progenitor cell markers. In IVD, Notch1 expression pattern resembled that of BrdU positive areas [25], and also correlated with the disc progenitor cell niche [30]. Noteworthy, the Notch signaling pathway is induced by hypoxia in disc cells [31]. Hiyama et al. [31] confirmed expression of Notch receptors and ligands in rat NP and AF tissues, and demonstrated that hypoxia increased expression of Notch1, Notch4, and Jagged2 mRNA in both tissues, and Jagged1 expression in AF. Inhibition of Notch signaling significantly reduced NP cell proliferation independent of oxemic tension. Interestingly, Hes1, a Notch target and also an important regulator of stem cell maintenance, was also induced by hypoxia in AF cells. Although not yet evaluated in NP cells, an earlier study by Gustafsson et al. [32] shed a light on how Notch signaling may control progenitor cell differentiation in a hypoxia-dependent manner. In their study, the authors showed that hypoxia dependent inhibition of myogenic and neural precursor differentiation required activation of the Notch signaling pathway. Importantly, hypoxic activation of Notch signaling involved interaction between HIF-1α and notch intracellular domain (NICD), leading to subsequent inhibition of myogenic and neural differentiation.
Furthermore, Hiyama et al. [31] observed a trend of increased expression of Jagged1 and Jagged2, and possibly Notch4 and Hes1 in NP tissues from degenerative human IVDs, despite their probable low progenitor cell content. This observation can be explained by a recent study demonstrating that inflammatory cytokines, IL-1β and TNF-α, induced Notch signaling in NP cells by up-regulating Notch receptors and ligands by activating NF-κB and MAPK signaling [33]. Importantly, in degenerative human discs, Notch1, Notch2, and Hey2 were up-regulated, and the increase in Notch2 was highest in mid-grade degenerate discs corresponding to the highest level of inflammatory cytokines. Thus, Notch signaling may be induced in response to inflammatory cytokines in the degenerative disc and act as a compensatory mechanism to revive endogenous cell population through proliferation [31, 33].
These studies indicate that Notch signaling molecules can possibly be used as disc progenitor markers. Furthermore, Notch signaling may be essential in disc progenitor maintenance in hypoxic microenvironment, and targeting the involved molecules can be a therapeutic strategy for preventing IVD degeneration.
CELL-BASED THERAPY FOR DISC DEGENERATION
Cell-based therapy for disc degeneration involves promoting endogenous progenitor cells or transplanted MSCs to differentiate into mature disc cells for functional regeneration of the native tissue [16]. As described in studies demonstrating pluripotency of disc progenitor cells [23, 24, 34], disc progenitor cells can be a valuable source for tissue specific stem cell, or they can be stimulated endogenously to take the role of resident NP cells.
Transplantation of MSCs has been widely investigated and the results of several studies have shown benefits of this strategy [35-42]. Various studies have described induction of MSC differentiation into disc-like cells; growth factor treatment, specific culture conditions, co-culture with mature disc cells, and seeding onto a tissue scaffold have all been attempted [16]. Treatment of MSCs with TGFβ, IGF1, FGF2, or PDGF-BB can induce differentiation of these cells into NP-like cells [43, 44]. Co-culturing MSCs with mature NP cells for 7 days resulted in up-regulation of NP marker genes in MSCs [45]. Interestingly, this induction of MSC differentiation occurred only when there was a cell-cell contact between the two cell types. When NP cells from degenerative discs were co-cultured with MSCs, the MSCs differentiated into NP-like cells, while the degenerative NP cells increased their expression of matrix genes, TGFB1, and growth and differentiation factor 5 (GDF5) [46]. These studies suggest that introducing MSCs into the IVD will not only cause MSC differentiation into NP-like cells, but also will induce the endogenous degenerative cells to increase their own matrix production. Another method of inducing MSCs to NP-like cell differentiation by using notochordal cell conditioned medium (NCCM). Culturing human MSCs in porcine NCCM resulted in greater accumulation of GAGs, increased collagen III, and decreased collagen II and Sox9 gene expression [47]. However, NCCM derived from notochordal cells in native tissue (NCT) as opposed to in alginate beads (NCA) induced higher GAG production and increased Sox9 and collagen II gene expression [48]. Interestingly, NCA-derived medium had anti-fibrotic and minimally hypertrophic effect on MSCs evidenced by decreased collagen I and II and low collagen X gene expression [48]. On the other hand, when BMSCs or NP cells were co-cultured with notochordal cells in 3D hydrogel for 4 weeks, no increase in matrix production was observed, possibly due to loss of notochordal cell phenotype during long-term culture [49]. Another recent study showed co-culturing porcine notochordal cells encapsulated in alginate beads with NP cells without direct cell-cell contact in hypoxia resulted in higher aggrecan to collagen II gene expression ratio. The authors identified CCN2 in the medium of notochordal cell and NP cell co-culture as well as NCCM derived from 3D alginate bead-encapsulated notochordal cells [50]. While NCCM or notochordal cells can potentially direct MSCs differentiate into NP-like cells, further investigation is necessary to identify specific factors involved in this process.
One of the most distinct features of NP cells is their adaptation to their hypoxic microenvironment (discussed in detail below). It follows that hypoxia is essential in directing MSCs toward native disc cell phenotypes. Risbud et al. [51] encapsulated rat MSCs in 3-dimensional (3D) alginate hydrogels and cultured them in medium with TGFβ1 under hypoxia, finding that this culture condition induced MSC differentiation towards an NP-like cell phenotype; the differentiated MSCs formed large aggregates and up-regulated GLUT-3, SOX9, MMP-2, collagen II, XI, and ACAN expression. Hypoxia also up-regulated the expression of CD44, CD166 and CD105. Stoyanov et al. [52] also reported that hypoxia with concurrent treatment of GDF5 induced human BM-MSCs to increase their ACAN and collagen II expression and GAG production, indicating the importance of hypoxia in MSC differentiation to disc cells. In a study of rabbit MSCs, when seeded into 3D nanofibrous poly(l-lactide) scaffold and treated with TGFβ1 under hypoxia, cells differentiated toward NP-like cells [53]. Importantly, the cells continuously expressed the functional NP-specific marker, HIF-1α.
Another important feature of NP cells for consideration in differentiation schemes is their origin. Resident cells of the NP are derived from the embryonic notochord, which is formed by convergence and extension of the chordamesoderm [54, 55]. An intriguing recent study suggests how notochord may have emerged through evolution [55]. The authors identified a group of mesodermal cells in the midline of Platynereis dumerilii, an invertebrate marine annelid, during development that expressed notochord-specific combination of genes. Expression of transcription factors (foxA, foxD, twist, soxD, soxE), signaling molecules (noggin, hedgehog), and guidance factors (netrin, slit), in combination with expression of brachyury was unique to the mesodermal midline cells in annelid, which strongly supported the similarity between these cells to the vertebrate chordamesoderm. Interestingly, the annelid midline mesodermal cells differentiated into a longitudinal muscle, the axochord, which secreted ECM abundant of collagen [55]. In NP, the fate of the notochord-derived cells and whether they are replaced by another cell type in the adult NP has been a debated topic [15]. A recent report by Merceron et al., however, disproved the idea that NP cells trans-differentiate into chondrocyte-like cells. By lineage studies and TUNEL assay, the authors clearly showed that hypoxia inducible factor (HIF)-1α deletion in embryonic notochordal cells resulted in complete NP cell death before they were replaced by non-notochordal cells [56]. Some groups have shown the cell population of the NP as heterogeneous, composed of both notochordal and chondrocyte-like cells [57, 58]. Several studies have pointed to a switch in the ratio of these notochordal to chondrocytic cells with aging or degeneration of the disc [57, 59, 60]. As such, it may be a better strategy to recapitulate features specific to the young, healthy NP cell. Indeed, a recent study by Liu et al. [61] showed that human induced pluripotent stem cells (hiPSCs) could be differentiated into notochordal cell (NC)-like cells by culturing them with pulverized porcine NP matrix, either with or without direct contact between the cells and the matrix. The differentiated cells increased expression of notochordal marker genes including brachyury, CK-8 (K8), and CK-18 (K18), and could be further differentiated to express ACAN and collagen II.
One NP marker critical in formation of the NP is Sonic hedgehog (SHH) that is secreted from the notochord during development. SHH and its receptor, patched (PTC) are expressed in the developing mouse notochord [62]; a fate mapping study later showed that it was specifically SHH-expressing cells of the notochord that will give rise to NP in mice [63]. Further work from Dahia lab has demonstrated that SHH plays an important role in signaling in the postnatal NP [64]. Interestingly, SHH signaling in the NP responds to canonical Wnt signaling, however activity of both signaling pathways is down-regulated in IVDs with ageing [65]. Re-activation of the Wnt signaling pathway in older IVDs increased expression levels of COL1A1, SOX9, and, chondroitin sulfate (CHSO4), ACAN, and brachyury. Another notochordal marker, brachyury [54], is required for differentiation and survival of the notochord [66], and its expression persists in mature NP cells of many species including humans [67-69]. However, in postnatal NP, the function of brachyury remains unknown, as do many of its transcriptional targets. Therefore, further work is necessary to elucidate their role in the adult disc.
Many in vivo studies using different degenerative IVD models have shown that transplantation of BM-MSCs into the disc can decelerate the degenerative process and promote regeneration [35-40]. Transplanted MSCs survive, proliferate, and acquire morphologic and functional phenotypes of disc cells. Studies by Sakai and colleagues demonstrated that transplanted MSCs expressed HIF-1/2α, HIF-1β, MMP-2, GLUT-1 and GLUT-3, as well as chondroitin sulfate, keratan sulfate, and collagens I, II, IV, all of which are similarly expressed by native NP cells [35-37]. In studies where human MSCs were transplanted into rat or porcine IVDs, results confirmed survival and differentiation of transplanted cells into disc-like cells [41,42]. Importantly, Yoshikawa et al. [70] reported successful transplantation of autologous BM-MSCs into IVDs of two human patients who were experiencing vacuum phenomenon with instability and were undergoing decompression surgery for spinal stenosis. During the surgery, autologous BM-MSCs seeded in a collagen sponge were transplanted into the degenerative IVD. Radiograph and CT showed improvement in the vacuum phenomenon at two years post-surgery. Discs that had undergone transplantation showed signs of regeneration as evaluated by high T2-weighted MRI signal intensity, indicative of high water content. More importantly, the patients’ symptoms were alleviated, strongly supporting the favorable results of MSC transplantation studies. In another clinical study, 26 patients with moderate to severe discogenic pain were injected with autologous bone marrow concentrate (BMC), which contained average 2,713 MSCs/mL [71]. At 12 months post-injection, the average Oswestry Disability Index (ODI) was reduced to 25.0 from over 30.0, and Visual Analogue Scale (VAS) to 33.2 mm from over 40.0 mm. Importantly, although all patients experienced pain alleviation, those who received BMC with greater than 2,000 MSCs/mL had significantly faster and greater decrease in ODI and VAS, indicating a correlation between MSC concentration and discogenic pain alleviation [71]. Additionally, studies using MSCs derived from adipose [72-74] and synovium [75], or using MSC-seeded tissue constructs [76-80] have confirmed the validity of MSCs in disc regeneration therapy.
It should be mentioned, however, that there are still many issues to be addressed before this therapeutic strategy can be successfully implemented in clinical settings. For example, Vadalà et al. [81] showed that MSCs injected into degenerative rabbit discs leaked and migrated out of the NP, forming large anterolateral osteophytes. This indicates the needs to develop more precise cell delivery systems or to use annulus-sealing techniques. Another important issue to consider in MSC transplantation is availability of nutrients and the degenerative state of the IVD microenvironment, which will significantly influence survival and activity of transplanted MSCs [82]. Nutrient deprivation is important factor in disc degeneration, and as such nutrient supply and demand is tightly regulated in healthy IVD. Addition of growth factors to supplement transplanted MSCs can thus further exacerbate already low nutrient level in degenerate disc by stimulating cell metabolism [83]. Considering this, MSC transplantation may be better suited as an early interventional therapy for disc degeneration.
The overall results of the in vitro and in vivo studies from using MSC and progenitor cells in disc regeneration are promising. However, it is important to note that most of these studies investigating MSC differentiation toward an NP cell have evaluated the state of differentiation using markers that are either not NP-specific or using an incomplete list of NP markers. For example, expression levels of COL2A1, ACAN, and SOX9 are commonly used to determine MSC acquisition of NP phenotypes [42, 43, 45, 46, 51-53]. Despite the fact that these genes are known to be expressed by healthy adult human NP cells [84], they are also typically expressed by chondrocytes. Minogue et al. [67] reported results of a microarray study comparing bovine NP, AF, and articular cartilage (AC) cells. The authors identified 34 genes specific to NP and 49 specific to either type of IVD-derived cell. Out of those chosen for qRT-PCR validation in human NP cells, 11 genes (SNAP25, K8, K18, K19, CDH2, IBSP, VCAN, TNMD, BASP1, FOXF1, and FBLN1) were confirmed. In addition, the study identified several putative “negative NP markers” – genes with lower expression in NP than the other tissues studied. The same group went on to use microarray-identified markers to distinguish NP-like differentiation in BM-MSCs and AD-MSCs [85]. Here, the authors differentiated both types of MSCs in collagen I gels before measuring expression levels of marker genes. They identified and validated five NP marker genes: PAX1, FOXF1, HBB, CAXII, and OVOS2 to validate differentiation to an NP-like phenotype. Differentiated MSCs showed significant increase in expression levels of classical markers COL2A1 and ACAN as well as PAX1 and FOXF1. In addition, both types of MSCs lacked expression of at least one AC marker following differentiation. This study again demonstrated the utility of a negative NP marker: IBSP, which was differentially expressed in the human samples. Since in this study differentiated BM-MSCs expressed negative NP markers FBLN1 and IBSP as opposed to AD-MSCs, the authors suggest that AD-MSCs may represent a superior cell type for differentiation to an NP-like phenotype. Some studies investigating transplantation of AD-MSCs also suggest their advantage over using BM-MSCs due to convenience of isolation and ready availability of starting material [72-74].
Establishing a clear and complete definition of NP cell phenotype is therefore required in order to utilize the concept of cell “markers” for differentiation.
MARKERS SPECIFIC TO CELL FUNCTION IN THE NP MICROENVIRONMENT
Two characteristic features of the nucleus pulposus microenvironment are 1) lack of vascularization, which creates a physiologically hypoxic environment, and 2) high content of water-binding proteoglycans, which elevates osmotic pressure outside of cells. It is therefore critical that cells transplanted into the NP are able to perform under these conditions. The ability of resident NP cells to survive, proliferate, and function within either unique microenvironment has been largely attributed to expression of two transcription factors: HIF-1α and tonicity-responsive enhancer binding protein (TonEBP/NFAT5).
The healthy IVD is physiologically hypoxic [86, 87] and vascular invasion of the disc is, in fact, associated with nerve ingrowth and pain [88, 89]. Therefore, cells transplanted into the NP must have mechanisms to cope with a low oxygen environment. The HIF family of transcription factors are responsible for activating an adaptive cellular response in cells exposed to hypoxia [90]. Under normoxic conditions, the HIF-α subunit, necessary for transcriptional activity, is targeted for proteasome-mediated degradation by selective hydroxylation of conserved proline residues by members of the prolyl hydroxylase family (PHDs). In hypoxia, prolyl hydroxylation cannot proceed, leaving the α subunit to dimerize with the β subunit and bind DNA in the nucleus [91]. Careful work by Risbud and coworkers has shown that NP cells constitutively express HIF-1α and HIF-2α under both normoxia and hypoxia [92, 93]. Consistent with this observation, alternative oxygen-independent means of HIF-α degradation and activity have been shown to impact protein levels in NP cells [94-96]. Taken together, unique regulation of HIF protein turnover and activity in NP cells suggests its importance in NP physiology. Further evidence for this comes from a recent mouse model of selective HIF-1α loss in the NP, demonstrating the necessity of this protein for cell survival [56, 97]. In their study, Merceron et al. demonstrated that tissue-specific conditional knockout of HIF-1α in notochordal cells resulted in morphologic changes of NP cells at E15.5, and complete disappearance by 1-month of age [56]. This was attributed to necessity of HIF-1 in driving metabolic and synthetic activities of NP cells in vivo, as was shown by previous in vitro studies [92, 98]. In addition to these pro-glycolysis targets, HIF has been shown to regulate expression of key matrix related genes in the NP such as ACAN [98, 99]. β−1,3-glucuronyltransferase 1 (GlcAT-I) [100], galectin 3 [101]. CAXII has been proposed as a marker of healthy human NP tissue [102]. This is not unexpected, as carbonic anhydrases play an important role in controlling pH balance, critical in a hypoxic tissues such as the solid tumors or the NP [103]. CAXII is hypoxia inducible in tumor cells, although a HIF-responsive element (HRE) has not yet been found [104]. Therefore, it is possible that other members of the CA family can serve as functional NP markers. Noteworthy, expression of CAII, CAIII, and CAVI has been shown in embryonic mouse notochord and/or NP [105]. In addition, robust expression of CAIII transcripts in early notochordal and NP has been shown [106].
In the healthy disc, water content of the NP is approximately 77% [107]. It is this hydrostatic pressure, deriving from the high concentration of water-binding aggrecan and other proteoglycans, which gives the disc its ability to resist compressive forces. As a result of this architecture, normal daily movement causes frequent fluctuations in extracellular osmolarity of the disc [108, 109] with values ranging from 430 to 496 mOsm [110, 111]. Robust expression of the osmosensitive transcription factor TonEBP allows NP cells to sustain normal cellular activities under this daily hypertonic fluctuations [112, 113]. Normally, mammalian cells in a hypertonic environment activate membrane electrolyte transporters to balance inter- and extracellular solute concentrations. This response increases the osmotic pressure inside the cell, which can damage DNA and lead to autophagy, senescence or apoptosis if sustained [114]. To relieve intracellular osmotic pressure, TonEBP drives expression of several genes essential in the exchange of accumulated charged ions for small organic non-ionic osmolytes [115, 116]. Indeed, TonEBP expression is required for NP cell survival in hypertonic medium and occurs most likely via transcriptional activation of canonical target genes sodium myoinositol transporter (SMIT), betaine-γ-amino butyric acid transporter (BGT1), and taurine transporter (TauT) [113]. In addition, TonEBP in NP cells drives expression of ACAN [113, 117], GlcAT-I, needed for chondroitin sulfate chain synthesis [118], and the water channel protein aquaporin 2 (AQP2) [119]. Although not a classic “cell marker,” the robust expression and responsiveness of TonEBP in the NP is certainly required for survival under physiological conditions of the disc. In addition, TonEBP allows NP cells to uniquely modify their extracellular matrix in response to osmotic stimuli [112]. As such, any cell destined for the stressful NP microenvironment should exhibit similar expression of these transcription factors and activation of their targets.
MARKERS OF EXTRACELLULAR MATRIX CONTENT
As described above, the matrix produced by cells of the NP and resulting hydrostatic pressure is of utmost functional importance for the disc. It is therefore prudent to consider the major matrix molecules produced by resident cells of the NP. The most abundant proteoglycan in the NP is ACAN, with lesser amounts of versican (VCAN), biglycan (BGN), decorin (DCN), and fibromodulin (FMOD) present in the tissue [9]. In addition to proteoglycans, collagens make up a substantial component of the NP matrix, mainly collagen II [10]. It is important to note that, despite expression of ACAN and collagen II in both chondrocytes and NP cells, the ratios of these molecules can be used to differentiate between matrices produced by the two cell types. This ratio has been measured as the GAG to hydroxyproline ratio, which Mwale et al. showed to be about 27:1 in the young adult NP and about 2:1 in hyaline cartilage [120]. Given that the GAG to hydroxyproline ratio decreased with increased histological grade of disc degeneration in this study, maintenance of a proteoglycan-rich matrix may signify ideal NP cell function. With the importance of cell-matrix interaction in mind, Chen et al [121] investigated expression levels of lamin chains, integrins, and other matrix binding proteins in NP tissues, finding that compared to surrounding AF, NP tissue had elevated expression of laminin α5 chain, integrin subunits α3, α6, and β4, CD239, and CD151. In a follow-up study, the same group showed integrins α3, α5, and β1 were important for NP attachment to laminin isoforms LM-111 and LM-511 [122].
CONCLUSIONS
Degeneration of the intervertebral discs is a ubiquitous and chronic condition. While there have been strides in the field to better understand cell function and pathophysiology, complete recovery of disc function and alleviation of pain still remains a major problem. Regenerative therapies, both those that harness the intrinsic capacity of native progenitor cells in the NP and those that rely on transplanted cells, may lead the way in disease treatment. However, the success of these therapies is dependent upon recapitulation of a healthy NP cell and tissue phenotype. From a standpoint of cell introduction to the NP, it is crucial that the cells express factors allowing for their survival, such as HIF-1α and TonEBP. Therefore, consideration of both functional markers and cell surface markers, such as Tie2, GD2, or CD24 and others for cell sorting, will be useful in future investigations on cell-based therapy for disc degeneration.
Figure 2.
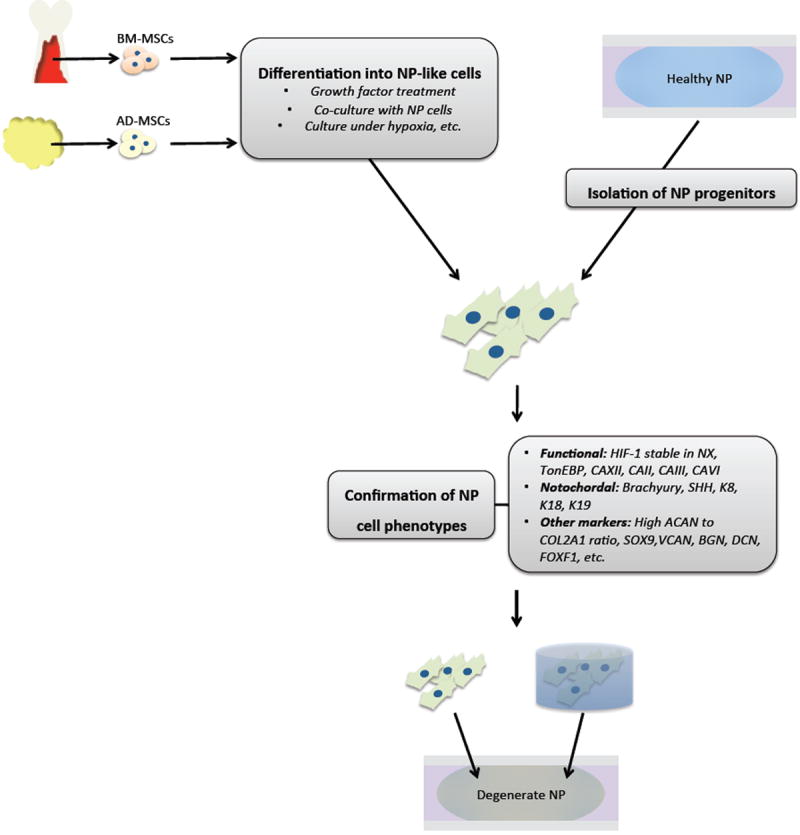
Schematic diagram showing cell based therapy strategy for disc regeneration. Mesenchymal stem cells (MSCs) can be derived from either bone marrow (BM-MSCs) or adipose (AD-MSCs), before undergoing differentiation into NP-like cells through various treatments. Alternately, NP progenitor cells may be identified with markers and isolated directly from healthy disc tissue. Confirmation of NP cell phenotype as defined by functional, tissue-of-origin, and other markers unique to NP cells before transplantation is necessary for successful disc regeneration.
Acknowledgments
Studies are supported by grants from National Institutes of Arthritis and Musculoskeletal and Skin Diseases AR055655, AR064733 and AR050087. ZIJ is supported by T32 AR052273. The authors wish to thank Zachary Schoepflin for help with literature search.
References
- 1.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363–70. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 2.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48(1):5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 3.The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656–64. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 5.Phillips FM, Reuben J, Wetzel FT. Intervertebral disc degeneration adjacent to a lumbar fusion. An experimental rabbit model. J Bone Joint Surg Br. 2002;84(2):289–94. doi: 10.1302/0301-620x.84b2.11937. [DOI] [PubMed] [Google Scholar]
- 6.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98(4):996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20(11):1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Kraemer J, Kolditz D, Gowin R. Water and electrolyte content of human intervertebral discs under variable load. Spine (Phila Pa 1976) 1985;10(1):69–71. doi: 10.1097/00007632-198501000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Cs-Szabo G, Ragasa-San Juan D, Turumella V, et al. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine (Phila Pa 1976) 2002;27(20):2212–9. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 10.Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35(Pt 4):652–5. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura K, Mochida J. Percutaneous reinsertion of the nucleus pulposus. An experimental study Spine (Phila Pa 1976) 1998;23(14):1531–8. doi: 10.1097/00007632-199807150-00006. discussion 1539. [DOI] [PubMed] [Google Scholar]
- 12.Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res. 2001;(389):94–101. doi: 10.1097/00003086-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Gorensek M, Jaksimović C, Kregar-Velikonja N, et al. Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol Biol Lett. 2004;9(2):363–73. [PubMed] [Google Scholar]
- 14.Acosta FL, Metz L, Adkisson HD, et al. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A. 2011;17(23-24):3045–55. doi: 10.1089/ten.tea.2011.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21(1):29–41. doi: 10.1615/critreveukargeneexpr.v21.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai D, Mochida J. Use of Stem Cells for Regeneration of the Intervertebral Disc. In: Shapiro IM, Risbud MV, editors. The Intervertebral Disc: Molecular and Structural Studies of the Disc in Health and Disease. Vienna: Springer; 2014. pp. 373–83. [Google Scholar]
- 17.Risbud MV, Schoepflin ZR, Mwale F, et al. Defining the Phenotype of Young Healthy Nucleus Pulposus Cells. J Orthop Res; Recommendations of the Spine Research Interest Group at the 2014 Annual ORS Meeting; 2014. pp. 283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melrose J, Smith SM, Fuller ES, et al. Biglycan and fibromodulin fragmentation correlates with temporal and spatial annular remodelling in experimentally injured ovine intervertebral discs. Eur Spine J. 2007;16(12):2193–205. doi: 10.1007/s00586-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JW, Walmsley R. Experimental incision of the intervertebral disc. J Bone Joint Surg Br. 1951;33-B(4):612–25. doi: 10.1302/0301-620X.33B4.612. [DOI] [PubMed] [Google Scholar]
- 20.Korecki CL, Costi JJ, Iatridis JC. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine (Phila Pa 1976) 2008;33(3):235–41. doi: 10.1097/BRS.0b013e3181624504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risbud MV, Guttapalli A, Tsai T-T, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 2007;32(23):2537–44. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 22.Brisby H, Papadimitriou N, Brantsing C, et al. The presence of local mesenchymal progenitor cells in human degenerated intervertebral discs and possibilities to influence these in vitro: a descriptive study in humans. Stem Cells Dev. 2013;22(5):804–14. doi: 10.1089/scd.2012.0179. [DOI] [PubMed] [Google Scholar]
- 23.Blanco JF, Graciani IF, Sanchez-Guijo FM, et al. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila Pa 1976) 2010;35(26):2259–65. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- 24.Feng G, Yang X, Shang H, et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am. 2010;92(3):675–85. doi: 10.2106/JBJS.H.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976) 2009;34(21):2278–87. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- 26.Henriksson HB, Svala E, Skioldebrand E, Lindahl A, Brisby H. Support of concept that migrating progenitor cells from stem cell niches contribute to normal regeneration of the adult mammal intervertebral disc: a descriptive study in the New Zealand white rabbit. Spine (Phila Pa 1976) 2012;37(9):722–32. doi: 10.1097/BRS.0b013e318231c2f7. [DOI] [PubMed] [Google Scholar]
- 27.Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasen M, Fei Q, Hutton WC, et al. Changes of number of cells expressing proliferation and progenitor cell markers with age in rabbit intervertebral discs. Acta Biochim Biophys Sin (Shanghai) 2013;45(5):368–76. doi: 10.1093/abbs/gmt019. [DOI] [PubMed] [Google Scholar]
- 29.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 30.Shu C, Hughes C, Smith SM, et al. The ovine newborn and human foetal intervertebral disc contain perlecan and aggrecan variably substituted with native 7D4 CS sulphation motif: spatiotemporal immunolocalisation and co-distribution with Notch-1 in the human foetal disc. Glycoconj J. 2013;30(7):717–25. doi: 10.1007/s10719-013-9475-9. [DOI] [PubMed] [Google Scholar]
- 31.Hiyama A, Skubutyte R, Markova D, et al. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: implications in degenerative disc disease. Arthritis Rheum. 2011;63(5):1355–64. doi: 10.1002/art.30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustafsson MV, Zheng X, Pereira T, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9(5):617–28. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Tian Y, Wang J, et al. Inflammatory cytokines induce NOTCH signaling in nucleus pulposus cells: implications in intervertebral disc degeneration. J Biol Chem. 2013;288(23):16761–74. doi: 10.1074/jbc.M112.446633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erwin WM, Islam D, Inman RD, Fehlings MG, Tsui FWL. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011;13(6):R215. doi: 10.1186/ar3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakai D, Mochida J, Yamamoto Y, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen® gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24(20):3531–41. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 36.Sakai D, Mochida J, Iwashina T, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27(3):335–45. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 37.Sakai D, Mochida J, Iwashina T, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 2005;30(21):2379–87. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 38.Crevensten G, Walsh AJL, Ananthakrishnan D, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004;32(3):430–4. doi: 10.1023/b:abme.0000017545.84833.7c. [DOI] [PubMed] [Google Scholar]
- 39.Hiyama A, Mochida J, Iwashina T, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26(5):589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Drapeau S, Howard SA, Thonar EJMA, Anderson DG. Transplantation of goat bone marrow stromal cells to the degenerating intervertebral disc in a goat disc injury model. Spine (Phila Pa 1976) 2011;36(5):372–7. doi: 10.1097/BRS.0b013e3181d10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei A, Tao H, Chung SA, et al. The fate of transplanted xenogeneic bone marrow-derived stem cells in rat intervertebral discs. J Orthop Res. 2009;27(3):374–9. doi: 10.1002/jor.20567. [DOI] [PubMed] [Google Scholar]
- 42.Henriksson HB, Svanvik T, Jonsson M, et al. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976) 2009;34(2):141–8. doi: 10.1097/BRS.0b013e31818f8c20. [DOI] [PubMed] [Google Scholar]
- 43.Steck E, Bertram H, Abel R, et al. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23(3):403–11. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 44.Ehlicke F, Freimark D, Heil B, Dorresteijn A, Czermak P. Intervertebral disc regeneration: influence of growth factors on differentiation of human mesenchymal stem cells (hMSC) Int J Artif Organs. 2010;33(4):244–52. [PubMed] [Google Scholar]
- 45.Richardson SM, Walker RV, Parker S, et al. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24(3):707–16. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 46.Strassburg S, Richardson SM, Freemont AJ, Hoyland JA. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med. 2010;5(5):701–11. doi: 10.2217/rme.10.59. [DOI] [PubMed] [Google Scholar]
- 47.Korecki CL, Taboas JM, Tuan RS, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1(2):18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Purmessur D, Schek RM, Abbott RD, et al. Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthritis Res Ther. 2011;13(3):R81. doi: 10.1186/ar3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potier E, Ito K. Using notochordal cells of developmental origin to stimulate nucleus pulposus cells and bone marrow stromal cells for intervertebral disc regeneration. Eur Spine J. 2014;23(3):679–88. doi: 10.1007/s00586-013-3107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gantenbein B, Calandriello E, Wuertz-Kozak K, et al. Activation of intervertebral disc cells by co-culture with notochordal cells, conditioned medium and hypoxia. BMC Musculoskelet Disord. 2014;15(1):422. doi: 10.1186/1471-2474-15-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Risbud MV, Albert TJ, Guttapalli A, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine (Phila Pa 1976) 2004;29(23):2627–32. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 52.Stoyanov JV, Gantenbein-Ritter B, Bertolo A, et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater. 2011;21:533–47. doi: 10.22203/ecm.v021a40. [DOI] [PubMed] [Google Scholar]
- 53.Feng G, Jin X, Hu J, et al. Effects of hypoxias and scaffold architecture on rabbit mesenchymal stem cell differentiation towards a nucleus pulposus-like phenotype. Biomaterials. 2011;32(32):8182–9. doi: 10.1016/j.biomaterials.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shapiro IM, Risbud MV. Transcriptional profiling of the nucleus pulposus: say yes to notochord. Arthritis Res Ther. 2010;12(3):117. doi: 10.1186/ar3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lauri a, Brunet T, Handberg-Thorsager M, et al. Development of the annelid axochord: Insights into notochord evolution. Science. 2014;345(6202):1365–8. doi: 10.1126/science.1253396. [DOI] [PubMed] [Google Scholar]
- 56.Merceron C, Mangiavini L, Robling A, et al. Loss of HIF-1a in the Notochord Results in Cell Death and Complete Disappearance of the Nucleus Pulposus. PLoS One. 2014;9(10):e110768. doi: 10.1371/journal.pone.0110768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec. 1982;204(4):307–14. doi: 10.1002/ar.1092040403. [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15(Suppl 3):S303–11. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205(5):357–62. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pazzaglia UE, Salisbury JR, Byers PD. Development and involution of the notochord in the human spine. J R Soc Med. 1989;82(7):413–5. doi: 10.1177/014107688908200714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Rahaman MN, Bal BS. Modulating notochordal differentiation of human induced pluripotent stem cells using natural nucleus pulposus tissue matrix. PLoS One. 2014;9(7):e100885. doi: 10.1371/journal.pone.0100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DiPaola CP, Farmer JC, Manova K, Niswander LA. Molecular signaling in intervertebral disk development. J Orthop Res. 2005;23(5):1112–9. doi: 10.1016/j.orthres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Choi K-S, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237(12):3953–8. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dahia CL, Mahoney E, Wylie C. Shh signaling from the nucleus pulposus is required for the postnatal growth and differentiation of the mouse intervertebral disc. PLoS One. 2012;7(4):e35944. doi: 10.1371/journal.pone.0035944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winkler T, Mahoney EJ, Sinner D, Wylie CC, Dahia CL. Wnt signaling activates shh signaling in early postnatal intervertebral discs, and re-activates shh signaling in old discs in the mouse. PLoS One. 2014;9(6):e98444. doi: 10.1371/journal.pone.0098444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herrmann BG, Kispert A. The T genes in embryogenesis. Trends Genet. 1994;10(8):280–6. doi: 10.1016/0168-9525(90)90011-t. [DOI] [PubMed] [Google Scholar]
- 67.Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12(1):R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang X, Jing L, Chen J. Changes in the molecular phenotype of nucleus pulposus cells with intervertebral disc aging. PLoS One. 2012;7(12):e52020. doi: 10.1371/journal.pone.0052020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dahia CL, Mahoney EJ, Durrani AA, Wylie C. Postnatal growth, differentiation, and aging of the mouse intervertebral disc. Spine (Phila Pa 1976) 2009;34(5):447–55. doi: 10.1097/BRS.0b013e3181990c64. [DOI] [PubMed] [Google Scholar]
- 70.Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine (Phila Pa 1976) 2010;35(11):E475–80. doi: 10.1097/BRS.0b013e3181cd2cf4. [DOI] [PubMed] [Google Scholar]
- 71.Pettine KA, Murphy MB, Suzuki RK, Sand TT. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015;33(1):146–56. doi: 10.1002/stem.1845. [DOI] [PubMed] [Google Scholar]
- 72.Hoogendoorn RJW, Lu ZF, Kroeze RJ, et al. Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future. J Cell Mol Med. 2008;12(6A):2205–16. doi: 10.1111/j.1582-4934.2008.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ganey T, Hutton WC, Moseley T, Hedrick M, Meisel H-J. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: experiments in a canine model. Spine (Phila Pa 1976) 2009;34(21):2297–304. doi: 10.1097/BRS.0b013e3181a54157. [DOI] [PubMed] [Google Scholar]
- 74.Chun H-J, Kim YS, Kim BK, et al. Transplantation of human adipose-derived stem cells in a rabbit model of traumatic degeneration of lumbar discs. World Neurosurg. 2012;78(3-4):364–71. doi: 10.1016/j.wneu.2011.12.084. [DOI] [PubMed] [Google Scholar]
- 75.Chen W-H, Liu H-Y, Lo W-C, et al. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials. 2009;30(29):5523–33. doi: 10.1016/j.biomaterials.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 76.Miyamoto T, Muneta T, Tabuchi T, et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12(6):R206. doi: 10.1186/ar3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaetani P, Torre ML, Klinger M, et al. Adipose-derived stem cell therapy for intervertebral disc regeneration: an in vitro reconstructed tissue in alginate capsules. Tissue Eng Part A. 2008;14(8):1415–23. doi: 10.1089/ten.tea.2007.0330. [DOI] [PubMed] [Google Scholar]
- 78.Nesti LJ, Li W-J, Shanti RM, et al. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng Part A. 2008;14(9):1527–37. doi: 10.1089/ten.tea.2008.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Driscoll TP, Nerurkar NL, Jacobs NT, Elliott DM, Mauck RL. Fiber angle and aspect ratio influence the shear mechanics of oriented electrospun nanofibrous scaffolds. J Mech Behav Biomed Mater. 2011;4(8):1627–36. doi: 10.1016/j.jmbbm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.See EY-S, Toh SL, Goh JCH. Simulated intervertebral disc-like assembly using bone marrow-derived mesenchymal stem cell sheets and silk scaffolds for annulus fibrosus regeneration. J Tissue Eng Regen Med. 2012;6(7):528–35. doi: 10.1002/term.457. [DOI] [PubMed] [Google Scholar]
- 81.Vadalà G, Sowa G, Hubert M, et al. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6(5):348–55. doi: 10.1002/term.433. [DOI] [PubMed] [Google Scholar]
- 82.Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24(2):416–25. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 83.Urban JPG, Smith S, Fairbank JCT. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29(23):2700–9. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 84.Sive JI, Baird P, Jeziorsk M, et al. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55(2):91–7. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Minogue BM, Richardson SM, Zeef LAH, Freemont AJ, Hoyland JA. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum. 2010;62(12):3695–705. doi: 10.1002/art.27710. [DOI] [PubMed] [Google Scholar]
- 86.Bartels EM, Fairbank JC, Winlove CP, Urban JP. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine (Phila Pa 1976) 1998;23(1):1–7. doi: 10.1097/00007632-199801010-00001. discussion 8. [DOI] [PubMed] [Google Scholar]
- 87.Rudert M, Tillmann B. Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop Scand. 1993;64(1):37–40. doi: 10.3109/17453679308994524. [DOI] [PubMed] [Google Scholar]
- 88.Repanti M, Korovessis PG, Stamatakis MV, Spastris P, Kosti P. Evolution of disc degeneration in lumbar spine: a comparative histological study between herniated and postmortem retrieved disc specimens. J Spinal Disord. 1998;11(1):41–5. [PubMed] [Google Scholar]
- 89.Sun Z, Wan Z-Y, Guo Y-S, Wang H-Q, Luo Z-J. FasL on human nucleus pulposus cells prevents angiogenesis in the disc by inducing Fas-mediated apoptosis of vascular endothelial cells. Int J Clin Exp Pathol. 2013;6(11):2376–85. [PMC free article] [PubMed] [Google Scholar]
- 90.Palmer BF, Clegg DJ. Oxygen sensing and metabolic homeostasis. Mol Cell Endocrinol. 2014;397(1-2):51–8. doi: 10.1016/j.mce.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 91.Webb JD, Coleman ML, Pugh CW. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell Mol Life Sci. 2009;66(22):3539–54. doi: 10.1007/s00018-009-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agrawal A, Gajghate S, Smith H, et al. Cited2 modulates hypoxia-inducible factor-dependent expression of vascular endothelial growth factor in nucleus pulposus cells of the rat intervertebral disc. Arthritis Rheum. 2008;58(12):3798–808. doi: 10.1002/art.24073. [DOI] [PubMed] [Google Scholar]
- 93.Risbud MV, Guttapalli A, Stokes DG, et al. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98(1):152–9. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 94.Fujita N, Chiba K, Shapiro IM, Risbud MV. HIF-1α and HIF-2α degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc. J Bone Miner Res. 2012;27(2):401–12. doi: 10.1002/jbmr.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fujita N, Markova D, Anderson DG, et al. Expression of prolyl hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: distinct roles of PHD2 and PHD3 proteins in controlling HIF-1α activity in hypoxia. J Biol Chem. 2012;287(20):16975–86. doi: 10.1074/jbc.M111.334466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gogate SS, Fujita N, Skubutyte R, Shapiro IM, Risbud MV. Tonicity enhancer binding protein (TonEBP) and hypoxia-inducible factor (HIF) coordinate heat shock protein 70 (Hsp70) expression in hypoxic nucleus pulposus cells: role of Hsp70 in HIF-1α degradation. J Bone Miner Res. 2012;27(5):1106–17. doi: 10.1002/jbmr.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mangiavini L, Wilson TL, Robling A, et al. HIF-1α is Essential for the Development of the Nucleus Pulposus. J Bone Miner Res. 27(Suppl) [Google Scholar]
- 98.Agrawal A, Guttapalli A, Narayan S, et al. Normoxic stabilization of HIF-1α drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293(2):C621–31. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- 99.Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308(3):401–7. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- 100.Gogate SS, Nasser R, Shapiro IM, Risbud MV. Hypoxic regulation of β-1,3-glucuronyltransferase 1 expression in nucleus pulposus cells of the rat intervertebral disc: role of hypoxia-inducible factor proteins. Arthritis Rheum. 2011;63(7):1950–60. doi: 10.1002/art.30342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeng Y, Danielson KG, Albert TJ, Shapiro IM, Risbud MV. HIF-1 alpha is a regulator of galectin-3 expression in the intervertebral disc. J Bone Miner Res. 2007;22(12):1851–61. doi: 10.1359/jbmr.070620. [DOI] [PubMed] [Google Scholar]
- 102.Power KA, Grad S, Rutges JPHJ, et al. Identification of cell surface-specific markers to target human nucleus pulposus cells: expression of carbonic anhydrase XII varies with age and degeneration. Arthritis Rheum. 2011;63(12):3876–86. doi: 10.1002/art.30607. [DOI] [PubMed] [Google Scholar]
- 103.Parks SK, Chiche J, Pouyssegur J. pH control mechanisms of tumor survival and growth. J Cell Physiol. 2011;226(2):299–308. doi: 10.1002/jcp.22400. [DOI] [PubMed] [Google Scholar]
- 104.Chiche J, Ilc K, Laferrière J, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69(1):358–68. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 105.Reibring C-G, El Shahawy M, Hallberg K, et al. Expression patterns and subcellular localization of carbonic anhydrases are developmentally regulated during tooth formation. PLoS One. 2014;9(5):e96007. doi: 10.1371/journal.pone.0096007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lyons GE, Buckingham ME, Tweedie S, Edwards YH. Carbonic anhydrase III, an early mesodermal marker, is expressed in embryonic mouse skeletal muscle and notochord. Development. 1991;111(1):233–44. doi: 10.1242/dev.111.1.233. [DOI] [PubMed] [Google Scholar]
- 107.Roberts S, Urban JP. Intervertebral Discs. In: Riihimäki H, Viikari-Juntura E, editors. Musculoskeletal System. Encyclopedia of Occupational Health and Safety. 6. Geneva: International Labor Organization; 2011. [Google Scholar]
- 108.Roberts N, Hogg D, Whitehouse GH, Dangerfield P. Quantitative analysis of diurnal variation in volume and water content of lumbar intervertebral discs. Clin Anat. 1998;11(1):1–8. doi: 10.1002/(SICI)1098-2353(1998)11:1<1::AID-CA1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 109.Urban JP. The chondrocyte: a cell under pressure. Br J Rheumatol. 1994;33(10):901–8. doi: 10.1093/rheumatology/33.10.901. [DOI] [PubMed] [Google Scholar]
- 110.Ishihara H, Warensjo K, Roberts S, Urban JP. Proteoglycan synthesis in the intervertebral disk nucleus: the role of extracellular osmolality. Am J Physiol. 1997;272(5 Pt 1):C1499–506. doi: 10.1152/ajpcell.1997.272.5.C1499. [DOI] [PubMed] [Google Scholar]
- 111.Van Dijk B, Potier E, Ito K. Culturing bovine nucleus pulposus explants by balancing medium osmolarity. Tissue Eng Part C Methods. 2011;17(11):1089–96. doi: 10.1089/ten.TEC.2011.0215. [DOI] [PubMed] [Google Scholar]
- 112.Johnson ZI, Shapiro IM, Risbud MV. Extracellular Osmolarity Regulates Matrix Homeostasis in the Intervertebral Disc and Articular Cartilage: Evolving role of TonEBP. Matrix Biol. 2014;40:10–6. doi: 10.1016/j.matbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsai T-T, Danielson KG, Guttapalli A, et al. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem. 2006;281(35):25416–24. doi: 10.1074/jbc.M601969200. [DOI] [PubMed] [Google Scholar]
- 114.Cheung CY, Ko BC. NFAT5 in cellular adaptation to hypertonic stress - regulations and functional significance. J Mol Signal. 2013;8(1):5. doi: 10.1186/1750-2187-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garcia-Perez A, Burg MB. Renal medullary organic osmolytes. Physiol Rev. 1991;71(4):1081–115. doi: 10.1152/physrev.1991.71.4.1081. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1924548. [DOI] [PubMed] [Google Scholar]
- 116.Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217(4566):1214–22. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 117.Tsai T-T, Guttapalli A, Agrawal A, et al. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res. 2007;22(7):965–74. doi: 10.1359/jbmr.070322. [DOI] [PubMed] [Google Scholar]
- 118.Hiyama A, Gajghate S, Sakai D, et al. Activation of TonEBP by calcium controls {beta}1,3-glucuronosyltransferase-I expression, a key regulator of glycosaminoglycan synthesis in cells of the intervertebral disc. J Biol Chem. 2009;284(15):9824–34. doi: 10.1074/jbc.M807081200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gajghate S, Hiyama A, Shah M, et al. Osmolarity and intracellular calcium regulate aquaporin2 expression through TonEBP in nucleus pulposus cells of the intervertebral disc. J Bone Miner Res. 2009;24(6):992–1001. doi: 10.1359/JBMR.090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–63. doi: 10.22203/ecm.v008a06. discussion 63–4. [DOI] [PubMed] [Google Scholar]
- 121.Chen J, Jing L, Gilchrist CL, et al. Expression of laminin isoforms, receptors, and binding proteins unique to nucleus pulposus cells of immature intervertebral disc. Connect Tissue Res. 2009;50(5):294–306. [PMC free article] [PubMed] [Google Scholar]
- 122.Bridgen DT, Gilchrist CL, Richardson WJ, et al. Integrin-mediated interactions with extracellular matrix proteins for nucleus pulposus cells of the human intervertebral disc. J Orthop Res. 2013;31(10):1661–7. doi: 10.1002/jor.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]


