Abstract
In recent decades the application of bioreactors has revolutionized the concept of culturing tissues and organs that require mechanical loading. In intervertebral disc (IVD) research, collaborative efforts of biomedical engineering, biology and mechatronics have led to the innovation of new loading devices that can maintain viable IVD organ explants from large animals and human cadavers in precisely defined nutritional and mechanical environments over extended culture periods. Particularly in spine and IVD research, these organ culture models offer appealing alternatives, as large bipedal animal models with naturally occurring IVD degeneration and a genetic background similar to the human condition do not exist. Latest research has demonstrated important concepts including the potential of homing of mesenchymal stem cells to nutritionally or mechanically stressed IVDs, and the regenerative potential of “smart” biomaterials for nucleus pulposus or annulus fibrosus repair. In this review, we summarize the current knowledge about cell therapy, injection of cytokines and short peptides to rescue the degenerating IVD. We further stress that most bioreactor systems simplify the real in vivo conditions providing a useful proof of concept. Limitations are that certain aspects of the immune host response and pain assessments cannot be addressed with ex vivo systems. Coccygeal animal disc models are commonly used because of their availability and similarity to human IVDs. Although in vitro loading environments are not identical to the human in vivo situation, 3D ex vivo organ culture models of large animal coccygeal and human lumbar IVDs should be seen as valid alternatives for screening and feasibility testing to augment existing small animal, large animal, and human clinical trial experiments.
Keywords: Bioreactor, cell viability, mechanical loading, intervertebral disc, mesenchymal stem cell, torsional loading, engineering, nutrition
1. INTRODUCTION
Low back pain is often related to intervertebral disc (IVD) degeneration. While painful IVD degeneration is not a lethal disease, it has been recognized as a major social burden with a high socioeconomic impact with many people often not being able to return to work for several weeks [1,2].
Two main hypotheses for the onset of painful IVD degeneration have been raised: The first hypothesis comes from Michael Adams and his colleagues, who investigated if IVDs would fail and degenerate based on pure mechanical overloading [3,4]. This hypothesis implies that the mechanical damage, i.e. micro-fissures in the tissue, occurs first and is followed by cells failing to maintain the homeostasis, and matrix breakdown. On the other hand, there is the limited nutrition hypothesis raised by Jill Urban and others [5,6], who state that at first there is a shortage of nutrition induced for the disc cells by blockage of diffusion through the cartilaginous endplate (CEP) route, which then leads to cell loss over the years and subsequently, the tissue homeostasis cannot be maintained, which then leads to mechanical failure of the IVD. The initiation and progression of IVD degeneration is a complex interaction of mechanical, biological, and physiological factors that are modulated by inflammatory responses, the genetic background and general health of the individual, which seems to be the most appropriate explanation for the development of IVD degeneration [7].
In order to test such hypotheses in IVD research many studies have been undertaken, including in vitro diffusion studies [8,9] and in vivo studies [10,11]. These studies demonstrated the importance of solute transport distances in defining cell density and metabolic rates. As a result, studies addressing nutritional compromise in the IVD require the use of IVDs from large animal models. Many issues were raised concerning in vivo animal studies regarding their limited application for the human situation [12]. For example, several biological differences exist among animal models, and quadrupeds may have distinct biomechanical properties from the upright human spine [12], however the relative contributions of gravitational forces and muscular contraction to spinal loading has not been thoroughly quantified in quadrupeds. Also in terms of the anatomy there are important differences, which make a direct comparison questionable. For instance, the IVDs of the porcine spine are thickest in the celebral region [13]. For the porcine model there seems to be biomechanical similarity of thoracic and human lumbar IVDs [13,14]. Recent studies also proposed the minipig as an animal model for studying IVD regeneration approaches such as injection of mesenchymal stem cells (MSCs) [15]. There are a number of mechanical and biological parameters to be considered, including cell density, which is considerably higher in many animal models than in human IVDs [16,17]. From a biomechanical perspective, there is some evidence that rodent and lagomorph (i.e. rabbit) IVDs have mechanical parameters that scale similarly to human IVDs although some important differences exist [17-19]. Some animals keep the notochordal cells throughout their life-time and other animals such as the human lose these cells when they are mature. In this respect, the presence or absence of notochordal cells has been discussed and their presence might produce outcomes that are more favourable in promoting regeneration and repair [20]. Their function has not yet been fully clarified but their regenerative potential has been described based on co-culture studies where these cells up-regulate glycosaminoglycan production [21-24] and even seem to release protective proteins that down-regulate apoptotic pathways [25]. In terms of range of motion (ROM) and creep, the coccygeal IVDs are very similar to lumbar discs [19,26-28].
3D organ culture models for the IVD are very appealing [12], as the effect of specific treatment strategies can be investigated in a controlled environment while in vivo animal studies add complexity and thus, might result in higher variance. Further, due to the absence of true upright spine animal models, ex vivo culture of human cadaveric IVDs in bioreactors represents a valid alternative to animal models. Monkeys, kangaroos and giraffes are not true upright walking animals, and there are also important functional and anatomical differences to the human. Thus, besides the ethical concerns one has to conclude that there is no good animal model for the human spine. In the following sections we will briefly discuss the basic concepts, which are needed to culture IVDs successfully.
2. OVERVIEW OF BIOREACTOR SYSTEMS
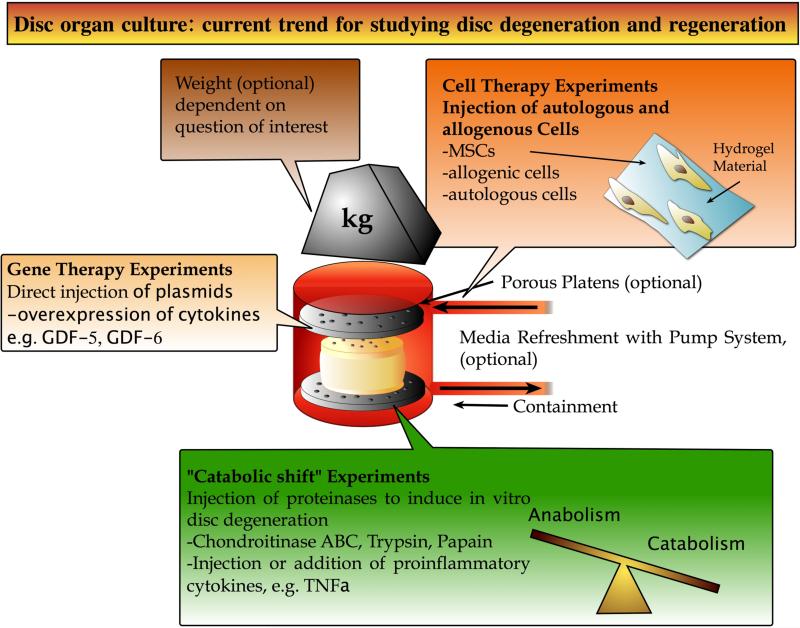
In recent years, several research groups have realized a variety of organ culture systems, which have been optimized over the years based on user experience. Here, we provide an almost complete list of these systems and summarize in short the major findings and limitations in terms of cell survival and function (Table 1). A number of bioreactor systems exist [29-35], all devoted to maintain the IVD explant, and designed specifically to fulfil the need of the different IVD sizes. Especially, the distance in the sagittal orientation from cartilaginous endplate to endplate determines the number of cells to be maintained and also the nutritional limits due to passive diffusion. In the last decade we have seen the emerging of several actively diffused bioreactors with tubing systems [7,31,36-39] as well as the development of static culture systems which do not require additional media tubing if the nutritional pathways can be maintained and the media to tissue volume ratio is high enough [35,36,40] (Table 1). Considering uni-axial compression, it becomes obvious that disc surface area is relevant and thus determines the force range of the bioreactor force cell and the dimensions of the culture chambers. Disc height and creep determines the required range of displacement [33,41]. Figure 1 depicts a general design of a bioreactor for IVD culture and summarizes current research foci for the establishment of in vitro degeneration and regeneration models. It has also been shown repeatedly that some sort of “physiological” loading of the IVD is essential to maintain the disc matrix content and the continuous solute exchange. Free-swelling culture, on the other hand, mimics the condition of spines and IVDs under low or microgravity [42,43] and could possibly affect cell viability by changes in osmotic pressure [44]. Recently, a wedge-loaded biomechanical system has been tested using bovine IVDs [45]. With this system it was found that asymmetric loading involving excessive bending can induce delamination of the annulus fibrosus, up-regulate metalloproteinases (MMPs) and pro-inflammatory cytokines and this lead to increased cell death. The simulated hyperflexion in this system is known to cause IVD injury [46]. Jim et al. [33] and Haglund et al. (2011) [34] have well demonstrated that the load distribution across the IVD is not homogenous in the bovine coccygeal discs as compared to a human lumbar disc. This discrepancy likely arises from the anatomy of the cartilaginous endplate (CEP) of the bovine tail, which is concave in structure whereas the human lumbar disc has a flat CEP [34]. Considering the anatomy of the bovine disc, the team of Haglund et al. (2011) developed a novel bioreactor with exchangeable part for fitting the anatomy of both human and bovine discs [34] (Figure 2). However, in order to enable torsional loading, a flat bone surface was needed to prevent slippage and to obtain a smooth hysteresis curve of the IVDs during the loading cycles; thus, no such adjustments considering the concave shape of the CEPs could be realized [40]. An overview of bioreactor systems published within the last decade by research groups around the globe is depicted in Figure 3. Obviously, all bioreactors have in common that they use biocompatible materials, which are supposedly noncytotoxic and easy to clean after culture. These materials are either polycarbonate (Figure 3, A, B) or combinations of glass and polyoxymethylene (POM) (Figure 3, D, E) PEEK or even of ULTEM® (Figure 3, C). Most bioreactors use a fluidic muscle system for force application, while hydraulic valves were used to apply the high forces in the range of 4kN to harbour cadaveric human IVDs [29]. For smaller disc specimens (e.g. rabbit), a cost-effective alternative is a multi-station loading system, which is integrated into a standard materials testing machine [47], providing the advantage of high throughput for simultaneous stimulation of multiple samples. In terms of loading, most bioreactors focus on uniaxial compressive loading except one that can also investigate torsion as a 2nd degree of freedom (DoF) by using rough-surface titanium plates to enable torsion [41]. More complex loadings by increasing the DoF up to six have been recently undertaken with formalin fixed samples [48] and studies are under way to use also unfixed live tissue (Costi, personal communication). Recently, it was also reported that bioreactor studies could be used to validate numerical modelling data [49,50]. Castro et al. [50] predicted the disc height variation of chondroitinase ABC injected IVDs under a given diurnal disc loading by their osmo-poro-hyper-viscoelastic and fiber-reinforced finite element model; data validation was performed by comparing the data obtained from ex-vivo loading of the goat discs in a bioreactor. The studies by Paul et al. (2012; 2013) [7,51] demonstrated that the creep of the IVD is happening in the first 4h of “high” loading. They also provide a finite element model of the enzymatic digestion process, which better predicts the enzymatic break-down of the ECM.
Table 1.
Overview of Intervertebral Disc Organ Culture Systems.
| Loading | Preparation of discs |
Model | Media Refreshment |
Culture System | Findings | Reference |
|---|---|---|---|---|---|---|
| Unloaded systems (free-swelling) | ||||||
| Unloaded, free swelling | Embedding of disc in alginate gel | Rabbit | Static culture | Culture well (standard lab plastics) | ↑ proteoglycan synthesis and maintained a higher content of extracellular matrix components | [55] |
| No loading, free swelling | With CEP, | Bovine coccygeal, Human cadaveric IVD | Static culture | Sterile plastic beaker culture | Injection of LINK N and or MSCs | [122] |
| Mechanically loaded systems | ||||||
| Static loading 0, 0.2, 0.4, 0.8, and 1 MPa | With CEP | Mouse lumbar | Static culture | Culture bottles (glass) | Apoptosis was absent in discs without load, but was particularly ↑ noticeable in loaded discs (load weight, 1.0 MPa). |
[123] |
| Static loading with ~0.4MPa | NEP | Bovine coccygeal | Pump | PEEK reactor, media refreshment with pumping | ↑Cell survival without CEP good, keeping CEP and BEP very ↓ cell viability | [54] |
| Papain injection free-swelling and then static loading with ~0.4MPa, injection of pNIPAM material and with MSCs | with BEP | Bovine coccygeal | Static culture | Glass/POM chamber | MSCs differentiated toward IVD cells, cell viability of MSCs stayed high, hydrogel valume was drastically reduced | [78] |
| Diurnal loading, 0.2MPa for 16h and 0.8Mpa for 8h | with BEP | Ovine | Pump | Polycarbonate | day 7 loading, diurnal superior over static, ↑ cell viability, ↑ diffusion | [36] |
| Cyclic compression unloaded (UL) or loaded (L) (0.1MPa for 16h/day and 2 intervals for 2h/day at 0.1Hz with 0.3MPa ± 0.1MPa | with CEP | Bovine, Ovine, Mouse | Pump or static | Custom-made glass/POM reactor, pressfit design | The discs were cultured for 14 days ↑ cell survival with CEP than with BEP |
[33,34,109] |
| Cyclic compression, static loading, simulated physiological loading, (SPL = ~0.1MPa for 16h/day, high 3× 30min/day blocks of dynamic loading from 0.1-0.6MPa, compared to high dynamic and high static loading. | with BEP (including CEP) | Bovine coccygeal, Ovine | Pump or static | Custom-made polycarbonate reactor | The ↑ cell viability with CEP than with bony endplate ↑ cell viability if |
[45,98,124] |
| Cyclic compression, static loading, simulated physiological loading, (SPL = ~0.1MPa for 16h/day high 3× 30min/day blocks of dynamic loading from 0.1-0.6MPa, compared to high dynamic and high static loading. | with BEP (including CEP) | Caprine | Pump | Custom-made polycarbonate reactor | The ↑ cell viability with CEP than with bony endplate ↑ cell viability if |
[7,31,49-51] |
| Cyclic compression physiological loading (0.2MPa for 16h/day, 0.6MPa±0.2MPa for 3×2h/day with 2h resting/day, test of low and high frequency, i.e. 0.2 vs 5 Hz | With BEP | Ovine, Bovine coccygeal | Pump or static | Custom-made polycarbonate reactor | Cell survival depends on loading frequency and nutrition, synergistic effects of nutrition and frequency causes ↓ cell viability | [37,38,125] |
| Complex cyclic torsio -compression loading, i.e. 0.6±0.1MPa for 8h and 16h free-swelling or low load 0.2MPa | With BEP | Bovine | Static | Custom-made glass/POM reactor | ↓ cell survival with extended complex loading in NP but not in AF (=twisting, combined compression and torsion | [32,98] |
| Cyclic compression 0.1Hz 0.4-0.6 MPa for 8h, recovery at 0.2 Mpa for 16h | With BEP | Human cadaveric IVD | Pump | Custom-made chambers, made of Ultem | System is capable of monitoring IVD height and mechanics during experiment | [29] |
| Cyclinc compression, 1 Hz, 1 MPa, 2500 cycles per day | With hemi-vertebrae | Rabbit | Static | Loading inserts in Falcon tubes, 6-station system mounted in Instron dynamic testing frame | Persistent degenerative changes after spinal trauma with dynamic loading | [47] |
| Osmotically adjusted systems | ||||||
| Osmotically adjusted with salt to iso-osmotic media (335 mosmol/kg) or were diurnally exposed for 8 hours to hyper-osmotic conditions (485 mosmol/kg | with BEP | Rabbit | static | well-plate design free-swelling or with osmotic pressure | ↑ cell viability for osmotically loaded IVDs compared to iso-tonic IVDs | [60,126,127] |
| Osmotically adjusted using PEG and dialysis membrane ((430 mOsm/kg): standard medium 8.2% w/v PEG; Hypertonic to native in situ NP (570 mOsm/kg | NP explants | Bovine | static | Culture well , standard lab plastics | Cultured for up to 42 days, ↑ cell viability, ↑ GAG/DNA ratio of hypertonic medium. | [58,128] |
| Osmolality adjusted by increasing NaCl to 410 mOsm/kg | IVD explants | Rat | static | Culture well, standard lab plastics | Biochemical and molecular assays reveal viable and functional nucleus pulposus cells; TGF-betal, TGF-beta3 elevated the expression of critical matrix genes, ↑ GAG/DNA ratio |
[129,130] |
Figure 1.
General set-up of a loading chamber and overview of current main questions addressed for degeneration and regenerative approaches. The material of the containment is ideally glass or cytocompatible biomaterial that can be cleaned and sterilized easily. A media refreshment pump and media tubing can help to maintain cell viability longer. Loading of the disc can be achieved by application of uni-axial compression or complex multiple degree of freedom loading.
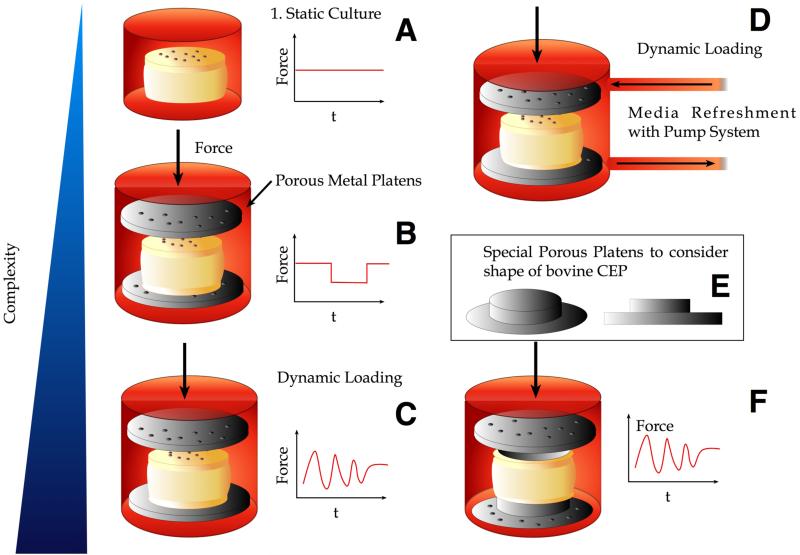
Figure 2. The evolution of intervertebral disc organ culture systems. From relatively simple designs with “free-swelling static” to dynamically loaded systems including media refreshment.
A. free-swelling organ culture in a simple plastic beaker. B. Organ culture using porous platens and uni-axial compression. Possibility to apply diurnal loading by using defined weights on top of the culture system. C. Cyclic compression system allowing for frequency control . D. Cyclic compression and media refreshment system using a peristaltic pump. E. Inlet: Adjusted platens to adapt the concave shape of the CEP in the case of the coccygeal bovine IVD. F. Culture chamber fitting the special porous platens.
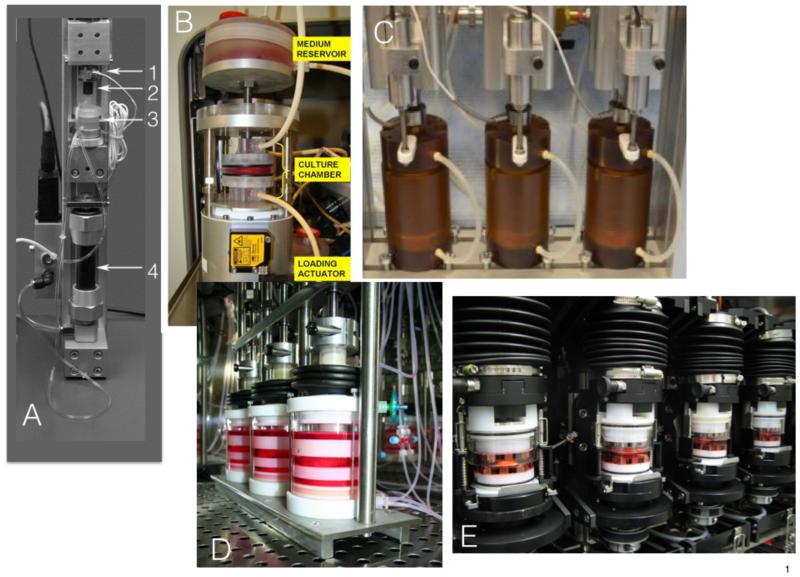
Figure 3. Five examples of Bioreactors recently published by different research teams.
A. Polycarbonate chamber with gas-permeable silicon membrane [36,37]. Key feature: media refreshment system with tubing; culture of IVD with BEP, a steel ball in the axis to ensure parallel and even force transition. 1. Force cell, 2. Coupling with steel ball, 3. Polycarbonate culture chamber, 4. Pneumatic actuator “fluidic muscle”. B. Polycarbonate culture system to allow IVD culture with BEP; key feature: perfusion of culture media through large medium containers [31]. C. Key feature: high force hydraulically actuated bioreactor, Instrumented with load cells and inductive way sensors to allow measurements of IVD height and mechanics throughout testing and culture, chambers are made from Ultem® [29]. D. Glass and Polyoxymethylen (POM) press fit design with uniaxial compression. key feature: specialized adjustments to allow bovine and human IVD culture [33]. E. Glass and POM bioreactor [32]; key feature: presence of serrated titanium plates to allow torsion and compression, special release mechanism to hook and un-hook the bioreactor stations.
So, there is a general trend from uni-axial compressive loading to more complex loading using multiple DoF loading. In addition, there is a trend to keep more structures around the motion segments such as ligaments and the facet joints, which also contribute significantly to a more realistic load distribution [52]. Nevertheless, these complex motion segments also require more nutrition, as there are much more cells involved, and could be limited by a reduced cell viability of the tissue overall especially the IVD.
3. MINIMAL REQUIREMENTS TO GET STARTED
One of the most frequent questions is what is required to maintain IVD explants in vitro? The tissue obtained from the slaughterhouse should be transported to the laboratory as quickly as possible as the tissue will start to decay. However, the cells in the inner part will stay unaffected during this processing time [40]. One of the first steps before starting experiments is to certify that the biological material has a high cell viability at so-called “day 0” of the experiment. This is especially advisable in case of obtaining material from an abattoir one is not very familiar with. Secondly, during the preparation and dissection process the end plate needs to be maintained permeable or made permeable again for nutrients diffusion from and into the organ explant. Here, different approaches have been undertaken. In an ovine study it was shown that cell viability could be improved by flushing of the lower extremities by systemic injection of heparin before euthanasia and immediate post-mortem flushing with ringer solution containing heparin [36]. Bovine coccygeal IVDs harvested from a local abattoir within 3-24 h post-mortem have advanced to a more available and reproducible model [53]. This turned out to be a very reliable animal source of healthy IVDs with the possibility to select for specific sizes of segments for various purposes [40,54]. A significant challenge for all IVD research, regardless of species, is to maintain the original tissue structure during the preparation of the tissue. Obviously, removing tissue, which is always under a certain hydrostatic pressure, causes swelling [55]. When muscular loading is removed and IVDs are isolated, the tissue exhibits a propensity to swell in standard culture media as a result of development of large osmotic gradients [56]. Notably, if bovine coccygeal disc is isolated without the superior and inferior end plates, its weight increases drastically compared to discs with bone-covered end plates. This rapid increase in wet weight of IVD without end plates is apparent within the first 1.5 hours (% increase of initial wet weight for discs after 10, 20, and 90 minutes was 10%, 16%, and 22% for IVDs without any end plates = no endplate, NEP), while the change was 2%, 4%, and 5% for discs with bone-covered end plates. After 15 hours, the weight of IVDs without end plates increased around 22% compared to 10% when cultured with bone-covered end plates (= BEP). A similar finding was observed by Gawri et al. (2011) [57] who compared swelling of human IVDs with cartilage end plates (= CEP) versus IVDs without end plates; after 66 hours in culture, the observed increase in wet weight was 21% in the cartilage end plates group, compared with 44% for the IVDs without end plates [54,57]. When the same experiment was done using bovine discs a 20% increase was found in CEP-prepared disc whereas NEP discs increased by 60-100% of the initial weight [33].
Another appealing approach is to use punched-out tissue of a pre-defined shape and surface area instead of an intact disc. This can be achieved for instance with biopsy punches. For the culture, it is important to maintain the glycosaminoglycans (GAGs) inside the tissue over time [58]. Such a system was established by Potier et al. (2014) [59]. This group proposed to maintain the GAGs content of the tissue by containing the tissue in dialysis membranes with a molecular cut-off of 15 kD or by raising the osmolality by addition of polyethyleneglycol (PEG) to the medium [58]. Their data also showed a better outcome in terms of cell viability and GAG/DNA ratio if the tissue was contained with the dialysis membrane (Table 1). A similar approach was reported by Haschtmann et al. (2006) [60] to achieve “diurnal” swelling and shrinking by increasing osmolality by addition of sucrose: They created a diurnal environment and cultured the IVDs daily for 8 h in iso-osmotic media followed by 16 h in hyperosmotic media. An improved cell viability was achieved by culturing the IVDs daily for 8 h in iso-osmotic media, followed by 16 h in hyperosmotic media (test group). In this way a convective transport could be enabled.
4. ORGAN CULTURE, BIOMATERIALS AND STEM CELL THERAPY
Adult mesenchymal stem cells (MSCs) have been shown to be a promising cell source for regenerative therapies for several decades [61,62]. MSCs have the potential to differentiate along various linages, including the skeletal lineages [63] and bone marrow derived MSCs are the most studied MSCs. While differentiation into cartilaginous cell types is well established, recent in vitro and in vivo studies indicated the potential of MSCs to differentiate also towards a phenotype similar to nucleus pulposus cells [63-72].
The regenerative potential of MSCs was further indicated by their migration potential towards injured and compromised (stressed) tissues such as bone defects [73]. Recent data also indicated that MSCs can actively “home” towards stressed IVDs by migrating through the CEP [74]. In this bovine IVD organ culture model fluorescently labelled primary human bone marrow MSCs were applied onto “stressed” IVDs and their migration potential towards the disc center was determined by confocal laser scanning microscopy. IVD cell stress was initiated through nutritional deprivation and/or unphysiological biomechanical loading [30,37] and annular puncture of the IVD with a needle. MSC migration potential was further confirmed in migration assays; MSCs migrated towards the conditioned medium of “stressed” IVDs, demonstrating the chemo-attractive effect of the medium [74]. One potential initiator of the observed cell migration is the chemokine RANTES/CCL5. This chemokine is expressed by NP cells and AF cells and is thought to play a central role in the biology of attracting stromal cells [75].
Yet, undifferentiated MSCs may have difficulties to survive in an IVD environment, which is typically depleted of oxygen, is more acidic, and has a higher osmolarity compared to other tissues [76,77]. Chan et al. [77] demonstrated in a papain-induced bovine caudal IVD degeneration organ culture model that MSCs could not thrive even in the healthy IVD matrix and many of the MSCs actually died as demonstrated by LIVE/DEAD stain and confocal microscopy [77] after a 10-day culture. Later, the same group [78] demonstrated MSC proliferation in IVD organ cultures under relatively mild loading conditions when the MSCs were suspended in a thermo-reversible hyaluronan-based hydrogel, i.e. [poly(N-isopropylacrylamide), HA-pNIPAM] prior injection into papain digested cavities. However, the HA-pNIPAM hydrogel was found to lose a large portion of the initial volume after 7 days of mild static compressive loading at 0.2 MPa [78]. Though the compromised stability under mechanical load is a limitation of the HA-pNIPAM hydrogel, other investigations demonstrated its potential to support MSC differentiation towards a disc-like phenotype. Indeed, human MSCs encapsulated within the HA-pNIPAM hydrogel survived and differentiated within an IVD cavity without the need for pre-culture and/or growth factor supplementation [79]. MSC survival was further investigated in an in vivo rat-tail model: Crevensten et al. (2004) [64] injected MSCs suspended in a 15% hyaluronan gel and observed that while the cell number of surviving MSCs significantly decreased after 7 and 14 days, after 28 days the MSC population returned to the initial amount of injected cells and viability was 100%. Further, a trend of increased IVD height compared to blank gel suggested an increase in matrix synthesis. From these findings the authors concluded that MSCs could survive and proliferate in IVDs [64].
Other recently published data by the Canadian disc researcher team by Mwale et al. (2014) [80] could show successful MSC survival without any pre-conditioning with growth factors if injected into bovine coccygeal organ culture model along with the short peptide link-N. They injected only about 105 cells into the bovine disc and found a high cell viability after 21 days of organ culture. However, a crucial factor seems to be number of MSCs that are supplied and that the IVDs are kept under high nutrition. On the effect of the number of injected MSCs there has been only one study systematically undertaken so far in an in vivo canine model [81]. Serigano et al. (2010) [81] injected 105, 106, or 107 per canine lumbar disc and found only after injecting at least 106 cells the expected regenerative effects by partial restoring disc height on MRI. Live/dead stain also confirmed higher cell viability if > 106 cells were applied.
During recent years the application of various hydrogels for NP and AF repair has been widely followed-up [80,82-85]. One approach is the injection of fibrin-genipin adhesive hydrogel to seal large AF defects [86,87]. In a bovine organ culture model, bovine-derived fibrin cross-linked with genipin successfully closed an AF wound that was induced by a stab with a scalpel blade [86,87]. The hydrogel with gelation times of less than 15 min successfully sealed large AF defects, promoted functional restoration with improved motion segment biomechanics, and served as a biocompatible adhesive biomaterial that had greatly enhanced in vivo longevity compared to fibrin alone. The authors concluded that fibrin-genipin would offer promise for AF repair strategies and would warrant further material development and evaluation for clinical application [86]. Thus, hydrogels might find their way into the operation rooms as commercially available products are already FDA-approved such as Tisseel© from Baxter, inc. Another approach is the combination of genipin reinforced hydrogels with adherence molecules or anti-inflammatory drugs such as anti-TNFα. In this respect, drug delivery systems consisting of hydrogel carriers have been investigated such as a hyaluronan-gel releasing stromal cell derived factor-1 (SDF-1) to enhance homing of MSCs [88]. Many other 3D hydrogels were investigated in particular for NP regeneration. These are summarized in [89,90].
Recently, viral and non-viral gene delivery has been investigated for their feasibility to be applied directly to the IVD [89,91]. DNA approaches have traditionally been undertaken using viral vectors onto monolayer cells [89] or in some cases using pellet or alginate bead cultures [91,92]. Wallach et al. (2006) [92] investigated adenovirus Ad-TGF-β1 and Ad-BMP-2 as therapeutic factors for the IVD, as a potential treatment strategy for degenerative disc disease. However, non-viral transfection methods might be key for future translational medicine as their acceptance is much higher and toxic issues to cells are much less than with viral methods. Such an approach has been recently investigated in a pilot study using a papain-cavity bovine organ culture model where transiently GDF-5-transfected MSCs embedded in a polyethylene glycol hydrogel (PEG) hydrogel were injected [91]. This study showed potential for such a gene therapy approach by achievement of increased GAG after injection of the transfected cells into the bovine organ culture model [91].
Bioreactors could serve as a “priming tool” if they achieved FDA approval and/or CE label. In vitro and in vivo studies have demonstrated that MSCs can be directed towards the discogenic pathway by application of growth factors such as GDF5 and GDF6 or hypoxic conditions [71,72,93]. It has been multiple times demonstrated that MSCs injected into the microenvironment of the disc start to differentiate into disc-like or more precisely into NPC-like cells either after preconditioning of the MSCs with suitable growth factors or directly driven by the transplantation into the microenvironment [78]. Other studies confirm direct discogenic differentiation by injection of undifferentiated MSCs after a prolonged culture time of 14 days into AFC-like precursor cells [94]. Concerning the “priming” of naïve MSCs towards a discogenic phenotype, there has been to the best of our knowledge no clinical trial so far involving an ex vivo step using bioreactors. Of course, the idea would be appealing to inject patient's autologous MSCs, which were expanded in vitro on plastics and then “primed” into IVD precursors by simulating the IVD-specific microenvironment. This could be accomplished by culturing MSCs under hypoxic conditions and application of specific mechanical or hydrostatic loading. It is hypothesized that MSCs “primed” by a mechanical bioreactor mimicking relevant loading conditions may show improved survival and activity after injection into the disc. The risk remains that the “primed” MSCs may be injected into an inflammatory microenvironment; this could have a negative or a beneficial influence on the MSCs, though the role of the inflammatory milieu is not very clear yet [95].
The results from these presented studies confirm that organ culture is a useful tool to investigate regenerative approaches such as cell therapy, gene therapy or biomaterial injection. Furthermore, these devices could hold the potential to “prime” MSCs or other stem cells for re-injection into the patient.
5. ANIMAL MODELS FOR ORGAN CULTURE
Much concern has been raised whether tail IVDs, the IVDs from the elongation of the spine should serve as a model system. From a mechanical point of view it was demonstrated that tail IVDs of different species could be used as model systems as their creep behaviour and compressive stiffness was comparable to the lumbar IVD in principle [26,96]. Beckstein et al. (2008) [27] compared nine animal species and concluded after correction for geometry that the disc tissue material properties are largely conserved across animal species, which were calf, pig, baboon, sheep, rabbit, rat, and mouse lumbar discs, in addition to the cow and rat tail IVD [27]. The coccygeal IVDs have a similar range of motion as lumbar IVDs in flexion and extension, bending and torsion [27]. Torsion angle of failure of coccygeal discs for rat and cattle was found to be very high at ~30° [97,98] in contrast to the human lumbar disc where the failure range is smaller ~10° [52]. Biochemical composition, cell density and the cell population lacking notochordal cells of the bovine tail IVD are highly similar to human lumbar IVD [99,100].
6. ENZYMATIC DISC DEGENERATION MODELS
In recent years, several approaches were undertaken to develop disc degeneration models involving proteinases that digest mainly GAGs or collagenases. Among various enzymes, chondroitinase ABC, trypsin and papain (serine protease) have been used to induce IVD degeneration. Chondroitinase ABC has been widely used to remove painful tissue in disc herniation but was also intensively investigated to induce disc degeneration in vitro and in vivo [101-107] (Table 2). Papain has been repeatedly shown to induce cavity formation if injected in various doses [77,108]. Recently, it has been demonstrated that loss of disc integrity and stiffness induced by injection of trypsin or by non-physiological loading can be partially recovered by applying a physiological loading [109]. Recently, Furtwängler et al. (2013) [110] injected more physiologically relevant enzymes such as aggreganase (ADAMTS4) collagenase (MMP3) and high temperature requirement serine protease A1 (HTRA1) into the IVD [110,111]. It has been repeatedly demonstrated that ADAMTses and MMPs are up-regulated in IVD degeneration. However, in vitro injection of higher doses of these enzymes did not induce major matrix break down as compared to papain and trypsin. Thus, neither injection of activated MMPs nor HTRA1 did cause visible matrix depletions as it was found with papain [110] with diurnal loading with 0.4 MPa and free-swelling recovery for 16 hrs (Table 2). It was noticed that different enzymatic treatment of IVDs caused similar T2* or T1ρ MRI sequences as different stages of IVD degeneration [106,112-114]. Several studies demonstrated a correlation between severity of disc degeneration and MRI signal. Along this line Purmessur et al. (2013) [115] investigated a role of TNFα, which is known to alert cells as a pro-inflammatory cytokine initiator of degenerative disease. They found that a 7-day incubation period with elevated levels of recombinant TNFα (200 ng/mL) induced a non-recoverable catabolic shift under static loading conditions [115], although it was noted that physiological loading may have reduced these effects. Gawri et al. (2014) [109] could also demonstrate that there is a connection between matrix homeostasis and the applied compressive loading. When they digested bovine coccygeal IVD with trypsin and did not apply any loading the matrix break-down was very high with a decrease of the proteoglycan content. However, with the application of physiological loading the matrix-breakdown could be recovered, which confirmed that some physiological loading is beneficial for intervertebral disc culture and homeostasis. It is likely that this dynamic physiological loading directly stimulates anabolic metabolism of the cells while also enhanced convective transport of large metabolites into the disc.
Table 2.
Summary of enzymatic and mechanical disc degeneration (= “catabolic shift”) experiments.
| Stress factor | Organ Culture | Animal Model | Outcome | Reference |
|---|---|---|---|---|
|
Enzymatic Degeneration Models
| ||||
| Injection of Catabolic Enzymes: Papain | BEP | Bovine coccygeal | ↑ Injection of MSC, cell survival was improved in papain digested IVDs, MSC differentiated toward IVD phenotype | [77,78,91,108] |
| Injection of Catabolic Enzymes: Trypsin | BEP | Bovine coccygeal | ↑ cell viability for osmotically loaded IVDs compared to isotonic IVDs | [33,80,108,109] |
| Injection of HTRA1, MMP3 and ADAMTS4 | BEP | Bovine coccygeal | No major cavity formed, no major changes in disc matrix observed in histology | [110] |
| Injection of chodroitinase ABC | BEP | Coat, rabbit, rat coccygeal | Altered mechanical loading, secondary to biochemical changes in the NP | [106,107,131,132] |
| Injection of proinflammatory Cytokines, TNFα | BEP | Bovine coccygeal | Addition of TNFα resulted in a ctabolic shift, ↑ MMPs, ↑ ADAMTses | [115] |
|
Mechanical Injury Models
| ||||
| Disc endplate fracture model, customized droptower to induce burst fractures in BEP | BEP, preserving 1/3 of a vertebral body | Rabbit | induced IVD degeneration much higher in burst fractures, ↑apoptosis, ↑MMPs, ↑interleukins in burst fractures, ↑LDH activity released to media | [35,47,120] |
| Single-ramp compression consistently cracks cartilaginous CEPs of healthy human IVDs, 5 % (non-injured) or 30 % (injured) strain | CEP | Bovine coccygeal | Mechanical injury resulted in about 40-50 % cell death viability in the NP, ↑Apoptosis, ↑inflammatory cytokines, ↑MMPS, ↑ADAMTses | [121] |
| Needle-stab or stab incision induced IVD degeneration | BEP | Bovine coccygeal | Needle stab models express ↑nitrite oxide (factor for stress), ↑LDH, stressed IVD releases factors that attract homing of MSCs | [74,87,116,133-135] |
7. Mechanical Disc Degeneration Models
Beside enzymatic break-down there is also the possibility to induce mechanical damage to the IVD. This is often performed by creating an annular defect. Bovine coccygeal disc organ cultures showed localized cell death and altered disc mechanics when punctured with large and small needles [116]. A 22-Gauge needle puncture showed increased signs of degeneration when combined with high intensity, or ‘degenerative’ loading conditions. This was demonstrated by increased cell death and elevated MMP gene expression in punctured IVD and was also associated with enhanced MSC migration into the tissue [74]. Organ cultures have also been utilized to investigate new AF repair strategies. Injection of platelet-rich plasma stimulated ECM synthesis in a bovine AF defect model [94]. A bovine annulotomy model was used to investigate a combined cell and biomaterial approach for AF rupture repair. Implantation of MSC-seeded scaffolds with a sutured membrane could preserve the disc height and prevent NP protrusion under bioreactor guided mechanical load [94]. Modified fibrin was also able to seal the AF defects and restore disc height following substantial compressive loading in a one week organ culture experiment [86].
An alternative access route, the transpedicular approach, has been proposed rather than injection of enzymes or cells through the outer and inner annulus fibrosus into the center of the IVD [117]. This approach involved the penetration of the vertebral body and the cartilaginous endplate. This approach has been tested in vitro in bovine coccygeal organ culture nucleotomy models. In particular, this endplate approach has been valuable and successful for testing the survival and differentiation of NP cells or MSCs encapsulated in various injectable hydrogels and implanted into partially nucleotomized discs. Examples include a thermo-reversible HA-pNIPAM hydrogel [79,118] and a biomimetic fibrinhyaluronan hydrogel for NP regeneration [119]. The advantage of the endplate approach is that the AF remains intact, minimizing the risk for leakage of injected cells or hydrogels under mechanical load, provided that the endplate is properly sealed, although the clinical translation of endplate routes for delivery of therapies is substantially more complicated.
8. ORGAN CULTURE & ENDPLATE FRACTURE MODEL
In the case of trauma, endplate damage has been proposed as a major cause for subsequent IVD degeneration. Dudli et al. (2011; 2014a; 2014b) [35,47,120] cultured rabbit IVD explants from the thoracolumbar region and analyzed the biological consequences of high-energy burst fractures. In these studies, impact energy was delivered by a dropped weight. A threshold impact energy value of approx. 0.75J was found to produce bone fractures in 50% of specimens. In vitro culture of rabbit IVDs following impact demonstrated that a fracture is needed in the BEP was required to induce degeneration and inflammation; an equi-energetic impact but without burst fracture yielded a significantly milder degenerative response [120]. Subsequent studies with this model have shown that nuclear depressurisation alone is not the mechanism for post-traum degeneration, pointing towards an immune-regulated response [35], and that degeneration persists also in the presence of post-traumatic dynamic loading [47]. Recently, injury-loaded IVDs by Alkhatib et al. (2014) [121] were able to induce acute mechanical trauma and to cause the catabolic cascade of disc degeneration. Thus, there is evidence that the CEP is of key importance to maintain a healthy disc and more investigations should be undertaken to better understand the relevance of disc injury/trauma for accelerated disc degeneration and the development of pain.
9. ORGAN CULTURE INVOLVING HUMAN IVDS
Recently, human lumbar IVD organ culture was established by Gawri et al. (2011) [57] and by Walter et al. (2014) [29]. Both groups managed to keep cells throughout the discs viable and metabolically active for more than 4 weeks. The usage of human cadaveric IVDs has to be considered of high clinical relevance and directly translational to human disease. Important advantages compared to other model systems are that the discs come with naturally occurring degeneration of varying grade, and they have a cell and matrix composition directly comparable to live human patients. As in other ex vivo model systems the effect of loading, inflammatory environment and nutritional status can be manipulated, allowing the effect of these factors to be factored into the regenerative potential of various treatment modalities. As explained earlier the upright position of the spine is unique for the human and cannot easily be reproduced in any animal model [12]. Of course, the culture of cadaveric human IVD requires the collaboration and the permissive law of transplantation since IVD should be obtained within 24h postmortem for successful organ culture. Two isolation methods have been used for the culture of human IVDs Walter et al. (2014) [29] applied a preparation method leaving a thin layer of BEP attached. A jet lavage system was used to clean the trabecular bone to enable nutrient diffusion. This method has been proven to be successful also for bovine coccygeal IVD [29,40]. Gawri et al. (2011) [57] removed the calcified parts of the endplates leaving a disc with intact CEP. In this case no further manipulation is necessary to enable sufficient nutrient supply. Discs isolated using this method have been shown to survive for a up to 4 moths in culture enabling long term studies to be conducted. More recently, such an in vitro cadaveric human IVD culture has been used to investigate the effect of peptide therapeutic treatment. The injection of short peptide sequences could be much more straightforward approach to be clinically feasible than the more risky methods involving autologous differentiated or progenitor cells. Here, Link N, a peptide sequence of Link protein, has been demonstrated to be a possible target for direct drug delivery [57,80]. Recent work by Mwale et al. (2014) [80] demonstrated that a single injection of link-N could increase proteoglycan production in the NP region of degenerated IVDs in organ culture. The same effect was observed when isolated cells were exposed to the peptide [122].
10. CONCLUSION
We conclude that organ culture of IVDs is highly appealing for the study of the interplay of mechanical forces and biological responses. IVD organ culture bioreactors are mainly force-controlled devices that are able to mimic the natural forces, so far mainly in uni-axial compression. There are small- and large animal, as well as cadaveric human IVD models. Investigations requiring simulation in a large, nutritionally compromised IVD can be modelled using organ cultures of IVDs that are large in size. The characteristic dimension of interest is the sagittal disc height from CEP-disc-CEP, which determines the number of transport rate of nutrients and metabolites and ultimately has a profound effect on the density of live cells in a IVDs [9,99]. Bovine coccygeal IVDs are appealing to meet this constraint because of their large IVD height. Physiological loading on IVDs from all regions, including coccygeal IVDs, are dominated by muscle contraction forces, and result in similar resting pressure as in the IVDs. However, peak forces are lower and ragens of motion are greater in caudal IVDs than lumbar IVDs so that caution should be exercised used when designing experiments and interpreting results. With this limitation, however, cell therapy using allogenic or autologous mesenchymal stem cells, injection of peptides such as link N, and injection of biomaterial hydrogels can be tested in vitro in an environment with many similarities to human IVDs. Enzymatic matrix break-down, addition of key inflammatory cytokines or mechanical damage have been investigated as IVD degeneration models to better similate the diseased human IVD state. These 3D organ culture models bridge basic science and translational medicine and allow in vitro experiments with substantial control while maintaining cells in a native tissue microenvironment that approaches the in vivo state more closely than other monolayer and 3D culture environments can achieve.
ACKNOWLEDGEMENTS
This work was supported by a grant of the Swiss National Science Project number SNF #310030_153411 and by two grants of the Lindenhof Foundation, Bern, Switzerland, Projects no. 13-02-F and 14-03-F.
LIST OF ABBREVIATIONS
- AF
Annulus fibrosus
- BEP
Bony endplate (bone form vertebral body)
- CEP
Cartilaginous Endplate
- DoF
Degree of freedom
- EP
Endplate
- IVD
intervertebral disc
- Link N
short peptide sequence, which bridges the hyaluronic acid with the polysaccharide chains in the aggrecan molecule, naturally occurring peptide that can stimulate proteoglycan synthesis
- MSC
mesenchymal stem cells
- MRI
Magnetic resonance imaging
- NEP
No endplates, i.e. detachment of cartilaginous endplates and free-swelling condition
- NP
Nucleus pulposus
- pNIPAM
polyamide poly-N-isopropylacrylamide
- POM
Polyoxymethylene
- ULTEM®
polyetherimide, which is an amorphous thermoplastic polyetherimide (PEI) material which combines exceptional mechanical, thermal, and electrical properties
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Wieser S, Horisberger B, Schmidhauser S, et al. Cost of low back pain in Switzerland in 2005. Eur J Health Econ. 2011;12:455–67. doi: 10.1007/s10198-010-0258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricci JA, Stewart WF, Chee E, et al. Back pain exacerbations and lost productive time costs in United States workers. Spine (Phila Pa 1976) 2006;31:3052–60. doi: 10.1097/01.brs.0000249521.61813.aa. [DOI] [PubMed] [Google Scholar]
- 3.Adams MA, Dolan P. Could sudden increases in physical activity cause degeneration of intervertebral discs? Lancet. 1997;350:734–5. doi: 10.1016/S0140-6736(97)03021-3. [DOI] [PubMed] [Google Scholar]
- 4.Adams MA, Freeman BJ, Morrison HP, et al. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25:1625–1636. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Grunhagen T, Shirazi-Adl A, Fairbank JC, et al. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42:465–77. vii. doi: 10.1016/j.ocl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–9. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 7.Paul CP, Schoorl T, Zuiderbaan HA, et al. Dynamic and static overloading induce early degenerative processes in caprine lumbar intervertebral discs. PLoS One. 2013;8:e62411. doi: 10.1371/journal.pone.0062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibby SR, Jones DA, Ripley RM, et al. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine. 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 9.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Werf M, Lezuo P, Maissen O, et al. Inhibition of vertebral endplate perfusion results in decreased intervertebral disc intranuclear diffusive transport. J Anat. 2007;211:769–74. doi: 10.1111/j.1469-7580.2007.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutton WC, Murakami H, Li J, et al. The effect of blocking a nutritional pathway to the intervertebral disc in the dog model. Journal of Spinal Disorders. 2004;17:53–63. doi: 10.1097/00024720-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busscher I, van der Veen AJ, van Dieën JH, et al. In vitro biomechanical characteristics of the spine: a comparison between human and porcine spinal segments. Spine (Phila Pa 1976) 2010;35:E35–42. doi: 10.1097/BRS.0b013e3181b21885. [DOI] [PubMed] [Google Scholar]
- 14.van Deursen DL, Snijders CJ, Kingma I, et al. In vitro torsion-induced stress distribution changes in porcine intervertebral discs. Spine (Phila Pa 1976) 2001;26:2582–6. doi: 10.1097/00007632-200112010-00011. [DOI] [PubMed] [Google Scholar]
- 15.Omlor GW, Bertram H, Kleinschmidt K, et al. Methods to monitor distribution and metabolic activity of mesenchymal stem cells following in vivo injection into nucleotomized porcine intervertebral discs. Eur Spine J. 2010;19:601–12. doi: 10.1007/s00586-009-1255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi S, Meir A, Urban J. Effect of cell density on the rate of glycosaminoglycan accumulation by disc and cartilage cells in vitro. J Orthop Res. 2008;26:493–503. doi: 10.1002/jor.20507. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki T, Kobayashi S, Takeno K, et al. A Phenotypic Comparison of Proteoglycan Production of Intervertebral Disc Cells Isolated from Rats, Rabbits, and Bovine Tails; Which Animal Model is Most Suitable to Study Tissue Engineering and Biological Repair of Human Disc Disorders? Tissue Eng Part A. 2009;15:3835–46. doi: 10.1089/ten.tea.2009.0250. [DOI] [PubMed] [Google Scholar]
- 18.Gantenbein B, Calandriello E, Wuertz-Kozak K, et al. Activation of intervertebral disc cells by co-culture with notochordal cells, conditioned medium and hypoxia BMC Musculoskeletal. Disorders. 2014;15:422. doi: 10.1186/1471-2474-15-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Showalter BL, Beckstein JC, Martin JT, et al. Comparison of Animal Discs Used in Disc Research to Human Lumbar Disc: Torsion Mechanics and Collagen Content. Spine (Phila Pa 1976) 2012;37:E900–7. doi: 10.1097/BRS.0b013e31824d911c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues-Pinto R, Richardson SM, Hoyland JA. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur Spine J. 2014;23:1803–14. doi: 10.1007/s00586-014-3305-z. [DOI] [PubMed] [Google Scholar]
- 21.de Vries S, Potier E, Doeselaar MV, et al. Conditioned medium derived from notochordal cell-rich nucleus pulposus tissue stimulates matrix production by canine nucleus pulposus cells and bone marrow derived stromal cells. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2014.0309. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purmessur D, Guterl CC, Cho SK, et al. Dynamic pressurization induces transition of notochordal cells to a mature phenotype while retaining production of important patterning ligands from development. Arthritis Res Ther. 2013;15:R122. doi: 10.1186/ar4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potier E, Ito K. Using notochordal cells of developmental origin to stimulate nucleus pulposus cells and bone marrow stromal cells for intervertebral disc regeneration. Eur Spine J. 2013;23:679–88. doi: 10.1007/s00586-013-3107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purmessur D, Cornejo MC, Cho SK, et al. Notochordal Cell-Derived Therapeutic Strategies for Discogenic Back Pain. Global Spine J. 2013;3:201–218. doi: 10.1055/s-0033-1350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erwin WM, Islam D, Inman RD, et al. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R215. doi: 10.1186/ar3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demers CN, Antoniou J, Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine. 2004;29:2793–9. doi: 10.1097/01.brs.0000147744.74215.b0. [DOI] [PubMed] [Google Scholar]
- 27.Beckstein JC, Sen S, Schaer TP, et al. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine (Phila Pa 1976) 2008;33:E166–73. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 28.Wilke HJ, Kettler A, Wenger KH, et al. Anatomy of the sheep spine and its comparison to the human spine. Anat Rec. 1997;247:542–55. doi: 10.1002/(SICI)1097-0185(199704)247:4<542::AID-AR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 29.Walter BA, Illien-Jünger S, Nasser PR, et al. Development and validation of a bioreactor system for dynamic loading and mechanical characterization of whole human intervertebral discs in organ culture. J Biomech. 2014;47:2095–101. doi: 10.1016/j.jbiomech.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Illien-Jünger S, Gantenbein-Ritter B, Grad S, et al. The Combined Effects of Limited Nutrition and High-Frequency Loading on Intervertebral Discs With Endplates. Spine (Phila Pa 1976) 2010;35(19):1744–1752. doi: 10.1097/BRS.0b013e3181c48019. [DOI] [PubMed] [Google Scholar]
- 31.Paul CP, Zuiderbaan HA, Zandieh Doulabi B, et al. Simulated-physiological loading conditions preserve biological and mechanical properties of caprine lumbar intervertebral discs in ex vivo culture. PLoS One. 2012;7:e33147. doi: 10.1371/journal.pone.0033147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan SCW, Walser J, Käppeli P, et al. Region Specific Response of Intervertebral Disc Cells to Complex Dynamic Loading: An Organ Culture Study Using a Dynamic Torsion-Compression Bioreactor. PLoS ONE. 2013;8:e72489. doi: 10.1371/journal.pone.0072489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jim B, Steffen T, Moir J, et al. Development of an intact intervertebral disc organ culture system in which degeneration can be induced as a prelude to studying repair potential. Eur Spine J. 2011;20:1244–54. doi: 10.1007/s00586-011-1721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haglund L, Moir J, Beckman L, et al. Development of a Bioreactor for Axially Loaded Intervertebral Disc Organ Culture. Tissue Eng Part C Methods. 2011;17:1011–9. doi: 10.1089/ten.TEC.2011.0025. [DOI] [PubMed] [Google Scholar]
- 35.Dudli S, John Ferguson S, Haschtmann D. Severity and pattern of posttraumatic intervertebral disc degeneration depends on the type of injury. Spine J. 2014 doi: 10.1016/j.spinee.2013.07.488. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Gantenbein B, Grünhagen T, Lee CR, et al. An in vitro organ culturing system for intervertebral disc explants with vertebral endplates: a feasibility study with ovine caudal discs. Spine (Phila Pa 1976) 2006;31:2665–73. doi: 10.1097/01.brs.0000244620.15386.df. [DOI] [PubMed] [Google Scholar]
- 37.Jünger S, Gantenbein-Ritter B, Lezuo P, et al. Effect of limited nutrition on in situ intervertebral disc cells under simulated-physiological loading. Spine (Phila Pa 1976) 2009;34:1264–71. doi: 10.1097/BRS.0b013e3181a0193d. [DOI] [PubMed] [Google Scholar]
- 38.Illien-Jünger S, Gantenbein-Ritter B, Grad S, et al. The Combined Effects of Limited Nutrition and High-Frequency Loading on Intervertebral Discs With Endplates. Spine (Phila Pa 1976) 2010;35(19):1744–1752. doi: 10.1097/BRS.0b013e3181c48019. [DOI] [PubMed] [Google Scholar]
- 39.Chan SCW, Gantenbein-Ritter B, Leung VY, et al. Cryopreserved intervertebral disc with injected bone marrow-derived stromal cells: a feasibility study using organ culture. Spine J. 2010;10(6):486–96. doi: 10.1016/j.spinee.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Chan SC, Gantenbein-Ritter B. Preparation of intact bovine tail intervertebral discs for organ culture. J Vis Exp. 2012;60:e3490. doi: 10.3791/3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walser J, Ferguson SJ, Gantenbein-Ritter B. Design of a mechanical loading device to Culture intact Bovine Caudal Motional Segments of the Spine under Twisting Motion. In: Davies J, editor. Replacing animal models: a practical guide to creating and using biomimetic alternatives. John Wiley & Sons, Ltd.; Chichester, UK: 2012. pp. 89–105. [Google Scholar]
- 42.Bailey JF, Hargens AR, Cheng KK, et al. Effect of microgravity on the biomechanical properties of lumbar and caudal intervertebral discs in mice. J Biomech. 2014;47:2983–8. doi: 10.1016/j.jbiomech.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Jin L, Feng G, Reames DL, et al. The effects of simulated microgravity on intervertebral disc degeneration. Spine J. 2013;13:235–42. doi: 10.1016/j.spinee.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohshima H, Tsuji H, Hirano N, et al. Water diffusion pathway, swelling pressure, and biomechanical properties of the intervertebral disc during compression load. Spine. 1989;14:1234–1244. doi: 10.1097/00007632-198911000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Walter BA, Korecki CL, Purmessur D, et al. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage. 2011;19:1011–8. doi: 10.1016/j.joca.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams MA, Hutton WC. Prolapsed intervertebral disc. A hyperflexion injury 1981 Volvo Award in Basic Science. Spine (Phila Pa 1976) 1982;7:184–91. [PubMed] [Google Scholar]
- 47.Dudli S, Haschtmann D, Ferguson SJ. Persistent degenerative changes in the intervertebral disc after burst fracture in an in vitro model mimicking physiological post-traumatic conditions. Eur Spine J. 2014 doi: 10.1007/s00586-014-3301-3. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Lawless IM, Ding B, Cazzolato BS, et al. Adaptive velocity-based six degree of freedom load control for real-time unconstrained biomechanical testing. J Biomech. 2014;47:3241–7. doi: 10.1016/j.jbiomech.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Vergroesen P-PA, Veen AJVD, Royen BJV, et al. Intradiscal pressure depends on recent loading and correlates with disc height and compressive stiffness. European Spine Journal. 2014;23:2359–2368. doi: 10.1007/s00586-014-3450-4. [DOI] [PubMed] [Google Scholar]
- 50.Castro APG, Paul CPL, Detiger SEL, et al. Long-Term Creep Behavior of the Intervertebral Disk: Comparison between Bioreactor Data and Numerical Results. Frontiers in Bioengineering and Biotechnology. 2014:2. doi: 10.3389/fbioe.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paul CP, Strijkers GJ, de Graaf M, et al. Changes in Biomechanical, Histological and Quantitative MRI Parameters in Lumbar Caprine Intervertebral Discs Subjected to Chondroitinase-Induced Degeneration. Global Spine Journal. 2012;2:P86. [Google Scholar]
- 52.White AA, Panjabi MM. Clinical Biomechanics of the Spine. Pennsylvania: B Lippincott Company; Philadelphia: 1990. [Google Scholar]
- 53.Oshima H, Ishihara H, Urban JP, et al. The use of coccygeal discs to study intervertebral disc metabolism. J Orthop Res. 1993;11:332–8. doi: 10.1002/jor.1100110304. [DOI] [PubMed] [Google Scholar]
- 54.Lee CR, Iatridis JC, Poveda L, et al. In vitro organ culture of the bovine intervertebral disc: effects of vertebral endplate and potential for mechanobiology studies. Spine (Phila Pa 1976) 2006;31:515–22. doi: 10.1097/01.brs.0000201302.59050.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiba K, Andersson GB, Masuda K, et al. A new culture system to study the metabolism of the intervertebral disc in vitro. Spine. 1998;23:1821–1827. doi: 10.1097/00007632-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 56.Bayliss MT, Urban JP, Johnstone B, et al. In vitro method for measuring synthesis rates in the intervertebral disc. Journal of Orthopaedic Research. 1986;4:10–17. doi: 10.1002/jor.1100040102. [DOI] [PubMed] [Google Scholar]
- 57.Gawri R, Mwale F, Ouellet J, et al. Development of an organ culture system for long term survival of the intact human intervertebral disc. Spine (Phila Pa 1976) 2011;36:1835–42. doi: 10.1097/BRS.0b013e3181f81314. [DOI] [PubMed] [Google Scholar]
- 58.van Dijk BG, Potier E, Ito K. Long-term culture of bovine nucleus pulposus explants in a native environment. Spine J. 2013 doi: 10.1016/j.spinee.2012.12.006. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Potier E, de Vries S, van Doeselaar M, et al. Potential application of notochordal cells for intervertebral disc regeneration: an in vitro assessment. Eur Cell Mater. 2014;28:68–81. doi: 10.22203/ecm.v028a06. [DOI] [PubMed] [Google Scholar]
- 60.Haschtmann D, Stoyanov JV, Ferguson SJ. Influence of diurnal hyperosmotic loading on the metabolism and matrix gene expression of a whole-organ intervertebral disc model. J Orthop Res. 2006;24:1957–66. doi: 10.1002/jor.20243. [DOI] [PubMed] [Google Scholar]
- 61.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 62.Evans CH. Advances in regenerative orthopedics. Mayo Clin Proc. 2013;88:1323–39. doi: 10.1016/j.mayocp.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Risbud MV, Albert TJ, Guttapalli A, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine (Phila Pa 1976) 2004;29:2627–32. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 64.Crevensten G, Walsh AJ, Ananthakrishnan D, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004;32:430–4. doi: 10.1023/b:abme.0000017545.84833.7c. [DOI] [PubMed] [Google Scholar]
- 65.Meisel HJ, Siodla V, Ganey T, et al. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Ganey T, Hutton WC, Moseley T, et al. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: experiments in a canine model. Spine (Phila Pa 1976) 2009;34:2297–304. doi: 10.1097/BRS.0b013e3181a54157. [DOI] [PubMed] [Google Scholar]
- 67.Sakai D, Mochida J, Iwashina T, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 2005;30:2379–87. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 68.Sakai D, Mochida J, Iwashina T, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27:335–45. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 69.Hiyama A, Mochida J, Iwashina T, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26:589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 70.Steck E, Bertram H, Abel R, et al. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–11. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 71.Gantenbein-Ritter B, Benneker LM, Alini M, et al. Differential response of human bone marrow stromal cells to either TGF-β(1) or rhGDF-5. Eur Spine J. 2011;20:962–971. doi: 10.1007/s00586-010-1619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stoyanov JV, Gantenbein-Ritter B, Bertolo A, et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater. 2011;21:533–47. doi: 10.22203/ecm.v021a40. [DOI] [PubMed] [Google Scholar]
- 73.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–23. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 74.Illien-Jünger S, Pattappa G, Peroglio M, et al. Homing of Mesenchymal Stem Cells in Induced Degenerative Intervertebral Discs in a Whole Organ Culture System. Spine (Phila Pa 1976) 2012;37:1865–73. doi: 10.1097/BRS.0b013e3182544a8a. [DOI] [PubMed] [Google Scholar]
- 75.Pattappa G, Peroglio M, Sakai D, et al. CCL5/RANTES is a key chemoattractant released by degenerative intervertebral discs in organ culture. Eur Cell Mater. 2014;27:124–36. doi: 10.22203/ecm.v027a10. discussion 136. [DOI] [PubMed] [Google Scholar]
- 76.Wuertz K, Godburn K, Iatridis JC. MSC response to pH levels found in degenerating intervertebral discs. Biochem Biophys Res Commun. 2009;379:824–9. doi: 10.1016/j.bbrc.2008.12.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan SC, Bürki A, Bonél HM, et al. Papain-induced in vitro disc degeneration model for the study of injectable nucleus pulposus therapy. Spine J. 2013;13:273–83. doi: 10.1016/j.spinee.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 78.Malonzo C, Chan SCW, Kabiri A, et al. A papain-induced disc degeneration model for the assessment of thermo-reversible hydrogel-cells therapeutic approach. Journal of Tissue Engineering and Regenerative Medicine. 2013 doi: 10.1002/term.1667. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 79.Peroglio M, Eglin D, Benneker LM, et al. Thermoreversible hyaluronan-based hydrogel supports in vitro and ex vivo disc-like differentiation of human mesenchymal stem cells. Spine J. 2013;13:1627–39. doi: 10.1016/j.spinee.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 80.Mwale F, Wang HT, Roughley P, et al. Link N and mesenchymal stem cells can induce regeneration of the early degenerate intervertebral disc. Tissue Eng Part A. 2014;20:2942–9. doi: 10.1089/ten.tea.2013.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serigano K, Sakai D, Hiyama A, et al. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28:1267–75. doi: 10.1002/jor.21147. [DOI] [PubMed] [Google Scholar]
- 82.Omlor GW, Nerlich AG, Lorenz H, et al. Injection of a polymerized hyaluronic acid/collagen hydrogel matrix in an in vivo porcine disc degeneration model. Eur Spine J. 2012;21:1700–8. doi: 10.1007/s00586-012-2291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collin EC, Grad S, Zeugolis DI, et al. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials. 2011;32:2862–70. doi: 10.1016/j.biomaterials.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 84.Cheng YH, Yang SH, Lin FH. Thermosensitive chitosan-gelatin-glycerol phosphate hydrogel as a controlled release system of ferulic acid for nucleus pulposus regeneration. Biomaterials. 2011;32:6953–61. doi: 10.1016/j.biomaterials.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 85.Hu J, Chen B, Guo F, et al. Injectable silk fibroin/polyurethane composite hydrogel for nucleus pulposus replacement. J Mater Sci Mater Med. 2012;23:711–22. doi: 10.1007/s10856-011-4533-y. [DOI] [PubMed] [Google Scholar]
- 86.Likhitpanichkul M, Dreischarf M, Illien-Junger S, et al. Fibrin-genipin adhesive hydrogel for annulus fibrosus repair: performance evaluation with large animal organ culture, in situ biomechanics, and in vivo degradation tests. Eur Cell Mater. 2014;28:25–38. doi: 10.22203/ecm.v028a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guterl CC, Torre OM, Purmessur D, et al. Characterization of Mechanics and Cytocompatibility of Fibrin-Genipin Annulus Fibrosus Sealant with the Addition of Cell Adhesion Molecules. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2012.0714. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pereira CL, Gonçalves RM, Peroglio M, et al. The effect of hyaluronan-based delivery of stromal cell-derived factor-1 on the recruitment of MSCs in degenerating intervertebral discs. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.06.017. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 89.Mern DS, Beierfuß A, Thomé C, et al. Enhancing human nucleus pulposus cells for biological treatment approaches of degenerative intervertebral disc diseases: a systematic review. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1583. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 90.Sakai D, Grad S. Advancing the Cellular and Molecular Therapy for Intervertebral Disc Disease. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.06.009. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 91.Bucher C, Gazdhar A, Benneker LM, et al. Nonviral Gene Delivery of Growth and Differentiation Factor 5 to Human Mesenchymal Stem Cells Injected into a 3D Bovine Intervertebral. Disc Organ Culture System Stem Cells International. 2013;2013:326828. doi: 10.1155/2013/326828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wallach CJ, Kim JS, Sobajima S, et al. Safety assessment of intradiscal gene transfer: a pilot study. Spine J. 2006;6:107–12. doi: 10.1016/j.spinee.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Clarke LE, McConnell JC, Sherratt MJ, et al. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014;16:R67. doi: 10.1186/ar4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pirvu T, Blanquer SB, Benneker LM, et al. A combined biomaterial and cellular approach for annulus fibrosus rupture repair. Biomaterials. 2015;42:11–9. doi: 10.1016/j.biomaterials.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 95.Krock E, Rosenzweig DH, Haglund L. The Inflammatory Milieu of the Degenerate Disc: is Mesenchymal Stem Cell-Based Therapy for Intervertebral Disc Repair a Feasible Approach? Curr Stem Cell Res Ther. 2015 doi: 10.2174/1574888x10666150211161956. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 96.Parolin M, Gawri R, Mwale F, et al. Development of a whole disc organ culture system to study human intervertebral disc. Evid Based Spine Care J. 2010;1:67–8. doi: 10.1055/s-0028-1100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barbir A, Godburn KE, Michalek AJ, et al. Effects of Torsion on Intervertebral Disc Gene Expression and Biomechanics, Using a Rat Tail Model. Spine (Phila Pa 1976) 2010;36:607–614. doi: 10.1097/BRS.0b013e3181d9b58b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan SC, Ferguson SJ, Wuertz K, et al. Biological response of the intervertebral disc to repetitive short term cyclic torsion. Spine (Phila Pa 1976) 2011;36:2021–30. doi: 10.1097/BRS.0b013e318203aea5. [DOI] [PubMed] [Google Scholar]
- 99.Boubriak OA, Watson N, Sivan SS, et al. Factors regulating viable cell density in the intervertebral disc: blood supply in relation to disc height. J Anat. 2013;222:341–8. doi: 10.1111/joa.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maroudas A, Stockwell RA, Nachemson A, et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–30. [PMC free article] [PubMed] [Google Scholar]
- 101.Kato F, Iwata H, Mimatsu K, et al. Experimental chemonucleolysis with chondroitinase ABC. Clin Orthop Relat Res. 1990:301–8. [PubMed] [Google Scholar]
- 102.Fry TR, Eurell JC, Johnson AL, et al. Radiographic and histologic effects of chondroitinase ABC on normal canine lumbar intervertebral disc. Spine (Phila Pa 1976) 1991;16:816–9. doi: 10.1097/00007632-199107000-00022. [DOI] [PubMed] [Google Scholar]
- 103.Sugimura T, Kato F, Mimatsu K, et al. Experimental chemonucleolysis with chondroitinase ABC in monkeys. Spine (Phila Pa 1976) 1996;21:161–5. doi: 10.1097/00007632-199601150-00001. [DOI] [PubMed] [Google Scholar]
- 104.Ishikawa H, Nohara Y, Miyauti S. Action of chondroitinase ABC on epidurally transplanted nucleus pulposus in the rabbit. Spine (Phila Pa 1976) 1999;24:1071–6. doi: 10.1097/00007632-199906010-00005. [DOI] [PubMed] [Google Scholar]
- 105.Sakuma M, Fujii N, Takahashi T, et al. Effect of chondroitinase ABC on matrix metalloproteinases and inflammatory mediators produced by intervertebral disc of rabbit in vitro. Spine. 2002;27:576–80. doi: 10.1097/00007632-200203150-00004. [DOI] [PubMed] [Google Scholar]
- 106.Hoogendoorn RJ, Wuisman PI, Smit TH, et al. Experimental intervertebral disc degeneration induced by chondroitinase ABC in the goat. Spine (Phila Pa 1976) 2007;32:1816–25. doi: 10.1097/BRS.0b013e31811ebac5. [DOI] [PubMed] [Google Scholar]
- 107.Hoogendoorn R, Doulabi BZ, Huang CL, et al. Molecular changes in the degenerated goat intervertebral disc. Spine. 2008;33:1714–21. doi: 10.1097/BRS.0b013e31817d2468. [DOI] [PubMed] [Google Scholar]
- 108.Roberts S, Menage J, Sivan S, et al. Bovine explant model of degeneration of the intervertebral disc. BMC Musculoskelet Disord. 2008;9:24. doi: 10.1186/1471-2474-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gawri R, Moir J, Ouellet J, et al. Physiological Loading Can Restore the Proteoglycan Content in a Model of Early IVD Degeneration. PLoS One. 2014;9:e101233. doi: 10.1371/journal.pone.0101233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Furtwängler T, Chan SC, Bahrenberg G, et al. Assessment of the Matrix Degenerative Effects of MMP-3, ADAMTS-4 and HTRA1 injected into a bovine Intervertebral Disc Organ Culture Model. Spine (Phila Pa 1976) 2013;38:E1377–87. doi: 10.1097/BRS.0b013e31829ffde8. [DOI] [PubMed] [Google Scholar]
- 111.Tiaden AN, Klawitter M, Lux V, et al. A detrimental role for human high temperature requirement serine protease A1 (HTRA1) in the pathogenesis of intervertebral disc (IVD) degeneration. J Biol Chem. 2012;287:21335–45. doi: 10.1074/jbc.M112.341032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lü DS, Shono Y, Oda I, et al. Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine (Phila Pa 1976) 1997;22:1828–34. doi: 10.1097/00007632-199708150-00006. discussion 1834-5. [DOI] [PubMed] [Google Scholar]
- 113.Park JS, Ahn JI. The effect of chondroitinase ABC on rabbit intervertebral disc. Radiological, histological and electron microscopic findings. Int Orthop. 1995;19:103–9. doi: 10.1007/BF00179970. [DOI] [PubMed] [Google Scholar]
- 114.Watanabe A, Benneker LM, Boesch C, et al. Classification of intervertebral disk degeneration with axial T2 mapping. AJR Am J Roentgenol. 2007;189:936–42. doi: 10.2214/AJR.07.2142. [DOI] [PubMed] [Google Scholar]
- 115.Purmessur D, Walter BA, Roughley PJ, et al. A role for TNFα in intervertebral disc degeneration: a non-recoverable catabolic shift. Biochem Biophys Res Commun. 2013;433:151–6. doi: 10.1016/j.bbrc.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Korecki CL, Costi JJ, Iatridis JC. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine (Phila Pa 1976) 2008;33:235–41. doi: 10.1097/BRS.0b013e3181624504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vadalà G, Russo F, Pattappa G, et al. The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine (Phila Pa 1976) 2013;38:E319–24. doi: 10.1097/BRS.0b013e318285bc4a. [DOI] [PubMed] [Google Scholar]
- 118.Peroglio M, Grad S, Mortisen D, et al. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur Spine J. 2012;21(Suppl 6):839–49. doi: 10.1007/s00586-011-1976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Z, Kaplan KM, Wertzel A, et al. Biomimetic fibrinhyaluronan hydrogels for nucleus pulposus regeneration. Regen Med. 2014;9:309–26. doi: 10.2217/rme.14.5. [DOI] [PubMed] [Google Scholar]
- 120.Dudli S, Haschtmann D, Ferguson SJ. Fracture of the vertebral endplates, but not equienergetic impact load, promotes disc degeneration in vitro. J Orthop Res. 2011;30:809–16. doi: 10.1002/jor.21573. [DOI] [PubMed] [Google Scholar]
- 121.Alkhatib B, Rosenzweig DH, Krock E, et al. Acute mechanical injury of the human intervertebral disc: link to degeneration and pain. Eur Cell Mater. 2014;28:98–110. doi: 10.22203/ecm.v028a08. discussion 110-1. [DOI] [PubMed] [Google Scholar]
- 122.Gawri R, Antoniou J, Ouellet J, et al. Best paper NASS 2013: link-N can stimulate proteoglycan synthesis in the degenerated human intervertebral discs. Eur Cell Mater. 2013;26:107–19. doi: 10.22203/ecm.v026a08. discussion 119. [DOI] [PubMed] [Google Scholar]
- 123.Ariga K, Yonenobu K, Nakase T, et al. Mechanical stress-induced apoptosis of endplate chondrocytes in organ-cultured mouse intervertebral discs: an ex vivo study. Spine (Phila Pa 1976) 2003;28:1528–33. [PubMed] [Google Scholar]
- 124.Korecki CL, MacLean JJ, Iatridis JC. Characterization of an in vitro intervertebral disc organ culture system. Eur Spine J. 2007;16:1029–37. doi: 10.1007/s00586-007-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Illien-Jünger S, Pattappa G, Peroglio M, et al. Homing of Mesenchymal Stem Cells in Induced Degenerative Intervertebral Discs in a Whole Organ Culture System. Spine (Phila Pa 1976) 2012;37:1865–73. doi: 10.1097/BRS.0b013e3182544a8a. [DOI] [PubMed] [Google Scholar]
- 126.Haschtmann D, Stoyanov JV, Gédet P, et al. Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J. 2008;17:289–99. doi: 10.1007/s00586-007-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haschtmann D, Stoyanov JV, Ettinger L, et al. Establishment of a novel intervertebral disc/endplate culture model: analysis of an ex vivo in vitro whole-organ rabbit culture system. Spine. 2006;31:2918–25. doi: 10.1097/01.brs.0000247954.69438.ae. [DOI] [PubMed] [Google Scholar]
- 128.van Dijk B, Potier E, Ito K. Culturing Bovine Nucleus Pulposus Explants by Balancing Medium Osmolarity. Tissue Eng Part C Methods. 2011;17:1089–96. doi: 10.1089/ten.TEC.2011.0215. [DOI] [PubMed] [Google Scholar]
- 129.Risbud MV, Izzo MW, Adams CS, et al. An organ culture system for the study of the nucleus pulposus: description of the system and evaluation of the cells. Spine. 2003;28:2652–2658. doi: 10.1097/01.BRS.0000099384.58981.C6. [DOI] [PubMed] [Google Scholar]
- 130.Risbud MV, Di Martino A, Guttapalli A, et al. Toward an optimum system for intervertebral disc organ culture: TGF-beta3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine. 2006;31:884–90. doi: 10.1097/01.brs.0000209335.57767.b5. [DOI] [PubMed] [Google Scholar]
- 131.Chiba K, Masuda K, Andersson GB, et al. Matrix replenishment by intervertebral disc cells after chemonucleolysis in vitro with chondroitinase ABC and chymopapain. Spine J. 2007;7:694–700. doi: 10.1016/j.spinee.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 132.Yerramalli CS, Chou AI, Miller GJ, et al. The effect of nucleus pulposus crosslinking and glycosaminoglycan degradation on disc mechanical function. Biomech Model Mechanobiol. 2007;6:13–20. doi: 10.1007/s10237-006-0043-0. [DOI] [PubMed] [Google Scholar]
- 133.Iatridis JC, Nicoll SB, Michalek AJ, et al. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13:243–62. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Michalek AJ, Funabashi KL, Iatridis JC. Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur Spine J. 2010;19:2110–6. doi: 10.1007/s00586-010-1473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iatridis JC, Michalek AJ, Purmessur D, et al. Localized Intervertebral Disc Injury Leads to Organ Level Changes in Structure, Cellularity, and Biosynthesis. Cellular and Molecular Bioengineering. 2009;2:437–447. doi: 10.1007/s12195-009-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]