SUMMARY
Objective
Deficits in social cognition are common and significant in people with temporal lobe epilepsy (TLE), but the functional and structural underpinnings remain unclear. The present study investigated how the side of seizure focus impacts face processing networks in temporal lobe epilepsy.
Methods
We used functional magnetic resonance imaging (fMRI) of a face processing paradigm to identify face responsive regions in 24 individuals with unilateral temporal lobe epilepsy (Left = 15; Right = 9) and 19 healthy controls. fMRI signals of face responsive regions ispilateral and contralateral to the side of seizure onset were delineated in TLE and compared to the healthy controls with right and left side combined. Diffusion tensor images were acquired to investigate structural connectivity between face regions that differed in fMRI signals between the two groups.
Results
In temporal lobe epilepsy, activation of the cortical face processing networks varied according to side of seizure onset. In temporal lobe epilepsy, the laterality of amygdala activation was shifted to the side contralateral to the seizure focus while controls showed no significant asymmetry. Furthermore, compared to controls, patients with TLE showed decreased activation of the occipital face responsive region in the ipsilateral side and an increased activity of the anterior temporal lobe in the contralateral side to the seizure focus. Probabilistic tractography revealed that the occipital face area and anterior temporal lobe are connected via the inferior longitudinal fasciculus, which in individuals with temporal lobe epilepsy showed reduced integrity.
Significance
Taken together, these findings suggest that brain function and white matter integrity of networks subserving face processing are impaired on the side of seizure onset, accompanied by altered responses on the side contralateral to the seizure.
Keywords: Face processing, Temporal lobe epilepsy, Inferior longitudinal fasciculus, Brain network alteration
1. INTRODUCTION
Facial expressions provide important socially meaningful incentives1, playing an important role in behavior during interpersonal exchanges. The study of interactions involving face perception not only constitute a central approach to understanding social functions in the human brain 2, but also provide important insights into more general mechanisms underlying basic cognitive processes 3. The cognitive abilities involved in recognition and response to socially relevant information are hypothesized to be mediated by a network of interconnected brain regions 4 which have been delineated using functional brain imaging studies.
Specifically, there exists a hierarchical organization branching from core anatomical regions for visual analysis of faces to an extended system that extracts social valence of facial expressions 5. The face-selective regions in the lateral fusiform gyrus (fusiform face area, FFA) 6 and occipital face area (OFA) in the inferior occipital gyrus 7, as well as regions in the superior temporal sulcus (STS), constitute core face processing areas responsible for early perceptions of basic facial features, identities, and changeable aspects of face (e.g., eye gaze and lip movements). This information is further integrated with biographical and emotional content in the extended face processing system including the anterior temporal lobe (ATL) and amygdala (for a review see 5).
Temporal lobe epilepsy (TLE), the most common epilepsy in adults, effects similar limbic and extra-limbic networks as those involved in face processing. Specifically, the most frequently identified epileptogenic regions in TLE, the temporal lobe and associated limbic structures, have direct connections to brain networks involved in face processing including fusiform gyri and extrastriate occipital lobe 8. Thus, recurrent seizures associated with TLE may alter these overlapping brain networks 9 and contribute to abnormal processing of faces. For example, individuals with TLE have difficulties recognizing fearful facial expressions, with the magnitude of deficits similar to patients with structural damage to these brain regions 10. These deficits may lead to abnormal social cognition and contribute to the anxiety, depression, and poor quality of life that have commonly been associated with TLE 11. Therefore, understanding if and how brain networks that mediate face processing are affected by TLE can provide insight into the pathophysiology of social problems found in individuals with this condition, such as difficulties with peer-to-peer interactions, independent living, and employment12 .
In this study, we examined the possibility that the overlap between the circuitry affected by TLE and the networks involved in face processing may contribute to epilepsy-provoked disruption of processing of stimuli with emotional content. We tested the hypothesis that the presence of epilepsy as well as the side of seizure onset will influence functional and structural networks of face processing in individuals with pharmacoresistant TLE compared to healthy controls. We used a multimodal approach including functional MRI and diffusion tensor imaging (DTI) to ask the following questions: 1) How are the core and extended face processing regions differentially impacted by the side of seizure onset; and 2) For those face processing regions that are altered in TLE, are the white matter tracts connecting these regions also impacted?
2. METHODS
2.1 Human Subjects
This study was reviewed and approved by the Institutional Review Boards of the University of California Irvine. All subjects gave their written informed consents prior to being included in this study. All procedures were consistent with the Declaration of Helsinki 13.
2.2. Participants
We recruited 24 individuals with pharmacoresistant TLE (seizure onset: Left = 15; Right = 9; Age = 37.4±2.4 years; female: 13) through the University of California Irvine Medical Center and 19 healthy controls (HC, 31.8±2.7 years; female: 10) from the surrounding community. Age and gender were not significantly different between the two groups (age: p= .13; ANOVA; gender: p= .92; Chi-square). Entry criteria for subjects included the ability to undergo an MRI, plus a diagnosis of unilateral pharmacoresistant TLE 14. This diagnosis was made by board-certified neurologists with expertise in epileptology (JJL) according to the criteria defined by the International League Against Epilepsy based on seizure semiology, video EEG, and neuroimaging. Where clinically indicated, subjects underwent phase II intracranial EEG monitoring where unilateral temporal onset was confirmed. The exclusion criteria included 1) evidence of extratemporal epilepsy, demonstrated either with brain MRI or EEG, 2) evidence of brain abnormalities that might confound normal brain development such as a) cognitive impairment (I.Q. < 70), b) history of hypoxic ischemic encephalopathy, c) intrauterine exposure to recreational drugs or alcohol, and 3) psychiatric history of chronic psychosis or suicidality. Control subjects were recruited through response to advertisements. Criteria for control subjects included 1) ability to undergo MRI, 2) no family member with epilepsy, 3) no history of a loss of consciousness, and 4) no neurological or psychiatric diagnoses. Participant characteristics can be viewed in Table I.
Table 1.
Patient Characteristics
| Patient | Age (years)/Gender | Handedness | Seizure Focus | Age at onset (years) | Duration of epilepsy (years) | Clinical MRI |
|---|---|---|---|---|---|---|
| 1 | 43/M | R | L | 20 | 23 | HS |
| 2 | 53/F | R | L | 1 | 51 | HS |
| 3 | 44/M | R | L | 26 | 27 | HS |
| 4 | 58/F | R | L | 9 | 49 | HS |
| 5 | 21/F | R | L | 16 | 5 | HS |
| 6 | 46/M | R | R | 44 | 2 | HS |
| 7 | 50/M | R | R | 6 | 44 | HS |
| 8 | 46/F | R | R | 17 | 29 | HS |
| 9 | 34/M | R | R | 25 | 9 | HS |
| 10 | 29/F | R | R | 14 | 15 | HS |
| 11 | 19/F | R | L | 7 | 12 | Normal |
| 12 | 24/F | R | L | 14 | 11 | Normal |
| 13 | 46/M | R | L | 36 | 9 | Normal |
| 14 | 30/M | R | L | 26 | 4 | Normal |
| 15 | 51/F | R | L | 5 | 46 | Normal |
| 16 | 28/M | R | L | 17 | 10 | Normal |
| 17 | 30/F | R | L | 2 | 26 | Normal |
| 18 | 21/F | R | L | 8 | 12 | Normal |
| 19 | 39/F | R | L | 36 | 3 | Normal |
| 20 | 31/M | R | L | 30 | 1 | Normal |
| 21 | 47/F | R | R | 10 | 38 | Normal |
| 22 | 55/M | R | R | 15 | 40 | Normal |
| 23 | 23/M | R | R | 21 | 2 | Normal |
| 24 | 29/F | R | R | 1 | 28 | Normal |
2.3. MRI Acquisition
MRI data was acquired using a 3-T scanner (Achieva, Philips Medical Systems, Best, The Netherlands) using an 8 channel head coil. Functional MR images were acquired using an echo-planar T2*-weighted sequence of 12 coronal slices oriented orthogonal to the hippocampal formation, 5 mm slice thickness with interslice gap of 1 mm, TR 1490 ms, TE 60 ms, 90 degree flip angle, FOV = 250 mm, matrix 64 x 64 (voxel 3.9 x 3.9 x 5 mm). DTI data were obtained using an echo planar sequence with the following parameters: TR = 11194 ms, TE = 55 ms, 60 axial slices, acquisition matrix = 112 mm × 110 mm (FOV = 224 mm), 64 diffusion directions with a b value of 800 seconds/mm2. Two sets of DTI images were averaged to increase signal-to-noise ratio (SNR). Anatomical images were collected using a coronal T1-weighted MP-RAGE sequence, slice thickness of 1mm, TR of 8.4 ms, TE of 3.7 ms, flip angle of 8 degrees, SENSE factor of 2.4.
2.4. fMRI Stimuli
We used a face paradigm provided by Schacher and colleagues 15 to map face-sensitive regions in each participant. This is a block design of 8 activation blocks (silent video of dynamic fearful face) and 8 baseline blocks (silent video of dynamic landscape), each lasting 24 s. This paradigm was chosen because it has been previously shown to elicit greater levels of activation than static faces16 and is effective at lateralizing activation to the side of seizure focus17, which was the focus of the current study. Prior to entering the scanner, subjects were informed of the content of the video, and were instructed to focus on the actors’ eyes.
2.5. Functional MRI processing and statistical analysis
Functional images were analyzed with the general linear model 18 for block designs using Statistical Parametric Mapping (SPM8; Wellcome Department of Imaging Neuroscience, London, UK) in the Matlab environment (Mathworks, Natick, MA, USA). All images were unwarped, corrected for slice timing, segmentation-based normalized to MNI space, spatially smoothed full-width at half-maximum 10 mm Gaussian kernel), and high-pass filtered (cutoff 1/120 Hz). Movement analysis and correction were performed on all functional images with the Artifact Detection Tool (ART) to identify motion outliers. Image outliers (> 2mm) were excluded for both linear motion parameters (X, Y, Z) in mm as well as rotational (angular) motion parameters (roll, pitch, yaw) in radians as a function of time.
First level analysis of functional MRI data was specified with a boxcar regression for the 1) faces and 2) places conditions. Parameter estimates for the general linear model were then generated at each voxel of every participant. Contrast images for faces > places were calculated to reveal neural activations related to facial processing. In each ANOVA, clusterwise significance was determined at the level of p < 0.05 corrected for family-wise error (FWE), following voxelwise thresholding p < 0.001 (uncorrected).
For each individual, we selected regions of activation previously shown to be involved in face processing, namely anterior temporal lobe (ATL), superior temporal sulcus (STS), occipital face area (OFA), fusiform face area (FFA), and amygdala (AMYG) 5. Within these regions, we calculated the % signal change from the peak activation voxel specific to each individual (in MNI space), a well-established procedure similar to previous studies of face processing 6, 19, consistent with other fMRI studies using ROI approaches, to examine face processing networks19-22. In a few instances where there was no significant activation for one of these regions, the voxel located at the coordinates of the respective group (TLE or control) results were used, as also employed in other studies 22.
As we were interested in the impact of side of seizure onset on face processing, the data from patients with TLE was classified as either ipsilateral or contralateral to the side of seizure focus. Because no differences were noted in the % signal change between the right and left hemisphere for any of the five ROIs in healthy controls, the signals were averaged for control subjects. A three-way (ipsilateral peak activation vs. contralateral peak activation vs. healthy control average) ANOVA was performed to identify group differences in % signal change of peak voxel activation within the five ROIs. Statistical differences were further subjected to post-hoc t-tests.
Previous work by Schacher and colleagues using this paradigm demonstrated that amygdala activation is significantly decreased on the side of seizure onset in TLE. We examined the reproducibility of the paradigm in our cohort by calculating a laterality index (LI) 23 based on Schacher and colleagues’ method of counting the number of significant activated voxels (P < 0.05, FWE corrected) within the amygdala ROIs with the following formula: LI = (right - left)/(right + left) 15.
2.7. Diffusion tensor imaging processing and statistical analysis
DTI processing and analysis were performed using FMRIB's Diffusion Toolbox (FDT Version 2.0) 24, part of FSL (FMRIB, FSL Version 4.1.5, http://www.fmrib.ox.ac.uk/fsl). First, the two sets of DTI images were corrected for eddy current distortions and head motion artifacts using an affine registration to the first scan's b0 volume, and then were averaged to increase SNR. FSL's BEDPOSTX program was then used to generate probability distributions of diffusion parameters at each voxel, including modeling for diffusion of crossing fibers along two directions.
To examine whether functional differences between TLE and controls are mediated through known anatomical connections, probabilistic tractography was conducted between fMRI regions of interest that exhibited significant group differences between TLE and controls. We used methodology outlined in Blank and colleagues 22 to establish the seed regions used for probabilistic tractography. Specifically, we transferred each subject's individual coordinates of peak voxel of functional activation as described above for each region into the individual subject's DTI space, using FMRIB's Linear Image Registration Tool (FLIRT). To ensure that only white matter voxels were tracked, the transformed coordinates were moved to the nearest point of gray / white junction based on fractional anisotropy maps (FA >0.25) and a 5mm sphere radius was centered on these coordinates. These spheres were then used as the seed points for probabilistic tractograpy. We used standard parameters from FSL's PROBTRACKX module for DTI probabilistic tractography (5000 streamline samples per seed voxel, step length = 0.5 mm, curvature threshold = 0.2). For each subject, fiber tracing was performed between ROIs in both directions, originating from one ROI with the other ROI acting as a waypoint. Results from each direction were averaged together and then transformed into MNI space. All tracts were then summed, and the resultant group tract was conservatively thresholded to include only those voxels common to at least 95% of the robust range of values, equal to approximately 66% of subjects. This group map was then transformed back into native space for each subject and mean fractional anisotropy (FA) was determined. The Johns Hopkins University White Matter Atlas was used as an anatomical template to characterize the identity of the white matter tracts 25. Ipsilateral and contralateral FA values of this tract in TLE were then compared to the average FA values of this tract in healthy controls using a two-sample t-test. Significance level was set at 0.025, to account for multiple comparisons (using Bonferroni correction). Mean FA of the white matter tract was correlated with percent signal change of face-responsive regions using Spearman's rho.
2.8. Clinical characteristics and statistical analysis
We also evaluated whether clinical characteristics of epilepsy were associated with fMRI/DTI signals. We correlated amygdala LI, % signal change of face processing peak voxel activation, and FA of the white matter tract with epilepsy duration and age of seizure onset. Correlation analyses were performed using Spearman's rho. To control for type 1 errors, only correlations with a p value <0.01 were considered significant.
As this study includes individuals with TLE both with and without hippocampal sclerosis, we further investigated if % signal change of peak voxel activation were influenced by hippocampal volumes. Hippocampal volumes were calculated from segmentation of T1-weighted anatomical images, using the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). The accuracy of hippocampal segmentation and was confirmed by visual inspection 26. Volumes of left and right hippocampus were then corrected for TIV and correlated with % signal change of peak voxel activation for all ROIs using Pearson product-moment correlation.
3. RESULTS
3.1. Brain activity during face processing
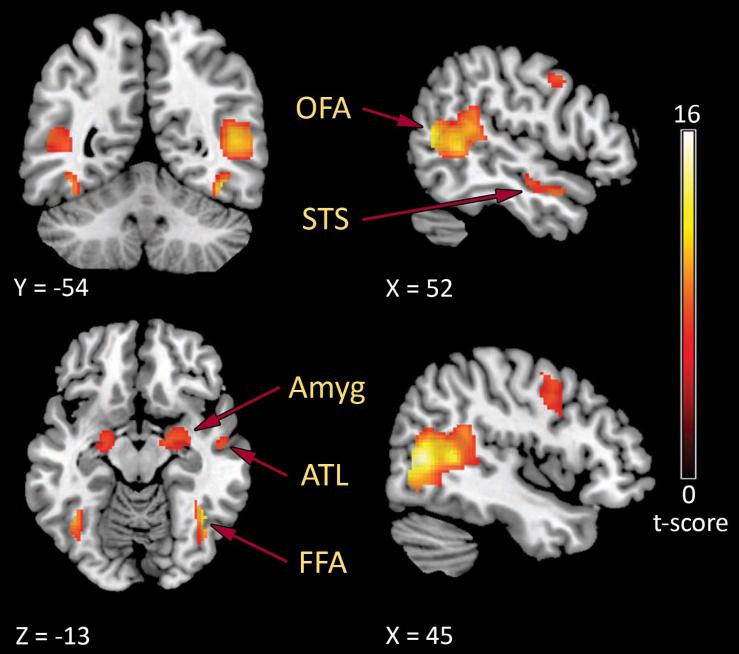
To identify brain activity in the core and extended face processing network, we contrasted fMRI signals between face and landscape in all participants (TLE and controls). Processing of faces led to significant activity (p < 0.05 FWE cluster corrected) in face-responsive regions (Figure 1) including the right amygdala (peak activation: x = 26, y = -2, z = -20), the left STS (peak activation: x = -54, y = -50, z = 10), the right OFA (peak activation: x = 46, y = -66, z = 6), and the right FFA (peak activation: x = 42, y = -56, z = -14) (see Supplemental Material Table 1). At the individual level, face-responsive regions could be localized in a majority of participants, with signal change values similar to previous studies 19,22. As we were also interested in how patterns of brain activity differed between individuals with TLE and healthy controls, we next identified regions of face processing that showed greater activation in healthy controls than in RTLE and LTLE, as well as regions of greater activity in RTLE and LTLE than in healthy controls (Table 2). In general, the TLE groups activated less cortical face processing regions but recruited greater subcortical brain regions than healthy controls.
Figure 1.
Group activation maps of face-responsive regions overlayed on MNI 152 standard space brain. Functional MRI activations demonstrates fearful face-responsive regions (face > place) in a distributed network including the occipital face area (OFA), fusiform face area (FFA), posterior superior temporal sulcus (STS), anterior temporal lobe (ATL), and the amygdala (Amyg) (one sample t-test at the second (random effects) level, p < 0.05 FWE corrected). Values on color bar = t-scores.
Table 2.
List of clusters showing significantly greater facial processing brain activity when comparing healthy controls to RTLE or LTLE.
| Face > Place peak location | Side | x | y | z | t value |
|---|---|---|---|---|---|
| HC > RTLE | |||||
| Cluster 1: 2306 voxels | |||||
| Mid occipital gyrus (BA 37) | R | 46 | −66 | 6 | 12.1 |
| Lingual gyrus (BA 18) | R | 40 | −74 | −4 | 10.3 |
| Sup temporal gyrus (BA 39) | R | 44 | −50 | 8 | 9.53 |
| Cluster 2: 1231 voxels | |||||
| Mid occipital gyrus (BA 19) | L | −40 | −68 | 10 | 10.65 |
| Fusiform gyrus (BA 37) | L | −42 | −58 | −14 | 9.02 |
| Mid temporal gyrus (BA 21) | L | −54 | −48 | 10 | 8.11 |
| Cluster 3: 538 voxels | |||||
| Amygdala | R | 30 | −6 | −16 | 7.58 |
| Lentiform Nucleus | R | 22 | −8 | −14 | 7.48 |
| Cluster 4: 597 voxels | |||||
| Mid frontal gyrus (BA 6) | R | 48 | 6 | 44 | 7.01 |
| Mid frontal gyrus (BA 6) | R | 40 | 2 | 54 | 5.72 |
| Mid frontal gyrus (BA 6) | R | 34 | 0 | 40 | 4.36 |
| Cluster 5: 955 voxels | |||||
| Thalamus | L | −6 | −30 | −8 | 6.26 |
| Thalamus | R | 10 | −28 | −4 | 6.24 |
| Amygdala | L | −20 | −8 | −16 | 6.07 |
| Cluster 6: 205 voxels | |||||
| Sup temporal gyrus (BA 22) | R | 54 | −10 | −14 | 5.68 |
| Sup temporal gyrus (BA 38) | R | 50 | 8 | −22 | 5.66 |
| Cluster 7: 197 voxels | |||||
| Precuneus | R | 28 | −42 | 50 | 5.43 |
| Cluster 8: 326 voxels | |||||
| Mid temporal gyrus (BA 46) | L | −44 | 18 | 16 | 5.34 |
| Inf frontal gyrus (BA 9) | L | −36 | 12 | 24 | 5.14 |
| HC > LTLE | |||||
| Cluster 1: 4133 voxels | |||||
| Inf temporal gyrus | R | 48 | −72 | 2 | 13.0 |
| Sup temporal gyrus (BA 13) | R | 56 | −42 | 16 | 9.4 |
| Fusiform gyrus (BA 37) | R | 40 | −52 | −14 | 7.65 |
| Cluster 2: 2648 voxels | |||||
| Mid temporal gyrus (BA 37) | L | −46 | −68 | 12 | 11.65 |
| Inf temporal gyrus | L | −46 | −76 | 4 | 10.8 |
| Cerebellum | L | −40 | −58 | −16 | 8.17 |
| Cluster 3: 511 voxels | |||||
| Mid frontal gyrus | R | 46 | 4 | 44 | 7.31 |
| Cluster 4: 1722 voxels | |||||
| Thalamus | L | −8 | −28 | −6 | 6.79 |
| Amygdala | L | −20 | −10 | −14 | 6.54 |
| Amygdala | R | 20 | −6 | −18 | 6.41 |
| Cluster 5: 388 voxels | |||||
| Precentral gyrus (BA 6) | L | −36 | 12 | 20 | 4.95 |
| Precentral gyrus (BA 6) | L | −44 | 4 | 26 | 4.0 |
| Insula (BA 13) | L | −36 | 18 | 10 | 3.54 |
| RTLE > HC | |||||
| Cluster 1: 183 voxels | |||||
| Globus Pallidus | L | −16 | −8 | −10 | 5.01 |
| LTLE > HC | |||||
| Cluster 1: 2550 voxels | |||||
| Inf temporal gyrus | R | 50 | −74 | 2 | 10.05 |
| Mid temporal gyrus (BA 18) | R | 36 | −84 | −4 | 7.88 |
| Fusiform gyrus (BA 37) | R | 38 | −52 | −16 | 6.64 |
| Cluster 2: 309 voxels | |||||
| Hypothalamus | L | −6 | −8 | −8 | 5.36 |
| Globus Pallidus | L | −16 | −10 | −8 | 4.58 |
| Thalamus | L | −10 | −14 | 2 | 4.08 |
| Cluster 3: 358 voxels | |||||
| Putamen | R | 20 | −8 | −14 | 5.28 |
| Cuneus (BA 18) | R | 32 | −8 | −14 | 3.81 |
| Cluster 4: 251 voxels | |||||
| Thalamus | R | 14 | −6 | 16 | 4.8 |
| Thalamus | R | 6 | −12 | 6 | 4.5 |
| Caudate | R | 12 | 4 | 8 | 3.72 |
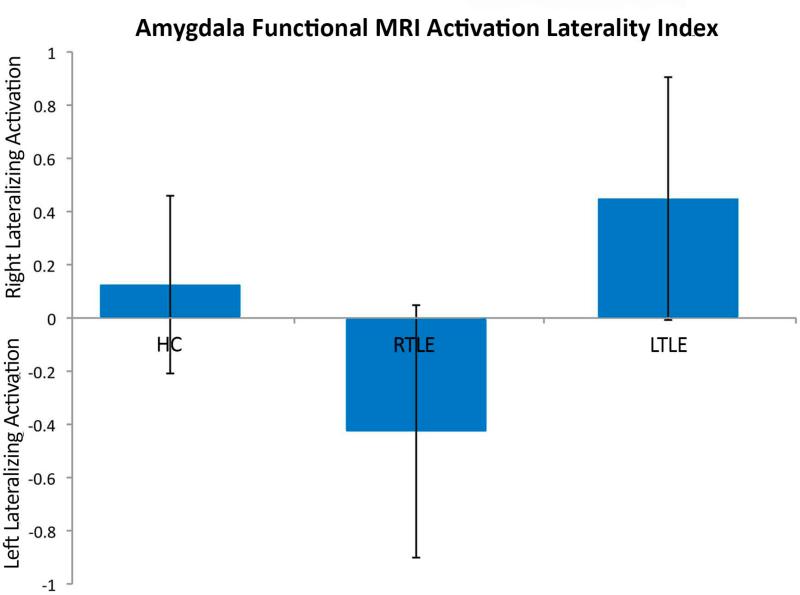
3.2. Amygdala Laterality Index
The amygdala laterality index (LI) lateralized to the side of seizure onset in TLE, replicating the findings of Schacher and colleagues. A positive LI indicates right hemisphere lateralized activation, while negative numbers indicate left hemisphere lateralized activation. There was a significant main effect among the three groups (F(2,40) = 13.76; P < 0.0001); post hoc t-tests show that the control group's LI (0.09 ± 0.08) was significantly different from both the TLE group with right-sided seizure focus (-0.43 ± 0.16; p < 0.01) as well as the TLE group with left-sided seizure focus (0.49 ± 0.12; p < 0.001). Thus, in individuals with TLE, regardless of whether seizures originated from the right or left side, the laterality of amygdala activation was consistently shifted to the side contralateral to the seizure focus (Figure 2).
Figure 2.
Amygdala fMRI laterality index (LI). Positive LI indicating right hemisphere lateralized activation, and negative LI indicating left hemisphere lateralized activation. Individuals with TLE demonstrated a shift of amygdala activation to the side contralateral to the seizure focus in both right and left TLE.
3.3. Altered brain activity in TLE individuals
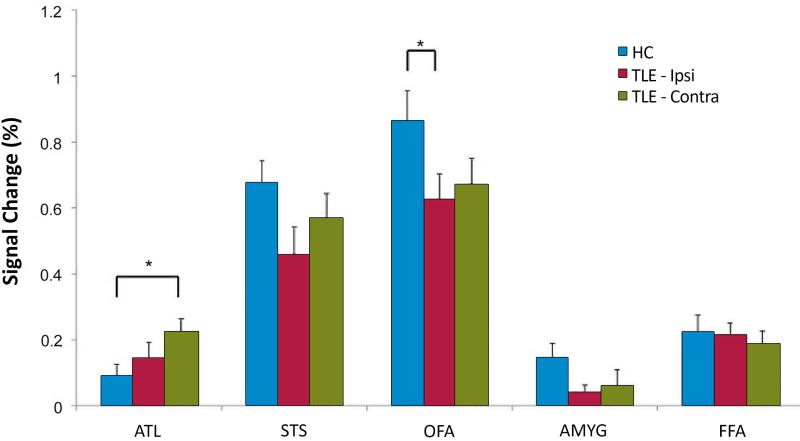
The fMRI analyses for face-responsive ROIs were performed according to the side of seizure onset (ipsilateral vs. contralateral) in individuals with TLE. Significant main effects were observed for group (i.e., ipsilateral peak activation vs. contralateral peak activation vs. healthy control average; F2,64 = 6.7; p < 0.001) and ROI (F4,61 = 2.9; p = 0.001). Post-hoc univariate comparisons among groups demonstrated TLE subjects had significantly decreased activation in the ipsilateral OFA (p < 0.02), and a trend toward decreased activation in the ipsilateral STS (p = 0.06) compared to healthy controls (Figure 3). Conversely, TLE subjects activated the contralateral ATL significantly more than healthy controls (p < 0.02). No group differences were noted for activation in either the amygdala or FFA in terms of percent signal change in peak voxel.
Figure 3.
Altered activation of face-responsive regions in TLE compared to healthy controls. Right and left TLE subjects were grouped together and analyses are performed according to the side of seizure onset (ipsilateral vs. contralateral). Individuals with TLE demonstrated significantly decreased activation in the ipsilateral occipital face area (OFA, *p < 0.02) and, a trend level decreased activation in the ipsilateral superior temporal sulcus (STS, p < 0.06) compared to healthy controls. Conversely, TLE subjects activated the contralateral anterior temporal lobe (ATL) significantly more than controls (*p < 0.02). Data are mean ± SEM.
3.4. Integrity of white matter connections vary in parallel with fMRI changes
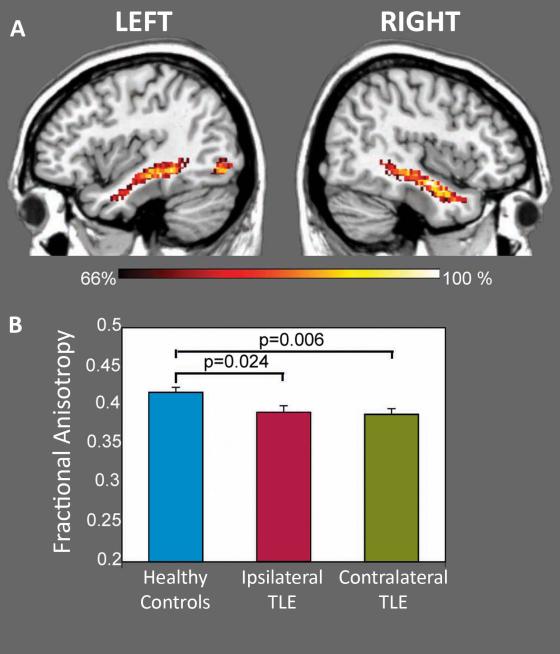
Given that the TLE group showed significantly altered brain activation in the OFA and ATL, we next examined if there is an anatomical connection between these regions, and if the integrity of this connection is altered in TLE. A single subject was excluded as an outlier because the individual's FA of this white matter tract was greater than 3 standard deviations above the group's mean (ipsilateral, contralateral: individual FA=0.74, 0.66; Group FA=0.41±0.08, 0.40±0.07). In the remaining subjects, DTI tractography produced a consistent white matter connection in each hemisphere, which in relation to an anatomical atlas, corresponded to the inferior longitudinal fasciculus (ILF) (Figure 4). The FA of the ILF, both ipsilateral and contralateral to the side of seizure onset, was significantly lower in the TLE group than controls’ ILF FA, averaged across the two hemispheres (ipsilateral p = 0.024, contralateral p = 0.006). There was no significant correlation between FA of the ILF and percent signal change of face responsive regions.
Figure 4.
White matter connectivity between face-responsive regions. Diffusion tensor imaging with probabilistic tractography delineated the inferior longitudinal fasciculus (ILF) connecting the occipital face area and anterior temporal lobe in TLE and healthy controls (top). Dark orange color indicates 66% of the group members contain these voxels and yellow color indicates 100% of the group contains these voxels within the ILF. Fractional anisotropy was significantly reduced in individuals with TLE, both ipsilateral (p = 0.024) and contralateral (p = 0.006) to side of seizure onset, compared to healthy controls (averaged across both hemispheres).
3.5 Relationship between fMRI/DTI changes, hippocampal volume and clinical characteristics
There were no significant correlations among fMRI signals, DTI indices (FA), age of seizure onset and duration of epilepsy in face processing brain regions, after correcting for multiple comparisons. Furthermore, there were no significant correlations between hippocampal volumes (corrected for TIV) and % signal change of peak voxel of activation (All p > 0.05).
4. DISCUSSION
The current study investigated functional and structural networks related to processing of faces in individuals with pharmacoresistant TLE and in healthy controls. In the subcortical regions, the laterality of the amygdala activation was shifted to the contralateral hemisphere in relation to the seizure focus, consistent with previous studies17, 27. In the cortical network, TLE subjects had decreased brain activity in the OFA, and to a lesser degree STS, ipsilateral to the side of seizure onset. On the contralateral side of the seizure focus, an unanticipated and very interesting finding was an increased ATL activation compared to healthy controls. In general, TLE subjects had greater activation in subcortical regions when viewing faces, as compared to controls; in contrast, controls subjects tended to have greater activation that TLE subjects in cortical regions. In addition, an anatomical connection within the inferior longitudinal fasciculus linked face processing regions altered in TLE (i.e. OFA and ATL), the integrity of which is also altered in TLE. Taken together, these findings suggest that brain function and white matter integrity of networks subserving face processing are impaired on the side of seizure onset, accompanied by altered responses on the side contralateral to the seizure. These results highlight the underlying neuroplasticity of TLE whereby reduced network function near the epileptogenic foci is accompanied by an altered organization of the contralateral network.
4.1. Attenuation of activation in the occipital face area ipsilateral to the side of seizure onset
The OFA forms part of the core face processing network and is thought to preferentially respond to shape and features of faces (e.g. nose, mouth) 7. The region receives early inputs from primary visual cortex to process increasingly complex shapes for further analysis in the extended face network 28. As such, the OFA likely represents the early locus for categorical encoding (i.e. face versus place) in the hierarchical face perceptual network 5. Indeed, higher-level visual-perceptual tasks (e.g. Face Recognition Test and Judgment of Line Orientation Test) are significantly impaired in individuals with TLE compared to controls 29, although low demand visual tasks are not altered 30. Structural MRI studies of individuals with TLE also consistently revealed pronounced atrophy in the extrastriate cortex (for a review see 31), a region implicated for higher-level visuospatial encoding. In line with these studies, we found that individuals with TLE showed a significantly weaker than normal activity in the OFA, ipsilateral to the side of seizure onset, during face processing.
TLE participants also showed a trend toward decreased activity in the STS, ipsilateral to the side of seizure onset. The STS is involved in the processing of dynamic aspects of faces, similar to the stimuli used in our study 7. In addition to dynamic images of eye and mouth movements, the STS also responds to static images of implied motion 32, which is important to decode human facial expression and thus the representation of emotion 33. Taken together, these findings suggest that the epileptogenic network in TLE may adversely impact face processing, with problems in part arising from deficits in both basic feature recognition (OFA) as well as dynamic aspects of movement and expression (STS).
Notably, we did not find differences in peak voxel percent signal change for the amygdala between TLE and healthy controls, a region that has been previously shown to be involved in face processing using this stimulus. This lack of a difference is likely due to our choice to use the peak voxel in our fMRI analyses. When we analyzed the activation in the amygdala, using methodology similar to Schachter et. al's study, we independently reproduced results that were in line with their findings. Specifically, TLE individuals demonstrated reduced amygdala activation on the contralateral hemisphere with respect to side of seizure onset.
Interestingly, we did not find significant group differences in percent signal change in peak voxel for the FFA, a brain region that is commonly activated in fMRI studies using a face-processing task. This discrepancy is likely due to our method of combining of right and left TLE groups for these analyses. While this approach served to focus on the impact of side of seizure onset, it may have removed the differential influences of right versus left TLE. This possibility is supported by a recent study by Labudda and colleagues using the same dynamic face paradigm27. They demonstrated that compared to healthy controls, the right TLE group showed decreased activation in the contralateral (left) FFA, while the left TLE group showed no significant differences.
4.2. Augmentation of signal in the anterior temporal lobe
In addition to the weaker than normal activity in the ipsilateral OFA and STS, individuals with TLE showed a significantly stronger activity during processing of face in the ATL contralateral to the seizure focus and nonsignificant increased activation in the ipsilateral ATL. The ATL has dense connections with a number of ‘social’ regions including limbic structures such as the amygdala and hippocampus, the prefrontal cortex, and fusiform gyrus 19. This region appears to be primarily involved in individualization of face perception 34 such as biographical information 5 and identity encoding 35. We speculate that these findings may indicate that brain responses to injury of face processing in the posterior brain areas (e.g. OFA) might include an increased reliance on the ATL, more pronounced on the contralateral side with respect to the side of seizure onset.
4.3. White matter connections between face processing regions altered in TLE
In order to test for structural underpinnings of these functional changes, we next examined the white matter tract mediating signals between regions with functional alterations in TLE; the OFA and ATL. Much of the existing data support a posterior to anterior hierarchical model in which the basic shape and components of face are extracted in the extrastriate visual cortex and sent forward to the ATL and subsequent regions such as the prefrontal and orbitofrontal lobe for further analysis. Our findings of a structural connection between these regions, the ILF, provide a conduit for this type of information transfer 36. Indeed, the ILF is likely important for visual perception and face recognition, as disconnections have lead to impaired facial processing 37. The ILF may also have an important role in processing of emotional content. Adults who witnessed domestic violence in childhood had a significantly lower FA in the left ILF, and the integrity of this white matter tract inversely correlated with depression and anxiety scores 38. Finally, in line with our study, several studies have revealed decreased FA of the ILF in individuals with TLE 39, 40. In summary, these findings imply that the epileptogenic activity may disrupt occipital-temporal anatomical connections, which may contribute to deficits in processing of visuospatial and emotional contents.
4.4. The influence of side of seizure onset on network organization
The reduction in network function and white matter integrity ipsilateral to the seizure focus, and the increase in activity in contralateral face-responsive regions relative to the benchmark of healthy controls, are consistent with findings in other brain networks described in subjects with TLE. Indeed there is now substantial evidence that seizure onset in one hemisphere influences cerebral organization in the contralateral hemisphere, including reorganization of language functions 41 with greater functional recruitment of the contralateral hemisphere, along with reduced ipsilateral and increased contralateral integrity of white matter connections 42, and increased rates of atypical (i.e. right hemisphere or bilateral) language 43. Additionally, memory reorganization is also evident in individuals with TLE, with both right and left TLE showing less hippocampal activation in the hemisphere ipsilateral to the seizure focus compared to the contralateral side, while viewing word, pictures, and face stimuli 44. However, such reorganization of language and memory do not necessarily result in effective language and memory performance, as a greater degree of contralateral hemisphere activation was associated with poorer cognitive performances 44, 45.
4.5. Limitations and future directions
The current study focused on the hypothesis that the face processing network is differentially altered in TLE, depending on the side of seizure onset. Thus, we examined functional and structural networks ipsilateral or contralateral to the seizure focus rather than analyzing right and left TLE as separate groups. The modest sample size of our study does not provide sufficient power to compare percent signal change of peak voxel in right and left TLE separately to controls, though we did note a general trend of greater activation in subcortical regions in right and left TLE, as compared to controls. We also included TLE patients with and without hippocampal sclerosis but their epileptogenic zone was rigorously determined with video EEG monitoring. Further, we did not find a correlation between hippocampal volumes and % signal change of peak voxel of activation. Although we use a conservative p value (p<0.001) to isolate our face responsive regions, voxel-wise thresholding was not corrected for multiple comparisons, and thus, the findings should be interpreted with caution. Furthermore, the stimuli used in this study contain several features between dynamic face and dynamic landscape conditions that are not controlled, and therefore future studies using stimuli containing more precisely defined contrasts in order to isolate face processing networks are needed. We also did not ask the study participants to rate their level of fear while viewing the emotional faces or to quantify their level of recognition of famous faces, and thus we are unable to link these behavioral measures to the fMRI signals. Future studies involving a larger sample size is needed to address these important research issues.
Supplementary Material
ACKNOWLEDGMENT
We thank Professor Hennric Jokeit and colleagues at the Swiss Epilepsy Center, Hirslanden Hospital, Zurich, Switzerland for providing the fMRI video stimulus. This work was supported grants from the NIH National Institute of Neurological Disorders and Stroke (K23 NS060993, PI: Lin, JJ; T32 NS45540 PI: Baram, TZ, Trainee: Fling, BW), and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000153.
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
JJL has received speaker's honorarium from UCB-Pharma and Sunovion Pharmaceutical. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001 Jul;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 2.Adolphs R. Cognitive neuroscience of human social behaviour. Nature reviews. 2003 Mar;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 3.Drevets WC, Raichle ME. Reciprocal Suppression of Regional Cerebral Blood Flow during Emotional versus Higher Cognitive Processes: Implications for Interactions between Emotion and Cognition. Cognition and Emotion. 1998;12:353–85. [Google Scholar]
- 4.Blakemore SJ. The social brain in adolescence. Nature reviews. 2008 Apr;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 5.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000 Jun;4:223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 6.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997 Jun;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000 Jan;3:80–4. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- 8.Powell HWR, Guye M, Parker GJM, et al. Noninvasive in vivo demonstration of the connections of the human parahippocampal gyrus. Neuroimage. 2004;22:740–7. doi: 10.1016/j.neuroimage.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Lanteaume L, Guedj E, Bastien-Toniazzo M, et al. Cognitive and metabolic correlates of emotional vulnerability in patients with temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2012 May;83:522–8. doi: 10.1136/jnnp-2011-301219. [DOI] [PubMed] [Google Scholar]
- 10.Reynders HJ, Broks P, Dickson JM, et al. Investigation of social and emotion information processing in temporal lobe epilepsy with ictal fear. Epilepsy Behav. 2005 Nov;7:419–29. doi: 10.1016/j.yebeh.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Broicher SD, Kuchukhidze G, Grunwald T, et al. “Tell me how do I feel”--emotion recognition and theory of mind in symptomatic mesial temporal lobe epilepsy. Neuropsychologia. 2012 Jan;50:118–28. doi: 10.1016/j.neuropsychologia.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. The Lancet. 2012;380:1180–92. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Declaration of Helsinki. Law Med Health Care. 1991 Fall-Winter;19:264–5. [PubMed] [Google Scholar]
- 14.Risinger MW, Engel J, Jr., Van Ness PC, et al. Ictal localization of temporal lobe seizures with scalp/sphenoidal recordings. Neurology. 1989 Oct;39:1288–93. doi: 10.1212/wnl.39.10.1288. [DOI] [PubMed] [Google Scholar]
- 15.Schacher M, Haemmerle B, Woermann FG, et al. Amygdala fMRI lateralizes temporal lobe epilepsy. Neurology. 2006 Jan;66:81–7. doi: 10.1212/01.wnl.0000191303.91188.00. [DOI] [PubMed] [Google Scholar]
- 16.LaBar KS, Crupain MJ, Voyvodic JT, et al. Dynamic perception of facial affect and identity in the human brain. Cereb Cortex. 2003 Oct;13:1023–33. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- 17.Schacher M, Haemmerle B, Woermann FG, et al. Amygdala fMRI lateralizes temporal lobe epilepsy. Neurology. 2006 Jan 10;66:81–7. doi: 10.1212/01.wnl.0000191303.91188.00. [DOI] [PubMed] [Google Scholar]
- 18.Friston KJ, Josephs O, Rees G, et al. Nonlinear event-related responses in fMRI. Magn Reson Med. 1998 Jan;39:41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- 19.Gschwind M, Pourtois G, Schwartz S, et al. White-matter connectivity between faceresponsive regions in the human brain. Cereb Cortex. 2012 Jul;22:1564–76. doi: 10.1093/cercor/bhr226. [DOI] [PubMed] [Google Scholar]
- 20.Sabatinelli D, Fortune EE, Li Q, et al. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011 Feb 1;54:2524–33. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Herrington JD, Taylor JM, Grupe DW, et al. Bidirectional communication between amygdala and fusiform gyrus during facial recognition. Neuroimage. 2011 Jun 15;56:2348–55. doi: 10.1016/j.neuroimage.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blank H, Anwander A, von Kriegstein K. Direct structural connections between voice- and face-recognition areas. J Neurosci. 2011 Sep 7;31:12906–15. doi: 10.1523/JNEUROSCI.2091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramer SC, Nelles G, Benson RR, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke; a journal of cerebral circulation. 1997 Dec;28:2518–27. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson M, Beckmann CF, Behrens TE, et al. Fsl. Neuroimage. 2012 Aug 15;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004 Jan;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001 Jan;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 27.Labudda K, Mertens M, Steinkroeger C, et al. Lesion side matters - an fMRI study on the association between neural correlates of watching dynamic fearful faces and their evaluation in patients with temporal lobe epilepsy. Epilepsy Behav. 2014 Feb;31:321–8. doi: 10.1016/j.yebeh.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Pitcher D, Walsh V, Duchaine B. The role of the occipital face area in the cortical face perception network. Exp Brain Res. 2011 Apr;209:481–93. doi: 10.1007/s00221-011-2579-1. [DOI] [PubMed] [Google Scholar]
- 29.Oyegbile TO, Dow C, Jones J, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004 May 25;62:1736–42. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- 30.Grant AC, Donnelly KM, Chubb C, et al. Temporal lobe epilepsy does not impair visual perception. Epilepsia. 2008 Apr;49:710–3. doi: 10.1111/j.1528-1167.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- 31.Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008 May;49:741–57. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 32.Kourtzi Z, Kanwisher N. Activation in human MT/MST by static images with implied motion. J Cogn Neurosci. 2000 Jan;12:48–55. doi: 10.1162/08989290051137594. [DOI] [PubMed] [Google Scholar]
- 33.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000 Jul;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 34.Nestor A, Plaut DC, Behrmann M. Unraveling the distributed neural code of facial identity through spatiotemporal pattern analysis. Proc Natl Acad Sci U S A. 2011 Jun 14;108:9998–10003. doi: 10.1073/pnas.1102433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews TJ, Ewbank MP. Distinct representations for facial identity and changeable aspects of faces in the human temporal lobe. Neuroimage. 2004 Nov;23:905–13. doi: 10.1016/j.neuroimage.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 36.Galimberti CA, Diegoli M, Sartori I, et al. Brain pseudoatrophy and mental regression on valproate and a mitochondrial DNA mutation. Neurology. 2006 Nov 14;67:1715–7. doi: 10.1212/01.wnl.0000242882.58086.9a. [DOI] [PubMed] [Google Scholar]
- 37.Tusa RJ, Ungerleider LG. The inferior longitudinal fasciculus: a reexamination in humans and monkeys. Ann Neurol. 1985 Nov;18:583–91. doi: 10.1002/ana.410180512. [DOI] [PubMed] [Google Scholar]
- 38.Choi J, Jeong B, Polcari A, et al. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage. 2012 Jan 16;59:1071–9. doi: 10.1016/j.neuroimage.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmadi ME, Hagler DJ, Jr., McDonald CR, et al. Side Matters: Diffusion Tensor Imaging Tractography in Left and Right Temporal Lobe Epilepsy. Ajnr. 2009 Jun 9; doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Govindan RM, Makki MI, Sundaram SK, et al. Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res. 2008 Jul;80:30–41. doi: 10.1016/j.eplepsyres.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waites AB, Briellmann RS, Saling MM, et al. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006 Feb;59:335–43. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- 42.Powell HW, Parker GJ, Alexander DC, et al. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007 May 15;36:209–21. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 43.Berl MM, Zimmaro LA, Khan OI, et al. Characterization of atypical language activation patterns in focal epilepsy. Ann Neurol. 2014 Jan;75:33–42. doi: 10.1002/ana.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell HW, Richardson MP, Symms MR, et al. Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia. 2007 Aug;48:1512–25. doi: 10.1111/j.1528-1167.2007.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helmstaedter C, Kurthen M, Linke DB, et al. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings, EEG, sex, and age at onset of epilepsy. Brain and cognition. 1997 Mar;33:135–50. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.