Abstract
Mental imagery is a fundamental cognitive process of interest to basic scientists and clinical researchers. This study examined large-scale oscillatory brain activity in the alpha band (8–12 Hz) during language-driven mental imagery using dense-array EEG. Three experiments demonstrated relative increases in alpha amplitude (1) during imagery prompted by words compared to fixation without imagery instruction, (2) during imagery of word content compared to imagery of geometric shapes, and (3) during imagery of emotionally evocative words compared to imagery of less emotionally arousing content. Alpha increases for semantically loaded imagery were observed in parieto-occipital regions, sustained throughout the imagery period. Findings imply that alpha oscillations index active memory and internal cognitive processing, reflecting neural communication in cortical networks representing motor, semantic, and perceptual aspects of the imagined scene.
The process of mental imagery has engaged the interest of philosophers and scientists for centuries (Waller, Schweitzer, Brunton & Knudson, 2012). Modern cognitive neuroscience approaches to this phenomenon suggest that mental imagery and perception share common neural real estate and entail similar functional processes (e.g., Cichy, Heinzle, & Haynes, 2012; Kosslyn, 2005). Since mental imagery is, ipso facto, an internally oriented brain state, it is considerably more dependent on endogenous signaling in contrast to external perception, which involves a greater degree of bottom-up activation impinging via sensory processes. Evidence from effective connectivity analyses indicates that visual imagery can be differentiated from visual perception by a reversal in the dominant direction of cortical information flow, with greater top-down signal flow to parieto-occipital cortical regions being observed during imagining than perceiving (Dentico et al., 2014). It is well known that in some contexts, imagery can interfere with perception (Craver-Lemley & Reeves, 1992) and that active inhibition of irrelevant sensory cortical regions may be observed during imagery to protect effortful processing from interference (Amedi et al., 2005). Current theorizing about the role of large-scale brain activity reflected in the alpha band of the human electroencephalogram (EEG), suggests that these neuronal oscillations play an important role in active cortical inhibition and gating of information flow (e.g., Klimesch, 2006). Here, we aimed to characterize the changes in brain electrical activity induced by word-evoked mental imagery recorded from high-density EEG. In a series of experiments we manipulated the complexity of imagery content and contrasted verbally prompted imagery differing in the amount of affective arousal.
As an active element of clinical intervention, imagery of emotional scenes is widely used in the treatment of fear, anxiety, depression, and many other diagnoses (Foa, McNally & Murdock, 1989; Kraft, 1970; Rubin, Spates, Johnson & Jouppi, 2009; Wiederhold, Jang, Gevirtz, Kim, Kim & Wiederhold, 2002). Much of this work has been guided by a bio-informational theory of imagery (Lang, 1979) which holds that verbal event descriptions of emotional situations activate a broad cognitive network in the brain, coding not just sensory information, but also associated semantic and efferent response representations. Subsequent research has shown, furthermore, that imagery word-cues engage facial, autonomic and somatic reflexes and that for emotionally arousing imagery these responses parallel reactions to actual pleasant or unpleasant events (Miller, Levin, Kozak, Cook, McLean & Lang, 1987). Hemodynamic neuroimaging research (e.g., functional magnetic resonance imaging, fMRI) provides further support for a network model, demonstrating that emotionally arousing imagery activates brain circuitry mediating fear/defensive and appetitive/reward responses. Arousing narratives prompt activation of supplementary motor area and lateral cerebellum (Sabatinelli, Lang, Bradley & Flaisch, 2006), consistent with observations regarding reflex physiology. BOLD activation of the insula, amygdala, and frontal cortex is found for distressing imagery (Britton, Phan, Taylor, Fig & Liberzon, 2005; Sinha Lacadie, Skudlarski, Fulbright, Rounsaville, Kosten & Wexler, 2005), and Costa and collaborators (2010) reported that pleasant content selectively activated the nucleus accumbens and the ventral medial prefrontal cortex.
While hemodynamic BOLD data have revealed much about metabolic changes in brain circuits active during imagery processing, monitoring EEG oscillations during imagery episodes has the advantage of directly assessing neural activity from large populations of pyramidal cortical cells in real-time. The focus here is on variations in alpha amplitude (8–12 Hz), the human brain’s most evident large-scale oscillatory activity, visible prima vista in the raw EEG recordings (Berger, 1929, 1969).
When participants are at rest, without engaging in active sensory processing (for instance, with eyes closed), substantial oscillatory amplitude increases in the alpha-band are apparent (Berger, 1929; Pfurtscheller, Stancak & Neuper, 1996). This has sometimes been interpreted as evidence of an idling brain--alpha in this view being the signature of cortical tissue that neither receives nor processes sensory input (Adrian & Matthews, 1934). Decreases in alpha spectral amplitude reliably occur with sensory processing of external stimuli (Bollimunta, Mo, Schroeder & Ding, 2011; Keil, Mussweiler & Epstude, 2006; Ray & Cole, 1985), and the degree of reduction has been viewed as an inverse measure of cortical arousal (Aftanas, Reva, Varlamov, Pavlov & Makhnev, 2004; De Cesarei & Codispoti, 2011; Neuper, Grabner, Fink & Neubauer, 2005a).
In a comprehensive review of the literature, Klimesch et al. (2006) reported that alpha increase is most often observed in tasks that involve top-down processing in the absence of external stimulation, for instance when participants maintain an internal representation in working memory. According to this view the waxing and waning of alpha-band power might index decreased and increased receptivity of brain circuits to external inputs, creating temporal windows of excitability (Hanslmayr, Gross, Klimesch, & Shapiro, 2011). It has been suggested, furthermore, that regional alpha changes reflect cortically mediated “pulses” of inhibition that are flexibly deployed as determined by task demands (Foxe and Snyder, 2011; Jensen and Mazaheri, 2010; Mathewson, Lleras, Beck, Fabiani, Ro & Gratton, 2011). This alpha-band inhibition putatively performs an active gating function by channeling or facilitating the organization of different types of information processing (Romei, Gross & Thut, 2010). In studies of spatial attention, spatial cues prompt fluctuations of alpha oscillations, such that alpha power is decreased in the hemisphere that is contralateral to the attended stimulus and increased in the ipsilateral hemisphere, implying that alpha-band oscillations optimize information processing through the suppression of distracting information (see e.g., Worden, Foxe, Wang & Simpson, 2000). In line with this notion, parametric amplitude increases of alpha-range oscillations have been demonstrated in a variety of experimental designs that manipulate the degree of internally focused processing, including motor process imagery (Neuper et al., 2005b; Pfurtscheller and Berghold, 1989), working memory load (Jensen, Gelfand, Kounios & Lisman, 2002; Klimesch, Sauseng & Hanslmayr, 2006), and music imagery (Schaefer, Vlek, & Desain, 2011). Summarizing this literature, the question arises as to whether mental imagery – a process in which distracting information from external inputs may be actively suppressed to protect effortful processing from harmful interference (e.g., Amedi et al., 2005) – prompts heightened alpha oscillations over widespread cortical areas.
In the present research, participants were instructed to generate a mental image subsequent to linguistic cues. The brain’s time-varying oscillatory changes were measured with dense-array EEG. In consideration of previous findings regarding alpha enhancement in tasks involving internal processing, three experiments addressed the following hypotheses: (1) in contrast to passive visual fixation, word prompted mental imagery should induce sustained alpha amplitude increases; (2) language-cued imagery should prompt greater alpha amplitude compared to imagery of semantically less-loaded, geometrical shapes in light of the increased demands on internal processing loads in the former; and (3) emotionally arousing pleasant and unpleasant word-cues should occasion greater alpha amplitude than affective neutral word cues – potentially reflecting heightened working memory processing, consequent on the broader cognitive/neural networks reported for emotional imagery (Lang, 1979).
General Method
Participants
A total of 36 undergraduate students (21 female, 15 male) participated in one of three experiments. No participant was included in more than one study. Their age ranged from 18 to 21 years and one female participant (Experiment 3) reported being left-handed. They had normal or corrected-to-normal vision and volunteered for the study for course credit. Participants gave written consent after being given a short description of the study. All procedures were approved by the institutional review board of the University of Florida.
Procedure
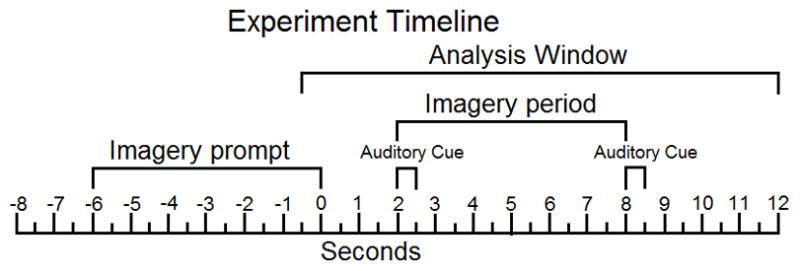
In all three experiments, participants were greeted, gave informed consent, were led to a sound-attenuated and shielded recording chamber and seated in a comfortable chair. Then a dense array electrode net was fitted and participants were presented with a list of imagery cues (words and geometric shapes) used in the experiment, printed on paper. Prior to the recording session, aspects of vivid imagery were explained to the participants, emphasizing that imagery often prompts stimulus visualization but could also engage one’s own bodily reactions (muscle tension, sweating, changes in respiration, heart rate). Participants were also instructed to maintain focus on the fixation cross throughout the experiment and asked to avoid blinking and head movements while either stimuli or the fixation cross were being presented. After instructions, the experiment began. In each experimental trial, a visual stimulus (word/shape) was presented for 6000 ms, followed by a blank screen (2000 ms) that ended with an auditory tone (500 ms). This tone cue signaled the onset of the imagery period (6000 ms), terminated by a second, identical, tone. Time between trials varied from 6000 ms to 9000 ms. A fixation cross was present on the screen as a center of focus throughout the imagery period and the inter-trial interval. The time course of stimulus and cue presentation during each trial is also represented in Figure 1. All stimuli were presented to participants on a 21-inch computer screen with 60 Hz frame rate, placed 1.0 m in front of the viewer. Auditory signals were 800 Hz sine tones, presented through free-field computer speakers at an intensity of approximately 70 dB SPL.
Figure 1.
Apparatus and EEG Data Collection
During all experiments, electrophysiological data were collected from the scalp using a 257-channel EEG system (EGI, Eugene, OR). Scalp impedance for each sensor was kept below 50 KOhms, which is recommended for this high input impedance amplifier (200 MOhms input impedance). The EEG was collected continuously with a sampling rate of 250 Hz and band-pass filtered to the 0.1–100 Hz frequency range using a Butterworth 6th order band-pass filter implemented in Matlab, having both cutoffs defined as the 3dB points of the filter response function.
Artifact Handling and Preprocessing
A statistical approach was applied for artifact correction of high density EEG, which included the transformation of the recorded data to an average reference. Continuous data were low-pass filtered offline at a frequency of 80 Hz (again, defined as the 3dB point, 4th order Butterworth filter with 12 dB/octave roll-off implemented in Matlab) and then submitted to the procedure proposed by Junghöfer and colleagues (2000) for segmentation and artifact correction. This procedure uses distributions of amplitudes, standard deviations and change values to identify channels and trials that contain artifacts. Recording artifacts are first detected using the recording reference (i.e. Cz), and subsequently global artifacts are detected using the average reference. In a next interactive step, distinct sensors from particular trials are removed and interpolated based on the distribution. Data at eliminated electrodes are replaced with a statistically weighted spherical spline interpolation from the full channel set (Junghofer, Elbert, Tucker, Rockstroh, 2000). Stimulus-locked epochs were extracted between 800 ms pre- and 12000 ms post-onset of the auditory cue that signaled the onset of the imagery period.
The mean number of interpolated channels across conditions and subjects was 12 (range 3–30). With respect to the spatial arrangement of the approximated sensors, it was ensured that the rejected sensors were not located within one region of the scalp, as this would make interpolation for this area invalid. Spherical spline interpolation was used throughout both for approximation of sensors and illustration of voltage maps (Junghöfer, Elbert, Leiderer, Berg, & Rockstroh, 1997). To avoid the impression of extra-cranial effects, sensors of the EGI 257-channel net covering extra-cranial tissue (i.e., the neck region) are displayed, but not color-coded in the illustrations. Single trials with excessive eye-movements and blinks, or with more than 30 channels containing artifacts were discarded. The validity of this procedure was further tested by visually inspecting the vertical and horizontal EOG computed from a subset of the electrodes that were part of the electrode net. Trials that showed remaining ocular artifacts were dismissed at this step of the analysis. Trial counts are reported for each experiment separately.
Time-Frequency Analysis
Preprocessed segments for each individual subject were transformed to time-varying spectral amplitude using a family of complex Morlet wavelets with a wavelet constant of 7 (see e.g., Tallon-Baudry and Bertrand, 1999). This resulted in a frequency resolution of 1.43 Hz and a time resolution (full width at half maximum) of 112 ms at a center frequency of 10 Hz. Wavelets were calculated for frequencies between 0.65 and 23.9 Hz, in steps of 0.65 Hz, for full coverage of the lower-frequency spectrum of interest in the present study. Because of the low trial count, high-beta and gamma range oscillations (> 30 Hz), which tend to be small in amplitude, were not considered. Absolute levels of time-varying spectral amplitude are shown in figures and were used for statistical analyses throughout. A baseline correction was not employed to avoid biasing of the signal towards one specific aspect during the recording epoch, in which a true baseline did not exist. Rather, we explicitly tested differences in pre-stimulus segments using permutation controlled t-tests (detailed below), to avoid spurious statistical results and address any differences between conditions in terms of overall level of oscillatory activity.
Statistical Analysis
Two approaches were used to evaluate differences in time-varying alpha amplitude. First, wavelet amplitude data were averaged for each subject and condition across a posterior electrode cluster containing POz and its 12 nearest neighbors (see Figure 2, right panel, for electrode positions), for the wavelets in the alpha range (i.e., center frequencies between 9.03–10.96 Hz), and across the time points during the imagery period (see Figure 2, top panel). This cluster represented the expected topography of alpha oscillatory activity typically observed in studies of scalp EEG (Keil et al., 2006; Lehmann, 1971b). It is also consistent with the locations showing maximum alpha amplitude, observed across the three experiments of which this study is comprised (see below). These regional averages were then compared according to the experimental design of each experiment, by means of paired samples t-tests (Experiments 1 and 2) or repeated measures ANOVA (Experiment 3) with a factor of content (pleasant, neutral, unpleasant). For illustration purposes, these test statistics were also obtained for each sensor and plotted as topographical maps. Finally, a linear mixed model was calculated on posterior alpha amplitude means across the three experiments, with participant (nested in experiment) modeled as a random effect variable, as well as fixed effects of experiment and condition, where the control conditions (fixation point, shape, and neutral words) were contrasted against the active conditions of (emotional) word imagery. The purpose of this analysis was to quantify the reliability of the hypothesized main effect across the three experiments. This is important because each experiment had a small sample and was intended to provide an initial proof of concept rather than explore specific interactions of imagery instruction with stimulus type, instruction, or hedonic valence etc.
Figure 2.
In a second step, time-frequency plots reflective of posterior oscillatory activity were created by averaging the entire time-frequency planes, across the twelve electrodes surrounding POz, as described above, for each participant and condition. Then, t-values were determined for each time and frequency point, reflecting differences related to experimental conditions across the entire time-frequency plane. Thresholds for statistical significance were determined using a permutation technique as recommended by Blair and Karniski (1993; see also Keil et al., 2005). To this end, 5000 t-planes were generated based on data in which the condition was randomly shuffled. These t-value planes were used to create a distribution of t-values for each time/frequency point. The top and bottom 2.5% tails of the resulting distribution served as the critical values for determining statistical significance of a t-value at a given time-frequency point. This analysis allows addressing questions regarding changes of spectral amplitude over time and effects in frequency bands other than the alpha band. Importantly, adopting a t-max procedure allowed us to control for the possibility of Type I errors as a result of performing multiple time-frequency comparisons.
Experiment 1
Procedure
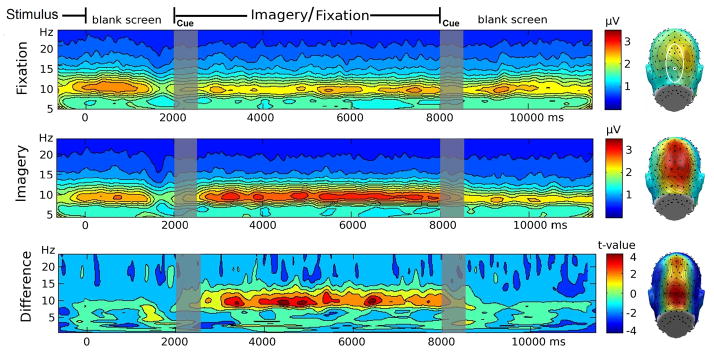
Words with neutral affective ratings were selected from the Affective Norms for English Words (ANEW; Bradley and Lang, 1999) database and presented to participants using the experimental setup described above. The ANEW words used in the study are given in Appendix A. They were selected to (i) be verbs and (ii) have clear (unambiguous) semantic content, to maximize the participants ability to actively engage in imagery of a rich scene that included motor, perceptual, and emotional information, as per instructions. Two sets of twenty trials were run: All trials had the same visual format: A word appeared on the screen for 6 seconds, terminated by a two second blank screen. A tone cue then sounded and a central crosshair was presented. For the first 20 trials (fixation condition), participants were under instruction to simply focus their gaze on the central fixation cross on hearing the tone cue. For the second 20 trials (imagery condition), participants were instructed that when they heard the tone cue, they were to vividly imagine a scene or event suggested by the word content. The imagery condition was always presented second, to avoid possible carryover of the imagery instruction to subsequent fixation-only trials. The same set of words was presented in each condition, in randomized order, with two presentations per word. For EEG analyses, on average, 16.2 trials were retained for analysis in the imagery condition and 16.0 in the fixation point condition for each subject.
Results
As expected, time-frequency decomposition showed a maximum of the spectral amplitude in the 9–11 Hz range. Amplitude modulation of this frequency band began 500 ms after the onset of the imagery cue and lasted for about 6000 ms as seen in Figure 2a (top panel), thus being sustained throughout the imagery period. Regional occipito-parietal alpha activity averaged across the time window of interest was significantly higher in the imagery condition compared to the fixation point condition (middle panel), t(11)=3.0, p<0.05. The time- and frequency specificity of this difference was examined using permutation t-tests of the entire time-frequency plane, as shown in Figure 2, bottom panel. This analysis demonstrated that instruction-related differences at parieto-occipital sites were constrained to the frequency band around 10 Hz, and temporally confined to the imagery period. The right panel of Figure 2 demonstrates that the topography of the spectral amplitude in the alpha range for both conditions and the difference topography were most pronounced at parietal and occipital electrode sites.
Discussion
This experiment examined the extent to which imagery-induced brain activity differed from brain activity exhibited during an initial block of trials in which observers were instructed to fixate on a central cross. We observed a pronounced enhancement of alpha-band activity selectively during word-prompted imagery over parieto-occipital recording sites. As discussed above, Experiment 2 was designed to replicate and extend this finding, by manipulating the semantic imagery “load”. Specifically, we contrasted the degree of alpha amplitude fluctuation during imagery periods prompted by semantic content (word cues) versus imagining simple non-linguistic geometric shapes with no semantic content.
Experiment 2
Procedure
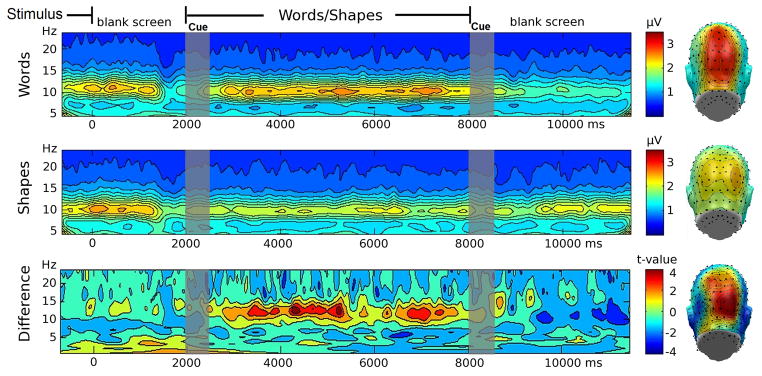
The experimental setup was the same for this experiment as for experiment 1. Stimulus presentation was performed as described in the General Methods. Two different groups of stimuli were used as imagery cues in this experiment: 20 different geometric shapes (shape imagery condition) and the same words from ANEW used in experiment 1 (word imagery condition). Shapes were circles, triangles, squares, and polygons, shown at different orientations, drawn in gray on a black background and matching the luminance of the word cues (approximately 70 candela/m2). Twenty trials of each condition were presented to participants in pseudo-randomized order where neither condition was presented for more than 3 trials in a row. An average of 14.8 trials were retained for analysis in the imagery condition and 16.0 in the geometric shapes condition. This difference did not reach statistical significance (p>0.2)
Results
Alpha activity averaged across posterior electrode sites and across the imagery period was significantly greater in the word condition compared to the shape condition, t(11)=11.8, p<0.01 (see Figure 3). In the word imagery condition, time-frequency decomposition showed a strong amplitude increase in the 9–11 Hz range beginning 500 ms after the auditory imagery onset cue and lasting for about 6000 ms as seen in the top panel of Figure 3. For comparison, the middle panel shows the shape imagery condition, which displayed less activity. The bottom panel displays the comparison of the two conditions in the time- and frequency domains computed using permutation t-tests, highlighting that effects of imagery instruction at parieto-occipital sensors were specific to the alpha range. Topographical mapping of the spectral amplitude in the alpha range during the imagery period showed comparable topographies as in Experiment 1, with alpha amplitude showing maxima around electrode site POz.
Figure 3.
Discussion
This experiment examined the extent to which semantic load affected the amplitude of alpha oscillations by contrasting imagery of geometric shapes with imagery based on more complex word prompts. The results imply that semantic content of the mental image heightens parietal alpha amplitude, corroborating the findings from Experiment 1. In a final experiment, we examined this hypothesis in the context of emotional imagery, which has been proposed to reflect heightened connectivity and intensity of cortical processing in areas representing sensory, motor, and motivational aspects of the imagined scene, compared to imagining affectively neutral scenes. Based on Experiments 1 and 2, we expected that emotionally engaging cues would prompt greater alpha amplitude increases than neutral word cues.
Experiment 3
Procedure
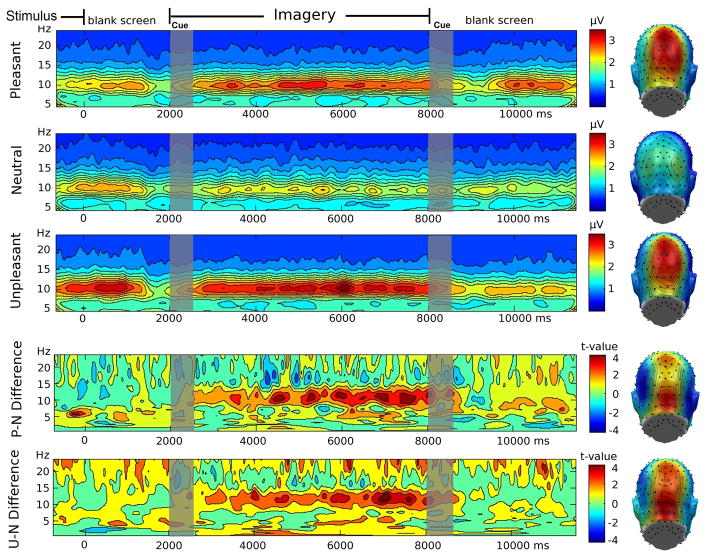
15 pleasant, 15 neutral and 15 unpleasant words were selected from the ANEW. The words are listed in Appendix 2. They were selected to be different in normative ratings with respect to pleasure (pleasant, neutral, and unpleasant words being rated 7.6, 6.4, and 2.8 respectively) and emotional arousal (6.5, 4.9, and 6.3 respectively, on the 9-point scale of the Self –Assessment Manikin, (Lang, 1980)). This, together with the need for matching the conditions for word frequency (mean word frequency 68.6 per million, SD = 171.0), could only be achieved by using nouns and adjectives. Again, participants viewed the words for 6 seconds using the experimental setup described above and were asked to vividly imagine the word content upon being cued with the same soft tone. 15 trials of each condition (pleasant, neutral and unpleasant) were presented in pseudo-randomized order, with neither condition presented for more than 3 times in a row; each word was presented only once during the experiment. Across subjects, on average 12.0 trials were retained for analysis in the pleasant condition, 12.3 trials in the neutral condition, and 12.3 trials in the unpleasant condition.
Results
Paralleling Experiments 1 and 2, visual inspection of the time-frequency decomposition showed pronounced posterior alpha activity in the 9–11 Hz range (see Figure 4). Repeated measures ANOVA indicated that emotional word content affected mean alpha amplitude in the time region of interest, F(2,22)=6.31, p<0.01. Following up this main effect, paired t-tests revealed that alpha amplitude was greater for the pleasant t(11)=2.42, p<0.05 and unpleasant t(11)=2.97, p<0.05 conditions, compared to the neutral condition, but pleasant and unpleasant scene imagery did not differ, t(11)<1.3. The bottom panel of Figure 4 displays the permutation t-planes for these comparisons, where red color implies that t-values exceeded the permutation-controlled threshold of 3.18. Again, a sustained increase of alpha amplitude was observed when imagining pleasant and unpleasant, compared to neutral content. The other frequency ranges did not show sustained and robust modulation resulting from the imagery instructions.
Figure 4.
Joint Analysis of Experiments 1 to 3
The mixed model analysis of the joint data from the three experiments showed that, across experiments, active conditions involving semantic or emotionally engaging imagery content were associated with greater alpha amplitude than control conditions involving fixation, shapes, or neutral words, F(1, 44.6)=16.1, p<0.01. By contrast, alpha amplitude did not vary by experiment, nor did experiment and condition interact, Fs < 1, ps> .4. Thus, across experiments, imagery with greater semantic and affective load resulted in greater alpha amplitude compared to the various control conditions.
General Discussion
The present series of three experiments set out to examine the sensitivity of time-varying alpha oscillations to text-driven, semantically loaded, imagery. Predictions were based on findings of heightened alpha amplitude when experimental tasks require active maintenance of internal representations (e.g., working memory load) as compared to tasks that involve processing external stimuli (reviewed in Klimesch et al., 2006).
Experiments 1 and 2 demonstrated that word-prompted imagery of semantic content elicits greater posterior alpha activity than an eyes-open fixation instruction (Experiment 1), or imagining geometric shapes (Experiment 2). Modulation as a function of imagery was most pronounced over parieto-occipital cortices, which is in line with the classical topography of alpha amplitude when comparing rest and active conditions (Lehmann, 1971a). Experiment 3 expanded on these findings, demonstrating that imagining words with emotional content prompted greater alpha-band amplitude than imagery of affectively neutral words. In line with Experiments 1 and 2, these results support the view that posterior alpha amplitude reflects internal processing, potentially indicating top-down control (Klimesch et al., 2006) and active memory networks (Bonnefond and Jensen, 2012; Jensen et al., 2002). The joint analyses of the three experiment in a mixed linear model showed strong evidence of a main effect of imagery content, but did not point towards an interaction effect of imagery instruction with stimulus type, instruction, or hedonic valence. This negative result should be treated with caution given the small sample size in each of the three experiments, which aimed to provide initial proof of concept rather than explore specific of task stimuli or instruction. These questions may be explored in future research, using appropriate sample sizes.
Many recent models of alpha oscillations emphasize their role in suppressing/inhibiting neural excitation by task-irrelevant, distracting stimuli (Foxe and Snyder, 2011), as well as indexing excitability of neural tissue (Rajagovindan and Ding, 2011). The finding of heightened alpha amplitude during semantically loaded imagery is generally consistent with the suggested roles for alpha oscillations during task processing. Notably, the interpretation of alpha amplitude enhancement as reflecting an active cognitive process is in stark contrast with traditional interpretations of alpha as an index of inactive tissue (Davidson et al., 1990) or cortical idling (Pfurtscheller et al., 1996) first described by Adrian and Matthews (Adrian and Matthews, 1934). Future work might attempt to parametrically manipulate semantic content of imagined scenes along a continuum of semantic complexity (e.g., word association value or meaningfulness as proposed by Paivio (Paivio et al., 1968).
It is interesting to consider that the interpretations stated above ground language and concepts in perception-action systems of the human brain (Pulvermuller and Fadiga, 2010). Empirical work linking imagery and language representation (Paivio et al., 1968) has shown that processing action-related words engages regions involved in actual actions, but words designating objects engage higher order sensory cortices (Hauk et al., 2008). Language prompted imagery of complex scenes is expected to engage many types of representations – action as well as object memories together with the temporal and semantic content associated with the imagined scene. Thus, a rich pattern of distributed neural communication would be expected, paralleling language processing (Pulvermuller, 1996). Future studies may utilize imagery prompts similar to those in the present study to investigate wide-range connectivity during mental imagery of complex scenes.
Imagery is by definition a private mental event, so questions regarding compliance with the task during experimentation are warranted. The increased activation directly after onset of imagery cues strongly suggests that participants responded to the imagery cue. Thus, prior studies in both clinical and research settings utilizing paradigms with similar or identical language cues (McTeague et al., 2009; McTeague et al., 2011) have repeatedly reported that text-driven imagery prompts heightened autonomic and somatic reactions consistent with emotional engagement (Borkovec and Hu, 1990; Cuthbert et al., 2003; Lang and Bradley, 2010).
The event-related time-frequency analyses conducted in this study suggested that imagery-related differences were specific to the alpha range, but it is conceivable that some effects in higher frequency bands were not detected because of the limited trial count used in the present study. Although appropriate to estimate robust and large alpha oscillations, trial counts of 20 or less may not be suitable for reliably quantifying events in the beta or gamma frequency ranges, which tend to be substantially lower in amplitude than alpha-band activity in the human EEG (Nunez & Silberstein, 2000). Oscillatory activity recorded from neural populations has been related to a variety of neurophysiological processes in animal models and other imaging modalities in humans. Alpha phase (relative latency of alpha waves) in particular appears to play a major role in the temporal organization and alignment of cortical high gamma oscillations (> 50 Hz), involved in basic perceptual and cognitive processes (Schroeder and Lakatos, 2009). Recent evidence indicates that the amplitude of cortical gamma can become coupled to the phase of thalamic alpha oscillations (Roux et al., 2013). Gamma oscillations embedded in lower-frequency rhythms have also been discussed as a potential mechanism of predictive coding (Neymotin et al., 2013) and active sampling of multimodal sensory events (Schroeder et al., 2010) as well as motor interactions with the environment (Haig and Gordon, 1998). To the extent that mental imagery could be seen as purely top-down driven (see Dentico et al., 2014) and thus as pure prediction from memory in a Bayesian sense (Melloni et al., 2011), explorations of alpha phase and gamma amplitude –while outside of the scope of this report– may represent an interesting avenue for future research.
In conclusion, the present study demonstrates reliable enhancement of parietal alpha amplitude when participants are instructed to imagine complex scenes prompted by language cues. In light of the extant theoretical and neurophysiological work, our findings may be interpreted as reflecting active neural communication in cortical networks representing motor, semantic, and perceptual aspects of the imagined scene. Future work may explore the usefulness of such an index, based on inexpensive and accessible EEG recordings, to monitor imagery-related brain activity in translational and clinical research.
Acknowledgments
This research was supported in part by grants from the National Institute of Mental Health to AK (R01MH097320 and R01MH084932) and to PJL (R01MH094386 and R01MH098078). The authors would like to thank Stephanie Babcock for assistance in data acquisition.
Appendix A: list of Anew Words from (Bradley & Lang, 1999) used in experiments 1 and 2
to dance, to run, to throw, to climb, to descent, to exit, to draw, to exercise, to fold, to lock, to organize, to pour, to relax, to reserve, to sew, to sit, to sleep, to wind, to listen, to contemplate
Appendix B: List of ANEW words from (Bradley & Lang, 1999) used in experiment 3
Pleasant: skijump, circus, fireworks, party, rollercoaster, beach, exercise, game, bouquet, wedding, birthday, kiss, romantic, flirt, fragrance
Neutral: nun, golfer, teacher, girl, boy, man, astronaut, woman, bride, dancer, baby, brother, child, doctor, gymnast
Unpleasant: alcoholic, beggar, criminal, invader, traitor, poison, vomit, putrid, gangrene, slime, rancid, garbage, scum, pus, rotten
References
- Adrian ED, Matthews BH. The Berger rhythm: potential changes from the occipital lobes in man. Brain. 1934:355–385. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- Aftanas LI, Reva NV, Varlamov AA, Pavlov SV, Makhnev VP. Analysis of evoked EEG synchronization and desynchronization in conditions of emotional activation in humans: temporal and topographic characteristics. Neurosci Behav Physiol. 2004;34:859–867. doi: 10.1023/b:neab.0000038139.39812.eb. [DOI] [PubMed] [Google Scholar]
- Amedi A, Malach R, Pascual-Leone A. Negative BOLD differentiates visual imagery and perception. Neuron. 2005;48:859–872. doi: 10.1016/j.neuron.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Ben-Simon E, Podlipsky I, Arieli A, Zhdanov A, Hendler T. Never Resting Brain: Simultaneous Representation of Two Alpha Related Processes in Humans. Plos One. 2008:3. doi: 10.1371/journal.pone.0003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Über das Elektrenkephalogramm des Menschen. Archiv für Psychiatrie und Nervenkrankheiten. 1929;87:527–570. [Google Scholar]
- Berger H. On the electroencephalogram of man. Electroencephalogr Clin Neurophysiol suppl. 1969;28:37–73. [PubMed] [Google Scholar]
- Blair RC, Karniski W. An alternative method for significance testing of waveform difference potentials. Psychophysiology. 1993;30:518–524. doi: 10.1111/j.1469-8986.1993.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J Neurosci. 2011;31:4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr Biol. 2012;22:1969–1974. doi: 10.1016/j.cub.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Hu S. The effect of worry on cardiovascular response to phobic imagery. Behav Res Ther. 1990;28:69–73. doi: 10.1016/0005-7967(90)90056-o. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Technical Report C-1. The Center for Research in Psychophysiology, University of Florida; Gainesvill, FL: 1999. Affective norms for English words (ANEW): Instruction manual and affective ratings. [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, Morris PG. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proceedings of the National Academy of Sciences. 2011;108(40):16783–16788. doi: 10.1073/pnas.1112685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugelski BR. Words and Things and Images. American Psychologist. 1970;25:1002. [Google Scholar]
- Cichy RM, Heinzle J, Haynes JD. Imagery and perception share cortical representations of content and location. Cerebral Cortex. 2012;22:372–380. doi: 10.1093/cercor/bhr106. [DOI] [PubMed] [Google Scholar]
- Costa VD, Lang PJ, Sabatinelli D, Versace F, Bradley MM. Emotional imagery: assessing pleasure and arousal in the brain’s reward circuitry. Hum Brain Mapp. 2010;31:1446–1457. doi: 10.1002/hbm.20948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craver-Lemley C, Reeves A. How visual imagery interferes with vision. Psychological Review. 1992;99:633–649. doi: 10.1037/0033-295x.99.4.633. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: fear memory imagery. Psychophysiology. 2003;40:407–422. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology. I. J Pers Soc Psychol. 1990;58:330–341. [PubMed] [Google Scholar]
- Dentico D, Cheung BL, Chang JY, Guokas J, Boly M, Tononi G, et al. Reversal of cortical information flow during visual imagery as compared to visual perception. NeuroImage. 2014;100:237–243. doi: 10.1016/j.neuroimage.2014.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesarei A, Codispoti M. Affective modulation of the LPP and alpha-ERD during picture viewing. Psychophysiology. 2011;48:1397–1404. doi: 10.1111/j.1469-8986.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, Corbetta M. Temporal dynamics of spontaneous MEG activity in brain networks. Proceedings of the National Academy of Sciences. 2010;107(13):6040–6045. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ. The neural basis of mental imagery. Trends Neurosci. 1989;12:395–399. doi: 10.1016/0166-2236(89)90079-9. [DOI] [PubMed] [Google Scholar]
- Foa EB, McNally R, Murdock TB. Anxious mood and memory. Behav Res Ther. 1989;27:141–147. doi: 10.1016/0005-7967(89)90072-7. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front Psychol. 2011;2:154. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruberger M, Ben-Simon E, Levkovitz Y, Zangen A, Hendler T. Towards a neuroscience of mind-wandering. Front Hum Neurosci. 2011;5:56. doi: 10.3389/fnhum.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig AR, Gordon E. EEG alpha phase at stimulus onset significantly affects the amplitude of the P3 ERP component. Int J Neurosci. 1998;93:101–115. doi: 10.3109/00207459808986416. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Gross J, Klimesch W, Shapiro KL. The role of alpha oscillations in temporal attention. Brain Research Reviews. 2011;67:331–343. doi: 10.1016/j.brainresrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Hauk O, Davis MH, Kherif F, Pulvermuller F. Imagery or meaning? Evidence for a semantic origin of category-specific brain activity in metabolic imaging. Eur J Neurosci. 2008;27:1856–1866. doi: 10.1111/j.1460-9568.2008.06143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp JF, Hawellek DJ, Corbetta M, Siegel M, Engel AK. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nature Neuroscience. 2012;15(6):884–890. doi: 10.1038/nn.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Leiderer P, Berg P, Rockstroh B. Mapping EEG-potentials on the surface of the brain: a strategy for uncovering cortical sources. Brain Topogr. 1997;9:203–217. doi: 10.1007/BF01190389. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–532. [PubMed] [Google Scholar]
- Keil A. Electro- and Magnetoencephalography in the study of emotion. In: Armony J, Vuilleumier P, editors. The Cambridge Handbook of Human Affective Neuroscience. Cambridge University Press; Cambridge: 2013. pp. 107–132. [Google Scholar]
- Keil A, Moratti S, Sabatinelli D, Bradley MM, Lang PJ. Additive effects of emotional content and spatial selective attention on electrocortical facilitation. Cereb Cortex. 2005;15:1187–1197. doi: 10.1093/cercor/bhi001. [DOI] [PubMed] [Google Scholar]
- Keil A, Mussweiler T, Epstude K. Alpha-band activity reflects reduction of mental effort in a comparison task: A source space analysis. Brain Res. 2006;1121:117–127. doi: 10.1016/j.brainres.2006.08.118. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res Brain Res Rev. 2006 doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Slobodskoj-Plusnin JY, Bocharov AV, Pylkova LV. The default mode network and EEG alpha oscillations: an independent component analysis. Brain Res. 2011;1402:67–79. doi: 10.1016/j.brainres.2011.05.052. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM. Mental images and the Brain. Cogn Neuropsychol. 2005;22:333–347. doi: 10.1080/02643290442000130. [DOI] [PubMed] [Google Scholar]
- Kraft T. Systematic desensitization using emotional imagery only. Percept Mot Skills. 1970;30:293–294. doi: 10.2466/pms.1970.30.1.293. [DOI] [PubMed] [Google Scholar]
- Lang PJ. A bioinformational theory of emotional imagery. Psychophysiology. 1979;16:495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: Computer applications. In: Sidowski JB, Johnson JH, William TA, editors. Technology in mental health care delivery systems. Ablex; Norwood, NJ: 1980. pp. 119–137. [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biol Psychol. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D. Multichannel topography of human alpha EEG fields. Electroencephalogr Clin Neurophysiol. 1971a;31:439–449. doi: 10.1016/0013-4694(71)90165-9. [DOI] [PubMed] [Google Scholar]
- Lehmann D. Topography of spontaneous alpha EEG fields in humans. Electroencephalogr Clin Neurophysiol. 1971b;30:161–162. [PubMed] [Google Scholar]
- Martin KA, Moritz SE, Hall CR. Imagery use in sport: A literature review and applied model. Sport Psychologist. 1999;13:245–268. [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, Gratton G. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Psychol. 2011;2:99. doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: Generalization, comorbidity, and physiological reactivity. Biological Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Bradley MM. Aversive imagery in panic disorder: agoraphobia severity, comorbidity, and defensive physiology. Biol Psychiatry. 2011;70:415–424. doi: 10.1016/j.biopsych.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni L, Schwiedrzik CM, Muller N, Rodriguez E, Singer W. Expectations change the signatures and timing of electrophysiological correlates of perceptual awareness. J Neurosci. 2011;31:1386–1396. doi: 10.1523/JNEUROSCI.4570-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Levin DN, Kozak MJ, Cook EWI, McLean AJ, Lang PJ. Individual differences in imagery and the psychophysiology of emotion. Cognition and emotion. 1987;1:367–390. [Google Scholar]
- Neuper C, Grabner RH, Fink A, Neubauer AC. Long-term stability and consistency of EEG event-related (de-)synchronization across different cognitive tasks. Clin Neurophysiol. 2005a;116:1681–1694. doi: 10.1016/j.clinph.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Neuper C, Scherer R, Reiner M, Pfurtscheller G. Imagery of motor actions: differential effects of kinesthetic and visual-motor mode of imagery in single-trial EEG. Brain Res Cogn Brain Res. 2005b;25:668–677. doi: 10.1016/j.cogbrainres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Neymotin SA, Hilscher MM, Moulin TC, Skolnick Y, Lazarewicz MT, Lytton WW. Ih Tunes Theta/Gamma Oscillations and Cross-Frequency Coupling in an In-Silico CA3 Model. PLoS One. 2013;8:e76285. doi: 10.1371/journal.pone.0076285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL, Silberstein RB. On the relationship of synaptic activity to macroscopic measurements: does co-registration of EEG with fMRI make sense? Brain Topogr. 2000;13:79–96. doi: 10.1023/a:1026683200895. [DOI] [PubMed] [Google Scholar]
- Paivio A, Yuille JC, Madigan SA. Concreteness, imagery, and meaningfulness values for 925 nouns. Journal of Experimental Psychology. 1968;76(suppl 1):1–25. doi: 10.1037/h0025327. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Berghold A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol. 1989;72:250–258. doi: 10.1016/0013-4694(89)90250-2. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band--an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Hebb’s concept of cell assemblies and the psychophysiology of word processing. Psychophysiology. 1996;33:317–333. doi: 10.1111/j.1469-8986.1996.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Fadiga L. Active perception: sensorimotor circuits as a cortical basis for language. Nat Rev Neurosci. 2010;11:351–360. doi: 10.1038/nrn2811. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagovindan R, Ding M. From prestimulus alpha oscillation to visual-evoked response: an inverted-U function and its attentional modulation. J Cogn Neurosci. 2011;23:1379–1394. doi: 10.1162/jocn.2010.21478. [DOI] [PubMed] [Google Scholar]
- Ray WJ, Cole HW. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228:750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci. 2010;30:8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Wibral M, Singer W, Aru J, Uhlhaas PJ. The Phase of Thalamic Alpha Activity Modulates Cortical Gamma-Band Activity: Evidence from Resting-State MEG Recordings. J Neurosci. 2013;33:17827–17835. doi: 10.1523/JNEUROSCI.5778-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin S, Spates CR, Johnson DA, Jouppi L. Dosed versus prolonged exposure in the treatment of fear: an experimental evaluation and review of behavioral mechanisms. J Anxiety Disord. 2009;23:806–812. doi: 10.1016/j.janxdis.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Bradley MM, Flaisch T. The neural basis of narrative imagery: emotion and action. Prog Brain Res. 2006;156:93–103. doi: 10.1016/S0079-6123(06)56005-4. [DOI] [PubMed] [Google Scholar]
- Schaefer RS, Vlek RJ, Desain P. Music perception and imagery in EEG: alpha band effects of task and stimulus. Int J Psychophysiol. 2011;82:254–259. doi: 10.1016/j.ijpsycho.2011.09.007. S0167-8760(11)00273-X [pii] [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Wilson DA, Radman T, Scharfman H, Lakatos P. Dynamics of Active Sensing and perceptual selection. Curr Opin Neurobiol. 2010;20:172–176. doi: 10.1016/j.conb.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JL. Imagery in Psychotherapy. American Psychological Association; Washington, DC: 2006. [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Cuthbert BN, Lang PJ. Processing fearful and neutral sentences: Memory and heart rate change. Cognition and Emotion. 1989;3:179–195. [Google Scholar]
- Waller D, Schweitzer JR, Brunton JR, Knudson RM. A Century of Imagery Research: Reflections on Cheves Perky’s Contribution to Our Understanding of Mental Imagery. The American Journal of Psychology. 2012:291–305. doi: 10.5406/amerjpsyc.125.3.0291. [DOI] [PubMed] [Google Scholar]
- Wiederhold BK, Jang DP, Gevirtz RG, Kim SI, Kim IY, Wiederhold MD. The treatment of fear of flying: a controlled study of imaginal and virtual reality graded exposure therapy. IEEE Trans Inf Technol Biomed. 2002;6:218–223. doi: 10.1109/titb.2002.802378. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:1–6. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]