Abstract
Enhanced leukocytic infiltration into pancreatic islets contributes to inflammation-based diminutions in functional β-cell mass. Insulitis (aka islet inflammation), which can be present in both T1DM and T2DM, is one factor influencing pancreatic β-cell death and dysfunction. IL-1β, an inflammatory mediator in both T1DM and T2DM, acutely (within 1h) induced expression of the CCL20 gene in rat and human islets and clonal β-cell lines. Transcriptional induction of CCL20 required the p65 subunit of NF-κB to replace the p50 subunit at two functional κB sites within the CCL20 proximal gene promoter. The NF-κB p50 subunit prevents CCL20 gene expression during unstimulated conditions and overexpression of p50 reduces CCL20, but enhances cyclooxygenase-2 (COX-2), transcript accumulation after exposure to IL-1β. We also identified differential recruitment of specific co-activator molecules to the CCL20 gene promoter, when compared with the CCL2 and COX2 genes, revealing distinct transcriptional requirements for individual NF-κB responsive genes. Moreover, IL-1β, TNF-α and IFN-γ individually increased the expression of CCR6, the receptor for CCL20, on the surface of human neutrophils. We further found that the chemokine CCL20 is elevated in serum from both genetically obese db/db mice and in C57BL6/J mice fed a high-fat diet. Taken together, these results are consistent with a possible activation of the CCL20-CCR6 axis in diseases with inflammatory components. Thus, interfering with this signaling pathway, either at the level of NF-κB-mediated chemokine production, or downstream receptor activation, could be a potential therapeutic target to offset inflammation-associated tissue dysfunction in obesity and diabetes.
Keywords: Chemokine, Cytokine, Diabetes, Inflammation, Innate Immunity, Transcription
1.1 Introduction
Type 1 (T1DM) and Type 2 diabetes mellitus (T2DM) are endocrine diseases associated with inflammation in several tissues, including pancreatic islets [1–3]. Pro-inflammatory cytokines, such as IL-1β and IFN-γ, initially impair islet β-cell function and are ultimately cytotoxic [4–6]. Pancreatic β-cells express high levels of the interleukin-1 receptor I (IL-1RI), making them exquisitely sensitive to IL-1β [5, 7]. Indeed, transcriptional reprogramming of pancreatic β-cells begins as early as one hour after exposure to IL-1β and induces expression of genes encoding inducible nitric oxide synthase, cyclooxygenase-2 [8–10] and various chemokines [2, 11–14]. Activation of the NF-κB pathway is central to transcriptional initiation of these genes and corresponding inflammatory responses.
The NF-κB family of transcription factors participates in diverse conditions associated with pathological outcomes, including aging [15], arthritis [16], cancer [17], neuroinflammation [18, 19], and metabolic disease [1]. There are five NF-κB subunits, designated as p65 (RelA), RelB, c-Rel, p50 (NFKB1), and p52 (NFKB2). Homo- and hetero-dimers consisting of combinations of these individual subunits are assembled after cellular exposure to specific stimuli [20]. Prior to such a stimulus, inhibitors of kappa B proteins (IκBs) specifically sequester NF-κB transcriptional regulators in the cytoplasm. Receptor activation promotes signaling cascades that lead to phosphorylation-induced degradation of the IκBs. This degradation exposes a nuclear localization signal within particular subunits (e.g., p65), culminating in nuclear accumulation of transcriptionally competent dimers [21]. Once in the nucleus, these dimeric NF-κB transcription factors recognize defined genomic response elements (i.e., κB sites) to initiate transcription.
The highly specific and controlled transcriptional activation of NF-κB target genes is a major route to tissue inflammation. Examples of such genes include the chemokine family of soluble secreted proteins that coordinate immune cell recruitment and activation in sites of inflammation [22, 23]. For example, elevation of CCL2 is associated with an increase in adipose tissue macrophages and CCL2 abundance positively correlates with body mass index [24, 25]. In addition, transgenic overexpression of CCL2 directly from pancreatic β-cells is sufficient to produce insulitis (i.e., islet inflammation) and diabetes [26]. In addition to CCL2, a wide variety of other chemokines are induced in rodent and human islets exposed to IL-1β [11, 27]. The ensuing increase in global chemokine receptor signaling upon response to these chemokines is associated with both pathological inflammation and poor islet transplant outcomes [28, 29].
The gene encoding the chemokine CCL20 (previously called exodus-1, LARC, and MIP-3α) was originally cloned from human pancreatic islet tissue [30]. Since its initial cloning, CCL20 has been implicated in a diverse set of inflammatory conditions, including rheumatoid arthritis [31], multiple sclerosis [32] and islet viral infection [33]. Indeed, CCL20 is able to chemoattract both adaptive and innate immune cells, including CD4+ and CD8+ T-cells [34, 35], dendritic cells [36], B-lymphocytes [37, 38] and natural killer (NK) cells [39]. Collectively, these data place CCL20 as a probable contributor to the development of T1DM, T2DM, or both endocrine diseases. However, very little is known about the role of CCL20 in obesity and diabetes and the molecular mechanisms mediating CCL20 gene regulation in pancreatic β-cells are poorly understood.
Herein, we demonstrate that CCL20 abundance is increased in islets from obese mice and that IL-1β strongly and rapidly promotes expression of the CCL20 gene in rat and human pancreatic β-cells. This transcriptional activation results from the highly-regulated and opposing activity of the p65 and p50 subunits of NF-κB. The fact that serum levels of CCL20 are elevated in both genetically obese db/db mice as well as in C57BL6/J mice on a high-fat diet provides links between obesity, CCL20, islet inflammation, and diabetes. Finally, we also report that inflammatory cytokines promote surface expression of CCR6, the only known receptor for CCL20 [40], in human peripheral blood neutrophils. Neutrophils are critical for initiating autoimmune-mediated β-cell destruction in NOD mice [41] and also accumulate in the human pancreas prior to diabetes onset [42, 43]. Thus, our results provide evidence that inflammatory signals presently linked with the development of obesity and diabetes also enhance CCL20 production in islet β-cells and induce the corresponding receptor (CCR6) on human neutrophils.
2.1 Materials and Methods
2.1.1 Cell culture, islet isolation and reagents
Culture and passage of the 832/13[44] and INS-1E [45] rat insulinoma cell lines have been described. Human islets were from obtained via Lonza (Clonetics™ Fresh Human Pancreatic Islets). All reagents were from Fisher Scientific, unless otherwise noted.
2.1.2 Animal Models, Pancreatic Islet Isolation, and RNA extraction from Isolated Mouse Islets
Seven week old male lean (db/+) and obese (db/db) mice (B6.BKS(D)-Leprdb/J) were obtained from the Jackson Laboratory and housed for one week with free access to food and water prior to isolation of islets. For the islet isolation, eight week old mice were first euthanized via CO2 asphyxiation followed by a cervical dislocation. Blood was collected via cardiac sticks and was used in ELISA assays for determination of serum CCL2 and CCL20 levels. Blood was also collected from 24 week old C57BL6/J mice fed ad libitum for 18 weeks with purified diets containing different fat contents (10, 25, and 60% kcal). All procedures were approved by the appropriate PBRC, Duke University, or UT Medical Center animal care and use committees.
For isolation of mouse islets, a 20 mL collagenase solution was prepared containing 1X HBSS, 4 mM sodium bicarbonate, 375 µL cold 1M HEPES buffer (Gibco, Grand Island, NY), 90 µg DNase 1 (Roche, Indianapolis, IN), and 0.18 mg Liberase TL (Roche). The ampulla of Vater was cannulated and the collagenase solution slowly infused into the common bile duct to perfuse the pancreas. After perfusion, the pancreas was excised and placed in a 50 mL Falcon tube containing 7 mL of the enzyme solution. The pancreas was periodically shaken while incubating for 15 minutes in a 37°C water bath. After 15 minutes, the pancreas was shaken vigorously for ten seconds before digestion was stopped with ~40 mL of quenching buffer (1X HBSS, 4 mM sodium bicarbonate, 27.5 mL fetal bovine serum (FBS), and 50 µL DNase at 100 mg/mL). This mixture was plunged several times through a 14 g blunt tipped needle to further homogenize the tissue. The homogenate was passed through a 400 µm screen (Bellco, Vineland, NJ) into a new Falcon tube to filter out large pieces of undigested exocrine tissue. The tube was spun at 250 × g at 4°C for 3 minutes, after which the supernatant was discarded. The pellet was suspended in a separate Falcon tube with 7 mL of a Polysucrose 400 (Fisher Scientific, Pittsburgh, PA) solution at 1.109 g/mL. A gradient was then produced by carefully layering 7 mL of 1.096 g/mL on top, followed by 1.070 g/mL and 0.570 g/mL. The gradient was spun at 1000 × g for 20 minutes at 4°C. The islets were located at the interface between the 1.070 g/mL and 0.570 g/mL layers, and a bulb pipette was used to extract the islets from the layer and into a new tube containing 40 mL of quenching buffer. The islets were washed in quenching buffer by centrifuging at 250 x g for 3 minutes. The supernatant was discarded and the wash step was repeated. The resulting pellet was suspended in 10 mL of RPMI 1640 media (11 mM glucose) supplemented with 10% FBS, penicillin (100 units/mL), streptomycin (100 µg/mL), and amphotericin B (2.5 µg/mL), and subsequently plated. Isolated islets were imaged using an Olympus CK40 inverted microscope and incubated at 37°C and 5% CO2 for ~24 hours. Isolation of rat islets has been described previously [46].
After 24 h of incubation following isolation, the islets were hand-picked into a clean 6-well plate, cultured an additional 24 h, then treated as described in the figure legends, suspended in 300 µL of RLT buffer and frozen at −80°C. RNA was isolated using Qiagen RNeasy microkit according to the manufacturer.
2.1.3 Construction of plasmids
A 3kb PCR product corresponding to −3kb of the rat CCL20 promoter was generated using the following primer sequences: (F): 5’aaaaaACGCGTTGTGGCCAATGTTCTAGTTGAC 3’ and (R): 5’aaaaa CTCGAGTTACACACTCCTTAGCAGGTTG 3’. The PCR product was digested with MluI and XhoI restriction enzymes and then inserted into the pGL3 luciferase reporter plasmid between the MluI (ACGCGT) and XhoI (CTCGAG) restriction sites. The cloned and verified −3kb promoter sequence was deposited in GenBank (Accession # KC572139.1).
2.1.4 Site-directed Mutagenesis
Proximal and distal NF-κB mutations in the −3kb-luciferase plasmid were generated by site-directed mutagenesis using the QuikChange Site-Directed Mutagenesis kit according to manufacturer’s instructions (Agilent Technologies). The following primer pairs were used to incorporate these mutations (mutations are in bold, underlined, and lowercase): proximal NF-κBm: (F) 5’CAGATTAATCAATGGGG cc AAACCCCGGGTGAGAAC 3’ and (R) 5’ GTTCTCACCCGGGGTTT gg CCATTGATTAATCTG 3’, distal NF-κBm #1: (F) 5’GCTCTCATTGGTGAGGG cc CTTTACTTCCTGTCTTC 3’ and (R) 5’GAAAGACAGGAAGTAAAG gg CCCTCACCAATGAGAGC 3’ and distal NF-κBm #2: (F) 5’ CTCTCATTGGTGAG tt GA aa TTACTTCCTGTCTTTCTCG 3’ and (R) 5’ CGAGAAAGACAGGAAGTAA tt TC aa CTCACCAATGAGAG 3’. Mutations were confirmed by DNA sequencing at the Pennington Biomedical Research Center Genomics Core Facility.
| WT | Mutant | |
|---|---|---|
| proximal NF-κB | GGGAAAACCC | GGccAAACC |
| distal NF-κB | GGGACTTTAC | Mut #1: GGccCTTTAC |
| Mut #2: ttGAaaTTAC |
2.1.5 Transfection and Luciferase Assays
Transient transfection of plasmid vectors into 832/13 cells was performed using TransFectin Lipid Reagent (Biorad) according to the manufacturer’s instructions. Following the transfection, cells were lysed in 1x Passive Lysis Buffer (Promega) and luciferase activity was measured using the Luciferase Assay System (Promega) in a GloMax plate reading luminometer (Promega). Luciferase activity was normalized to total protein content as determined by BCA assay (Promega).
2.1.6. RNA isolation, cDNA synthesis and real-time RT-PCR
RNA was isolated from cells using Isol-RNA Lysis Reagent (5 Prime Inc., Gaithersburg, MD) and from human, rat and mouse islets using the RNeasy kit (QIAGEN). cDNA synthesis and real-time PCR analysis were as described (AJP). Primers used to detect mRNA abundance of RS9, Ccl20, Ccl2 and Cox2 were designed using Primer3Plus software and are available upon request. Transcript abundance was normalized to the housekeeping gene Ribosomal S9 (RS9).
2.1.7 ELISA
Circulating levels of CCL2 and CCL20 were analyzed using serum samples from C57BL/J and db/db mice obtained from the Jackson Laboratories. For in vitro experiments, the release of CCL2 and CCL20 into cell culture medium was detected. All samples were analyzed using R&D Quantikine ELISA kits (R&D systems, Minneapolis, MN) according to the manufacturer’s protocol. Release of CCL2 and CCL20 into the culture medium was normalized to total protein to account for any potential variability in cell number.
2.1.8 Protein isolation and immunoblotting
Whole-cell lysate preparation and immunoblotting were performed as described [13]. Antibodies used were from the following sources: anti-p65 (#8242), anti-p50/105 (#12540), and anti- β-Actin (#8457) were all from Cell Signaling Technology while anti-IκBα (sc-371) was from Santa Cruz.
2.1.9 Chromatin Immunoprecipitation
832/13 cells were cultured in 15-cm dishes, using one dish per treatment condition. After treatment of cells as indicated in the figure legends, the culture medium was aspirated, formaldehyde was added at a final concentration of 1% in PBS and incubated at room temperature for 10 minutes. At the end of the crosslinking, glycine was added to a final concentration of 125 mM for 5 minutes at room temperature. Cells were washed once with cold PBS and scraped into 1-mL prechilled PBS with 1× protease inhibitors (Halt Protease Inhibitor Cocktail; Thermo Fisher Scientific). Cell pellets were collected by centrifugation at 1000g for 5 minutes at 4°C. Supernatants were discarded, and pellets were reconstituted in 0.5 mL of SDS lysis buffer containing 1× protease inhibitors (1% SDS, 10 mM EDTA, and 50 mM Tris-HCl; pH 8.0). Lysates were incubated on ice for 15 minutes, and then DNA was sheared by sonication using a Bioruptor Pico (Diagenode). The following sonication conditions generated fragments of DNA between 100 and 500 bp in length: cycle on: 30 seconds, cycle off: 30 seconds for a total of 90 cycles. Sonicated fragments were centrifuged at 12,000g for 10 minutes at 4°C, and supernatants were transferred to a clean microcentrifuge tube. Dynabeads were prepared as follows: once resuspended, 10 µL of beads were transferred to a new microcentrifuge tube for each condition. The tubes were placed in a magnetic rack to separate the beads out of solution, and supernatants were removed. 1 µg antibody in 200 µL PBS with 0.02% Tween-20 was added to the beads and incubated for 10 mins at RT with rotation. Tubes were placed on the magnetic rack and supernatants were removed. Bead-antibody complexes were washed with 200 µL PBS with 0.02% Tween-20. 250 µg of sheared cross-linked DNA was diluted 10-fold in dilution buffer containing 1 × protease inhibitors (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl, and 17 mM Tris, pH 8.0), added to tubes containing dynabead-antibody complexes and incubated overnight at 4°C with rotation. The following day, dynabead-antibody-chromatin complexes were washed sequentially with 0.5 mL of the following buffers: 1× low-salt buffer, (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl, and 20 mM Tris; pH 8.0), 1× high-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 500 mM NaCl, and 20 mM Tris; pH 8.0); and 1× LiCl buffer (0.25 M LiCl, 1% NP-40, 1 mM EDTA, and 10 mM Tris; pH 8.0), and 2× Tris EDTA. Once the last wash buffer was completely removed, 100 µL of 10% Chelex-100 resin in PBS was added, vortexed for 10 s, and heated to 100°C for 10 minutes. After centrifugation at 17,000g for 1 minute at 4°C, the supernatants were transferred to new tubes. Then 120 µL of RNase and DNase-free water was added, vortexed for 10 s, and centrifuged at 17,000g for 1 minute at 4°C. Supernatants were pooled. Inputs were processed as followed: 20 µL of sonicated, precleared DNA was incubated overnight at 65°C with NaCl to a final concentration of 200 mM. Input DNA was then treated with RNase A for 30 minutes at 37°C and proteinase K for 1 hour at 45°C and then purified using a QIAGEN Cleanup kit (QIAGEN); 2.5 µL of the purified DNA was used as a template for PCR. Primer sequences for amplifying the Ccl20, Ccl2 and Cox2 NF-κB elements and coding regions were generated using Primer3Plus software and are available upon request. Antibodies used for immunoprecipitation were from Santa Cruz Biotechnology, Inc. (p65 and p50) while normal rabbit serum (IgG) was obtained from Sigma-Aldrich.
2.1.10 Pancreas Histology and Immunohistochemistry
Pancreata were fixed via immersion in 10% neutral buffered formalin for 48 hours prior to paraffin processing, embedding, and sectioning at three microns. All immunohistochemistry steps were performed on a Leica Bond Max auto-immunostainer running a modified IHC protocol. Briefly, slides were dewaxed and subjected to HIER using Leica’s epitope retreival solution 2 (ER2, pH 9.0) for 20 minutes prior to primary antibody incubation (abcam anti-CCL20 [ab9829], 1:100 dilution) for 1 hr. Detection was accomplished using Leica’s Bond Polymer Refine Detection Kit and a modified “Protocol F” by incubating with anti-rabbit polymer for 8 minutes followed by a peroxide block for 5 minutes, 10 minutes of DAB reagent, and then counterstain with hematoxylin for 5 minutes.
2.1.11 Human PBN Isolation and Stimulation
Human PBNs were isolated from EDTA-treated blood from healthy human volunteers using dextran sedimentation and density gradient centrifugation as previously described [47]. PBNs were re-suspended in PBS and stimulated with 1ng/ml of either IL-1β, TNF-α, or IFN-γ or left unstimulated for 30min at 37°C. Cell surface expression of CCR6 and CXCR2 was measured by a FACScalibur (BD Biosciences) instrument after staining with monoclonal antibodies (Biolegend) and data analyzed using FlowJo software (v10). The use of human subjects has been approved by the University of Tennessee Institutional Review Board (IRB# 6476B).
2.1.12 Statistics
One way ANOVA with Tukey’s post hoc test or Student’s t-test was performed using GraphPad Prism 6.0. The corresponding p values are given in the figure legends.
3.1 Results
3.1.1 IL-1β increases CCL20 gene transcription in rat and human islets and β-cell lines
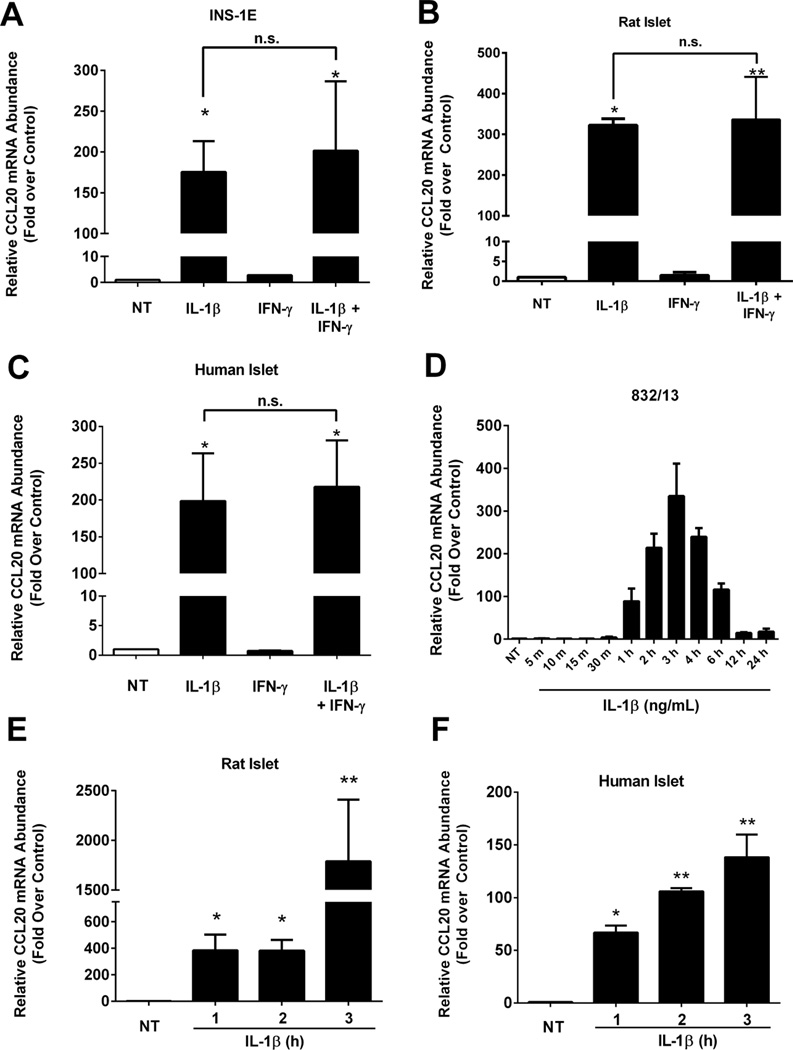
IL-1β is one of the major cytokines leading to islet inflammation in T1DM and T2DM [48] and global deletion of the IL-1RI reduces metabolic sequelae that arise due to high-fat feeding [49]. In INS-1E cells, a rat insulinoma cell line, 1ng/mL IL-1β increased the abundance of CCL20 mRNA 175-fold over the untreated control (Figure 1A). Similar results were obtained using isolated rat islets (322-fold increase; Figure 1B) and isolated human islets (198-fold increase; Figure 1C). Exposure to IFN-γ alone did not induce expression of the CCL20 gene and the combination of IFN-γ plus IL-1β was unable to augment transcript levels over IL-1β alone (Figure 1A–C).
Figure 1. IL-1β induces expression of the CCL20 gene in human and rat islets and β-cell lines.
A. INS-1E rat insulinoma cells were untreated (NT) or treated with 1ng/mL IL-1β, 100 U/mL IFN-γ or both cytokines for 3 h. *, P < 0.05 vs. NT, n.s. = not significant. B. Isolated islets from Wistar rats were untreated (NT) or treated for 3 h with 10 ng/mL IL-1β, 100 U/mL IFN-γ or both cytokines. **, P < 0.01 vs. NT, *, P < 0.05 vs. NT, n.s. = not significant. C. Human islets were untreated (NT) or stimulated for 3 h with 10 ng/mL IL-1β, 100 U/mL IFN-γ or both cytokines.*, P < 0.05 vs. NT, n.s. = not significant. D. 832/13 rat insulinoma cells were untreated (NT) or treated with 1 ng/mL IL-1β for the indicated times. E. Rat islets were untreated (NT) or stimulated for 1, 2 or 3 h with 10 ng/mL IL-1β. **, P < 0.01 vs. NT, *, P < 0.05 vs. NT. F. Human islets were untreated (NT) or stimulated for 1, 2 or 3 h with 10 ng/mL IL-1β. **, P < 0.01 vs. NT, *, P < 0.05 vs. NT. A–F. Transcript data are shown as means ± SEM from three individual experiments.
Time course analysis revealed that the CCL20 gene was induced as early as one hour after exposure to IL-1β (Figure 1D). This observation was confirmed in both isolated rat islets (Figure 1E) and human islets (Figure 1F), where CCL20 mRNA synthesis increased as early as 1h with a peak at 3h post-IL-1 exposure. This rapid and robust increase in CCL20 expression is not dependent on new protein synthesis, indicating CCL20 is a primary response gene in pancreatic β-cells. Indeed, inhibition with cycloheximide had no effect on IL-1β-mediated transcript accumulation (data not shown). Because the two clonal rat β-cell lines, rat islets and human islets all display similar responses after IL-1β exposure, the 832/13 rat β-cell line was used for the remainder of the studies presented below.
3.1.2 The RelA/p65 subunit of NF-κB is required for expression of the CCL20 gene
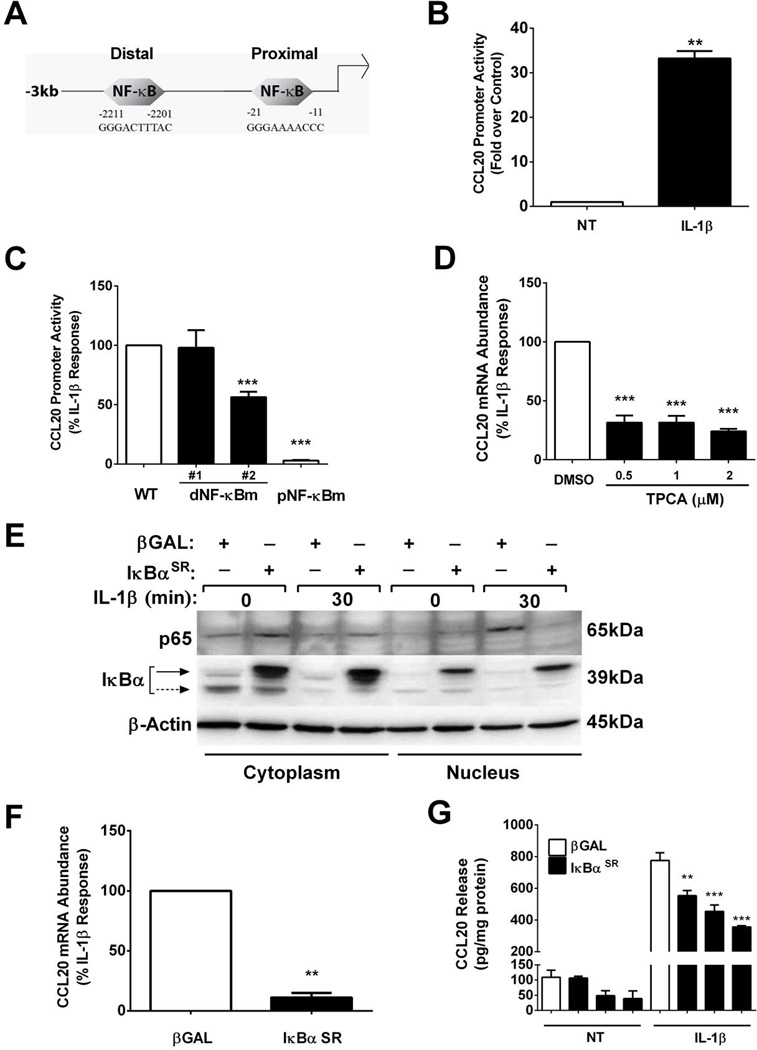
The CCL20 gene is a primary response gene in pancreatic β-cells (Figure 1). Using in silico analysis, we identified two potential κB genomic elements within −3kb (relative to the transcriptional start site) of the CCL20 gene promoter (Figure 2A). We cloned this −3kb proximal promoter region, which contains both of the predicted κB responsive elements shown in Figure 2A, into a luciferase reporter vector. This reporter construct displayed a 33-fold increase in transcriptional activity after exposure to IL-1β (Figure 2B).
Figure 2. IL-1β-mediates upregulation of the CCL20 gene through IκKβ, IκBα and p65.
A. Schematic representation of −3kb region of the CCL20 promoter, showing the distal and proximal κB sites. B. 832/13 cells were transfected with a luciferase reporter construct containing −3kb of the CCL20 proximal promoter. 24 h after the transfection cells were exposed to IL-1β (1 ng/mL) for 4 h. **, P < 0.01 vs. NT. C. 832/13 cells were transfected with the wild-type (WT) CCL20 −3kb promoter- luciferase construct or −3kb promoter-luciferase constructs containing mutations in either the distal (dNF-κBm) or proximal (pNF-κBm) NF-κB response elements. 24 h post-transfection cells were untreated or treated for 4 h with 1 ng/mL IL-1β (maximal IL-1β response is set at 100%). ***, P < 0.001 vs. WT. B–C. Promoter activity is shown as means + S.E.M. D. 832/13 cells were pre-treated for 1 h with either DMSO (vehicle control) or the indicated concentrations of TPCA. Cells were subsequently treated for 3 h with 1 ng/mL IL-1β. ***, P < 0.001 vs. DMSO. E. 832/13 cells were transduced with adenoviruses overexpressing β-Galactosidase (βGAL) or IκBα superrepressor (SR). After a 24 h exposure to adenovirus, cells were stimulated with 1 ng/mL IL-1β for 30 mins. Cells were fractionated and immunoblotted to determine relative abundance of p65 and IκBα in these respective subcellular fractions. β-Actin served as a loading control. Solid arrow= adenovirally overexpressed IκBα; dashed arrow = endogenous IκBα. A representative image of two immunoblots is shown in E. F. 832/13 cells were transduced with adenoviruses expressing βGAL or IκBαSR Following a 24 h exposure to adenoviruses, cells were stimulated for 3 h with 1 ng/mL IL-1β. **, P < 0.01. D–F. Relative mRNA abundance of CCL20 is shown. G. 832/13 cells were exposed to adenoviruses expressing either βGAL or IκBαSR for 12 h, followed by 12 h stimulation with 1 ng/mL IL-1β. CCL20 release into the media was quantified by ELISA assay and normalized to total protein content. **, P < 0.01 vs. βGAL-IL-1β control, ***, P < 0.001 vs. βGAL-IL-1β control. For promoter activity, mRNA and ELISA experiments three individual replicates were generated and expressed as means ± SEM.
To investigate the importance of the κB responsive elements, we generated multiple mutations within these two predicted κB sites (Figure 2C). The wild-type promoter-luciferase construct was induced 24.4-fold (set at 100% in Figure 2C). The transcriptional activity of the CCL20 gene promoter sequence was unaffected by conservative mutations within the distal κB element (dNF-κBm #1; Figure 2C; shown as a percentage of the wild-type promoter). However, further divergence from a consensus κB sequence (dNF-κBm #2) produced a 50% decrease in IL-1β-driven promoter activity. By contrast, mutations in the proximal κB genomic element (pNF-κBm), a response sequence closer to the transcriptional start site, abolished reporter expression in response to IL-1β (Figure 2C).
Having established that functional κB sites are required to drive transcription in response to IL-1β, we next examined the requirement for signaling through IKKβ, a known component of the canonical NF-κB pathway linked to IL-1R activation [50]. Using 2-[(Aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide (TPCA), an IκKβ inhibitor, there was a 69–76% decrease in IL-1β-mediated CCL20 mRNA accumulation over a 0.5 – 2µM concentration range (Figure 2D). We note that this concentration range of TPCA is sufficient to protect against cytokine-induced β-cell death [5] and also illustrates the importance of IκKβ in regulating transcription of the CCL20 gene.
The IKK complex phosphorylates the IκBα proteins, leading to their degradation, with subsequent release of p65 for translocation to the nucleus to initiate transcriptional activation. In order to further define the how NF-κB pathway proteins control CCL20 expression, we expressed a mutant form of IκBα (IκBαSR; SR= super-repressor) containing S32A/S36A amino acid substitutions that render the protein resistant to phosphorylation-induced degradation [51]. As expected, adenoviral delivery of the IκBαSR construct to 832/13 cells prevented the IL-1β-mediated increase in p65 nuclear accumulation (Figure 2E). Blocking p65 movement to the nucleus resulted in an 89% decrease in CCL20 mRNA abundance after cellular exposure to IL-1β (Figure 2F). Overexpression of the IκBαSR, relative to the βGal control, reduced CCL20 promoter activity by 77% (data not shown), while CCL20 secretion into the media was diminished by 54% (Figure 2G). siRNA-mediated decreases in p65 also prevented IL-1β-mediated increases in CCL20 synthesis and secretion (not shown). These data demonstrate the importance of the IκKβ/IκBα control of NF-κB transcription factors for regulation of the CCL20 gene.
3.1.3 IL-1β signaling displaces the inhibitory p50 subunit concomitant with binding of the p65 subunit of NF-κB to the gene promoters of CCL2 and CCL20, but not COX2
We observed increased expression of the CCL2 [14] and CCL20 (Figure 2) genes in pancreatic β-cells after IL-1β exposure. Because the p65 and p50 NF-κB protein subunits are considered to be the prototypical dimerization partners promoting stimulus-specific transcription [50], their occupancy at sites containing either the distal or proximal κB sites within the CCL20 gene promoter region was measured using chromatin immunoprecipation assays. CCL2 and COX2 were examined in comparison because they either do (COX2) or do not (CCL2) require p50 for their expression in response to IL-1β [9, 14].
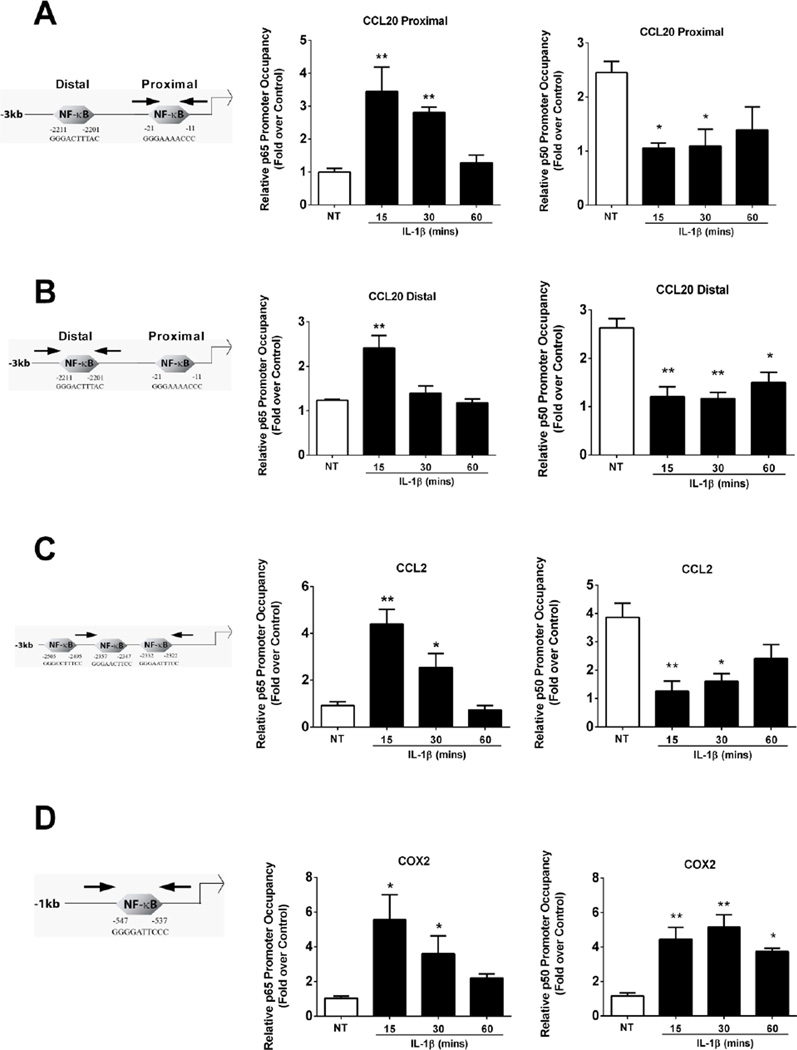
We observed a time dependent increase in p65 occupancy at each of the predicted κB elements within the CCL20 gene promoter, which was maximal at 15 min after exposure to IL-1β (Figure 3A; arrows denote the regions spanned by PCR primers). By contrast, the p50 subunit occupied these same κB genomic regions in the basal (unstimulated) state, but was displaced concomitant with the IL-1β-mediated arrival of p65 (compare Figures 3A and 3B middle panels with right panels).
Figure 3. p50 is displaced from CCL2 and CCL20, but recruited to the COX2 gene promoter, in response to IL-1β.
A–D. 832/13 cells were untreated (NT) or stimulated with 1 ng/mL IL-1β for 15, 30 or 60 mins. ChIP assays were performed to determine relative occupancy of p65 (middle panels) and p50 (right panels) on the CCL20 proximal (A) and distal promoter (B), and on the CCL2 (C) and COX2 (D) promoters. **, P < 0.01 vs. NT, *, P < 0.05 vs. NT. Data are shown as means ± SEM from 3-4 individual experiments. A- D left panels are schematic representations of the indicated promoters. PCR primers amplifying a given gene promoter region are denoted schematically by the left and right facing arrows.
To understand whether this pattern of promoter residence is consistent for other NF-κB target genes induced by IL-1β, we also measured the p65 and p50 occupancy on the CCL2 and COX2 promoters. CCL2 [14, 52] and COX2 [9, 53] both contain κB responsive elements within their gene promoters. For CCL2, we detected a similar NF-κB subunit occupancy pattern as that observed for CCL20, with p65 robustly recruited to κB sites (Figure 3C) while p50 occupancy was lost upon IL-1β exposure (Figure 3C middle panel vs. right panel). By contrast, p65 and p50 were simultaneously recruited to the COX2 gene promoter in response to IL-1β (Figure 3D; middle and right panels). No p50 occupancy was detected at κB sites in the COX2 gene promoter in the basal state (Figure 3D; right panel). These data are consistent with our previous observations, that in response to IL-1β, p50 is required for expression of the COX2 [9], but not the CCL2 gene [14]. Thus, in terms of NF-κB subunit specificity at these particular gene promoters, which are all responsive to IL-1β, CCL20 shows similarities for subunit binding with CCL2, while COX2 clearly displays a different NF-κB subunit requirement when compared to the two chemokine genes.
3.1.4 IκBαSR prevents p65, but not p50, binding to IL-1β responsive gene promoters
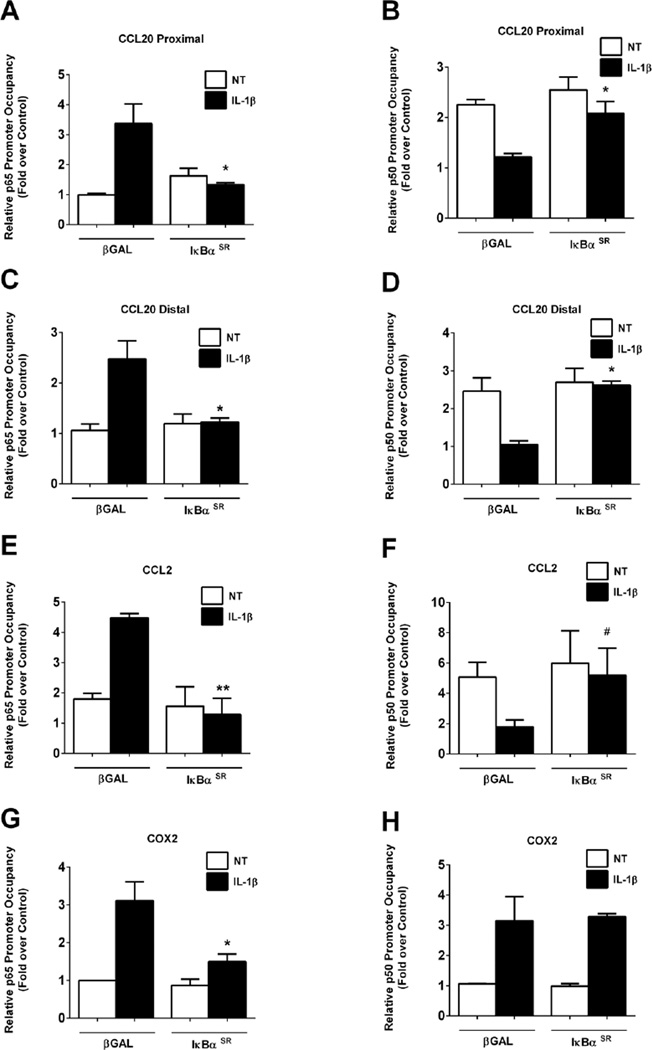
Because IL-1β induces rapid binding of p65 to specific κB response elements concomitant with displacement of p50 (Figure 3), we investigated whether restricting p65 translocation to the nucleus with the IκBαSR would modify the differential NF-κB subunit occupancy at the CCL20 and CCL2 gene promoters. IL-1β induces a 3.4-fold increase in p65 binding to the proximal CCL20 κB site, which is inhibited in the presence of the IκBαSR (Figure 4A). In addition, there is a 46% decrease in p50 occupancy at the proximal κB site after exposure to IL-1β; this displacement of p50 from the κB site is blocked in the presence of the IκBαSR (Figure 4B). Almost identical results were obtained at the CCL20 distal κB site (Figures 4C and 4D). We interpret these results to indicate the p65 replaces p50 at the CCL20 gene promoter, an event that is prevented by restricting p65 to cytosol.
Figure 4. Restricting p65 nuclear entry prevents the displacement of p50 from chemokine gene promoters.
A–H. 832/13 cells transduced with adenoviruses overexpressing βGAL or IκBαSR 24 h post-transduction cells were untreated (NT) or stimulated with 1 ng/mL IL-1β for 15 mins. ChIP assays were performed to determine relative occupancy of p65 (A, C, E, G) and p50 (B, D, F, H) on the CCL20 proximal (A, B) and distal promoters (C, D), and on the CCL2 (E, F) and COX2 (G, H) promoters. **, P < 0.01 vs. βGAL (IL-1β), *, P < 0.05 vs. βGAL (IL-1β), #P < 0.1 vs. βGAL (IL-1β). ChIP assay data are expressed as means ± SEM from 3–4 individual experiments.
For the CCL2 gene, IκBαSR also blocked p65 recruitment to κB sites (Figure 4E), which is consistent with the decreased expression of the CCL2 gene by this maneuver [14]. The p50 subunit binding to the CCL2 in the basal state was reduced by 65% after exposure to IL-1β and this IL-1β-mediated displacement of p50 was fully reversed in the presence of the IκBαSR (Figure 4F). In contrast, the COX2 promoter had a 38% reduction in p65 occupancy during overexpression of the IκBαSR (Figure 4G) with no alterations in p50 occupancy (Figure 4H). Thus, there are consistent requirements for CCL20 and CCL2 responses to IL-1β, which differ from NF-κB subunit occupancy during COX2 transcriptional activation.
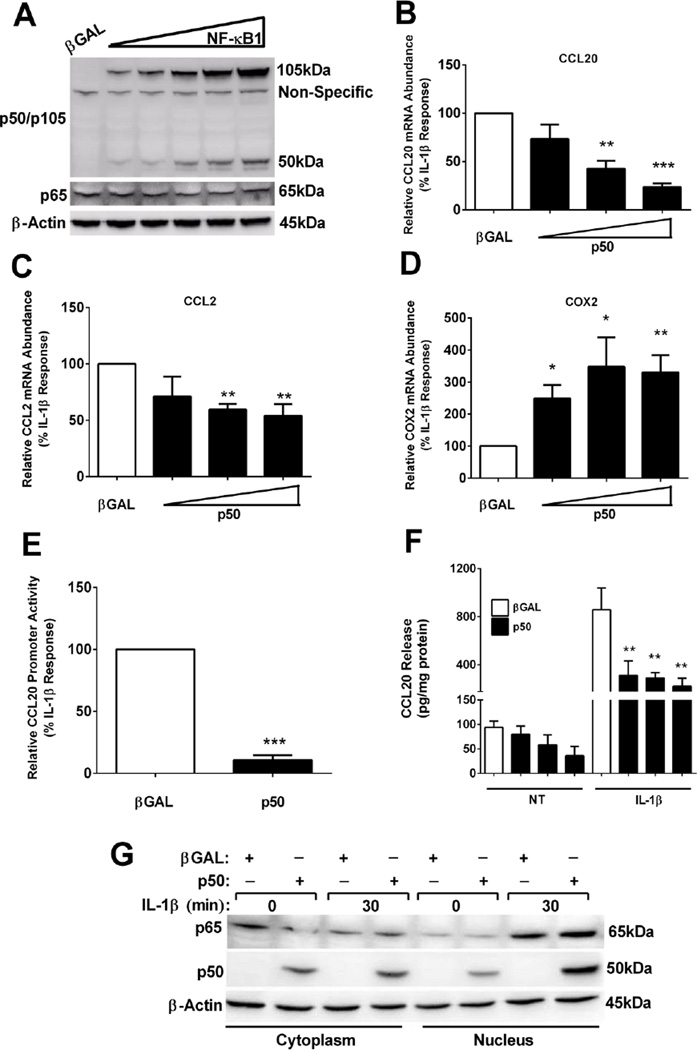
3.1.5 Overexpression of p50 markedly decreases the IL-1β-mediated expression of the CCL20 gene, but enhances the expression of the gene encoding COX2
Overexpression of the NFKB1 gene produces the 105kDa protein that is constitutively processed into the 50kDa p50 subunit (see ref [54] and Figure 5A). Because p50 occupies the promoter region of CCL20 and CCL2 during the basal state, we next investigated whether enhancing the abundance of p50 was sufficient to block the IL-1β-mediated gene expression. To test this possibility, we overexpressed the p50 subunit using adenoviral transduction. CCL2 and COX2 were once again used for comparison purposes. Using the three highest viral concentrations driving expression of p50 (shown in Figure 5A), we observed a dose-dependent reduction (26%, 58%, and 77%, respectively) in IL-1β-mediated CCL20 mRNA levels (Figure 5B) and a 29%, 41%, and 46% suppression of CCL2 gene expression (Figure 5C). By contrast, expression of the COX2 gene was enhanced by 2.49-, 3.47, and 3.31-fold by the combination of p50 overexpression and IL-1β stimulation (Figure 5D). There was a 90% reduction in IL-1β– driven CCL20 promoter activity with p50 overexpression (Figure 5E). This decrease in transcription resulted in a 64%, 66%, and 74% reduction in secreted CCL20 protein (Figure 5F).
Figure 5. p50 overexpression diminishes CCL2 and CCL20 gene expression, but enhances expression of COX2, in response to IL-1β.
A. 832/13 cells were transduced with adenoviruses expressing either βGAL or five increasing concentrations of NF-κB1. 24 h post-transduction whole cell lysates were harvested and immunoblotted for p50/p105 (expressed from the NF-κB1 gene) with p65 and β-Actin shown as the loading controls. The image shown is representative of two independent experiments. B–D. 832/13 cells were exposed to adenoviruses expressing βGAL or increasing concentrations of p50 (as shown in immunoblot in A) for 24 h. Cells were then exposed to 1 ng/mL IL-1β for 3 h. Relative mRNA abundance of CCL20 (B), CCL2 (C) and COX2 (D) are shown. ***, P < 0.001 vs. βGAL, **, P < 0.01 vs. βGAL, *, P < 0.05 vs. βGAL. E. 832/13 cells were transfected with −3kb CCL20 promoter-luciferase construct. 4 h post-transfection cells were transduced with adenoviruses expressing either βGAL or p50 adenovirus overnight, then stimulated for 4 h with 1 ng/mL IL-1β. ***, P < 0.001 vs. βGAL. F. Following a 12 h incubation with adenoviruses expressing βGAL or p50, 832/13 cells were untreated (NT) or stimulated with 1 ng/mL IL-1β for an additional 12 h. CCL20 release into the media is shown. **, P < 0.01 vs. βGAL (IL-1β). G. 832/13 cells were transduced with either βGAL or p50 for a 12 h exposure, followed by a 30 min stimulation with 1 ng/mL IL-1β. Cells were fractionated and immunoblots completed to determine the subcellular location of p65 and p50. β-Actin served as the loading control. The experiment in G was repeated on two separate occasions, and a representative image is shown. Promoter activity, mRNA abundance and ELISA data are expressed as means ± SEM from 3 individual experiments.
The ability of p50 overexpression to repress the expression of the CCL20 gene cannot be explained by simply restricting p65 to the cytoplasm (Figure 5G). Instead, overexpression of the NFKB1 gene, which leads to elevated p50 abundance, blunted the ability of IL-1β to drive expression of the CCL20 and CCL2 genes, while enhancing the expression of the COX2 gene. This is a clear demonstration of distinct functional outcomes that occur via placement of NF-κB subunits at discrete IL-1β-inducible, NF-κB regulated genes.
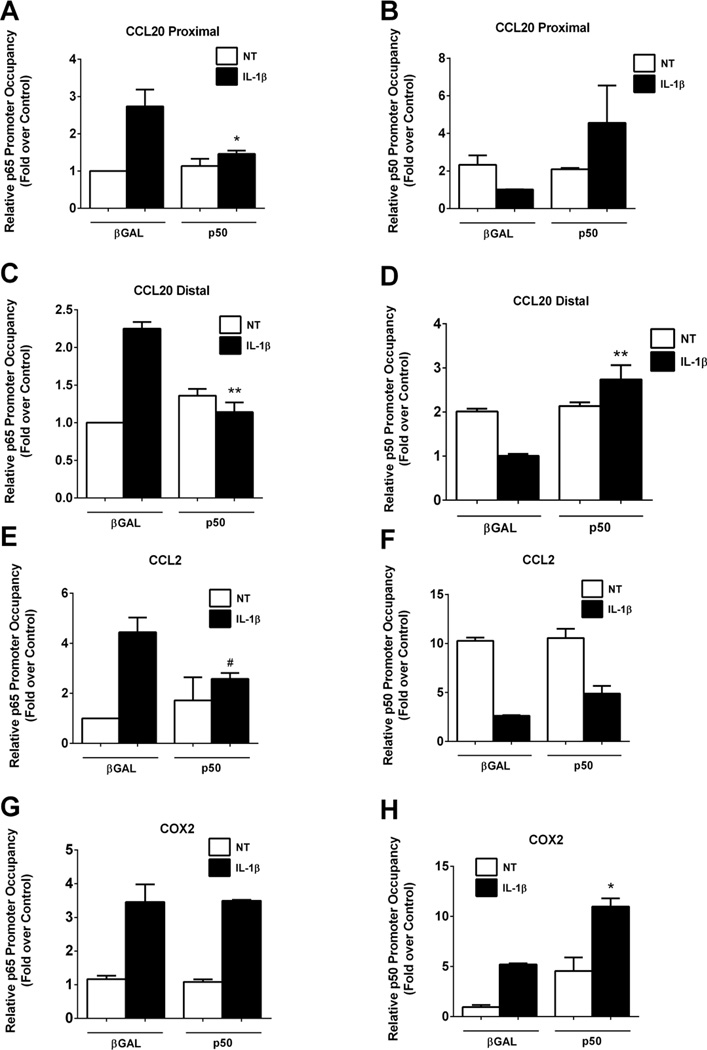
In order to discern whether elevations in p50 abundance modify p65/p50 occupancy at κB genomic elements, we next measured occupancy of each subunit in the presence or absence of p50 overexpression. Overexpression of p50 completely blocked the IL-1β-induced recruitment of p65 to the proximal κB site within the CCL20 gene promoter region (Figure 6A). The displacement of p50 by IL-1β is also prevented by the presence of overexpressed p50 (Figure 6B). Similar results were observed at the distal κB site where overexpression of p50 blocked p65 recruitment (Figure 6C) as well as reversed the IL-1β-mediated exclusion of p50 (Figure 6D). Similarly, at the CCL2 gene promoter, p50 overexpression blocked the p65 recruitment normally induced by IL-1β (Figure 6E). Indeed, there is 46% more p50 associated with the CCL2 promoter after p50 overexpression when compared to the βGal control (Figure 6F). We note that there is not a complete restoration of p50 associated with the κB containing regions within the CCL2 gene promoter as was observed for the CCL20 gene promoter (Figures 6B and 6D). By contrast, the COX2 gene retained IL-1β-stimulated p65 recruitment in both the presence and absence of p50 overexpression (Figure 6G). Moreover, p50 recruitment to the COX2 gene promoter occurred in the absence of IL-1β (basal state) and was equivalent to the IL-1β-induced condition during p50 overexpression (Figure 6H). Furthermore, p50 overexpression plus IL-1β doubled the amount of p50 bound to the COX2 gene promoter (Figure 6H; black bars), consistent with p50 overexpression enhancing the expression of the COX2 gene (Figure 5D).
Figure 6. p50 overexpression blocks recruitment of p65 to the CCL2 and CCL20, but not COX2, gene promoters.
A–H. 832/13 cells were transduced with adenoviruses overexpressing βGAL or p50 (NF-κB1). 24 h post-transduction cells were untreated (NT) or stimulated with 1 ng/mL IL-1β for 15 mins. ChIP assays were performed to determine relative occupancy of p65 (A, C, E, G) and p50 (B, D, F, H) on the CCL20 proximal (A, B) and distal promoters (C, D), and on the CCL2 (E, F) and COX2 (G, H) promoters. **, P < 0.01 vs. βGAL (IL-1β), *, P < 0.05 vs. βGAL (IL-1β), #P < 0.1 vs. βGAL (IL-1β). ChIP data are shown as means ± SEM from 3 individual experiments.
3.1.6 Differential co-activator recruitment to κB target genes in response to IL-1β
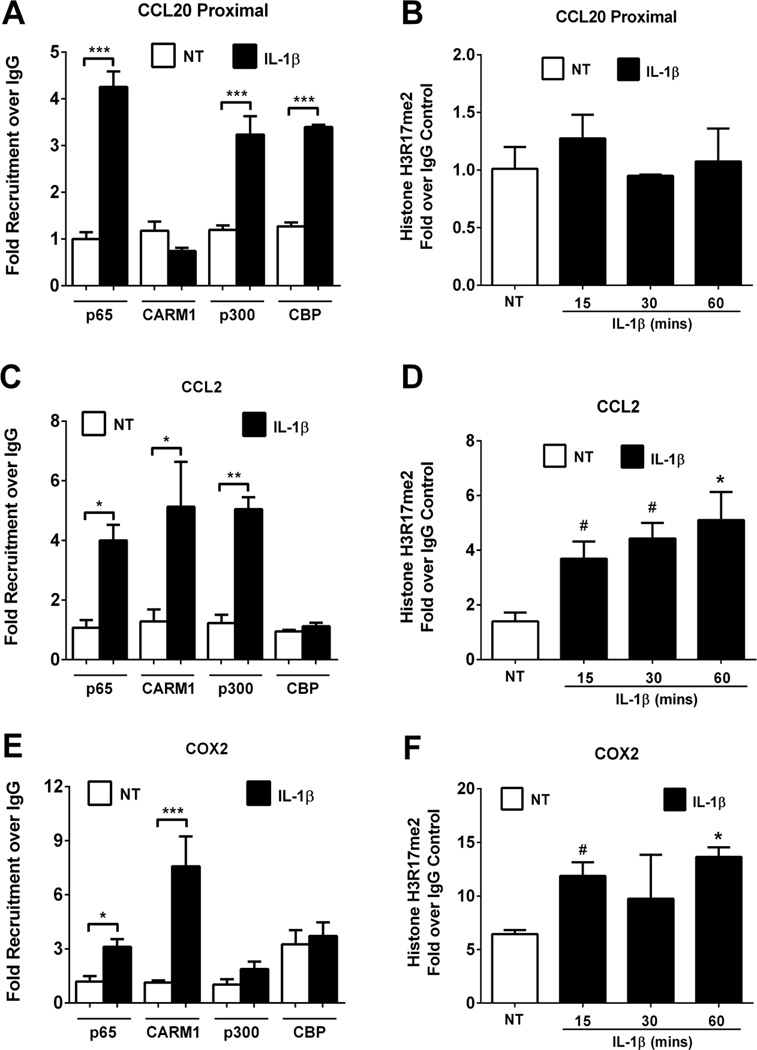
Recruitment of co-activator complexes to a given gene promoter is a critical part of the transcriptional regulatory process [55]. As a starting point to determine how selectivity of NF-κB subunits controls the expression of specific genes in response to IL-1β, we investigated the recruitment of three distinct proteins with known co-regulatory function: coactivator arginine methyltransferase (CARM1), CREB-binding protein (CBP), and p300. The recruitment of each of these proteins was monitored on the CCL20, CCL2, and COX2 gene promoters. These co-activators were chosen based on their established involvement in stimulus-induced transcriptional regulation [56, 57].
Cellular exposure to IL-1β for 15min induced a 4.25 fold increase in p65 binding, but no occupancy of CARM1 at the CCL20 gene promoter (Figure 7A). However, p300 and CBP were both recruited to the CCL20 promoter region in response to IL-1β (Figure 7A). Consistent with a lack of CARM1 occupancy, the CCL20 promoter shows no enrichment in H3R17 methylation, a known target of CARM1 (Figure 7B). By contrast, CARM1 is enriched at the CCL2 gene promoter after cellular exposure to IL-1β (Figure 7C), which is congruent with increased levels of H3R17 methylation (Figure 7D). Interestingly, the CCL2 gene shows enrichment for p300, but not CBP (Figure 7C). Alternatively, the COX2 promoter does not show increased occupancy of either p300 or CBP in response to IL-1β. However, we do note a higher basal level of CBP (~3-fold above background) associated with the COX2 gene promoter, which is unaltered by signaling through the IL-1β pathway (Figure 7E). In addition, the IL-1β-mediated recruitment of CARM1 to the COX2 gene is also associated with attendant elevations in H3R17 methylation at the corresponding COX2 proximal promoter region (Figure 7F). Thus, IL-1β-mediated control of NF-κB target genes occurs via differential co-activator specificities, which are in turn linked to their specific histone post-translational modifications.
Figure 7. Differential co-activator recruitment in response to IL-1β.
A, C, E. 832/13 cells were untreated (NT) or stimulated with 1 ng/mL IL-1β for 15 mins. ChIP assays were used to determine relative occupancy of p65, CARM1, p300 and CBP on the CCL20 proximal promoter (A), and on the CCL2 (C) and COX2 (E) promoters. ***, P < 0.001 vs. NT, **, P < 0.01 vs. NT, *, P < 0.05 vs. NT. B, D, F. 832/13 cells were untreated (NT) or stimulated with 1 ng/mL IL-1β for 15, 30 or 60 mins. ChIP assay of histone H3 dimethylated on Arginine 17 (H3R17me2) is shown at the CCL20 proximal promoter (B), and on the CCL2 (D) and COX2 (F) promoters. *, P < 0.05 vs. NT, #P < 0.1 vs. NT. Data are shown as means ± SEM from 3 individual experiments.
3.1.7 Pro-inflammatory cytokines promote cell surface expression of CCR6 in human peripheral blood neutrophils
Peripheral blood neutrophils (PBNs) are important innate immune cells that contribute to the development of T1DM [41] and are also elevated during obesity [58]. We observed elevated expression of chemokines capable of recruiting neutrophils and other immune cells within pancreatic islets isolated from obese (db/db) mice relative to their lean (db/+) controls (Table 1). In addition, Cox2 and Pges, which are responsible for production of prostaglandin E2, are also elevated in obese islets concomitant with a decrease in the expression of the transcription factors MafA, Nkx6.1, and Pdx-1 (Table 1). These data are consistent with a decrease in islet transcription factor abundance associated with obesity and T2DM in mice and humans [59] and further show that chemokines and other inflammatory genes are coordinately upregulated in pancreatic islets during obesity.
Table 1. Islets from obese (db/db) mice display enhanced expression of inflammatory genes and a decrease in the expression of transcription factors that regulate β-cell identity.
Expression of genes associated with inflammation are shown as the fold increase in obese islets relative to lean (middle column), while islet β-cell transcription factor abundance is given as the percent decrease in obese islets relative to the lean controls (right hand column).
| Gene | Fold Increase (Obese/Lean) |
% Decrease in Obese Islet (Lean = 100%) |
|---|---|---|
| Ccl2 | 8.9 | - |
| Ccl20 | 4.5 | - |
| Cox2 | 3.2 | - |
| Cxcl1 | 6.1 | - |
| Ptges | 5.2 | - |
| Mafa | - | 74 |
| Nkx6.1 | - | 90 |
| Pdx1 | - | 78 |
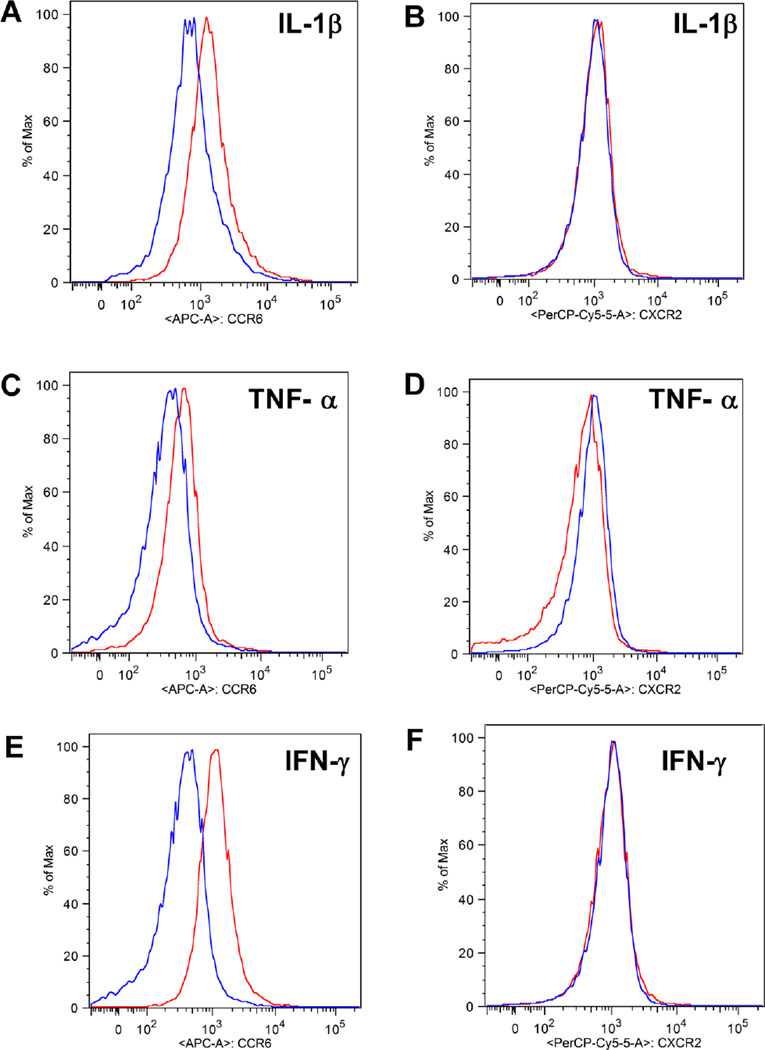
Because the CCL20 ligand is a primary response gene induced by IL-1β in rodent and human islets and clonal β-cell lines, we next investigated whether human peripheral blood neutrophils (PBNs) responded to inflammatory signals by altering their expression of specific chemokine receptors, such as CCR6 and CXCR2. To mimic a local inflammatory state, human PBNs were exposed to IL-1β, TNF-α, or IFN-γ for 30 minutes. All of these cytokines have been linked to islet inflammation, obesity, and/or diabetes [3, 60–63]. We detected elevated levels of CCR6 after exposure to each of these pro-inflammatory cytokines, with IFN-γ producing the largest increase in CCR6 expression (Figure 8A, C, & E). A combination of IL-1β, TNF-α, and IFN-γ did not further induce CCR6 expression over the response seen with the individual cytokines (not shown). By contrast, CXCR2 expression on PBNs was essentially unaltered under identical conditions (Figure 8B, D, & F). Thus, obesity is associated with both elevated cytokine [64, 65] and chemokine expression (Table 1) within pancreatic islets, which could potentially influence the expression of neutrophil chemokine receptors. This change in receptor expression is expected to modify the neutrophil capacity to respond to the local microenvironment.
Figure 8. Exposure to inflammatory cytokines increases expression of CCR6 on PBNs.
Human PBNs were stimulated with 1ng/ml of IL-1β, TNF-α, or IFN-γ or unstimulated for 30min at 37°C. Cell surface detection of CCR6 (A, C, E) and CXCR2 (B, D, F) is shown. The blue line represents cell surface expression during the unstimulated state while the red line represents expression after cytokine stimulation. This is a representative experiment from 5 total experiments.
3.1.8 Circulating CCL2 and CCL20 levels are elevated in mouse models of obesity
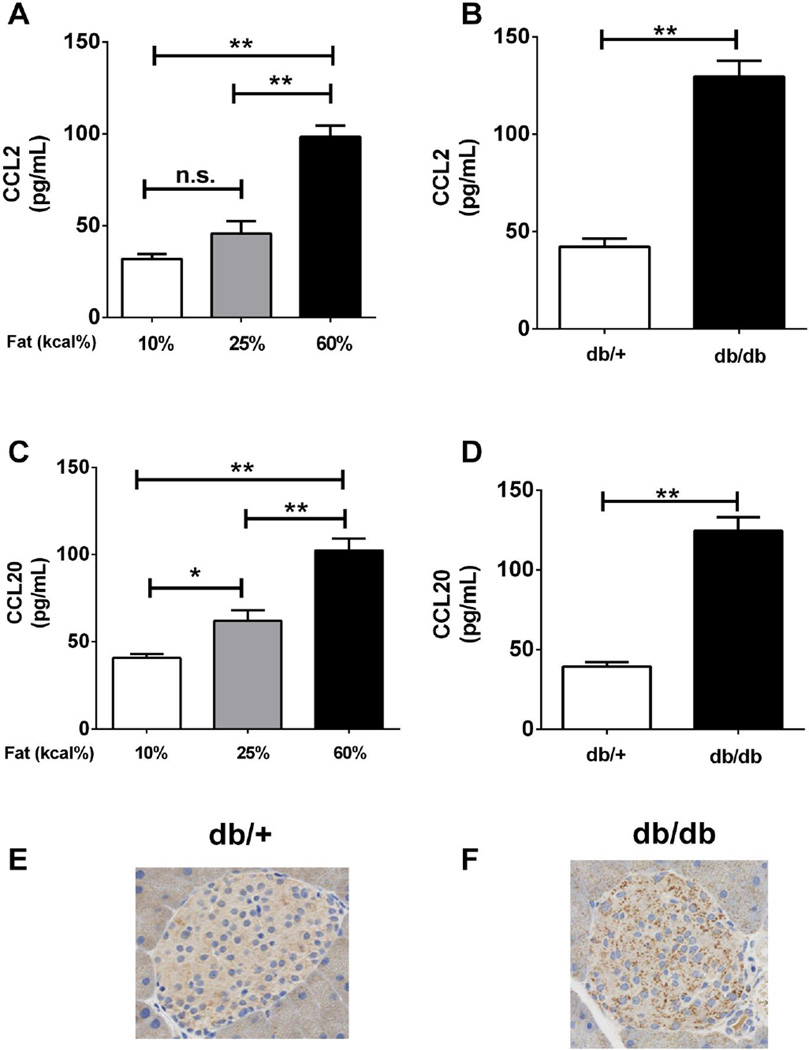
Obesity is associated with both local and systemic inflammation [66, 67] and CCL2 levels are elevated in human obesity [24]. Thus, we first measured CCL2 as a control for chemokine elevation during obesity. Using serum obtained from C57BL6/J mice, we detected a 3.09-fold increase in circulating CCL2 in mice fed a high-fat diet (containing 60% kcal from fat) for 18 weeks relative to animals on a 10% fat (kcal) diet (Figure 9A). In addition, db/db mice, a genetic model of obesity induced by leptin resistance, also had 3.06-fold more CCL2 in the serum relative to their lean (db/+) counterparts (Figure 9B).
Figure 9. CCL20 is elevated in pancreatic islets and serum from obese mice.
Serum samples from 24 week-old C57BL6/J mice fed for 18 weeks with the diets containing the indicated fat content (A,C) or 8 week old db/db mice fed a diet containing 6% fat (B,D) were analyzed for CCL2 (A,B) and CCL20 (C,D) protein levels by ELISA. Data represent means + S.E.M. from 6-8 animals per group. Pancreata isolated from 8 week old lean (db/+; panel E) or obese (db/db; panel F) mice were stained for the presence of CCL20 and counterstained with hematoxylin to identify nuclei. Note the increase in punctate non-nuclear granules indicating immunoreactive CCL20 in the obese (db/db) mice (panel F). **, P < 0.01, *, P < 0.05, n.s. = not significant.
The expression of the CCL20 gene is enhanced in human islets cultured in the presence of palmitate [68], which may indicate a link between elevated CCL20 expression and obesity. Thus, we compared serum levels of CCL20 from C57BL6/J mice after 18 weeks on diets containing different percentages of their calories from fat. Mice fed 25% (grey bar) or 60% fat (black bar) had 1.55-fold and 2.58-fold increases, respectively, in serum CCL20 abundance when compared to control mice on a diet with 10% fat (Figure 9C). The db/db mice, which readily develop obesity on a chow (< 10% kcal from fat) diet, exhibited a 3.18-fold elevation in serum CCL20 relative to their lean counterparts (db/+; Figure 9D). Moreover, histological analysis of islets within the db/db pancreas revealed noticeably higher levels of CCL20 protein when compared with islets from db/+ mice (compare Figure 9E with 9F; notice the increase in non-nuclear punctate staining of CCL20 granules in islets from the obese db/db mice). Collectively, these data reveal that the circulating levels of both CCL2 and CCL20 are elevated during obesity and that the CCL20 protein is readily detectable in pancreatic islets from obese mice.
4.1 Discussion
The pro-inflammatory cytokine IL-1β is a key contributor to the development of both T1DM and T2DM [62, 69]. Decreases in function and mass of the pancreatic islet β-cells are the foremost causes of both T1DM and T2DM [2, 70, 71]. IL-1β is a pro-inflammatory cytokine, secreted by a variety of immune cells, that elicits powerful transcriptional and functional effects on islet β-cells [5, 6, 8, 12, 13]. Children positive for islet autoantibodies, indicating a genetic predisposition to diabetes, display elevated circulating IL-1β [72]. In addition, IL-1β is also increased in children recently diagnosed with T1DM [73].
Because IL-1β induces a host of chemokine and other inflammatory genes in pancreatic β-cells, interfering with its activity has been targeted to relieve inflammation-induced tissue dysfunction. Interestingly, clinical interventions targeting the IL-1 signaling pathway improved islet β-cell function in human subjects with impaired glucose tolerance [74, 75] and those with overt diabetes [76] but did not alleviate peripheral insulin resistance in either group. This therapeutic outcome could be explained by decreasing the availability of the ligand(s) activating IL-1RI, which is highly expressed on islet β-cells relative to other tissues [7]. We report here that expression of the CCL20 gene in pancreatic β-cells is exquisitely sensitive to IL-1β. In addition, we found that IL-1R activation in β-cells led to differential control of the CCL2, CCL20, and COX2 genes via recruitment of precise NF-κB transcription factor subunits to specific genomic elements.
Analysis of global transcriptome changes revealed that CCL20 is one of the most highly expressed genes in response to pro-inflammatory stimuli [11]. Our present data adds several novel and mechanistic results to this original observation, which include: 1) Two distinct and functional κB elements, located within 3kb upstream of the transcriptional start site, are present in the CCL20 gene promoter. 2) IL-1β induces expression of the CCL20 gene within 1h and this response does not require new protein synthesis, indicating that CCL20 is a primary response gene. 3) The CCL20 gene is a bona fide NF-κB target gene that is differentially regulated by the p65 and p50 subunits. 4) The COX2 gene, while highly responsive to IL-1β, is regulated by a different mechanism than that for the CCL2 and CCL20 genes. 5) PBNs display increased cell surface CCR6, the receptor for CCL20, after exposure to the inflammatory cytokines IL-1β, TNF-α, or IFN-γ. 6) CCL20 is elevated in the serum of both db/db mice and in C57BL6 mice fed a high-fat diet.
To our knowledge, this is the first study to report elevated circulating CCL20 in two distinct mouse models of obesity and insulin resistance. We speculate that obese humans will display similar increases in serum CCL20. Because CCL20 promotes chemotaxis of CD4+ and CD8+ T-cells [34, 35], dendritic cells [36], B-lymphocytes [37, 38], natural killer (NK) cells [39], and neutrophils [38], elevated expression of this chemokine could be a clinical marker of obesity-induced inflammation. Many of the aforementioned immune cells are known to be associated with T1DM, T2DM or both diseases [41, 77, 78].
Adipocytes, which are a major contributor to glucose homeostasis and endocrine function [79], also synthesize CCL20. Interestingly, CCL20 expression in mature adipocytes is positively correlated with BMI and lymphocytes infiltrating the adipose tissue express CCR6 [80]. CCR6 is currently the only known receptor for CCL20. Therefore, the rise in circulating CCL20 we observe in obese mouse models (Figure 9) could potentially be due to several tissue sources, including adipocytes and pancreatic β-cells.
Collectively, these data place CCL20 in a prime position to be a significant contributor to the development or progression of both T1DM and T2DM based on circulating levels during obesity, direct expression from pancreatic β-cells, and the ability to influence the immune cell types associated with both major forms of diabetes. Therefore, the CCL20-CCR6 interaction should be considered as a potential novel target for therapeutic intervention in obesity and diabetes. Indeed, inhibitors of this axis have shown promise in mouse models and preclinical trials of other autoimmune diseases [81, 82].
Unlike most chemokine receptors, CCR6 has only one known ligand (i.e., CCL20). We found that IL-1β, TNF-α, and IFN-γ were all able to induce CCR6 expression on the surface of human neutrophils (Figure 8). This data complements a previous study, which reported on a Th17+ subpopulation of human CD4+ T-cells, which were also positive for CCR6 [83]. Thus, tissue-derived CCL20 may be impacting several specific immune cell populations in the context of autoimmune and auto-inflammatory diseases. In addition to CCL20, IL-1β induces the expression of many different chemokines in pancreatic islets and β-cell lines, including CCL2, CXCL1, CXCL2, CXCL10, and CCL20 (refs [12–14] and data herein). The expression of all of these genes requires specific signaling molecules within the IL-1R/NF-κB signaling pathways along with the associated transcription factors.
A key finding in the present study is that the individual gene promoters controlling expression of the CCL2 and CCL20 genes display p50 occupancy in the basal (unstimulated) state (Fig. 5), indicating a likely repressor function of this subunit in this particular context. After IL-1β exposure, p50 is replaced by p65 on the CCL2 and CCL20 gene promoters prior to the increase in transcript accumulation. However, both p65 and p50 are recruited to the COX2 gene in response to IL-1β, which is similar to what occurs with the CXCL1 and CXCL2 genes [12]. Because the temporal recruitment of p65 is similar between all three genes, we speculate that the COX2 gene requires a p65/p50 heterodimer for activation while CCL2 and CCL20 transcriptional activation most likely involves p65 homodimers.
The biological significance of the differential NF-κB subunit usage is not entirely clear at the present time but may indicate a constraint on signal-specific co-activator recruitment to each gene. Indeed, although CCL2, CCL20, and COX2 are all induced by IL-1β and require p65 for their expression, we detected differential co-activator requirements at the promoter regions of each of these genes. This may be one way in which timing, specificity, and duration of genes involved in the inflammatory response are controlled. If so, it may thus allow for selective targeting of individual genes, or groups of genes, by small molecule inhibitors of enzymatic activity contained within each particular co-activator protein.
Moreover, our novel data reveals that the CCL2 and CCL20 genes are differentially regulated when compared with COX2 and other chemokine genes (e.g., CXCL10). The IL-1β-induced increase in CXCL10 transcription is magnified when IFN-γ is present [13]. While CCL20 and CXCL10 are both elevated during obesity [see ref [84] and Fig 1 of the present study] and both require NF-κB for increased expression after cellular exposure to IL-1β (see Fig. 3 and 4 and ref [13]), CCL20 expression is not sensitive to IFN-γ (Figure 1). Thus, based on our work and the work of others, we postulate that dysfunctional islets from obese rodents and humans may be due to a decrease in the expression and abundance of transcription factors that control islet β-cell function concomitant with a coordinated increase in synthesis and secretion of various chemokines (e.g., Table 1). Our observations of increased expression of the COX2 and PTGES genes in islets from obese mice (Table 1) are also congruent with transgenic expression of COX2 and mPGES promoting losses in functional β-cell mass in vivo [85].
In summary, pancreatic β-cells express CCL20 in response to inflammatory signals, such as IL-1β, while neutrophils upregulate expression of CCR6, the only known receptor for CCL20, in response to several pro-inflammatory cytokines. These findings offer the intriguing possibility that the CCL20-CCR6 axis may be a novel therapeutic target to offset pathologies either associated with obesity and/or leading to diabetes. Our data suggest that therapeutic interventions may be able to target either specific components of the NF-κB pathway (to stop overall production of chemokine ligands) or CCL20 signaling through CCR6 (e.g., by directly targeting CCR6). Combination therapies aimed at both of these sites would also be feasible.
Highlights.
CCL20 mRNA and protein are increased by IL-1β in pancreatic [3-cells.
Induction of CCL20 requires IκKβ, a component of the NF-κB signaling pathway.
p65, a NF-κB transcriptional subunit, is recruited to the CCL20 gene promoter.
Circulating CCL20 is elevated in mouse models of obesity and insulin resistance.
Human neutrophils elevate CCR6 protein in response to pro-inflammatory cytokines.
Acknowledgments
This project used the PBRC Genomics Core Facility and the Cell Biology and Bioimaging Core Facilities that are supported in part by COBRE (P20-GM103528) and NORC (P30-DK072476) center grants from the National Institutes of Health. In addition, a grant from the Physicians Medical Education and Research Foundation (to J.J.C. and M.D.K.) and NIH grant P20-GM103528 (J.J.C.) provided funding for this project. This work was also partially supported by NIH grant R01AI071042-01A2 (T.E.S.). The authors thank Matthew Goff and Tiantain Jiang for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke SJ, Collier JJ. Insulitis and Diabetes: A Perspective on Islet Inflammation. Immunome Research. 2014;10 In Press. [Google Scholar]
- 3.Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31(Suppl 2):S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 4.Corbett JA, Wang JL, Sweetland MA, Lancaster JR, Jr, McDaniel ML. Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J Clin Invest. 1992;90:2384–2391. doi: 10.1172/JCI116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collier JJ, Burke SJ, Eisenhauer ME, Lu D, Sapp RC, Frydman CJ, Campagna SR. Pancreatic beta-Cell Death in Response to Pro-Inflammatory Cytokines Is Distinct from Genuine Apoptosis. PLoS One. 2011;6:e22485. doi: 10.1371/journal.pone.0022485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993;90:1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, Kerr-Conte J, Pattou F, Ehses JA, Schuit FC, Donath MY. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150:5218–5229. doi: 10.1210/en.2009-0543. [DOI] [PubMed] [Google Scholar]
- 8.Burke SJ, Updegraff BL, Bellich RM, Goff MR, Lu D, Minkin SC, Jr, Karlstad MD, Collier JJ. Regulation of iNOS Gene Transcription by IL-1beta and IFN-gamma Requires a Coactivator Exchange Mechanism. Mol Endocrinol. 2013;27:1724–1742. doi: 10.1210/me.2013-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke SJ, Collier JJ. The gene encoding cyclooxygenase-2 is regulated by IL-1beta and prostaglandins in 832/13 rat insulinoma cells. Cell Immunol. 2011;271:379–384. doi: 10.1016/j.cellimm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Heitmeier MR, Scarim AL, Corbett JA. Interferon-gamma increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem. 1997;272:13697–13704. doi: 10.1074/jbc.272.21.13697. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar SA, Lee CE, Victorino F, Nguyen TT, Walters JA, Burrack A, Eberlein J, Hildemann SK, Homann D. Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes. 2012;61:436–446. doi: 10.2337/db11-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke SJ, Lu D, Sparer TE, Masi T, Goff MR, Karlstad MD, Collier JJ. NF-kappaB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Endocrinol Metab. 2014;306:E131–E149. doi: 10.1152/ajpendo.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke SJ, Goff MR, Lu D, Proud D, Karlstad MD, Collier JJ. Synergistic Expression of the CXCL10 Gene in Response to IL-1beta and IFN-gamma Involves NF-kappaB, Phosphorylation of STAT1 at Tyr701, and Acetylation of Histones H3 and H4. J Immunol. 2013;191:323–336. doi: 10.4049/jimmunol.1300344. [DOI] [PubMed] [Google Scholar]
- 14.Burke SJ, Goff MR, Updegraff BL, Lu D, Brown PL, Minkin SC, Jr, Biggerstaff JP, Zhao L, Karlstad MD, Collier JJ. Regulation of the CCL2 Gene in Pancreatic beta-Cells by IL-1beta and Glucocorticoids: Role of MKP-1. PLoS One. 2012;7:e46986. doi: 10.1371/journal.pone.0046986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-kappaB in Aging and Disease. Aging and disease. 2011;2:449–465. [PMC free article] [PubMed] [Google Scholar]
- 16.Brown KD, Claudio E, Siebenlist U. The roles of the classical and alternative nuclear factor-kappaB pathways: potential implications for autoimmunity and rheumatoid arthritis; Arthritis research &. therapy. 2008;10:212. doi: 10.1186/ar2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 18.Ellrichmann G, Thone J, Lee DH, Rupec RA, Gold R, Linker RA. Constitutive activity of NF-kappa B in myeloid cells drives pathogenicity of monocytes and macrophages during autoimmune neuroinflammation. Journal of neuroinflammation. 2012;9:15. doi: 10.1186/1742-2094-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab. 2013;24:40–47. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 21.Beg AA, Baldwin AS., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 22.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 24.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. International journal of obesity. 2006;30:1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- 26.Martin AP, Rankin S, Pitchford S, Charo IF, Furtado GC, Lira SA. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes. 2008;57:3025–3033. doi: 10.2337/db08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowley MJ, Weinberg A, Zammit NW, Walters SN, Hawthorne WJ, Loudovaris T, Thomas H, Kay T, Gunton JE, Alexander SI, Kaplan W, Chapman J, O'Connell PJ, Grey ST. Human islets express a marked proinflammatory molecular signature prior to transplantation. Cell Transplant. 2012;21:2063–2078. doi: 10.3727/096368911X627372. [DOI] [PubMed] [Google Scholar]
- 28.Merani S, Truong WW, Hancock W, Anderson CC, Shapiro AM. Chemokines and their receptors in islet allograft rejection and as targets for tolerance induction. Cell Transplant. 2006;15:295–309. [PubMed] [Google Scholar]
- 29.Citro A, Cantarelli E, Maffi P, Nano R, Melzi R, Mercalli A, Dugnani E, Sordi V, Magistretti P, Daffonchio L, Ruffini PA, Allegretti M, Secchi A, Bonifacio E, Piemonti L. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012;122:3647–3651. doi: 10.1172/JCI63089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hromas R, Gray PW, Chantry D, Godiska R, Krathwohl M, Fife K, Bell GI, Takeda J, Aronica S, Gordon M, Cooper S, Broxmeyer HE, Klemsz MJ. Cloning and characterization of exodus, a novel beta-chemokine. Blood. 1997;89:3315–3322. [PubMed] [Google Scholar]
- 31.Matsui T, Akahoshi T, Namai R, Hashimoto A, Kurihara Y, Rana M, Nishimura A, Endo H, Kitasato H, Kawai S, Takagishi K, Kondo H. Selective recruitment of CCR6-expressing cells by increased production of MIP-3 alpha in rheumatoid arthritis. Clin Exp Immunol. 2001;125:155–161. doi: 10.1046/j.1365-2249.2001.01542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jafarzadeh A, Bagherzadeh S, Ebrahimi HA, Hajghani H, Bazrafshani MR, Khosravimashizi A, Nemati M, Gadari F, Sabahi A, Iranmanesh F, Mohammadi MM, Daneshvar H. Higher Circulating Levels of Chemokine CCL20 in Patients with Multiple Sclerosis: Evaluation of the Influences of Chemokine Gene Polymorphism, Gender, Treatment and Disease Pattern. Journal of molecular neuroscience : MN. 2014 doi: 10.1007/s12031-013-0214-2. [DOI] [PubMed] [Google Scholar]
- 33.Ylipaasto P, Smura T, Gopalacharyulu P, Paananen A, Seppanen-Laakso T, Kaijalainen S, Ahlfors H, Korsgren O, Lakey JR, Lahesmaa R, Piemonti L, Oresic M, Galama J, Roivainen M. Enterovirus-induced gene expression profile is critical for human pancreatic islet destruction. Diabetologia. 2012;55:3273–3283. doi: 10.1007/s00125-012-2713-z. [DOI] [PubMed] [Google Scholar]
- 34.Sato K, Kawasaki H, Nagayama H, Enomoto M, Morimoto C, Tadokoro K, Juji T, Takahashi T. Chemokine receptor expressions and responsiveness of cord blood T cells. J Immunol. 2001;166:1659–1666. doi: 10.4049/jimmunol.166.3.1659. [DOI] [PubMed] [Google Scholar]
- 35.Christopherson K, 2nd, Brahmi Z, Hromas R. Regulation of naive fetal T-cell migration by the chemokines Exodus-2 and Exodus-3. Immunol Lett. 1999;69:269–273. doi: 10.1016/s0165-2478(99)00099-1. [DOI] [PubMed] [Google Scholar]
- 36.Kucharzik T, Hudson JT, 3rd, Waikel RL, Martin WD, Williams IR. CCR6 expression distinguishes mouse myeloid and lymphoid dendritic cell subsets: demonstration using a CCR6 EGFP knock-in mouse. Eur J Immunol. 2002;32:104–112. doi: 10.1002/1521-4141(200201)32:1<104::AID-IMMU104>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Brandes M, Legler DF, Spoerri B, Schaerli P, Moser B. Activation-dependent modulation of B lymphocyte migration to chemokines. Int Immunol. 2000;12:1285–1292. doi: 10.1093/intimm/12.9.1285. [DOI] [PubMed] [Google Scholar]
- 38.Krzysiek R, Lefevre EA, Bernard J, Foussat A, Galanaud P, Louache F, Richard Y. Regulation of CCR6 chemokine receptor expression and responsiveness to macrophage inflammatory protein-3alpha/CCL20 in human B cells. Blood. 2000;96:2338–2345. [PubMed] [Google Scholar]
- 39.Robertson MJ, Williams BT, Christopherson K, 2nd, Brahmi Z, Hromas R. Regulation of human natural killer cell migration and proliferation by the exodus subfamily of CC chemokines. Cell Immunol. 2000;199:8–14. doi: 10.1006/cimm.1999.1601. [DOI] [PubMed] [Google Scholar]
- 40.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 41.Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, Lehuen A. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 42.Battaglia M. Neutrophils and type 1 autoimmune diabetes. Curr Opin Hematol. 2014;21:8–15. doi: 10.1097/MOH.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 43.Valle A, Giamporcaro GM, Scavini M, Stabilini A, Grogan P, Bianconi E, Sebastiani G, Masini M, Maugeri N, Porretti L, Bonfanti R, Meschi F, De Pellegrin M, Lesma A, Rossini S, Piemonti L, Marchetti P, Dotta F, Bosi E, Battaglia M. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes. 2013;62:2072–2077. doi: 10.2337/db12-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 45.Janjic D, Maechler P, Sekine N, Bartley C, Annen AS, Wolheim CB. Free radical modulation of insulin release in INS-1 cells exposed to alloxan. Biochem Pharmacol. 1999;57:639–648. doi: 10.1016/s0006-2952(98)00346-3. [DOI] [PubMed] [Google Scholar]
- 46.Collier JJ, Fueger PT, Hohmeier HE, Newgard CB. Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes. 2006;55:1398–1406. doi: 10.2337/db05-1000. [DOI] [PubMed] [Google Scholar]
- 47.Markert M, Andrews PC, Babior BM. Measurement of O2- production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Methods Enzymol. 1984;105:358–365. doi: 10.1016/s0076-6879(84)05048-5. [DOI] [PubMed] [Google Scholar]
- 48.Donath MY, Boni-Schnetzler M, Ellingsgaard H, Halban PA, Ehses JA. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol Metab. 2010;21:261–267. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 49.McGillicuddy FC, Harford KA, Reynolds CM, Oliver E, Claessens M, Mills KH, Roche HM. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes. 2011;60:1688–1698. doi: 10.2337/db10-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner DA, Sartor RB. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- 52.Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, Okubo T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052–2063. [PubMed] [Google Scholar]
- 53.Newton R, Kuitert LM, Bergmann M, Adcock IM, Barnes PJ. Evidence for involvement of NF-kappaB in the transcriptional control of COX-2 gene expression by IL-1beta. Biochem Biophys Res Commun. 1997;237:28–32. doi: 10.1006/bbrc.1997.7064. [DOI] [PubMed] [Google Scholar]
- 54.Moorthy AK, Savinova OV, Ho JQ, Wang VY, Vu D, Ghosh G. The 20S proteasome processes NF-kappaB1 p105 into p50 in a translation-independent manner. EMBO J. 2006;25:1945–1956. doi: 10.1038/sj.emboj.7601081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 56.Covic M, Hassa PO, Saccani S, Buerki C, Meier NI, Lombardi C, Imhof R, Bedford MT, Natoli G, Hottiger MO. Arginine methyltransferase CARM1 is a promoter-specific regulator of NF-kappaB-dependent gene expression. EMBO J. 2005;24:85–96. doi: 10.1038/sj.emboj.7600500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 58.Olza J, Aguilera CM, Gil-Campos M, Leis R, Bueno G, Martinez-Jimenez MD, Valle M, Canete R, Tojo R, Moreno LA, Gil A. Myeloperoxidase is an early biomarker of inflammation and cardiovascular risk in prepubertal obese children. Diabetes Care. 2012;35:2373–2376. doi: 10.2337/dc12-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific beta cell transcription factors in type 2 diabetes. J Clin Invest. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li CR, Mueller EE, Bradley LM. Islet antigen-specific Th17 cells can induce TNF-alpha-dependent autoimmune diabetes. J Immunol. 2014;192:1425–1432. doi: 10.4049/jimmunol.1301742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 62.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 63.Rabinovitch A, Suarez-Pinzon WL, Sorensen O, Bleackley RC, Power RF. IFN-gamma gene expression in pancreatic islet-infiltrating mononuclear cells correlates with autoimmune diabetes in nonobese diabetic mice. J Immunol. 1995;154:4874–4882. [PubMed] [Google Scholar]
- 64.Boni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, Pattou F, Halban PA, Weir GC, Donath MY. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93:4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring) 2015;23:512–518. doi: 10.1002/oby.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grant RW, Dixit VD. Mechanisms of disease: inflammasome activation and the development of type 2 diabetes. Front Immunol. 2013;4:50. doi: 10.3389/fimmu.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cnop M, Abdulkarim B, Bottu G, Cunha DA, Igoillo-Esteve M, Masini M, Turatsinze JV, Griebel T, Villate O, Santin I, Bugliani M, Ladriere L, Marselli L, McCarthy MI, Marchetti P, Sammeth M, Eizirik DL. RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes. 2014;63:1978–1993. doi: 10.2337/db13-1383. [DOI] [PubMed] [Google Scholar]
- 69.Nepom GT, Ehlers M, Mandrup-Poulsen T. Anti-cytokine therapies in T1D: Concepts and strategies. Clin Immunol. 2013;149:279–285. doi: 10.1016/j.clim.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 72.Yeung WC, Al-Shabeeb A, Pang CN, Wilkins MR, Catteau J, Howard NJ, Rawlinson WD, Craig ME. Children with islet autoimmunity and enterovirus infection demonstrate a distinct cytokine profile. Diabetes. 2012;61:1500–1508. doi: 10.2337/db11-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez F, Oyarzun A, Carrasco E, Angel B, Albala C, Santos JL. [Plasma levels of interleukin-1beta, interleukin-2 and interleukin-4 in recently diagnosed type 1 diabetic children and their association with beta-pancreatic autoantibodies] Revista medica de Chile. 2004;132:413–420. doi: 10.4067/s0034-98872004000400002. [DOI] [PubMed] [Google Scholar]
- 74.van Poppel PC, van Asseldonk EJ, Holst JJ, Vilsboll T, Netea MG, Tack CJ. The interleukin-1 receptor antagonist anakinra improves first-phase insulin secretion and insulinogenic index in subjects with impaired glucose tolerance. Diabetes Obes Metab. 2014 doi: 10.1111/dom.12357. [DOI] [PubMed] [Google Scholar]
- 75.van Asseldonk EJ, Stienstra R, Koenen TB, Joosten LA, Netea MG, Tack CJ. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2119–2126. doi: 10.1210/jc.2010-2992. [DOI] [PubMed] [Google Scholar]
- 76.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 77.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, Roep BO, von Herrath MG. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarikonda G, Pettus J, Phatak S, Sachithanantham S, Miller JF, Wesley JD, Cadag E, Chae J, Ganesan L, Mallios R, Edelman S, Peters B, von Herrath M. CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J Autoimmun. 2013 doi: 10.1016/j.jaut.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 79.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 80.Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, Sengenes C, Lafontan M, Galitzky J, Bouloumie A. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 81.Mackay CR. Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat Immunol. 2008;9:988–998. doi: 10.1038/ni.f.210. [DOI] [PubMed] [Google Scholar]
- 82.Liston A, Kohler RE, Townley S, Haylock-Jacobs S, Comerford I, Caon AC, Webster J, Harrison JM, Swann J, Clark-Lewis I, Korner H, McColl SR. Inhibition of CCR6 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response. Journal of immunology. 2009;182:3121–3130. doi: 10.4049/jimmunol.0713169. [DOI] [PubMed] [Google Scholar]
- 83.Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, Knip M, Otonkoski T, Vaarala O. IL-17 immunity in human type 1 diabetes. J Immunol. 2010;185:1959–1967. doi: 10.4049/jimmunol.1000788. [DOI] [PubMed] [Google Scholar]
- 84.Schulthess FT, Paroni F, Sauter NS, Shu L, Ribaux P, Haataja L, Strieter RM, Oberholzer J, King CC, Maedler K. CXCL10 impairs beta cell function and viability in diabetes through TLR4 signaling. Cell Metab. 2009;9:125–139. doi: 10.1016/j.cmet.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Oshima H, Taketo MM, Oshima M. Destruction of pancreatic beta-cells by transgenic induction of prostaglandin E2 in the islets. J Biol Chem. 2006;281:29330–29336. doi: 10.1074/jbc.M602424200. [DOI] [PubMed] [Google Scholar]