Abstract
Background
Given that odors enhance the retrieval of autobiographical memories, induce physiological arousal, and trigger trauma-related flashbacks, it is reasonable to hypothesize that odors play a significant role in the pathophysiology of posttraumatic stress disorder (PTSD). For these reasons, this preliminary study sought to examine self-reported, odor-elicited distress in PTSD.
Methods
Combat veterans with (N=30) and without (N=22) PTSD and healthy controls (HC: N=21), completed an olfactory questionnaire that provided information on the hedonic valence of odors as well as their ability to elicit distress or relaxation.
Results
Two main findings were revealed: Compared to HC, CV+PTSD, but not CV-PTSD, reported a higher prevalence of distress to a limited number of select odors that included fuel (p=.004), blood (p=.02), gunpowder (p=.03), and burning hair (p=.02). In contrast to this increased sensitivity, a blunting effect was reported by both groups of veterans compared to HC that revealed lower rates of distress and relaxation in response to negative hedonic odors (p=.03) and positive hedonic odors (p<.001), respectively.
Limitations
The study is limited by its use of retrospective survey methods, whereas future investigations would benefit from laboratory measures taken prior, during, and after deployment.
Conclusion
The present findings suggest a complex role of olfaction in the biological functions of threat detection. Several theoretical models are discussed. One possible explanation for increased sensitivity to select odors with decreased sensitivity to other odors is the co-occurrence of attentional bias toward threat odors with selective ignoring of distractor odors. Working together, these processes may optimize survival.
Keywords: olfaction, odor threat cues, odor sensitivity, attentional bias, fear, PTSD
1. Introduction
Exposure to an event or situation that has the potential for causing death or serious bodily harm may lead to the development of post-traumatic stress disorder (PTSD; Kessler et al., 1995, 2005), a condition characterized by intrusive thoughts or memories, avoidance of stimuli and contextual reminders, as well as increased startle, hypervigilance, and focused attention to potential threat cues. Current, non-pharmacological PTSD treatments are largely based upon a conditioned fear and deficits in extinction learning model of pathogenesis. For example, the persistence of fear responses and physiological hyper-arousal in the presence of a conditioned stimulus (e.g. roadside trash or overpass) in a safe environment (after the person returns from the war theatre) represents a failure in extinction learning. These observations have led to the development of prolonged exposure treatments (PE), which employ imagery and/or in vivo exposure to threat-related cues (Foa, 2006; Schnurr et al., 2007).
Current PE strategies almost exclusively utilize visual and/or auditory cues associated with past traumatic events. Many individuals with PTSD, however, report that trauma-related odors are particularly potent reminders of past traumatic events (e.g. odors associated with explosions or “burning” materials). Incorporating trauma-related odors into exposure treatments may be particularly important given that odors have been increasingly recognized as perhaps the most poignant cause of the spontaneous re-experiencing of vivid, autobiographical memories (e.g. Proustian phenomenon; Chu & Downes, 2002). Within this context, odor-triggered memories are highly emotional and such memories, even distant ones, are often experienced as if they are taking place in the “here-and-now” (Masaoka et al., 2012; Willander & Larsson, 2006). Such odor-evoked time misperceptions can be brief and as such resemble short-lived dissociative flashbacks.
Given that highly-emotional and involuntary memories of traumatic events are characteristic of PTSD, that odors can enhance the retrieval of autobiographical memories and trigger physiological arousal (Chu & Downes, 2002; Herz, 2004; Herz & Chupik, 1995; Willander & Larsson, 2006; Saive et al., 2014; Masaoka et al., 2012), that olfactory hallucinations are associated with deeply personal, mood-congruent, distant memories (Nickell & Uhde, 1994–1995), and that olfactory stimuli can trigger flashbacks (Kline & Rausch, 1985; Vermetten & Bremner, 2003), it is reasonable to hypothesize that odors in general and traumarelated odors in particular may play a significant and under-appreciated role in the pathophysiology of PTSD. More specifically, these observations suggest a possible role for previously neutral odors becoming conditioned threat cues when they are paired with traumatic or life-threatening events.
Combat veterans exposed to unique odors under dangerous circumstances offer an ideal population to investigate odor-related fear and anxiety. While the long-term goal of our research team is to design more effective therapies that take into account evidence-based data on the role of odors in the pathogenesis and treatment of PTSD, there is very little systematically-collected information on this topic. Therefore, as a first attempt to evaluate behavioral responses to a range of odors with different qualities, we developed a self-report survey and examined prevalence rates of odor-elicited distress in combat veterans with and without PTSD and healthy controls without exposure to combat.
The specific questions we examined were as follows: 1) What odor categories (e.g. burning-, death/decay-, drug-, food-, flammables-, floral-, garbage-, human body fluids/excretions-, environment-related) and specific individual odors within odor categories commonly elicit distress in healthy controls? and 2) Is there a difference in the proportion of individuals reporting distress in response to categories of odors, specific individual odors, and/or odors with a specific hedonic valence (i.e. unpleasant versus pleasant) among combat veterans, with and without PTSD, and healthy controls?
2. Methods
2.1 Participants
Fifty-two combat veterans, with and without posttraumatic stress disorder [CV+PTSD (n=30) and CV-PTSD (n=22), respectively], were recruited through local advertisement to participate in a larger study investigating odor-elicited anxiety. To meet initial eligibility for the larger study, which included an MRI exam, potential participants were required to 1) have served in a combat zone in Iraq or Afghanistan [Operation Enduring Freedom (OEF), Iraqi Freedom (OIF), or New Dawn (OND)], 2) have a DSM-IV primary diagnosis of PTSD related to combat or have no history of any DSM-IV disorder including alcohol or other substance use disorder, assessed by the Mini International Neuropsychiatric Interview (MINI; Sheehan et al 1998), 3) have no history of head injury/trauma given its high association with olfactory dysfunction, 4) be psychotropic medication-free, 5) be able to undergo an MRI exam (e.g. contraindications such as shrapnel injuries or claustrophobia), and 6) pass a urine drug screen (CLIAwaivedTM, San Diego, CA). Levels of trait anxiety were collected using the State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983).
Twenty-one healthy controls (HC) were recruited anonymously by distributing study materials to a community sample of adults, who then returned completed forms in pre-addressed and stamped envelopes. General demographics, as well as detailed information regarding physical and mental health, including all prescription medications taken in the past year and current or past diagnoses and/or treatment of all psychiatric disorders, were utilized to identify the HC included in this preliminary investigation. All methods were approved by the Institutional Review Board for Human Research at the Medical University of South Carolina. All participants completed written informed consent as required.
2.2. Olfactory Questionnaire (OQ)
Information pertaining to hedonic valence (i.e. pleasantness/unpleasantness) and ability to modulate mood (i.e. elicit relaxation/distress) was acquired for forty-nine common odors through a detailed olfactory questionnaire (OQ). The specific odors listed in the OQ were selected, in part, from previous PTSD literature (Vermetten & Bremner, 2003), as well as from a library of odorants provided by ScentAir™. The odor list was not intended to be exhaustive of all odors and odor categories, nor did it include all potential trauma-related odors. For the present study, participants recorded whether each odor, listed in alphabetical order, was pleasant, unpleasant, or neutral, and if each odor elicited relaxation, distress, or did not modulate mood in either direction.
2.3. Analysis of Odor-elicited Distress
2.3.1. Distress by Odor Categories
Odor-elicited distress was first assessed for 9 different odor categories: 1) burning-, 2) death/decay-, 3) drug-, 4) food-, 5) flammable-, 6) floral-, 7) garbage-, 8) human body fluids/excretion-, and 9) environmental-related odors (Table 1). Odor-elicited distress ratings for odor categories were calculated for each participant using the following method: If one or more odor within a particular category was rated to elicit distress by an individual, the entire category was given a “distress” rating for that individual.
Table 1.
Odor categories and the individual odors included in each category.
| Burning | Food | Flammables | Human Body |
|---|---|---|---|
| Burning rubber | *Almond | Fuel | Fluids/Excretions |
| Burning hair | Apple cider | Floral | Blood |
| Burning wire | Banana | *Baby powder | #Body odor/Sweat |
| Diesel exhaust | *Bread | Eucalyptus | Feces |
| Engine exhaust | Bubblegum | Jasmine | #Raw sewage |
| Gun powder | Chocolate | Juniper | #Urine |
| Wood fire | *Cinnamon | Lavender | #Vomit |
| Death/Decay | Citrus | Lemongrass | Environmental |
| #Dead body | *Coconut | *Lilac | Animal farm |
| Drugs | *Coffee | Magnolia | *Cedar |
| Tobacco | *French vanilla | Sage | *Forest |
| Marijuana | Grapefruit | Garbage | *Fresh cut grass |
| Mocha | Garbage | Musty | |
| Peppermint | *Ocean saltwater | ||
| Pomegranate | |||
| Sugar |
=odor rated "pleasant" by at least 50% of healthy controls and was given no "unpleasant" ratings
=odor rated "unpleasant" by at least 50% of healthy controls and was given no "pleasant" ratings
2.3.2. Behavioral Response to Individual Odors
When examining the response to individual odors, each odor was rated as to whether it elicited “distress”, “relaxation”, or did not modulate mood in any direction and was thus considered “neutral”.
2.3.3. Statistical Analysis
Chi-square analyses were employed to assess differences across diagnostic groups in the frequency at which odor categories, and individual odors within categories, were rated to elicit distress.
2.4. Analysis of Hedonic Valence
Each of the 49 odors were classified as either “positively-valenced”, “negativelyvalenced”, or “mixed-valenced” based on ratings provided by the HC group.
2.4.1. Pleasant Odors
If ≥50% of the HC group reported an odor to be “pleasant” and no HC reported that odor to be “unpleasant”, the odor was classified “pleasant”. This method resulted in twelve “pleasant” odors that were clustered into the “positive hedonic valence group” (Table 1 identifies these odors with *).
2.4.2. Unpleasant Odors
If ≥50% of the HC group reported an odor to be “unpleasant” and no HC rated that odor as “pleasant”, the odor was designated an “unpleasant” odor. This resulted in the identification of five individual “unpleasant” odors that became the “negative hedonic valence group” (Table 1 identifies these odors with #).
2.4.3. Mixed Odors
Thirty-two odors failed to meet our criteria for either the positive or negative hedonic valence groups and were not considered further in the analyses.
2.4.4. Statistical Analysis
When coding the positive and negative hedonic valence groups for each individual using the same method described for coding odor-elicited distress within “odor categories”, ceiling effects were found. For example, 100% of all study participants rated at least 1 odor in the positive hedonic valence group to be relaxing. Therefore, the percent of odors within the positive and negative hedonic valence groups that were rated “relaxing” and “distressing”, respectively, were used, along with univariate ANOVA, to assess differences across diagnostic groups.
3. Results
3.1. Demographics
The CV+PTSD group (N=30) was comprised of almost all males (M/F=28/2), as was the CV-PTSD group (N=22; M/F=21/1). While the male to female ratio for these 2 groups of combat veterans did not differ from each other, they were significantly different than the HC group [N=21; M/F=6/15; χ2 (2, N=73) = 34.7, p<.001]. There was also a main effect of age (F2,70=16.14, p<.001), as the HC group (M=47.0, SD=12.7) was significantly older than both the CV+PTSD (M=33.4, SD=11.0; p<.001) and CV-PTSD (M=30.0, SD=7.0; p<.001) groups. Trait anxiety, acquired in most, but not all, of the combat veterans was significantly different in those with (M=52.5, SD=11.2) compared to without (M=31.8, SD=11.2) PTSD (t44=6.3, p<.001).
3.2. Odor Categories
The prevalence of odor-elicited distress for each of the nine odor categories listed in Table 1 was first assessed for significant differences across diagnostic groups. A main effect of diagnosis on the frequency of odor-elicited distress was revealed for four of the nine odor categories, including “burning”, “flammables”, “garbage”, and “human body fluids/excretions”. Post-hoc group comparisons revealed that CV+PTSD reported a significantly higher prevalence of distress to flammable-related odors compared to HC (p=.004) and a significantly higher prevalence of distress to burning-related odors compared to both HC (p=.005) and CV-PTSD (p=.001). Table 2 lists all Chi-square results.
Table 2.
Prevelance of distress for odor categories among combat veterans with PTSD (CV+PTSD), without PTSD (CV-PTSD), and healthy controls (HC).
|
HC (N=21) |
CV-PTSD (N=22) |
CV+PTSD (N=30) |
Main effect of Dx | HC vs CV-PTSD | Post-hoc group comparisons HC vs CV+PTSD |
CV-PTSD vs CV+PTSD | |
|---|---|---|---|---|---|---|---|
| Odor Type | % distressed | % distressed | % distressed | χ2 (2, N=73) | p-value | p-value | p-value |
| Human Body Fluids/Excretions | 100.0 | 68.2 | 80.0 | χ2=7.60, p=.022 | 0.005a | 0.029b | |
| Garbage | 61.9 | 13.6 | 23.3 | χ2=13.18, p=.001 | 0.001c | 0.005d | |
| Burning | 61.9 | 54.5 | 93.3 | χ2=11.30, p=.004 | 0.005e | 0.001f | |
| Death/Decay | 52.4 | 59.1 | 76.7 | ns | |||
| Environmental | 57.1 | 22.7 | 33.3 | ns | |||
| Drugs | 33.3 | 18.2 | 13.3 | ns | |||
| Flammables | 4.8 | 18.2 | 40.0 | χ2=9.05, p=.011 | 0.004g | ||
| Floral | 4.8 | 0.0 | 6.7 | ns | |||
| Food | 0.0 | 9.1 | 0.0 | ns | |||
The entire odor category was rated "distressing" for an individual if he/she reported 1 or more odors within that category to elicit distress. ns = nonsignificant with a p-value >.05
= χ2 (1, N=43) = 7.98
= χ2 (1, N=51) = 4.76
= χ2 (1, N=43) = 10.71
= χ2 (1, N=51) = 7.71
= χ2 (1, N=51) = 7.74
= χ2 (1, N=52) = 10.76
= χ2 (1, N=51) = 8.08
In contrast to higher prevalence rates in CV+PTSD for “burning” and “flammable” odor-related distress, lower prevalence rates of distress for human body fluids/excretions-related odors were reported in combat veterans with and without PTSD compared to HC (p=.029, p=.005, respectively). Significantly lower prevalence rates of distress for garbage-related odors were also noted [CV+PTSD and CV-PTSD compared to HC (p=.005, p=.001, respectively). Table 2 lists all Chi-square results.
3.3. Individual Odors within Categories
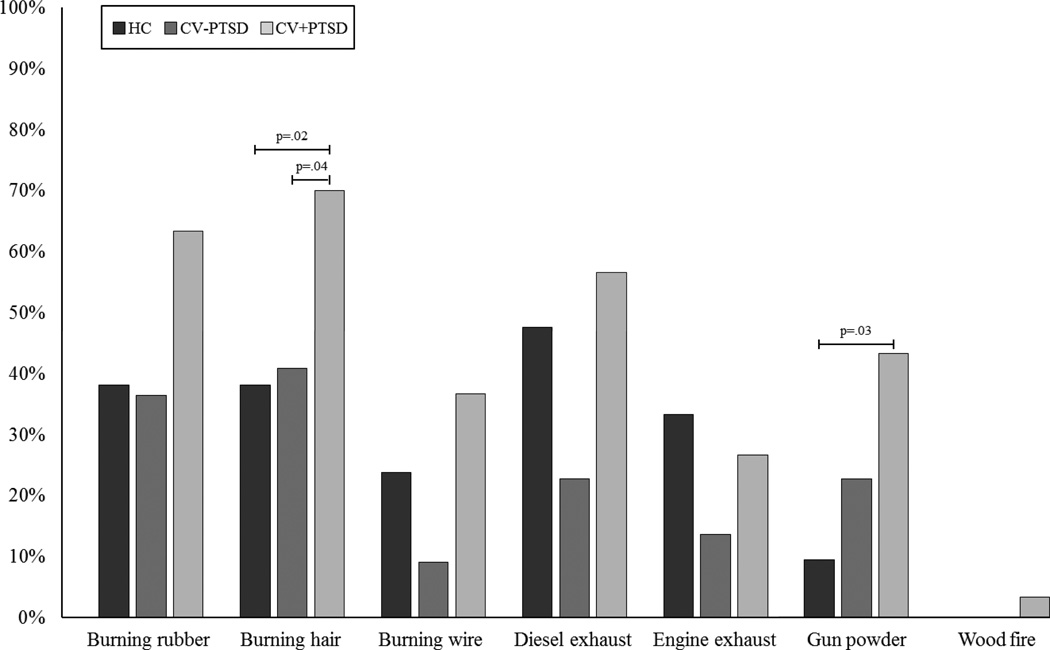
Individual odors within “burning” and “flammables” were assessed to determine if specific odors within these categories were particularly distressing. Chi-square analyses revealed that while the CV+PTSD reported a higher prevalence of odor-elicited distress than both CV-PTSD and HC for nearly every burning-related odor, a main effect of diagnosis was found for just “burning hair” [χ2 (2, N=73) = 6.61, p=.037] and “gunpowder” [χ2 (4, N=73) = 10.78, p=.029]. Post-hoc group comparisons revealed that CV+PTSD reported a significantly higher prevalence of distress to “gunpowder” compared to HC (p=.031) and “burning hair” compared to both HC (p=.024) and CV-PTSD (p=.036). See Figure 1for full Chi-square results. The “flammable” category contained just one odor, that of fuel, and thus no further assessment was needed at an individual odor level.
Figure 1. Prevalence rates of odor-elicited distress for “burning” odors.
Combat veterans with PTSD reported increased sensitivity on 6 of the 7 burning-related odors compared to healthy HC and an increased sensitivity on all 7 burning odors compared to combat veterans without PTSD. As calculated by binomial statistics, this pattern of increased sensitivity across these numbers of individual burning odors in CV+PTSD compared to HC and CV-PTSD subjects have probabilities of p=.054 and p=.0078, respectively, of taking place by chance. In terms of individual odors, a significantly higher prevalence of combat veterans with PTSD (CV+PTSD) compared to combat veterans without PTSD (CV-PTSD) [χ2 (1, N=52) = 4.40, p=.036] or healthy controls (HC) [χ2 (1, N=51) = 5.13, p=.024] specifically reported burning hair to be distressing. Gun powder was also distressing for significantly more CV+PTSD than HC [χ2 (2, N=51) = 6.93, p=.031].
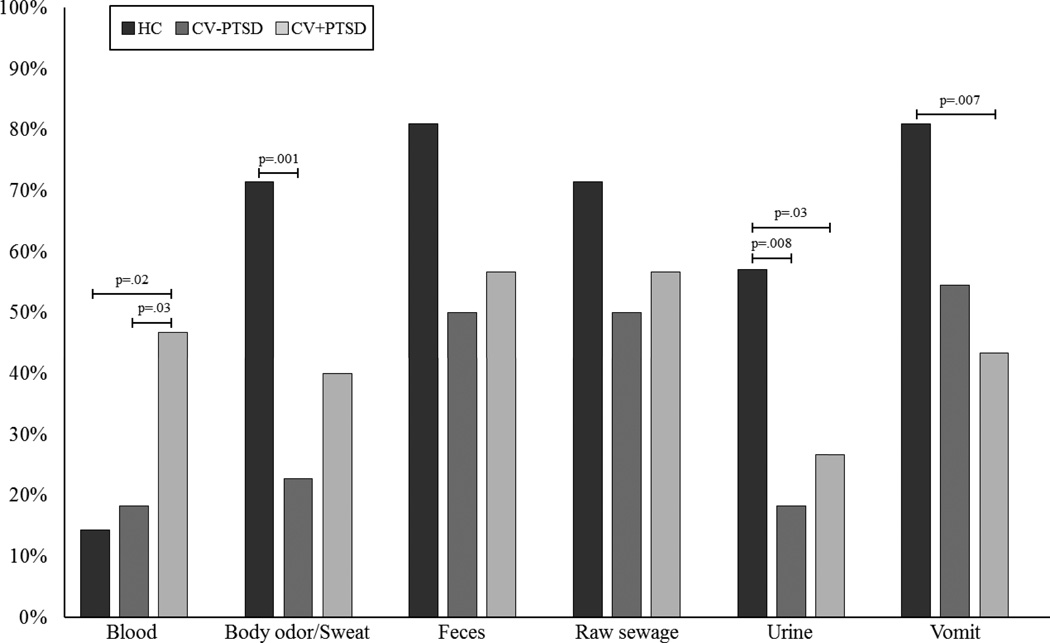
The two odor categories that revealed lower prevalence rates of distress in combat veterans, compared to healthy controls, were “human body fluids/excretions” and “garbage”. Individual odors within these categories were assessed to determine which odors contributed to this effect. Chi-square analyses revealed a main effect of diagnosis for “body odor/sweat” [χ2 (4, N=73) = 12.10, p=.017], “urine” [χ2 (2, N=73) = 8.28, p=.016], “vomit” [χ2 (2, N=73) = 7.27, p=.026], and “blood” [χ2 (2, N=73) = 8.04, p=.018]. Post-hoc group comparisons revealed that CV+PTSD, compared to HC, reported a significantly lower prevalence of distress to “urine” (p=.028) and “vomit” (p=.007), and that CV-PTSD, compared to HC, reported a significantly lower prevalence of distress to “urine” (p=.008) and “body odor/sweat” (p=.001). Only one human body fluids/excretions-related odor, namely “blood”, was associated with an increased prevalence of distress in CV+PTSD compared to both HC (p=.016) as well as CV-PTSD (p=.033). See Figure 2 for full Chi-square results. The fourth and final significant odor category, “garbage”, also contained just one odor and thus no further assessment was needed.
Figure 2. Prevalence rates of odor-elicited distress for “human body fluids/excretions” odors.
depicts reduced sensitivity to odors in the human body fluids/excretions category for combat veterans with and without PTSD (CV+PTSD and CV-PTSD, respectively) compared to healthy controls (HC). Specifically, a lower prevalence of distress to “urine” [χ2 (1, N=51) = 4.81, p=.028] and “vomit” [χ2 (1, N=51) = 7.22, p=.007] was reported in CV+PTSD compared to HC. Similarly, CV-PTSD, compared to HC, reported a significantly lower prevalence of distress to “urine” [χ2 (1, N=43) = 6.98, p=.008] and “body odor/sweat” [χ2 (1, N=43) = 10.24, p=.001]. The only odor that did not fit this profile was blood, an odor likely to be present during traumatic combat experiences. Significantly more CV+PTSD, than CV-PTSD or HC reported the smell of blood to be distressing [χ2 (1, N=52) = 4.55, p=.033; χ2 (1, N=51) = 5.83, p=.016, respectively).
3.4. Odor Hedonic Valence Groups
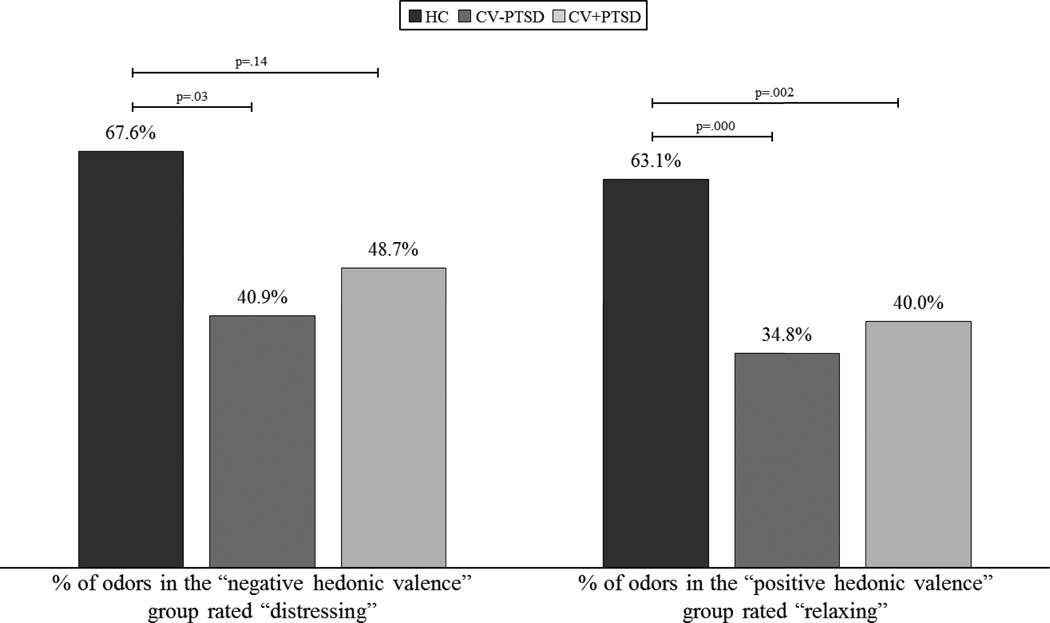
Univariate ANOVA revealed a main effect of diagnosis for the percentage of odors rated “distressing” within the negative hedonic valence group (F2,70=3.77, p=.028) and “relaxing” within the positive hedonic valence group (F2,70=9.46, p<.001). Figure 3 demonstrates that HC rated a higher percentage of odors in the negative hedonic valence group “distressing” compared to both CV-PTSD (p=.029) and CV+PTSD (p=.14) and a higher percentage of odors in the positive hedonic valence group “relaxing” compared to both CV+PTSD (p=.002) and CV-PTSD (p<.001).
Figure 3. Group differences in the percentage of odors rated to be “distressing” or “relaxing” within the negative and positive hedonic valence groups.
demonstrates that combat veterans with and without PTSD (CV+PTSD, CV-PTSD, respectively) compared to healthy controls (HC) report overall reduced sensitivity not only to the distressing properties of odors with negative hedonic valence (that are non-trauma-related), but to the relaxing properties of odors with positive hedonic valence.
4. Discussion
Although case reports have reported that trauma-associated odors can induce anxiety, including flashbacks (Hinton et al., 2004; Vermetten & Bremner 2003), this is to our knowledge the first study to survey the emotional impact of odors within nine different categories (burning-, death/decay-, drug -, food-, flammable-, floral-, garbage-, human body fluids/excretions-, and environmental-related odors) in civilians and combat veterans with and without PTSD. Given the high levels of trait anxiety in our combat veterans, one might have reasonably predicted a global increased sensitivity to odors in general or an increased sensitivity to only unpleasant odors or an increased sensitivity to odors that are novel in Iraq and Afghanistan. Our findings suggest, however, that the role of the olfactory system in the biological functions of threat detection is highly sophisticated. While no single mechanism can explain all of our findings, several theoretical models have direct relevance to our observations. These factors are outlined below and deserve attention in future research.
4.1. Hyposmia
Our results demonstrated a blunted response by combat veterans compared to healthy controls to a large number of odors that ranged across odor categories and hedonic valence. Given this broad finding, general dysfunction of the olfactory system might be a first consideration. For instance, could indigenous environmental factors and/or war-related chemical hazards in Iraq and Afghanistan (e.g. sand, dust, particulates, toxins released from burn pits, etc.) cause irreversible damage to the olfactory system and result in a decreased ability to detect odors in general? While there is strong evidence for an increase in both acute (Korzeniewski et al., 2014) and chronic (Abraham et al., 2014) respiratory disease in deployed and post-deployed OIF military personnel, specific insults to the olfactory system (e.g. olfactory mucosa/epithelium, receptors, nerve, etc.) have yet to be investigated and are thus unknown.
Preliminary research from our laboratory (Cortese et al., 2014) demonstrated mild hyposmia in combat veterans. In that study, combat veterans with and without PTSD, compared to published age norms, had reduced sensitivity to a neutral odor, meaning they needed higher odor concentrations for reliable detection. Although the cause and relationship of hyposmia and odor experiences have not been adequately studied and thus remain unknown, it is possible that even mild hyposmia may contribute in part to decreased hedonic valence sensitivity. While hyposmia might theoretically explain decreased sensitivity to positive hedonic odors, it would not explain the increased sensitivity to some, but not all, odors with negative hedonic valence. Additional or other mechanisms beyond hyposmia would be required to explain such a differential profile.
4.2. Selective Ignoring of Distractor Odors
Our findings of general blunting to a wide range of both positively- and negatively-valenced odors might also be explained as a highly adaptive, pro-survival mechanism for threat detection within environments with unfamiliar olfactory stimuli, i.e. the likely odor environments of our OEF/OIF/OND combat veterans. In fact, many unpleasant odors (e.g. garbage, feces, raw sewage, etc.) reported by our combat veterans to be extensively present in deployed areas of Iraq and Afghanistan are not associated with imminent danger in active combat zones. Our findings of an overall decreased prevalence of distress in veterans to these unpleasant odors (or strong pleasant odors for that matter) opens up the possibility that combat veterans, both with and without PTSD, learned during combat to ignore non-life-threatening “distractor” odors. The ability to ignore distracting odors and to concentrate on odor threat cues of real danger or threat to life has pro-survival benefits and is consistent with a highly adaptive mechanism for threat detection.
Increasing evidence suggests that the tasks of ignoring and attending to sensory stimuli represent two independent but interactive brain processes (Chait et. al, 2010; Lenartowicz et al., 2014). Theoretically, a person could have a deficit in one or both of these processes. In the case of an inability to ignore distracting odor stimuli, such as unpleasant odors or, even, strongly pleasant odors, one would predict a reduced ability to focus on the identification of cues associated with real danger. To the extent that a post-deployment decreased prevalence of distress to unpleasant odors reflects the same abilities during combat, our findings support the idea that our veterans had access to an important odor-processing function (i.e. ignoring distractor odors). Our findings further suggest that the veterans without PTSD were particularly skilled at ignoring distractor odor cues. Whether the inability to selectively ignore non-life-threatening distractor odors might predict PTSD vulnerability is an interesting question for future research.
Additional research that builds upon Lenartowicz and colleagues (2014) and Chait and colleagues (2010) work is necessary to differentiate the mediators of selective ignoring versus attentional avoidance. Both processes probably involve automatic, as well as focused/voluntary efforts, to achieve what might appear to be the same endpoint. From our perspective, however, selective ignoring represents a healthy process, which allows the person to achieve a positive outcome by ignoring distractors (e.g. bad body odor) and concentrating on signals of real danger. In contrast, and, as proposed by Mogg and coworkers (1987) attentional avoidance likely represents an unhealthy process, which results in increased anxiety and failure to habituate.
4.3. Trait Anxiety & Attentional Bias
Trait anxiety has been associated with self-reported increased sensitivity to a wide-range of environmental odorants (Bailer et al., 2008). Given the higher levels of trait anxiety in our PTSD veterans, one might expect them to report a global increased sensitivity to a wide-range of negative hedonic valenced odors. However, this was not at all the case. There was absolutely no evidence, even at a trend level, to suggest a global over-sensitivity to negative hedonic odors in general, even to odors considered “disgusting” by most individuals. Instead, our PTSD veterans reported increased levels of distress to a very small and selective number of odors (i.e. burnt hair, discharged gunpowder, blood, and fuel odors).
A separate line of research has shown an association between anxiety or stress-related disorders and attentional bias toward threat (Bryant & Harvey, 1995; Cisler & Koster, 2010). While our survey method was not designed to examine attentional bias to odor threats per se, our data parallel those of McNally and colleagues (1990) who found that Vietnam combat veterans with PTSD preferentially focused their attention on syndrome-specific threat cues (i.e. PTSDrelated word like “body bags” compared to OCD-related words like “germs”). Analogous to this, our PTSD veterans reported an increased sensitivity to selective and specific combat-related odors. Limitations in our self-report methodology did not allow us to determine whether there were pre-deployment increased sensitivities to these threat-related odors. It would be useful in future research to determine whether different dimensions of attentional bias such as facilitated attention, delayed disengagement and attentional avoidance (for review of these attentional bias components using visual threat cues, see Cisler & Koster, 2010) can be demonstrated to odor stimuli. It should be emphasized that prior research investigating attentional bias to threat has employed laboratory tests [e.g. Visual Search (Pineles et al., 2007), Modified Stroop (Ashley et al., 2013), and Spatial Cueing (Mogg et al., 2008) tasks] that rely exclusively on visual stimuli and cannot easily be adapted for the study of odors. Promising research (La Buissonniere-Ariza et al., 2013), however, has recently demonstrated the feasibility of investigating attentional bias to odor stimuli using odor detection response times as a measure of facilitated attention.
4.4. Conditioned Odor Threat
Our findings of a greater prevalence of distress to the odors of blood, fuel, burnt hair and the scent of discharged gunpowder, but not to other equally unpleasant and negative hedonic odors (even intensely unpleasant odors unique to Iraq/Afghanistan), suggest that these particular odors have personal salience as signals of danger. Although it is unknown how, or for that matter when, our combat veterans with PTSD developed an increased sensitivity to these specific odors, associative odor-threat learning (Li, 2014) is a likely mechanism.
The animal literature provides overwhelming evidence on the effectiveness of odors as conditioned threat cues (Walker et al., 2005, Kass et al., 2013). In fact, the strength of odor-conditioned threat has been demonstrated through single trial odor-shock training (Paschall & Davis, 2002a), second-order conditioning (Paschall & Davis, 2002b), as well as transgenerational inheritance (Dias & Ressler, 2014). A growing body of evidence reveals that these behavioral changes are accompanied by neuroplasticity along the olfactory pathway, not only in higher-level association areas of the piriform cortex (Li et al., 2008), but even in structures (i.e. olfactory receptors and olfactory bulb) involved in the earliest stages of olfactory processing (Jones et al., 2008; Kass et al., 2013).
While associative learning through the pairing of burning, blood, and fuel odors with life-threatening combat experiences may underlie our findings of increased odor sensitivity to selective odors, information on the specific relationship between these odors and the actual traumatic events experienced by our veterans was not assessed in this report. We also cannot rule out that the veterans with PTSD were more sensitive to burning, blood, and fuel odors prior to their combat experiences.
4.5. Unconditioned Odor Threat
Of particular interest, and controversy, is the possible role of unconditioned threat odors in humans. In animals, specific odors are clearly hardwired to elicit defensive and/or fear behaviors. For example, natural predator odors elicit species-specific fear behaviors (e.g. “tail-flag, flight and jump behaviors in deer exposed to wolf urine; Osada et al, 2014; for review see Apfelbach et al., 2005).Fear behaviors associated with exposure to predator odors in animals represent an innate response conserved through evolution. In humans, there are auditory (e.g. loud sounds), visual (e.g. flashes of light), and somatosensory stimuli (e.g. electric shock) that produce startle and immediate alarm and vigilance behaviors (Lang & Davis, 2006; Grillon, 2008). These alerting responses are also hard-wired, conserved through evolution, and galvanize pro-survival behaviors. Whether evolutionarily conserved odor threat cues exist in humans is yet to be determined. However, if they do exist, one would expect an unconditioned odor threat cue to meet the following criteria: a) the odor stimulus occurs naturally in the human environment, b) the identification of the odor and avoidance behaviors have pro-survival benefits, c) the odor elicits an alarm response in all or almost all healthy humans and d) the alarm and fear response is immediate and automatic in nature. Although the category of human body fluids/excretions met these criteria, no single odor was consistently reported to be distressing by all healthy normal controls, which seems unexpected given the evolutionary significance of threat odors in the biological functions of alarm, vigilance, and fear in animals.
4.6. Conditioned-Unconditioned Odor Threat Interactions
What is probably under-appreciated by many mental health professionals is that odors are processed via two neural pathways: a) the more familiar olfactory pathway and b) the less commonly acknowledged trigeminal pathway. Odors that preferentially activate the trigeminal pathway (e.g. CO2, ammonia, etc.) produce an irritating sensation (i.e. burning, pungent, and stinging) in the nose, the fundamental sensations that could easily serve as unconditioned stimuli. Among the odorants we surveyed that are also suspected of being highly present in combat zones of Iraq and Afghanistan, fuel and intense burning odors come closest to having strong trigeminal characteristics. And, a significantly higher prevalence of combat veterans with PTSD reported these odors to be distressing compared to those without PTSD and health controls. Given that several lines of evidence indicate olfactory perception can be potentiated by trigeminal activation (Bensafi et al., 2007; Hummel & Livermore, 2002; Moessnang et al., 2013), we speculate that odorants with strong trigeminal properties may be more likely to become conditioned to lifethreatening, traumatic, events.
4.7. Impaired Extinction Learning
For warriors in combat zones, the automatic startle, fear, and alarm that is triggered by exposure to auditory and visual threat cues should have pro-survival advantages (LeDoux, 1996; 2012). To the extent our PTSD veterans had the same levels of self-perceived alarm to burnt hair, fuel, gunpowder and blood odors (i.e. odors signaling true danger) during deployment as they did at the time of our assessment, we would expect these individuals to have been especially skilled in identifying and responding to true threat odors during combat. This would appear to be a valuable advantage for individuals operating in highly dangerous, life-threatening combat situations and, therefore, seems somewhat incompatible with the negative attributes of PTSD. A possible explanation for this apparent paradox is failure with extinction learning in those warriors who later develop PTSD. We propose that all warriors learn during combat to associate blood, fuel, and burning odors with life-threatening situations. In this model, all warriors, including those who do and do not develop PTSD, would have comparable increased sensitivity to these odors during deployment. What would distinguish the warriors who later develop PTSD is an inability to extinguish the conditioned fear response (Norrholm et al., 2011) after returning to civilian life.
Information supporting delayed extinction in PTSD is based almost exclusively on visual and/or auditory stimuli. We speculate that impaired extinction to odor threat cues parallels the delayed extinction to visual/auditory stimuli that has already been established by several independent research teams. Several factors, however, may make trauma-related odors particularly resistant to extinction: 1) odors may facilitate fear conditioning through multimodal enhanced learning, fear generalization, and avoidance (Sauerhofer et al., 2012), 2) many of the more distressing odor threat cues (e.g. blood, burnt hair) in PTSD are uncommon in civilian life and, therefore, less available for habituation and 3) burning-related and fuel type odors may be primed for evolving into second-order, unconditioned stimuli and, on their own, begin to elicit fear behaviors (Wessa and Flor, 2007).
4.8. Limitations and Future Directions
To our knowledge, this is the first study to ascertain whether there are certain odor categories or specific individual odors that elicit distress in combat veterans with PTSD. While prior research in our laboratory found a strong relationship between subjective distress using similar survey methods and later objective findings under double-blind, placebo-controlled in anxiety patients (Boulenger & Uhde, 1984; Uhde, 1995), there are understandable limitations with the survey methods. What is needed is the development of an attentional bias odor threat test, which tracks and quantifies detection speed, disengagement, and avoidance of odor threat cues. Such a research tool would identify the extent to which, if any, the positive biological function of “ignoring” distractor odors versus “avoiding” odor threat cues play an interactive role in the pathophysiology of PTSD. Even taking into account the limitations of self-report methods and the extrapolation of prevalence data as an index of behavioral sensitivity, our findings indicate that veterans with PTSD and, to a lesser extent, even healthy veterans without PTSD, appear to have different responses to certain individual odors and odor categories. Future investigations are required to determine whether such differences represent at-risk factors prior to deployment and/or a marker of illness severity.
The long-term goal of our research team is to design more effective therapies that take into account evidence-based data on the role of odors in the pathogenesis and treatment of PTSD. Recognizing the role of trauma-related odors in the triggering of distress and flashbacks, our team and others are beginning to incorporate odors into prolonged exposure treatment. Although it was not the focus of our study, it is evident that some individuals with PTSD report trauma-related odors as their primary source of distress, rather than visual and/or auditory stimuli. Thus, it is conceivable that exposure techniques using exclusively trauma-related odors are not only essential, but perhaps even sufficient, to achieve a therapeutic response in selective cases.
There is only a paucity of data to suggest how to go about selecting odors as part of a treatment. While our findings offer a valuable starting point (i.e. by probing veterans for distress in response to burning-related, fuel, and blood odors), there remains a great deal of variability in terms of individual degrees of distress even to these odors. Our findings clearly indicate that one cannot simply select odors that were novel or unpleasant reminders of the combat zone because such a strategy would identify many odors that have little, if any salience, to the combat veterans in terms of representing true life-threatening odor threat cues. Therefore, in terms of picking trauma-related odors for inclusion into an exposure treatment paradigm, “unpleasant” odors in general, or even, odors that are highly unique to the combat zone itself, may be ineffective. The challenge is to identify odors for exposure treatment not that the person “ignores” but, rather, “avoids” because they represent true, life-threatening odor cues. Ultimately, for these treatments to achieve optimal effectiveness, it will be necessary to identify odors on an individual basis that have personal salience to the index traumatic event(s). On a related note, the selection of odors to elicit relaxation, as opposed to being incorporated into exposure-habituation paradigms, may be even more challenging, insofar as odors with a high degree of positive “relaxing” properties in normal healthy adults may be considerably less likely to be helpful in combat veterans, with or without PTSD.
Acknowledgement
We would like to thank Drs. Anouk Grubaugh and Ron Acierno for their assistance in the recruitment of subjects for this study.
Funding for this study was provided by NIMH Grant K01 MH090548 (BMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare that they have no conflicts of interest.
Some of these findings will be presented in June 2015 at the annual meeting of the American Society of Clinical Psychopharmacology (ASCP), Miami Beach, FL.
References
- Abraham JH, Eick-Cost A, Clark LL, Hu Z, Baird CP, DeFraites R, Tobler SK, Richards EE, Sharkey JM, Lipnick RJ, Ludwig SL. A retrospective cohort study of military deployment and postdeployment medical encounters for respiratory conditions. Mil. Med. 2014;179:540–546. doi: 10.7205/MILMED-D-13-00443. [DOI] [PubMed] [Google Scholar]
- Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Ashley V, Honzel N, Larsen J, Justus T, Swick D. Attentional bias for trauma-related words: exaggerated emotional Stroop effect in Afghanistan and Iraq war veterans with PTSD. BMC Psychiatry. 2013;13 doi: 10.1186/1471-244X-13-86. 86-244X-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailer J, Witthoft M, Rist F. Psychological predictors of short- and medium term outcome in individuals with idiopathic environmental intolerance (IEI) and individuals with somatoform disorders. J. Toxicol. Environ. Health. A. 2008;71:766–775. doi: 10.1080/15287390801985562. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Frasnelli J, Reden J, Hummel T. The neural representation of odor is modulated by the presence of a trigeminal stimulus during odor encoding. Clin. Neurophysiol. 2007;118:696–701. doi: 10.1016/j.clinph.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Boulenger JP, Uhde TW, Wolff EA, 3rd, Post RM. Increased sensitivity to caffeine in patients with panic disorders. Preliminary evidence. Arch. Gen. Psychiatry. 1984;41:1067–1071. doi: 10.1001/archpsyc.1983.01790220057009. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Processing threatening information in posttraumatic stress disorder. J. Abnorm. Psychol. 1995;104:537–541. doi: 10.1037//0021-843x.104.3.537. [DOI] [PubMed] [Google Scholar]
- Chait M, de Cheveigne A, Poeppel D, Simon JZ. Neural dynamics of attending and ignoring in human auditory cortex. Neuropsychologia. 2010;48:3262–3271. doi: 10.1016/j.neuropsychologia.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Downes JJ. Proust nose best: odors are better cues of autobiographical memory. Mem. Cognit. 2002;30:511–518. doi: 10.3758/bf03194952. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clin. Psychol. Rev. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese BM, Leslie KR, Grubaugh A, Yang QX, Uhde TW. Olfactory function and odor cue-reactivity in combat veterans with and without PTSD. Neuropsychopharmacology. 2014;39:S229. [Google Scholar]
- Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB. Psychosocial therapy for posttraumatic stress disorder. J. Clin. Psychiatry. 2006;67(Suppl 2):40–45. [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology (Berl) 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz RS, Cupchik GC. The emotional distinctiveness of odor-evoked memories. Chem. Senses. 1995;20:517–528. doi: 10.1093/chemse/20.5.517. [DOI] [PubMed] [Google Scholar]
- Herz RS. A naturalistic analysis of autobiographical memories triggered by olfactory visual and auditory stimuli. Chem. Senses. 2004;29:217–224. doi: 10.1093/chemse/bjh025. [DOI] [PubMed] [Google Scholar]
- Hinton DE, Pich V, Chhean D, Pollack MH, Barlow DH. Olfactory-triggered panic attacks among Cambodian refugees attending a psychiatric clinic. Gen. Hosp. Psychiatry. 2004;26:390–397. doi: 10.1016/j.genhosppsych.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Hummel T, Livermore A. Intranasal chemosensory function of the trigeminal nerve and aspects of its relation to olfaction. Int. Arch. Occup. Environ. Health. 2002;75:305–313. doi: 10.1007/s00420-002-0315-7. [DOI] [PubMed] [Google Scholar]
- Jones SV, Choi DC, Davis M, Ressler KJ. Learning-dependent structural plasticity in the adult olfactory pathway. J. Neurosci. 2008;28:13106–13111. doi: 10.1523/JNEUROSCI.4465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MD, Rosenthal MC, Pottackal J, McGann JP. Fear learning enhances neural responses to threat-predictive sensory stimuli. Science. 2013;342:1389–1392. doi: 10.1126/science.1244916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline NA, Rausch JL. Olfactory precipitants of flashbacks in posttraumatic stress disorder: case reports. J. Clin. Psychiatry. 1985;46:383–384. [PubMed] [Google Scholar]
- Korzeniewski K, Gregulski R. Reasons for medical evacuations of soldiers serving in International Security Assistance Force (ISAF) operation in Afghanistan. Int. Marit. Health. 2014;65:210–215. doi: 10.5603/IMH.2014.0040. [DOI] [PubMed] [Google Scholar]
- La Buissonniere-Ariza V, Lepore F, Kojok KM, Frasnelli J. Increased odor detection speed in highly anxious healthy adults. Chem. Senses. 2013;38:577–584. doi: 10.1093/chemse/bjt028. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog. Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. The Emotional Brain. New York, NY: Simon and Schuster; 1996. [Google Scholar]
- Lenartowicz A, Simpson GV, Haber CM, Cohen MS. Neurophysiological signals of ignoring and attending are separable and related to performance during sustained intersensory attention. J. Cogn. Neurosci. 2014;26:2055–2069. doi: 10.1162/jocn_a_00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319:1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. Learning to smell danger: acquired associative representation of threat in the olfactory cortex. Front. Behav. Neurosci. 2014;8:98. doi: 10.3389/fnbeh.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka Y, Sugiyama H, Katayama A, Kashiwagi M, Homma I. Slow breathing and emotions associated with odor-induced autobiographical memories. Chem. Senses. 2012;37:379–388. doi: 10.1093/chemse/bjr120. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Kaspi SP, Riemann BC, Zeitlin SB. Selective processing of threat cues in posttraumatic stress disorder. J. Abnorm. Psychol. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- Moessnang C, Pauly K, Kellermann T, Kramer J, Finkelmeyer A, Hummel T, Siegel SJ, Schneider F, Habel U. The scent of salience--is there olfactory-trigeminal conditioning in humans? Neuroimage. 2013;77:93–104. doi: 10.1016/j.neuroimage.2013.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Weinman J. Memory bias in clinical anxiety. J. Abnorm. Psychol. 1987;96:94–98. doi: 10.1037//0021-843x.96.2.94. [DOI] [PubMed] [Google Scholar]
- Mogg K, Holmes A, Garner M, Bradley BP. Effects of threat cues on attentional shifting, disengagement and response slowing in anxious individuals. Behav. Res. Ther. 2008;46:656–667. doi: 10.1016/j.brat.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell PV, Uhde TW. Dose-response effects of intravenous caffeine in normal volunteers. Anxiety. 1994;1:161–168. doi: 10.1002/anxi.3070010403. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada K, Miyazono S, Kashiwayanagi M. Pyrazine analogs are active components of wolf urine that induce avoidance and fear-related behaviors in deer. Front. Behav. Neurosci. 2014;8:276. doi: 10.3389/fnbeh.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschall GY, Davis M. Second-order olfactory-mediated fear-potentiated startle. Learn. Mem. 2002b;9:395–401. doi: 10.1101/lm.50602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschall GY, Davis M. Olfactory-mediated fear-potentiated startle. Behav. Neurosci. 2002a;116:4–12. doi: 10.1037//0735-7044.116.1.4. [DOI] [PubMed] [Google Scholar]
- Pineles SL, Shipherd JC, Welch LP, Yovel I. The role of attentional biases in PTSD: is it interference or facilitation? Behav. Res. Ther. 2007;45:1903–1913. doi: 10.1016/j.brat.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Saive AL, Royet JP, Ravel N, Thevenet M, Garcia S, Plailly J. A unique memory process modulated by emotion underpins successful odor recognition and episodic retrieval in humans. Front. Behav. Neurosci. 2014;8:203. doi: 10.3389/fnbeh.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerhofer E, Pamplona FA, Bedenk B, Moll GH, Dawirs RR, von Horsten S, Wotjak CT, Golub Y. Generalization of contextual fear depends on associative rather than non-associative memory components. Behav. Brain Res. 2012;233:483–493. doi: 10.1016/j.bbr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, Resick PA, Thurston V, Orsillo SM, Haug R, Turner C, Bernardy N. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA. 2007;297:820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Uhde TW. Caffeine-Induced Anxiety: An Ideal Checmical Model of Panic Disorder? In: Asnis GM, van Praag HM, editors. Panic Disorder: Clinical, Biological, and Treatment Aspects. New York, NY: John Wiley & Sons, Inc; 1995. pp. 181–205. [Google Scholar]
- Vermetten E, Bremner JD. Olfaction as a traumatic reminder in posttraumatic stress disorder: case reports and review. J. Clin. Psychiatry. 2003;64:202–207. doi: 10.4088/jcp.v64n0214. [DOI] [PubMed] [Google Scholar]
- Walker DL, Paschall GY, Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn. Mem. 2005;12:120–129. doi: 10.1101/lm.87105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am. J. Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Willander J, Larsson M. Smell your way back to childhood: autobiographical odor memory. Psychon. Bull. Rev. 2006;13:240–244. doi: 10.3758/bf03193837. [DOI] [PubMed] [Google Scholar]