Abstract
Post-transcriptional events regulate herpesvirus gene expression, yet few herpesvirus RNA-binding proteins have been identified. We used an unbiased approach coupling oligo(dT) affinity capture with proteomics to identify viral RNA-associated proteins during infection. Using this approach, we identified and confirmed changes in the abundance or activity of two host RNA-associated proteins, DHX9 and DDX3, in cells infected with human cytomegalovirus (HCMV). We also identified and confirmed previously unreported activities for the HCMV US22 and pp71 proteins as RNA-associated viral proteins and confirmed that a known viral RNA-binding protein, pTRS1, associates with RNA in infected cells. Further, we found that HCMV pp71 co-sedimented with polysomes, associated with host and viral RNAs, and stimulated the overall rate of protein synthesis. These results demonstrate that oligo(dT) affinity capture coupled with proteomics provides a rapid and straightforward means to identify RNA-associated viral proteins during infection that may participate in the post-transcriptional control of gene expression.
Keywords: Human cytomegalovirus (HCMV), herpesvirus, proteomics, RNA-binding, post-transcriptional regulation, translation, messenger RNA (mRNA)
Introduction
Human cytomegalovirus (HCMV) is a ubiquitous human pathogen that infects the majority of the population (12). Healthy individuals carry HCMV asymptomatically for the rest of their lives. However, immune-compromised individuals are at risk for severe life threatening disease caused by reactivated HCMV infections (74). In addition, HCMV can be congenitally passed from mother to child during pregnancy and HCMV is a leading cause of infectious birth defects (49).
Regulation of HCMV gene expression is complex and occurs at both the transcriptional and post-transcriptional levels. Like host mRNAs, viral precursor RNAs are transcribed and mature in the nucleus, and then transported to the cytosol where they are translated into protein. Ribonucleoprotein (RNP) complexes play a critical role in each step of host mRNA maturation and quality control (1, 22) and therefore play important roles in the post-transcriptional control of gene expression. Herpesvirus transcripts are also subject to post-transcriptional regulation. However, unlike host transcripts, many herpesvirus transcripts are unspliced and therefore require a distinct complement of RNPs for their maturation and export (85). For example human herpesviruses encode homologs of the HCMV UL69 protein (pUL69), which interacts with the host RNA export machinery and facilitates the transport of unspliced viral mRNAs into the cytoplasm (62, 63). In addition herpesvirus proteins manipulate host post-transcriptional regulatory systems in order to modify the expression of antiviral host messages and miRNAs (10, 54, 61, 86). For HCMV, post-transcriptional regulation plays an essential role in the kinetics of viral gene expression. mRNAs for several late genes are transcribed early in infection; however, late mRNAs are not translated into protein until viral DNA replication occurs (26, 92). Whether HCMV encodes additional proteins that impact post-transcriptional control of viral gene expression remains unknown.
One approach to identify proteins involved in post-transcriptional regulation of gene expression involves the identification of RNA-associated proteins. For example, RNA-affinity chromatography coupled with mass spectrometry has been used to identify RNA binding proteins in mammalian cells (4, 14). Similar strategies have been used to define host and viral proteins that associate with specific viral RNAs (56, 60, 76). Such approaches have proven useful in defining the complement of RNA binding proteins that might participate in RNA metabolism, trafficking, or translation.
In order to identify HCMV proteins that might function in post-transcriptional regulation, we used an unbiased proteomics approach based on oligo(dT) affinity purification to identify mRNA-associated proteins during HCMV infection. We find that infection alters the abundance and activity of two host RNA-binding proteins, DHX9 and DDX3X. In addition, we identified and confirmed previously undescribed mRNA-binding activities for the HCMV US22 protein and the viral tegument protein pp71. Further studies revealed that pp71 bound mRNA throughout a time course of infection and associated with polysomes in HCMV infected cells. Both host and viral mRNAs co-purified with pp71 from infected cell lysates and pp71 associated with mRNA in the absence of additional viral proteins. Together these results demonstrate the utility of oligo(dT) affinity capture coupled with proteomics to identify new RNA-binding activities for herpesvirus proteins.
Materials and Methods
Cells and viruses
Human foreskin fibroblasts (HFFs) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) with 10% Newborn Calf Serum (NCS) at 37°C with 5% CO2 and used between passages 7 and 14. HEK 293T and HeLa cells were maintained in DMEM with 10% Fetal Bovine Serum (FBS). Mutant HCMV AD169 strains expressing pTRS1 (BADinTRS1GFP) and pUS22 (BADinUS22GFP) with C-terminal GFP fusion tags under the control of their native promoters were made by lambda/red-mediated recombineering (107) (87) using the BADwt BAC as the parental strain. HCMV BADinGFP (ADGFP), BADinTRS1GFP and BADinUS22GFP viruses were grown on HFFs. Unless otherwise noted, HFFs were infected at an MOI of three for one hour at 37°C and 5% CO2 in DMEM plus 10% NCS.
Cloning and transfections
The pp71-HA plasmid was constructed by amplifying the UL82 open reading frame from the BADinGFP BAC using gene specific primers that include restriction sites for BamHI and EcoRI as well as a HA tag sequence for the C terminus of the protein. The amplicon was ligated into the pcDNA3.1 multi-cloning site in between the BamHI and EcoRI sites. The pUL35A-GFP mammalian expression vector was produced by amplifying the UL35A open reading frame from the BADinGFP BAC using gene specific primers (Forward: GGAGATAGAACCATGATGATCGAGGGCGCCTCTCGGCAGACG, Reverse: CAAGAAAGCTGGGTCGAGATGCCGTAGGTTTTCGGCCAGATCG) The amplicon was recombined into pDEST47 using Gateway Cloning (Life Technologies).
Oligo(dT) capture and mass spectrometry
Confluent HFFs were mock infected or infected with ADGFP at an MOI of three. At 72 hpi the cells were washed with PBS and harvested by scraping. The cells were pelleted by centrifugation at 2200 rpm for 10 minutes and resuspended in 1 mL oligo(dT) buffer (40 mM HEPES pH 7.6, 500 mM NaCl, 1 mM EDTA, 0.3% CHAPS, Complete EDTA-free protease inhibitor (Roche)) and incubated on ice for 10 minutes. Insoluble material was removed by centrifugation at 4°C and 15,000 rpm. Where indicated, the supernatant was treated with micrococcal nuclease for 30 minutes at room temperature with rocking. The supernatant was mixed with 10 mg of Oligo(dT)-Cellulose Type 7 (GE Health Sciences) beads that were rehydrated in 100 µL oligo(dT) buffer plus 20 mg/mL yeast tRNA prior to mixing. The slurry was nutated for one hour at 4°C. The beads were pelleted and washed three times with oligo(dT) buffer and twice with wash buffer (250 mM NaCl, 40 mM HEPES pH 7.6, 1 mM EDTA, Complete Protease inhibitors, 20 mg/mL yeast tRNA). The mRNA and bound proteins were eluted in 100 µL elution buffer (40 mM HEPES pH 7.6, 1 mM EDTA, Complete Protease inhibitors, 40 mg/mL soluble oligo(dT) with mixing for one hour at 4°C. Protein levels were estimated by resolving a 10 µL aliquot of the eluate on a 10% SDS-PAGE gel and staining with GelCode Blue (Pierce). The proteins were digested with trypsin in solution and purified over C-18 columns. The resulting peptides were identified using liquid chromatography coupled tandem mass spectrometry (LC-MS/MS). MS spectra were searched using the MASCOT algorithm against the current version of the Human IPI database.
Oligo(dT) capture of RNA binding proteins and western blots
Infected HFFs or transfected HEK 293T or HeLa cells were harvested by scraping and pelleted by centrifugation at 1500 rpm. Cells were lysed in 1 mL oligo(dT) buffer and incubated on ice for 10 minutes. Insoluble material was removed by centrifugation at 4°C and 15,000 rpm. Where indicated the supernatant was treated with micrococcal nuclease for 30 minutes at room temperature with rocking. The supernatant was then mixed with Oligo (dT)-Cellulose Type 7 (GE Health Sciences) beads and nutated for one hour at 4°C. The beads were washed three times in oligo(dT) buffer and resuspended in denaturing protein sample buffer (0.1 M Tris-HCl pH 6.8, 6% Glycerol, 2% SDS, 0.1 M DTT, 0.002% Bromophenol Blue). Proteins were separated by 10% SDS-PAGE gels and transferred to Protran nitrocellulose membrane (Whatmann). Blots were probed with primary antibodies specific for PABP (1:2000; Cell Signaling), GFP (1:1000; Roche cat# 11814460001) or pp71 (45) followed by HRP-conjugated secondary antibodies (KPL) followed by ECL (Advansta) and visualization on x-ray film or using a digital chemiluminesence detection system (Bio-Rad).
Immunoprecipitations
Transfected HEK 293T or HeLa cells were lysed in 1 mL RIPA buffer (50 mM Tris-HCl pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1mM EDTA) containing protease inhibitors (Complete EDTA-free protease inhibitor; Roche) followed by incubation on ice for 10 minutes. Insoluble material was removed by centrifugation at 4°C and 15,000 rpm. The supernatants were then treated with or without micrococcal nuclease for 30 minutes at room temperature with rocking. Supernatants were pre-cleared by nutating with Protein A/G beads (Santa Cruz Biotechnology) at 4°C for 30 minutes. The pre-clear beads were then pelleted and discarded. The pre-cleared lysates were then incubated with 2 µg GFP antibody (Roche) at 4°C with nutating for one hour. Immune complexes were captured on Protein A/G sepharose beads by nutating at 4°C for one hour. The beads were pelleted and washed three times in RIPA buffer and resuspended in denaturing protein sample buffer. Immune complexes were resolved on 10% SDS-PAGE gels and analyzed by Western blot.
Identification of mRNAs associated with pp71 immune complexes
Infected HFFs were lysed and pre-cleared for immunoprecipitation as above. A portion of the lysate was removed for the isolation of total RNA. The pre-cleared lysates were then incubated with 100 µL Protein A/G beads that had been pre-conjugated to pp71 or IE1 antibodies. The immune complexes were formed at 4°C with nutating for one hour. The beads were then pelleted and washed three times in RIPA buffer before being resuspended in 100 µL RIPA buffer. 10 µL aliquots of resuspended beads were removed, mixed with protein sample buffer (0.5 M Tris-HCl pH 6.8, 30% Glycerol, 10% SDS, 0.6 M DTT, 0.012% bromophenol blue) and probed by western blot for pp71 and Tubulin (Sigma). RNA was extracted from the remaining beads with 1 mL Trizol and RNA. cDNA was prepared from the captured RNAs and total RNA using random hexamer primers and the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative RT-PCR (qRT-PCR) was performed using a Lightcycler 480 with SYBR Green Master Mix (Roche) and primers for IE1 (Forward: CAAGTGACCGAGGATTGCAA, Reverse: CACCATGTCCACTCGAACCTT), UL44 (Forward: GCGTGCAAGTCTCGACTAAGGAGC, Reverse: AAGTACTGTGCCTCTTAGTCGGGGG), pp28 (Forward: GTGTCCCATTCCCGACTCG, Reverse: TTCACAACGTCCACCCACC) and GAPDH (Forward: CTGTTGCTGTAGCCAAATTCGT, Reverse: ACCCACTCCTCCACCTTTGAC). In order to normalize detected mRNA levels for each gene, threshold cycles (CT) from immunoprecipitated samples were normalized back to the CT obtained from total RNA. The normalized CT for each gene was then compared between the two conditions: the nonspecific IE1 control IP and the pp71 IP. The Fold increase for each gene was calculated from the difference in normalized CT between these two conditions.
Confocal Microscopy
HFFs were plated on glass cover slips and infected at a multiplicity of one with the indicated virus strain. At the indicated times post infection cells were washed and fixed using 2% paraformaldehyde, blocked in 1% BSA for at least one hour at 4°C and then probed with pp71 antibodies and fluorescent anti-mouse secondary antibodies. Coverslips were mounted onto slides with Vectashield mounting solution (Vector Laboratories). Cells were imaged using a Zeiss CLSM 710 Spectral Confocal Laser Scanning Microscope.
Metabolic labeling of nascent proteins
HeLa cells plated in 6-well dishes were transfected with the indicated expression plasmids and harvested 24 hours after transfection. Where indicated samples were treated with Torin1 (96) (250 nM) or cycloheximide (100 µg/mL) for 16 hours prior to harvest. Forty-five minutes prior to harvest cell growth media was replaced with media lacking methionine and cysteine. After fifteen minutes 125 µCi of 35S-methionine/cysteine was added (Perkin Elmer) to the culture media. Cells were labeled for 30 minutes at 37°C and then washed twice with ice-cold PBS prior to harvest by scraping. Cell pellets were lysed in RIPA buffer, insoluble material was removed by centrifugation at 4°C and 15,000 rpm, and equal portions of lysate were precipitated in a solution containing 0.1 mg/mL BSA and 20% TCA. The proteins were precipitated on ice for thirty minutes and the precipitated proteins were bound to glass fiber filters (Whatmann) using a vacuum manifold. The filters were washed twice with 20% TCA followed by two washes with 100% ethanol and allowed to air dry for twenty minutes. Radioactive proteins retained on the filters were quantified in a scintillation counter. Protein concentrations were determined by Bradford assay. To visualize changes in nascent protein synthesis, 10 µg of each sample were separated on 10% SDS-PAGE gels, which were subsequently dried and visualized by autoradiography.
Results
Unbiased proteomic profiling of RNA-associated proteins in HCMV-infected cells
In order to identify proteins associated with mRNA during infection, we performed an unbiased screen for proteins that co-purify with oligo(dT) cellulose from HCMV infected cells. Figure 1 shows the basic details of the assay. Human foreskin fibroblasts (HFFs) were infected with HCMV for 72 hours and then lysed in a buffer containing high salt (500 mM NaCl) in order to limit non-specific protein:protein interactions. mRNA:protein complexes were isolated by incubating the lysates with oligo(dT) conjugated cellulose beads. As high salt concentrations lower the base pairing stringency of RNA:DNA interactions (95), we decreased the salt concentration during the wash and elution steps to limit non-specific RNA binding to the oligo(dT) DNA oligomers. A substantial source of false positives in proteomics experiments arises from nonspecific binding of proteins to the affinity matrix (82). Therefore the mRNA:protein complexes were eluted from the beads by the addition of excess soluble oligo(dT), leaving non-specific interactors bound to the matrix. The eluted lysates were separated on SDS-PAGE gels and visualized by Coomassie staining (Fig 1B). Numerous bands were enriched in abundance in the oligo (dT) eluates as compared to whole cell lysates. The eluates from HCMV infected cells appeared to contain all of the bands found in uninfected eluates, along with several unique bands (Fig 1B). We performed the assay in the presence of micrococcal nuclease in order to visualize levels of nonspecific proteins bound to the beads in the absence of RNA (Fig 1B). While some non-specific interactors remained, micrococcal nuclease treatment reduced the number and intensity of bands, suggesting that many of the captured proteins were retained in an RNA-dependent manner.
Figure 1. Oligo(dT) capture combined with mass spectrometry identifies HCMV proteins associated with mRNA.
(A) Flow chart of the method used to identify HCMV mRNA associated proteins. (B) Proteins isolated using the method in A were separated by SDS-PAGE and visualized using GelCode Blue staining. Left panel; the effect of micrococcal nuclease treatment on the complement and intensity of oligo(dT)-associated proteins. Right Panel; Gel showing oligo(dT)-associated proteins isolated from uninfected or HCMV-infected fibroblasts. Arrows denote bands specific to infected cells. (C) Gene ontogeny analysis shows that cellular proteins detected by LC-MS/MS after oligo (dT) capture are significantly enriched for RNA-associated functions.
The complement of proteins in the oligo(dT) eluate was determined using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Initial inspection of the eluted proteins revealed that both the infected and uninfected samples contained many known RNA-binding proteins (Table 1, Table S1). Gene ontogeny analysis revealed that the set of proteins enriched in the oligo(dT) eluate were significantly associated with RNA-binding, post-transcriptional regulation, and splicing (Fig. 1C). This analysis suggests that our assay enriched for proteins that interact with RNA in both uninfected and infected primary fibroblasts.
Table 1. Host RNA-associated proteins identified by oligo(dT) proteomics.
The uniprot accession numbers (http://www.uniprot.org/) and gene names for the top thirty identified host proteins. The third column notes previous studies demonstrating an RNA-associated activity for the specified protein.
| Uniprot # | Gene Description | RNA associated activity |
|---|---|---|
| P52272 | Heterogeneous nuclear ribonucleoprotein M | 21, 25, 44, 65 |
| P11940 | Polyadenylate-binding protein 1 | 19, 20, 30, 65, 103 |
| P22626 | Heterogeneous nuclear ribonucleoproteins A2/B1 | 6, 43, 79, 104 |
| P23246 | Splicing factor, proline- and glutamine-rich | 29, 77, 88 |
| P27694 | Replication protein A 70 kDa DNA-binding subunit | |
| Q00839 | Heterogeneous nuclear ribonucleoprotein U | 23, 50, 108 |
| P07910 | Heterogeneous nuclear ribonucleoproteins C1/C2 | 6, 9, 39, 79, 104 |
| P08670 | Vimentin | |
| Q13310 | Poly(A) binding protein 4 | 43 |
| P14866 | Heterogeneous nuclear ribonucleoprotein L | 32, 40, 41, 64, 80, 90 |
| P16989 | Y-box-binding protein 3 | |
| O43390 | heterogeneous nuclear ribonucleoprotein R | 37, 43 |
| O15523 | ATP-dependent RNA helicase DDX3Y | 3, 27, 47, 51, 70, 89, 93, 105 |
| O00571 | ATP-dependent RNA helicase DDX3X | 3, 27, 47, 51, 70, 89, 93, 105 |
| Q04837 | Single-stranded DNA-binding protein, mitochondrial | |
| Q96G97 | Seipin | |
| Q15233 | Non-POU domain-containing octamer-binding protein | 5, 88, 109 |
| Q96AE4 | Far upstream element-binding protein 1 | 42, 58 |
| O14979 | Heterogeneous nuclear ribonucleoprotein D-like | 48, 75 |
| P51991 | Heterogeneous nuclear ribonucleoprotein A3 | 67 |
| P67809 | Nuclease sensitive element binding protein-1 | 36, 43, 66, 91, 94, 101 |
| O75534 | Cold shock domain-containing protein E1 | 7, 15, 31 |
| Q9Y3F4 | Serine-threonine kinase receptor-associated protein | 16, 100 |
| P09651 | Heterogeneous nuclear ribonucleoprotein A1 | 6, 43, 79, 104 |
| Q9Y2W1 | Thyroid hormone receptor-associated protein 3 | 53, 99 |
| Q08211 | ATP-dependent RNA helicase A (DHX9) | 24, 35, 52, 59, 78, 102 |
| Q15717 | ELAV-like protein 1 | 8, 102 |
| P11142 | Heat shock cognate 71 kDa protein | 43 |
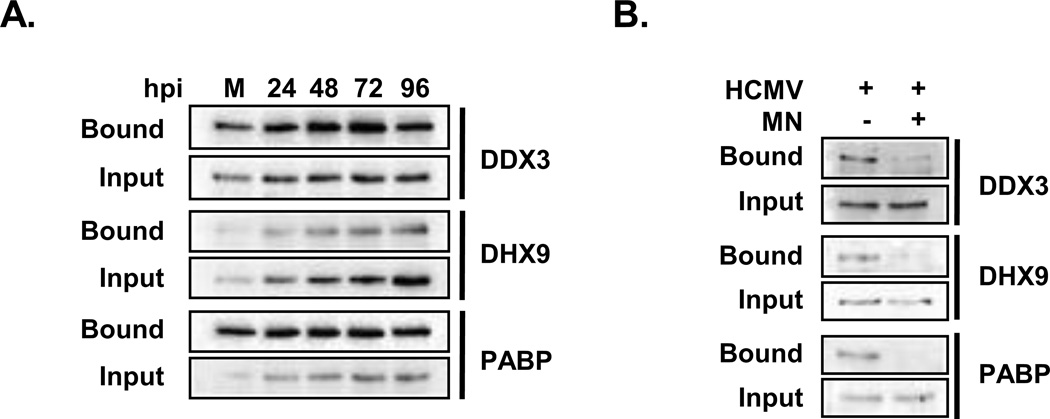
To gain a sense of whether infection changes the abundance of host proteins associated with mRNA, we compared total spectral counts for individual host proteins detected in the mock and HCMV infected samples. Our data suggested that infection might increase the association of several host proteins with mRNA. Two such host proteins, the RNA helicases DDX3X and DHX9 were selected for further analysis. Both proteins co-purified with oligo(dT) from uninfected cell lysates (Fig. 2A), consistent with previous results (52, 105). Micrococcal nuclease treatment inhibited binding of both proteins, demonstrating that the association was dependent on the presence of RNA and not the result of non-specific interactions with the cellulose beads (Fig. 2B). Infection increased the amount of each protein associated with mRNA, stimulating the binding of DHX9 to a greater extent than DDX3X (Fig. 2A). The increased association was likely due to the increased levels of DDX3X and DHX9 observed in HCMV-infected cells. We conclude that HCMV infection changes the abundance of two host RNA-binding proteins associated with mRNA during infection. While our data suggests similar changes may occur for additional host proteins, we caution that further studies are needed to confirm these changes for individual host proteins.
Figure 2. HCMV infection changes the activity of host RNA-binding proteins.
(A) HFFs were infected with HCMV AD169 at a multiplicity of three and harvested at the indicated times following infection. The abundance of the host DDX3X and DHX9 proteins in oligo(dT) precipitates (bound) or total cell lysate (input) was measured by Western blot. (B) Seventy two hours after infection oligo(dT) precipitates were isolated from infected cell lysates and analyzed by Western blot. Where indicated the samples were treated with micrococcal nuclease (+MN) to confirm that the interaction is RNA dependent. The results in A and B are representative of at least two independent experiments.
HCMV pTRS1 and pUS22 associate with mRNA in HCMV infected cells
In addition to cellular proteins, oligo(dT) eluates from infected cells also contained viral proteins (Table S1). Two of the viral proteins, pTRS1 and pUS22, contain a US22 superfamily domain, yet the proteins differ in their subcellular localization. pTRS1 is found predominantly in the cytoplasm throughout infection (Fig. 3A and (81)). Conversely, pUS22 is primarily found in the nucleus (Fig. 3A, bottom (71)). pTRS1 was previously shown to bind mRNA in vitro (33), although the association of pTRS1 with mRNA during HCMV infection has not been reported. Both pTRS1 and pUS22 co-purified with oligo(dT) throughout a time course of HCMV infection (Fig. 3B). Micrococcal nuclease treatment diminished the amount of pTRS1 and pUS22 that co-purified with oligo(dT), demonstrating an RNA-dependent interaction with the beads (Fig 3C,D). The host poly (A) binding protein (PABP1), a well-characterized cellular RNA-binding protein (28), was used as a control in these experiments (Fig 3C). These data are the first report of pTRS1 binding to mRNA in the context of HCMV infection and also validate the ability of oligo(dT) coupled proteomics to capture and identify both nuclear and cytoplasmic HCMV proteins associated with mRNA. In addition, these data identify the first molecular activity for HCMV pUS22 as an RNA-associated protein.
Figure 3. Confirmation that HCMV pUS22 and pTRS1 associate with mRNA during HCMV infection.
(A) HFFs were infected with HCMV strains expressing GFP-tagged TRS1 (TRS1-GFP) or US22 (US22-GFP). Seventy-two hours after infection protein localization was visualized by confocal microscopy. (B) Oligo(dT) precipitates from cells infected with a US22GFP virus throughout a time course of infection were analyzed by Western blot with GFP (US22GFP) or TRS1-specific antibodies. (C) Cells were infected with the US22GFP virus and oligo(dT) associated proteins were analyzed by Western blot using GFP and PABP specific antibodies at seventy two hours after infection. Where indicated the lysates were treated with micrococcal nuclease (+MN) prior to incubation with oligo(dT) beads. (D) Cells were infected with the TRS1GFP virus for seventy two hours and the presence of pTRS1 in the oligo(dT) precipitate was measured by Western blot (C). All results are representative of three independent experiments.
HCMV pp71 is an RNA-associated protein
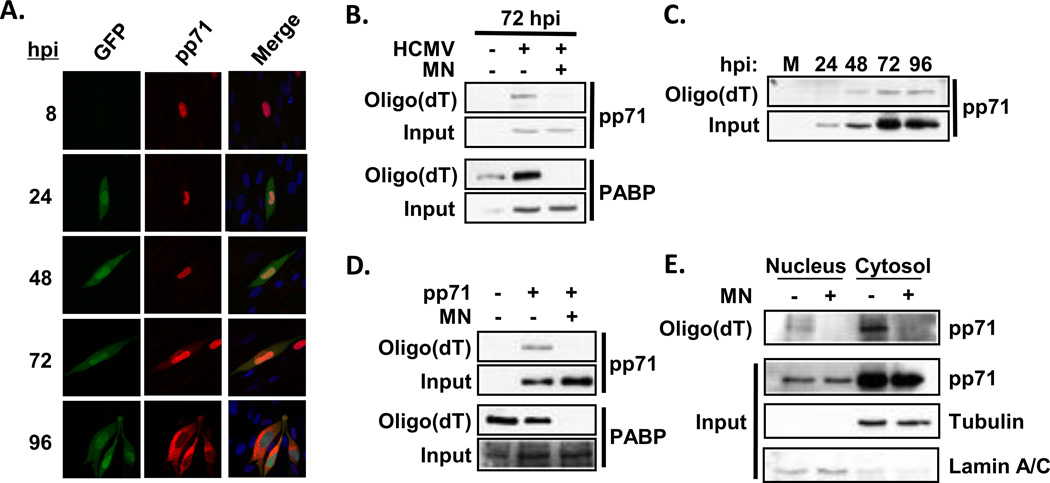
We also identified the HCMV pp71 protein in our proteomics analysis. While several functions have been ascribed to pp71 (13, 45, 46, 98) it has not previously been shown to associate with mRNA. To examine the association of pp71 with mRNA in more detail, we measured the association of pp71 with oligo(dT) cellulose throughout a time course of HCMV infection (Fig. 4C). pp71 co-purified with oligo(dT) cellulose as early as 24 hours after infection, and the amount of pp71 associated with the resin increased as infection progressed. The increase in pp71 binding over time was proportional to the increase in pp71 expression. Micrococcal nuclease treatment reduced pp71 co-purification with oligo(dT) beads, indicating that co-purification with the beads is dependent on the presence of mRNA (Fig. 4B)
Figure 4. HCMV pp71 associates with mRNA during HCMV infection.
(A) The location of GFP or pp71 was determined throughout a time course of infection by confocal microscopy. (B) Oligo(dT) precipitates from HCMV infected cells (72 hpi) were analyzed by Western blot. Where indicated, the lysates were incubated with micrococcal nuclease (+MN) prior to oligo(dT) capture. (C) Oligo(dT) precipitates were isolated from HCMV infected cell lysates at the indicated times after infection and analyzed by Western blot using a pp71-specfic antibody. (D) Oligo(dT) precipitates were isolated from HeLa cells transfected with a pp71 expression vector and analyzed by Western blot. (E) Seventy two hours after HCMV infection oligo(dT) precipitates were isolated from nuclear or cytoplasmic fractions and analyzed by Western blot. All experiments are representative of at least three independent experiments.
In order to determine if other viral factors are required for the association of pp71 with mRNA, we determined if pp71 could associate with mRNA outside of the context of infection. Cells were transfected with a vector expressing pp71, and the ability of pp71 to co-purify with oligo(dT) cellulose was determined by Western blot (Fig. 4D). As during infection, pp71 co-purified with oligo(dT) and micrococcal nuclease treatment inhibited the interaction. These data confirm our proteomics data and show that pp71 associates with mRNA in HCMV-infected cells. In addition the interaction between pp71 and mRNA is not mediated by additional viral factors.
pp71 associates with mRNA in the nucleus and cytoplasm
In newly infected cells, tegument-derived pp71 localizes in the nucleus. In contrast, nascent pp71 localizes primarily to the cytoplasm where it contributes to HCMV replication through an unknown mechanism (38). In order to visualize the location of pp71 at various times during infection we performed indirect immunofluorescence for pp71 at various times after infection with HCMV (Fig. 4A). As previously reported pp71 was only found in the nucleus until 48 hours post infection, when some pp71 began to appear in the cytoplasm. By 96 hours after infection pp71 was primarily found in the cytoplasm of infected cells. To determine if pp71 preferentially associates with mRNA in the nucleus or cytosol, we measured the association of pp71 with oligo(dT) in nuclear and cytosolic extracts from infected cells. We analyzed samples at 72 hours after infection for this experiment, a time when pp71 is present in both cellular compartments. Both nuclear and cytosolic pp71 co-purified with the oligo(dT) cellulose and binding in both locations was decreased by micrococcal nuclease treatment (Fig. 4E). We conclude that pp71 can bind mRNA in both the nuclear and cytoplasmic compartments.
pp71 associates with host and viral mRNAs
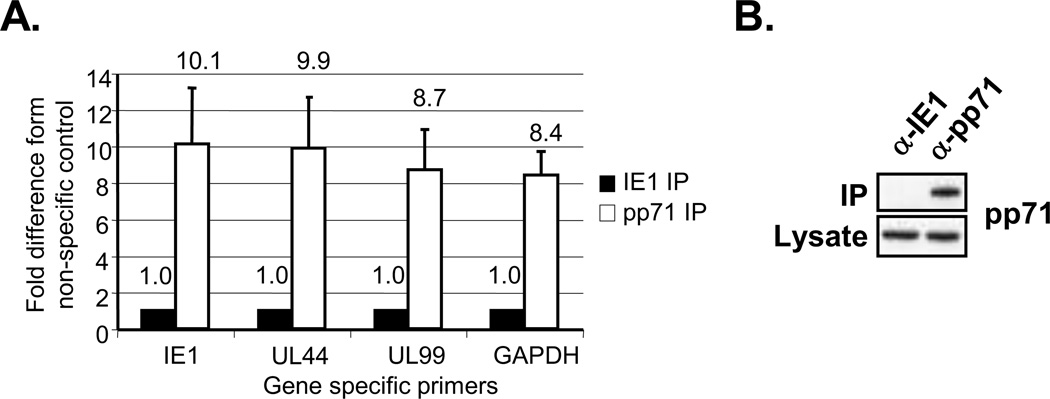
Our finding that pp71 co-purified with oligo(dT) cellulose in an RNA-dependent manner suggested that pp71 might associate with viral transcripts during infection. We therefore measured the association of pp71 with specific host and viral mRNAs in HCMV-infected cells. pp71-specific immune complexes were isolated from infected cell lysates and the associated RNAs were extracted and analyzed by quantitative reverse-transcriptase real time PCR (qRT-PCR). As a control we measured the abundance of specific mRNAs in immune complexes specific for the HCMV IE1 protein, which is not known to associate with mRNA. pp71-specific immune complexes were enriched for the HCMV IE1, UL44 and UL99 mRNAs compared to the IE1 control (Fig. 5A). The host GAPDH mRNA was also enriched in pp71 immune complexes, demonstrating that pp71 associates with a host mRNA during infection. Ribosomal RNAs were not found in pp71 immune complexes, and no signal was observed when the reverse transcription step was omitted (data not shown). We conclude that pp71 associates with mRNAs in HCMV infected cells. These results also suggest that pp71 may not discriminate between host and viral transcripts.
Figure 5. pp71 associates with host and viral mRNAs during infection.
(A) Lysates from infected HFFs (72hpi) were immunoprecipitated with antibodies specific for pp71 or IE1. The abundance of IE1, UL44, UL99 or GAPDH transcript in the immune complexes was quantified by qRT-PCR. Error bars indicate the standard error of the mean (n=3). (B) Western blots were performed on immunoprecipitated fractions from A with antibodies specific to pp71.
A portion of pp71 associates with polysomes during infection
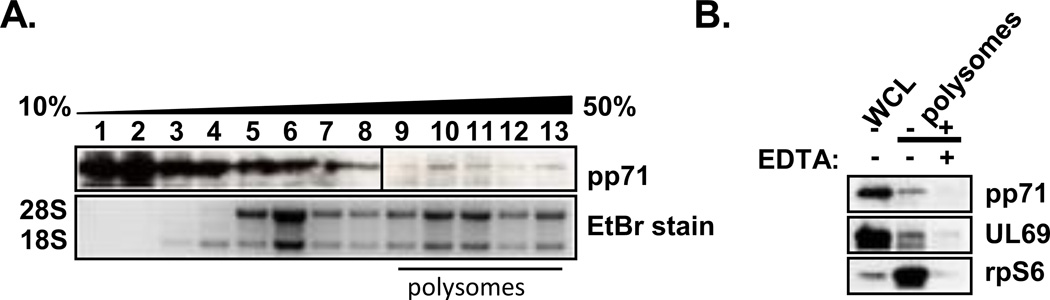
We next determined if pp71 associates with actively translating mRNA in HCMV infected cells. mRNAs undergoing high levels of translation are bound by multiple ribosomes, or polysomes, which migrate into the dense fractions of linear sucrose gradients. We reasoned that if pp71 were associated with actively translating mRNAs then pp71 should co-migrate with polysomes in a sucrose gradient. Cytoplasmic lysates from HCMV-infected cells were resolved through 10–50% linear sucrose gradients to separate ribosomal subunits, monosomes, and polysomes. The sucrose gradients were fractionated, and the distribution of pp71 throughout the gradient was determined by Western blot (Fig. 6A). The majority of pp71 localized to lighter fractions of the gradient, which contains RNP complexes and free mRNAs (68). However, a portion of the pp71 was detectable throughout the gradient, including the heavier fractions containing polysomes.
Figure 6. pp71 associates with polysomes during HCMV infection.
(A) Lysate from infected HFFs (72 hpi) was resolved through a 10–50% linear sucrose gradient and the gradient was fractionated. Aliquots from each fraction were either TCA precipitated and analyzed by Western blot (top) or total RNA was extracted and separated on an agarose gel to visualize ribosomal RNA (bottom). The black bar denotes the location of polysome-containing fractions. (B) Seventy two hours after infection cells were lysed in buffer with or without added EDTA. The lysates were separated by sucrose gradient centrifugation as in A and aliquots of the polysomes-containing fractions were pooled, precipitated and analyzed by Western blots with antibodies specific to pp71, UL69 or rpS6. The data are representative of at least two independent experiments.
In order to confirm that the migration of pp71 in the gradient reflected an association with polysomes, we determined the effect of disrupting ribosomes on the abundance of pp71 in the polysome-containing fractions. High concentrations of EDTA disrupt ribosomes and thus deplete polysomes from the denser fractions of the gradient (11). EDTA treatment reduced the abundance of ribosomes in the more dense gradient fractions as demonstrated by the reduced abundance of the ribosomal protein S6 (rpS6) (Fig. 6B). Consistent with previous results, the HCMV UL69 protein (pUL69) was also present in polysome-containing fractions and its abundance in these fractions was also diminished by EDTA treatment (Fig. 6B) (2). EDTA treatment also reduced the abundance of pp71 in the polysome-containing gradient fractions. As cytosolic lysates were used in the polysome analysis, these data support our above conclusion that pp71 associates with mRNA in the cytosol. We conclude that a portion of pp71 associates with polysomes in infected cells and therefore likely interacts with translation competent mRNAs.
The pp71-binding partner pUL35A also associates with mRNA, however, its interaction with pp71 is RNA-independent
Another HCMV tegument protein, pUL35, has been shown to interact with pp71 in the nucleus of infected cells (84). Recently, however, the smaller isoform of pUL35, pUL35A, was also found to co-localize with pp71 in the cytoplasm. Proteomic analysis of pUL35A found that pUL35A associates with several RNA binding proteins during HCMV infection (83), suggesting that pUL35A might also associate with RNA. To determine if pUL35A associates with mRNA, we measured the ability of pUL35A to co-purify with oligo(dT) cellulose in transfected cells. pUL35A co-purified with oligo(dT) cellulose in an RNA-dependent manner, as micrococcal nuclease treatment diminished the interaction (Fig 7A). pUL35A also co-purified with oligo(dT) in the presence of pp71, however the amount of pUL35A co-purifying with oligo(dT) was slightly reduced when pp71 was present (Fig. 7A). Two isoforms of pUL35A were consistently detected in cells transfected with both pp71 and pUL35A: the larger isoform was only observed in the presence of pp71. While the nature of the larger isoform is currently unknown, this result suggests that pp71 might induce a post-translational modification of pUL35A that is not necessary for pUL35A to associate with RNA. These results show that pUL35A can associate with mRNA outside of infection. In addition these results demonstrate that our proteomics dataset is not complete, and that HCMV encodes additional RNA-associated proteins not identified by our approach.
Figure 7. pp71 and UL35A associate with mRNA, but do not require RNA for their interaction.
(A) Oligo(dT) precipitates were isolated from HeLa cells transfected with combinations of empty vector, pp71-HA, and pUL35A-GFP. Where indicated the lysate was treated with micrococcal nuclease (+MN) prior to capture. The bound proteins were analyzed by Western blot. (B) HeLa cells were transfected with a pp71-HA expression plasmid with or without a vector expressing pUL35A as a carboxyl-terminal GFP fusion protein (pUL35A-GFP). GFP-specific immune complexes were isolated and analyzed by Western blot using the indicated antibodies. Where indicated, lysates were treated with micrococcal nuclease (+MN) prior to immunoprecipitation. The data are representative of at least three independent experiments.
Our finding that both pp71 and pUL35A associate with mRNA suggested that perhaps the interaction of pUL35A and pp71 is bridged by an RNA intermediate. In order to determine if pp71 binding to pUL35A is mRNA dependent, we determined if micrococcal nuclease digestion prevented the interaction in transfected cells. pp71 co-precipitated with pUL35A and the interaction was not inhibited by micrococcal nuclease treatment (Fig. 7B). We conclude that while both pp71 and pUL35A interact with mRNA, the pp71:pUL35A interaction does not require an RNA intermediate.
Protein synthesis is increased in cells expressing pp71 and pUL35A
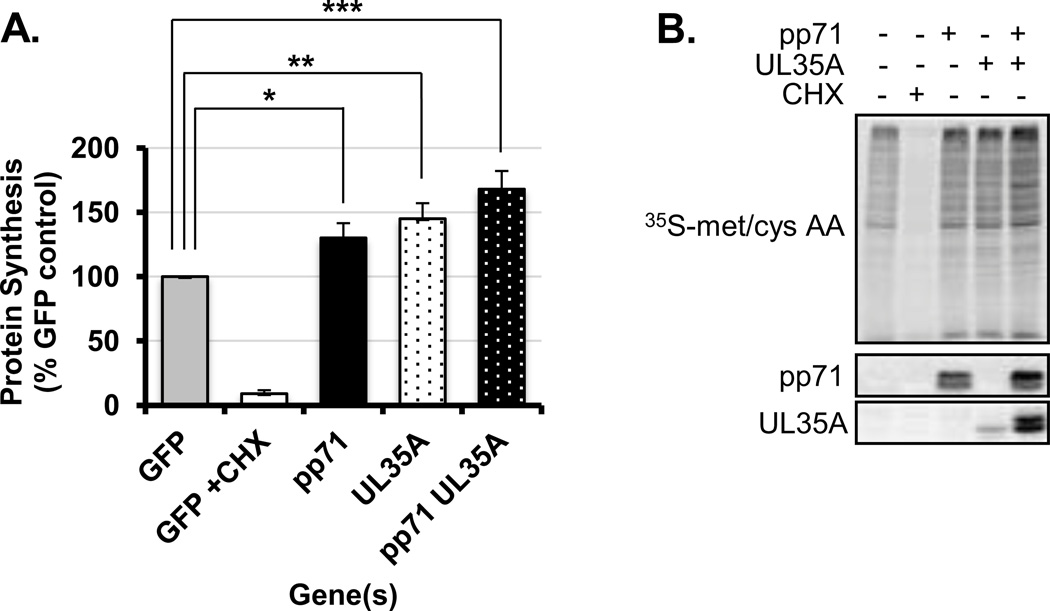
Since pp71 and pUL35A both associate with mRNA we investigated the impact of these proteins on the overall rate of protein synthesis. When expressed alone, pp71 and pUL35A increased total protein synthesis (Fig. 8A, B) as compared to control cells expressing GFP. Co-expressing pp71 and pUL35A together increased protein synthesis to a greater extent than either protein alone. However, pp71 and pUL35A did not confer stress resistant protein synthesis as observed during infection (18, 57, 72), as inhibiting the host translation initiation complex eIF4F with an mTOR inhibitor limited protein synthesis in pp71 and pUL35A expressing cells (data not shown). While these data do not exclude the possibility that pp71 and UL35A may globally alter transcription or RNA stability, the increase in protein synthesis in the presence of pp71 and pUL35A together with our finding that pp71 associates with polysomes and mRNAs during infection suggest that these proteins act in concert to increase translation.
Figure 8. pp71 and UL35A expression increases total protein synthesis.
(A) HeLa cells were transfected with plasmids expressing pEGFP, pp71, pUL35A or both pp71 and pUL35A. Newly synthesized proteins were labeled metabolically and quantified by liquid scintillation. The results were normalized to the amount of total protein in each sample and are expressed as percent of counts detected in the GFP control. Error bars indicate the standard error of the mean (n=6). * = p < 0.05, ** = p < 0.01, *** = p < 0.001 (B) Radiolabeled proteins from A were separated on SDS-PAGE gels and visualized by autoradiography.
Discussion
We have used a rapid, unbiased proteomics method to identify RNA-associated proteins during viral infection. Our approach identified known host RNA-associated proteins and for two such proteins, DDX3X and DHX9, identified changes in their abundance during infection. Importantly, our approach also identified both known and previously undescribed HCMV RNA-associated proteins. Using this approach we identified and confirmed the novel association of two HCMV proteins, pUS22 and pp71, with mRNA in infected cells. We also demonstrate for the first time that pTRS1, a known HCMV RNA-binding protein (33), associates with mRNA during infection. Together these results demonstrate that this approach captures both nuclear and cytosolic HCMV RNA-associated proteins. We also demonstrate a new activity for the HCMV pp71 protein as an RNA-associated protein that associates with actively translating host and viral mRNAs during infection. These results demonstrate that oligo(dT) affinity chromatography coupled with mass spectrometry is an efficient means for identifying herpesvirus proteins associated with RNA during viral infection.
Past studies using oligo(dT) capture coupled with proteomics have identified many factors involved in mRNA expression and metabolism (4, 14). In this study we used a similar approach to identify previously undetected interactions of HCMV proteins with mRNA during lytic infection. We previously used a similar screen to identify host proteins that specifically interact with poliovirus genomic RNA during infection (56). In contrast, HCMV infected cells express hundreds of viral transcripts as well as thousands of host RNAs. Thus in this study, oligo(dT) affinity purification captures host and viral proteins associated with either host or HCMV transcripts. The viral proteins identified by this approach could therefore be involved in post-transcriptional regulation of viral transcripts, or alternatively the expression and metabolism of host mRNAs.
Several aspects of our approach make it easily adaptable for use in other viral systems. Our approach relies on commercially available reagents, and comparable mass spectrometers are available in many academic proteomics core facilities. The relatively small sample size required suggests this approach could be adapted to identify viral RNA-binding proteins expressed during lytic or latent infection. While we could confirm changes in the abundance of host proteins associated with mRNA, more quantitative mass spectrometry techniques (e.g. SILAC, iTRaQ, reviewed in (73)) could be incorporated to precisely measure changes in the abundance of host or viral RNA-associated proteins throughout a time course of infection. Applying oligo(dT) coupled proteomics should prove useful in many viral culture systems for quantitatively identifying proteins, either host or viral, associated with RNA.
In its current form our approach was sufficient to identify new activities for pp71 and pUS22 as HCMV RNA-associated proteins. In addition we could confirm that a known HCMV RNA-binding protein, HCMV pTRS1, associates with mRNA during HCMV infection. However, our results also show that our approach is not comprehensive, as we did not detect the known viral RNA-binding pUL69 and the pUL35A protein co-purified with oligo(dT) cellulose in transfected cells but was not detected in our proteomics screen. In the case of pUL69 this could reflect a relatively low affinity of pUL69 for RNA, as its RNA-binding ability is not required for its function as a nuclear export factor for HCMV transcripts (63). Perhaps the use of more sensitive instrumentation would provide greater depth. Alternatively, the stringent binding and wash conditions of our approach were designed to limit false positives, perhaps giving rise to false negatives. Nonetheless, our current approach was sufficiently sensitive to identify known and unknown interactions that we could subsequently confirm using traditional molecular biology techniques.
Our results also identify new RNA-associated roles for previously characterized HCMV proteins. The HCMV pUS22 protein is an abundant nuclear protein that is also found in the supernatant of infected cells (71). While pUS22 is the namesake member of a family of related betaherpesvirus proteins (17), no function has been described for HCMV pUS22. Thus our finding that pUS22 associates with mRNA is the first functional annotation for pUS22. We hypothesize that pUS22 may play a role in RNA splicing or export based on its presence at high levels in the nucleus of infected cells, however additional experiments are needed to test this hypothesis. Of note is the fact that HCMV pTRS1 is a member of the US22 family of proteins and also associates with mRNA (33). Perhaps US22 family members share a common function in the post-transcriptional control of viral gene expression. This idea is consistent with previous studies demonstrating a role for pTRS1 and its MCMV homologs as antagonists of the antiviral kinase PKR (34). It is also possible that some of the RNA-associated proteins we identified may interact with polyadenylated RNAs that are not mRNAs, as some non-coding RNAs such as lncRNAs can also be polyadenylated (reviewed in (106)).
We also identified a new function for HCMV pp71 as an mRNA associated protein. We found that pp71 interacts with mRNA in infected and transfected cells, associates with polysomes during infection and stimulates protein synthesis both alone and in conjunction with its binding partner pUL35A. How the association of pp71 and pUL35A with mRNA might affect viral gene expression or replication is currently unclear. pp71 binds to the host BclAF1 protein (55), which is a component of an RNP complex that regulates mRNA processing and transport (70). Perhaps pp71 modulates BclAF1 activity, thereby effecting host or viral gene expression at the post-transcriptional level. Another possibility is that pp71 and pUL35A together inhibit cellular stress responses that would otherwise limit viral protein synthesis. pUL35A associates with the host G3BP1 protein (83), which limits translation during viral infections by sequestering mRNAs in cytoplasmic puncta termed stress granules (97) (69). Perhaps pUL35A and pp71 limit stress granule formation or sequestration of viral mRNAs into stress granules, thereby promoting viral protein synthesis. However it is also possible that pp71 and UL35A increase protein synthesis through a global effect on transcription or RNA stability. In any case, our finding that pp71 binds to mRNA provides direction for future studies to define a role for pp71 in the post-transcriptional control of gene expression during HCMV infection.
In summary, we show that oligo(dT) affinity capture coupled with proteomics is a useful approach to identify proteins that associate with RNA, either host or viral, during infection. Given recent insights into the surprising complexity of the HCMV transcriptome, our approach provides a straightforward and practical means of identifying viral proteins that may manipulate mRNA processing, export, stability or translation. Our results identify new activities for viral proteins and also provide new directions for defining the underlying processes HCMV uses to regulate viral gene expression.
Supplementary Material
Highlights.
We used an unbiased proteomics approach to identify RNA-associated proteins during HCMV infection.
We confirmed changes in abundance and activity for two host RNA-binding proteins, DHX9 and DDX3.
We identified and confirmed three HCMV-RNA binding proteins during infection, pUS22, pTRS1 and pp71.
pp71 associated with host and viral mRNAs and associated with polysomes in infected cells, and increased protein synthesis.
Acknowledgments
We thank members of the Moorman lab and the laboratory of Cary Moody for helpful discussions and insights. We also thank Steve Bachenheimer for critical reading of the manuscript and Blossom Damania, Dirk Dittmer, and Nancy Raab-Traub for making this work possible. This study was supported by grant 1R01AI103311-01 from the U.S. National Institutes of Health to N.J.M. and funds from the North Carolina University Cancer Research Fund to N.J.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends in biochemical sciences. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Aoyagi M, Gaspar M, Shenk TE. Human cytomegalovirus UL69 protein facilitates translation by associating with the mRNA cap-binding complex and excluding 4EBP1. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2640–2645. doi: 10.1073/pnas.0914856107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariumi Y. Multiple functions of DDX3 RNA helicase in gene regulation, tumorigenesis, and viral infection. Frontiers in genetics. 2014;5:423. doi: 10.3389/fgene.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltz AG, Munschauer M, Schwanhausser B, Vasile A, Murakawa Y, Schueler M, Youngs N, Penfold-Brown D, Drew K, Milek M, Wyler E, Bonneau R, Selbach M, Dieterich C, Landthaler M. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Molecular cell. 2012;46:674–690. doi: 10.1016/j.molcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Basu A, Dong B, Krainer AR, Howe CC. The intracisternal A-particle proximal enhancer-binding protein activates transcription and is identical to the RNA- and DNA-binding protein p54nrb/NonO. Molecular and cellular biology. 1997;17:677–686. doi: 10.1128/mcb.17.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer AL, Christensen ME, Walker BW, LeStourgeon WM. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977;11:127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- 7.Boussadia O, Niepmann M, Creancier L, Prats AC, Dautry F, Jacquemin-Sablon H. Unr is required in vivo for efficient initiation of translation from the internal ribosome entry sites of both rhinovirus and poliovirus. Journal of virology. 2003;77:3353–3359. doi: 10.1128/JVI.77.6.3353-3359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan CM, Steitz JA. HuR and mRNA stability. Cellular and molecular life sciences : CMLS. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunner JE, Ertel KJ, Rozovics JM, Semler BL. Delayed kinetics of poliovirus RNA synthesis in a human cell line with reduced levels of hnRNP C proteins. Virology. 2010;400:240–247. doi: 10.1016/j.virol.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant HE, Wadd SE, Lamond AI, Silverstein SJ, Clements JB. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome-associated protein 145 and inhibits splicing prior to the first catalytic step. Journal of virology. 2001;75:4376–4385. doi: 10.1128/JVI.75.9.4376-4385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burny A, Huez G, Marbaix G, Chantrenne H. On a messenger ribonucleoprotein complex from rabbit reticulocytes. Biochimica et biophysica acta. 1969;190:228–231. doi: 10.1016/0005-2787(69)90176-2. [DOI] [PubMed] [Google Scholar]
- 12.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Reviews in medical virology. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 13.Cantrell SR, Bresnahan WA. Interaction between the human cytomegalovirus UL82 gene product (pp71) and hDaxx regulates immediate-early gene expression and viral replication. Journal of virology. 2005;79:7792–7802. doi: 10.1128/JVI.79.12.7792-7802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, Krijgsveld J, Hentze MW. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Chang TC, Yamashita A, Chen CY, Yamashita Y, Zhu W, Durdan S, Kahvejian A, Sonenberg N, Shyu AB. UNR, a new partner of poly(A)-binding protein, plays a key role in translationally coupled mRNA turnover mediated by the c-fos major coding-region determinant. Genes & development. 2004;18:2010–2023. doi: 10.1101/gad.1219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chari A, Golas MM, Klingenhager M, Neuenkirchen N, Sander B, Englbrecht C, Sickmann A, Stark H, Fischer U. An assembly chaperone collaborates with the SMN complex to generate spliceosomal SnRNPs. Cell. 2008;135:497–509. doi: 10.1016/j.cell.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Chee MS, Bankier AT, Beck S, Bohni R, Brown CM, Cerny R, Horsnell T, Hutchison CA, 3rd, Kouzarides T, Martignetti JA, et al. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Current topics in microbiology and immunology. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 18.Clippinger AJ, Maguire TG, Alwine JC. The changing role of mTOR kinase in the maintenance of protein synthesis during human cytomegalovirus infection. Journal of virology. 2011;85:3930–3939. doi: 10.1128/JVI.01913-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coller JM, Gray NK, Wickens MP. mRNA stabilization by poly(A) binding protein is independent of poly(A) and requires translation. Genes & development. 1998;12:3226–3235. doi: 10.1101/gad.12.20.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosson B, Couturier A, Chabelskaya S, Kiktev D, Inge-Vechtomov S, Philippe M, Zhouravleva G. Poly(A)-binding protein acts in translation termination via eukaryotic release factor 3 interaction and does not influence [PSI(+)] propagation. Molecular and cellular biology. 2002;22:3301–3315. doi: 10.1128/MCB.22.10.3301-3315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datar KV, Dreyfuss G, Swanson MS. The human hnRNP M proteins: identification of a methionine/arginine-rich repeat motif in ribonucleoproteins. Nucleic acids research. 1993;21:439–446. doi: 10.1093/nar/21.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreyfuss G, Kim VN, Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nature reviews. Molecular cell biology. 2002;3:195–205. doi: 10.1038/nrm760. [DOI] [PubMed] [Google Scholar]
- 23.Fackelmayer FO, Dahm K, Renz A, Ramsperger U, Richter A. Nucleic-acid-binding properties of hnRNP-U/SAF-A, a nuclear-matrix protein which binds DNA and RNA in vivo and in vitro. European journal of biochemistry / FEBS. 1994;221:749–757. doi: 10.1111/j.1432-1033.1994.tb18788.x. [DOI] [PubMed] [Google Scholar]
- 24.Fuller-Pace FV. DExD/H box RNA helicases: multifunctional proteins with important roles in transcriptional regulation. Nucleic acids research. 2006;34:4206–4215. doi: 10.1093/nar/gkl460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gattoni R, Mahe D, Mahl P, Fischer N, Mattei MG, Stevenin J, Fuchs JP. The human hnRNP-M proteins: structure and relation with early heat shock-induced splicing arrest and chromosome mapping. Nucleic acids research. 1996;24:2535–2542. doi: 10.1093/nar/24.13.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geballe AP, Leach FS, Mocarski ES. Regulation of cytomegalovirus late gene expression: gamma genes are controlled by posttranscriptional events. Journal of virology. 1986;57:864–874. doi: 10.1128/jvi.57.3.864-874.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geissler R, Golbik RP, Behrens SE. The DEAD-box helicase DDX3 supports the assembly of functional 80S ribosomes. Nucleic acids research. 2012;40:4998–5011. doi: 10.1093/nar/gks070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goss DJ, Kleiman FE. Poly(A) binding proteins: are they all created equal? Wiley interdisciplinary reviews. RNA. 2013;4:167–179. doi: 10.1002/wrna.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gozani O, Patton JG, Reed R. A novel set of spliceosome-associated proteins and the essential splicing factor PSF bind stably to pre-mRNA prior to catalytic step II of the splicing reaction. The EMBO journal. 1994;13:3356–3367. doi: 10.1002/j.1460-2075.1994.tb06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray NK, Coller JM, Dickson KS, Wickens M. Multiple portions of poly(A)-binding protein stimulate translation in vivo. The EMBO journal. 2000;19:4723–4733. doi: 10.1093/emboj/19.17.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grosset C, Chen CY, Xu N, Sonenberg N, Jacquemin-Sablon H, Shyu AB. A mechanism for translationally coupled mRNA turnover: interaction between the poly(A) tail and a c-fos RNA coding determinant via a protein complex. Cell. 2000;103:29–40. doi: 10.1016/s0092-8674(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 32.Guang S, Felthauser AM, Mertz JE. Binding of hnRNP L to the pre-mRNA processing enhancer of the herpes simplex virus thymidine kinase gene enhances both polyadenylation and nucleocytoplasmic export of intronless mRNAs. Molecular and cellular biology. 2005;25:6303–6313. doi: 10.1128/MCB.25.15.6303-6313.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakki M, Geballe AP. Double-stranded RNA binding by human cytomegalovirus pTRS1. Journal of virology. 2005;79:7311–7318. doi: 10.1128/JVI.79.12.7311-7318.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hakki M, Marshall EE, De Niro KL, Geballe AP. Binding and nuclear relocalization of protein kinase R by human cytomegalovirus TRS1. Journal of virology. 2006;80:11817–11826. doi: 10.1128/JVI.00957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartman TR, Qian S, Bolinger C, Fernandez S, Schoenberg DR, Boris-Lawrie K. RNA helicase A is necessary for translation of selected messenger RNAs. Nature structural & molecular biology. 2006;13:509–516. doi: 10.1038/nsmb1092. [DOI] [PubMed] [Google Scholar]
- 36.Hartmuth K, Urlaub H, Vornlocher HP, Will CL, Gentzel M, Wilm M, Luhrmann R. Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16719–16724. doi: 10.1073/pnas.262483899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassfeld W, Chan EK, Mathison DA, Portman D, Dreyfuss G, Steiner G, Tan EM. Molecular definition of heterogeneous nuclear ribonucleoprotein R (hnRNP R) using autoimmune antibody: immunological relationship with hnRNP P. Nucleic acids research. 1998;26:439–445. doi: 10.1093/nar/26.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hensel GM, Meyer HH, Buchmann I, Pommerehne D, Schmolke S, Plachter B, Radsak K, Kern HF. Intracellular localization and expression of the human cytomegalovirus matrix phosphoprotein pp71 (ppUL82): evidence for its translocation into the nucleus. The Journal of general virology. 1996;77(Pt 12):3087–3097. doi: 10.1099/0022-1317-77-12-3087. [DOI] [PubMed] [Google Scholar]
- 39.Huang M, Rech JE, Northington SJ, Flicker PF, Mayeda A, Krainer AR, LeStourgeon WM. The C-protein tetramer binds 230 to 240 nucleotides of pre-mRNA and nucleates the assembly of 40S heterogeneous nuclear ribonucleoprotein particles. Molecular and cellular biology. 1994;14:518–533. doi: 10.1128/mcb.14.1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung LH, Heiner M, Hui J, Schreiner S, Benes V, Bindereif A. Diverse roles of hnRNP L in mammalian mRNA processing: a combined microarray and RNAi analysis. Rna. 2008;14:284–296. doi: 10.1261/rna.725208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang B, Lim JH, Hahm B, Jang SK, Lee SW. hnRNP L is required for the translation mediated by HCV IRES. Biochemical and biophysical research communications. 2009;378:584–588. doi: 10.1016/j.bbrc.2008.11.091. [DOI] [PubMed] [Google Scholar]
- 42.Jacob AG, Singh RK, Mohammad F, Bebee TW, Chandler DS. The splicing factor FUBP1 is required for the efficient splicing of oncogene MDM2 pre-mRNA. The Journal of biological chemistry. 2014;289:17350–17364. doi: 10.1074/jbc.M114.554717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonson L, Vikesaa J, Krogh A, Nielsen LK, Hansen T, Borup R, Johnsen AH, Christiansen J, Nielsen FC. Molecular composition of IMP1 ribonucleoprotein granules. Molecular & cellular proteomics : MCP. 2007;6:798–811. doi: 10.1074/mcp.M600346-MCP200. [DOI] [PubMed] [Google Scholar]
- 44.Kafasla P, Patrinou-Georgoula M, Lewis JD, Guialis A. Association of the 72/74-kDa proteins, members of the heterogeneous nuclear ribonucleoprotein M group, with the pre-mRNA at early stages of spliceosome assembly. The Biochemical journal. 2002;363:793–799. doi: 10.1042/0264-6021:3630793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalejta RF, Bechtel JT, Shenk T. Human cytomegalovirus pp71 stimulates cell cycle progression by inducing the proteasome-dependent degradation of the retinoblastoma family of tumor suppressors. Molecular and cellular biology. 2003;23:1885–1895. doi: 10.1128/MCB.23.6.1885-1895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalejta RF, Shenk T. Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71 protein. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3263–3268. doi: 10.1073/pnas.0538058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasim V, Wu S, Taira K, Miyagishi M. Determination of the role of DDX3 a factor involved in mammalian RNAi pathway using an shRNA-expression library. PloS one. 2013;8:e59445. doi: 10.1371/journal.pone.0059445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawamura H, Tomozoe Y, Akagi T, Kamei D, Ochiai M, Yamada M. Identification of the nucleocytoplasmic shuttling sequence of heterogeneous nuclear ribonucleoprotein D-like protein JKTBP and its interaction with mRNA. The Journal of biological chemistry. 2002;277:2732–2739. doi: 10.1074/jbc.M108477200. [DOI] [PubMed] [Google Scholar]
- 49.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Reviews in medical virology. 2007;17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 50.Kiledjian M, Dreyfuss G. Primary structure and binding activity of the hnRNP U protein: binding RNA through RGG box. The EMBO journal. 1992;11:2655–2664. doi: 10.1002/j.1460-2075.1992.tb05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai MC, Lee YH, Tarn WY. The DEAD-box RNA helicase DDX3 associates with export messenger ribonucleoproteins as well as tip-associated protein and participates in translational control. Molecular biology of the cell. 2008;19:3847–3858. doi: 10.1091/mbc.E07-12-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CG, Hurwitz J. A new RNA helicase isolated from HeLa cells that catalytically translocates in the 3' to 5' direction. The Journal of biological chemistry. 1992;267:4398–4407. [PubMed] [Google Scholar]
- 53.Lee KM, Hsu Ia W, Tarn WY. TRAP150 activates pre-mRNA splicing and promotes nuclear mRNA degradation. Nucleic acids research. 2010;38:3340–3350. doi: 10.1093/nar/gkq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S, Song J, Kim S, Kim J, Hong Y, Kim Y, Kim D, Baek D, Ahn K. Selective degradation of host MicroRNAs by an intergenic HCMV noncoding RNA accelerates virus production. Cell host & microbe. 2013;13:678–690. doi: 10.1016/j.chom.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Lee SH, Kalejta RF, Kerry J, Semmes OJ, O'Connor CM, Khan Z, Garcia BA, Shenk T, Murphy E. BclAF1 restriction factor is neutralized by proteasomal degradation and microRNA repression during human cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9575–9580. doi: 10.1073/pnas.1207496109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenarcic EM, Landry DM, Greco TM, Cristea IM, Thompson SR. Thiouracil cross-linking mass spectrometry: a cell-based method to identify host factors involved in viral amplification. Journal of virology. 2013;87:8697–8712. doi: 10.1128/JVI.00950-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lenarcic EM, Ziehr B, De Leon G, Mitchell D, Moorman NJ. Differential role for host translation factors in host and viral protein synthesis during human cytomegalovirus infection. Journal of virology. 2014;88:1473–1483. doi: 10.1128/JVI.02321-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H, Wang Z, Zhou X, Cheng Y, Xie Z, Manley JL, Feng Y. Far upstream element-binding protein 1 and RNA secondary structure both mediate second-step splicing repression. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2687–E2695. doi: 10.1073/pnas.1310607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Tang H, Mullen TM, Westberg C, Reddy TR, Rose DW, Wong-Staal F. A role for RNA helicase A in post-transcriptional regulation of HIV type 1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:709–714. doi: 10.1073/pnas.96.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Masaki T, Shimakami T, Lemon SM. hnRNP L and NF90 interact with hepatitis C virus 5’-terminal untranslated RNA and promote efficient replication. Journal of virology. 2014;88:7199–7209. doi: 10.1128/JVI.00225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindberg A, Kreivi JP. Splicing inhibition at the level of spliceosome assembly in the presence of herpes simplex virus protein ICP27. Virology. 2002;294:189–198. doi: 10.1006/viro.2001.1301. [DOI] [PubMed] [Google Scholar]
- 62.Lischka P, Rosorius O, Trommer E, Stamminger T. A novel transferable nuclear export signal mediates CRM1-independent nucleocytoplasmic shuttling of the human cytomegalovirus transactivator protein pUL69. The EMBO journal. 2001;20:7271–7283. doi: 10.1093/emboj/20.24.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lischka P, Toth Z, Thomas M, Mueller R, Stamminger T. The UL69 transactivator protein of human cytomegalovirus interacts with DEXD/H-Box RNA helicase UAP56 to promote cytoplasmic accumulation of unspliced RNA. Molecular and cellular biology. 2006;26:1631–1643. doi: 10.1128/MCB.26.5.1631-1643.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Mertz JE. HnRNP L binds a cis-acting RNA sequence element that enables intron-dependent gene expression. Genes & development. 1995;9:1766–1780. doi: 10.1101/gad.9.14.1766. [DOI] [PubMed] [Google Scholar]
- 65.Lleres D, Denegri M, Biggiogera M, Ajuh P, Lamond AI. Direct interaction between hnRNP-M and CDC5L/PLRG1 proteins affects alternative splice site choice. EMBO reports. 2010;11:445–451. doi: 10.1038/embor.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyabin DN, Eliseeva IA, Ovchinnikov LP. YB-1 protein: functions and regulation. Wiley interdisciplinary reviews. RNA. 2014;5:95–110. doi: 10.1002/wrna.1200. [DOI] [PubMed] [Google Scholar]
- 67.Ma AS, Moran-Jones K, Shan J, Munro TP, Snee MJ, Hoek KS, Smith R. Heterogeneous nuclear ribonucleoprotein A3, a novel RNA trafficking response element-binding protein. The Journal of biological chemistry. 2002;277:18010–18020. doi: 10.1074/jbc.M200050200. [DOI] [PubMed] [Google Scholar]
- 68.Masek T, Valasek L, Pospisek M. Polysome analysis and RNA purification from sucrose gradients. Methods in molecular biology. 2011;703:293–309. doi: 10.1007/978-1-59745-248-9_20. [DOI] [PubMed] [Google Scholar]
- 69.Matsuki H, Takahashi M, Higuchi M, Makokha GN, Oie M, Fujii M. Both G3BP1 and G3BP2 contribute to stress granule formation. Genes to cells : devoted to molecular & cellular mechanisms. 2013;18:135–146. doi: 10.1111/gtc.12023. [DOI] [PubMed] [Google Scholar]
- 70.Merz C, Urlaub H, Will CL, Luhrmann R. Protein composition of human mRNPs spliced in vitro and differential requirements for mRNP protein recruitment. Rna. 2007;13:116–128. doi: 10.1261/rna.336807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mocarski ES, Pereira L, McCormick AL. Human cytomegalovirus ICP22, the product of the HWLF1 reading frame, is an early nuclear protein that is released from cells. The Journal of general virology. 1988;69(Pt 10):2613–2621. doi: 10.1099/0022-1317-69-10-2613. [DOI] [PubMed] [Google Scholar]
- 72.Moorman NJ, Shenk T. Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. Journal of virology. 2010;84:5260–5269. doi: 10.1128/JVI.02733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munday DC, Surtees R, Emmott E, Dove BK, Digard P, Barr JN, Whitehouse A, Matthews D, Hiscox JA. Using SILAC and quantitative proteomics to investigate the interactions between viral and host proteomes. Proteomics. 2012;12:666–672. doi: 10.1002/pmic.201100488. [DOI] [PubMed] [Google Scholar]
- 74.Nogalski MT, Collins-McMillen D, Yurochko AD. Overview of human cytomegalovirus pathogenesis. Methods in molecular biology. 2014;1119:15–28. doi: 10.1007/978-1-62703-788-4_2. [DOI] [PubMed] [Google Scholar]
- 75.Omnus DJ, Mehrtens S, Ritter B, Resch K, Yamada M, Frank R, Nourbakhsh M, Reboll MR. JKTBP1 is involved in stabilization and IRES-dependent translation of NRF mRNAs by binding to 5’ and 3’ untranslated regions. Journal of molecular biology. 2011;407:492–504. doi: 10.1016/j.jmb.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 76.Pacheco A, Lopez de Quinto S, Ramajo J, Fernandez N, Martinez-Salas E. A novel role for Gemin5 in mRNA translation. Nucleic acids research. 2009;37:582–590. doi: 10.1093/nar/gkn979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patton JG, Porro EB, Galceran J, Tempst P, Nadal-Ginard B. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes & development. 1993;7:393–406. doi: 10.1101/gad.7.3.393. [DOI] [PubMed] [Google Scholar]
- 78.Pellizzoni L, Charroux B, Rappsilber J, Mann M, Dreyfuss G. A functional interaction between the survival motor neuron complex and RNA polymerase II. The Journal of cell biology. 2001;152:75–85. doi: 10.1083/jcb.152.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pinol-Roma S, Choi YD, Matunis MJ, Dreyfuss G. Immunopurification of heterogeneous nuclear ribonucleoprotein particles reveals an assortment of RNA-binding proteins. Genes & development. 1988;2:215–227. doi: 10.1101/gad.2.2.215. [DOI] [PubMed] [Google Scholar]
- 80.Pinol-Roma S, Swanson MS, Gall JG, Dreyfuss G. A novel heterogeneous nuclear RNP protein with a unique distribution on nascent transcripts. The Journal of cell biology. 1989;109:2575–2587. doi: 10.1083/jcb.109.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romanowski MJ, Shenk T. Characterization of the human cytomegalovirus irs1 and trs1 genes: a second immediate-early transcription unit within irs1 whose product antagonizes transcriptional activation. Journal of virology. 1997;71:1485–1496. doi: 10.1128/jvi.71.2.1485-1496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rowles DL, Terhune SS, Cristea IM. Discovery of host-viral protein complexes during infection. Methods in molecular biology. 2013;1064:43–70. doi: 10.1007/978-1-62703-601-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salsman J, Jagannathan M, Paladino P, Chan PK, Dellaire G, Raught B, Frappier L. Proteomic profiling of the human cytomegalovirus UL35 gene products reveals a role for UL35 in the DNA repair response. Journal of virology. 2012;86:806–820. doi: 10.1128/JVI.05442-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salsman J, Wang X, Frappier L. Nuclear body formation and PML body remodeling by the human cytomegalovirus protein UL35. Virology. 2011;414:119–129. doi: 10.1016/j.virol.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 85.Sandri-Goldin RM. Viral regulation of mRNA export. Journal of virology. 2004;78:4389–4396. doi: 10.1128/JVI.78.9.4389-4396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sciabica KS, Dai QJ, Sandri-Goldin RM. ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. The EMBO journal. 2003;22:1608–1619. doi: 10.1093/emboj/cdg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharan SK, Thomason LC, Kuznetsov SG, Court DL. Recombineering: a homologous recombination-based method of genetic engineering. Nature protocols. 2009;4:206–223. doi: 10.1038/nprot.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shav-Tal Y, Zipori D. PSF and p54(nrb)/NonO--multi-functional nuclear proteins. FEBS letters. 2002;531:109–114. doi: 10.1016/s0014-5793(02)03447-6. [DOI] [PubMed] [Google Scholar]
- 89.Shih JW, Wang WT, Tsai TY, Kuo CY, Li HK, Wu Lee YH. Critical roles of RNA helicase DDX3 and its interactions with eIF4E/PABP1 in stress granule assembly and stress response. The Biochemical journal. 2012;441:119–129. doi: 10.1042/BJ20110739. [DOI] [PubMed] [Google Scholar]
- 90.Shih SC, Claffey KP. Regulation of human vascular endothelial growth factor mRNA stability in hypoxia by heterogeneous nuclear ribonucleoprotein L. The Journal of biological chemistry. 1999;274:1359–1365. doi: 10.1074/jbc.274.3.1359. [DOI] [PubMed] [Google Scholar]
- 91.Skabkin MA, Kiselyova OI, Chernov KG, Sorokin AV, Dubrovin EV, Yaminsky IV, Vasiliev VD, Ovchinnikov LP. Structural organization of mRNA complexes with major core mRNP protein YB-1. Nucleic acids research. 2004;32:5621–5635. doi: 10.1093/nar/gkh889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith IL, Hardwicke MA, Sandri-Goldin RM. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- 93.Soto-Rifo R, Rubilar PS, Limousin T, de Breyne S, Decimo D, Ohlmann T. DEAD-box protein DDX3 associates with eIF4F to promote translation of selected mRNAs. The EMBO journal. 2012;31:3745–3756. doi: 10.1038/emboj.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stickeler E, Fraser SD, Honig A, Chen AL, Berget SM, Cooper TA. The RNA binding protein YB-1 binds A/C-rich exon enhancers and stimulates splicing of the CD44 alternative exon v4. The EMBO journal. 2001;20:3821–3830. doi: 10.1093/emboj/20.14.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tan SC, Yiap BC. DNA, RNA, and protein extraction: the past and the present. Journal of biomedicine & biotechnology. 2009;2009:574398. doi: 10.1155/2009/574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. The Journal of biological chemistry. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tourriere H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. The Journal of cell biology. 2003;160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Trgovcich J, Cebulla C, Zimmerman P, Sedmak DD. Human cytomegalovirus protein pp71 disrupts major histocompatibility complex class I cell surface expression. Journal of virology. 2006;80:951–963. doi: 10.1128/JVI.80.2.951-963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Varia S, Potabathula D, Deng Z, Bubulya A, Bubulya PA. Btf and TRAP150 have distinct roles in regulating subcellular mRNA distribution. Nucleus. 2013;4:229–240. doi: 10.4161/nucl.25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vukmirovic M, Manojlovic Z, Stefanovic B. Serine-threonine kinase receptor-associated protein (STRAP) regulates translation of type I collagen mRNAs. Molecular and cellular biology. 2013;33:3893–3906. doi: 10.1128/MCB.00195-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watermann DO, Tang Y, Zur Hausen A, Jager M, Stamm S, Stickeler E. Splicing factor Tra2-beta1 is specifically induced in breast cancer and regulates alternative splicing of the CD44 gene. Cancer research. 2006;66:4774–4780. doi: 10.1158/0008-5472.CAN-04-3294. [DOI] [PubMed] [Google Scholar]
- 102.Weidensdorfer D, Stohr N, Baude A, Lederer M, Kohn M, Schierhorn A, Buchmeier S, Wahle E, Huttelmaier S. Control of c-myc mRNA stability by IGF2BP1-associated cytoplasmic RNPs. Rna. 2009;15:104–115. doi: 10.1261/rna.1175909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wells SE, Hillner PE, Vale RD, Sachs AB. Circularization of mRNA by eukaryotic translation initiation factors. Molecular cell. 1998;2:135–140. doi: 10.1016/s1097-2765(00)80122-7. [DOI] [PubMed] [Google Scholar]
- 104.Wilk HE, Werr H, Friedrich D, Kiltz HH, Schafer KP. The core proteins of 35S hnRNP complexes. Characterization of nine different species. European journal of biochemistry / FEBS. 1985;146:71–81. doi: 10.1111/j.1432-1033.1985.tb08621.x. [DOI] [PubMed] [Google Scholar]
- 105.Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell. 2004;119:381–392. doi: 10.1016/j.cell.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 106.Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. Journal of molecular biology. 2013;425:3723–3730. doi: 10.1016/j.jmb.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu D, Sawitzke JA, Ellis H, Court DL. Recombineering with overlapping single-stranded DNA oligonucleotides: testing a recombination intermediate. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7207–7212. doi: 10.1073/pnas.1232375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yugami M, Kabe Y, Yamaguchi Y, Wada T, Handa H. hnRNP-U enhances the expression of specific genes by stabilizing mRNA. FEBS letters. 2007;581:1–7. doi: 10.1016/S0014-5793(07)01283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Z, Carmichael GG. The fate of dsRNA in the nucleus: a p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell. 2001;106:465–475. doi: 10.1016/s0092-8674(01)00466-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.