Abstract
The role of vascular endothelial growth factor - A (VEGFA) in tumor angiogenesis is well established and accordingly, molecules targeting VEGFA or its receptors are being presently used in the clinics for treatment of several types of cancer. However, these anti-angiogenic agents are expensive and have serious side effects. Thus identification of newer drugs with manageable systemic side effects or toxicities is of immense clinical importance. Since we have reported earlier that dopamine (DA) inhibits VEGFA induced angiogenesis in experimental tumor models, we therefore sought to investigate whether DA treatment results in similar toxicities like other anti-angiogenic agents. Our results indicated that unlike sunitinib, another commonly used anti-angiogenic agent in the clinics which targets VEGF receptors, DA (50mg/kg/d × 7days i.p.) not only could inhibit tumor angiogenesis and growth of HT29 human colon cancer and LLC (Lewis lung carcinoma) in mice, it also did not cause hypertension, hematological, renal and hepatic toxicities in normal, HT29 and LLC tumor bearing animals. Furthermore and interestingly, in contrast to the currently used anti-angiogenic agents, DA also prevented 5-fluorouracil (5FU) induced neutropenia in HT29 colon cancer bearing mice. This action of DA was through inhibition of 5FU mediated suppression of CFU-GM colony forming units in the bone marrow. Thus our results indicate that DA may be safely used as an anti-angiogenic drug for the treatment of malignant tumors.
Keywords: Dopamine, anti-angiogenic drugs, 5-FU, toxicity, Lung Cancer, Colon Cancer
Introduction
Neovascularization or formation of new blood vessels from adult endothelial cells (angiogenesis) and bone marrow (BM) -derived endothelial progenitor cells (vasculogenesis) is essential for the growth, progression and metastasis of malignant tumors (1–3). This process is tightly regulated by both pro-and anti-angiogenic factors, and a shift of the balance towards the pro-angiogenic factors results in the generation of neovessels in tumor microenvironment (2). Among the pro-angiogenic factors, the role of VEGFA in mediating tumor angiogenesis is well established (2). VEGFA mainly binds to VEGF receptor-2 (VEGFR-2) to induce neovascularization in tumors (2). Accordingly, VEGFA-and VEGF receptor targeting monoclonal antibodies and tyrosine kinase inhibitors have been developed to treat several types of solid tumors (4, 5). Since these agents unlike conventional cytotoxic drugs usually target the functions of this growth factor in proliferating endothelial cells and because endothelial cells are normally quiescent in absence of stimuli, it is thus expected that these drugs will be generally well tolerated mainly due to their specific cellular effects (6, 7). However, these anti-VEGF agents are not only expensive, but they also have serious toxicities, which precludes their uses in many patients (8, 9). We and others have reported that the neurotransmitter DA can inhibit VEGFA induced angiogenesis and vasculogenesis in experimental tumors (10–14). DA acting through its D2 receptors present in the adult endothelial and BM-derived endothelial precursor cells suppresses pro-angiogenic signaling pathways of VEGFA (10, 13). Because DA inhibits the actions of VEGFA, it will therefore be important to investigate if the anti-angiogenic dose of DA has similar side-effects that are observed with the currently used anti-VEGF drugs (8, 9). The results of this study will further help to translate the anti-angiogenic action of DA for the treatment of cancer.
Materials and Methods
Cells and Reagents
HT29 cells (ATCC) were cultured in McCoy’s 5A (ATCC) medium, supplemented with 10% FBS (12). Highly angiogenic and VEGFA producing type LLC cells (clone D122-96) was provided by Dr. L. Eisenbach (Weizman Institute of Science, Rehovot, Israel) and were cultured in DMEM (ATCC) containing 10% FBS (15).
Mice, Tumors
Animal experiments were undertaken after approval by the Ohio State University Animal Care and Use Committee. Male athymic nude (Charles River Laboratory) and C57BL/6 (Jackson Laboratory) (6–8 wk old) mice were used. The HT29 orthotopic colon cancer model was developed as before (12). Briefly, 1 ×106 HT29 cells in 50 μL medium were injected into the cecal wall from the serosal side with a 30-gauge needle. After tumor cell transplantation, the cecum was returned to the abdominal cavity, and the wound was closed. The abdomen of the animals were surgically reopened approximately 60 days after tumor implantation to detect tumors in the cecum and then closed. (12). Tumor bearing animals were divided randomly into vehicle and DA treatment groups. DA (American Reagent Laboratories) was administered once daily intraperitoneally (i.p.) at a dose of 50 mg/kg for 7 consecutive days (12). This dose of DA corresponds to 5% of the LD50 in mice (11, 12) and raises the plasma DA level in mice to 1.2 ± 0.01 nmol/mL in 1 min from 1.5 ± 0.01 pmol/mL (normal plasma level in mice) (11). Furthermore, this raised level of DA is comparable to the plasma level of DA reached in human subjects treated with an intravenous infusion of the dopaminergic dose of DA (16). Sunitinib malate (Selleckchem) was administered at a dose 40mg/kg once daily by oral gavage for 7 consecutive days as this dose (40mg/kg orally once daily) was reported to inhibit angiogenesis in mice bearing HT29 and LLC tumors (17). 5-FU (APP Pharmaceuticals) was administered at a dose of 20 mg/kg once daily for 5 consecutive days (18) either alone or after DA treatment (50mg/kg) of HT 29 tumor bearing mice for 7 days.
For LLC tumors, the dorsal skin of the C57/BL6 mice was shaved and 200 μL of cells at a concentration of 1X 107 cells /ml, were injected subcutaneously into the right flank of mice. When tumors reached a size of 100mm3 (~ 10 days) mice were randomly divided into vehicle, DA and sunitinib treatment groups (17, 18). For CFU-GM assay, DA was administered for 7 days (50mg/kg), 5-FU was administered at a dose of 20 mg/kg once daily for 5 consecutive days (18) either alone or after DA treatment of normal mice for 7 days and then BM was collected.
Tumor growth and angiogenesis
For orthotopic HT29, tumors were collected and growth was determined by weighing the tumor mass (18). For LLC tumors, growth was determined by caliper measurement of the largest diameter and its perpendicular and tumor volume was calculated (12). Tumor angiogenesis was determined by immunohistochemical staining of CD31 positive vessels (11, 12)
Hematology and Biochemical Assays
Blood was collected by cardiac puncture after anesthetizing the mice. Complete blood counts with 6-part white blood cell differential were performed on a portion of EDTA anti-coagulated whole blood (FORCYTE Autosampler10, Oxford Science, Inc., Oxford, CT). Following coagulation of the remaining whole blood at room temperature for 30 min., the clotted blood was centrifuged at 3000 rpm for 5-10 min. at 4C. Biochemical profiles were performed on serum samples (VetACE, Alfa Wasserman, West Caldwell, NJ)
Measurement of Blood Pressure
Systolic, Diastolic and Mean blood pressures (BP) were measured in untreated, DA and sunitinib treated mice using a computerized tail-cuff system (CODA System, Kent Scientific). Prior to treatment mice were trained daily and the BP were recorded at the end of treatment. This method of BP measurement corresponds with direct measurements of intra-arterial pressure (19).
Colony Forming Assay
Mouse BM colony forming assay was performed using Methocult products for CFU assays (StemCell Technologies). BM was collected from femurs and tibia in Iscove’s MDM from normal, 5 FU, DA and DA+5FU treated normal mice. Cells were seeded in culture dishes with 1×105 cells/ml MethoCult M3434 medium (StemCell Technologies, Inc.) (total volume, 1.1 ml) and incubated for 12 days at 37° C with 5 % CO2 in air and 95 % humidity. CFU-GM colonies were identified and counted using an inverted microscope (20).
Statistics
For statistical analysis the differences among the groups were evaluated by ANOVA and unpaired student’s test or Dunn’s multiple comparison tests. Mean and SE were calculated and P<0.05 was considered significant (12)
Results and Discussion
Effect on tumor growth and angiogenesis
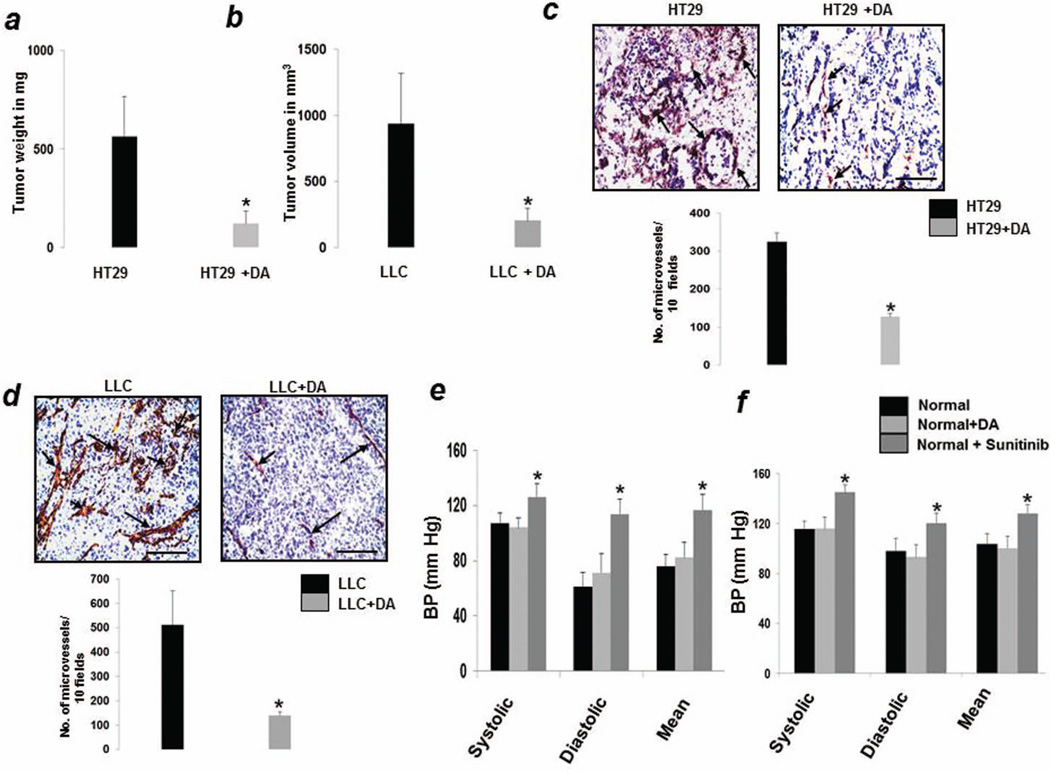
In HT29 colon (Fig1a) and LLC tumors (Fig1b), DA treatment showed significant reduction in tumor growth when compared with vehicle-treated controls. Angiogenic response as assessed by counting the numbers of CD31 positive vessels were also significantly less in DA treated tumors (Fig 1c and d).
Figure 1.
Dopamine (DA) treatment significantly reduced the growth of (a) orthotopic HT29 colon cancer and (b) subcutaneous lewis lung carcinoma (LLC) tumors as compared to untreated tumors. Tumor angiogenesis was also significantly reduced in dopamine treated (c) HT29 and (d) LLC tumors compared to respective untreated controls. In contrast to sunitinib, dopamine treatment had no significant effect on the diastolic, systolic and mean blood pressures of normal (e) athymic nude and (f) C57/BL6 mice. Results are mean ± SE of three separate experiments; n=13 per group;*P < 0.05 when compared to respective controls.
Effect on Blood Pressure
Among the several toxicities that have been reported following the use of anti-VEGF agents, hypertension is most commonly observed with recent reports indicating that the incidence of hypertension following use of these agents varies between 20%–87% (8, 21). Therefore, detection and management of anti-VEGF therapy induced hypertension has become a major challenge. Hence while testing the possible side effects which the anti-angiogenic dose of DA might have, we first studied the effect of DA on the BPs of animals. After establishing baseline BPs, normal nude (Fig1e) and C57/BL6 (Fig1f) were injected with either DA (50mg/kg i.p.) or sunitinib (40mg/kg orally) or vehicle (PBS) for 7days. Following completion of treatment, no change in BP (systolic, diastolic and mean) was noted between DA treated and vehicle treated groups (Fig 1e and f). However, sunitinib caused significant increase in the BPs of the animals (Fig 1e and f). Similar effects were also observed in HT29 and LLC bearing animals (data not shown). These results indicated that the anti-angiogenic dose of DA does not cause hypertension.
Liver function tests
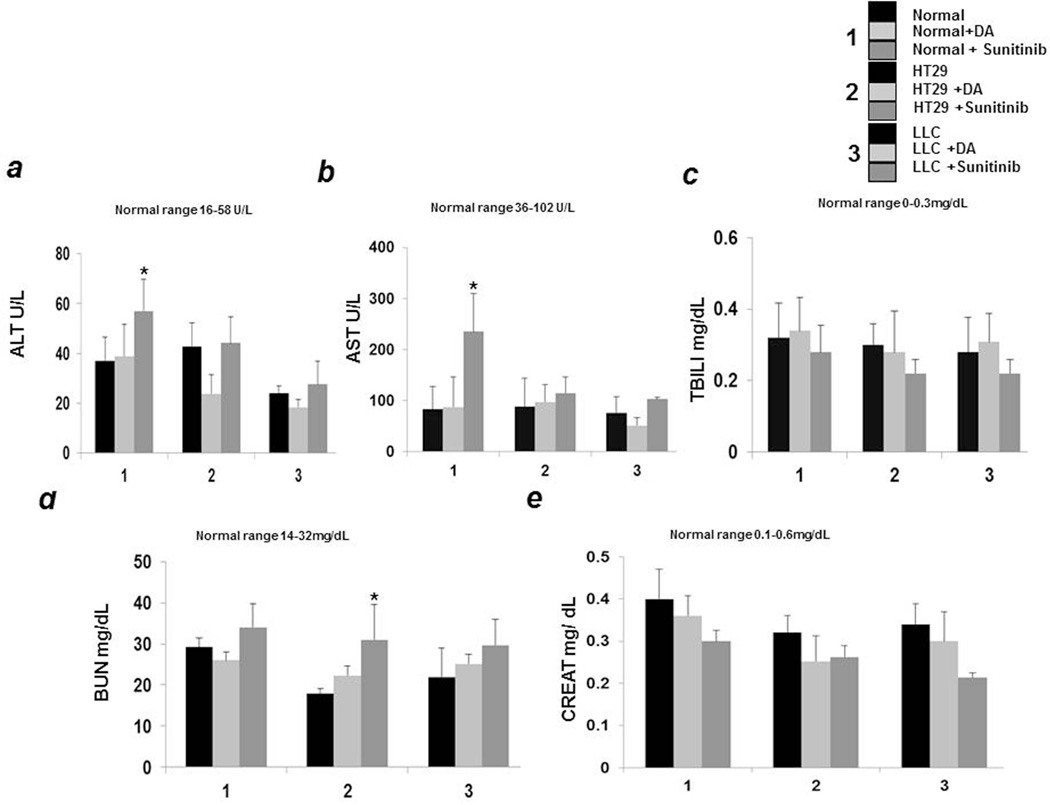
Severe hepatic toxicity has been reported in patients receiving currently available anti-VEGF drugs (22). Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) elevations were reported in cancer patients treated with these angiogenic inhibitors. Transaminase elevations usually occur early during the treatment and they are reversible with patients generally being asymptomatic. However, such elevations may also lead to severe liver damage, which in turn may lead to interruptions in treatment and significant morbidity (22). We therefore tested whether DA can cause hepatic toxicity in normal, HT29 and LLC tumor bearing mice. Our results demonstrate that there were no abnormal changes in the serum ALT (Fig2a) and AST levels (Fig2b) in DA treated normal and tumor bearing mice. In addition, there was also no elevation of serum bilirubin level (Fig2c) after DA treatment, thereby indicating that unlike other anti-angiogenic drugs, DA administration does not adversely affect liver functions. However, in contrast to DA, significant increase in these two liver enzymes was noted in normal mice treated with sunitinib. ALT and AST levels were also increased in sunitinib treated tumor bearing animals in comparison to untreated controls (Fig 2a and b). We did not detect any significant changes in the serum bilirubin levels among the different groups (Fig 2c)
Figure 2.
In contrast to sunitinib, dopamine (DA) treatment had no significant toxic effect on liver and renal functions compared to untreated controls as was determined by (a) alanine aminotransferase (ALT) (b) aspartate aminotransferase (AST) (c) total bilirubin (TBILI) (d) blood urea nitrogen (BUN) and (e) Creatinine (Creat) levels in serum of untreated and treated (DA and sunitinib) normal, HT29 and LLC tumor bearing mice. Results are mean ± SE of three separate experiments; n=13 per group;*P < 0.05 when compared to respective controls.
Renal effects
The association between VEGF inhibitors and renal damage has been commonly reported (21). We therefore tested whether DA treatment has any impact on renal functions of animals. Our results demonstrate that serum blood urea nitrogen (BUN) (Fig 2d) and creatinine (Fig2e) levels were normal after DA treatment in normal, HT29 and LLC tumor bearing mice. On the contrary, serum BUN was increased in sunitinib treated animals (Fig 2d). No significant differences were noted in the serum creatinine levels between the groups (Fig 2e).
Hematological effects
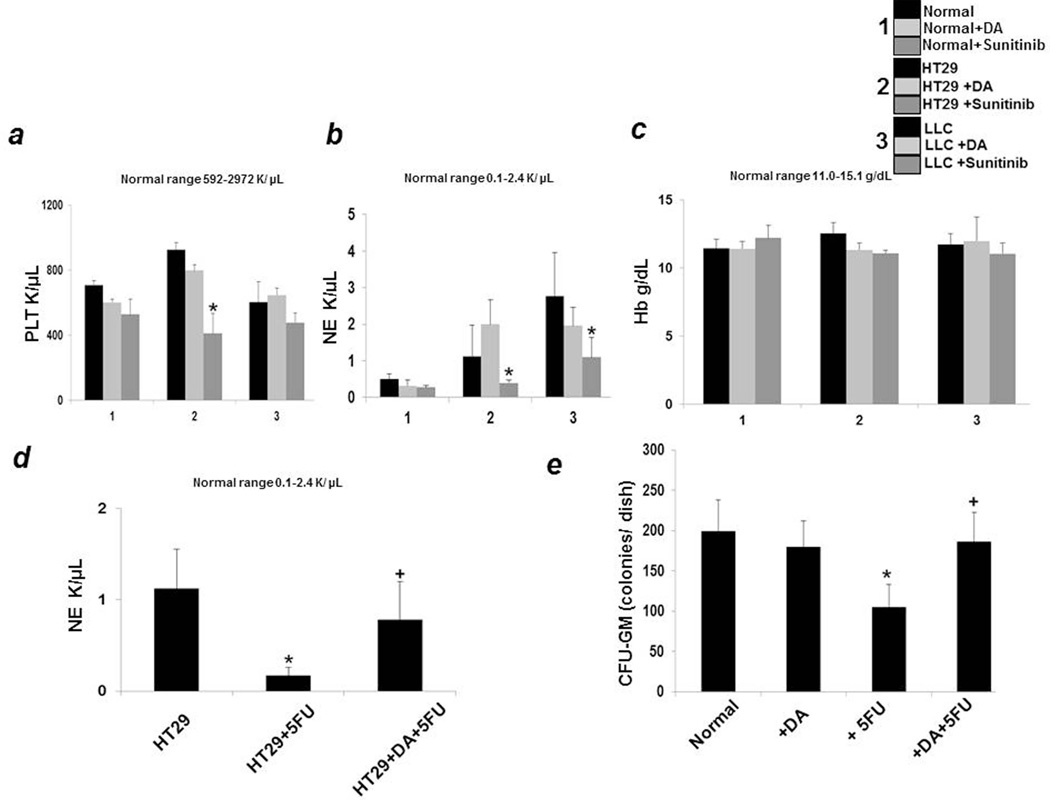
Myelosuppression and thrombocytopenia are common problems in cancer patients receiving anti-angiogenic drugs (23). Drugs that inhibit the actions of VEGFA can cause myelosuppression and thrombocytopenia as VEGFRs expressed in hematopoietic cells play a role in myelopoiesis and thrombopoiesis (23). However, the degree of suppression varies between drugs, with some causing both thrombocytopenia and neutropenia (23). We therefore determined the platelet (Fig 3a) and neutrophil (NE) (Fig3b) counts in normal and tumor bearing mice treated with DA. Administration of DA did not cause thrombocytopenia in normal, HT29 human colon cancer and LLC bearing mice (Fig 3a). In addition, DA administration also did not induce neutropenia in these animals (Fig3b). It is also important to note here that there were also no significant changes in the hemoglobin levels of the animals treated with DA (Fig3c). Sunitinib in contrast caused decrease in both platelet and neutrophil counts (Fig 3a and b). We did not find any significant differences in hemoglobin (Hb) levels between the different groups (Fig 3c).
Figure 3.
In comparison to sunitinib, dopamine (DA) treatment showed no hematological toxicity as determined by measuring the (a) platelet (PLT) (b) neutrophil (NE) and (c) hemoglobin (Hb) levels in blood of DA and sunitinib treated normal, HT29 and LLC tumor bearing mice and comparing to untreated controls. (d).Combination treatment of DA and 5FU of HT29 tumor bearing mice prevented 5 FU induced significant reduction of NE counts (*P < 0.05 when compared to control; + P < 0.05 when compared to 5FU treated group). (e) The number of CFU-GM colonies in culture significantly increased when bone marrow cells were collected from mice receiving combination treatment of DA and 5FU compared to 5 FU alone. *P < 0.05 when compared to normal; + P < 0.05 when compared to 5FU treated group. Results are mean ± SE of three separate experiments; n=13 per group.
Furthermore, we had previously reported that combination treatment of DA with 5FU can significantly inhibit colon cancer growth in mice (12). Because one of the serious side effects of 5FU alone and combining anti-VEGF drugs with 5FU is low NE count in colon cancer patients (24), we therefore examined whether administration of DA together with 5FU has any effect on NE counts in colon cancer bearing mice. Mice receiving only 5 FU injections had significantly lowered NE counts (Fig3d). However, when these tumor bearing animals were treated with DA followed with 5 FU, DA was able to prevent this suppressive action of 5 FU (Fig3d).
Effect on CFU-GM colony formation by dopamine
Thereafter to elucidate the mechanism by which DA prevented 5-FU induced myelosuppression, we determined the number of colony forming unit-granulocyte macrophage (CFU-GM) colonies in normal, DA, 5 FU and DA+5FU treated normal mice because there are several reports which indicate that 5-FU administration suppresses the CFU-GM colony forming cells in the BM (25). Our results indicated that treatment with 5-FU resulted in significantly lower CFU-GM colonies in comparison to normal (Fig3e). On the contrary, the number of CFU-GM colonies formed on treatment with DA and then with 5FU was significantly more than that formed by 5FU alone; thereby indicating that DA could inhibit 5FU mediated suppression of CFU-GM colony forming units in the BM (Fig3e).
In summary, there are now several reports which indicate that anti-VEGF agents can cause hypertension, abnormal AST, ALT and BUN levels suggesting hepatic and renal damage (8, 9). In addition, these drugs can also cause neutropenia and thrombocytopenia (8, 9). On the contrary, our results demonstrate that the anti-angiogenic dose of DA is devoid of serious toxicities usually observed with the use of anti-VEGF agents in the clinics such as sunitinib (23).
Importantly, our experiments also indicate that DA can ameliorate neutropenia induced by commonly used anti-cancer drugs, such as 5FU. This information together with the safety profile of DA known due to its use in the treatment of other disorders demonstrate that this small molecule can not only be used as an effective anti-angiogenic drug, it may also be utilized to prevent bone-marrow suppression observed with 5FU when used in combination with anti-VEGF agents for the treatment of colon cancer in the clinics. Finally, the cost of treatment with DA is significantly less and this in turn is also very important in perspectives of health economics for proper utilization of the funds allocated for the treatment of cancer.
What’s new?
This report for the first time indicates that the anti-angiogenic dose of DA does not cause any untoward side effects commonly observed with the presently used anti-VEGF agents. In addition, DA can also prevent 5FU induced neutropenia in tumor bearing animals. Therefore this inexpensive drug which is being used in the clinics for many years for the treatment of cardiovascular and renal disorders may also be safely administered as an anti-angiogenic agent for the treatment of malignant tumors.
Acknowledgements
Grant Sponsor: NIH/NCI, R01 CA124763 to S.B. and CSIR 21(0895)/12EMR-II to PSD. We also thank the Comparative Pathology and Mouse Phenotyping Shared Resource of the Ohio State University for hematological and clinical chemistry tests.
References
- 1.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak HF. Angiogenesis: update 2005. J Thromb Haemos. 2005;3:1835–1842. doi: 10.1111/j.1538-7836.2005.01361.x. [DOI] [PubMed] [Google Scholar]
- 3.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 4.Sloan B, Scheinfeld NS. Pazopanib, a VEGF receptor tyrosine kinase inhibitor for cancer therapy. Curr Opin Investig Drugs. 2008;9:1324–1335. [PubMed] [Google Scholar]
- 5.Sullivan LA, Brekken RA. The VEGF family in cancer and antibody-based strategies for their inhibition. MAbs. 2010;2:165–175. doi: 10.4161/mabs.2.2.11360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 7.Davis DW, McConkey DJ, Zhang W, Herbst RS. Antiangiogenic tumor therapy. Biotechniques. 2003;34:1048–1050. doi: 10.2144/03345dd01. [DOI] [PubMed] [Google Scholar]
- 8.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- 9.Gressett SM1, Shah SR. Intricacies of bevacizumab-induced toxicities and their management. Ann Pharmacother. 2009;43:490–501. doi: 10.1345/aph.1L426. [DOI] [PubMed] [Google Scholar]
- 10.Basu S, Nagy JA, Pal S, Vasile E, Eckelhoefer IA, Bliss VS, Manseau EJ, Dasgupta PS, Dvorak HF, Mukhopadhyay D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med. 2001;7:569–574. doi: 10.1038/87895. [DOI] [PubMed] [Google Scholar]
- 11.Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–4356. doi: 10.1158/1078-0432.CCR-04-0059. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. 2008;14:2502–2510. doi: 10.1158/1078-0432.CCR-07-1778. [DOI] [PubMed] [Google Scholar]
- 13.Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Invest. 2008;118:1380–1389. doi: 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Smith M, Lu C, Shahzad MM, Pena GN, Allen JK, Stone RL, Mangala LS, Han HD, Kim HS, Farley D, Berestein GL, Cole SW, Lutgendorf SK, Sood AK. Dopamine blocks stress-mediated ovarian carcinoma growth. Clin Cancer Res. 2011;17:3649–3659. doi: 10.1158/1078-0432.CCR-10-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 16.MacGregor DA, Smith TE, Prielipp RC, Butterworth JF, James RL, Scuderi PE. Pharmacokinetics of dopamine in healthy male subjects. Anesthesiology. 2000;92:338–346. doi: 10.1097/00000542-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Sounni NE, Cimino J, Blacher S, Primac I, Truong A, Mazzucchelli G, Paye A, Calligaris D, Debois D, De Tullio P, Mari B, De Pauw E, Noel A. Blocking lipid synthesis overcomes tumor regrowth and metastasis after antiangiogenic therapy withdrawal. Cell Metab. 2014;20:280–294. doi: 10.1016/j.cmet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Chakroborty D, Sarkar C, Yu H, Wang J, Liu Z, Dasgupta PS, Basu S. Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc Natl Acad Sci U S A. 2011;108:20730–20735. doi: 10.1073/pnas.1108696108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009;54:652–658. doi: 10.1161/HYPERTENSIONAHA.109.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezeh PC, Lauer FT, MacKenzie D, McClain S, Liu KJ, Hudson LG, Gandolfi AJ, Burchiel SW. Arsenite selectively inhibits mouse bone marrow lymphoid progenitor cell development in vivo and in vitro and suppresses humoral immunity in vivo. PLoS One. 2014;9:e93920. doi: 10.1371/journal.pone.0093920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayman SR, Leung N, Grande JP, Garovic VD. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep. 2012;14:285–294. doi: 10.1007/s11912-012-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard SA, Krajewski KM, Thornton E, Jagannathan JP, O'Regan K, Cleary J, Ramaiya N. Decade of molecular targeted therapy: abdominal manifestations of drug toxicities--what radiologists should know. AJR Am J Roentgenol. 2012;199:58–64. doi: 10.2214/AJR.11.7432. [DOI] [PubMed] [Google Scholar]
- 23.Grenon NN. Managing toxicities associated with antiangiogenic biologic agents in combination with chemotherapy for metastatic colorectal cancer. Clin J Oncol Nurs. 2013;17:425–433. doi: 10.1188/13.CJON.425-433. [DOI] [PubMed] [Google Scholar]
- 24.Gianola FJ, Sugarbaker PH, Barofsky I, White DE, Meyers CE. Toxicity studies of adjuvant intravenous versus intraperitoneal 5-FU in patients with advanced primary colon or rectal cancer. Am J Clin Oncol. 1986;9:403–410. doi: 10.1097/00000421-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Djazayeri K, Szilvássy Z, Peitl B, Németh J, Nagy L, Kiss A, Szabó B, Benko I. Accelerated recovery of 5-fluorouracil-damaged bone marrow after rosiglitazone treatment. Eur J Pharmacol. 2005;522:122–129. doi: 10.1016/j.ejphar.2005.08.053. [DOI] [PubMed] [Google Scholar]