Abstract
The forkhead domain FOXP2 and FOXP1 transcription factors are implicated in several cognitive disorders with language deficits, notably autism, and thus play a central role in learned vocal motor behavior in humans. Although a similar role for FoxP2 and FoxP1 is proposed for other vertebrate species, including songbirds, the neurodevelopmental expression of these genes are unknown in a species with lifelong vocal learning abilities. Like humans, budgerigars (Melopsittacus undulatus) learn new vocalizations throughout their entire lifetime. Like songbirds, budgerigars have distinct brain nuclei for vocal learning, which include the magnocellular nucleus of the medial striatum (MMSt), a basal ganglia region that is considered developmentally and functionally analogous to Area X in songbirds. Here we used in situ hybridization and immunohistochemistry to investigate FoxP2 and FoxP1 expression in the MMSt of juvenile and adult budgerigars. We found FoxP2 mRNA and protein expression levels in the MMSt that were lower than the surrounding striatum throughout development and adulthood. In contrast, FoxP1 mRNA and protein had an elevated MMSt/striatum expression ratio as birds matured, regardless of their sex. These results show that life-long vocal plasticity in budgerigars is associated with persistent low-level FoxP2 expression in the budgerigar MMSt, and suggests the possibility that FoxP1 plays an organizational role in the neurodevelopment of vocal motor circuitry. Thus, developmental regulation of the FoxP2 and FoxP1 genes in the basal ganglia appears essential for vocal mimicry in a range of species that possess this relatively rare trait.
Keywords: basal ganglia, budgerigar, FoxP2, FoxP1, gene expression, vocal learning
INTRODUCTION
Increasing evidence suggests that the underlying genetic mechanisms for vocal learning are shared between such divergent taxa as humans and several lineages of birds. The neurogenetic basis for vocal learning is not understood completely, but activity of the P2 and P1 forkhead box transcription factors, FOXP2 and FOXP1, in the basal ganglia plays a central role (Scharff and White, 2004; White et al., 2006; Bolhuis et al., 2010; White, 2010). FOXP2 activity during human embryonic brain development is necessary for the organization of cortical and basal ganglia structures involved in sensorimotor integration and fine orofacial motor control. Mutations in this gene in humans produce speech and language pathologies, and neuroanatomical abnormalities, notably in a striatal region of the basal ganglia (Vargha-Khadem et al., 1998; Lai et al., 2001; Watkins et al., 2002; Belton et al., 2003; Lai et al., 2003; MacDermot et al., 2005). Similar to FOXP2, expression of FOXP1 is linked to CNS development and organogenesis (Ferland et al., 2003; Tamura et al., 2003; Jepsen et al., 2008). Moreover, specific mutations and altered FOXP1 expression levels were found in patients with general cognitive dysfunctions, including intellectual disability and autism spectrum disorders, along with speech related impairments (Hamdan et al., 2010; Horn et al., 2010; Bacon and Rappold, 2012; Chien et al., 2013; Le Fevre et al., 2013; Tsang et al., 2013).
The avian homologs of the FoxP transcription factors appear to regulate neural development and plasticity underlying vocal learning abilities in songbirds and possibly other avian vocal learners. FoxP2 and FoxP1 show overlapping expression in the basal ganglia of both songbirds and parrots, including a striatal subregion (Area X in songbirds, magnocellular nucleus of the medial striatum or MMSt in budgerigars) that is necessary for vocal learning in both species (Haesler et al., 2004; Teramitsu et al., 2004). In zebra finches (Taeniopygia guttata), FoxP2 expression in Area X peaks late during sensory motor learning, which suggests a positive association with long-term behavioral consolidation (Haesler et al., 2004). Furthermore, during juvenile sensorimotor learning and adulthood in zebra finches, levels of FoxP2 mRNA in the striatal vocal control region decrease as birds produce a variable “practice” song that is thought to facilitate vocal motor learning (Teramitsu and White, 2006; Teramitsu et al., 2010). The extent of FoxP2 mRNA and protein downregulation in the striatal vocal control nucleus is related to the amount of singing (Teramitsu and White, 2006; Miller et al., 2008) and associated with co-regulation of thousands of genes (Hilliard et al., 2012). Knockdown of FoxP2 expression in Area X of zebra finches at the onset of sensorimotor learning and continuing into adulthood or during adulthood only resulted in poor learning (Haesler et al., 2007; Murugan et al., 2013), decreased dendritic spine density (Schulz et al., 2010), and abolished dopaminergic (D1R) modulation of vocal variability (Murugan et al., 2013). These investigations in songbirds suggest that FoxP2 regulates transcription that is associated with structural changes in the basal ganglia that generate vocal variability. FoxP1 in the adult zebra finch brain circuit for vocal control is thought to be involved in the formation of circuits for learned vocal control since its expression closely matches this circuit’s well-known sexual dimorphism (Haesler et al., 2004; Teramitsu et al., 2004).
Zebra finch males are close-ended vocal learners in which males learn to sing during an early-life critical period and then lose that ability and cannot learn new vocal patterns in adulthood (Zann, 1996). In contrast, budgerigars (Melopsittacus undulatus), are a small parrot that like humans (Ellis, 1994), are open-ended learners that are capable of using auditory feedback to learn new vocalizations throughout adult life (Brittan-Powell et al., 1997; Heaton and Brauth, 1999; Heaton et al., 1999; Hile and Striedter, 2000; Dahlin et al., 2014). Moreover, humans, songbirds, and parrots are thought to share a homologous basal ganglia substrate for vocal learning (Figure 1A,B; (Hall et al., 1999; Jarvis and Mello, 2000; Petkov and Jarvis, 2012).
Figure 1.
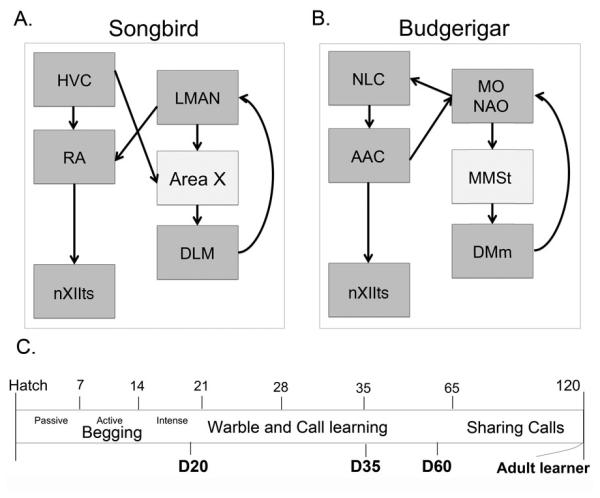
Shown here is a general schematic of interconnected vocal control nuclei in (A) the songbird brain and (B) the budgerigar brain (Nottebohm et al., 1976; Striedter, 1994). Area X and MMSt in the basal ganglia are part of a cortico-basal ganglia-thalamo-cortical loop important for learning acoustic gestures (Petkov and Jarvis, 2012). The songbird and budgerigar CNS via nXIIts projects to the syringeal muscles that produce sound. (C). Vocal development of budgerigars begins after hatch with food begging calls followed by a transitional period around D20 when socially-learned vocalizations first appear. Adult like vocalizations begin to emerge around D35, and at around D60 birds begin to join social groups and imitate conspecifics (Brittan-Powell et al., 1997; Hall et al., 1999). Vocal learning in new social groups occurs frequently in adults. The present study used birds that were isolated and recorded at the ages shown in bold, D20, D35, D60 and adults D>120. Abbreviations: Songbird: Area X and HVC are used as proper names: DLM, medial portion of the dorsolateral thalamic nucleus; LMAN, lateral magnocellular nucleus of the anterior nidopallium; RA, robust nucleus of the archipallium. Budgerigar: AAc, central nucleus of the anterior arcopallium; MO, oval nucleus of the mesopallium; MMSt, magnocellular nucleus of the medial striatum; NAO, oval nucleus of the anterior nidopallium; DMm magnocellular nucleus of the dorsomedial thalamus; nXIIts, tracheosyringeal motor nucleus, a portion of the 12th (hypoglossal) nucleus.
Behavioral phenotype differences between close-ended vocal learners and open-ended vocal learners could arise from neurogenetic differences in their basal ganglia center for vocal control. Here we examine developmental patterns of FoxP2 and FoxP1 in budgerigars to test whether the expression of these genes is developmentally regulated and whether these patterns differ from those found in the zebra finch. We used in situ hybridization and immunohistochemistry to detect FoxP2 and FoxP1 mRNA and protein expression in the MMSt of juvenile and adult budgerigars of both sexes during 4 distinct developmental periods that coincide with these distinct behavioral stages: (1) at the start of their development of “transitional” immature calls beginning ~20 days post hatch; (2) shortly after fledging ~35 days, around which time these birds produce their first adult-like contact call; (3) ~60 days when these birds typically join their first social group; (4) during adulthood, a period during which birds continually learn novel group specific calls (Figure 1C).
METHODS
Animals and acoustic recording
The budgerigars, used for this study were from our breeding colony at NMSU and maintained on a natural light dark cycle, with ad libitum access to food and water. We used a total of 45 budgerigars, 33 at three developmental timepoints, 11 each at 20, 35, and 60 days old (D). In addition, we used 12 adult male and female budgerigars that were all >D120. Developmental studies show that motor learning begins ~D20, when non-learned begging calls transition successively into adult-like contact calls for the first time; this is typically completed 2 to 3 weeks later (Brittan-Powell et al., 1997; Hall et al., 1999). The neural control centers for vocal mimicry also appear as distinct nuclei starting around D20 (Heaton and Brauth, 1999). All of the birds in each age group were used for in situ hybridization, and 5-6 birds from these age groups were used for immunohistochemistry. All budgerigars were euthanized within a two-day window of reaching the targeted developmental time periods. Of these birds, 18 juveniles were male, 14 juveniles were female, 6 adults were male and 6 adults were female, as determined by sex genotyping using PCR (Pease et al., 2012). Sex genotyping was inconclusive for 1 D60 bird. The birds were individually housed overnight in lab-constructed sound attenuation chambers, the following morning recorded for 2 hours after lights-on at 6 AM, and then immediately euthanized. These birds were acoustically recorded using microphones linked to an 8 channel mixer with digital output to a Windows 7 based PC running Sound Analysis Pro Software (Tchernichovski et al., 2000). The computer digitally captured continuous recordings of all sound events from the chambers The captured files were then visually inspected using spectrogram analysis, filtered for bird vocalization events, and quantified using Raven Pro software (Cornell Ornithology Lab, Ithaca, NY). Within the 2-hour period of observation most birds in the D35, D60 and D120 groups did not vocalize and no bird in these groups had more than 2 short vocalization events (warbles or contact calls). Some of the D20 birds did vocalize. Five of these birds produced 1381, 322, 210, 95 and 11 vocalizations each, which all contained a mix of warble and call-like elements. We noted that the call like elements resembled the “transitional” patterned food begging calls found in budgerigars at the earliest stage of sensory- motor learning.
Tissue preparation
Immediately after being acoustically recorded for at least 2 hours in the morning, the birds were weighed and then euthanized via isoflurane inhalation. The whole brain was extracted within 5 minutes and flash frozen using liquid nitrogen. The brain was then stored at −80° C and later sectioned at −20° C using a Leica CM1850 cryostat microtome (Leica Microsystems, Buffalo Grove, IL). Sections of 20 μm were then mounted onto positively charged glass microscope slides (Fisher Scientific, Waltham, MA, #12-550-20) in 7 replicate series. One series was stained with thionin to enable identification of neuroanatomical structures and to help guide localization of the protein expression patterns for FoxP1 and FoxP2 in the MMSt (magnocellular nucleus of the medial striatum) while referencing the budgerigar brain atlas (http://www.brauthlab.umd.edu/atlas.htm). Briefly, this staining procedure involved a series of 1- 2 minute slide baths in decreasing concentrations of ethanol, 1.5 min in thionin stain, and a water rinse followed by 2 min baths in increasing concentrations of ethanol. Slides were then dipped in xylenes (Sigma-Aldrich, St. Louis, MO, #534056) for 10 minutes, coverslipped with DPX Mountant (Sigma-Aldrich, St. Louis, MO, #06522) and left to dry overnight. The remaining slides were stored at −80° C until analyzed further using in situ hybridization and immunohistochemistry.
In situ hybridization and analysis
In situ hybridizations were performed using riboprobes as described previously (Teramitsu et al., 2004; Chen et al., 2013). The probes were designed to hybridize to the 3’ region of zebra finch FoxP1 and FoxP2. The FoxP2 probe corresponded to bp 1870-2127 in budgerigar FoxP2 coding sequence (GenBank# AY466101.1) and the FoxP1 probe corresponded to 1731-2035 bp in a predicted budgerigar FoxP1 coding sequence (NCBI RefSeq XM_005149417.1). The zebra finch FoxP2 3’ probe and FoxP1 3’ probe show 98.8% and 97.4% coding sequence identity to their corresponding budgerigar FoxP2 and FoxP1 3’ regions, respectively. In contrast, the FoxP2 3’ probe was only 63.6% identical to budgerigar FoxP1 sequence and the FoxP1 3’ probe was also only 63.1% identical to budgerigar FoxP2 at the coding sequence level. The pattern of expression we found in budgerigars with the FoxP1 and FoxP2 probes was consistent with those reported previously in adult parrots using full-length probes (Haesler et al., 2004). We noted that our zebra finch FoxP1 3’ probe sequence did not overlap with a different zebra finch FoxP1 3’ probe (Wada et al., 2006) that did not generate a specific hybridization signal in budgerigar brain. Further, specificity of the antisense probes was determined by the absence of a hybridization signal with the corresponding sense probes. To generate probes, the FoxP cDNA fragments were amplified by PCR from the pCR 4-TOPO vector (Invitrogen, Carlsbad, CA) using m13F and reverse primers for subsequent in vitro translation with T3 (antisense probes) or T7 (sense probes) RNA polymerase. To hybridize these probes, thaw-mounted 20 um frozen sections were air-dried at RT for 1 hour, quickly rinsed in 1X PBS, and postfixed with 4% paraformaldehyde, pH 7.4. Following acetylation and dehydration, the tissue slides were prehybridized for 1 hour in an oven at 55° C while coverslipped in solution containing 50% formamide, 1X Denhardt’s, 0.2% SDS, 10 mM EDTA (pH 8.0), 200 mM Tris (pH=7.8), 1.5 mM NaCl, 250 μg/ml tRNA, and 25 μg/ml poly A. Slides were then hybridized at 55° C overnight in a similar solution that included 10% dextran sulfate and [33P]UTP-labeled RNA probes. Equivalent 8 X 106 counts per minute of riboprobes were loaded on each slide for both FoxP1 and FoxP2. Post-hybridization slides were de-coverslipped and rinsed at 55° C for 15 min in 4X SSC, washed at RT for 2 hours in 2X SSC, treated with RNase A (Sigma) for 30 mins, washed twice in 2X SSC for 15 min each at 37° C, and finally washed for 1 hour in 0.25X SSC at 60° C before dehydration in graded ethanols, air-drying and exposure to autoradiographic film (BioMax MR film; Eastman Kodak, Rochester, NY). Slides were exposed to autoradiographic film for ~1 or 2 weeks for FoxP1 or FoxP2, respectively. Developed films were digitized at 600 dpi using a CanoScan 4400P scanner and software (Canon, Ōta, Tokyo, Japan) controlled by a PC running Windows. Film images produced by the 33P decay emissions of the probes were consistent in consecutive tissue sections and similar expression patterns were observed in multiple birds, confirming probe specificity. Adobe Photoshop (Adobe Systems, San Jose, CA) was used to measure mean pixel intensities of the areas of interest after saving the digital image in a tiff format, which allowed for 8 bits per sampled pixel or 256 different shades of gray to be analyzed. These values for 2 different sections of each brain region in each hemisphere for each animal were imported into JMP software for statistical analysis (SAS Institute Inc., Cary, NC). Mesopallial measurements included both dorsal and ventral regions. One-way ANOVAs and Tukey-Kramer HSD were used to analyze group data.
Immunohistochemistry
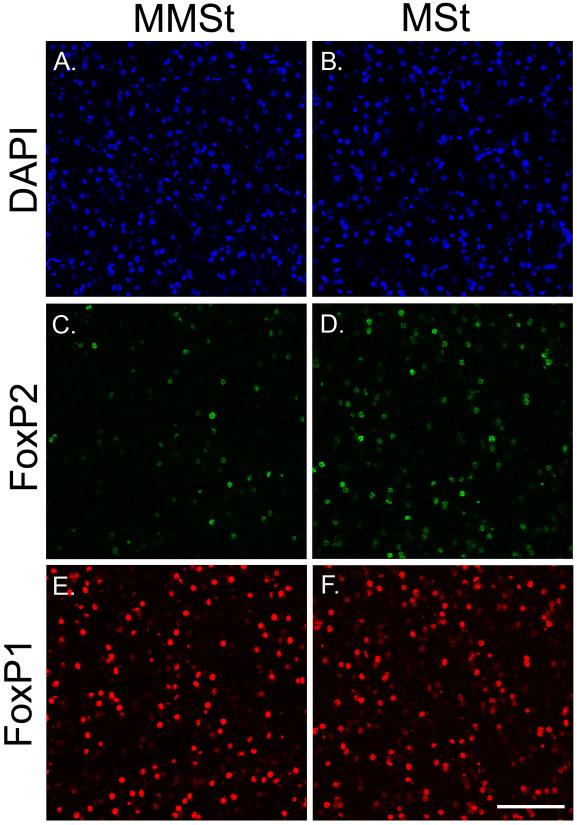
Fresh-frozen brain sections containing MMSt and adjoining striatum on microscope slides were used to measure FoxP2 and FoxP1 protein expression. Brain sections were first submerged in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, #P6148) for 5 min and rinsed with 1X phosphate buffered saline (PBS) 3 times for 5 min each. To block nonspecific binding, tissue was incubated in PBST (1X PBS with 0.3% Triton X-100) with 5% donkey serum (Jackson Immuno, West Grove, PA, #107175) for 1 hour at 4° C. Tissue slides were incubated overnight at 4° C in a PBST/1% donkey serum solution containing the polyclonal goat antibody to FoxP2 (Santa Cruz, Dallas, TX, #sc-21069) at 1:1000, and the polyclonal rabbit antibody to FoxP1 (Abcam, Cambridge, MA, #ab16645) at 1:500. Primary antibody was omitted for negative controls. Target specificity of the primary antibody for FoxP2 had been previously verified in zebra finches (Soderstrom and Luo, 2010), while the primary antibody for FoxP1 was previously verified in rats (Bowers et al., 2013). We note that the staining pattern for FoxP2 closely matched that for FoxP1; overlapping confocal images show co-expression of FoxP2 and FoxP1 (see Figure 3). Following overnight incubation at 4° C, sections were washed 3 times for 5 min each with 1X PBS, then incubated for 2 hours at room temperature in PBST/1% donkey serum and 1:200 dilutions of two fluorescence-tagged secondary antibodies (Life Technologies, Carlsbad, CA) against goat or rabbit IgG, each with distinct excitation spectra (AlexaFluor 488 nm to detect FoxP2, Alexa Fluor 594 nm to detect FoxP1). Slides were then washed with 1X PBS 3 times for 5 min each and coverslipped using Vectashield with DAPI (excited by 405 nm; Vector, Burlingame, CA, #H-1200) as a counterstain. Slides were stored overnight at room temperature before confocal imaging.
Figure 3.
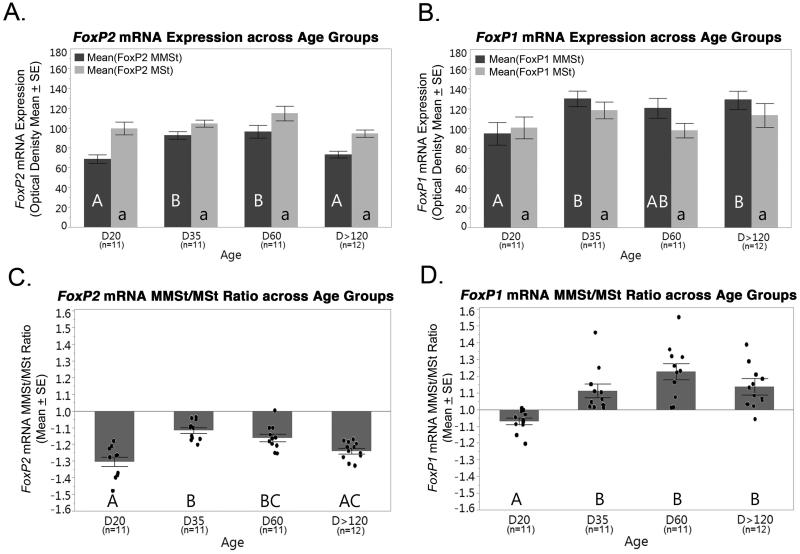
(A) FoxP2 mRNA expression in the MMSt (dark grey) and MSt (light grey) across age groups. No significant differences were found between groups in the MSt (p<0.05). (B) FoxP1 mRNA expression in the MMSt and MSt across age groups. No significant differences were found between age groups for both the MSt. FoxP1 mRNA expression in the MMSt was significantly lower at D20 compared to D35 and D>120. (C, D) FoxP2 and FoxP1 mRNA expression ratios (MMSt/MSt) show significant differences between age groups (p<0.05). Points in (C) and (D) represent individual birds. For all graphs, significant mRNA expression differences in the MMSt, MSt, (A, B, respectively) or a ratio thereof (C, D), between the four age groups is denoted with different letters. Bars with different letters are significantly different (p<05). The letter case is used to denote significant differences between age groups separately for the MMSt (uppercase) and MSt (lowercase). Error bars = SE.
Confocal microscopy and quantification
Fluorescent images of protein expression after immunohistochemistry were captured using a Leica TCS SP5 II Broadband Confocal microscope (Leica, Solms Germany). Cytoarchitectural boundaries were determined using the adjacent thionin stained and FoxP1 and FoxP2 in situ hybridized slides. Coronal sections were imaged with at 40X. Optimal beam settings were used for each channel (405 nm for DAPI, 594 nm for FoxP1, 488 nm for FoxP2). For each channel, images of 3 different tissue sections containing the same brain regions (MMSt and the adjoining striatum) were taken for both brain hemispheres of each animal. These confocal images were converted to an 8-bit gray scale, threshold was manually adjusted, and the image was then made into a binary file. Outliers with a radius of < 3 pixels were removed and cell counts were automatically obtained and manually checked using ImageJ software (NIH, Bethesda, MD). The values obtained from cells counts for four brain sections (two from each hemisphere) of each the MMSt and adjoining medial striatum (MSt) were recorded. All FoxP1 and FoxP2 counts were normalized by DAPI to control for varying cell densities. The counts were then averaged for each individual bird. These individual averages were then used to calculate the MMSt/adjoining medial striatum ratio for each animal. The ratios of FoxP1 and FoxP2 expression passed Shapiro-Wilk normality tests, and were analyzed further using a one- way ANOVA with age group as a fixed factor, followed by a post hoc pairwise comparison (Tukey-Kramer HSD). JMP software (SAS Institute Inc., Cary, NC) was used for all statistical analyses.
RESULTS
FoxP2 mRNA and protein expression
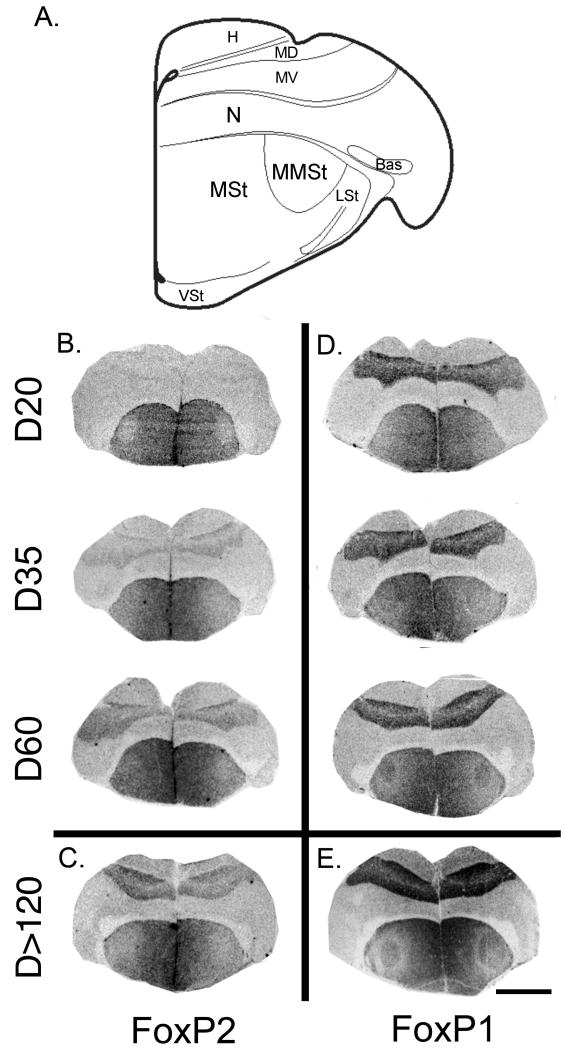
FoxP2 mRNA expression appeared to be consistently elevated in the medial striatum (MSt) compared to the hyperpallium and nidopallium across all age groups (Table 1; Figure 2B, C). Moreover, mesopallial expression of FoxP2 mRNA appeared to increase with age. Juvenile D20 animals showed low mesopallial expression levels similar to that in the nidopallium, while adults (D>120) had higher mesopallial expression similar to that found in the striatum at this age (Figure 2A-C). Further analysis of FoxP2 mRNA expression in the MSt revealed comparably high levels across all age groups (Figure 3A). In the MMSt, FoxP2 mRNA expression varied across development (ANOVA, F(3,41)=8.98, p<0.001). MMSt FoxP2 mRNA expression was low at D20 and in adults compared to D35 (p=0.003) and D60 (p<0.001) (Figure 3A). Although FoxP2 mRNA expression in the MMSt was highest at D35 and D60, the ratio of FoxP2 mRNA expression in the MMSt expression relative to MSt was below 1 across all age groups (Figure 3C). We also found group differences in the FoxP2 MMSt/MSt expression ratio (ANOVA, F(3,41)=15.75, p<0.001), with a lower MMSt/MSt ratio found at D20 compared to D35 (p<0.001) and D60 (p<0.001). The FoxP2 MMSt/MSt ratio was also lower in adults compared to D35 (p=0.001). Some D20 birds produced immature vocalizations within the 2-hour period before sacrifice (n=5 of 11), and although the FoxP2 MMSt/MSt ratio at D20 negatively correlated with the amount of vocal production, this relationship only approached significance (Spearman p= −0.616, p=0.057). Sex differences in FoxP2 mRNA in the MMSt/MSt (ANOVA, F(1)>0.01, p=0.984) and its interaction with age (F(3)=0.462, p=0.710) were not significant.
Table 1.
Mean optical density values of FoxP mRNA expression in budgerigar brains normalized to background
| D20 FoxP1 |
D20 FoxP2 |
D35 FoxP1 |
D35 FoxP2 |
D60 FoxP1 |
D60 FoxP2 |
Adult FoxP1 |
Adult FoxP2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F |
| Hyperpallium | 57 | 58 | 36 | 37 | 71 | 74 | 52 | 46 | 77 | 80 | 57 | 55 | 78 | 77 | 61 | 68 |
| Mesopallium | 119 | 128 | 48 | 47 | 143 | 146 | 71 | 77 | 147 | 146 | 78 | 78 | 152 | 158 | 99 | 103 |
| Nidopallium | 49 | 54 | 50 | 42 | 49 | 49 | 42 | 33 | 60 | 53 | 48 | 47 | 59 | 54 | 53 | 60 |
| Basorostral pallial nucleus |
18 | 24 | 33 | 39 | 21 | 22 | 24 | 18 | 24 | 19 | 28 | 31 | 22 | 28 | 18 | 27 |
| Medial striatum (MSt) | 106 | 93 | 92 | 114 | 118 | 122 | 100 | 118 | 107 | 95 | 127 | 110 | 111 | 119 | 91 | 102 |
| Magnocellular nucleus of the medial striatum (MMSt) |
100 | 87 | 64 | 78 | 130 | 133 | 90 | 101 | 127 | 118 | 104 | 94 | 130 | 139 | 70 | 79 |
Figure 2.
A. Location of the MMSt and adjoining striatum (MSt) in a schematic section from the budgerigar brain atlas at http://www.brauthlab.umd.edu/atlas.htm (Brauth SE; Jarvis et al., 2013). (B) In situ hybridized FoxP2 mRNA in which the MMSt can be found at (B) D20, D35, D60, and (C) in adults, D>120 (all birds are male). Sections of similar male brains show in situ hybridized FoxP1 mRNA in (D) and (E). Scale bar in (E) = 4 mm. Abbreviations: H, Hyperpallium; MD, dorsal Mesopallium; MV, ventral Mesopallium; N, Nidopallium; Bas, Basorostral pallial nucleus; MMSt, Magnocellular nucleus of the medial striatum; MSt, Medial striatum; LSt, Lateral striatum; VSt, Ventral striatum.
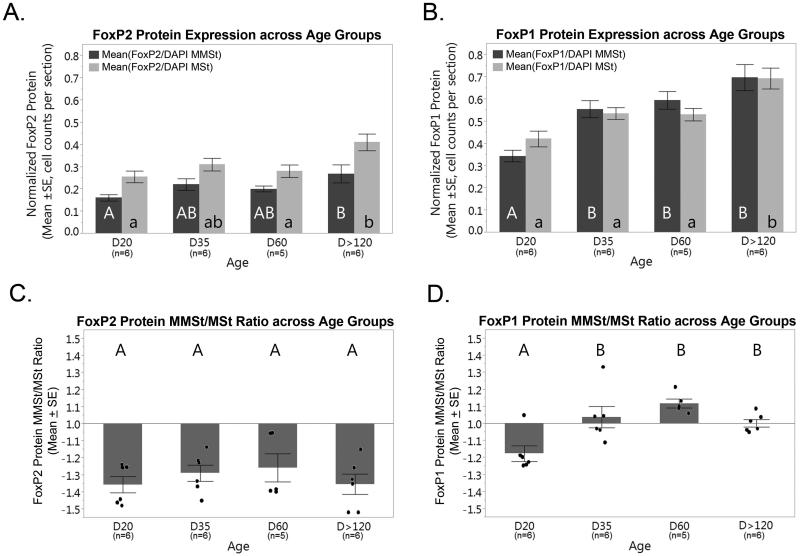
We next evaluated whether the proportion of cells expressing FoxP2 protein also was reduced in the MMSt during development and adulthood (Figures 4 and 5). FoxP2 protein expression in the MSt and MMSt across age groups was comparable to that of FoxP2 mRNA. However, there were differences between age groups in FoxP2 protein expression in the MSt (ANOVA, F(3,19) Ratio=5.08, p=0.009; Figure 5A). MSt expression in adults was significantly higher than that of both D20 (p=0.008) and D60 (p=0.040) birds. A one-way ANOVA of protein levels in MMSt with age group as a fixed factor approached significance F(3,19)=2.84, p=0.065). Post hoc tests showed FoxP2 protein expression in the MMSt at D20 was significantly lower than that from adults (p=0.043). A direct examination of the degree of downregulation in the MMSt using the ratio of FoxP2 protein expression in the MMSt/MSt found no significant differences between the ratios at each age group (ANOVA, F(3,19)=0.067, p=0.580; Figure 5C). Thus, we find that similar to FoxP2 mRNA, expression of FoxP2 protein in the MMSt remains lower than that in the surrounding medial striatum throughout periods of learning in both juveniles and adults, albeit with a slight increase in overall protein levels as birds reach adulthood. A main effect for sex on MMSt/MSt FoxP2 protein expression and its interaction with age was not significant (ANOVA, F(1)=0.072, p=0.792, and F(2)=0.229, p=0.798).
Figure 4.
Confocal images taken with a 40X objective to detect FoxP2 and FoxP1 protein within the MSt and MMSt. Example images are shown from a female animal at D35. From top to bottom: (A, B) DAPI stained cells in 405 nm within the MMSt and MSt; (C, D) FoxP2 protein expressing neurons in 488 nm within the MMSt and MSt; (E, F) FoxP1 expressing neurons in 594 nm within the MMSt and MSt; Scale bar in H = 50 μM.
Figure 5.
(A) DAPI normalized FoxP2 protein expression across age groups in the MMSt (dark grey) and MSt (light grey). Significant differences between age groups in the MMSt and MSt. are shown with bars not connected by the same letter (p<0.05). (B) DAPI normalized FoxP1 protein expression across age groups in the MMSt (dark grey) and MSt (light grey) show significant differences in D20 and adult birds using bars with different letters (p<0.05). (C) FoxP2 and (D) FoxP1 MMSt/MSt protein expression ratios across age groups. No significant differences in FoxP2 expression ratios were found between groups. Ratios were <1 for all age groups. (D) MMSt/MSt FoxP1 protein expression ratios were significantly lower at D20 (p<0.05). Points in (C) and (D) represent individual birds. Error bars = SE.
FoxP1 mRNA and protein expression
We observed increased FoxP1 mRNA expression in the mesopallium and striatum relative to nidopallial and hyperpallial brain regions across all age groups (Table 1; Figure 2D, E). Although FoxP1 mRNA expression in the MSt appeared to increase over development, these differences did not reach statistical significance (ANOVA, F(3,41)=1.43, p=0.246; Figure 3B. However FoxP1 mRNA expression in the MMSt was significantly different across the age groups (ANOVA, F(3,41)=3.23, p=0.320; Figure 3B). D20 birds had significantly less expression than birds at D35 (p=0.048) and D>120 (p=0.046). Moreover, the ratio of FoxP1 mRNA expression in the MMSt relative to the MSt was significantly lower at D20 compared to all other age groups (p<0.001-0.007). FoxP1 mRNA expression showed no relationship to vocalizing in D20 birds (Spearman p= 0.058, p= 0.873). A main effect of sex and its interaction with age for MMSt/MSt FoxP1 mRNA expression was also not significant (ANOVA, F(1)=0.004, p=0.286, and F(3)=1.096, p=0.363).
Comparable differences were found in FoxP1 protein expression between age groups. Significant differences were found in FoxP1 protein expression between age groups in the MSt (ANOVA, F(3, 19)=10.19, p<0.001), where adult birds had higher expression compared to D60 (p=0.026), D35 (p=0.024), and D20 birds (p<0.001). Furthermore, there was an age group difference in FoxP1 protein expression in the MMSt (ANOVA, F(3,19) Ratio=12.64, p<0.001), and post-hoc tests revealed that FoxP1 expression in D20 birds was significantly lower than that of D35 (p=0.010), D60 (p=0.003) and adult birds (p<0.001). D35, D60 and adult birds did not differ from one another (Figure 5B). A ratio of FoxP1 expression in the MMSt compared to the MSt showed the degree of FoxP1 protein expression was also different between the age groups (ANOVA, F(3,19)=7.96, p=0.001; Figure 5D). The ratios of D20 birds were significantly lower than that of D35 (p=0.010), D60 (p=0.001) and adults birds (p=0.038). Thus, in MMSt, FoxP1 mRNA and protein expression appears to increase after D20. We did not find a significant main effect of sex on MMSt/MSt FoxP1 protein expression (ANOVA, F(1)=0.083, p=0.778), and its interaction with age was also not significant, and F(2)=0.557, p=0.587).
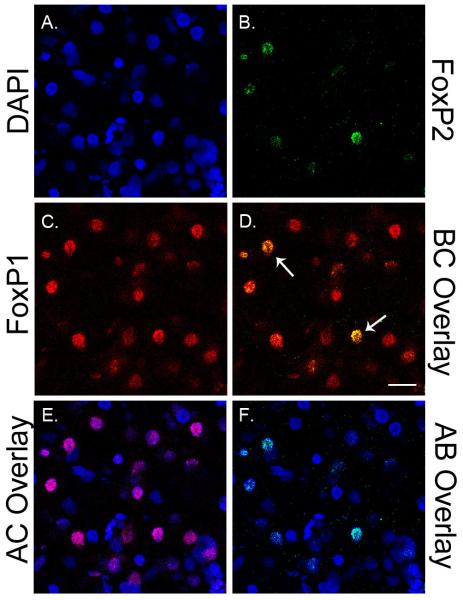
FoxP2 and FoxP1 co-expression in the MMSt
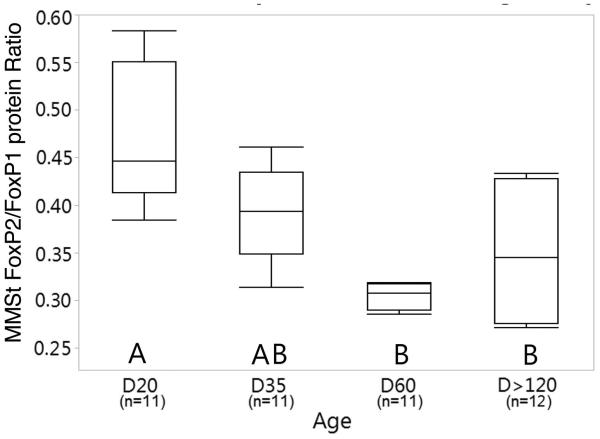
As expected, most of the cells expressing FoxP1 protein in the MMSt and MSt overlapped with FoxP2 protein expressing cells (Figure 6). To further explore the possibility of a potential interaction between FoxP2 and FoxP1 in the MMSt we analyzed an expression ratio of FoxP2/FoxP1 protein and found a significant difference across age groups (ANOVA, F(3, 19)=6.429, p=0.004). In D20 bird the FoxP2/FoxP1 protein expression ratio was significantly higher compared to that ratio in birds at D60 (p=0.004) and D120 (p=0.026). Although the FoxP2/FoxP1 ratio was highest at D20, it remained <1 across the age groups; mean expression was 0.47, 0.39, 0.30, and 0.35 for D20-D>120, respectively (Figure 7).
Figure 6.
Representative confocal images taken with a 63X objective show overlapping FoxP2 and FoxP1 protein expression within the MMSt of a female animal at D20. A, B and C show DAPI, FoxP2 and FoxP1 labeled cells. D, E, and F show different combinations of overlapping expression between A, B, and C. In (D) arrows indicate example cells co-expressing FoxP2 and FoxP1; scale bar = 20 μM.
Figure 7.
Box plots show a ratio of FoxP2/FoxP1 protein expression in the MMSt. FoxP expression is DAPI normalized. Top and bottom whisker lines, and box line represent maximum, minimum and median values, respectively.
DISCUSSION
We investigated developmental FoxP2 and FoxP1 mRNA and protein expression within a basal ganglia vocal learning nucleus in the budgerigar, a parrot species with open-ended vocal learning. Our results suggest that these genes play a conserved role for vocal learning in evolutionarily diverse species. Moreover, the developmental FoxP2 expression pattern we observe here in budgerigars differs from that found during zebra finch development, and is consistent with persistent vocal plasticity in budgerigars. The developmental FoxP1 expression we observed in the MMSt provides support for its role, as previously suggested, in the development of vocal motor neural circuitry. Thus, the divergent developmental expression patterns we find for FoxP2 and FoxP1 suggests the possibility that these genes may have distinct contributions to the processes underlying vocal ontogeny in species with vocal learning.
Functional implications of FoxP2 expression in budgerigars
Previous research in adult and juvenile zebra finches found that downregulation of FoxP2 in Area X is related to the production of undirected songs that lack a particular social target (Teramitsu and White, 2006; Miller et al., 2008); such singing is understood to be a form of vocal practice (Olveczky et al., 2005). This singing-dependent downregulation of FoxP2 is consistent with a post-organizational role for FoxP2 in the modulation of neural vocal motor circuits for learning. We could not determine whether learned vocal production in fledgling juveniles or adults downregulates FoxP2 expression, as these budgerigars rarely vocalized during the 2-hour period of observation prior to euthanization. Moreover the relationship between vocal practice and FoxP2 protein expression in D20 birds could not be fully explored here due to a lack of statistical power as only 3 of 6 D20 birds vocalized, and only 2 of these 3 D20 birds produced >11 vocalizations within the 2 hour period before sacrifice. Nonetheless, we did find a trend in D20 birds towards lower FoxP2 mRNA expression as vocal production increased, consistent with a role for FoxP2 in modulating plasticity in the budgerigar.
Downregulation of FoxP2 mRNA and protein expression within the MMSt relative to the MSt was seen in both juvenile and adult budgerigars, and both are capable of learning new vocal patterns. We hypothesize that the persistent low level FoxP2 expression in the MMSt maintains this region in a state that allows for persistent plasticity; thus permitting mature vocal learning circuits to encode the necessary motor patterns to produce learned vocalizations. If true, then perhaps the extent of vocal learning in budgerigars correlates with the low level of FoxP2 expression in the MMSt. Although it is beyond the scope of this study, we are pursing the question of whether vocal modification in the budgerigar may be influenced by social contexts such as group membership status (novel or stable), and its potential to influence FoxP2 expression. We should point out here that we are not proposing that FoxP2 is not necessary for vocal learning. Rather, our results are consistent with results from zebra finches showing that downregulation of FoxP2 during undirected singing is associated with greater plasticity in song (Teramitsu and White, 2006), and that FoxP2 is a transcriptional regulator of a suite of other genes in their Area X (Hilliard et al., 2012). Together, these results suggest the hypothesis that downregulation of FoxP2 in the budgerigar MMSt is a key regulatory event that allows for the vocal plasticity seen in both juvenile and adult of this species.
Previous studies in budgerigars suggested that FoxP2 expression would be elevated in the MMSt relative to the adjoining striatum during early vocal learning periods and then decline as birds entered adulthood, as was found in developing zebra finches (Teramitsu et al., 2004) (Haesler et al., 2004). Such regulation in zebra finches suggested a role for FoxP2 in the formation of circuits for learned vocalizations. Developmental expression studies in human brain also support a role for FoxP2 in the development of motor-related circuits (Lai et al., 2003). One explanation for the developmental FoxP2 expression differences between zebra finches and budgerigars, suggests an alternative, though not mutually exclusive, role for FoxP2. That is, the upregulation of FoxP2 in D35 and D50 zebra finches may be related to long-term consolidation within the neural circuits underlying a specific behavioral performance, e.g. the crystallization of a stereotyped song. We found that FoxP2 mRNA expression was increased at D35 and D60 compared to D20 and adults, but this expression did not equal or exceed the surrounding striatum at any developmental timepoint we observed. Since budgerigars are open-ended vocal learners they may experience this crystallization to a lesser degree; thus there may be no point in their development during which FoxP2 expression is upregulated in the MMSt relative to the MSt. This hypothesis is consistent with a finding in mice, showing that FoxP2 regulates gene expression crucial for modulating synapse formation (Sia et al., 2013).
The role of FoxP1 expression and interactions with FoxP2
The FoxP1 mRNA and protein expression ratio for the MMSt relative to the MSt was <1 in the D20 birds, but increased significantly in D35, D60, and adult birds, showing that FoxP1 in the MMSt is being upregulated relative to the adjoining striatum as birds matured. This finding is similar to those found in vocal learning songbirds, where FoxP1 mRNA expression in zebra finches was also upregulated in Area X relative to the MSt in juvenile and adult male birds. However in zebra finches, Area X/outlying striatum FoxP1 mRNA expression appeared to peak in younger (D35) birds, whereas in budgerigars, this FoxP1 mRNA and protein ratio was highest at D60. This expression pattern was unlike that of FoxP2 and suggests that upregulation of FoxP1 expression in the MMSt (or Area X) plays a role in the development and adult function of basal ganglia circuitry that is required for vocal plasticity. A similar role for FoxP1 has been described in developing mouse brain (Ferland et al., 2003). Differences in the timing of peak FoxP1 expression in the motor circuitry could reflect differences in the corresponding rates of maturation in different species.
FoxP1 may interact with FoxP2 and other genes in the FoxP family to regulate genes involved in the development and maintenance of vocal learning circuits. FoxP2 and FoxP1 act in cooperation to regulate development of mouse lung and esophageal tissues (Shu et al., 2007) so perhaps these genes cooperate to establish and modify connections in the brain as well. In songbirds, FoxP2 and FoxP1 are co-expressed in the striatum (Chen et al., 2013). Here we found in budgerigars overlapping FoxP2 and FoxP1 protein expression in the same MMSt cells and that the ratio of MMSt FoxP2/FoxP1 protein expression was highest in nestling birds, significantly decreasing as birds aged. The deceasing ratio was due to a prodigious increase in FoxP1 protein expression as birds aged. These results suggest that a primary role for FoxP1 expression, during early development and not later, could be interacting with FoxP2 for the cooperative regulation of gene expression.
Lack of sex differences but a mesopallial increase in FoxP expression
Unlike in the zebra finch, where FoxP2 and FoxP1 mRNA expression is sexually dimorphic, we detected no differences in FoxP2 and FoxP1 gene expression between male and female budgerigars. This result is consistent with the vocal learning behavior observed in this species, as both sexes have been shown to learn new vocalizations, even as adults (Farabaugh and Dooling, 1996; Hile and Striedter, 2000; Dahlin et al., 2014). Interestingly our data suggest a role for the FoxP genes outside of the striatum. We found a striking difference in FoxP2 and FoxP1 mRNA expression in the mesopallium, where expression gradually increased throughout development into adulthood, in parallel with the acquisition and increase of the vocal repertoire. Although this region of the mesopallium does not contain vocal control nuclei, in general the mesopallium is enlarged in birds with high cognitive abilities, like parrots (Lefebvre et al., 2004; Iwaniuk and Hurd, 2005; Chen et al., 2013). Cognitive complexity in birds may be dependent on mesopallial brain organization and, as our data suggests, its underlying gene activity that includes the FoxP2 and FoxP1 genes.
Conclusions
Vocal learning has evolved independently in various groups of birds and mammals (Petkov and Jarvis, 2012), yet the exact physiological components of this complex behavior are not completely understood. The results from these experiments shed light on some of the neuromolecular mechanisms that allow vocal learning in juvenile and adult animals, and add to the increasing evidence for common neurogenetic mechanisms underlying learned vocal communication. Further investigation of FoxP gene regulation in budgerigars is a promising route for increasing our understanding of the neurogenetic processes underlying vocal learning in both juveniles and adults.
Acknowledgements
This work was supported by NIH NICHD grant SC1HD068128 to T. Wright. T. Voyles was supported by a Howard Hughes Medical Institute Science Education grant 52006932 to New Mexico State University, work by S. White and T. Wright at the Marine Biological Laboratory was supported by the Grass Foundation, and the material was prepared on equipment supported by the National Science Foundation under Grant Number MRI-DBI-095817.We thank Alfredo Montoya and the staff of the NMSU Animal Care Facility for expert bird care. Special thanks to Jemima Perez, Esteban Lucero, Patricia Duarte-Hash, Keely Brown, Jon Heston, Dr. Julie Miller, Dr. Peter Cooke, and Dr. Anna Young for their contributions to this project.
REFERENCES
- Bacon C, Rappold GA. The distinct and overlapping phenotypic spectra of FOXP1 and FOXP2 in cognitive disorders. Hum Genet. 2012;131:1687–1698. doi: 10.1007/s00439-012-1193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belton E, Salmond CH, Watkins KE, Vargha-Khadem F, Gadian DG. Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum Brain Mapp. 2003;18:194–200. doi: 10.1002/hbm.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nat Rev Neurosci. 2010;11:747–759. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- Bowers JM, Perez-Pouchoulen M, Edwards NS, McCarthy MM. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J Neurosci. 2013;33:3276–3283. doi: 10.1523/JNEUROSCI.0425-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauth SEHJ, Roberts TF, Liang W. Budgerigar Brain Atlas. http://www.brauthlab.umd.edu/atlas.htm. accessed Nov 2014.
- Brittan-Powell EF, Dooling RJ, Farabaugh SM. Vocal development in budgerigars (Melopsittacus undulatus): Contact calls. J. Comp. Psych. 1997;111:226–241. doi: 10.1037/0735-7036.111.3.226. [DOI] [PubMed] [Google Scholar]
- Chen Q, Heston JB, Burkett ZD, White SA. Expression analysis of the speech-related genes FoxP1 and FoxP2 and their relation to singing behavior in two songbird species. Journal of Experimental Biology. 2013;216:3682–3692. doi: 10.1242/jeb.085886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien WH, Gau SS, Chen CH, Tsai WC, Wu YY, Chen PH, Shang CY, Chen CH. Increased gene expression of FOXP1 in patients with autism spectrum disorders. Mol Autism. 2013;4:23. doi: 10.1186/2040-2392-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin CR, Young AM, Cordier B, Mundry R, Wright TF. A test of multiple hypotheses for the function of call sharing in female budgerigars, Melopsittacus undulatus. Behavioral Ecology and Sociobiology. 2014;68:145–161. doi: 10.1007/s00265-013-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. The study of second language acquisition. Oxfrord University Press; 1994. [Google Scholar]
- Farabaugh SM, Dooling RJ. Acoustic communication in parrots: laboratory and field studies of Budgerigars, Melopsittacus undulatus. In: Kroodsma DE, Miller EH, Kroodsma DE, Miller EHs, editors. Ecology and Evolution of Acoustic Communication in Birds. Cornell University Press; Ithaca, New York: 1996. pp. 97–117. [Google Scholar]
- Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA. Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460:266–279. doi: 10.1002/cne.10654. [DOI] [PubMed] [Google Scholar]
- Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus area X. PLoS Biology. 2007;5:2885–2897. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C. FoxP2 expression in avian vocal learners and non-learners. J Neurosci. 2004;24:3164–3175. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WS, Cookson KK, Heaton JT, Roberts TF, Shea SD, Amateau SK, Brauth SE. Cytoarchitecture of vocal control nuclei in nestling budgerigars: relationships to call development. Brain Behav Evol. 1999;53:198–226. doi: 10.1159/000006595. [DOI] [PubMed] [Google Scholar]
- Hamdan FF, Daoud H, Rochefort D, Piton A, Gauthier J, Langlois M, Foomani G, Dobrzeniecka S, Krebs MO, Joober R, Lafreniere RG, Lacaille JC, Mottron L, Drapeau P, Beauchamp MH, Phillips MS, Fombonne E, Rouleau GA, Michaud JL. De novo mutations in FOXP1 in cases with intellectual disability, autism, and language impairment. Am J Hum Genet. 2010;87:671–678. doi: 10.1016/j.ajhg.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton JTJ, Brauth SES. Effects of deafening on the development of nestling and juvenile vocalizations in budgerigars (Melopsittacus undulatus) J Comp Psychol. 1999;113:314–320. doi: 10.1037/0735-7036.113.3.314. [DOI] [PubMed] [Google Scholar]
- Heaton JTJ, Dooling RJR, Farabaugh SMS. Effects of deafening on the calls and warble song of adult budgerigars (Melopsittacus undulatus) J Acoust Soc Am. 1999;105:2010–2019. doi: 10.1121/1.426734. [DOI] [PubMed] [Google Scholar]
- Hile AG, Striedter GF. Call convergence within groups of female budgerigars (Melopsittacus undulatus) Ethology. 2000;106:1105–1114. doi: 10.1006/anbe.1999.1438. [DOI] [PubMed] [Google Scholar]
- Hilliard AT, Miller JE, Fraley ER, Horvath S, White SA. Molecular microcircuitry underlies functional specification in a basal ganglia circuit dedicated to vocal learning. Neuron. 2012;73:537–552. doi: 10.1016/j.neuron.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D, Kapeller J, Rivera-Brugués N, Moog U, Lorenz-Depiereux B, Eck S, Hempel M, Wagenstaller J, Gawthrope A, Monaco AP, Bonin M, Riess O, Wohlleber E, Illig T, Bezzina CR, Franke A, Spranger S, Villavicencio-Lorini P, Seifert W, Rosenfeld J, Klopocki E, Rappold GA, Strom TM. Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits. Human Mutation. 2010;31:E1851–E1860. doi: 10.1002/humu.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniuk AN, Hurd PL. The evolution of cerebrotypes in birds. Brain Behav Evol. 2005;65:215–230. doi: 10.1159/000084313. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Mello CV. Molecular mapping of brain areas involved in parrot vocal communication. J. Comp. Neurol. 2000;419:1–31. doi: 10.1002/(sici)1096-9861(20000327)419:1<1::aid-cne1>3.0.co;2-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen K, Gleiberman AS, Shi C, Simon DI, Rosenfeld MG. Cooperative regulation in development by SMRT and FOXP1. Genes Dev. 2008;22:740–745. doi: 10.1101/gad.1637108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Fisher SE, Hurst JA, Vargha-Khadem F. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;6855:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126:2455–2462. doi: 10.1093/brain/awg247. [DOI] [PubMed] [Google Scholar]
- Le Fevre AK, Taylor S, Malek NH, Horn D, Carr CW, Abdul-Rahman OA, O'Donnell S, Burgess T, Shaw M, Gecz J, Bain N, Fagan K, Hunter MF. FOXP1 mutations cause intellectual disability and a recognizable phenotype. Am J Med Genet A. 2013;161A:3166–3175. doi: 10.1002/ajmg.a.36174. [DOI] [PubMed] [Google Scholar]
- Lefebvre L, Reader SM, Sol D. Brains, innovations and evolution in birds and primates. Brain Behav Evol. 2004;63:233–246. doi: 10.1159/000076784. [DOI] [PubMed] [Google Scholar]
- MacDermot KD, Bonora E, Sykes N, Coupe A-M, Lai CSL, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, Fisher SE. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. American journal of human genetics. 2005;76:1074–1080. doi: 10.1086/430841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, Spiteri E, Condro MC, Dosumu-Johnson RT, Geschwind DH, White SA. Birdsong decreases protein levels of FoxP2, a molecule required for human speech. J Neurophysiol. 2008;100:2015–2025. doi: 10.1152/jn.90415.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan M, Harward S, Scharff C, Mooney R. Diminished FoxP2 levels affect dopaminergic modulation of corticostriatal signaling important to song variability. Neuron. 2013;80:1464–1476. doi: 10.1016/j.neuron.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease SM, Salinas-Melgoza A, Renton K, Escalante P, Wright TF. Brood sex ratio of the lilac-crowned parrot (Amazona finschi) Wilson Journal of Ornithology. 2012;124:393–396. [Google Scholar]
- Petkov CI, Jarvis ED. Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front Evol Neurosci. 2012;4:12. doi: 10.3389/fnevo.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Yu J, Rivas MV, Horita H, Feenders G, Whitney O, Jarvis SC, Jarvis ER, Kubikova L, Puck AE, Siang-Bakshi C, Martin S, McElroy M, Hara E, Howard J, Pfenning A, Mouritsen H, Chen CC, Wada K. Global view of the functional molecular organization of the avian cerebrum: mirror images and functional columns. J Comp Neurol. 2013;521:3614–3665. doi: 10.1002/cne.23404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, White SA. Genetic components of vocal learning. Ann N Y Acad Sci. 2004;1016:325–347. doi: 10.1196/annals.1298.032. [DOI] [PubMed] [Google Scholar]
- Schulz SB, Haesler S, Scharff C, Rochefort C. Knockdown of FoxP2 alters spine density in Area X of the zebra finch. Genes Brain Behav. 2010;9:732–740. doi: 10.1111/j.1601-183X.2010.00607.x. [DOI] [PubMed] [Google Scholar]
- Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- Sia GM, Clem RL, Huganir RL. The human language-associated gene SRPX2 regulates synapse formation and vocalization in mice. Science. 2013;342:987–991. doi: 10.1126/science.1245079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Luo B. Late-postnatal cannabinoid exposure persistently increases FoxP2 expression within zebra finch striatum. Developmental Neurobiology. 2010;70:195–203. doi: 10.1002/dneu.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF. The vocal control pathways in budgerigars differ from those in songbirds. J Comp Neurol. 1994;343:35–56. doi: 10.1002/cne.903430104. [DOI] [PubMed] [Google Scholar]
- Tamura S, Morikawa Y, Iwanishi H, Hisaoka T, Senba E. Expression pattern of the winged-helix/forkhead transcription factor Foxp1 in the developing central nervous system. Gene Expr Patterns. 2003;3:193–197. doi: 10.1016/s1567-133x(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Bijan P, Mitra PP. A procedure for an automated measurement of song similarity. Animal Behaviour. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA. Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. Journal of Neuroscience. 2004;24:3152–3163. doi: 10.1523/JNEUROSCI.5589-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramitsu I, Poopatanapong A, Torrisi S, White SA. Striatal FoxP2 is actively regulated during songbird sensorimotor learning. PLoS ONE. 2010;5:e8548. doi: 10.1371/journal.pone.0008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramitsu I, White SA. FoxP2 regulation during undirected singing in adult songbirds. J Neurosci. 2006;26:7390–7394. doi: 10.1523/JNEUROSCI.1662-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang KM, Croen LA, Torres AR, Kharrazi M, Delorenze GN, Windham GC, Yoshida CK, Zerbo O, Weiss LA. A genome-wide survey of transgenerational genetic effects in autism. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, Frackowiak RS, Friston KJ, Pembrey ME, Mishkin M, Gadian DG, Passingham RE. Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci U S A. 1998;95:12695–12700. doi: 10.1073/pnas.95.21.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Howard JT, McConnell P, Whitney O, Lints T, Rivas MV, Horita H, Patterson MA, White SA, Scharff C, Haesler S, Zhao S, Sakaguchi H, Hagiwara M, Shiraki T, Hirozane-Kishikawa T, Skene P, Hayashizaki Y, Carninci P, Jarvis ED. A molecular neuroethological approach for identifying and characterizing a cascade of behaviorally regulated genes. Proc Natl Acad Sci U S A. 2006;103:15212–15217. doi: 10.1073/pnas.0607098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Vargha-Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, Frackowiak RS, Mishkin M, Gadian DG. MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain. 2002;125:465–478. doi: 10.1093/brain/awf057. [DOI] [PubMed] [Google Scholar]
- White SA. Genes and vocal learning. Brain Lang. 2010;115:21–28. doi: 10.1016/j.bandl.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SA, Fisher SE, Geschwind DH, Scharff C, Holy TE. Singing mice, songbirds, and more: Models for FOXP2 function and dysfunction in human speech and language. Journal of Neuroscience. 2006;26:10376–10379. doi: 10.1523/JNEUROSCI.3379-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann RA. Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford University Press; New York: 1996. [Google Scholar]