Abstract
Cognition, negative symptoms, and depression are potential predictors of disability in schizophrenia. We present analyses of pooled data from four separate studies (all n>169; total n=821) that assessed differential aspects of disability and their potential determinants. We hypothesized that negative symptoms would predict social outcomes, but not vocational functioning or everyday activities and that cognition and functional capacity would predict vocational functioning and everyday activities but not social outcomes. The samples were rated by clinician informants for their everyday functioning in domains of social and vocational outcomes, and everyday activities, examined with assessments of cognition and functional capacity, rated clinically with the Positive and Negative Syndrome Scale (PANSS) and self-reporting depression. We computed a model that tested the hypotheses described above and compared it to a model that predicted that negative symptoms, depression, cognition, and functional capacity had equivalent influences on all aspects of everyday functioning. The former, specific relationship model fit the data adequately and we subsequently confirmed a similar fit within all four samples. Analyses of the relative goodness of fit suggested that this specific model fit the data better than the more general, equivalent influence predictor model. We suggest that treatments aimed at cognition may not affect social functioning as much as other aspects of disability, a finding consistent with earlier research on the treatment of cognitive deficits in schizophrenia, while negative symptoms predicted social functioning. These relationships are central features of schizophrenia and treatment efforts should be aimed accordingly.
1. Introduction
Everyday functioning is commonly impaired in schizophrenia, affecting domains of social functioning, vocational performance, and performance of everyday activities. Even among those patients classified as ‘responders’ to available pharmacological and psychosocial treatments, disability rates are high and functional outcomes have changed minimally compared to success in treating psychosis (Hegarty et al., 1994). Cognitive deficits and negative symptoms are thought to represent the main drivers of disability, (Breier et al., 1991; Carone et al., 1991) although influences outside of the individual such as opportunities and disincentives such as disability compensation meaningfully affect certain domains of functioning (Rosenheck et al., 2006; Harvey et al., 2009). While positive symptoms usually improve with treatment, or can otherwise be compensated for (Ventura et al. 2009) both cognitive deficits and negative symptoms receive minimal benefit despite the fact that current antipsychotics control psychosis to the point that clinical remission rates are close to 50% in some studies (Harvey and Bellack, 2009).
Moreover, negative and cognitive symptoms of schizophrenia, often present prior to the emergence of frank psychosis (Meyer et al., 2014), appear to be related but separable domains with different functional implications (Harvey et al., 2006; Couture et al., 2011). Ventura et al (2009) concur that cognitive and negative symptoms both predict outcome, but note that negative symptoms partially mediate the longitudinal relationship between cognition and outcome, and suggest therefore that cognition has both direct and indirect effects on functioning. Similarly, Lin et al. (2013) suggest that negative symptoms mediate the influence of cognition on outcome. Some of our own work has suggested that different domains of everyday functioning may not be as highly intercorrelated as previously thought and may also have different potential determinants. For instance, we previously reported that cognitive and functional capacity deficits predicted impairments in everyday activities (with the exception of social outcomes) and that negative symptoms were related to poor social outcomes to a greater extent than to other aspects of everyday outcome (Leifker et al., 2009). Meta-analyses have suggested that non-social cognitive deficits show less relation to social deficits when compared to the influence of social cognition (Green et al., 2000; Green et al., 2004; Fett et al., 2011), and we have found that social deficits were less responsive to interventions aimed at treatment of cognition and functional skills deficits compared to work and instrumental functions (Bowie et al., 2012). Finally, achievement of different functional milestones (work, residence, and social achievements) is minimally intercorrelated in schizophrenia, suggesting that global indices of disability may lack the requisite specificity (Harvey et al., 2012) and that there are likely specific predictors of impairments in different domains of everyday functioning.
In this paper we present analyses of a unique set of data: four separately collected datasets with similar methodological strategies that allowed for the evaluation of the relationship between three different aspects of real-world functional outcomes: social functioning, vocational skills, and performance of everyday activities, assessed with the same scales and informant strategies, and an identical set of potential determinants of functioning: neuropsychological test performance, performance-based measures of functional capacity, and negative and depressive symptoms. All four separate large-scale (n=169 in the smallest; total n=821) studies contribute information on the correlational relationships between everyday outcomes and an array of potential predictors.
These studies were conducted in five separate geographical areas (New York, Atlanta, San Diego, Miami, and Dallas) have no overlap of patients or clinicians, and reflect a wide range of demographic and ethnic variation in the patients assessed, while the same real-world functional outcome measure, clinical ratings, self-reports of symptoms, and functional capacity measures were used. Cognition was assessed with batteries that have overlap of identical tests in 3/4 studies and a highly similar battery in the other study. While some results regarding correlational aspects between symptoms, functional capacity, and everyday outcomes have been published from three of the studies (See below), there has never been a systematic comparison of the influences of negative symptoms, depression, cognition, and functional capacity on everyday functioning across sequentially completed studies with the same assessment strategies.
Based on previous research delineated above, we hypothesized that negative symptoms would predict social deficits, but not impairments in everyday activities and vocational outcomes, while cognition and functional capacity would predict deficits in everyday activities and vocational outcomes, but not social outcomes. This is a cross-sectional hypothesis in a sample of relatively chronic patients. Previous research (Ventura et al, 2015) has found that negative symptoms early in the course of illness predict both social functioning and work/school functioning at a year after initial contact, suggesting that influences of reductions in motivation or emotional expression have broad impacts. However, in a sample where the illness is already fully developed, we hypothesized that negative symptoms would exert a greater influence on social outcomes than everyday activities. We also hypothesized that depression, negative symptoms, and cognition and functional would exert independent influences on the real-world outcomes of interest. We tested this model in the sample as a whole, as well as in each of the individual subsamples. We also computed a generic model, wherein cognition and functional capacity, depression, and negative symptoms were hypothesized to be equally important for the prediction of all elements of real-world outcomes in the database.
We tested several hypotheses in these analyses of the substantive model and its comparator model;
A model specifying that negative symptoms will be have a more substantial predictive influence on social deficits than cognition or functional capacity will be the best fit to that data.
A model specifying that cognition and functional capacity will predict everyday activities and vocational outcomes more substantially than social outcomes will be the best fit to the data.
Depression will impact all aspects of functional outcomes.
2. Methods
2.1. Participants
The data are part of four study cohorts collected in five different geographical areas, aimed at identifying the course and correlates of change in functional status as well as the optimal method for rating everyday functioning among schizophrenia outpatients.
The study participants were patients (n=821) with schizophrenia or schizoaffective disorder receiving treatment at one of several different outpatient service delivery systems in Atlanta, Dallas, Miami, San Diego and New York City. Atlanta patients were either recruited at a private psychiatric rehabilitation program (Skyland Trail) or from the outpatient population at the Atlanta VA Medical Center. San Diego patients were recruited from the UCSD Outpatient Psychiatric Services clinic, a large public mental health clinic and other local community clinics, or by self-referral. Miami patients were recruited from the outpatient services at the University of Miami Miller School of Medicine. The Mount Sinai Sample recruitment was conducted at the Bronx VA Medical Center, an outpatient clinic at a New York State Psychiatric Hospital, or Mount Sinai School of Medicine. The Dallas sample was collected from Metrocare Services, a large non-profit provider of mental health services in Dallas County, and other outpatient services associated with the University of Texas Southwestern Medical (UTSW) Center. All research participants provided signed informed consent according to standards approved by the responsible local Institutional Review Boards.
Patients from Atlanta, San Diego, and Miami were participants in one of two phases of the Validation of Everyday Real World Outcomes Study (VALERO), parts 1 or 2. UCSD and Atlanta patients participated in VALERO 1, and UCSD, Atlanta and Miami patients participated in VALERO 2, which was started 6 months after the conclusion of data analysis of VALERO 1. Dallas patients, as well as a completely new sample of Miami patients were participants in phase I of SCOPE (Pinkham et al., 2014) study. We examined the data from these studies based on the study in which they were collected. These data were collected between July 2007 and May 2014. The Mount Sinai Sample was collected between March 2003 and June of 2008.
All enrollees completed a structured diagnostic interview, administered by a trained interviewer. The Structured Clinical Interview for the DSM (SCID; First et al., 2002) was used at the Atlanta sites, the Mini International Neuropsychiatric Interview, 6th Edition (Sheehan et al., 1998) in Dallas, San Diego, and Miami, and the Comprehensive Assessment of Symptoms and History (CASH) (Andreasen et al., 1992) in New York; all diagnoses were verified in local consensus procedures. Screening also included global cognitive function and premorbid functioning measured with the Mini-Mental State Examination (Folstein et al., 1975) and the Wide Range Achievement Test, 3rd Edition (WRAT3; Wilkinson, 1993) Recognition Reading subtest. Patients were excluded for a history of traumatic brain injury, brain disease such as seizure disorder or neurodegenerative condition, a MMSE score below 18 in the Mt. Sinai Sample, a reading score below the 6th grade in all samples, or the presence of another DSM-IV diagnosis that would exclude the diagnosis of schizophrenia. To capture a comprehensive array of participants reflective of real-world realities, comorbid substance use disorders were not an exclusion criterion. Rather, patients who appeared intoxicated were rescheduled. No inpatients were recruited but patients who resided in a variety of residential facilities including unsupported, supported, or supervised facilities were eligible. Informants were not screened for psychopathology or substance abuse. These procedures were described in previous publications (Pinkham et al., 2014; Durand et al., 2014; Bowie et al., 2008).
2.2. Assessment Strategy
Following screening, the test battery was completed. All raters received extensive training in performing all of the assessments and every three months their performance was re-evaluated. Although the SCOPE study was aimed at social cognition, social cognition measures are not presented in this report.
Real world functioning was rated with the same rating scale. In the Mt Sinai study and in VALERO 2, ratings were generated by a high-contact clinician, either a case manager, a residential facility manager, or a psychotherapist who stated that they knew patient “very well”. In VALERO 1 and SCOPE, high contact clinicians and friends or relatives of the patients provided information to a clinical rater. The rater then generated ratings for the patients’ everyday functioning.
2.2.1. Cognition
For the Mt. Sinai study, which preceded the finalization of the MATRICS consensus cognitive battery (MCCB), a comprehensive assessment of cognitive performance was completed (Bowie et al., 2012). For VALERO parts 1 and 2, a modified version of the MATRICS consensus cognitive battery (MCCB) was used (Bowie et al., 2008; Durand et al., 2015). In the SCOPE study, we used a further abbreviation of the MCCB, based on our interest in social cognition and our goal of examining cognition as a composite predictor. In the SCOPE study, we used the BACS Symbol coding test, Trail-making test part A, Animal naming fluency, The Maryland Letter-Number span test, and the Hopkins Verbal learning test as our cognitive battery. This decision was made empirically, on the basis of previously published results regarding the best predictors of composite scores on neuropsychological assessments (Keefe et al., 2006) and an analysis of the VALERO I data (unpublished) identifying the best predictors of composite performance. In the Mt. Sinai study, we examined similar constructs with some slightly different tests: we used the WAIS-3 digit symbol task (processing speed), the WAIS-3 letter number span test (working memory), and the Hopkins verbal learning test (verbal episodic memory), with animal naming and trail-making part A the same as the other studies.
For the analyses developing the cognitive performance latent trait, we used the common tests from VALERO I and II and Scope (BACS Symbol coding test, Trail-making test part A, Animal naming fluency, The Maryland Letter-Number span test, and the Hopkins Verbal learning) and the 5 Mt. Sinai tests to model the cognition latent trait. We chose to model a single latent trait because of the limited set of cognition measures and the previous findings that these measures had previously been found to be the major contributors to a unifactorial factor structure in a large sample of patients with schizophrenia (Keefe et al., 2006). We then examined whether the indicators of the latent trait had the same factor loadings, correlations with functional capacity and real-world outcomes measures despite subtle differences in the tests employed. All cognitive tests results were entered as t-scores, with the scores for VALERO I and II and SCOPE based on the MCCB norms and the Mt. Sinai t-scores based on our previously published normative procedures (Bowie et al., 2008). Supplemental Table 1 presents the tests as administered in each of the samples.
2.2.2. Functional Capacity
The brief version of the UCSD Performance-based Skills Assessment (UPSA-B) was used to assess functional capacity in all four studies. Participants performed everyday tasks related to communication and finances (Patterson et al., 2001). During the Communication role-play subtest, participants perform tasks using a telephone (e.g., making an emergency call; dialing a number from memory; calling to reschedule a doctor’s appointment). For the Finance subtest, participants count change, read a utility bill and write and record a check for the bill. The UPSA-B requires approximately 10 minutes to complete, and raw scores are converted into a total score ranging from 0–100. Higher scores indicate better functional capacity.
2.2.3. Real-World Functional Outcomes
The rating scale employed in these studies was the Specific Levels of Functioning (SLOF) scale (Schneider & Struening, 1983). The original SLOF is a 43-item self- or informant-rated scale of a person’s behavior and functioning which was abbreviated to assess the following domains: Interpersonal Functioning (e.g., initiating, accepting and maintaining social contacts; effectively communicating), independent participation in Everyday Activities (shopping, using telephone, paying bills, use of leisure time, use of public transportation), and Vocational Functioning (e.g., employable skills, level of supervision required to complete tasks, ability to stay on task, completes tasks, punctuality). The dependent variables for the statistical analyses were the scores on these three different subscales. We did not attempt to generate an overall composite score because our previous studies with this scale suggested that the subscales were differentially correlated with real-world functional milestones (Harvey et al., 2011) and our interest was to identify a predictor model aimed at identifying the predictors of each aspect of functioning.
2.2.4. Clinical Symptoms
The 21-item Beck Depression Inventory-II (BDI-II; Beck et al., 1996) was used to assess the self-reported severity of depression. Severity of psychotic and negative symptoms was assessed using the Positive and Negative Syndrome Scale (Kay, 1991). In a previous study of 3 of the four current samples, we found that only 2 of the 30 PANSS items were correlated with any of the three elements of functional outcome (Robertson et al., 2014). As both of those were negative symptoms, we focused on negative symptoms in the present study.
2.3. Statistical Approach
All of the factor analyses performed used the robust full information maximum likelihood method of model fitting and parameter estimation (Raykov & Marcoulides, 2008). This method fits the model to all subjects and all available data in a data set and accounts for some possible violations of normality. Thereby, each subject contributes all his or her available data to the model fitting and estimation process (under the widely made assumption of data missing at random; e.g., Little & Rubin, 2002). Thus, the fact that some subjects had missing values on some of the observed variables involved in the models did not lead to dropping any subject from the overall data set.
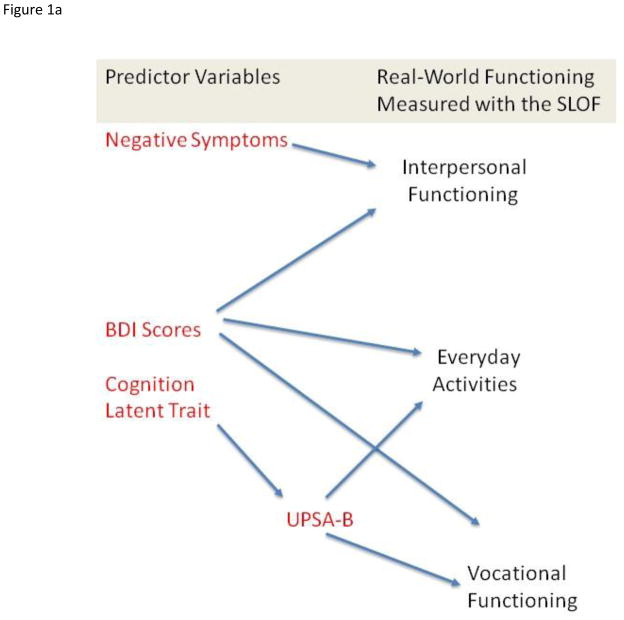
The strategy we used to examine the predictive relationships of the variables across the samples was confirmatory factor analysis. We developed latent traits that reflected constructs measured by more than one indicator (e.g., cognition and negative symptoms) and examined the similarity of their fit across samples. We then used those latent traits and other predictors that were indexed by a single measure (functional capacity and depression) to test an a priori theoretical model of the prediction of three observer rated aspects of real-world functioning in the community: social functioning, everyday activities, and vocational performance, as presented in Figure 1a. For fitting all models, we specified that these everyday outcomes were statistically independent from each other. However, the models allowed the predictor variables to conform to their observed levels of correlation as they were entered in the model.
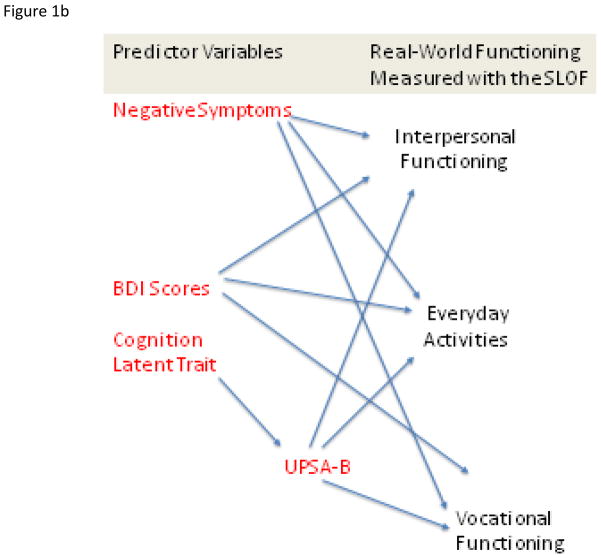
Figure 1.
Figure 1a. Overall Theoretical Model Tested in Four Samples of Patients with Schizophrenia. Figure 1b. Generic Prediction Model
We also computed a “generic” model, presented in Figure 1b, wherein cognition and functional capacity, depression, and negative symptoms were hypothesized to be equally important for the prediction of all elements of real-world outcomes in the community. In this model we also specified that the real-world outcomes were independent of each other.
The first analyses addressed the single trait model for the cognitive performance variables and for the negative symptoms measures. Then the hypothesis driven overall model presented in Figure 1a was fitted simultaneously to the data in all 4 groups and tested for plausibility. The model was then examined for measurement invariance (i.e., an identical fit) in each of the 4 groups. Finally, we fit our “generic prediction model” presented in Figure 1b in the entire sample and in each subsample. Throughout these analyses for both hypothesis driven and generic models, we evaluated fit using the popular root mean square error of approximation (RMSEA) and the Comparative fit index (CFI). According to a widely adopted ‘rule-of-thumb’, the value for RMSEA is particularly informative when assessing model fit. Specifically, a finding of this index being considerably below .06 is indicative of a plausible model (e.g., Raykov & Marcoulides, 2006; Hu & Bentler, 1999). CFI indexes are generally seen to be good at over 0.90 and excellent at over 0.95. A nonsignificant Chi-square value supports the failure to reject the null hypothesis of model fit and usually can be considered an added piece of evidence for plausibility of a model. However, we de-emphasize its importance in the present study due to its sizable overall sample because the chi-square value with large samples has a tendency to be spuriously high (e.g., Bollen, 1989).
In order to compare fit of the generic and hypothesis-driven models, we use the strategy of sequential chi-square tests, wherein the chi-square values and degrees of freedom are subtracted to directly compare the significance of the difference in fits of the two models, both in the entire sample and in each subsample.
3. Results
Demographic data on the four samples are presented in Table 1. Mt. Sinai patients were all older than 50, in line with the goal of studying the course of cognitive functioning in older patients with a variable history of institutional stay. Other demographic variables are similar other than a higher proportion of Hispanic research participants (coming from Miami) in VALERO II and SCOPE.
Table 1.
Demographic and Clinical Variables, in four Different Patient Samples with Schizophrenia
| Mt. Sinai Sample | Valero I | Valero II | SCOPE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n=233 | n=195 | n=214 | n=179 | ||||||||
| Characteristic | n | % | n | % | n | % | n | % | x2 | p | |
| Male | 161 | 70 | 134 | 69 | 139 | 65 | 107 | 60 | 1.36 | 0.51 | |
| Race | |||||||||||
| Caucasian | 133 | 57 | 106 | 54 | 117 | 55 | 102 | 57 | 2.02 | 0.36 | |
| African American | 89 | 38 | 74 | 38 | 77 | 36 | 72 | 40 | 0.89 | 0.64 | |
| Other or more than 1 | 11 | 5 | 14 | 8 | 20 | 9 | 5 | 3 | 5 | 0.08 | |
| Hispanic Ethnicity | 23 | 10 | 23 | 12 | 50 | 23 | 43 | 24 | 19.32 | <0.001 | |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | df | p | |
| Age (Years) | 58.4 | 7.3 | 44.3 | 11.7 | 41.0 | 12.4 | 38.54 | 13.45 | 158.04 | 2 | <0.001 |
| Education | 12.5 | 2.4 | 12.8 | 2.7 | 12.3 | 2.2 | 12.4 | 2.4 | 2.86 | 2 | 0.115 |
Note. Mt. Sinai Patients are from New York; Valero I patients are from Atlanta and San Diego; Valero II patients are from Atlanta, San Diego, and Miami; SCOPE patients are from Miami and Dallas.
Scores for all real-world functioning variables and their predictors are presented in Table 2. All of the between groups differences, calculated with one-way ANOVAs, were statistically significant. However, many of the group differences were minimal, and significant only because of the substantial sample sizes. The Mt. Sinai patients were less impaired than the other samples on social functioning and also had less severe depression and negative symptoms. Performance-based variables were remarkably similar across the samples. The cognition t scores differed by less than 2 points (0.2 SD) across the samples and the UPSA-B mean scores were within two points across the samples, despite statistically significant differences between the groups. Thus, the clinical, performance-based, and functional outcomes variables were minimally variable across the three samples. Pearson correlations between all predictor variables are presented in Table 3.
Table 2.
Scores on Everyday Outcomes and Predictor Variables In Four Samples of People with Schizophrenia
| Mt. Sinai Sample | VALERO I | VALERO II | SCOPE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n=233 | n=195 | n=214 | n=179 | |||||||
| Variable | ||||||||||
| M | SD | M | SD | M | SD | M | SD | F | p | |
| Everyday Functioning | ||||||||||
| SLOF Interpersonal Functions | 29.78 | 5.37 | 24.84 | 6.31 | 22.45 | 5.97 | 23.03 | 6.23 | 72.02 | .001 |
| SLOF Activities Subscale | 49.06 | 8.54 | 48.35 | 8.61 | 44.40 | 10.66 | 47.52 | 8.55 | 34.34 | .001 |
| SLOF Vocational Subscale | 24.23 | 5.64 | 23.92 | 4.67 | 20.06 | 5.29 | 24.92 | 4.34 | 30.53 | .001 |
| Predictor Variables | ||||||||||
| Cognition Composite Score | 36.02 | 9.83 | 37.90 | 9.94 | 37.41 | 8.71 | 37.36 | 8.08 | 5.27 | .005 |
| UPSA-B Score | 69.03 | 19.64 | 70.66 | 13.14 | 70.57 | 14.98 | 70.08 | 13.97 | 15.54 | .001 |
| Negative Symptoms | 13.16 | 5.65 | 15.35 | 6.09 | 15.61 | 6.64 | 14.36 | 5.32 | 28.86 | .001 |
| BDI-II | 11.90 | 10.07 | 15.80 | 12.02 | 15.33 | 11.68 | 16.83 | 12.18 | 56.24 | .001 |
Note. Mt. Sinai Patients are from New York; Valero I patients are from Atlanta and San Diego; Valero II patients are from Atlanta, San Diego, and Miami; SCOPE patients are from Miami and Dallas.
Table 3.
Correlations Between Real World Functioning Variables and Predictor Variables
| Mt. Sinai Sample | VALERO I | VALERO II | SCOPE | |||||
|---|---|---|---|---|---|---|---|---|
| n=233 | n=195 | n=214 | n=179 | |||||
| Everyday Functioning Variable | ||||||||
| r | p | r | p | r | p | r | p | |
| SLOF Interpersonal Functions | ||||||||
| Negative Symptoms | −.42 | .001 | −.41 | .001 | −.39 | .001 | −.38 | .001 |
| UPSA-B | −.10 | .15 | .13 | .07 | .16 | .06 | .19 | .05 |
| Cognition Composite | .10 | .15 | .13 | .07 | .16 | .06 | .15 | .06 |
| BDI total Scores | − .14 | .08 | − .34 | .001 | .00 | .99 | −.18 | .05 |
| SLOF Activities Subscale | ||||||||
| Negative Symptoms | −.10 | .15 | −.11 | .16 | −.08 | .27 | −.09 | .20 |
| UPSA-B | .42 | .001 | .36 | .001 | .39 | .001 | .29 | .001 |
| Cognition Composite | .30 | .001 | .32 | .001 | .29 | .001 | .26 | .001 |
| BDI total Scores | .06 | .46 | .25 | .002 | .04 | .83 | −.07 | .36 |
| SLOF Vocational Subscale | ||||||||
| Negative Symptoms | −.08 | .27 | −.12 | .11 | −.10 | .15 | −.11 | .16 |
| UPSA-B | .30 | .001 | .32 | .001 | .28 | .001 | .21 | .003 |
| Cognition Composite | .20 | .005 | .26 | .002 | .23 | .005 | .28 | .001 |
| BDI total Scores | .10 | .15 | .23 | .004 | .07 | .48 | −.04 | .57 |
Note. Mt. Sinai Patients are from New York; Valero I patients are from Atlanta and San Diego; Valero II patients are from Atlanta, San Diego, and Miami; SCOPE patients are from Miami and Dallas.
As a next step, we examined the structure of the cognitive performance variables, with the aim to find out whether they defined a single latent trait and exhibited measurement invariance (a necessary condition for measuring the same trait in the groups: Millsap, 2012). The single-trait cognitive hypothesis was found plausible with partial measurement invariance. Specifically, within the four groups all 5 factor loadings were constrained for equality as were all intercepts except those of trail making part A and multi-trial list learning. The corresponding 4-group single-trait cognitive model was associated with tenable fit: chi-square (χ2) = 69.198, degrees of freedom (df) = 34, RMSEA = .071 with 90%-confidence interval (.047, .095), and CFI = .957. We concluded that the dimensional structure of our cognitive tests were consistent with a single-latent trait associated with essentially the same measurement structure in all 4 groups, despite the fact that different tests were used in the Mt. Sinai sample.
Following these analyses, we examined the latent structure of the negative symptoms measures. For these measures, we did not find a version of the single-factor model associated with tenable fit. Exploratory factor analyses suggested a lack of unidimensionality of these tests in each of the 4 groups, with a consistent finding across the groups of a common trait indexed by the symptoms passive-apathetic social withdrawal and active social avoidance with no other viable factor structure involving the other 4 items. In order to ensure that there were no relationships with other negative symptoms and any of the outcomes measures, we performed three stepwise regression analyses in the entire patient sample, predicting all three of the outcomes variables with the 7 negative symptoms. For everyday activities, no negative symptom measure entered the equation, F(1,818)=1.66, p=.06. For vocational outcomes, only active social avoidance was a significant predictor in the regression, F(1,818)=12.99, p<.001. Finally, for interpersonal functioning, passive-apathetic social withdrawal and active social avoidance both entered the equation, F(2, 817)=91.39, <.001. For these reasons, we included this pair of variables in our confirmatory factor analyses as joint indicators of a negative symptoms latent trait.
We next tested the model in Figure 1a. Note that the indicators of real-world community functioning are hypothesized to be uncorrelated with each other and that cognitive performance is specified as a fully mediated predictor of real-world community outcomes. This overall 4-sample model was found to be associated with tenable fit: χ2 = 318.3 df = 180, RMSEA = .061, CFI = .944. The variance accounted for in interpersonal functioning was 23%, while the variance accounted for in everyday activities was 28%, and variance accounted for in work functioning was 19%. This model was found to be tenable in each of the four samples, as can be seen from the fit indexes presented in Table 4. Thus, the hypothesis-driven model was found to fit the data in the entire sample and in each subsample.
Table 4.
Chi-square, degrees of freedom, RMSEA, and CFI for the hypothesis driven model in the 4 groups
| Group | χ2 | df | RMSEA | 90%-CI | CFI |
|---|---|---|---|---|---|
| Mt. Sinai | 70.884 | 39 | .059 | (.037, .081) | .957 |
| VALERO 1 | 62.755 | 39 | .056 | (.028, .081) | .958 |
| VALERO 2 | 54.153 | 39 | .043 | (0, .068) | .977 |
| SCOPE | 71.196 | 39 | .068 | (.042, .093) | .933 |
Note. 90%-CI = 90%-confidence interval (associated with the RMSEA).
Note. Mt. Sinai Patients are from New York; Valero I patients are from Atlanta and San Diego; Valero II patients are from Atlanta, San Diego, and Miami; SCOPE patients are from Miami and Dallas.
We then fit the generic model presented in Figure 1b. This model fit the data slightly less well than the hypothesis-driven model: χ2 = 355.6, df=192, RMSEA = .064, CFI = .91. Variance accounted for in interpersonal functioning was essentially identical at 23%, with variance accounted for in everyday activities at 27%, and work functioning at 18%. Thus, the more complex model appears to fit the data slightly less well and accounts for the same amount of variance in the outcomes variables as the model that hypothesizes more specific patterns of correlational influence.
Using the sequential chi-square subtraction procedure, the difference in model fit was statistically significant: χ2 =37.3 df=12 (Difference), p=.0002, indicating that the hypothesis-driven model was confirmed to be a significantly better fit to the data than the generic model. These sequential chi-square subtraction analyses were performed in each of the subsamples. In every subsample, the hypothesis driven model was a significantly better fit than the generic model, all χ2 (3) > 87.35, all p<.05, suggesting that in the sample as a whole and in each subsample, the hypothesis-driven model was a better fit to the data. Note that the generic model, if tested alone, would have been viewed as a reasonable fit to the data in these four samples (CFI>0.9; RMSEA<.07).
4. Discussion
Analyses of data from four different samples of people with schizophrenia yielded results that may inform attempts to reduce disability in schizophrenia. An overall model was found that fit the data and did not differ in its fit across the four separately collected samples. This model which specified that negative symptoms were a predictor of the severity of social deficits in the overall sample and in each subsample, but did not contribute to predicting real-world performance of everyday activities and vocational outcomes, and was a significantly better fit than a model that suggested that negative symptoms predicted all aspects of functional outcome. Similarly, cognition and functional capacity were predictors of the severity of deficits in performing everyday activities and vocational outcomes, but would not predict social functioning. Negative symptoms that measure social motivation and engagement were related to social outcomes, with other no other negative symptoms correlating with any aspect of real world outcomes. Further, no negative symptoms manifested any significant correlations with either everyday activities or vocational outcomes in zero-order correlational analyses.
The best fitting model also specified that functional capacity exerted its influences on the performance of everyday activities and vocational outcomes in a direct manner with cognitive deficits exerting their influence entirely through their correlation with functional capacity. The hypothesis driven model fit the data significantly better than a generic prediction model in in the sample as a whole and in each subsample. However, the generic prediction model, with negative symptoms, depression, and cognition/functional capacity predicting each element of everyday functioning, would have been viewed as an adequate model on its own. Thus, these data should not be interpreted to suggest that negative symptoms are completely unrelated to other aspects of outcome, but in our large database, the only real-world outcomes that correlated with any of the measured negative symptoms was SLOF interpersonal functioning.
The present research methods have several strengths. Individuals who were not aware of the results of performance-based assessments and clinical symptoms rated all the real-world outcomes. Thus, the resulting correlations are not in any way due to rater overlap. Further, the consistent methods across the studies, with quite different characteristics of the samples of subjects (Mt. Sinai Sample: all over 50; Miami Samples from VALERO 2 and SCOPE: Substantial numbers of Hispanic participants), suggest that the results are generalizable to patients with schizophrenia across a variety of demographic sites and treatment settings. These results may not apply to inpatients with a more adverse course of illness; however, these patients are current rare, typically quite old, or hospitalized for forensic reasons. We found a cognitive performance latent trait that was similar across all 4 samples, even though slightly different cognitive tests were used in one of the studies. This latent trait is based on a small set of cognitive tests and larger cognitive assessment batteries might lead to more complex factor structures.
There are some limitations of these data and these analyses. All patients had to be able to participate in an extensive in person assessment. This may have eliminated some patients with lower levels of functioning. All patients were selected for having a high contact clinician. More extensive cognitive assessments may have led to a more complex factor structure. There was no attempt to make the individual samples representative in terms of everyday functional outcomes; this concern is obviated by the results of studies of milestone achievements in both the Mt. Sinai Sample (Leung et al., 2008), and Valero 1 sample (Harvey et al, 2011; Gould et al., 2013) which were quite consistent with normative expectations based on multiple earlier studies. Depression was rated with self-report, although the correlation with the PANSS depression item in the entire sample was quite substantial, r=.64, suggesting that clinical ratings would not have led to different results. Meta-analyses have suggested that neurocognitive deficits are less strongly related to social deficits, when compared to the influence of social cognition (Fett et al., 2011). We did not address social cognition measures because we did not measure social cognition in all of the four samples analyzed, cautioning that this dimension (social cognition) might have been somewhat related to the negative symptoms’ relationship with interpersonal functioning.
The item content of the negative symptoms and SLOF interpersonal subscale used to assess interpersonal skills, and the PANSS negative symptom construct (i.e., initiating and maintaining social contacts vs. active social avoidance, blunted affect) has overlap in item content, which could clearly affect the strength of the association despite being rated by different sources (i.e., clinical raters and other informants). This overlap cannot explain why ratings of interpersonal functioning on the SLOF are not correlated with cognition and functional capacity in any of the four samples and why cognition and functional capacity so consistently correlate with SLOF everyday activities and vocational outcomes. The model suggesting that all of the predictor variables (cognition, functional capacity, depression, and negative symptoms) are associated with all aspects of outcome (social, vocational, and everyday activities) fits the data well enough such that if we had not tested our hypothesis-driven model we could still have concluded that this model was a good fit to the data.
There are some recent data to which these results could be compared. Galderisi et al. (2014) recently performed a multi-site study of real-world community outcomes in a large (N>800) sample of Italian outpatients with schizophrenia. They found, similar to our findings, that depression was not a major predictor of outcomes and that the influence of cognition on everyday outcomes was generally mediated by the influences of functional capacity. A critical difference between their study and ours is that they treated everyday outcomes as a single latent trait, determined by scores on the same three SLOF subscales that we examined in this study. As a result, their findings need to considered in the contest that some studies have suggested that the three domains of the SLOF are not highly intercorrelated and that the everyday functional milestones underlying those ratings can be unrelated to each other (Harvey et al., 2012).
Treatment implications based on these results may be substantial and include proper matching of treatments with outcomes targeted. Negative symptoms predicted social functioning and cognitive deficits did not in the best fitting model. Thus, cognitive rehabilitation treatments might not be expected to improve social functioning if negative symptoms are prominent or not included in the treatment protocol. This finding is consistent with the lack of symptomatic benefits, including negative symptoms, found in previous meta-analytic studies of cognitive remediation (Wykes et al., 2011). The inverse may be true for vocational functioning and the performance of everyday activities. Reductions of negative symptoms might not have an immediate beneficial impact on these two everyday functional domains. The differential relationships between negative and cognitive symptoms, and the outcome domains examined may warrant further research into combining various available treatment approaches targeting cognitive and negative symptoms more efficiently, perhaps achieving synergistic benefits.
Supplementary Material
Supplemental Table 1 Cognitive and Functional Capacity Measures used in four Different Patient Samples with Schizophrenia: Relationships to MATRICS Cognitive Domains
Acknowledgments
All individuals who contributed to this paper are listed as authors. No professional medical writer was involved in any portion of the preparation of the manuscripts. Data were collected by paid research assistants who did not contribute to the scientific work in this paper.
This research was supported by Grants MH 63116 and MH078775 to Dr. Harvey, MH 93432 to Drs. Harvey, Penn, and Pinkham, and MH078737 to Dr. Patterson from the National Institute of Mental Health. Supplemental funding was provided by Genentech.
Role of Funding Source.
This research was funded by the National Institute of Mental Health, who provided no input into the analyses and presentation of these data. Genentech Pharma provided additional funding used to compile the data into a single database, although the funding source provided no input into the writing of the paper.
Footnotes
Disclosure:
Dr. Strassnig has received consulting fees from Janssen Pharmaceuticals.
Dr. O’Gorman was a full time employee of Genentech.
Dr. Harvey has received consulting fees from Boehringer Ingelheim, Forum Pharma, Genentech, Otsuka America, Roche Pharma, Sanofi Pharma, Sunovion Pharma, and Takeda Pharma during the past year.
Dr. Bowie has received consulting fees from Abbvie, Lundbeck, and Takeda.
Dr. Durand has received consulting fees for Teva Pharmaceutical during the past year.
None of the other authors have any commercial interests to report.
Contributions of the Authors.
Drs. Bowie, Harvey, Patterson, Penn, and Pinkham designed the overall studies and obtained funding. Drs. Strassnig and O’Gorman conceptualized the current analyses and wrote the first draft of the paper. Dr. Raykov designed and performed the specific statistical analyses. Dr. Harvey provided scientific oversight throughout the project and edited the manuscript. All authors provided detailed comments to the paper across several drafts of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Flaum M, Arndt S. The comprehensive assessment of symptoms and history (CASH): An instrument for assessing psychopathology and diagnosis. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual. 2. San Antonio, Tex: Psychological Corp; 1996. Beck Depression Inventory. [Google Scholar]
- Bollen KA. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63 (5):505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, McGurk SM, Mausbach BT, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for Schizophrenia: Effects on Cognition, Functional Competence, and Real-world behavior. Am J Psychiatry. 2012;169:710–718. doi: 10.1176/appi.ajp.2012.11091337. [DOI] [PubMed] [Google Scholar]
- Breier A, Schreiber JL, Dyer J, Pickar D. National Institute of Mental Health longitudinal study of chronic schizophrenia. Prognosis and predictors of outcome. Arch Gen Psychiatry. 1991;48:239–246. doi: 10.1001/archpsyc.1991.01810270051007. [DOI] [PubMed] [Google Scholar]
- Carone BJ, Harrow M, Westermeyer JF. Posthospital course and outcome in schizophrenia. Arch Gen Psychiatry. 1991;48:247–253. doi: 10.1001/archpsyc.1991.01810270059008. [DOI] [PubMed] [Google Scholar]
- Couture SM, Granholm EL, Fish SC. A Path Model Investigation of Neurocognition, Theory of Mind, Social Competence, Negative Symptoms and Real-World Functioning in Schizophrenia. Schizophr Res. 2011;125:152–160. doi: 10.1016/j.schres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Strassnig M, Sabbag S, Twamley EW, Patterson TL, Harvey PD. Factors Influencing Self-Assessment of Cognition and Functioning in Schizophrenia: Implications For Treatment Studies. Eur Neuropsychopharmacol. 2015;25:185–191. doi: 10.1016/j.euroneuro.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- First B, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gould F, Sabbag S, Durand D, Patterson TL, Harvey PD. Self-assessment of functional ability in schizophrenia: milestone achievement and its relationship to accuracy of self-evaluation. Psychiatry Res. 2013;207 (1–2):19–24. doi: 10.1016/j.psychres.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophr Bull. 2006;32 :250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Helldin L, Bowie CR, Heaton RK, Olsson AK, Hjärthag F, Norlander T, Patterson TL. Performance-based measurement of functional disability in schizophrenia: a cross-national study in the United States and Sweden. Am J Psychiatry. 2009;166:821–827. doi: 10.1176/appi.ajp.2009.09010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Bellack AS. Toward a terminology for functional recovery in schizophrenia: is functional remission a viable concept? Schizophr Bull. 2009;35 (2):300–306. doi: 10.1093/schbul/sbn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Raykov T, Twamley EW, Vella L, Heaton RK, Patterson TL. Validating the measurement of real-world functional outcome: Phase I results of the VALERO study. Am J Psychiatry. 2011;168:1195–2001. doi: 10.1176/appi.ajp.2011.10121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Sabbag S, Prestia D, Durand D, Twamley EW, Patterson TL. Functional milestones and clinician ratings of everyday functioning in people with schizophrenia: overlap between milestones and specificity of ratings. J Psychiatr Res. 2012;46(12):1546–1552. doi: 10.1016/j.jpsychires.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty JD, Baldessarini RJ, Tohen M, Waternaux C, Oepen G. One hundred years of schizophrenia: a meta-analysis of the outcome literature. Am J Psychiatry. 1994;151 (10):1409–1416. doi: 10.1176/ajp.151.10.1409. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cut-off criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equat Mod. 1999;6:55. [Google Scholar]
- Kay SR. Positive and negative syndromes in schizophrenia: Assessment and research. Brunner/Mazel; New York: 1991. [Google Scholar]
- Keefe RSE, Bilder RM, Harvey PD, et al. Baseline Neurocognitive Deficits in the CATIE Schizophrenia Trial. Neuropsychopharmacology. 2006;31:2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- Leifker FR, Bowie CR, Harvey PD. Determinants of everyday outcomes in schizophrenia: the influences of cognitive impairment, functional capacity, and symptoms. Schizophr Res. 2009;115(1):82–87. doi: 10.1016/j.schres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Leung WW, Bowie CR, Harvey PD. Functional implications of neuropsychological normality and symptom remission in older outpatients diagnosed with schizophrenia: A cross-sectional study. J Int Neuropsychol Soc. 2008;14 (3):479–488. doi: 10.1017/S1355617708080600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-H, Huang C-H, Chang YC, et al. Clinical Symptoms, mainly negative symptoms medicate the influence of neurocognition and social cognition on functional outcome in schizophrenia. Schizophr Res. 2013;146:231–237. doi: 10.1016/j.schres.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York: Wiley & Sons; 2002. [Google Scholar]
- Meyer EC, Carrion RE, Cornblatt BA, et al. The Relationship of Neurocognition and Negative Symptoms to Social and Role Functioning Over Time in Individuals at Clincal High Risk in the First Phase of the North American Prodrome Longitudinal Study. Schizophr Bull. 2013;40(6):1452–1461. doi: 10.1093/schbul/sbt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millsap RE. Statistical analysis of measurement invariance. New York: Taylor & Francis; 2012. [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27:235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Green MF, Buck B, Healey K, Harvey PD. The Social Cognition Psychometric Evaluation study: results of the expert survey and RAND panel. Schizophr Bull. 2014;40 (4):813–823. doi: 10.1093/schbul/sbt081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raykov T, Marcuolides GA. On Multilevel Model Reliability Estimation from the Perspective of Structural Equation Modeling. Struct Equat Mod. 2006;13:130–141. [Google Scholar]
- Raykov T, Marcoulides GA. An introduction to applied multivariate analysis. New York: Taylor & Francis. London; 2008. [Google Scholar]
- Robertson BR, Prestia D, Twamley EW, Patterson TL, Bowie RC, Harvey PD. Social competence versus negative symptoms as predictors of real world social functioning in schizophrenia. Schizophr Res. 2014;160:136–141. doi: 10.1016/j.schres.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheck R, Leslie D, Keefe R, McEvoy J, Swartz M, Perkins D, Stroup S, Hsiao JK, Lieberman J CATIE Study Investigators Group. Barriers to employment for people with schizophrenia. Am J Psychiatry. 2006;163 (3):411–417. doi: 10.1176/appi.ajp.163.3.411. [DOI] [PubMed] [Google Scholar]
- Sabbag S, Twamley EW, Vella L, Heaton RK, Patterson TL, Harvey PD. Predictors of the accuracy of self-assessment of everyday functioning in people with schizophrenia. Schizophr Res. 2012;137(1–3):190–195. doi: 10.1016/j.schres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LC, Struening EL. SLOF: a behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr. 1983;19:9–21. doi: 10.1093/swra/19.3.9. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Ventura J, Hellemann GS, Thames AD. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia. A meta-analysis Schizophr Res. 2009;113(2–3):189–199. doi: 10.1016/j.schres.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Subotnik KL, Gitlin MJ, et al. Negative symptoms and functioning during the first year after a recent onset of schizophrenia and 8 years later. Schizophr Res. 2015;161:407–411. doi: 10.1016/j.schres.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Wide-Range Achievement Test 3: Administration Manual. Wilmington, Del: Wide Range; 1993. [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Cognitive and Functional Capacity Measures used in four Different Patient Samples with Schizophrenia: Relationships to MATRICS Cognitive Domains