Abstract
Patients with Chronic Lymphocytic Leukemia (CLL) are known to have an increased incidence of second cancers, but the contribution of commonly used frontline therapies to the incidence of second cancers is unclear. We report on the characteristics, incidence, outcomes and factors associated with second cancers in 234 patients receiving Fludarabine, Cyclophosphamide, and Rituximab (FCR) based regimens in the frontline setting. The risk of second cancers was 2.38 times higher than the expected risk in the general population. Ninety three patients (40%) had other cancers before and 66 patients (28%) after FCR. The rates of t-AML/MDS (5.1%) and Richter’s transformation (RT) (9%) were high while solid tumors were not increased. Overall survival of patients with second cancers after frontline FCR was shorter (median of 4.5 years) compared to patients with and without prior cancers. Second cancer risk after frontline FCR is mainly due to high rates of t-AML/MDS and RT and as speculated the survival of affected patients is shorter.
Introduction
Increased risk of second cancers in patients with CLL was reported in some studies.[1-4] The risk of skin cancers is 8 fold higher and the risk of other malignancies is twofold higher than in the general population. Mechanisms which may explain increased risk of second cancers in CLL are largely unclear. Tsimberidou et al. reported on the frequencies, outcomes and risk factors for development of second cancers in CLL from MD Anderson cancer center (MDACC). Second cancers in CLL may be due to patient specific risk factors, pro-tumorigenic microenvironment in patients with CLL or due to chemotherapy related factors, such as immunosuppression. Some groups have also reported that the secondary neoplasms may occur with equal frequency whether they occurred concurrently or after the diagnosis of CLL.[5] Immunodeficiency in CLL may also predispose to the increased risk of other malignancies.[6] In addition, Monoclonal B cell lymphocytosis (MBL) a precursor state to CLL, is reportedly associated with increased risk of non-hematological malignancies.[7] Chemoimmunotherapy with FCR is the most commonly used frontline treatment modality in physically fit patients with CLL.[8,9] Development of second cancers after chemoimmunotherapy in lymphomas is reported in few studies.[10-12] It is particularly relevant to study the development of second cancers among patients with CLL as they achieve longer progression free survival (PFS) and overall survival (OS) after therapy with immunosuppressive fludarabine based regimens. Studies with alkylating agents did not demonstrate a clear evidence for higher risk of second cancers compared to untreated patients with CLL.[4,13] Cyclophosphamide is known to have weaker carcinogenicity compared to other alkylating agents however high dose cyclophosphamide based therapies increases the risk of second cancers in patients with CLL and Non-Hodgkin’s lymphoma.[14,15] Skin cancers which developed after therapy with fludarabine in patients with CLL were reported to be more aggressive, with higher growth rate, recurrence and distant metastasis.[16] Cheson et al compared the number of observed to the expected second cancers in 724 relapsed refractory patients with CLL who were treated with fludarabine for a median follow up of 7.4 years.[17] The risk of second cancers in patients with CLL was higher than in healthy individuals, however, this increased risk was consistent with rates expected in patients with CLL.[3,17] The addition of rituximab to fludarabine and cyclophosphamide (FC) increases immunosuppression.[8] Whether the FCR based regimens further increase the risk of second cancers is still unclear. Present study aims to evaluate the characteristics, incidence and outcomes of second cancers in patients with CLL before and after receiving FCR based regimens in the frontline setting. We compared CLL patients with and without cancers prior to FCR therapy with patients that developed second cancers after FCR. We have also compared the risk of other malignancies with the expected risk among healthy individuals.
Patients and methods
This is a retrospective analysis of patients with CLL who have received FCR based therapies in the front line setting, between January 2004 and March 2012 at MDACC. Diagnosis of CLL was made according to the 2008 IWCLL (International working group on CLL) criteria.[18] All patients signed informed consent as per the declaration of Helsinki and charts were reviewed after approval from MDACC Institutional Review Board. Patient charts were reviewed for clinical features, treatments outcomes, history of prior cancers before FCR treatment and development of second cancers after the completion of FCR regimen.[19]
FCR based regimens
Treatment regimens included in the analysis were standard FCR (FCR, n=131); FCR with granulocyte-macrophage colony-stimulating factor (FCR GM-CSF, n=13); FCR with thrice higher rituximab per cycle (FCR3, n=63); FCR with mitoxantrone (FCMR, n=3); FCR with alemtuzumab (CFAR, n=24). Details of treatment protocols have been reported previously.[20-23] Of note, the patients included in frontline CFAR regimen were high risk patients with β2 microglobulin ≥ 4.0 and del17p 6/24 (22%). Following the completion of the therapy, all patients were periodically followed up at MDACC by clinical assessment and relevant investigations. Second cancers were diagnosed with appropriate investigations.
Statistical methods
Analysis of clinical outcomes
Response criteria used were as defined by the National Cancer Institute (NCI) - Working Group.[24] Overall survival (OS) was determined from the time of starting therapy with FCR based regimens until death from any cause or last follow up available from patients records. PFS was measured from the time of starting treatment to the time of disease progression, death or last follow-up. The distribution of each continuous variable was summarized by its mean, standard deviation and range. The distribution of each categorical variable was summarized in terms of its frequencies and percentages. Continuous variables were compared between risk groups by Wilcoxon rank sum tests and categorical variables were compared by Fisher Exact tests. Kaplan-Meier curves were used to estimate unadjusted overall survival and progression free survival. Log rank test was used to compare time to event outcomes between each group. The logistic regression model was applied to evaluate the ability of the covariates to predict the presence or absence of second cancers. The multivariate logistic regression model was finalized by including the variables that showed a significant association (P≤0.01) with the second cancer status in the univariate model. All cancers were considered for the analysis, except in the comparisons with SEER database. Comparison with SEER data base excluded non-melanoma skin cancers.
Cancer and mortality risk analysis
To determine the number of patients who developed second cancers after FCR based regimens, we computed standardized incidence ratios (SIRs). This is a ratio of the number of patients who developed subsequent invasive cancers (excluding non-melanoma skin cancers; NMSC) in our population (O = observed) compared with the number of cases expected (E = expected) to occur if the US population rates were applied to the same cohort. The latter number was determined with age, sex, and calendar year-specific incidence from the SEER data applied to the relative person-years at risk from our population. To calculate the SIR (O/E) we used cohort analysis for Epidemiology and End Result program. For SIRs, person years at risk were calculated from the date of starting FCR based therapy to the date of diagnosis of second cancer, death, or date of last contact, whichever came first. The 95% confidence intervals (CIs) and P values for the SIRs were determined by assuming a Poisson distribution for the observed number of patients with subsequent cancers. A two sided test was used to test the equality of the O/E number of patients with cancer.
Results
Patient characteristics
Initial clinical characteristics of 234 patients who were treated with FCR based regimens are shown in Table 1.
Table 1.
Initial characteristics of 234 patients prior to receiving FCR treatment
| Variable | No of patients |
Total patients | % |
|---|---|---|---|
| Age ≥ 60 years | 127 | 234 | 54 |
| Male sex | 171 | 234 | 73 |
| Smoking history | 81 | 234 | 35 |
| ALC≥100 ×109/L | 92 | 232 | 40 |
| LDH≥1 X normal | 110 | 230 | 48 |
| β2-microglobulin ≥3.5g/dL | 133 | 230 | 58 |
| RAI stage III/IV | 71 | 234 | 30 |
| Cytogenetics | |||
| Normal | 139 | 219 | 64 |
| Abnormal | 80 | 219 | 36 |
| FISH | |||
| 17p deletion | 24 | 215 | 10 |
| 11q deletion | 49 | 215 | 23 |
| Trisomy 12 | 34 | 215 | 16 |
| Negative | 42 | 215 | 19 |
| 13q deletion | 64 | 215 | 30 |
| IGHV unmutated | 116 | 181 | 64 |
| Zap70-positive * | 112 | 180 | 62 |
| CD38-positive | 91 | 234 | 39 |
| Previous cancers | 93 | 234 | 40 |
| Previous chemotherapy / radiotherapy | 15 | 93 | 16 |
| Type of frontline chemotherapy | |||
| FCR ** | 207 | 234 | 88 |
| CFAR | 25 | 234 | 11 |
| FCMR | 3 | 234 | 1 |
| Number of FCR cycles | |||
| 1-3 | 38 | 232 | 16 |
| 4-6 | 194 | 232 | 84 |
Zap-70 in BM by immunohistochemistry (IHC);
FCR based therapy includes: FCR, FCR3, FCR-GM;
Abbreviations: LDH, Lactate dehydrogenase; ALC, absolute lymphocyte count; IGHV, Immunoglobulin variable heavy chain; FISH, Fluorescence in situ hybridization; ZAP-70, Zeta-chain associated protein kinase 70; CFAR: Cyclophosphamide, Fludarabine, Alemtuzumab, Rituximab; FCMR: Fludarabine, Cyclophosphamide, Mitoxantrone, Rituximab
Second Cancers
Out of the 234 patients with CLL included in this study, 159 (68%) of the patients had other cancers either prior to or after receiving front line FCR based therapies (n=93; 40% and n=66; 28% respectively).
Other cancers prior to FCR based therapies
Ninety three (40%) patients had a history of other cancer prior to treatment with FCR based regimens. Among them 34 patients (14%) had non-melanoma skin cancers (NMSC), 14 (6%) melanoma, 17 (7%) prostate cancer, 8 (3%) breast cancer, 5 (2%) liver and gastrointestinal tract, 4 patients (2%) Renal cell cancer and all other cancers at ≤ 1%. Six patients (3%) had more than one cancer type before they started treatment. Fifteen out of 93 patients (16%) with prior history of other cancers have received radiotherapy or chemotherapy or both for treatment of other cancers. Three patients received platinum compounds (cisplatin, n=1, carboplatin, n=2), one patient received alkylating agent (cyclophosphamide) and anthracycline (adriamycin) as a part of CHOP protocol and one patient received topoisomerase inhibitor (etoposide). None of these patients received purine analogues. Patients with other cancers prior to treatment were older as compared to patients without a history of prior cancers (p=0.0007). These patients received fewer cycles of chemotherapy than patients without history of prior cancers (p=0.006).
Second cancers after FCR based therapy
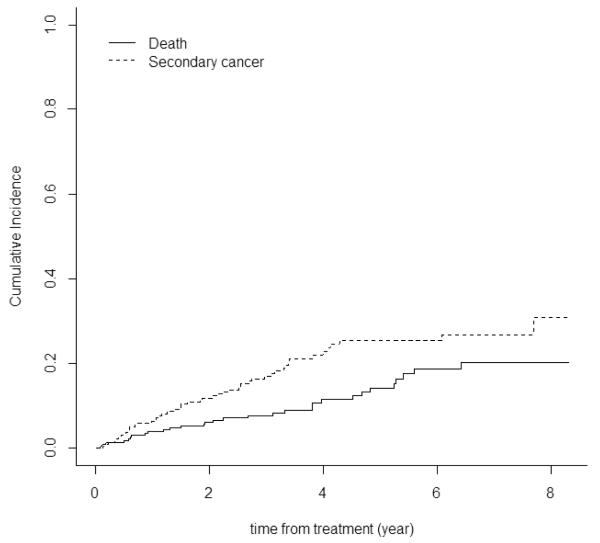
The median follow up of the patients after frontline FCR based therapies was 4.4 years (95% CI, 3.7 to 5.0). After excluding 22 patients (9%) who developed non-melanoma skin cancers 14 (5.9%) developed solid tumors, 12 (5%) hematological malignancies and 22 (9%) Richter’s transformation (RT). The distribution of cancers included MDS / AML 12 (5.1%), Lung cancer 3 (5%), prostate cancer 2 (1%), liver and gastrointestinal 4 (2%), melanoma 2 (3%) and other malignancies at lower rates. The median time to development of second cancers in the whole cohort was not reached (Figure 1). Among patients who developed second cancers, the median time to t-AML/MDS was 2.7 years (Range 1.1 to 7.8 years) compared to 1.5 years (Range 0.3 to 7.3 years) for solid tumors and 1.1 years (Range 0.1 to 6.2 years) for RT, however this difference was not statistically significant (p=0.38). Characteristics of patients who developed AML/MDS are summarized in supplementary table 1.
Figure 1. Probability of Second cancers after FCR based therapy.
Cumulative incidence of second cancers and death from initiation of treatment with FCR to last follow-up in all patients; median time was not reached. Cumulative death rate is shown for comparison.
Follow up and time to event analysis
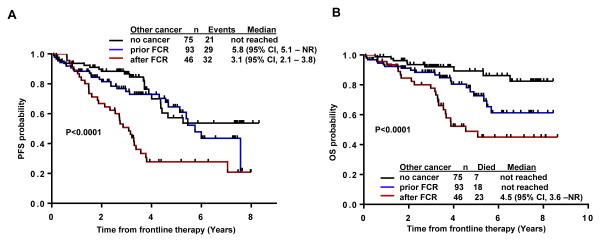
The median follow-up for all the 234 patients after FCR based therapy was 4.4 years (95% CI, 3.7 to 5.0). The estimated median PFS in the total cohort was 4.5 years (95% CI, 3.81 to 5.75) and 5.3 (95% CI, 4.4 to NR) when excluding 22 patients (9%) who developed RT after FCR. PFS in patients developing second cancers after FCR based therapies was 3.1 years (95% CI, 2.3 to 3.8) as compared to 5.8 years (95% CI: 5.1 to NR ) in patients with prior history of other cancers and NR in patients without prior history of other cancers (p<0.0001) (Figure 2A).
Figure 2. Progression free survival (PFS) and overall survival (OS) in patients after FCR with and without second cancers.
(A) PFS for patients with second cancers after FCR was shorter (3.1 years) compared to patients without second cancers (not reached) or cancer before FCR (5.8 years) (B) Overall survival from start of treatment in patients with second cancers after FCR was shorter (4.5 years) compared to patients who had cancers prior to treatment and patients without second cancer (not reached) (p < 0.0001). Patients who developed Richter’s transformation were excluded.
The median OS for the total cohort was not reached (NR), even after excluding higher risk patients treated with CFAR and FCMR (n=27). At the time of last follow up, 172 (74%) patients were alive and estimated 5 year OS rate was 73% (95% CI, 65-81) excluding the patients who developed Richter’s transformation (RT). The actuarial 5-year overall survival rate in patients who had second cancer after FCR therapy (19%; n=46) was 48% (95% CI, 35-67), 74% (95% CI, 63-88) for patients with history of prior cancers (40%; n=93) and 92% (95% CI, 86-98) for patients without history of other cancers (32%; n=75) (Figure 2B).
The median overall survival for patients who developed second cancer after FCR was 4.5 years (95% CI, 3.6 to NR) and not reached for patients with/without prior history of other cancers (Figure 2B). Seven patients (9%) died among the 75 patients without prior cancers as compared to 18/93 patients (19%) among the patients with prior cancers and 23/46 patients (50%) among those with second cancer after FCR based therapies (p <0.0001) Figure 2B.
The median survival from the time of diagnosis of RT was 7.3 months (0.1 to 4.8 years) (Figure 3). After exclusion of high risk patients treated with CFAR and FCMR (n=27) the estimated PFS after FCR based therapy was 4.85 years (95% CI, 3.81 -7.57) and OS not reached. Among the 24 patients treated with CFAR regimen, 7 patients (26%) developed RT whereas, among the 132 patients who were treated with standard FCR, 10 patients developed RT (7%).
Figure 3. Overall survival of patients who developed of Richter’ transformation (RT) after FCR based therapy.
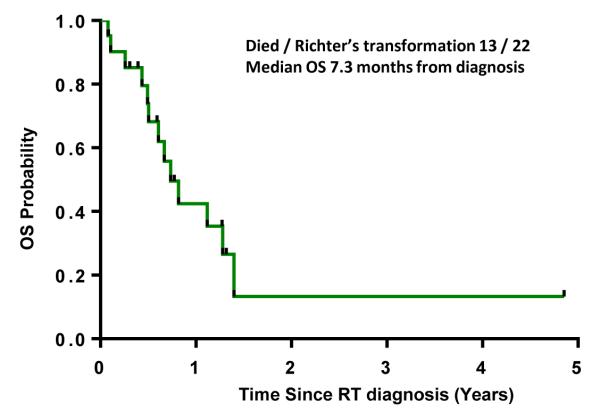
The time to transformation is calculated from RT diagnosis.
Comparison with SEER data
Second cancer developed in 66 (28%) patients after FCR based therapies. We have compared 26 patients who developed solid tumors ( 14) or AML/MDS (12) with the SEER database (Table 2) not included are patients with skin cancers and patients with Richter’s transformation. . The overall risk of developing second cancer after FCR based therapy was 2.38 times higher than the expected risk in general population, especially higher risk was observed in females and in patient age > 60 years. When compared to age and race matched general population from the SEER database, patients who develop second cancer after FCR had significantly higher observed to expected (O/E) ratio of AML and MDS - O/E- 240 (95% CI, 124.08 - 420). The risk of lung cancer 1.17(95% CI, 0.24 - 3.4), colon cancer 1.45(95% CI, 0.17 to 5.23) and prostate cancer 0.7 (95% CI, 0.08 to 2.53) were not statistically different. Two patients had more than one cancer (one had melanoma after lung cancer and one had prostate cancer after colon cancer). When patients with CFAR regimen (n=24) were excluded from the cohort, the overall O/E SIR for development of second cancers after FCR based therapies was 2.16 (95% CI, 1.34- 3.30). (data not shown)
Table 2.
Comparison of Frequency of Second Cancers in CLL Patients after Frontline FCR based therapies with SEER Data
| Variable* | Observed | Expected | O/E | 95% CI for O/E |
|---|---|---|---|---|
| Overall | 26 | 10.92 | 2.38 | 1.55 – 3.50 |
| Male | 20 | 9.03 | 2.21 | 1.35 – 3.41 |
| Female | 6 | 1.9 | 3.16 | 1.15 – 6.88 |
| Age ≥ 60 y | 19 | 7.3 | 2.60 | 1.56 – 4.06 |
| Age < 60 y | 7 | 3.6 | 1.94 | 0.78 – 4.00 |
| Second cancer type | ||||
| t-AML/MDS | 12 | 0.05 | 240 | 124.08 - 420 |
| Lung | 3 | 2.57 | 1.17 | 0.24 – 3.40 |
| Colon | 2 | 1.38 | 1.45 | 0.17 – 5.23 |
| Prostate | 2 | 2.85 | 0.70 | 0.08 – 2.53 |
Excluded Richter’s Transformation, non-melanoma skin cancers and cancer within 12 months of therapy.
Abbreviations: y,years; t-AML/MDS, therapy related acute myeloid leukemia or myelodysplastic syndrome
Correlation between Second Cancers after FCR and patients characteristics
In univariate analysis, parameters associated with development of second cancer after FCR included age > 60 years, past history of smoking, β2-microglobulin≥3.5mg/dL, LDH twice the upper limit of normal or higher, abnormal karyotype and treatment with CFAR/FCMR vs FCR (Table 3). In multivariate analysis, the factors that were independently associated with development of second cancers after FCR based therapies were - LDH higher than twice the upper limit of normal and therapy with CFAR or FCMR regimens compared to FCR, FCR3, and FCR-GM.
Table 3.
Factors predicting development of second cancers after frontline FCR chemotherapy.
| Parameters | Second cancer | Univariate analysis | Multivariate analysis* | ||
|---|---|---|---|---|---|
| Yes/all in category 66/234 (28%) |
OR | P value | OR | P value | |
| Age ≥60 yrs | 44/125 (35%) | 1.92 | 0.03 | 1.53 | 0.2 |
| <60 yrs | 24/109 (22%) | ||||
| Gender | |||||
| Male | 55/171 (32%) | 1.82 | 0.09 | ||
| Female | 13/63 (21%) | ||||
| Smoking History | |||||
| No | 38/153 (25%) | ||||
| Yes | 30/81(37%) | 1.78 | 0.05 | 1.77 | 0.08 |
| RAI stage | |||||
| 0 1,2 | 45/163 (27%) | ||||
| 3,4 | 23/71 (32%) | 1.26 | 0.46 | ||
| Bulky disease | |||||
| LN < 5cm | 28/112 (25%) | ||||
| LN≥5cm/ Splenomegaly | 40/122 (33%) | 1.46 | 0.19 | ||
| ALC | |||||
| <100,000 | 41/150 (27%) | ||||
| ≥100,000 | 27/92 (29%) | 1.0 | 0.99 | ||
| LDH | |||||
| < 2×ULN | 52/200 (26%) | ||||
| ≥ 2×ULN | 15/30 (50%) | 2.85 | 0.01 | 2.78 | 0.03 |
| β2 macroglobulin (mg/dL) | |||||
| <3.5 | 21/97 (22%) | ||||
| ≥3.5 | 47/133 (35%) | 1.98 | 0.03 | 1.42 | 0.32 |
| CD38 | |||||
| Negative | 39/143 (27%) | ||||
| Positive | 29/101 (29%) | 1.25 | 0.45 | ||
| ZAP70 | |||||
| Negative | 18/68 (26%) | ||||
| Positive | 35/112 (31%) | 1.26 | 0.5 | ||
| IGHV Status | |||||
| Mutated | 14/65 (22%) | ||||
| Unmutated | 37/116 (32%) | 1.69 | 0.14 | ||
| Cytogenetics | |||||
| Diploid | 34/139 (24%) | ||||
| Abnormal | 32/80 (40%) | 2.04 | 0.02 | 0.84 | 0.6 |
| FISH | |||||
| 13q | 15/64 (23%) | ||||
| Negative | 10/42 (24%) | ||||
| T12 | 11/34 (32%) | ||||
| 11q/ATM | 11/49 (22%) | ||||
| 17p/p53 | 13/26 (50%) | 0.08 | |||
| Protocol type | |||||
| FCR/FCR3/FCR+GM | 52/207 (25%) | ||||
| CFAR/FCMR | 16/27 (59%) | 3.56 | <0.01 | 2.78 | 0.01 |
| Number of Cycles | |||||
| 1-3 | 14/38 (37%) | ||||
| 4-6 | 14/38 (37%) | 0.66 | 0.66 | ||
Abbreviations: yrs,years;LN, lymph node; ALC, absolute lymphocyte count; 2×ULN,twice the upper limit of normal; IGHV, Immunoglobulin variable heavy chain; CFAR: Cyclophosphamide, Fludarabine, Alemtuzumab, Rituximab; FCMR: Fludarabine, Cyclophosphamide, Mitoxantrone, Rituximab; FISH, Fluorescence in situ hybridization; ZAP-70, Zeta-chain associated protein kinase 70
Discussion
Higher risk of second cancers exists in patients with CLL and second cancers are one of the causes of death in these patients.[3,25,26] Information regarding the impact of prior therapy and second cancers in CLL patients is derived mainly from patients treated with chlorambucil.[4] In one study no difference was observed between untreated and chlorambucil treated patients with CLL.[27] Data on the relationship of chemoimmunotherapy and development of second cancers in patients with CLL is sparse. Specifically, treatment of various hematological and non-hematological cancers with alkylating agents, radiation and purine analogues predispose to development of second cancers in patients with preexisting malignancy.[28,29]
In this study, we have demonstrated the occurrence of second cancers in a cohort of patients with CLL who received FCR based regimens in the frontline setting. The risk of secondary malignancies with FCR based regimens is important for the assessment of the long term safety of commonly used FCR based regimens in CLL. Our study demonstrates that CLL patients after frontline FCR based therapy have 2.38 times higher risk of second cancers than in the general population. Particularly, the incidence of AML and MDS was significantly higher after FCR based therapy with a crude rate of 5.1% during the follow up period of 4.4 years (95% CI, 3.7 to 5.0). We did not find a significantly higher rate of non-hematologic cancers. Solid tumors were found in 5.9% of patients in our cohort, however, the small number of events and short follow up time should be considered cautiously and confirmed with further studies. Preliminary result of the CLL8 of the GCLLSG reported 5.7% solid tumors combined in the FC and FCR. Comparison to the general population was not reported.[30] The risk of non-hematological malignancies is progressively increasing with no either plateau or decrease. (Figure 1A) Our analysis helps to understand the risk posed by FCR based regimens in the development of second cancers. Relatively shorter follow-up and small cohort of patients are the main limitations of this study. In addition, comparisons between FCR treated and untreated CLL patients could indicate the additive effect of FCR based therapies on the development of second cancers. Nevertheless, treated patients have more adverse disease characteristics posing other limitations for such comparison.
We also report that patients age > 60 years have a higher tendency for second cancers. This higher tendency in older patients was also observed in previous report in treated and untreated patients with CLL.[2] In our analysis, female patients had a higher rate of second cancers. Although most reports show higher rates of second cancers in male patients with CLL, excessive risk of second malignancies in female patients treated with fludarabine was reported.[17] Non-significantly higher rate myelodisplasia was reported in female patients after fludarabine based regimens.[31] One possible explanation for higher rate of second cancers in female patients could be higher myelotoxicity due to gender differences in chemoimmunotherapy pharmacodynamics. Elderly female patients were found to have slower rituximab clearance.[32] The higher rituximab blood levels and longer exposure may potentiate bone marrow toxicity. Whether female patients with CLL are more prone to develop second cancers after receiving any purine analogue needs to be confirmed in a larger cohort of patients. In the current study, patients with second cancer after FCR based therapy had shorter progression free survival compared to patients with and without prior cancers (Figure 2A). Four different types of second cancers in CLL patients after FCR based therapy deserve separate attention. These include skin cancers, secondary non-hematologic malignancies, t-AML/MDS and Richter’s transformation. Thirty two patients (14%) had skin cancer before FCR and additional 22 (9 %) had skin cancer after FCR. Skin cancers are most common second cancers in patients with CLL.[6,33] It is important to study further the association of skin cancer in patients with CLL after FCR therapy.[6,34] Patients with CLL have an increase in the incidence of skin cancers with three fold increased rate of malignant melanoma and up to 14 times higher rates of basal cell carcinomas.[13,35-37] Importantly, skin cancers may be more aggressive with higher rates of recurrence, regional metastasis and death in CLL patients as compared to skin cancers in general population.[35,36,38,39] Patients with CLL have an increased risk of death due to malignant melanoma and non-melanoma skin cancer, with standard mortality ratios 4.79 and 17.0 respectively.[40] In spite of high rate and aggressiveness of skin cancers in CLL patients, adherence to screening programs is inadequate.[41]
Previous studies did not demonstrate increased second cancer rates in treated CLL patients compared to those patients who have been observed and untreated, however the types of chemotherapy regimens varied and did not include the current standard FCR.[37]
One large retrospective study reported the increased risk for second cancers in CLL patients and included 16,367 patients from the SEER data between the years 1973 and 1996.[37] This report indicated modest but significantly elevated risk of solid tumors in CLL patients with overall O/E ratio of 1.2 (95% CI, 1.25-1.26). The risk of second cancers remained relatively constant between less than one year (O/E 1.25) and over 10 years (O/E 1.16) from the time of diagnosis of CLL. Second cancer risk for patients treated with chemotherapy was similar to untreated patients.[37] Most recent studies reported higher rates (> 2 fold) of second cancers than previously reported [2,3,25,36] One possible explanation is that higher risk of second cancers could be related to higher degree of immunosuppression caused by current therapies. In one report, an aggressive course of squamous cell carcinoma (SCC) with diffuse metastasis was noted after treatment with fludarabine.[42] The authors suggested that T cell depletion by fludarabine was responsible for the sudden transformation in the biological course and behavior of the SCC. Another study of cladribine and second cancers in patients with CLL did not demonstrate increase in risk of second malignancies in CLL except for lung cancer.[43]
The overall risk of therapy related myeloid neoplasms with a median follow up of 4.4 years after FCR based treatment was 5% with 8/127 (6.3%) patients (< 60 years) and 4/107 (3.7%) patients (≥ 60 years). The crude rates of MDS/AML were higher than previously reported for similar patients receiving alkylating agents or fludarabine monotherapy or both.[17,30,44,45] There is a steady increase of AML after chemotherapies used in the treatment of non-Hodgkin’s lymphoma in the last 3 decades.[12] However, patients receiving non-high dose cyclophosphamide based therapies (cumulative dose < 11250mg/m2) do not show a significantly elevated risk of MDS/AML.[15] Addition of fludarabine potentiates the DNA damage caused by alkylating agents and inhibit subsequent DNA repair.[46] This translates clinically in having a shorter time to develop into MDS developing after alkylator agents.[31,47,48] The CALGB 9011 trial showed that the risk of t-AML/MDS after the frontline treatment with fludarabine and chlorambucil combination was 3.5% as compared to 0.5% and 0% for fludarabine alone and chlorambucil respectively, with a median follow up of 4.2 years.[49] Higher risk of t-AML/MDS was also seen following fludarabine based combination therapy (FC, FCR, FCR with mitoxantrone) as a first line or salvage therapy in 137 patients with a crude rate of 2.5% for previously untreated (n=40) patients and 9.3% for pretreated ( n=97) patients (p=0.28).[50] On the contrary, among 210 patients treated at a single center with fludarabine and cyclophosphamide (FC) only four (1.9%) developed MDS/AML after a median follow up of 41 months.[45] Preliminary data from the largest trial on frontline FC and FCR, the CLL8, reported only 12 patients (1.5%) that developed AML/MDS, lower rate in comparison to our observation. [30] In three well controlled FCR frontline clinical trials 19 out of 426 CLL patients (4.5%) developed t-AML/MDS over a follow up period of 44 months,[51] having similar rate of t-AML/MDS as in our cohort.
Delayed cytopenias induced by chemotherapy, chemotherapy induced bone marrow damage with dysplastic changes and the effect of growth factors, can all mimic MDS.[51] Therefore overestimation of t-MDS after FCR due to conditions mimicking MDS should be considered. Relatively high rate of AML/MDS was seen in patients treated with FCR3 protocol (6 of 36 patients) containing 3 times higher dose of rituximab per cycle compare to standard FCR regimen (1 of 131 patients) (Supplementary table 1). This observation may suggest that higher doses of rituximab potentiate myelotoxicity of chemoimmunotherapy. On the contrary, compared to FC the addition of rituximab in CLL8 did not increase the rate of AML/MDS.[30]
Richter transformation (RT) i.e. development of an aggressive lymphoma, mostly diffuse large B-Cell lymphoma, is analyzed separately in the current study. In the classical RT, the malignant B cell in RT is clonally related to the original CLL clone. The remaining 20% of cases are unrelated to the CLL clone and are considered as second cancer arising in a patient with CLL.[52] The incidence of RT ranges from 2 to less than 10%.[44,53-55] The influence of prior treatment especially with purine analogues on the incidence of RT is unclear. Patients in our cohort may represent higher proportion of patients with poor risk disease due to referral from smaller centers. In some reports the incidence of RT among fludarabine treated patients was high (up to 12%).[56,57] On the contrary, an analysis of 511 patients with CLL who were randomized to initial fludarabine, chlorambucil or combined therapy on an intergroup trial (CALGB 9011), RT developed in 7% of the patients with no significant difference among treatment arms.[58] In our study 22 of 234 (9%) patients developed RT which is a higher rate of RT among other studies. Notably, patients treated with frontline CFAR (FCR+ Alemtuzumab) had higher incidence of RT (7/24, 30%) compared with patients treated with FCR regimen (10/132,7%). A possible explanation for this higher risk of RT is the pronounced immunosuppression with the addition of alemtuzumab to the FCR regimen. In addition, patients selected to participate in this trial required high β2-microglobulin (≥ 4mg/dL). This group of patients had high rates, 6/24 (25%) of TP53 gene deletion. Interestingly, post renal transplant patients under immunosuppressive therapy have increased risk for second malignancies. In this setting, alemtuzumab did not show further increased risk.[59]
In conclusion, our study indicates that the relatively high rate of second cancers after frontline FCR based therapies (SIR 2.38) is particularly contributed by t-AML/MDS and is less influenced by the rate of solid tumors. Importantly, this observation is limited by size of the cohort and duration of follow up and it requires further validation. Richter’s transformation (9%) was also relatively high compared to previous reports. The development of second cancers after FCR does affect the overall survival adversely and OS is significantly shorter in these patients as compared to patients with or without prior malignancies. Older age, female sex, are other predisposing factors for second cancers after FCR. The long term pro-tumorigenic effect of FCR based chemoimmunotherapy should be considered during the follow up of patients with CLL.
Supplementary Material
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232:267–269. [PubMed] [Google Scholar]
- 2.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–910. doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beiggi S, Johnston JB, Seftel MD, et al. Increased risk of second malignancies in chronic lymphocytic leukaemia patients as compared with follicular lymphoma patients: a Canadian population-based study. Br J Cancer. 2013 doi: 10.1038/bjc.2013.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dighiero G, Maloum K, Desablens B, et al. Chlorambucil in indolent chronic lymphocytic leukemia. French Cooperative Group on Chronic Lymphocytic Leukemia. N Engl J Med. 1998;338:1506–1514. doi: 10.1056/NEJM199805213382104. [DOI] [PubMed] [Google Scholar]
- 5.Santoro A, Rilke F, Franchi F, Monfardini S. Primary malignant neoplasms associated with chronic lymphocytic leukemia. Tumori. 1980;66:431–437. doi: 10.1177/030089168006600404. [DOI] [PubMed] [Google Scholar]
- 6.Greene MH, Hoover RN, Fraumeni JF., Jr Subsequent cancer in patients with chronic lymphocytic leukemia--a possible immunologic mechanism. J Natl Cancer Inst. 1978;61:337–340. [PubMed] [Google Scholar]
- 7.Solomon BM, Rabe KG, Moreira J, et al. Risk of Cancer in Patients with Clinical Monoclonal B-Cell Lymphocytosis (MBL): A Cohort Study of Newly Diagnosed Patients Compared to Controls. ASH Annual Meeting Abstracts. 2012;120:2893. [Google Scholar]
- 8.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 9.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarella C, Passera R, Magni M, et al. Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol. 2011;29:814–824. doi: 10.1200/JCO.2010.28.9777. [DOI] [PubMed] [Google Scholar]
- 11.Moser EC, Noordijk EM, van Leeuwen FE, et al. Risk of second cancer after treatment of aggressive non-Hodgkin’s lymphoma; an EORTC cohort study. Haematologica. 2006;91:1481–1488. [PubMed] [Google Scholar]
- 12.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121:2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travis LB, Curtis RE, Hankey BF, Fraumeni JF., Jr Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst. 1992;84:1422–1427. doi: 10.1093/jnci/84.18.1422. [DOI] [PubMed] [Google Scholar]
- 14.Travis LB, Curtis RE, Stovall M, et al. Risk of leukemia following treatment for non-Hodgkin’s lymphoma. J Natl Cancer Inst. 1994;86:1450–1457. doi: 10.1093/jnci/86.19.1450. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Wang H, Zhou S, et al. Risk of second malignant neoplasms after cyclophosphamide-based chemotherapy with or without radiotherapy for non-Hodgkin’s lymphoma. Leuk Lymphoma. 2012 doi: 10.3109/10428194.2012.743657. [DOI] [PubMed] [Google Scholar]
- 16.Rashid K, Ng R, Mastan A, Sager D, Hirschman R. Accelerated growth of skin carcinoma following fludarabine therapy for chronic lymphocytic leukemia. Leuk Lymphoma. 2005;46:1051–1055. doi: 10.1080/10428190500096989. [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD, Vena DA, Barrett J, Freidlin B. Second malignancies as a consequence of nucleoside analog therapy for chronic lymphoid leukemias. J Clin Oncol. 1999;17:2454–2460. doi: 10.1200/JCO.1999.17.8.2454. [DOI] [PubMed] [Google Scholar]
- 18.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badoux XC, Keating MJ, Wang X, et al. Cyclophosphamide, fludarabine, alemtuzumab, and rituximab as salvage therapy for heavily pretreated patients with chronic lymphocytic leukemia. Blood. 2011;118:2085–2093. doi: 10.1182/blood-2011-03-341032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strati P, Ferrajoli A, Lerner S, et al. Fludarabine, cyclophosphamide and rituximab plus granulocyte macrophage colony-stimulating factor as frontline treatment for patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2014;55:828–833. doi: 10.3109/10428194.2013.819574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parikh SA, Keating MJ, O’Brien S, et al. Frontline chemoimmunotherapy with fludarabine, cyclophosphamide, alemtuzumab, and rituximab for high-risk chronic lymphocytic leukemia. Blood. 2011;118:2062–2068. doi: 10.1182/blood-2011-01-329177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 23.Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 25.Kyasa MJ, Hazlett L, Parrish RS, Schichman SA, Zent CS. Veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have a markedly increased rate of second malignancy, which is the most common cause of death. Leuk Lymphoma. 2004;45:507–513. doi: 10.1080/10428190310001612939. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Maekawa I, Mikuni C, Yamaguchi T, Sakamaki H, Mori M. [Prognosis in 75 cases of chronic lymphocytic leukemia and second malignancies] Rinsho Ketsueki. 1997;38:740–744. [PubMed] [Google Scholar]
- 27.Callea V, Brugiatelli M, Stelitano C, Gentile M, Nobile F, Morabito F. Incidence of second neoplasia in patients with B-cell chronic lymphocytic leukemia treated with chlorambucil maintenance chemotherapy. Leuk Lymphoma. 2006;47:2314–2320. doi: 10.1080/10428190600880977. [DOI] [PubMed] [Google Scholar]
- 28.Kry SF, Salehpour M, Followill DS, et al. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:1195–1203. doi: 10.1016/j.ijrobp.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 29.Travis LB. Therapy-associated solid tumors. Acta Oncol. 2002;41:323–333. doi: 10.1080/028418602760169361. [DOI] [PubMed] [Google Scholar]
- 30.Fischer K, Bahlo J, Fink A-M, et al. Extended Follow up of the CLL8 Protocol, a Randomized Phase-III Trial of the German CLL Study Group (GCLLSG) Comparing Fludarabine and Cyclophosphamide (FC) to FC Plus Rituximab (FCR) for Previously Untreated Patients with Chronic Lymphocytic Leukemia (CLL): Results On Survival, Progression-Free Survival, Delayed Neutropenias and Secondary Malignancies Confirm Superiority of the FCR Regimen. ASH Annual Meeting Abstracts. 2012;120:435. [Google Scholar]
- 31.Niparuck P, Kanoksil W, Chuncharunee S, et al. Therapy-related myelodysplastic syndrome/acute myeloid leukemia following fludarabine therapy for non-Hodgkin lymphoma and chronic lymphocytic leukemia in Thai patients. Leuk Lymphoma. 2010;51:2120–2125. doi: 10.3109/10428194.2010.505675. [DOI] [PubMed] [Google Scholar]
- 32.Pfreundschuh M, Muller C, Zeynalova S, et al. Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood. 2014;123:640–646. doi: 10.1182/blood-2013-07-517037. [DOI] [PubMed] [Google Scholar]
- 33.Mehrany K, Weenig RH, Pittelkow MR, Roenigk RK, Otley CC. High recurrence rates of Basal cell carcinoma after mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985–988. doi: 10.1001/archderm.140.8.985. [DOI] [PubMed] [Google Scholar]
- 34.Levi F, Randimbison L, Te VC, La Vecchia C. Non-Hodgkin’s lymphomas, chronic lymphocytic leukaemias and skin cancers. Br J Cancer. 1996;74:1847–1850. doi: 10.1038/bjc.1996.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehrany K, Weenig RH, Lee KK, Pittelkow MR, Otley CC. Increased metastasis and mortality from cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. J Am Acad Dermatol. 2005;53:1067–1071. doi: 10.1016/j.jaad.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 36.Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105:1076–1081. doi: 10.1038/bjc.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hisada M, Biggar RJ, Greene MH, Fraumeni JF, Jr., Travis LB. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98:1979–1981. doi: 10.1182/blood.v98.6.1979. [DOI] [PubMed] [Google Scholar]
- 38.Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and Merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30:843–849. doi: 10.1200/JCO.2011.34.9605. [DOI] [PubMed] [Google Scholar]
- 39.Mehrany K, Weenig RH, Pittelkow MR, Roenigk RK, Otley CC. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38–42. doi: 10.1111/j.1524-4725.2005.31006. discussion 42. [DOI] [PubMed] [Google Scholar]
- 40.Davidovitz Y, Ballin A, Meytes D. Flare-up of squamous cell carcinoma of the skin following fludarabine therapy for chronic lymphocytic leukemia. Acta Haematol. 1997;98:44–46. doi: 10.1159/000203561. [DOI] [PubMed] [Google Scholar]
- 41.Mansfield AS, Rabe KG, Slager SL, et al. Skin cancer surveillance and malignancies of the skin in a community-dwelling cohort of patients with newly diagnosed chronic lymphocytic leukemia. J Oncol Pract. 2014;10:e1–4. doi: 10.1200/JOP.2013.000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsen CR, Hansen PB, Clausen NT. Aggressive growth of epithelial carcinomas following treatment with nucleoside analogues. Am J Hematol. 2002;70:48–50. doi: 10.1002/ajh.10080. [DOI] [PubMed] [Google Scholar]
- 43.Robak T, Blonski JZ, Gora-Tybor J, et al. Second malignancies and Richter’s syndrome in patients with chronic lymphocytic leukaemia treated with cladribine. Eur J Cancer. 2004;40:383–389. doi: 10.1016/j.ejca.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 44.Keating MJ, O’Brien S, Lerner S, et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998;92:1165–1171. [PubMed] [Google Scholar]
- 45.Colovic M, Suvajdzic N, Jankovic G, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia in patients with chronic lymphocytic leukemia treated with fludarabine and cyclophosphamide. Biomed Pharmacother. 2011;65:319–321. doi: 10.1016/j.biopha.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi T, Nowak BJ, Keating MJ, Plunkett W. DNA repair initiated in chronic lymphocytic leukemia lymphocytes by 4-hydroperoxycyclophosphamide is inhibited by fludarabine and clofarabine. Clin Cancer Res. 2001;7:3580–3589. [PubMed] [Google Scholar]
- 47.Misgeld E, Germing U, Aul C, Gattermann N. Secondary myelodysplastic syndrome after fludarabine therapy of a low-grade non-Hodgkin’s lymphoma. Leuk Res. 2001;25:95–98. doi: 10.1016/s0145-2126(00)00092-8. [DOI] [PubMed] [Google Scholar]
- 48.McLaughlin P, Estey E, Glassman A, et al. Myelodysplasia and acute myeloid leukemia following therapy for indolent lymphoma with fludarabine, mitoxantrone, and dexamethasone (FND) plus rituximab and interferon alpha. Blood. 2005;105:4573–4575. doi: 10.1182/blood-2004-08-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrison VA, Rai KR, Peterson BL, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20:3878–3884. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- 50.Tam CS, Seymour JF, Prince HM, et al. Treatment-related myelodysplasia following fludarabine combination chemotherapy. Haematologica. 2006;91:1546–1550. [PubMed] [Google Scholar]
- 51.Zhou Y, Tang G, Medeiros LJ, et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol. 2012;25:237–245. doi: 10.1038/modpathol.2011.158. [DOI] [PubMed] [Google Scholar]
- 52.Rossi D, Spina V, Forconi F, et al. Molecular history of Richter syndrome: origin from a cell already present at the time of chronic lymphocytic leukemia diagnosis. Int J Cancer. 2012;130:3006–3010. doi: 10.1002/ijc.26322. [DOI] [PubMed] [Google Scholar]
- 53.Robertson LE, Pugh W, O’Brien S, et al. Richter’s syndrome: a report on 39 patients. J Clin Oncol. 1993;11:1985–1989. doi: 10.1200/JCO.1993.11.10.1985. [DOI] [PubMed] [Google Scholar]
- 54.Foucar K, Rydell RE. Richter’s syndrome in chronic lymphocytic leukemia. Cancer. 1980;46:118–134. doi: 10.1002/1097-0142(19800701)46:1<118::aid-cncr2820460120>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 55.Mauro FR, Foa R, Giannarelli D, et al. Clinical characteristics and outcome of young chronic lymphocytic leukemia patients: a single institution study of 204 cases. Blood. 1999;94:448–454. [PubMed] [Google Scholar]
- 56.Thornton PD, Bellas C, Santon A, et al. Richter’s transformation of chronic lymphocytic leukemia. The possible role of fludarabine and the Epstein-Barr virus in its pathogenesis. Leuk Res. 2005;29:389–395. doi: 10.1016/j.leukres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Pocock C, Matutes E, Wotherspoon A, Cunningham D, Catovsky D. Fludarabine therapy may precipitate large cell transformation in chronic lymphocytic leukemia but not in follicular lymphoma. Blood. 1998;92:429a. [Google Scholar]
- 58.Solh M, Rai KR, Peterson BL, et al. The impact of initial fludarabine therapy on transformation to Richter syndrome or prolymphocytic leukemia in patients with chronic lymphocytic leukemia: analysis of an intergroup trial (CALGB 9011) Leuk Lymphoma. 2013;54:252–254. doi: 10.3109/10428194.2012.710327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puttarajappa C, Yabes J, Bei L, et al. Cancer risk with alemtuzumab following kidney transplantation. Clin Transplant. 2013;27:E264–271. doi: 10.1111/ctr.12094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.