Abstract
Circulation and oxygenation of blood outside the body is commonly required during complex surgical interventions involving coronary pulmonary bypass (CPB) and extracorporeal membrane oxygenation (ECMO). Both CPB and ECMO are life-supporting procedures utilizing a heart-lung machine, which subjects the blood to unphysiological conditions, potentially promoting undesirable blood coagulation. Traditionally, thrombotic complications from CPB and ECMO are resolved by heparin, an inexpensive broad spectrum anticoagulant that prevents blood clotting, but often results in bleeding. Despite hemostatic support therapy and constant monitoring, the lives of patients undergoing CPB and ECMO are often threatened by uncontrolled bleeding. There is an urgent need for novel strategies which provide safe anti-coagulation alternatives during CPB and ECMO procedures. Several non-traditional approaches, including nitric oxide donors as well as various protease and contact activation inhibitors, have been investigated and shown some success. More recently, Larsson et al. isolated a recombinant fully human (3F7) antibody inhibiting Factor XIIa. The antibody was shown to be both an efficacious and safe alternative to heparin. Below we will examine this study in more detail and offer considerations for translation of this novel concept to the clinic.
Keywords: Thrombosis, anticoagulant, blood coagulation, 3F7, neutralizing antibody, heparin, factor XII, preclinical safety, translation to clinic, nanotechnology
Background
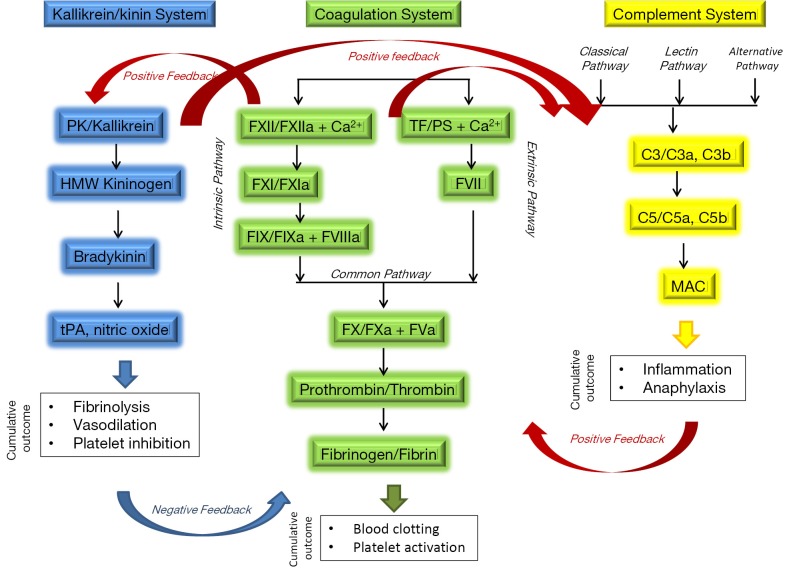
Hemostasis is a delicate balance between pro- and anti-coagulant processes in the blood. A shift in this balance in either direction can result in pathological conditions leading to thrombosis or hemorrhage. Coronary pulmonary bypass (CPB) and extracorporeal membrane oxygenation (ECMO), respectively, are used clinically to provide short- and long-term circulation and oxygenation of blood when the heart and/or lungs lose functionality (e.g., in sepsis) or have to be temporarily arrested (e.g., coronary artery bypass graft). Extracorporeal circulation subjects blood to unphysiological shear stress, osmotic forces, turbulence and contact with artificial surfaces. Altogether, these conditions can lead to activation of several systems involved in blood coagulation: (I) the plasma coagulation system, via the intrinsic pathway; (II) the kallikrein/kinin system; (III) the complement system. The close relationship between these systems and their role in blood coagulation are summarized in Figure 1. Activation of these systems results in thrombosis and inflammation. The latter has pro-coagulant activity, thus further contributing to thrombosis.
Figure 1.
Complex relationship between complement, coagulation and kallikrein/kinin systems. The complement, coagulation and kallikrein/kinin systems are working in concert with each other to provide both positive and negative feedback for hemostasis and inflammation. Positive feedback between coagulation and kininogen/kinin systems occurs through FXII ability to activate prekallikrein (PK) and reciprocal effect of PK on FXII activation. FXIIa activates complement system through activation of C1 component of complement triggering classical pathway of activation. A positive feedback also exists between Kallikrein/kinin and complement system. Inflammation promoted through activation of complement and bradykinin-generating arm of kallikrein/kinin system, in turn, facilitates coagulation through activation of extrinsic coagulation pathway. Certain products of kallikrein/kinin system (e.g., tPA) supply important negative feedback to coagulation system by initiating fibrinolysis and leading to clot dissolution and restoration of hemostasis. Contact activation of complement, FXII and kallikrein pathways is responsible for clotting complications during extracorporeal circulation. FXII is a common element providing positive feedback between the three systems. Selective inhibition of FXIIa allows safe anticoagulation without bleeding. HMW—high molecular weight; tPA, tissue plasminogen activator; TF, tissue factor (CD142); PS, phosphatidylserine; “a” following coagulation factor indicates activated form of the factor; C3 and C5 are complement components 3 and 5, respectively, “a” and “b” following complement components indicate split products of these components; membrane attack complex (MAC), also known as terminal complex.
The standard of care treatment for avoidance of thrombotic complications is heparin. Other traditional anticoagulants include vitamin K antagonist (e.g., warfarin), inhibitors of thrombin (e.g., lepirudin), and inhibitors of active factor X (FXa) (e.g., fondaparinux). While these compounds do provide the benefit of anticoagulation, all have severe side effects, including but not limited to neurological, hematological and immunological toxicities. When used during CPB and ECMO, the benefits of traditional anticoagulants are often counterbalanced by bleeding. Despite efforts including constant patient monitoring and hemostatic support therapies, hemorrhage remains a major life-threatening complication (1). Many researchers, including the authors of the paper discussed herein, have suggested the need for safer anticoagulants and have explored various experimental approaches to fulfill such need. Earlier studies include investigation of aprotinin, nitric oxide donors, contact activation, and FXa inhibitors, and have shown some success in experimental models of ECMO (2-7).
Most recently, a group led by Thomas Renne of the Karolinska Institute in Sweden hypothesized that targeting coagulation factor XII may offer a safe alternative to the standard of care anticoagulants (8). Factor XII, also known as Hageman factor, is a zymogen form of active factor XII (FXIIa). Contact between FXII and polyanionic surfaces results in formation of FXIIa. The latter is an active serine protease, which activates the so-called intrinsic pathway of coagulation by cleaving FXI to generate another active protease, FXIa. Factor XIa continues the intrinsic proteolytic cascade to culminate in thrombin generation and formation of blood clots. In addition, FXIIa activates the kallikrein/kinin system leading to generation of bradykinin, a pro-inflammatory mediator that causes dilation of blood vessels and hypotension. FXIIa also activates C1 component of complement thus contributing to formation of C3-convertase. Moreover, along with high molecular weight kininogen binding of FXII to C1qR triggers conversion of prekallikrein to kallikrein, a prerequisite for kinin generation and further activation of kallikrein/kinnin system (9).
While contact activation of FXII in vitro culminates in thrombin formation and is widely used to assess plasma coagulation time, activation of plasma coagulation in vivo is believed to be solely orchestrated by tissue factor (TF), also known as CD 142. The Renne group hypothesized that inhibiting FXII in vivo should not have a dramatic effect on hemostasis, but will prevent blood from clotting as long as the FXIIa inhibitor is present. This hypothesis was based on extensive studies that showed FXII deficiency in mice was similar to that in FXII-deficient human patients, which has no effect on normal hemostasis and protects from thrombotic complications (10-12). By screening a Dyax human Fab-based phage antibody library against plasma-derived FXIIa, Renne’s group isolated a fully human FXIIa-neutralizing antibody (3F7) (8). Results from the study are encouraging, and suggest that 3F7 may be a safer alternative to heparin for CPB and ECMO procedures in the clinic.
3F7 as a safe alternative to heparin for CPB and ECMO procedures
Discovery and characterization
Phages binding to FXIIa were isolated by elution with an inhibitor specific to the FXIIa catalytic site. The specificity was further confirmed by ligand binding and competitive in vitro assays using FXIIa and βFXIIa (a proteolytic cleavage fragment of FXIIa containing only the serine protease domain). The light and heavy chains isolated through phage display screening were reformatted to form human IgG4. Recombinant antibodies were further generated in HEK293T cells and their functionality was verified using an in vitro assay against a chromogenic FXIIa substrate conversion. Interestingly, although all antibodies isolated using this procedure bound to FXIIa, only the 3F7 antibody completely inhibited its activity. Another interesting observation was that 3F7 reacted with rabbit, mouse and human FXIIa, but not with rat protein. The authors employed labor intensive and sophisticated molecular approaches to uncover a two amino acid difference in the antibody binding epitope between rat and mouse FXIIa. More importantly, substitution of these two amino acids in the human FXII sequence with those specific to rat, eliminated 3F7 recognition of the human protein. Likewise, replacement of the mouse sequence with the rat’s amino acids conferred antibody binding to rat FXIIa.
A comprehensive functional analysis was done to show that 3F7 prolongs rabbit and human plasma coagulation time in the APTT assay, specific to the intrinsic, FXII-triggered pathway. Simultaneously, this showed no effect on the PT and thrombin time assays, specific to extrinsic (TF-triggered) and common pathways, respectively. They further demonstrated that 3F7 inhibits in vitro activation and adhesion of platelets to the collagen-coated surface under both arterial and venous shear flow rates, and prevents thrombosis in vivo in the mouse model of vascular endothelium injury. The results of the mouse study were also reproduced in a rabbit model.
When compared to heparin, 3F7 had similar anticoagulant activity; however, its effect on hemostasis was drastically better. Unlike heparin, 3F7 did not cause bleeding from skin and kidney wounds. Moreover, when the ECMO system, clinically used to support extracorporeal circulation and oxygenation of infants undergoing surgical procedures, was adapted to rabbits, both 3F7 and heparin prevented thrombin generation and occlusion of the catheter. However, only 3F7 prevented bleeding and a change in hemostasis. Taken together, these findings suggest that 3F7 may be a safer alternative to heparin during times when the heart and lungs are “on vacation”.
Why is 3F7 better than heparin and other FXIIa inhibitors?
The major advantage of 3F7 is its specificity to FXIIa. By inhibiting FXIIa, 3F7 affects only direct downstream targets of this protease (formation of C3 complement convertase and FXIa). In contrast, heparin has a broader spectrum of effects. In addition to affecting formation of C3 complement convertase and FXIa, it inhibits other elements of coagulation including thrombin, fibrin and kallikrein. Another advantage of 3F7 is that unlike heparin, 3F7 does not require neutralization when anticoagulation is no longer needed (e.g., at the end of surgery). When heparin is used as an anticoagulant, administration of protamine sulfate is needed to quickly neutralize heparin at the end of ECMO. Protamine sulfate is a cationic substance, and is associated with safety concerns such as severe hypotension, catastrophic pulmonary vasoconstriction, pulmonary hypertension, and noncardiogenic pulmonary edema (http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1964129-33f4-4e4e-86e3-8e6a4e65bd83).
There are also several other advantages of 3F7. Unlike other FXIIa inhibitors (rHA-infestin-4 and Ir-CPI) derived from arthropods, the human nature of 3F7 poses a lower risk of immunogenicity. 3F7 also inhibits amidolytic activity of proteases, unlike the monoclonal 2/215 antibody against FXIIa, and has a higher affinity to FXIIa than to the zymogen form of this protein. Therefore, unlike FXII zymogen inhbitors (P5-2-1, anti-HF and B7C9 antibodies), 3F7 is not expected to interfere with other functions of FXII zymogen (e.g., its mitogenic activity). When compared to inhibitors of zymogen and activated forms of FXI and FIX, 3F7 inhibits both pro-coagulant (thrombin-generating) and pro-inflammatory (bradykinin-generating) pathways. FXI and FIX inhibitors, on the other hand, target only the pro-coagulant arm of the coagulation cascade. Finally, 3F7 can interfere with both artificial surface-induced and platelet-produced FXIIa activities, making it a preferred anticoagulant for ECMO over heparin, phosphorylcholine and fibronectin. These advantages are recognized by the authors (8) and have been discussed to various degrees by others (13).
Lastly, 3F7 is thought to have a greater capacity to “knock-out” FXIIa as compared to respective siRNA therapies (8). 3F7 has another potential advantage over siRNA therapeutics: clinical translation of siRNAs is limited by systemic inflammatory responses resulting from recognition by Toll-like and other innate receptors (14). Induction of pro-inflammatory cytokines enhances expression of the active form of TF on leukocyte and endothelial cell surfaces (15). This is undesirable for ECMO/CPB procedures because pro-coagulant effects involving the extrinsic (TF-mediated) pathway are independent of FXII.
Unanswered questions
The work by Larsson et al. has demonstrated a safe utility for 3F7 as an anticoagulant for ECMO/CPB procedures. They suggested that this antibody may also be used to circumvent other coagulation-triggered conditions such as myocardial infarction, ischemic stroke, or pulmonary embolism. However, the complexity of blood coagulation mechanisms in these conditions warrants further investigation for a better understanding of the safety and efficacy aspects of using 3F7 in non-ECMO/CPB settings. The authors recognized that FXII deficiency results in a decrease in firmness of the blood clot, increasing the risk of clot destabilization, dislodgment, and accumulation in other organs leading to possible embolisms. D-dimer formation was monitored as a biomarker of pulmonary embolism which was elevated in 3F7 treated rabbits. Since excess fibrin formation in these animals was not detected, the authors suggested that elevation in D-dimer levels was a result of increased fibrinolysis. A detailed investigation of this arm of hemostasis, however, was beyond the scope of this study.
Since pulmonary embolism represents a significant potential safety concern, further investigation of the effects of 3F7 on fibrinolysis is required. Furthermore, a better understanding of the immunogenic potential of this antibody is also necessary, as well as investigation of its potential effects on other therapeutic compounds patients may be exposed to. Since complement activation related pseudoallergy (CARPA) is a common dose-limiting toxicity for many drugs, including small and macromolecules (16), and since 3F7 inhibits formation of C3 complement convertase, we are enthusiastic that this antibody may have added benefit in cases where patients undergoing ECMO/CPB are treated for other conditions associated with drug-mediated CARPA.
Translational challenges
Translational challenge 1—selection of appropriate species
Preclinical studies are normally conducted in rodents (most commonly rats) and non-rodents (most commonly dogs, but in some cases non-human primates) (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm074957.pdf). Since 3F7 is not reactive with rat FXIIa, preclinical studies would have to utilize a different animal model, e.g., mouse. Drug clearance rates and volume of distribution depend on animal body mass, making smaller animals less predictive. Reactivity to monkey and dog FXIIa would be required to support non-rodent toxicology.
Translational challenge 2—selection of appropriate model(s)
Estrogen-dependent and independent mutations in the FXII gene resulting in gain of protein function, which may be asymptomatic in males, have recently been described (17). Understanding how such differences would influence the safety and efficacy of 3F7, selection of appropriate models to test this hypothesis, as well as a study to address potential gender-dependent differences may be needed.
Translational challenge 3—understanding clearance rate and circulation half-life
Although 3F7 does not require neutralization and is expected to stay in circulation without bleeding side effects, a deeper understanding of the clearance rate and circulation half-life will be needed in view of the immunogenicity challenges (see translational challenge 4 below).
Translational challenge 4—potential immunogenicity
Decades of preclinical research and clinical experience with biological therapeutic products has shown that therapeutic proteins and antibodies can be effectively recognized by the immune system, building up a multi-stage response against them. Antibodies recognizing therapeutic proteins affect their efficacy and pharmacokinetics, and may also result in general immune and hypersensitivity reactions (18,19). Unfortunately, even fully human therapeutic antibodies are not excluded from this problem. Although fully human antibodies carry a lower risk for inducing immune responses (20), generation of anti-idiotypic antibodies to this category of biological therapeutics is still a valid safety concern (21). Therefore, clinical translation of the 3F7 antibody will require tests for immuno- and anti-genicity, especially if the antibody will be used for repeated administration.
Considerations for overcoming potential immunogenicity
If antigenicity problems are encountered, a safer version of the antibody will be required. Nanotechnology formulation may be one such avenue to thwart these issues. Several studies demonstrate that nanoparticles’ physicochemical properties (size, charge, surface chemistry and architecture) can be tuned to either enhance immune recognition (e.g., nanoparticle adjuvants) or to mask the particle from the immune system (e.g., nanoparticle drug delivery). The latter is an attractive property for therapeutic proteins and antibodies. There are several examples showing that nanoparticles prevent immune recognition and reduce immunogenicity of therapeutic proteins. For example, Libutti et al. described the lack of antigenic response to rhTNF in patients receiving repeated i.v.. administration of the PEG-gold-TNF nanomedicine (22). Other examples, including liposomal delivery of streptokinase (23) and recombinant factor VIII (24,25), reveal that encapsulation of a recombinant protein helps to overcome hurdles associated with patients developing neutralizing antibodies in response to a protein.
Nanotechnology may offer additional benefits for a 3F7 antibody as well, because some nanoparticles have intrinsic anticoagulant properties. In the experience of the Nanotechnology Characterization Lab (NCL) (http://ncl.cancer.gov), preclinical characterization of more than 300 nanoformulation concepts has shown that polyanionic synthetic or natural polymers often inhibit the intrinsic (FXII-triggered) coagulation pathway. Application of such materials for formulation of a 3F7 antibody may not only mask the antibody from immune recognition, but allow for a decreased dose while still providing therapeutic benefit. Care must be taken when considering nanoparticles for drug delivery, as some of these materials possess strong pro-coagulant activities. These and other challenges in nanoparticle characterization have been reviewed elsewhere (26).
Conclusions
In summary, 3F7 antibody neutralizing activity of FXIIa has been recently discovered and analyzed in vitro and in vivo in animal models of thrombosis and ECMO. The available experimental evidence suggests this antibody may be a safer alternative to heparin when extracorporeal circulation is required. 3F7 is as effective as heparin in inhibiting the coagulation cascade, but does not include the hemorrhage complications. The results published by Renne’s lab are encouraging and exciting, and warrant further research in this area to accelerate translation of this antibody into clinical settings.
Acknowledgements
We are grateful to Rachael M. Crist for help with manuscript preparation. This project has been funded in whole or in part with Federal funds from the Frederick National Laboratory for Cancer Research, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Disclosure: The authors declare no conflict of interest.
References
- 1.Esper SA, Levy JH, Waters JH, et al. Extracorporeal membrane oxygenation in the adult: a review of anticoagulation monitoring and transfusion. Anesth Analg 2014;118:731-43. [DOI] [PubMed] [Google Scholar]
- 2.Addonizio VP, Fisher CA, Bowen JC, et al. Prostacyclin in lieu of anticoagulation with heparin for extracorporeal circulation. Trans Am Soc Artif Intern Organs 1981;27:304-7. [PubMed] [Google Scholar]
- 3.Annich G, White T, Damm D, et al. Recombinant Kunitz protease inhibitory domain of the amyloid beta-protein precursor as an anticoagulant in venovenous extracorporeal circulation in rabbits. Thromb Haemost 1999;82:1474-81. [PubMed] [Google Scholar]
- 4.Fuhrer G, Gallimore MJ, Heller W, et al. Aprotinin in cardiopulmonary bypass--effects on the Hageman factor (FXII)--Kallikrein system and blood loss. Blood Coagul Fibrinolysis 1992;3:99-104. [DOI] [PubMed] [Google Scholar]
- 5.Gikakis N, Khan MM, Hiramatsu Y, et al. Effect of factor Xa inhibitors on thrombin formation and complement and neutrophil activation during in vitro extracorporeal circulation. Circulation 1996;94:II341-6. [PubMed] [Google Scholar]
- 6.Wachtfogel YT, Bischoff R, Bauer R, et al. Alpha 1-antitrypsin Pittsburgh (Met358-->Arg) inhibits the contact pathway of intrinsic coagulation and alters the release of human neutrophil elastase during simulated extracorporeal circulation. Thromb Haemost 1994;72:843-7. [PubMed] [Google Scholar]
- 7.Wachtfogel YT, Hack CE, Nuijens JH, et al. Selective kallikrein inhibitors alter human neutrophil elastase release during extracorporeal circulation. Am J Physiol 1995;268:H1352-7. [DOI] [PubMed] [Google Scholar]
- 8.Larsson M, Rayzman V, Nolte MW, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med 2014;6:222ra17. [DOI] [PubMed]
- 9.Ghebrehiwet B, CebadaMora C, Tantral L, et al. gC1qR/p33 serves as a molecular bridge between the complement and contact activation systems and is an important catalyst in inflammation. Adv Exp Med Biol 2006;586:95-105. [DOI] [PubMed] [Google Scholar]
- 10.Matafonov A, Leung PY, Gailani AE, et al. Factor XII inhibition reduces thrombus formation in a primate thrombosis model. Blood 2014;123:1739-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renné T, Pozgajová M, Grüner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med 2005;202:271-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renné T, Schmaier AH, Nickel KF, et al. In vivo roles of factor XII. Blood 2012;120:4296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmaier AH. Extracorporeal circulation without bleeding. Sci Transl Med 2014;6:222fs7. [DOI] [PubMed]
- 14.Robbins M, Judge A, MacLachlan I. siRNA and innate immunity. Oligonucleotides 2009;19:89-102. [DOI] [PubMed] [Google Scholar]
- 15.Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des 2012;18:1478-93. [DOI] [PubMed] [Google Scholar]
- 16.Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol 2014;61:163-73. [DOI] [PubMed] [Google Scholar]
- 17.Binkley KE. Factor XII mutations, estrogen-dependent inherited angioedema, and related conditions. Allergy Asthma Clin Immunol 2010;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Büttel IC, Chamberlain P, Chowers Y, et al. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals 2011;39:100-9. [DOI] [PubMed] [Google Scholar]
- 19.Büttel I, Völler K, Schneider C, et al. Immunogenicity and its impact on benefit/risk considerations in the authorisation of biopharmaceuticals. Curr Drug Saf 2010;5:287-92. [DOI] [PubMed] [Google Scholar]
- 20.Harding FA, Stickler MM, Razo J, et al. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs 2010;2:256-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Available online: http://regulations.justia.com/regulations/fedreg/2014/08/14/2014-19267.html
- 22.Libutti SK, Paciotti GF, Byrnes AA, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res 2010;16:6139-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkins WR, Vaughan DE, Plavin SR, et al. Streptokinase entrapment in interdigitation-fusion liposomes improves thrombolysis in an experimental rabbit model. Thromb Haemost 1997;77:1174-8. [PubMed] [Google Scholar]
- 24.Ramani K, Miclea RD, Purohit VS, et al. Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A. J Pharm Sci 2008;97:1386-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramani K, Purohit V, Miclea R, et al. Passive transfer of polyethylene glycol to liposomal-recombinant human FVIII enhances its efficacy in a murine model for hemophilia A. J Pharm Sci 2008;97:3753-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crist RM, Grossman JH, Patri AK, et al. Common pitfalls in nanotechnology: lessons learned from NCI's Nanotechnology Characterization Laboratory. Integr Biol (Camb) 2013;5:66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]