Abstract
Plants are exposed to various environmental stresses during their life cycle such as salt, drought and cold. Natural variation mediated plant growth adaptation has been employed as an effective approach in response to the diverse environmental cues such as salt stress. However, the molecular mechanism underlying this process is not well understood. In the present study, a collection of 82 Arabidopsis thaliana accessions (ecotypes) was screened with a view to identify variation for salinity tolerance. Seven accessions showed a higher level of tolerance than Col-0. The young seedlings of the tolerant accessions demonstrated a higher K+ content and a lower Na+/K+ ratio when exposed to salinity stress, but its Na+ content was the same as that of Col-0. The K+ transporter genes AtHAK5, AtCHX17 and AtKUP1 were up-regulated significantly in almost all the tolerant accessions, even in the absence of salinity stress. There was little genetic variation or positive transcriptional variation between the selections and Col-0 with respect to Na+-related transporter genes, as AtSOS genes, AtNHX1 and AtHKT1;1. In addition, under salinity stress, these selections accumulated higher compatible solutes and lower reactive oxygen species than did Col-0. Taken together, our results showed that natural variation in salinity tolerance of Arabidopsis seems to have been achieved by the strong capacity of K+ retention.
Introduction
Salinity is one of the most serious environmental constraints to plant growth and crop productivity [1]. It is responsible for a combination of ionic and osmotic stresses, which together inhibit leaf expansion, restrict photosynthesis and limit the accumulation of biomass [2–3]. The adaptive response by many plants to salinity stress includes the active exclusion of sodium (Na+) ions and/or their sequestration into the vacuole, the production of compatible solutes and reactive oxygen species (ROS) detoxification [1, 4–8].
An increasing number of genes specifying relevant salt tolerance have been identified in recent years in both the model plant Arabidopsis thaliana (hereafter Arabidopsis) and other species. One of the most prominent of these belongs to the Salinity Overly Sensitive (SOS) signaling pathway, which has been proposed as a key component in the process of Na+ exclusion and the maintenance of ion homeostasis [9–10]. The SOS pathway genes comprise SOS1 (encoding a plasma membrane Na+/H+ antiporter, also known as NHX7), SOS2 (a Ser/Thr kinase) and SOS3 (a Ca2+-binding protein), which transduce a salt stress-induced Ca2+ signal to regulate to Na+ efflux at cellular level and control long distance transport of Na+ from root to shoot [6, 11–20]. The over-expression of SOS1 in Arabidopsis has been shown to confer a heightened level of salinity tolerance [21–23]. The high-affinity K+ transporters (HKT1;1) is a further determinant of salinity tolerance in Arabidopsis, which controls Na+ entry into plant roots and Na+ recirculation by the phloem [24–30]. Besides, the tonoplast-localized Na+/H+ exchanger (NHX1) catalyzes Na+ sequestration in vacuoles [6, 31], and its C terminus is correlated with the regulation of the antiporter selectivity [32]. The importance of Na+ compartmentation to the vacuole by overexpressing AtNHX1 has been shown by improved performance in salt-stressed transgenic tomato [33], brassica [34], rice [35].
Potassium (K+) is also a major factor in resistance to salinity, drought and fungal diseases in plant [36–38]. Previously the growing bulk of evidence has demonstrated that there is a strong positive correlation between the ability to retain K+ and overall plant salt tolerance when challenged with NaCl in several species including barley [39–40] and wheat [41]. So far, a large number of genes encoding K+ transporters and channels have been identified for high/low-affinity K+ uptake in Arabidopsis [42–46]. The high affinity K+ transporter 5 (HAK5), belongs to KUP/HAK/KT families, located in the epidermis of primary and lateral roots, has been identified as high-affinity transporters [47–49], and is associated with K+ accumulation by genetic mapping of natural variations in K+ concentration in Arabidopsis [50]. In addition, the K+ uptake transporter 1 (KUP1) is referred to as a dual- or high-affinity transporter depending on the expression system [51–52]. Moreover, the cation/H+ exchanger 17 (CHX17), one of 28 CHX gene families, is also known to be induced in response to salinity, and may contribute towards K+ homeostasis [53].
Arabidopsis accessions are widely distributed and known to vary considerably with respect to their adaption to various surrounding environments. In response, considerable genetic variations and adaptive traits of them have also been described for their level of resistance/tolerance to both biotic and abiotic stress [54–58], as well as the various developmental traits such as the flowering time [59], circadian rhythms [60], trichome cell fate determination [61], seed size and dormancy [62]. Along with the allelic variation at the gene sequence exposed by ongoing re-sequencing of the Arabidopsis genome [63], global transcriptomic analyses have revealed that the response to several abiotic and biotic stresses can vary between Arabidopsis accessions at the level of specific transcript abundance [55, 64–65]. Some of the genes responsible for this variation have been successfully identified by genetic means [54, 66–69]. An extensive characterization of the existing natural genetic variation for salinity tolerance in Arabidopsis has identified a few quantitative trait loci (QTLs) and genomic regions as harboring relevant genes [56, 58]. Furthermore, DNA microarray based genotyping was utilized to identify AtHKT1;1 associated with salt tolerance as the casual locus driving the natural variation in shoot Na+ accumulation in Ts-1 and Tsu-1 accessions and it also been found that a coastal cline in sodium accumulation in Arabidopsis is driven by natural variation of AtHKT1;1 [68, 70]. However, salt tolerance is a complex trait and controlled by multiple genes, and involved with various molecular and biochemical mechanisms. So far, how Arabidopsis accessions achieve a particular level of salt tolerance in nature is not well known.
Here, we describe the variation for salinity tolerance shown by a set of 82 Arabidopsis accessions, and the identification of seven particularly tolerant accessions by comparison with a reference accession Col-0 as reported previously [71]. An analysis of the behavior of these salt-tolerant accessions has demonstrated that the high K+ content is a key factor for their adaptive response, and some relevant K+ transporter genes may play active roles for maintaining high K+ contents in these tolerant accessions, and our results provide important information for understanding the mechanisms underlying natural variation mediated salt tolerance in Arabidopsis. In addition, these selections also accumulated a higher compatible solutes concentration and a lower ROS than did Col-0 under salinity stress.
Materials and Methods
Plant materials and growing conditions
The set of 82 Arabidopsis accessions (S1 Table) screened for salinity tolerance comprised: Amel-3 (V10530), Asc-1 (CS22163), Be-0 (CS6613), Benk-3 (W10286), Blh-1 (CS6645), Borde (W10290), Boxmeer (W10545), Bs-1 (CS6627), Cal-0 (CS6659), Cam-3 (W40296), Can-0 (CS1065), Churchill (CS1072), Cnt (CS1635), Col-0 (A22625), Csh-1 (CS22419), Deil-1 (W10293), Driel-1 (CS75949), Ei-2 (CS6689), Esp-1 II (CS40021), Everlaan voor (W10170), Fet-1 (W40284), Fet-6 (W40286), Fi-1 (CS6705), Got-1 (CS22277), Hs-1 (CS22351), Ka-0 (CS6752), Kil-0 (CS6754), Kno-1 (CS22401), Kyo-1 (V10372), Kz-2 (CS22436), Lac-2 (W40288), Lac-4 (W40289), Lim (CS8070), Lm-2 (CS6784), Looe-2 (V10540), Lth-1 (CS22363), Mar 2–4 (W40278), Marb-1 (CS75968), Mh-1 (CS6793), Mib-3 (V40305), Mir-0 (N1379), Mog-1 (V40292), Mog-11 (V40293), Mog-12 (V40294), Nd-1 (CS1636), No-0 (CS1394), Oerd-2 (W10299), Oerd-4 (W10040), O-17 (V10451), Ovliel-1 (V10440), Oy-0 (N1437), Pa-1 (N1439), Penz-2 (V10543), Polp-1 (V10536), Pyl (V10370), Ren-1 (CS22253), Rhen-3 (V10498), Rhen-4 (V10499), Rsch-0 (CS6848), Sah-0 (CS6917), Sav-0 (N1514), Seat-1 (CS22341), Sf-2 (CS1516), Sij-2 (CS9655), Sij-4 (CS9656), St.yues-lik (V10537), Ta-0 (N1549), Tanz-1 (CS75924), Te-0 (CS6918), Terlet-1 (CS75950), Treb-1 (V10542), Ts-1 (A22647), Tsu-1 (N1640), Vliel-1 (V10438), Wag-11 (W10295), Wag-13 (V10428), Wei-1 (CS6182), Wil-1 (N1595), Ws-0 (N1595), Ws-2 (CS2360), Wt-4 (CS6895) and Wt-5 (CS6896). The seeds were surface-sterilized overnight in chlorine which was induced by NaClO and HCl, then dried for 20 min before plating on solidified Murashige and Skoog (MS) medium (0.8% (w/v) agar, pH 5.7) supplemented with 1% (w/v) sucrose. The seeds were exposed to 4°C in the dark for 48 h, and then removed to a 16 h photoperiod (350 μmol m-2 s-2) at 23°C and 60% relative humidity.
Salinity tolerance assays
Seedlings were exposed to salinity stress in three different ways. (1) Three-day-old seedlings germinated on solidified MS medium (see above) were transferred to a similar medium supplemented with 0, 150, 180 or 200 mM NaCl, and allowed to grow for a further six days under the conditions described above. (2) Five-day-old seedlings were floated on a nylon mesh suspended over liquid MS medium for three days, and exchanged thereafter on a daily basis over the next three days by liquid MS medium containing 0 or 200 mM NaCl. (3) Nine-day-old seedlings germinated on solidified MS medium were transplanted into an autoclaved mixture of perlite/vermiculite/peat (1/1/3, v/v) and left to establish for three weeks, after which they were watered to capacity every two days for the next twenty days with 500 mM NaCl (as a control, a set of similar plants was given just water).
Growth parameters, chlorophyll and malondialdehyde (MDA) content
Seeds was germinated and grown on solidified MS medium as above. To assay root growth, three-day-old seedlings were transferred to an MS medium containing 0 or 150 mM NaCl and left to grow for a further nine days. Root length was measured from digital images using ImageJ software (http://imagej.nih.gov/ij/). Measurements were expressed in the form of the ratio between the mean growth of primary root lengths achieved in the salinity treatment and the control treatment, based on at least 20 seedlings. Salinity-induced chlorosis of the cotyledons was assessed by eye after exposing three-day-old seedlings to a medium containing 200 mM NaCl for six days. Leaf chlorophyll content was measured on seedlings which had been germinated for three days on solidified MS medium, then transferred to the same medium containing 200 mM NaCl for a further five days. The leaf material was homogenized in cold 96% ethanol, and its chlorophyll content determined following [72]. The MDA assay was carried out on six-day-old seedlings grown on MS medium, then transferred to a liquid MS medium containing 0 or 200 mM NaCl for 36 h. MDA content was measured using a modified thiobarbituric acid (TBA) method [29]. Each experiment was replicated three times.
Measurements of Na+ and K+ content
Three-day-old seedlings grown on solidified MS medium were transferred to a liquid MS containing 0 or 200 mM NaCl. Plants were harvested after 12 h of salt treatment. The whole plants of control and salt-treated group for each accession were rinse separately in sterile distilled water timely, then dried quickly on the filter paper and fresh weights were recorded. Samples were digested in 1% (w/v) nitric acid and 30% (w/v) hydrogen peroxide, of which the ratio is 1:1 (v/v). Na+ and K+ contents were measured using flame atomic absorption spectrophotometer (AA-7000, Japan) following [73]. The results presented are the average and standard error of the mean (SE) for three biological replicates.
Ion flux measurement
Net K+ fluxes were measured at the YoungerUSA (Xuyue Beijing) NMT Service Center by using Non-invasive Micro-test Technology (NMT100 Series①, YoungerUSA LLC, Amherst, MA01002, USA; Xuyue (Beijing) Sci. & Tech. Co., Ltd., Beijing, China) and iFluxes/imFluxes 1.0 (YoungerUSA, LLC, Amherst, MA 01002, USA) Software.
Pre-pulled and silanized glass micropipettes (Φ5±1μm, XY-DJ-01, YoungerUSA) were first filled with a backfilling solution (100 mM KCl) to a length of approximately 1.0 cm from the tip. The micropipettes were front filled with 180 μm columns of selective liquid ion-exchange cocktails (K+ LIX: XY-SJ-K, Sigma 60031, YoungerUSA). An Ag/AgCl wire electrode holder (XY-DJGD, YoungerUSA) was inserted in the back of the electrode to make electrical contact with the electrolyte solution. YG003-Y05 (YoungerUSA) was used as the reference electrode. Prior to the flux measurement, the microelectrodes were calibrated with cultural media with different concentrations of K+, 0.05 mM and 0.5 mM respectively. Only electrodes with a Nernstian slope > 53 mV/decade were used in this study. The concentration gradients of specific K+ ions were found by means of selective micro-electrodes “vibrating” repeatedly between two points in a user predefined fashion. The molecular/ionic fluxes are calculated based on Fick’s law of diffusion: J = −D 0·(dc/dx), where J is the ion flux (unit: pico mole cm-2 s-1), dc is its concentration gradient, dx is the distance the microelectrode moved repeatedly from one point to another perpendicular to the surfaces of samples at a frequency of ca. 0.3 Hz and usually between 5–35 μm, and D 0 is its diffusion constant. The direction of the flux is derived from Fick’s law of diffusion.
To measure ion fluxes, 4-day-old Arabidopsis accession seedlings were pretreated with 100 mM NaCl for 0 or 3 h in liquid MS medium (without sucrose, pH 5.7). The seedlings were then washed 3 times with redistilled water and the roots were immobilized on the bottom of the chamber containing the 5–10 mL measuring buffer (0.1 mM KCl, 0.1 mM MgCl2, 0.1 mM NaCl, 0.1 mM CaCl2, 0.3 mM MES, pH 5.7) for 20 minutes. The control group was subjected to the same conditions for 10 minutes. The concentration gradients of the K+ ion were measured at the mature root zone (> 2 mm from the root tip, where the first root hair appeared) by moving the ion-selective microelectrode between two positions close to the plant root with a distance of 20 μm. 6 minutes of continuous recording were performed at each measurement point. The steady-state K+ flux rates were expressed as the mean of the measured points of 6–15 seedlings and the error bars indicated the SD.
Measurements of proline, soluble sugar and soluble protein content
The fresh weight of six-day-old seedlings raised on solidified MS medium, then exposed to a liquid MS medium containing 0 or 200 mM NaCl for 36 h, was measured and the material was snap-frozen in liquid nitrogen. Proline was extracted and estimated using the ninhydrin acid reagent method, employing proline as the standard [74]. The soluble sugar and soluble protein content of three-day-old seedlings raised on solidified MS medium and then exposed to a liquid MS medium containing 0 or 200 mM NaCl for 12 h was measured by first obtaining their fresh weight, then following the protocols described by Bailey and Bradford respectively [75–76]. At least 15 plants were included in each of the three replicate measurements.
Determination of ROS content and superoxide dismutase (SOD) activity
Five-day-old seedlings raised in solidified medium were transferred to MS medium containing 150 mM NaCl for ten days. The superoxide content of the extracted tissue was assessed using nitroblue tetrazolium (NBT) staining [77] and that of H2O2 by 3, 3’-diaminobenzidine (DAB) staining [78]. The necessary reagents were purchased from Sigma-Aldrich (SAINT LOUIS, USA).
For the analysis of SOD isozymes, three-day-old seedlings raised on solidified MS medium were transferred to a liquid MS medium containing 100 mM NaCl for 0, 3 and 9 h. The seedling was snap-frozen and ground to a powder, from which soluble protein was extracted by homogenization in the mix of 50 mM potassium phosphate buffer (pH 7.8), 1 mM EDTA, 1% w/v polyvinylpyrrolidone. The homogenate was centrifuged (15 000 g × 10 min) at 4°C and the supernatant assayed for SOD activity in accordance with [79]. The reactions were quantified spectrophotometrically at 560 nm.
Re-sequencing of AtSOS1-3, AtNHX1 and AtHKT1;1
Genomic DNA was extracted from the seven selected accessions and Col-0 plants using a CTAB-based protocol [57] and PCR-amplified using the primer sequences given in S2 Table. The resulting amplifications were purified and sequenced.
Total RNA extraction and Quantitative real-time PCR (qRT-PCR) analysis
Five-day-old seedlings were exposed to a liquid MS medium containing 100 mM NaCl for 0, 3 or 6 h. The tissue was snap-frozen, ground to a powder and used as a source of RNA by extracting with an RNeasy Plant Mini kit (Qiagen, Germany). The resulting total RNA was treated with RNase-free DNase I (Fermentas, USA) and then reverses transcribed using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA). qRT-PCRs were performed in Bio-RAD CFX Connect Real-time PCR Detection System (Bio-Rad, Hercules, CA) based on the SYBR Green I Master kit (Roche, Grossbasel, Switzerland) according to the manufacturer’s instructions. The Arabidopsis ACTIN2 gene was used as the reference sequence, and transcript abundances were determined by the 2-△△CT method [80]. The PCR temperature regime comprised a 95°C/3 min denaturation, followed by 40 cycles of 95°C/10 s, 59°C/30 s, 72°C/10 s. The quality of the PCR reactions was estimated based on melting curves. Data were analyzed using CFX Manager software. All primer sequences used are given in S3 Table. Each qRT-PCR was based on three biological replicates and three technical replicates.
Statistical analyses
The data were reported as mean values ± standard error (SE) from at least three independent experiments with three replicates. All data were analyzed using Student’s t-test. Significance between one of the selected accessions and Col-0 accession was determined by P-values (* means P < 0.05, ** means P < 0.01).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AtSOS1 (AT2G01980), AtSOS2 (AT5G35410), AtSOS3 (AT5G24270), AtNHX1 (AT5G27150), AtHKT1;1 (AT4G10310), AtHAK5 (AT4G13420), AtCHX17 (AT4G23700), AtKUP1 (AT2G30070), AtGORK (AT5G37500), AtProDH1 (AT3G30775), AtP5CDH (AT5G62530), AtP5CR (AT5G14800), AtP5CS1 (AT2G39800), AtP5CS2 (AT3G55610), AtCSD1 (AT1G08830), AtCAT2 (AT4G35090), AtAPX2 (AT3G09640), AtZAT10 (AT1G27730), AtZAT12 (AT5G59820) and AtACTIN2 (AT3G18780).
Results
Identification of salinity tolerant accessions and the physiological basis of their tolerance
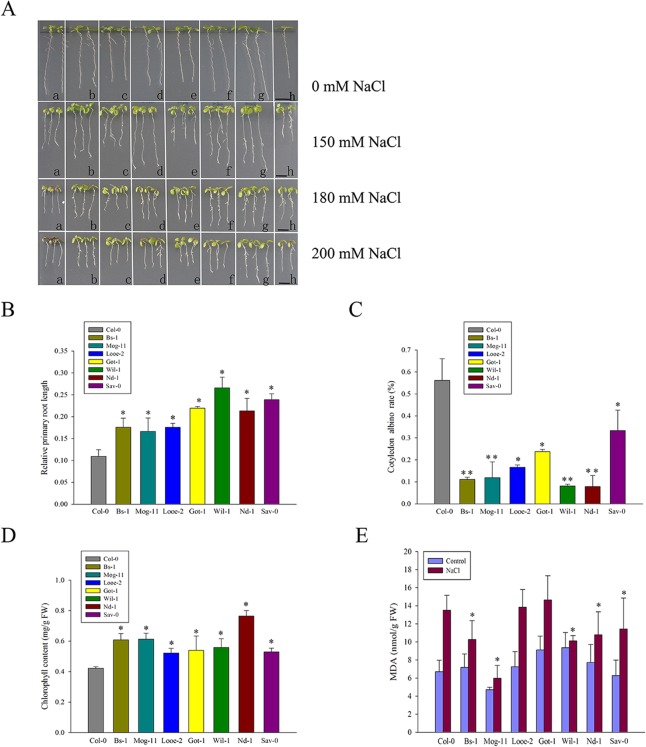
Eighty-two Arabidopsis accessions from natural populations, collected in islands or coastal habitats from Europe, Asia, East Africa and North America, were challenged by growing the plants on a medium containing 150 or 200 mM NaCl (S1 Table). With Col-0 as the reference accession, nineteen accessions showed severe sensitivity and the primary root of them were inhibited extremely under 150 mM NaCl (S1 Table). The other accessions showed less growth inhibition than Col-0 in response to 150 mM NaCl, however, under 200 mM NaCl, some were not able to withstand, showed increased chlorosis and even died, in view of this, these accessions was not considered as the salt-tolerant ones (S1 Table). Finally, seven entries from Europe comprised of Bs-1 (Switzerland), Mog-11 (France), Looe-2 (UK), Got-1 (Germany), Wil-1 (Russia), Nd-1 (Germany), and Sav-0 (Czech Rep), were selected as the salt-tolerant ones in this study. To further evaluate their response to salt stress, agar medium supplemented with four different NaCl concentrations (0, 150, 180 and 200 mM) were used, as a result, the selected accessions exhibited obvious tolerant phenotypes (Fig 1A). Moreover, the presence of 150 mM NaCl reduced both the rate of elongation of the primary root and the chlorophyll content, but both traits were less affected in the seven selected accessions than in Col-0 (Fig 1B–1D). In the presence of 200 mM NaCl, the selections had lower cotyledon albino rate compared to Col-0 (Fig 1C). Furthermore, the MDA were accumulated in all tested accessions when subjected to salt stress, but the levels of MDA in most of the selections were still less than in Col-0 (Fig 1E). In addition, the improved tolerance of the selections was also confirmed by using soil or hydroponics assay, as shown in S1 Fig, Col-0 was observed an increased chlorosis compared with the tolerant ones.
Fig 1. The effect of salinity stress on the selected accessions.
(A) Col-0 and the selected accessions seedlings exposed for 6 days to a medium containing 0 mM (uppermost panel), 150 mM (upper panel), 180 mM (middle panel) and 200 mM (lower panel) NaCl. a: Col-0; b: Mog-11; c: Looe-2; d: Wil-1; e: Bs-1; f: Nd-1; g: Sav-0; h: Got-1. Bar = 0.5 cm. (B) Elongation of the primary root of Col-0 and the selected accessions after a 9 day exposure to 0 or 150 mM NaCl. Values represent the mean percentages of root length achieved in the absence of NaCl stress. (C) Bleaching of the cotyledons of Col-0 and the selected accessions exposed for 6 days to 200 mM NaCl. (D) Chlorophyll content of Col-0 and the selected accessions exposed to 200 mM NaCl for 5 days. (E) MDA content of Col-0 and the selected accessions exposed to 0 or 200 mM NaCl for 36 h. All experiments were run in triplicate and involved at least 20 seedlings per assay. Values given as mean ± SE (n = 3). Significant differences from the performance of Col-0 accession are indicated by * or ** (P < 0.05, P < 0.01).
Arabidopsis salinity tolerance depended more on K+ contents
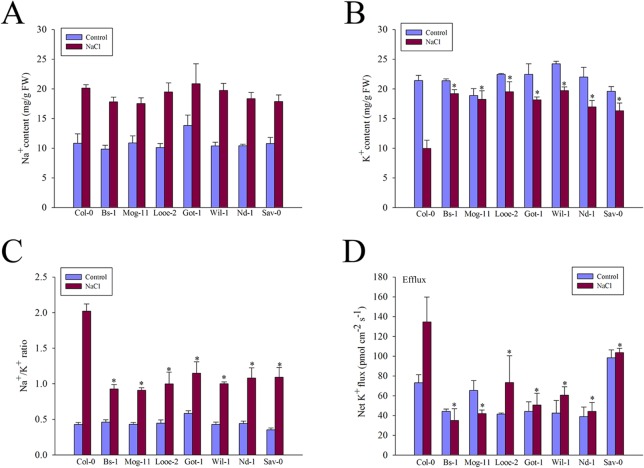
In order to investigate whether the change of Na+ and K+ contents is related to the underlying salinity tolerance in the selected Arabidopsis accessions, the amount of total plant Na+ and K+ contents were measured. The results showed that there was no discernible difference in the Na+ contents of the whole plants harvested from the selected accessions and Col-0, whether or not the plants had been exposed to salinity (Fig 2A). Despite the high level of Na+ in the selected accessions, they were able to tolerate the stress, which indicated that Na+ exclusion was not a special factor of their elevated salinity tolerance. Although the K+ contents of all seven accessions were reduced by salinity stress, they were still able to maintain a higher level than that was achieved by Col-0 (Fig 2B), resulting in a lower Na+/K+ ratio (Fig 2C).
Fig 2. Changes in ion contents, Na+/K+ ratio and net K+ fluxes of Col-0 and the selected accessions.
The whole plants treated with 0 or 200 mM for 12 h were collected and subjected to the determination. (A) Na+ content, (B) K+ content, (C) Na+/K+ ratio and (D) Net K+ fluxes in root tips. Different net K+ fluxes were measured respectively in mature zones of the accessions exposed to 100 mM NaCl for 0 or 3 h, Values are mean ± SE (n = 6–15 seedlings) from three independent experiments. Significant differences from the performance of Col-0 accession are indicated by * (P < 0.05).
K+ fluxes response
Significant differences were found when net K+ fluxes were measured from the mature root zone in response to 0 or 100 mM NaCl treatment (Fig 2D). Although all accessions showed significant net K+ efflux in response to salinity, the magnitude of this efflux was significantly different between the tolerant ones and Col-0. As shown in Fig 2D, average K+ efflux after salt application was only 30–70 pmol m-2 s-1 for the salt-tolerant accessions (Bs-1, Mog-11, Looe-2, Got-1, Wil-1 and Nd-1) and about 100 pmol m-2 s-1 for Sav-0, however, Col-0 was up to 130–140 pmol m-2 s-1 K+ efflux rate after salt stress. These results showed that these tolerant selections had lower K+ effluxes than the sensitive Col-0, which result in the strong K+ retention and enhanced K+ contents in these selections after salinity stress.
High transcript levels of K+ transporter genes in salt-tolerant accessions
Previously, some important K+ transporters such as HAK5, CHX17 and KUP1 were reported involved in K+ uptake and transport. Based on the high K+ content and low K+ efflux in the selected tolerant accessions, we tried to find that if the high K+ level were connected with the expression of the K+ transporters genes AtHAK5, AtCHX17 and AtKUP1. When the transcription levels of them were analyzed by qRT-PCR, it became clear that AtHAK5 was up-regulated in almost all tolerant accessions, especially in Mog-11 and Got-1, in which the AtHAK5 expression level were increased more than 10-fold compared to that of Col-0 (Fig 3A and S4 Table). The abundance of AtCHX17 transcript was also higher in the majority of the selections following a 6 h exposure to NaCl, especially in Mog-11 and Wil-1 (Fig 3B and S4 Table), whereas a similar response of AtKUP1 appeared at 3 h exposure to NaCl, the peak was in that of Wil-1 (Fig 3C and S4 Table). The level of their high transcript corresponded to the high K+ level and low K+ efflux in all tolerant accessions in response to salinity stress (Fig 2B and Fig 2D), implying that these K+ transporters are active in improving the K+ retention in them. Together with the measurements of K+ content and K+ flux, it was also supported that salt tolerance correlated positively with a high ability to retain K+ in the selected tolerant accessions.
Fig 3. Expression analysis of genes related to K+ transport by qRT-PCR.
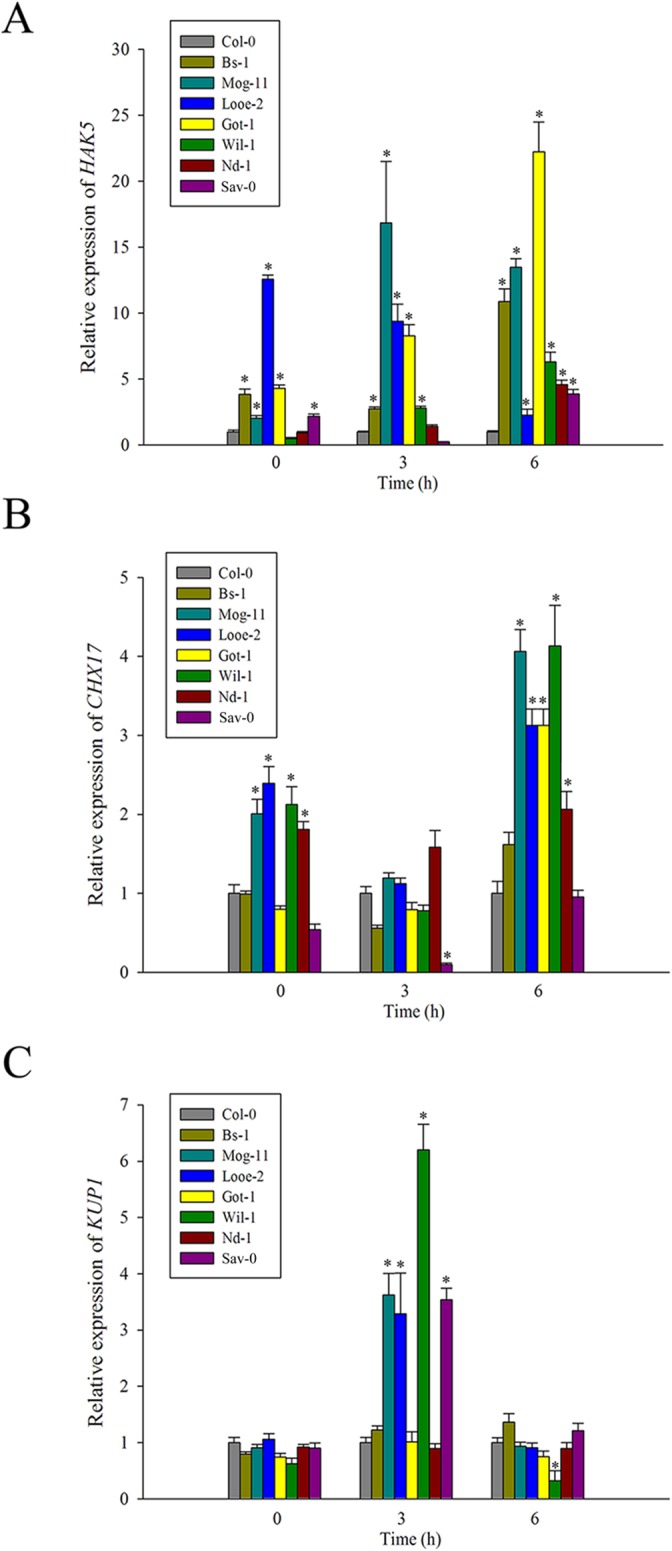
The transcript levels of (A) HAK5, (B) CHX17 and (C) KUP1 in Col-0 and the selected accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3). Significant differences from Col-0 accession at the same time point are indicated by * or ** (P < 0.05, P < 0.01).
Natural genetic variation and the transcription behavior of the SOS pathway genes
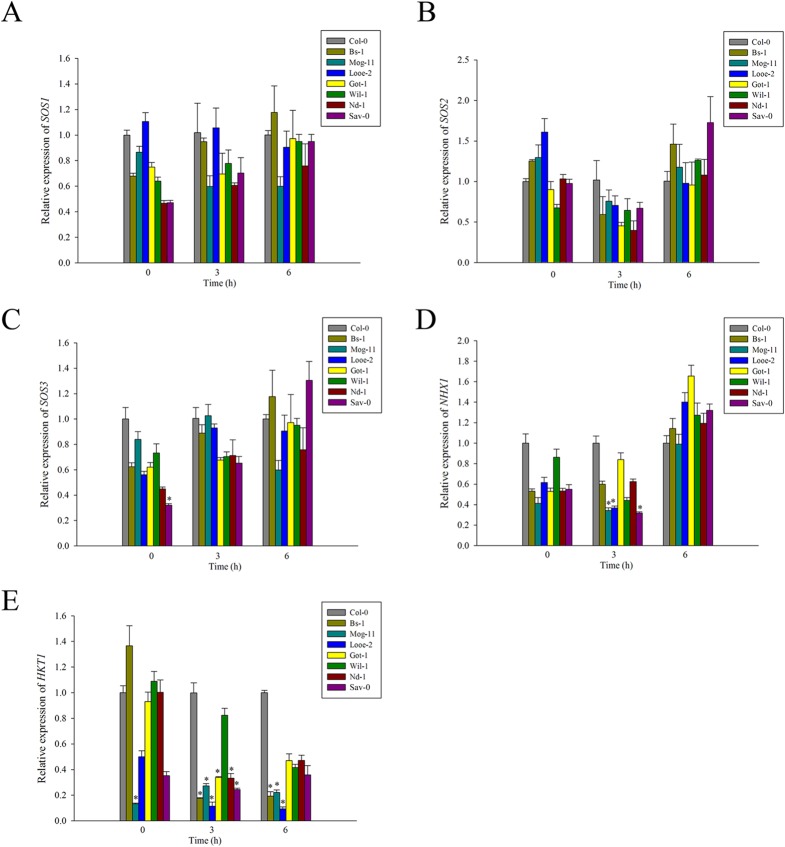
To examine whether those Arabidopsis variants for salt tolerance rely on polymorphism in the SOS genes, the coding regions of AtSOS1, AtSOS2, and AtSOS3 were re-sequenced from the seven tolerant accessions (S2 Fig and S5 Fig). Two sequence variants in the AtSOS1 C terminal auto-inhibitory domain were observed among the tolerant plants’ alleles, compared to that of the Col-0 allele (S2 Fig); the T116A variant was present in six of the seven selections (the exception was Nd-1), and the D1125E variant was present in one (Looe-2) (S2 Fig). Both variants were also carried by the salinity sensitive accession Mh-1 (S2–S3 Fig), as well as by a number of other sequenced accessions at http://1001genomes.org/ (S4 Fig). The DSPS motif, which is phosphorylated by SOS2, was invariant (S2 Fig). The AtSOS2 sequence was identical to that in Col-0 for all the selections (S5 Fig). AtSOS3, the only polymorphism observed was V138I, present exclusively in Mog-11 (S5 Fig). Thus, there is little genetic variation in the sequence variation of the SOS genes among the tested Arabidopsis accessions.
Furthermore, the re-sequencing was followed by an analysis of the AtSOS genes transcript abundance in salinity challenged plants. After both 3 and 6 h exposure to stress, the levels of AtSOS1, AtSOS2 and AtSOS3 transcript present were nearly similar and not high significantly in all seven entries to the respective ones present in Col-0 (Fig 4A and 4B and S5 Table), which could be in line with no significant difference in the Na+ contents of the selected accessions compared to that of Col-0 (Fig 2A). These data gave a hint that the SOS signaling pathway has highly conserved in Arabidopsis and play a fundamental role for the differential salinity tolerance among the selected accessions.
Fig 4. Expression analysis of genes related to Na+ transport by qRT-PCR.
The transcript levels of (A) SOS1, (B) SOS2, (C) SOS3, (D) NHX1 and (E) HKT1;1 in Col-0 and the selected accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3). Significant differences from Col-0 accession at the same time point are indicated by * (P < 0.05).
Natural genetic variation and the transcription behavior of both AtNHX1 and AtHKT1;1
Re-sequencing of the AtNHX1 coding region especially for its C-terminal domain revealed no polymorphisms among the tolerant accessions (S5 Fig), nor was there a significant increase in the transcription level of AtNHX1 in the tolerant selections compared to that of Col-0 with a exposure to NaCl treatment (Fig 4D and S5 Table). Moreover, re-sequencing of AtHKT1;1 revealed two variants (R003I and L024V) only in Wil-1 (S5 Fig), and the abundance of AtHKT1;1 transcript in response to NaCl stress was also not increased in most of the seven selected accessions compared to Col-0 (Fig 4E and S5 Table). Thus, there were little variation in the gene sequences and transcription behaviors of AtNHX1 and AtHKT1;1 in the tolerant selections. Alongside the lack of variation noted for Na+ content, it was concluded that Na+ seclusion or recirculation by AtNHX1 and AtHKT1;1 has also no positive relationship with differential salinity tolerance of the selected accessions.
The accumulation of compatible solutes
Next, the differences of compatible solutes contents between the selected tolerant accessions and Col-0 were investigated. When plants exposed to 200 mM NaCl, the contents of compatible solutes including proline, soluble protein and soluble sugar were measured respectively. The results showed that the seven selected accessions were different from each other, but all had a higher levels of proline, soluble protein and soluble sugar than Col-0 (Fig 5 and S8 Fig), which were associated differently with the NaCl-induced net K+ flux in each other (S6 Table).
Fig 5. The contents of compatible solutes.
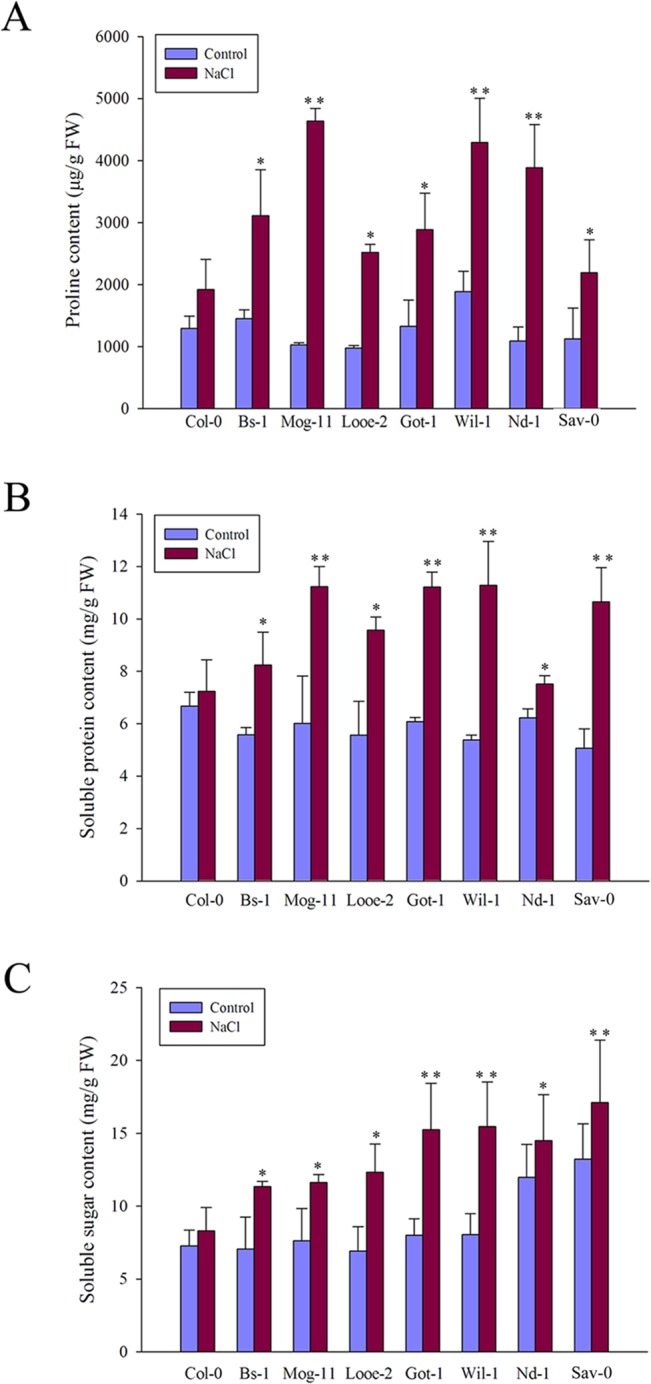
(A) The content of proline in Col-0 and the selected accessions exposed to 0 or 200 mM NaCl for 36 h. (B) The contents of soluble proteins and (C) soluble sugar in Col-0 and the selected accessions exposed to 0 or 200 mM NaCl for 12 h. Values given as mean ± SE (n = 3), each replicate comprised at least 15 seedlings. Significant differences from the performance of Col-0 accession are indicated by * or ** (P < 0.05, P < 0.01).
As for proline, the transcript abundance of AtProDH1 and AtP5CDH, which encode enzymes involved in the proline degradation pathway [81], was analyzed via qRT-PCR. The results showed that AtProDH1 and AtP5CDH were less strongly transcribed in the selected tolerant accessions than in Col-0, and nearly the same difference was observed in non-stressed plants (Fig 6), suggesting that low levels of both AtProDH1 and AtP5CDH expression were the main cause of higher proline levels in the tolerant accessions (Fig 5A). In addition, AtP5CR, AtP5CS1 and AtP5CS2 which encode enzymes involved in the proline biosynthsis pathway [81], were also analyzed via qRT-PCR at 3 and 6 h after salt treatment. However, these genes regulating proline biosynthesis were not changed positively compared to that of Col-0, except Looe-2 at 3 h after salt stress (S6 Fig). These results suggested that the higher proline content is correlated closely with the decreased AtProDH1 and AtP5CDH expression in the selected tolerant accessions.
Fig 6. Expression analysis of genes related to proline degradation by qRT-PCR.
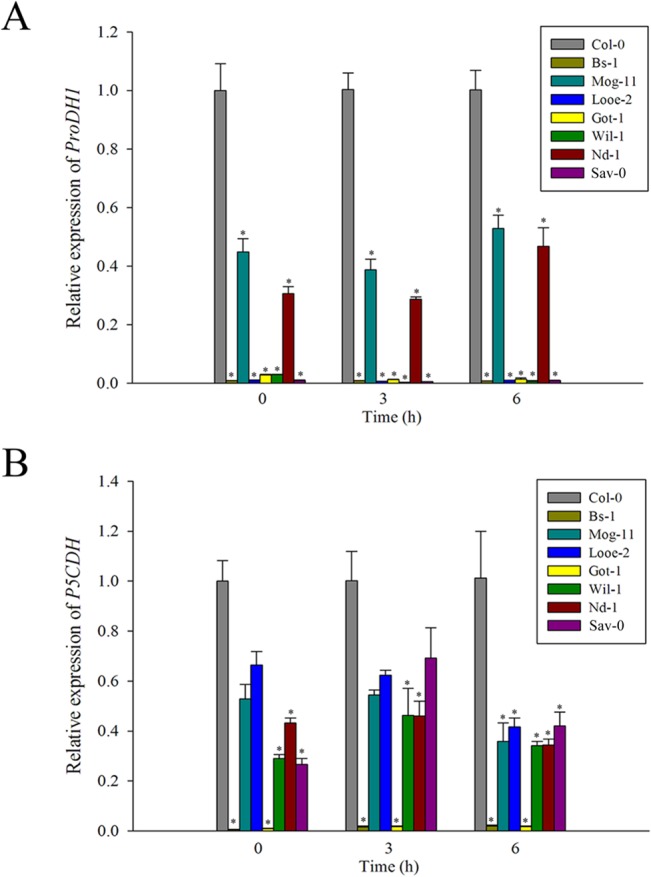
The transcript levels of (A) ProDH1 and (B) P5CDH in Col-0 and the selected accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3). Significant differences from Col-0 accession at the same time point are indicated by * (P < 0.05).
Redox response after salt treatment
In order to examine whether there are difference in ROS levels between the tolerant selections and Col-0 under salinity stress, NBT staining and DAB staining were carried out to assess the ROS contents. The results showed that salinity treatment promoted the accumulation of the superoxide radical and hydrogen peroxide, but to a lesser extent in the seven selected accessions than in Col-0 (Fig 7A and 7B). Moreover, the SOD activity was responsive to the stress, after a 3 or 9 h exposure to stress, there was a greater level of enzyme activity in the tolerant selections than in Col-0 (Fig 7C).
Fig 7. Accumulation of oxidants and superoxide dismutase (SOD) activity in response to salinity stress.
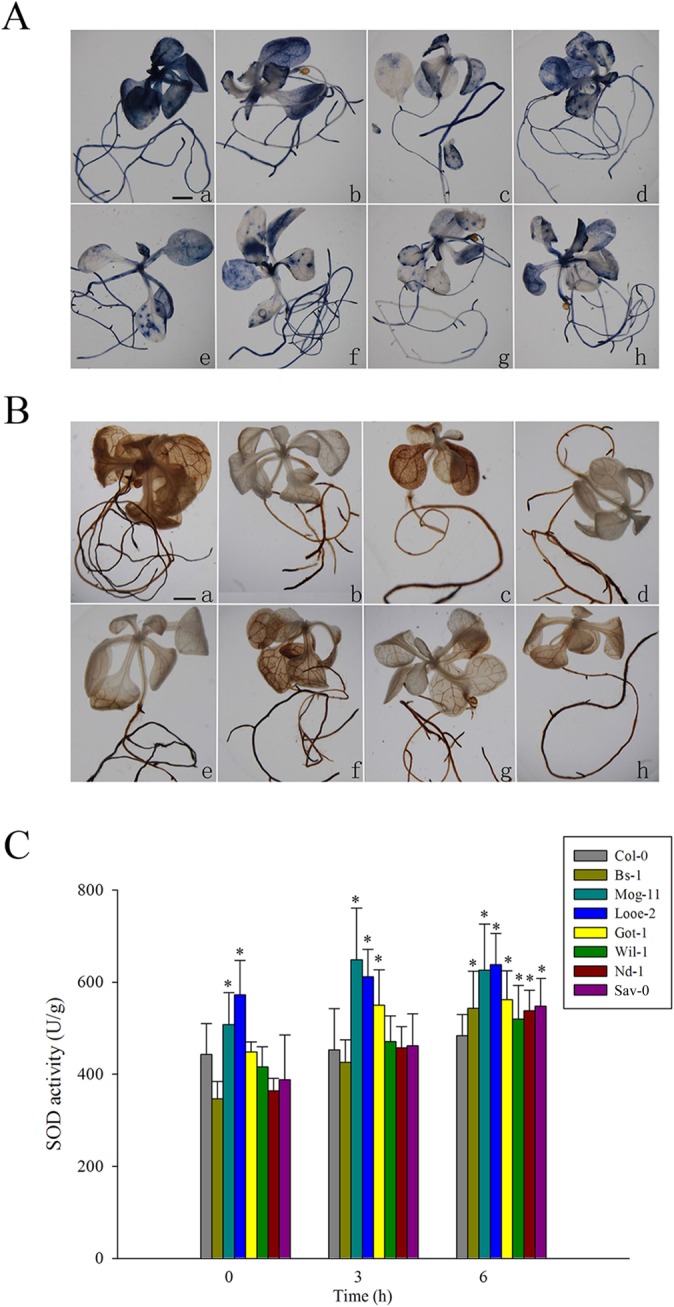
(A) Superoxide radical, detected by nitroblue tetrazolium (NBT) staining, (B) H2O2, detected by 3, 3’-diaminobenzidine (DAB) staining in Col-0 and the selected accessions exposed to 150 mM NaCl for 10 days. a: Col-0; b: Bs-1; c: Mog-11; d: Looe-2; e: Got-1; f: Wil-1; g: Nd-1; h: Sav-0. Bar = 0.1 cm. (C) SOD activity in Col-0 and the selected accessions exposed to 100 mM NaCl for 0, 3 and 9 h. Values given as mean ± SE (n = 3), each replicate comprised at least 15 seedlings. Significant differences from the performance of Col-0 accession at the same time point are indicated by * (P < 0.05).
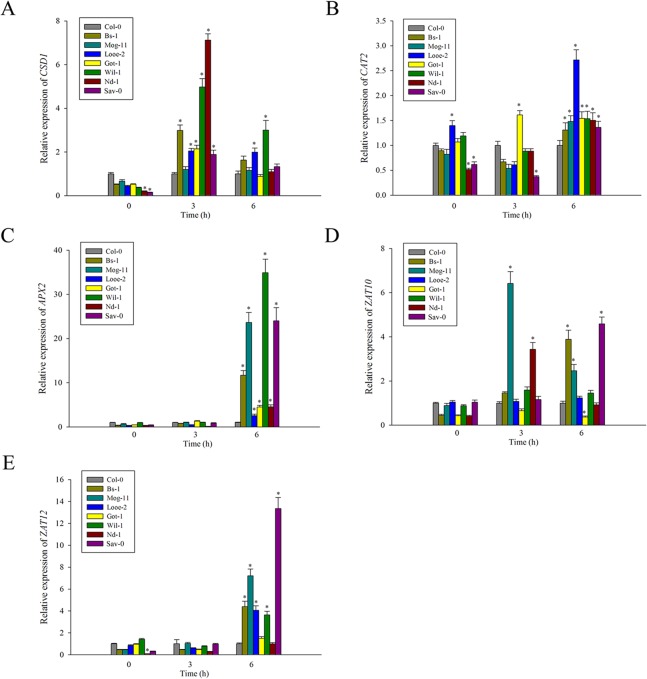
On the other hand, the transcription levels of AtCSD1, AtCAT2, AtAPX2 respectively encoding SOD, catalase (CAT) and ascorbate peroxidase (APX), as well as two transcription factors AtZAT10 and AtZAT12 participating in the ROS signaling pathways were several-times higher in most of the selections than in Col-0 (Fig 8). Interestingly, the high expression levels of AtAPX2 emerged remarkably in Mog-11, Wil-1 and Sav-0, more than 20-fold change relative to that of Col-0 (Fig 8C). In addition, AtCSD1, as one of three Cu/Zn SOD genes, its expression were higher in almost all the selections (except Mog-11) (Fig 8A), which could be correlated with their higher SOD activity compared to that of Col-0 (Fig 7C). Thus, these results demonstrated that the lower ROS amounts in the detected tolerant accessions could be attributed to their higher SOD activity together with the elevated transcription levels of those relevant genes in response to salinity stress.
Fig 8. Expression analysis of genes related to ROS metabolism by qRT-PCR.
The transcript levels of (A) CSD1, (B) CAT2, (C) APX2, (D) ZAT10 and (E) ZAT12 in Col-0 and the selected accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Significant differences from Col-0 accession at the same time point are indicated by * (P < 0.05).
Discussion
Arabidopsis accessions are highly variable in genotypes and phenotypes [82]. In the present study, a screen of 82 accessions, collected from diverse habitats, was carried out to investigate the extent of variation in the response to salinity stress (S1 Table), resulting in the identification of seven accessions noticeably more tolerant of salinity than the reference accession Col-0 (Fig 1A). The tolerance of the selected seven accessions was manifested by a better ability to maintain root growth (Fig 1B), a weaker tendency for the cotyledons to become bleached (Fig 1C) and higher chlorophyll content than that of Col-0 (Fig 1D). Moreover, the selections had less MDA accumulation under salt stress, indicated decreased cell membrane damage compared to Col-0 (Fig 1E). Therefore, these results indicated that the seven accessions tolerate salinity better than Col-0 (Fig 1 and S1 Table). Previously, Ts-1 and Tsu-1, directly located on the coastal regions, were selected as two salt tolerant accessions [68]. In the present study, we also screened them and found that these two accessions were more tolerant than Col-0, but less than the selected seven accessions evaluated by an assay using agar plates. The different results in salt tolerance probably due to the two different assays for the plants grown on the two media, the similar case also reported previously [56, 83]. However, in this study, the improved tolerance of the selections was in agreement with the evaluation by using soil or hydroponics assay (S1 Fig).
It has been found that hypersensitivity to salt stress was frequently associated with poor K+ absorption in Arabidopsis and tomato mutants [38, 84], and the maintenance of high K+ levels and a low Na+/K+ ratio in the cytoplasm could also be essential for salt tolerance [85–86]. This is consistent with our results, when exposed to salinity stress, the seven tolerant Arabidopsis selections had higher K+ contents, maintained a lower Na+/K+ ratio than that of the standard accession Col-0 (Fig 2B and 2C), suggesting that the maintenance of higher K+ contents in the tolerant selections contributed significantly to salinity tolerance of them. Further to measure the net K+ flux among them, it was interestingly found that K+ efflux for all the tested accessions even that in normal condition, but the salt-tolerant selections showed less K+ efflux than Col-0 (Fig 2D), suggesting that the higher K+ content in the selections was attributed to the decreased K+ efflux in them. Thus, the tolerant ecotypes possessed the strong ability to retain K+ or prevent K+ loss under salt stress. In fact, the positive relationship between K+ retention and salinity tolerance was also supported by increasing similar findings in other species such as barley, wheat and Thellungiella halophila [39–41, 87]. Some high-affinity K+ transporters including HAK5, CHX17 and KUP1 were previously reported to play active roles for K+ uptake, accumulation and homeostasis under a low K+ condition [47, 49, 52–53, 88–89]. Consistently, in this study, a comparative analysis of AtHAK5, AtCHX17 and AtKUP1 expression levels indicated that there is a positive relationship the correlation between these genes and high K+ content in the selected accessions. As such, AtHAK5 transcript abundance in the tolerant accessions was significantly higher than in Col-0, most markedly in Mog-11 and Got-1 after salt treatment (Fig 3A and S4 Table). AtCHX17 and AtKUP1 were both inducible obviously by salinity in different treated time point (Fig 3B and 3C and S4 Table), especially in the tolerant accessions including Mog-11, Looe-2 and Wil-1 (Fig 3B and 3C). The higher increased expression of AtHAK5, AtCHX17 and AtKUP1 after salt treatment is consistent with the high ability to retain K+ in the tolerant accessions, implying that AtHAK5, AtCHX17 and AtKUP1 may be essential to improve K+ uptake and results in more K+ to be retained in the tolerant accessions. Next, it could be interesting to further explore whether the up-regulation of these genes is caused by the polymorphisms of them. Moreover, a strong K+ retention is also able attributed to the smaller salt-induced K+ leakage in the control of K+ channels including some of Shaker-like outward-rectifying depolarization-activated K+ channels, K+-Ca2+ activated outward-rectifying channels (KCO) and nonselective cation channels (NSCCs) [37, 90–93], and these K+ channels could also work together with the above K+ transporters to retain K+ or prevent K+ loss under salt stress. Previously, K+ efflux from plant tissues in Arabidopsis was largely conferred by the gated outward-rectifying depolarization-activated K+ channel (GORK) [94], and the AtGORK expression levels in the selections were also detected in this study, but no significant difference were found among them in response to salinity (S7 Fig). Over all, it was concluded that the strong K+ retention in the tolerant selections was possibly attributed to the high-efficient K+ uptake mediated actively by the AtHAK5, AtCHX17 and AtKUP1 transporter, rather than the smaller K+ leakage regulated by the AtGORK channel.
Salinity tolerance has also commonly been associated with an enhanced ability of Na+ detoxification and thereby to maintain a low Na+ in cytoplasm [2, 6–7, 26, 95]. The SOS signaling pathway and AtNHX1, two major factors were reported to export Na+ out of the cell or sequester Na+ within the vacuole, and AtHKT1;1 are essential for root-to-shoot Na+ partitioning, also as a major player in plant salt tolerance [5–6, 16, 25, 31, 96]. Moreover, the critical functional region of the SOS1 protein is its auto-inhibitory C terminal SOS1 domain, which must be phosphorylated for the activation of the whole protein by the SOS2-SOS3 complex [97]. In the present study, the sequence comparison among the Arabidopsis accessions focused on this region identified that all but one carried the alternative allele to Col-0 at position 1116, which lies within the C terminal auto-inhibitory domain (S2 Fig). However, this allele cannot be critical for gene function, as it is also carried by both tolerant and sensitive accessions (S2–S4 Fig). Similarly, there were also no critical variations in the AtSOS2, AtSOS3, AtNHX1 and AtHKT1;1 of the selected accessions (S5 Fig). Moreover, there were little variations in the transcriptional behavior of the AtSOS genes, AtNHX1 and AtHKT1;1 genes (Fig 4 and S5 Table), which could be due to the polymorphism of the promoter regions of those genes among the selections. However, when challenged by NaCl, the transcription levels of AtSOS genes in the tolerant selections showed no significant changes (Fig 4A–4C and S5 Table), thus, it was concluded that there was no difference in the efficiency of regulating Na+ efflux at cellular level among the tested accessions, which correspond to the similar high Na+ levels of the tested accessions. Consistently, a similar transcriptional variation in response to salt stress in Arabidopsis were reported previously [71]. In addition, the transcription level of AtNHX1 decreased in Mog-11, Looe-2 and Sav-0 at 3 h after salt treatment (Fig 4D and S5 Table), and it was still not clearly why the AtHKT1;1 gene was expressed obviously lower in most of the tolerant ones than in Col-0 when exposed to salt stress (Fig 4E and S5 Table). In all, these results supported that the SOS signaling pathway, as well as AtNHX1 and AtHKT1;1, could be as a fundamental and conserved mechanism to the elevated salinity tolerance displayed by the seven accessions, but not a key special factor to distinct them from Col-0.
Salinity damage can be mitigated if the plant tissue effectively accumulates compatible solutes. The presence of these compounds relieves the osmotic pressure imposed by saline soil water and thus allows the plant to continue taking up water, stabilize the photosystem II complex, protect the structure of enzymes and proteins, maintain membrane integrity and protect cells against oxidative damage by scavenging ROS [6, 98–100]. In this study, an elevated level of such solutes, in particular that of proline, was found and could be as a feature of the tolerant accessions (Fig 5, S8 Fig and S5 Table), and consistent with this physiological difference, the lower transcription kinetics of AtProDH1 and AtP5CDH which encode enzymes involved in proline degradation [81], were noted between the tolerant accessions and Col-0 when exposed to salinity stress (Fig 5A and Fig 6). A similar conclusion was previously reached for the salinity tolerant accession Bu-5 [56]. However, it was not observed a positive salinity response of some key enzyme genes involved in proline biosynthesis (S6 Fig), for which the reasonable explanations are possibly that de novo proline comes at high carbon cost and is not preferentially adopted by plants [81]. On the other hand, ROS play a dual role in plants acting as both key regulators of many biological processes including growth and development, cell cycle, hormone signaling, and as toxic by-products that cause oxidative stress and cell damage during different stress conditions [101–104]. Salinity stress has long been known to cause the over-accumulation of ROS in plants [105–106], and the excessive ROS scavenging is also regarded as a basic strategy for plant adaptation to stress [2, 5–6, 107]. Previously, it has been reported that higher proline levels might be essential to detoxify salt-tress induced ROS produced in plant tissues [100, 108–112], and high K+ content would also help to reduce the ROS amount in plants [113]. As a consequence, in this study, the tolerant accessions indeed exhibited a lower level of ROS than Col-0 (Fig 7A and 7B). Furthermore, it has already been reported that a number of enzymatic and non-enzymatic mechanisms to detoxify ROS existed in plant cells, such as SODs, which dismutate O2 ·- to O2 and H2O2, is a key element of the enzymatic protective system [107, 114], and over-expression of various SOD genes in higher plants enhanced tolerance to salinity stress [115]. Consistently, we also found a higher SOD activity in the tolerant accessions compared to Col-0, and a higher transcript level of AtCSD1 (except Mog-11) (Fig 7C and Fig 8A). In addition, there were also higher transcript abundance of AtCAT2 and AtAPX2 (Fig 8B and 8C), suggesting that these protective enzymes participated actively in ROS scavenging. Notably, APX has a higher affinity for H2O2 than CAT, and could play a more active role in ROS scavenging by according to their elevated expression of AtAPX2, especially in Mog-11, Wil-1 and Sav-0 (Fig 8C). Moreover, two upstream transcription factors AtZAT10 and AtZAT12 in the ‘ROS gene network’ [2, 102], were also up-regulated significantly in the tolerant accessions (Fig 8D and 8E), and the data was in agreement with the previous report [71]. Therefore, we concluded that the tolerant accessions also possessed these adaptive mechanisms of slowing down proline degradation and improving ROS scavengers to enhance salinity tolerance.
In conclusion, several salt-tolerant accessions have been identified from a collection of over 80 diverse accessions, while their differential salt tolerance is independent to the essential SOS pathway and other well-known Na+-related transporter genes, as AtNHX1 and AtHKT1;1, as well as the Na+ content in plants, However, the high K+ content, which is the result of the low K+ efflux and high expression of the K+ transporters genes AtHAK5, AtKUP1 and AtCHX17, plays important role in the salinity response of these tolerant accessions. An elevated level of compatible solutes and a reduced level of ROS in plants provided further physiological indicators of the tolerant plants’ improved ability to adapt to the salinity stress. These data suggest that the tolerant accessions were better pre-conditioned to survive salinity stress through more responsive regulation of K+ homeostasis and more sensitive transcription of key stress response genes.
Supporting Information
(A) Soil-grown 3-week-old plants watered with 0 or 500 mM NaCl for 20 days, (B) Hydroponics-grown 5-day-old seedlings exposed to 0 or 200 mM NaCl for 3 days. a: Col-0; b: Looe-2; c: Mog-11; d: Wil-1; e: Nd-1; f: Bs-1; g: Sav-0; h: Got-1. Col-0 is marked by a red ellipse.
(TIF)
(A) The structure of SOS1. The transmembrane domain is shown in yellow, the cytosolic domain in blue, the cyclic nucleotide binding domain in green and the autoinhibitory domain in red. (B) Allelic variation for the SOS1 sequence among the selected accessions. SOS1 sequences of the indicated species were aligned using CLUSTAL-W. The SOS1 sequence of the salinity-sensitive accession Mh-1 is shown in S3 Fig Peptide changes are marked by vertical red lines and solid arrowheads (blue arrowhead: T1116A, grey arrowhead: D1125E). The DSPS motif is boxed in red.
(TIF)
The 4-day-old seedlings of Mh-1 and Col-0 accessions were exposed to 0 or 150 mM NaCl for 15 days. Bar = 0.5 cm.
(TIF)
SOS1 sequences of the indicated species were aligned using CLUSTAL-W. Peptide changes are marked by blue arrowhead: T1116A.
(TIF)
Domain structure of (A) SOS1 (see also S2 Fig), (B) SOS2, (C) SOS3, (D) NHX1 and (E) HKT1;1. The SOS2 FISL/NAF motif is displayed in cyan, the protein phosphatase interaction (PPI) motif in blue, and the rest of PPI domain in green. The four SOS3 EF-hand motifs are displayed in orange, and the vertical red line indicates variable residue V138I in EF-hand 3. NHX1 contains 12 transmembrane motifs and HKT1;1 eight. The vertical red lines in HKT1;1 represent variable residues R003I and L024V.
(TIF)
The transcript levels of (A) P5CR, (B) P5CS1 and (C) P5CS2 in Col-0 and the selected accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3). Significant differences from Col-0 accession at the same time point are indicated by * (P < 0.05).
(TIF)
The transcript level of GORK in Col-0 and the selected accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3), Significant differences from Col-0 accession at the same time point are indicated by * (P < 0.05).
(TIF)
(TIF)
(DOC)
(DOC)
(DOC)
The expression levels of these genes normalized to ACTIN2 gene respectively were analyzed in Col-0 and the selected tolerant accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3).
(DOC)
The expression levels of these genes normalized to ACTIN2 gene respectively were analyzed in Col-0 and the selected tolerant accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3).
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 31270327 and No. 31222005), the "1000-talents Plan" from China for young researchers (11200095551303) and the Research Foundation for Excellent Young Scientist in Shandong Province (No. BS2013SW005). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Greenway H, Munns R. Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol. 1980;31: 149–190. [Google Scholar]
- 2. Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59: 651–681. 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- 3. Rahnama A, James RA, Poustini K, Munns R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct Plant Biol. 2010;37: 255–263. [Google Scholar]
- 4. Chinnusamy V, Jagendorf A, Zhu JK. Understanding and improving salt tolerance in plants. Crop Sci. 2005;45: 437–448. [Google Scholar]
- 5. Deinlein U, Stephan AB, Horie T, Luo W, Xu GH, Schroeder JI. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19: 371–379. 10.1016/j.tplants.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta B, Huang B. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics. 2014;2014: 701596 10.1155/2014/701596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005;167: 645–663. [DOI] [PubMed] [Google Scholar]
- 8. Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6: 66–71. [DOI] [PubMed] [Google Scholar]
- 9. Zhu JK. Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol. 2001;4: 401–406. [DOI] [PubMed] [Google Scholar]
- 10. Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol. 2003;6: 441–445. [DOI] [PubMed] [Google Scholar]
- 11. Guo Y, Halfter U, Ishitani M, Zhu JK. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell. 2001;13: 1383–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishitani M, Liu J, Halfter U, Kim CS, Shi M, Zhu JK. SOS3 function in plant salt tolerance requires myristoylation and calcium binding. Plant Cell. 2000;12: 1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X. The Salt Overly Sensitive (SOS) pathway, established and emerging roles. Mol Plant. 2013;6: 275–286. 10.1093/mp/sst017 [DOI] [PubMed] [Google Scholar]
- 14. Liu J, Ishitani M, Halfter U, Kim CS, Zhu JK. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci USA. 2000;97: 3730–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Zhu JK. A calcium sensor homolog required for plant salt tolerance. Science. 1998;280: 1943–1945. [DOI] [PubMed] [Google Scholar]
- 16. Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA. 2002;99: 8436–8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi H, Ishitani M, Kim CS, Zhu JK. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA. 2000;97: 6896–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi H, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002;14: 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot. 2003;91: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53: 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feki K, Quintero FJ, Khoudi H, Leidi EO, Masmoudi K, Pardo JM, et al. A constitutively active form of a durum wheat Na+/H+ antiporter SOS1 confers high salt tolerance to transgenic Arabidopsis . Plant Cell Rep. 2014;33: 277–288. 10.1007/s00299-013-1528-9 [DOI] [PubMed] [Google Scholar]
- 22. Shi H, Lee BH, Wu SJ, Zhu JK. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana . Nat Biotechnol. 2003;21: 81–85. [DOI] [PubMed] [Google Scholar]
- 23. Yang Q, Chen ZZ, Zhou XF, Yin HB, Li X, Xin XF, et al. Overexpression of SOS (Salt Overly Sensitive) genes increases salt tolerance in transgenic Arabidopsis . Mol Plant. 2009;2: 22–31. 10.1093/mp/ssn058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berthomieu P, Conéjéro G, Nublat A, Brackenbury WJ, Lambert C, Savio C, et al. Functional analysis of AtHKT 1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003;22: 2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hauser F, Horie T. A conserved primary salt tolerance mechanism mediated by HKT transporters, a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ. 2010;33: 552–565. 10.1111/j.1365-3040.2009.02056.x [DOI] [PubMed] [Google Scholar]
- 26. Munns R, James RA, Xu B, Athman A, Conn SJ, Jordans C, et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat Biotechnol. 2012;30: 360–364. 10.1038/nbt.2120 [DOI] [PubMed] [Google Scholar]
- 27. Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee BH, Matsumoto TK, et al. AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc Natl Acad Sci USA. 2001;98: 14150–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rus A, Lee BH, Muñoz-Mayor A, Sharkhuu A, Miura K, Zhu JK, et al. AtHKT1 facilitates Na+ homeostasis and K+ nutrition in planta. Plant Physiol. 2004;136: 2500–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sunarpi, Horie T, Motoda J, Kubo M, Yang H, Yoda K, et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005;44: 928–938. [DOI] [PubMed] [Google Scholar]
- 30. Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, et al. The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol. 2000;122: 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ for its kinase activity and C-terminal regulatory domain for salt antiport in Arabidopsis . Science. 1999;285: 1256–1258. [DOI] [PubMed] [Google Scholar]
- 32. Yamaguchi T, Apse MP, Shi H, Blumwald E. Topological analysis of a plant vacuolar Na+/H+ antiporter reveals a luminal C terminus that regulates antiporter cation selectivity. Proc Natl Acad Sci USA. 2003;100: 12510–12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang HX, Blumwald E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol. 2001;19: 765–768. [DOI] [PubMed] [Google Scholar]
- 34. Zhang HX, Hodson JN, Williams JP, Blumwald E. Engineering salt-tolerant Brassica plants, characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci USA. 2001;98: 12832–12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochika H, et al. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004;45: 146–159. [DOI] [PubMed] [Google Scholar]
- 36. Amtmann A, Troufflard S, Armengaud P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant. 2008;133: 682–691. 10.1111/j.1399-3054.2008.01075.x [DOI] [PubMed] [Google Scholar]
- 37. Shabala S, Cuin TA. Potassium transport and plant salt tolerance. Physiol Plant. 2008;133: 651–669. [DOI] [PubMed] [Google Scholar]
- 38. Zhu JK, Liu J, Xiong L. Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell. 1998;10: 1181–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S. Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ. 2005;28: 1230–1246. [Google Scholar]
- 40. Chen Z, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007;145: 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cuin TA, Betts SA, Chalmandrier R, Shabala S. A root’s ability to retain K+ correlates with salt tolerance in wheat. J Exp Bot. 2008;59: 2697–2706. 10.1093/jxb/ern128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, et al. Phylogenetic relationships within cation transporter families of Arabidopsis . Plant Physiol. 2001;126: 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Véry A, Sentenac H. Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol. 2003;54: 575–603. [DOI] [PubMed] [Google Scholar]
- 44. Wang Y, Wu WH. Plant sensing and signaling in response to K+-deficiency. Mol Plant. 2010;3: 280–287. 10.1093/mp/ssq006 [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Wu WH. Potassium transport and signaling in higher plants. Annu Rev Plant Biol. 2013;64: 451–476. 10.1146/annurev-arplant-050312-120153 [DOI] [PubMed] [Google Scholar]
- 46. Ward JM, Mäser P, Schroeder JI. Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol. 2009;71: 59–82. 10.1146/annurev.physiol.010908.163204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gierth M, Maser P, Schroeder JI. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 2005;137: 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grabov A. Plant KT/KUP/HAK potassium transporters: single family-multiple functions. Ann Bot. 2007;99: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maathuis FJ, Sanders D. Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana . Proc Natl Acad Sci USA. 1994;91: 9272–9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harada H, Leigh RA. Genetic mapping of natural variation in potassium concentrations in shoots of Arabidopsis thaliana . J Exp Bot. 2006;57: 953–960. [DOI] [PubMed] [Google Scholar]
- 51. Fu HH, Luan S. AtKUP1: A dual-affinity K+ transporter from Arabidopsis . Plant Cell. 1998;10: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim EJ, Kwak JM, Uozumi N, Schroeder JI. AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell. 1998;10: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cellier F, Conejero G, Ricaud L, Luu DT, Lepetit M, Gosti F, et al. Characterization of AtCHX17, a member of the cation/H+ exchangers, CHX family, from Arabidopsis thaliana suggests a role in K+ homeostasis. Plant J. 2004;39: 834–846. [DOI] [PubMed] [Google Scholar]
- 54. Gollner K, Schweizer P, Bai Y, Panstruga R. Natural genetic resources of Arabidopsis thaliana reveal a high prevalence and unexpected phenotypic plasticity of RPW8-mediated powdery mildew resistance. New Phytol. 2008;177: 725–742. 10.1111/j.1469-8137.2007.02339.x [DOI] [PubMed] [Google Scholar]
- 55. Hannah MA, Wiese D, Freund S, Fiehn O, Heyer AG. Natural genetic variation of freezing tolerance in Arabidopsis . Plant Physiol. 2006;142: 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Katori T, Ikeda A, Iuchi S, Kobayashi M, Shinozaki K. Dissecting the genetic control of natural variation in salt tolerance of Arabidopsis thaliana accessions. J Exp Bot. 2010;61: 1125–1138. 10.1093/jxb/erp376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Loudet O, Chaillou S, Camilleri C, Bouchez D, Daniel-Vedele F. Bay-0 × Shahdara recombinant inbred line population: a powerful tool for the genetic dissection of complex traits in Arabidopsis . Theor Appl Genet. 2002;104: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 58. Quesada V, Garcia-Martinez S, Piqueras P, Ponce MR, Micol JL. Genetic architecture of NaCl tolerance in Arabidopsis . Plant Physiol. 2002;130: 951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kowalski SP, Lan TH, Feldmann KA, Paterson AH. QTL mapping of naturally-occurring variation in flowering time of Arabidopsis thaliana . Mol Gen Genet. 1994;245: 548–555. [DOI] [PubMed] [Google Scholar]
- 60. Swarup K, Alonso-Blanco C, Lynn JR, Michaels SD, Amasino RM, Koornneef M, et al. Natural allelic variation identifies new genes in the Arabidopsis circadian system. Plant J. 1999;20: 67–77. [DOI] [PubMed] [Google Scholar]
- 61. Symonds VV, Hatlestad G, Lloyd AM. Natural allelic variation defines a role for ATMYC1: trichome cell fate determination. PLoS Genet. 2011;7: e1002069 10.1371/journal.pgen.1002069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Koornneef M. Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana . Proc Natl Acad Sci USA. 1999;96: 4710–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weigel D, Mott R. The 1001 Genomes Project for Arabidopsis thaliana . Genome Biol. 2009;10: 107 10.1186/gb-2009-10-5-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jiang Y, Deyholos MK. Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol. 2006;6: 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Van Poecke RMP, Sato M, Lenarz-Wyatt L, Weisberg S, Katagiri F. Natural variation in RPS2-mediated resistance among Arabidopsis genotypes, correlation between gene expression profiles and phenotypic responses. Plant Cell. 2007;19: 4046–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alonso-Blanco C, Gomez-Mena C, Llorente F, Koornneef M, Salinas J, Martínez-Zapater JM. Genetic and molecular analyses of natural variation indicate CBF2 as a candidate gene for underlying a freezing tolerance quantitative trait locus in Arabidopsis . Plant Physiol. 2005;139: 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jha D, Shirley N, Tester M, Roy SJ. Variation in salinity tolerance and shoot sodium accumulation in Arabidopsis ecotypes linked to differences in the natural expression levels of transporters involved in sodium transport. Plant Cell Environ. 2010;33: 793–804. 10.1111/j.1365-3040.2009.02105.x [DOI] [PubMed] [Google Scholar]
- 68. Rus A, Baxter I, Muthukumar B, Gustin J, Lahner B, Yakubova E, et al. Natural variants of AtHKT1 enhance Na+ accumulation in two wild populations of Arabidopsis . PLoS Genet. 2006;2: e210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Y, Yang L, Zheng Z, Grumet R, Loescher W. Transcriptomic and Physiological Variations of Three Arabidopsis Ecotypes in Response to Salt Stress. PLoS One. 2013;8: e69036 10.1371/journal.pone.0069036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baxter I, Brazelton JN, Yu D, Huang YS, Lahner B, Yakubova E, et al. A coastal cline in sodium accumulation in Arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1 . PLoS Genet. 2010;6: e1001193 10.1371/journal.pgen.1001193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chan ZL, Loescher W, Grumet R. Transcriptional variation in response to salt stress in commonly used Arabidopsis thaliana accessions. Plant Physiol Bioch. 2013;73: 1–13. 10.1016/j.plaphy.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 72. Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents, verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA-Biomembranes. 1989;975: 384–394. [Google Scholar]
- 73. Storey R. Salt tolerance, ion relations and the effects of root medium on the response of Citrus to salinity. Aust J Plant Physiol. 1995;22: 101–114. [Google Scholar]
- 74. Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39: 205–207. [Google Scholar]
- 75. Bailey RW. The reaction of pentoses with anthrone. Biochem J. 1958;68: 669–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72: 248–254. [DOI] [PubMed] [Google Scholar]
- 77. Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J. 1999;17: 603–614. [DOI] [PubMed] [Google Scholar]
- 78. Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB. Subcellular localization of H2O 2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction, Plant J. 1997;11: 1187–1194. [Google Scholar]
- 79. Giannopolitis CN, Ries SK. Superoxide dismutase: I. Occurrence in higher plants. Plant Physiol. 1977;59: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT method. Method. 2001;25: 402–408. [DOI] [PubMed] [Google Scholar]
- 81. Funck D, Eckard S, Müller G. Non-redundant functions of two proline dehydrogenase isoforms in Arabidopsis . BMC Plant Biol. 2010;10: 70 10.1186/1471-2229-10-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shindo C, Bernasconi G, Hardtke CS. Natural genetic variation in Arabidopsis, tools, traits and prospects for evolutionary ecology. Ann Bot-London. 2007;99: 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Flowers TJ. Improving crop salt tolerance. J Exp Bot. 2004;55: 307–319. [DOI] [PubMed] [Google Scholar]
- 84. Borsani O, Cuartero J, Fernandez JA, Valpuesta V, Botella MA. Identification of two loci in tomato reveals distinct mechanisms for salt tolerance. Plant Cell. 2001;13: 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hu Y, Fricke W, Schmidhalter U. Salinity and the growth of non-halophytic grass leaves: the role of mineral nutrient distribution. Funct Plant Biol. 2005;32: 973–985. [DOI] [PubMed] [Google Scholar]
- 86. Maathuis FJM, Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot. 1999;84: 123–133. [Google Scholar]
- 87. Ali Z, Park HC, Ali A, Oh DH, Aman R, Kropornicka A, et al. TsHKT1;2, a HKT1 homolog from the extremophile Arabidopsis relative Thellungiella salsuginea, shows K+ specificity in the presence of NaCl. Plant Physiol. 2012;158: 1463–1474. 10.1104/pp.111.193110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Alemán F, Nieves-Cordones M, Martínez V, Rubio F. Root K+ acquisition in plants: the Arabidopsis thaliana model. Plant Cell Physiol. 2011;52: 1603–1612. 10.1093/pcp/pcr096 [DOI] [PubMed] [Google Scholar]
- 89. Shabala S, Pottosin I. Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiol Plant. 2014;151: 257–279. 10.1111/ppl.12165 [DOI] [PubMed] [Google Scholar]
- 90. Anschütz U, Becker D, Shabala S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J Plant Physiol. 2014;171: 670–687. 10.1016/j.jplph.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 91. Maathuis FJ. The role of monovalent cation transporters in plant responses to salinity. J Exp Bot. 2006;57: 1137–1147. [DOI] [PubMed] [Google Scholar]
- 92. Shabala S, Babourina O, Newman I. Ion-specific mechanisms of osmoregulation in bean mesophyll cells. J Exp Bot. 2000;51: 1243–1253. [PubMed] [Google Scholar]
- 93. Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, et al. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006;141: 1653–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, et al. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci. 2010;123: 1468–1479. 10.1242/jcs.064352 [DOI] [PubMed] [Google Scholar]
- 95. Volkov V, Amtmann A. Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, has specific root ion-channel features supporting K+/Na+ homeostasis under salinity stress. Plant J. 2006;48: 342–353. [DOI] [PubMed] [Google Scholar]
- 96. Zhang JL, Shi HZ. Physiological and molecular mechanisms of plant salt tolerance. Photosynth Res. 2013;115: 1–22. 10.1007/s11120-013-9813-6 [DOI] [PubMed] [Google Scholar]
- 97. Quintero FJ, Martinez-Atienza J, Villalta I, Jiang X, Kim WY, Ali Z, et al. Activation of the plasma membrane Na+/H+ antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc Natl Acad Sci USA. 2011;108: 2611–2616. 10.1073/pnas.1018921108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cuin TA, Shabala S. Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ. 2007;30: 875–885. [DOI] [PubMed] [Google Scholar]
- 99. Shen B, Hohmann S, Jensen RG, Bohnert aH. Roles of sugar alcohols in osmotic stress adaptation. Replacement of glycerol by mannitol and sorbitol in yeast. Plant Physiol. 1999;121: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15: 89–97. 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 101. Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci. 2000;57: 779–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9: 490–498. [DOI] [PubMed] [Google Scholar]
- 103. Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17: 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, et al. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9: 436–442. [DOI] [PubMed] [Google Scholar]
- 105. Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33: 453–467. 10.1111/j.1365-3040.2009.02041.x [DOI] [PubMed] [Google Scholar]
- 106. Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, et al. ROS signaling: the new wave? Trends Plant Sci. 2011;16: 300–309. 10.1016/j.tplants.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 107. Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48: 909–930. 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 108. Smirnoff N, Cumbes QJ. Hydroxyl radical-scavenging activity of compatible solutes. Phytochemistry. 1989;28: 1057–1060. [Google Scholar]
- 109. Shen B, Jensen RG, Bohnert HJ. Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol. 1997;115: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Khedr AHA, Abbas MA, Abdel Wahid AA, Paul Quick W, Abogadallah GM. Proline induces the expression of salt-stress responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J Exp Bot. 2003;54: 2553–2562. [DOI] [PubMed] [Google Scholar]
- 111. Székely G, Abrahám E, Cséplo A, Rigó G, Zsigmond L, Csiszáj R et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008;53: 11–28. [DOI] [PubMed] [Google Scholar]
- 112. Miller G, Honig A, Stein H, Suzuki N, Mittler R, Zilberstein A, et al. Unraveling △1-pyrroline-5-carboxylateproline cycle in plants by uncoupled expression of proline oxidation enzymes. J Biol Chem. 2009;284: 26482–26492. 10.1074/jbc.M109.009340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci. 2005;168: 521–530. [Google Scholar]
- 114. Bowler C, Van Camp W, Van Montagu M, Inzé D. Superoxide dismutase in plants. CRC Crit Rev Plant Sci. 1994;13: 199–218. [Google Scholar]
- 115. Tanaka Y, Hibino T, Hayashi Y, Tanaka A, Kishitani S, Takabe T, et al. Salt tolerance of transgenic rice overexpressing yeast mitochondrial Mn-SOD in chloroplasts. Plant Sci. 1999;148: 131–138. 11189764 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Soil-grown 3-week-old plants watered with 0 or 500 mM NaCl for 20 days, (B) Hydroponics-grown 5-day-old seedlings exposed to 0 or 200 mM NaCl for 3 days. a: Col-0; b: Looe-2; c: Mog-11; d: Wil-1; e: Nd-1; f: Bs-1; g: Sav-0; h: Got-1. Col-0 is marked by a red ellipse.
(TIF)
(A) The structure of SOS1. The transmembrane domain is shown in yellow, the cytosolic domain in blue, the cyclic nucleotide binding domain in green and the autoinhibitory domain in red. (B) Allelic variation for the SOS1 sequence among the selected accessions. SOS1 sequences of the indicated species were aligned using CLUSTAL-W. The SOS1 sequence of the salinity-sensitive accession Mh-1 is shown in S3 Fig Peptide changes are marked by vertical red lines and solid arrowheads (blue arrowhead: T1116A, grey arrowhead: D1125E). The DSPS motif is boxed in red.
(TIF)
The 4-day-old seedlings of Mh-1 and Col-0 accessions were exposed to 0 or 150 mM NaCl for 15 days. Bar = 0.5 cm.
(TIF)
SOS1 sequences of the indicated species were aligned using CLUSTAL-W. Peptide changes are marked by blue arrowhead: T1116A.
(TIF)
Domain structure of (A) SOS1 (see also S2 Fig), (B) SOS2, (C) SOS3, (D) NHX1 and (E) HKT1;1. The SOS2 FISL/NAF motif is displayed in cyan, the protein phosphatase interaction (PPI) motif in blue, and the rest of PPI domain in green. The four SOS3 EF-hand motifs are displayed in orange, and the vertical red line indicates variable residue V138I in EF-hand 3. NHX1 contains 12 transmembrane motifs and HKT1;1 eight. The vertical red lines in HKT1;1 represent variable residues R003I and L024V.
(TIF)
The transcript levels of (A) P5CR, (B) P5CS1 and (C) P5CS2 in Col-0 and the selected accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3). Significant differences from Col-0 accession at the same time point are indicated by * (P < 0.05).
(TIF)
The transcript level of GORK in Col-0 and the selected accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3), Significant differences from Col-0 accession at the same time point are indicated by * (P < 0.05).
(TIF)
(TIF)
(DOC)
(DOC)
(DOC)
The expression levels of these genes normalized to ACTIN2 gene respectively were analyzed in Col-0 and the selected tolerant accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3).
(DOC)
The expression levels of these genes normalized to ACTIN2 gene respectively were analyzed in Col-0 and the selected tolerant accessions exposed to 100 mM NaCl for 0, 3 and 6 h. Values given as mean ± SE (n = 3).
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.