Abstract
Celiac disease (CD) is an intestinal inflammatory disease that manifests in genetically susceptible individuals when exposed to dietary gluten. It is a common chronic disorder, with a prevalence of 1% in Europe and North America. Although the disease primarily affects the gut, the clinical spectrum of CD is remarkably varied, and the disease can affect many extraintestinal organs and systems, including the liver. The hepatic dysfunction presenting in CD ranges from asymptomatic liver enzyme elevations or nonspecific reactive hepatitis (cryptogenic liver disorders), to chronic liver disease. In this article, we review the clinical presentations and possible mechanisms of CD-related liver injury to identify strategies for the diagnosis and treatment of these disorders in childhood.
Keywords: Celiac disease, Cryptogenic hypertransaminasemia, Autoimmune liver disease, End-stage liver disease, Fatty liver
Core tip: Celiac disease (CD) is increasingly reported in children who are symptomless or present atypical symptoms and signs. Liver abnormalities are common extraintestinal manifestations in patients with CD and range from mild hepatic injury to severe liver disease. Awareness of this may help clinicians to improve strategies for the diagnosis and treatment of these disorders in childhood.
INTRODUCTION
Celiac disease (CD) is a chronic intestinal inflammatory disease that manifests in genetically susceptible individuals when exposed to dietary gluten[1]. The prevalence of CD is high in the European and North American population (1%), reaching 10% to 15% in patients who have first-degree relatives with this disease[1,2]. Genetic predisposition plays an important role in the development of CD. Ninety percent of affected individuals carry the HLA-DQ2 (e.g., DQA1*0501-DQB1*0201) haplotype, 5% the DQ8 haplotype (e.g., DQA1*0301-DQB1*0302), and the remaining 5% carry at least one of the two DQ2 alleles (frequently the DQB1*0201)[1,3]. Ingestion of gluten is necessary for the disease to develop[4]. Immunogenic peptides, created by deamidation of food-derived gliadin peptides by small intestinal tissue transglutaminase, are presented by antigen-presenting cells, mostly dendritic cells bearing HLA-DQ2 and DQ8 molecules, to proinflammatory CD4+ T cells, activating them[4]. Upon activation, the T cell produces a variety of cytokines like interferon-gamma as part of a Th1 response which results in clonal expansion of activated T cells, stimulation of cytotoxic T cells and B cell recruitment with subsequent production of anti-gliadin (AGA) and anti-transglutaminase antibodies (tTGA)[4]. Thus, intolerance to gluten is responsible for an immune-mediated damage of the intestinal mucosa, which resolves after a gluten-free diet (GFD)[4].
CD diagnosis still relies on serology and small intestinal biopsy. tTGA and anti-endomysial antibodies (EMA) of the immunoglobulin A (IgA) class have the highest diagnostic accuracy with a sensitivity of 98% and a specificity ranging from 90% to 99%. Deamidated gliadin peptide antibodies (DGP) of IgG class are a valuable diagnostic tool for identifying CD in patients with IgA deficiency and in children aged less than 2 years. Small bowel biopsy remains in adults the diagnostic gold standard, whereas in children and adolescents, as recently recommended, CD diagnosis can be accepted without the need for duodenal biopsy in symptomatic cases showing tTGA at high titer (> 10-times upper normal limit), backed up by EMA and HLA-DQ2 and/or positive DQ8[3].
Although CD primarily affects the gut, the clinical manifestations of the disease are remarkably wide, with many extraintestinal organs and systems, including the liver, affected[5,6]. Liver changes in patients with CD have been reported since 1977 by Hagander et al[7] who demonstrated that transaminases were often increased in untreated CD, normalizing upon a strict GFD. More recently, studies performed after CD was identified as an autoimmune disease, have underlined the strong relationship between CD and autoimmune liver disorders. In this article, we review the clinical presentations and possible mechanisms of CD-related liver injury in order to identify strategies for the diagnosis and treatment of these disorders in childhood.
CRYPTOGENIC LIVER DISORDER (CELIAC HEPATITIS)
An association between CD and cryptogenic liver damage was first reported in 1977 by Hagander et al[7] who found that 40% of adults with incipient CD had increased serum concentrations of transaminases, which returned to normal upon GFD in the majority of patients. One year later, Lindberg et al[8] reported elevation of serum aminotransferases in about one-third of pediatric patients with CD. Approximately one decade later, a mild to moderate hypertransaminasemia was observed in about 60% of symptomatic Italian children aged less than 2 years with newly diagnosed CD[9]. Prevalence studies have reported that transaminases are elevated in 39% to 47% of celiac adults[10-12] and in 26% to 57% of children at diagnosis of CD (Table 1)[9,13-15]. Frequently, elevation in transaminases is mild, and is not associated with hepatomegaly or splenomegaly. In those patients who had undergone liver biopsy[10,16-18], histological changes such as Kupffer cell hyperplasia, mononuclear cell infiltration, steatosis, and mild fibrosis have been reported. In most cases, transaminase values normalized upon a 1-year GFD.
Table 1.
Studies reporting the prevalence of cryptogenic hypertransaminasemia in children and adolescents with celiac disease
| Ref. | Study design | Study population with CD | Diagnosis of CD | Number of patients with elevated transaminases | Effect of GFD | Comment |
| Bonamico et al[9], 1986 | Observational | 65 untreated symptomatic children aged 6-mo to 18 yr | Intestinal biopsy | 37 (56.9%) had elevated (> 45 U/L) ALT (3.1%) or AST (29.2%) or both (24.6%) | Only 5 cases had a follow-up for 3-4 wk after GFD: normalization of transaminases was achieved in all | Excluded were Hepatitis A and B, but not other causes of liver disease |
| Farre et al[13], 2002 | Prospective | 114 untreated symptomatic children aged 9-mo to 17 yr | Serology (EMA IgA or IgG and tTGA IgA) and/or intestinal biopsy | 37 (32.0%) had elevated1 ALT-or- AST (14.9%) or both (14.9%) | 35 of 37 had a follow-up for 9-18 mo after GFD: normalization of transaminases was achieved in all | |
| Arslan et al[14], 2005 | Observational | 27 untreated symptomatic children with a mean age of 6 (SD 5) years | Serology (EMA IgA and AGA IgA/IgG) and/or intestinal biopsy | 7 (25.9%) had elevated ALT (> 45 U/L) | All patients had normalization of transaminases after 2-11 mo of GFD | |
| Di Biase et al[15], 2010 | Prospective | 350 untreated children with suspected CD aged 1 to 16 yr | Serology and intestinal biopsy according to the ESPGHAN criteria | 140 (40.0%) had elevated AST (≥ 38 U/L) and/or ALT (≥ 41 U/L); four with values > 5 times upper normal levels | Normalization of transaminases after 6 mo of GFD was achieved in 133 (97.8%) of 136 children with transaminase values < 5 times upper normal levels | The four children with transaminase values > 5 times upper normal levels as well as the 3 children with persistent elevated transaminases had further laboratory investigation and were found to be affected by autoimmune hepatitis |
Normal reference values for AST < 50 U/L from 1 to 6 years, < 38 U/L from 6 to 18 years; for ALT < 31 U/L from 1 to 18 years. CD: Celiac disease; GFD: Gluten-free diet; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; IgA: Immunoglobulin A; IgG: Immunoglobulin G; EMA: Anti-endomysial antibodies; tTGA: Anti-tissue transglutaminase antibodies; AGA: Anti-gliadin antibodies; ESPGHAN: European Society for Paediatric Gastroenterology, Hepatology and Nutrition.
Conversely, CD is present in patients investigated because of chronic unexplained hypertransaminasemia. Volta et al[18] for the first time reported that adults with elevated concentrations of aminotransferases of unknown origin were affected by symptomless CD. Five of the 55 study patients with cryptogenic elevation of transaminases fulfilled the criteria for CD diagnosis. Other common causes of liver disease were excluded. Three of these patients showed histologically a picture of reactive hepatitis typical of CD patients with elevated transaminases. The importance of these findings has been confirmed by other investigators, who found a similar prevalence of CD in large patient populations with cryptogenic hypertransaminasemia[19].
Recently, Sainsbury et al[20] conducted a meta-analysis to estimate the prevalence of CD in adults with cryptogenic hypertransaminasemia, as well as the prevalence of hypertransaminasemia in those with incipient CD. The combined proportion with positive celiac serology and biopsy-proven CD in unexplained hypertransaminasemia were 6% (95%CI: 3%-10%) and 4% (1%-7%), respectively. However, there was significant heterogeneity between studies (P < 0.001). This is about four times the risk of CD, in the general population (about 1%)[20]. The combined proportion with abnormal serum aminotransferases in incipient CD was 27% (13%-44%). A 12-mo GFD normalized serum transaminase values in 63%-90% of patients. Discordant results were reported by Korpimäki et al[21] in a large population-based study including celiac patients with minor or atypical symptoms, and with or without GFD, as well as subjects without CD. The authors estimated that only 11% of the untreated celiac patients had elevated transaminase values. This prevalence was about the same as was found in treated CD cases and controls without CD. Variation in the CD clinical presentation and severity, as well as definition of the upper normal limits for serum transaminases may account for such discrepancies.
Also in children, hypertransaminasemia may represent the only manifestation of CD. In 1986 an 11-year-old girl with a chronic and unexplained elevated aminotransferases was reported. Liver histology evidenced slight inflammation of the portal tract[22]. CD was diagnosed on the basis of antireticulin antibodies and subsequently by intestinal biopsy. Seven years later six children with chronic hypertransaminasemia and histologic findings ranging from reactive hepatitis to moderately active chronic hepatitis, were reported[23]. They were asymptomatic and had jejunal histology consistent with CD diagnosis. In all subjects, transaminases normalized on a GFD. Resolution of hepatic histologic lesions occurred in two children, whereas aminotransferases increased in three children upon a gluten challenge[23]. Finally, in a prospective study involving 425 children and adolescents with isolated hypertransaminasemia, Iorio et al[24] found 166 patients with persistently (more than 6 mo) elevated transaminases of whom three (1.8%) were identified as having CD. Therefore, routine screening for CD is to be recommended in children with otherwise unexplained hypertransaminasemia.
AUTOIMMUNE LIVER DISORDERS ASSOCIATED WITH CELIAC DISEASE
Autoimmune liver disorders (AILD), including autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), and primary biliary cirrhosis (PBC) have been shown to be associated with CD[25-28].
AIH is a progressive inflammatory liver disorder and is more common among females. It is associated serologically with high levels of aminotransferases and IgG, the presence of autoantibodies, and histologically with interface hepatitis in the absence of known etiology[29]. Hepatitis at the portal-parenchymal interface (“interface hepatitis”) is typical. The picture is characterized by a lymphoplasmacytic infiltrate crossing the limiting plate and invading the liver parenchyma. Other associated lesions are hepatocyte swelling and pycnotic necrosis. Fibrosis is found in all forms of the disease except the mildest ones[30]. Two types of AIH can be recognized: type 1 AIH is associated with antinuclear antibodies and/or smooth muscle antibodies and affects adult patients much more commonly, while type 2 AIH, characterized by antibodies to liver-kidney microsome type 1, is usually confined to childhood CD[18,31].
In the late 1970s, CD was occasionally reported in patients with AIH[18,32-34]. Then several studies established a relationship between CD and AIH of both types 1 and 2[26]. The first of these studies included the largest cohort of AIH patients (e.g., 181, of whom 157 with type 1 and 24 with type 2) who were screened for CD by serology[18]. Among these patients, eight [4.4% (3.8% with type 1 and 8.3% with type 2 AIH)] were found to have raised levels of EMA IgA. Of these 8 antibody-positive patients, five underwent jejunal biopsy which revealed a subtotal villous atrophy typical of CD. In a recent systematic review[26] performed in adults, the prevalence of CD in AIH ranged between 2% and 20% but was approximately 4% in most studies.
In children, at first the association between CD and AIH was only reported in isolated cases[35-37]. Subsequently pediatric surveys have reported a wide prevalence of CD in AIH ranging from 3.6% to 12% (Table 2)[38-43]. In an Italian retrospective (1990-2005) multicenter study, Caprai et al[39] found that among 140 children with AILD, 23 (16%) had CD [19 with AIH (12 with type 1; 4 with type 2; 3 seronegative), 2 with autoimmune cholangitis and 2 with overlap syndrome]. CD was diagnosed before liver disease in 18 of them, though raised aminotransferases were found in 16 at CD diagnosis. Conversely, five of the 23 patients had a diagnosis of AILD before the identification of CD. Nineteen patients had liver-related non-organ-specific autoantibodies. Hepatic biopsy showed inflammatory lesions with features of autoimmune damage and different degrees of fibrosis in all 19 subjects and cirrhosis in 4 of them. All patients on GFD achieved remission on immunosuppressive therapy, but 14 relapsed either because treatment ceased or because the GFD was not respected. Diamanti et al[40] retrospectively (1990-2006) evaluated the CD prevalence in 40 AIH children. There were five cases of CD in the 40 AIH patients (12.5%); all five CD patients had type 1 AIH. In four patients (80%), AIH preceded the diagnosis of CD. On GFD the level of transaminases mildly decreased, and never reached normal concentrations. Tosun et al[41] who retrospectively evaluated the presence of CD in 15 AIH patients, found a prevalence of 46% (95%CI: 21%-67%), being the highest ever reported in pediatric literature, although the sample size is small. In a prospective study involving 26 Egyptian patients (aged 3.5-21 years) with AIH, El-Shabrawi et al[42] reported an 11.5% prevalence of CD. Very recently, in a retrospective and prospective evaluation (1995-2000), Nastasio et al[43] reported that among 79 patients with AIH, CD was present in 15 (19%) of them (9 had type 1, 3 type 2, and 3 were seronegative). All these patients achieved sustained remission on a GFD when treated with immunosuppressive therapy.
Table 2.
Studies reporting the prevalence of positive celiac serology or biopsy-proven celiac disease in children and adolescents with autoimmune liver diseases
| Ref. | Study design | Study population with AILD | Number of patients with CD | Effect of GFD |
| Caprai et al[39], 2008 | Retrospective | 140 patients aged 7-125 mo with AILD | 23 (16.4%) (19 with AIH; 2 with AIC; and 2 with overlap syndrome) had CD on the basis of serology (EMA IgA and/or tTGA IgA) Diagnosis of CD preceded the diagnosis of liver disease in 18 of the 23 patients | All patients achieved remission on GFD and immunosuppressive therapy, but 14 relapsed because of discontinuation of therapy or during spontaneous gluten challenge |
| Diamanti et al[40], 2008 | Retrospective | 40 patients aged 3-13.2 yr with AIH | 5 (12.5%) had CD on the basis of serology and histological findings In four patients CD was diagnosed after AIH onset | On GFD four patients showed a mild decrease in transaminases, but never a complete normalization |
| Tosun et al[41], 2010 | Retrospective | 15 patients aged 4-15 yr with AIH | 7 (46.0%) had CD on the basis of serology and histological findings CD and AIH were diagnosed concomitantly | Not available |
| El-Shabrawi et al[42], 2011 | Prospective | 26 patients aged 3.5-21 yr with AIH | CD serology (tTGA IgA and/or EMA IgA) was positive in 4 (15.4%). Three out of these four AIH (11.5%) showed histological findings of CD | Not available |
| Nastasio et al[43], 2013 | Retrospective and Prospective | 79 children and adolescents with AIH | 15 (19.0%) had CD on the basis of serology and histological findings Diagnosis of CD preceded the diagnosis of liver disease in 8 of the 15 patients | All 15 patients on GFD achieved sustained remission when treated with immunosuppressive therapy |
AILD: Autoimmune liver diseases; CD: Celiac disease; GFD: Gluten-free diet; AIH: Autoimmune hepatitis; EMA: Anti-endomysial antibodies; tTGA: Anti-tissue transglutaminase antibodies.
There are two studies providing prospective data on AIH in children with CD (Table 3)[15,44]. Di Biase et al[15] showed that isolated hypertransaminasemia was present in 40% of CD subjects on a gluten-containing diet, and that 2% had AIH, while there were no other AILD. Liver tests became normal after GFD only in CD patients with isolated hypertransaminasemia, but not in AIH cases who required GFD plus immunosuppressant therapy. Ventura et al[44] showed that AILD were more frequent in adolescents and young adults with CD than in the general population. In particular, out of 374 CD patients 10 (1.1%) had a diagnosis of AIH. They also reported that in patients with CD, AILD rates increased as age at diagnosis increased, suggesting a possible relationship with duration of exposure to gluten[44].
Table 3.
Studies reporting the prevalence of autoimmune hepatitis in children and adolescents with celiac disease
| Ref. | Study design | Study population with CD | Diagnosis of CD | Number of patients with AIH | Effect of GFD |
| Ventura et al[44], 1999 | Prospective | 909 children and adolescents with CD (group 1, < 2 yr of age; group 2, 2-10 yr; group 3, > 10 yr) | Serology and intestinal biopsy according to the ESPGHAN criteria | 10 (1.1%) had AIH, of whom 2.9% in group 2 and 0.8% in group 3 | Not available |
| Di Biase et al[15], 2010 | Prospective | 350 untreated children with suspected CD aged 1 to 16 yr | Serology and intestinal biopsy according to the ESPGHAN criteria | 7 (2.0%) had AIH, of whom 5 type I AIH | During treatment with GFD, steroids and azathioprine for 5 yr, all AIH persistently normalized clinical and biochemical parameters. After withdrawal, 6 patients maintained a sustained remission (12-63 mo) |
CD: Celiac disease; AIH: Autoimmune hepatitis; GFD: Gluten-free diet; ESPGHAN: European Society for Paediatric Gastroenterology, Hepatology and Nutrition.
PSC is a cholestatic disorder characterized by inflammation and periductal fibrosis of the intrahepatic and/or extrahepatic bile ducts[45-47]. No characteristic autoantibody has been identified in PSC patients. The diagnosis depends on evidencing the characteristic biliary lesions in biopsy tissue or the intra and extrahepatic biliary tree abnormalities by cholangiography[47]. Many patients, especially children, have PSC-AIH overlap with features of both diseases, and this is termed autoimmune sclerosing cholangitis (ASC)[46,48]. ASC refers to cases with PSC who have positive autoantibodies and may have histological features overlapping with those seen in AIH[47]. In adults, PBC may also be found. This additional form of AILD is characterized by the presence of anti-mitochondrial antibodies. It progresses slowly and is more common in females. Histologically, PBC is characterized by portal inflammation and immune-mediated destruction of the intrahepatic bile ducts. Autoimmune cholangitis (AIC) is a cholestatic liver disorder with biochemical signs of cholestasis, histological features of inflammatory bile duct damage, and negativity for anti-mitochondrial antibodies. PSC, PBC, and AIC have been mainly described in adults with CD[21,49-53]. In children, the association between CD and PSC or AIH/ASC overlap syndrome or AIC has been only reported in two studies[39,54].
NONALCOHOLIC FATTY LIVER DISEASE/NONALCOHOLIC STEATOHEPATITIS
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver conditions ranging from simple, uncomplicated steatosis, to nonalcoholic steatohepatitis (NASH), with inflammation and liver cell injury progressive to cryptogenic cirrhosis. NAFLD has become the most common cause of chronic liver disease in children and adolescents. Case reports and cross-sectional studies describe the association of various forms of fatty liver with CD[55-60]. Wigg et al[55] found that 3 of 22 adult patients with NASH had positive AGA IgA and IgG, and one of them had a histological diagnosis of CD. Grieco et al[56] reported histologically-diagnosed CD in 4 (13.3%) of 30 patients with laboratory diagnosis of NASH. After one year on GFD, the transaminase levels were normalized, and duodenal histology was improved. Nehra et al[57] investigating the relationship between NASH and CD, found that only one (2.1%) of the 47 study obese patients with NASH was positive for EMA IgA. In a study of 59 overweight patients undergoing liver biopsy for persistent hypertransaminasemia, NASH was detected in 38 (64%) whereas simple steatosis was found in 21 (36%)[58]. Six (10%) of the 59 patients showed positivity for tTGA and two (3.4%) of them also positivity for EMA IgA. Histology confirmed CD in the two patients positive for both markers. In both cases, liver enzymes went back to normal after a 6-mo GFD. In a study involving 121 patients with biopsy-proven NAFLD, Lo Iacono et al[59] reported that the prevalence of histologically-confirmed CD was 3.3%. In an Iranian population of 116 patients with NAFLD (as diagnosed on the basis of elevated transaminase levels, liver ultrasound and/or liver biopsy), Rahimi et al[60] found the prevalence of histologically-confirmed CD to be 2.2%. Interestingly, CD was more commonly diagnosed among NAFLD patients having body mass index (BMI) < 27 kg/m² compared to those with BMI > 27 kg/m² (5.83% vs 0%, P = 0.001). Very recently, in a nationwide study of more than 26000 children and adults with CD, Reilly et al[61] found an increased risk of NAFLD compared to the general population. Excess risks were highest in the first year after CD diagnosis, but persisted through 15 years beyond diagnosis with CD.
On the basis of the above findings, we conclude that there is an association between CD and fatty liver. However, since fatty liver is not an unusual finding in the general population of developed countries, the association of hepatic steatosis with CD may be a coincidental finding rather than a true association. To complicate matters further, fatty infiltration of the liver may be secondary to rapid weight loss or malabsorption, both etiologically linked to fatty liver. Future investigations should be undertaken to resolve this issue and should include pediatric populations for whom there are very few data at present.
SEVERE LIVER DAMAGE
Although rarely, severe liver disease has been described in adults with CD[62-64]. In a Finnish study, 4 patients with severe liver failure awaiting liver transplantation were discovered to have CD (one had congenital liver fibrosis; one, a massive hepatic steatosis; and two patients had progressive hepatitis with no apparent cause)[62]. Their liver disease improved after GFD. The Authors then screened 185 patients undergoing liver transplantation and found that 8 (4.3%) of them had CD, which is 4-10 times the population prevalence of CD in Finland. Most of these patients had AILD. Only 1 patient was on GFD. This suggests that in some cases of CD, GFD help to avoid end-stage liver disease. Subsequently, in a study from United States involving an ample cohort of individuals with end-stage AILD (n = 310) and non-AILD (n = 178) who underwent liver transplantation[64], the prevalence of tTGA and EMA was significantly greater in HLA-DQ2- or HLA-DQ8-positive patients with end-stage AILD compared with those with end-stage non-AILD (14.2% vs 5.4%, P = 0.0001 and 4.3% vs 0.78%, P = 0.01, respectively), while the co-occurrence of tTGA and EMA was increased five-fold in end-stage AILD (3% vs 0.6%). However, the study was retrospective, and apart from two patients, intestinal tissues were not available for re-review. Thus, a definite diagnosis of CD was not possible for most of the patients positive for CD-related autoantibodies. When serum samples were tested 6-12 or ≥ 24 mo post-transplantation, tTGA and EMA became normal in 94% and 100% of patients, respectively. This occurred without excluding gluten from the diet which implies no relationship between gluten and autoantibody kinetics. The suppression of tTGA and EMA after the transplant suggests that the lack of autoantibody positivity of post-transplant sera cannot exclude a diagnosis of CD, therefore supporting the pre-transplantation screening of patients with end-stage AILD[64].
In children, severe liver disease has been described in association with CD[65-68]. Demir et al[65] reported five celiac children with cryptogenic cirrhosis. In three patients with chronic diarrhea and hepatosplenomegaly, the diagnoses of CD and cirrhosis were concomitant, whereas in two patients, CD was diagnosed following that of cirrhosis. One to five years later, three patients on strict GDF had normal values of serum aminotransferases, and clinical improvement. The other two patients with poor dietary compliance had no improvement in liver function. Al-Hussaini et al[66] reported an 11-year-old girl with liver failure due to sclerosing cholangitis associated with CD. Treatment with ursodeoxycholic acid and GFD, and steroid tapered over three months, normalized the liver function tests. A few cases of CD with severe liver involvement requiring liver transplant have been also reported[67,68]. In a case-report, Pavone et al[67] described a 14-year-old girl with CD and mild gastrointestinal symptoms developing, after a long exposure to gluten, severe hepatic dysfunction requiring liver transplantation. Casswall et al[68] reported six 13- to 36-mo-old girls who within 1-24 mo of the diagnosis of CD developed severe liver damage. Four of these girls had acute liver failure and two needed a liver transplant.
PATHOGENESIS OF LIVER DYSFUNCTION IN CD
The pathogenesis of the hypertransaminasemia and liver damage in CD remains poorly understood. Probably they involve increased intestinal permeability and alterations in gut microbiota, chronic intestinal inflammation, and genetic predisposition (Figure 1).
Figure 1.
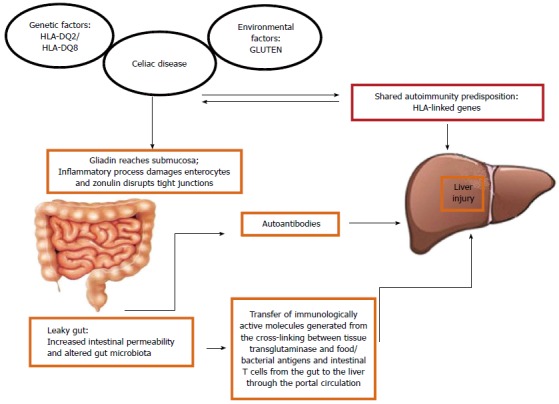
Possible pathogenetic mechanisms between celiac disease and liver abnormalities.
Since the liver receives three quarters of its blood supply from the intestine, it is one of the organs most exposed to gut-derived toxic factors[69-72]. Cross-talk between the gut and the liver is an intriguing hypothesis that may explain the hepatobiliary changes associated with many intestinal inflammatory diseases including CD. The suggestion that increased intestinal permeability and altered gut microbiota may contribute to the development of several diseases was made since 1890 (Llewellyn Jones: “Theory of auto-intoxication from gut bacteria”)[72]. Gut epithelial cells are linked to one another with tight junctions (TJs), which play an essential role in maintaining the integrity of the intestinal barrier and in demarcating microbes in the gut from the host immune system. Zonulin, a human protein known to reversibly regulate intestinal permeability by modulating intercellular TJs[73], is augmented in autoimmune conditions associated with TJ dysfunction including CD[74].
Patients with CD and hypertransaminasemia have an important increase in intestinal permeability compared with those whose liver enzymes are normal[11]. The increased intestinal permeability may ease the entry of toxins, antigens, and inflammatory substances (cytokines and/or autoantibodies) to the portal circulation and these mediators may play a part in the pathogenesis of hepatic involvement in CD. Interestingly, increased intestinal permeability caused by disruption of intercellular TJs in the intestine as well as increased prevalence of small intestinal overgrowth has been reported in adult patients with NAFLD[75]. Moreover, it has been found that serum zonulin concentration is increased in children and adolescents with NAFLD and correlates with the severity of steatosis[76]. This may also explain hepatic fat deposition in CD. Autoantibodies directed against tTG are present in the liver and other extraintestinal tissues in CD. This raises the possibility of a pathogenic role for the humoral-mediated immune responses in liver injury observed in CD. It has also been suggested that an aberrant T lymphocyte homing to the liver may contribute to trigger immune hepatic damage. As matter of fact, an increased number of lymphocytes expressing molecules of intestinal origin have been discovered in hepatic sinusoidal endothelial cells in individuals with liver abnormalities[77]. Moreover, liver-primed T cells have been demonstrated to migrate into the intestine and into the gut-associated lymphoid tissue, suggesting an enterohepatic lymphocyte circulation[78]. The ability of T cells of homing both to the liver and the intestine may explain the link between CD and liver diseases.
Considerable progress has been made toward understanding the role of genetics in autoimmune liver damage. It is well known that CD and some autoimmune liver disorders share HLA class II molecules and haplotypes. The main genetic marker of CD is HLA-DQ2, which is present in about 95% of CD patients. HLA-DQ2 is in strong linkage disequilibrium with HLA-DR3, which is the major HLA risk factor for AIH[79].
CONCLUSION
CD is increasingly reported in children who are symptomless or present atypical symptoms and signs. Liver abnormalities are common extraintestinal manifestations in patients with CD and range from mild hepatic injury to severe liver disease. The so-called celiac hepatitis is a frequent, benign, clinically silent condition which resolves on a GFD. Autoimmune liver diseases are less common and are associated in the majority of cases with clinical signs and symptoms of chronic liver disease, which need specific immunosuppressive therapy, rather than just GFD. Although rarely, CD may be also associated with severe liver involvement requiring liver transplant. In light of this background early diagnosis and treatment of CD-associated chronic and severe liver diseases may play an important role in the prognosis of this clinical entities. To this end, screening for liver involvement in celiac children and for CD by means of tTGA and EMA in children with liver diseases should become routine practice.
Footnotes
Conflict-of-interest: There are no potential conflicts of interest relevant to this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 24, 2015
First decision: February 10, 2015
Article in press: April 17, 2015
P- Reviewer: Dore MP, Francavilla R, Quigley EMM, Ukleja A S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419–2426. doi: 10.1056/NEJMcp1113994. [DOI] [PubMed] [Google Scholar]
- 2.Tack GJ, Verbeek WH, Schreurs MW, Mulder CJ. The spectrum of celiac disease: epidemiology, clinical aspects and treatment. Nat Rev Gastroenterol Hepatol. 2010;7:204–213. doi: 10.1038/nrgastro.2010.23. [DOI] [PubMed] [Google Scholar]
- 3.Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 4.Newton KP, Singer SA. Celiac disease in children and adolescents: special considerations. Semin Immunopathol. 2012;34:479–496. doi: 10.1007/s00281-012-0313-0. [DOI] [PubMed] [Google Scholar]
- 5.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–188. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 6.Duggan JM, Duggan AE. Systematic review: the liver in coeliac disease. Aliment Pharmacol Ther. 2005;21:515–518. doi: 10.1111/j.1365-2036.2005.02361.x. [DOI] [PubMed] [Google Scholar]
- 7.Hagander B, Berg NO, Brandt L, Nordén A, Sjölund K, Stenstam M. Hepatic injury in adult coeliac disease. Lancet. 1977;2:270–272. doi: 10.1016/s0140-6736(77)90954-0. [DOI] [PubMed] [Google Scholar]
- 8.Lindberg T, Berg NO, Borulf S, Jakobsson I. Liver damage in coeliac disease or other food intolerance in childhood. Lancet. 1978;1:390–391. doi: 10.1016/s0140-6736(78)91115-7. [DOI] [PubMed] [Google Scholar]
- 9.Bonamico M, Pitzalis G, Culasso F, Vania A, Monti S, Benedetti C, Mariani P, Signoretti A. [Hepatic damage in celiac disease in children] Minerva Pediatr. 1986;38:959–962. [PubMed] [Google Scholar]
- 10.Bardella MT, Fraquelli M, Quatrini M, Molteni N, Bianchi P, Conte D. Prevalence of hypertransaminasemia in adult celiac patients and effect of gluten-free diet. Hepatology. 1995;22:833–836. [PubMed] [Google Scholar]
- 11.Novacek G, Miehsler W, Wrba F, Ferenci P, Penner E, Vogelsang H. Prevalence and clinical importance of hypertransaminasaemia in coeliac disease. Eur J Gastroenterol Hepatol. 1999;11:283–288. doi: 10.1097/00042737-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen MB, Fausa O, Elgjo K, Schrumpf E. Hepatic lesions in adult coeliac disease. Scand J Gastroenterol. 1990;25:656–662. doi: 10.3109/00365529008997589. [DOI] [PubMed] [Google Scholar]
- 13.Farre C, Esteve M, Curcoy A, Cabré E, Arranz E, Amat LL, Garcia-Tornel S. Hypertransaminasemia in pediatric celiac disease patients and its prevalence as a diagnostic clue. Am J Gastroenterol. 2002;97:3176–3181. doi: 10.1111/j.1572-0241.2002.07127.x. [DOI] [PubMed] [Google Scholar]
- 14.Arslan N, Büyükgebiz B, Oztürk Y, Ozer E. The prevalence of liver function abnormalities in pediatric celiac disease patients and its relation with intestinal biopsy findings. Acta Gastroenterol Belg. 2005;68:424–427. [PubMed] [Google Scholar]
- 15.Di Biase AR, Colecchia A, Scaioli E, Berri R, Viola L, Vestito A, Balli F, Festi D. Autoimmune liver diseases in a paediatric population with coeliac disease - a 10-year single-centre experience. Aliment Pharmacol Ther. 2010;31:253–260. doi: 10.1111/j.1365-2036.2009.04186.x. [DOI] [PubMed] [Google Scholar]
- 16.Leonardi S, Bottaro G, Patané R, Musumeci S. Hypertransaminasemia as the first symptom in infant celiac disease. J Pediatr Gastroenterol Nutr. 1990;11:404–406. doi: 10.1097/00005176-199010000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Altuntaş B, Kansu A, Girgin N. Hepatic damage in gluten sensitive enteropathy. Acta Paediatr Jpn. 1998;40:597–599. doi: 10.1111/j.1442-200x.1998.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 18.Volta U, De Franceschi L, Lari F, Molinaro N, Zoli M, Bianchi FB. Coeliac disease hidden by cryptogenic hypertransaminasaemia. Lancet. 1998;352:26–29. doi: 10.1016/s0140-6736(97)11222-3. [DOI] [PubMed] [Google Scholar]
- 19.Bardella MT, Vecchi M, Conte D, Del Ninno E, Fraquelli M, Pacchetti S, Minola E, Landoni M, Cesana BM, De Franchis R. Chronic unexplained hypertransaminasemia may be caused by occult celiac disease. Hepatology. 1999;29:654–657. doi: 10.1002/hep.510290318. [DOI] [PubMed] [Google Scholar]
- 20.Sainsbury A, Sanders DS, Ford AC. Meta-analysis: Coeliac disease and hypertransaminasaemia. Aliment Pharmacol Ther. 2011;34:33–40. doi: 10.1111/j.1365-2036.2011.04685.x. [DOI] [PubMed] [Google Scholar]
- 21.Korpimäki S, Kaukinen K, Collin P, Haapala AM, Holm P, Laurila K, Kurppa K, Saavalainen P, Haimila K, Partanen J, et al. Gluten-sensitive hypertransaminasemia in celiac disease: an infrequent and often subclinical finding. Am J Gastroenterol. 2011;106:1689–1696. doi: 10.1038/ajg.2011.134. [DOI] [PubMed] [Google Scholar]
- 22.Maggiore G, De Giacomo C, Scotta MS, Sessa F. Celiac disease presenting as chronic hepatitis in a girl. J Pediatr Gastroenterol Nutr. 1986;5:501–503. doi: 10.1097/00005176-198605000-00031. [DOI] [PubMed] [Google Scholar]
- 23.Vajro P, Fontanella A, Mayer M, De Vincenzo A, Terracciano LM, D’Armiento M, Vecchione R. Elevated serum aminotransferase activity as an early manifestation of gluten-sensitive enteropathy. J Pediatr. 1993;122:416–419. doi: 10.1016/s0022-3476(05)83430-4. [DOI] [PubMed] [Google Scholar]
- 24.Iorio R, Sepe A, Giannattasio A, Cirillo F, Vegnente A. Hypertransaminasemia in childhood as a marker of genetic liver disorders. J Gastroenterol. 2005;40:820–826. doi: 10.1007/s00535-005-1635-7. [DOI] [PubMed] [Google Scholar]
- 25.Volta U. Pathogenesis and clinical significance of liver injury in celiac disease. Clin Rev Allergy Immunol. 2009;36:62–70. doi: 10.1007/s12016-008-8086-x. [DOI] [PubMed] [Google Scholar]
- 26.Mirzaagha F, Azali SH, Islami F, Zamani F, Khalilipour E, Khatibian M, Malekzadeh R. Coeliac disease in autoimmune liver disease: a cross-sectional study and a systematic review. Dig Liver Dis. 2010;42:620–623. doi: 10.1016/j.dld.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Schrumpf E, Abdelnoor M, Fausa O, Elgjo K, Jenssen E, Kolmannskog F. Risk factors in primary sclerosing cholangitis. J Hepatol. 1994;21:1061–1066. doi: 10.1016/s0168-8278(05)80618-x. [DOI] [PubMed] [Google Scholar]
- 28.Kingham JG, Parker DR. The association between primary biliary cirrhosis and coeliac disease: a study of relative prevalences. Gut. 1998;42:120–122. doi: 10.1136/gut.42.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vergani D, Mieli-Vergani G. Autoimmune hepatitis. In: Textbook of Hepatology: From Basic Science to Clinical Practice., editor. 3rd ed. Chichester, UK: Blackwell Publishing; 2007. pp. 1089–1101. [Google Scholar]
- 30.Moy L, Levine J. Autoimmune hepatitis: a classic autoimmune liver disease. Curr Probl Pediatr Adolesc Health Care. 2014;44:341–346. doi: 10.1016/j.cppeds.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Villalta D, Girolami D, Bidoli E, Bizzaro N, Tampoia M, Liguori M, Pradella M, Tonutti E, Tozzoli R. High prevalence of celiac disease in autoimmune hepatitis detected by anti-tissue tranglutaminase autoantibodies. J Clin Lab Anal. 2005;19:6–10. doi: 10.1002/jcla.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindberg J, Ahrén C, Iwarson S. Intestinal villous atrophy in chronic active hepatitis. Scand J Gastroenterol. 1979;14:1015–1018. [PubMed] [Google Scholar]
- 33.Swarbrick ET, Fairclough PD, Campbell PJ, Levison DA, Greenwood RH, Baker LR. Coeliac disease, chronic active hepatitis, and mesangiocapillary glomerulonephritis in the same patient. Lancet. 1980;2:1084–1085. doi: 10.1016/s0140-6736(80)92309-0. [DOI] [PubMed] [Google Scholar]
- 34.Lindberg J, Ahrén C, Jonsson J. Gluten-free diet in chronic active hepatitis associated with intestinal villous atrophy. Hepatogastroenterology. 1982;29:52–54. [PubMed] [Google Scholar]
- 35.Arvola T, Mustalahti K, Saha MT, Vehmanen P, Partanen J, Ashorn M. Celiac disease, thyrotoxicosis, and autoimmune hepatitis in a child. J Pediatr Gastroenterol Nutr. 2002;35:90–92. doi: 10.1097/00005176-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Alaswad B, Brosnan P. The association of celiac disease, diabetes mellitus type 1, hypothyroidism, chronic liver disease, and selective IgA deficiency. Clin Pediatr (Phila) 2000;39:229–231. doi: 10.1177/000992280003900406. [DOI] [PubMed] [Google Scholar]
- 37.Bridoux-Henno L, Dabadie A, Briard D, Bahon-Riedinger I, Jouan H, Le Gall E. A case of celiac disease presenting with autoimmune hepatitis and erythroblastopenia. J Pediatr Gastroenterol Nutr. 2001;33:616–619. doi: 10.1097/00005176-200111000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Vajro P, Paolella G, Maggiore G, Giordano G. Pediatric celiac disease, cryptogenic hypertransaminasemia, and autoimmune hepatitis. J Pediatr Gastroenterol Nutr. 2013;56:663–670. doi: 10.1097/MPG.0b013e31828dc5c5. [DOI] [PubMed] [Google Scholar]
- 39.Caprai S, Vajro P, Ventura A, Sciveres M, Maggiore G, SIGENP Study Group for Autoimmune Liver Disorders in Celiac Disease. Autoimmune liver disease associated with celiac disease in childhood: a multicenter study. Clin Gastroenterol Hepatol. 2008;6:803–806. doi: 10.1016/j.cgh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Diamanti A, Basso MS, Pietrobattista A, Nobili V. Prevalence of celiac disease in children with autoimmune hepatitis. Dig Liver Dis. 2008;40:965. doi: 10.1016/j.dld.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Tosun MS, Ertekin V, Selimoğlu MA. Autoimmune hepatitis associated with celiac disease in childhood. Eur J Gastroenterol Hepatol. 2010;22:898–899. doi: 10.1097/MEG.0b013e32832faf09. [DOI] [PubMed] [Google Scholar]
- 42.El-Shabrawi M, El-Karaksy H, Mohsen N, Isa M, Al-Biltagi M, El-Ansari M. Celiac disease in children and adolescents with autoimmune hepatitis: a single-centre experience. J Trop Pediatr. 2011;57:104–108. doi: 10.1093/tropej/fmq057. [DOI] [PubMed] [Google Scholar]
- 43.Nastasio S, Sciveres M, Riva S, Filippeschi IP, Vajro P, Maggiore G. Celiac disease-associated autoimmune hepatitis in childhood: long-term response to treatment. J Pediatr Gastroenterol Nutr. 2013;56:671–674. doi: 10.1097/MPG.0b013e31828b1dfa. [DOI] [PubMed] [Google Scholar]
- 44.Ventura A, Magazzù G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999;117:297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 45.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181–1188. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 46.Deneau M, Jensen MK, Holmen J, Williams MS, Book LS, Guthery SL. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: epidemiology and natural history. Hepatology. 2013;58:1392–1400. doi: 10.1002/hep.26454. [DOI] [PubMed] [Google Scholar]
- 47.Davison S. Coeliac disease and liver dysfunction. Arch Dis Child. 2002;87:293–296. doi: 10.1136/adc.87.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gregorio GV, Portmann B, Karani J, Harrison P, Donaldson PT, Vergani D, Mieli-Vergani G. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33:544–553. doi: 10.1053/jhep.2001.22131. [DOI] [PubMed] [Google Scholar]
- 49.Hay JE, Wiesner RH, Shorter RG, LaRusso NF, Baldus WP. Primary sclerosing cholangitis and celiac disease. A novel association. Ann Intern Med. 1988;109:713–717. doi: 10.7326/0003-4819-109-9-713. [DOI] [PubMed] [Google Scholar]
- 50.Venturini I, Cosenza R, Miglioli L, Borghi A, Bagni A, Gandolfo M, Modonesi G, Zeneroli ML. Adult celiac disease and primary sclerosing cholangitis: two case reports. Hepatogastroenterology. 1998;45:2344–2347. [PubMed] [Google Scholar]
- 51.Logan RF, Ferguson A, Finlayson ND, Weir DG. Primary biliary cirrhosis and coeliac disease: an association? Lancet. 1978;1:230–233. doi: 10.1016/s0140-6736(78)90480-4. [DOI] [PubMed] [Google Scholar]
- 52.Dickey W, McMillan SA, Callender ME. High prevalence of celiac sprue among patients with primary biliary cirrhosis. J Clin Gastroenterol. 1997;25:328–329. doi: 10.1097/00004836-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Volta U, Rodrigo L, Granito A, Petrolini N, Muratori P, Muratori L, Linares A, Veronesi L, Fuentes D, Zauli D, et al. Celiac disease in autoimmune cholestatic liver disorders. Am J Gastroenterol. 2002;97:2609–2613. doi: 10.1111/j.1572-0241.2002.06031.x. [DOI] [PubMed] [Google Scholar]
- 54.Lacaille F, Canioni D, Bernard O, Fabre M, Brousse N, Schmitz J. Celiac disease, inflammatory colitis, and primary sclerosing cholangitis in a girl with Turner’s syndrome. J Pediatr Gastroenterol Nutr. 1995;21:463–467. doi: 10.1097/00005176-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 55.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grieco A, Miele L, Pignatoro G, Pompili M, Rapaccini GL, Gasbarrini G. Is coeliac disease a confounding factor in the diagnosis of NASH? Gut. 2001;49:596. doi: 10.1136/gut.49.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46:2347–2352. doi: 10.1023/a:1012338828418. [DOI] [PubMed] [Google Scholar]
- 58.Bardella MT, Valenti L, Pagliari C, Peracchi M, Farè M, Fracanzani AL, Fargion S. Searching for coeliac disease in patients with non-alcoholic fatty liver disease. Dig Liver Dis. 2004;36:333–336. doi: 10.1016/j.dld.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Lo Iacono O, Petta S, Venezia G, Di Marco V, Tarantino G, Barbaria F, Mineo C, De Lisi S, Almasio PL, Craxì A. Anti-tissue transglutaminase antibodies in patients with abnormal liver tests: is it always coeliac disease? Am J Gastroenterol. 2005;100:2472–2477. doi: 10.1111/j.1572-0241.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 60.Rahimi AR, Daryani NE, Ghofrani H, Taher M, Pashaei MR, Abdollahzade S, Kalani M, Ajdarkosh H. The prevalence of celiac disease among patients with non-alcoholic fatty liver disease in Iran. Turk J Gastroenterol. 2011;22:300–304. doi: 10.4318/tjg.2011.0216. [DOI] [PubMed] [Google Scholar]
- 61.Reilly NR, Lebwohl B, Hultcrantz R, Green PH, Ludvigsson JF. Increased risk of non-alcoholic fatty liver disease after diagnosis of celiac disease. J Hepatol. 2015:Epub ahead of print. doi: 10.1016/j.jhep.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaukinen K, Halme L, Collin P, Färkkilä M, Mäki M, Vehmanen P, Partanen J, Höckerstedt K. Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology. 2002;122:881–888. doi: 10.1053/gast.2002.32416. [DOI] [PubMed] [Google Scholar]
- 63.Ojetti V, Fini L, Zileri Dal Verme L, Migneco A, Pola P, Gasbarrini A. Acute cryptogenic liver failure in an untreated coeliac patient: a case report. Eur J Gastroenterol Hepatol. 2005;17:1119–1121. doi: 10.1097/00042737-200510000-00017. [DOI] [PubMed] [Google Scholar]
- 64.Rubio-Tapia A, Abdulkarim AS, Wiesner RH, Moore SB, Krause PK, Murray JA. Celiac disease autoantibodies in severe autoimmune liver disease and the effect of liver transplantation. Liver Int. 2008;28:467–476. doi: 10.1111/j.1478-3231.2008.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demir H, Yüce A, Caglar M, Kale G, Kocak N, Ozen H, Gürakan F, Saltik-Temizel IN. Cirrhosis in children with celiac disease. J Clin Gastroenterol. 2005;39:630–633. doi: 10.1097/01.mcg.0000170734.49725.53. [DOI] [PubMed] [Google Scholar]
- 66.Al-Hussaini A, Basheer A, Czaja AJ. Liver failure unmasks celiac disease in a child. Ann Hepatol. 2013;12:501–505. [PubMed] [Google Scholar]
- 67.Pavone P, Gruttadauria S, Leonardi S, Sorge G, Minervini MI, Greco F, La Rosa M, Marino I. Liver transplantation in a child with celiac disease. J Gastroenterol Hepatol. 2005;20:956–960. doi: 10.1111/j.1440-1746.2005.03223.x. [DOI] [PubMed] [Google Scholar]
- 68.Casswall TH, Papadogiannakis N, Ghazi S, Németh A. Severe liver damage associated with celiac disease: findings in six toddler-aged girls. Eur J Gastroenterol Hepatol. 2009;21:452–459. doi: 10.1097/MEG.0b013e32830e1f12. [DOI] [PubMed] [Google Scholar]
- 69.Duseja A, Chawla YK. Obesity and NAFLD: the role of bacteria and microbiota. Clin Liver Dis. 2014;18:59–71. doi: 10.1016/j.cld.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, Nardone G. Gut--liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471–476. doi: 10.1016/j.numecd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, Day C, Grieco A. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19:5314–5324. [PubMed] [Google Scholar]
- 72.Bjarnason I, Takeuchi K, Bjarnason A, Adler SN, Teahon K. The G.U.T. of gut. Scand J Gastroenterol. 2004;39:807–815. doi: 10.1080/00365520410003326. [DOI] [PubMed] [Google Scholar]
- 73.Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915:214–222. doi: 10.1111/j.1749-6632.2000.tb05244.x. [DOI] [PubMed] [Google Scholar]
- 74.Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. 2012;1258:25–33. doi: 10.1111/j.1749-6632.2012.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 76.Pacifico L, Bonci E, Marandola L, Romaggioli S, Bascetta S, Chiesa C. Increased circulating zonulin in children with biopsy-proven nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:17107–17114. doi: 10.3748/wjg.v20.i45.17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grant AJ, Lalor PF, Salmi M, Jalkanen S, Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002;359:150–157. doi: 10.1016/S0140-6736(02)07374-9. [DOI] [PubMed] [Google Scholar]
- 78.Neumann K, Kruse N, Szilagyi B, Erben U, Rudolph C, Flach A, Zeitz M, Hamann A, Klugewitz K. Connecting liver and gut: murine liver sinusoidal endothelium induces gut tropism of CD4+ T cells via retinoic acid. Hepatology. 2012;55:1976–1984. doi: 10.1002/hep.24816. [DOI] [PubMed] [Google Scholar]
- 79.Czaja AJ, Doherty DG, Donaldson PT. Genetic bases of autoimmune hepatitis. Dig Dis Sci. 2002;47:2139–2150. doi: 10.1023/a:1020166605016. [DOI] [PubMed] [Google Scholar]


