Abstract
Background
Gynecological cancer is characterized by tumor hypoxia. However, the role of hypoxia-inducible factor 1α (HIF-1α) in gynecological cancer remains unclear.
Method
Electronic databases including Cochrane Library, PUBMED, Web of Knowledge and clinical trial registries were searched from inception through October 2014 for published, case-control studies assessing the association between HIF-1α and the clinicopathological characteristics of gynecological cancer. We pooled results from 59 studies using fixed or random-effects models and present results as odds ratios (ORs) following the PRISMA guidelines.
Results
Our meta-analysis, which included 6,612 women, demonstrated that the expression of HIF-1α was associated with the clinicopathological characteristics of gynecological cancer. The expression of HIF-1α in cancer or borderline tissue was significantly higher than that in normal tissue (cancer vs. normal: odds ratio (OR) =9.59, 95% confidence interval (CI): 5.97, 15.39, p<0.00001; borderline vs. normal: OR=4.13, 95% (CI): 2.43, 7.02, p<0.00001; cancer vs. borderline: OR=2.70, 95% (CI): 1.69, 4.31, p<0.0001). The expression of HIF-1α in III‒IV stage or lymph node metastasis was significantly higher than that in I‒II stage or that without lymph node metastasis, respectively (OR=2.66, 95% (CI): 1.87,3.79, p<0.00001; OR= 3.98, 95% (CI): 2.10,12.89, p<0.0001). HIF-1α was associated with histological grade of cancer (Grade 3 vs. Grade 1: OR=3.77, 95% (CI): 2.76,5.16, p<0.00001; Grade 3 vs. Grade 2: OR=1.62, 95% (CI): 1.20,2.19, p=0.002; Grade 2 vs. Grade 1: OR=2.34, 95% (CI): 1.82,3.00, p<0.00001),5-years disease free survival (DFS) rates (OR=2.93, 95% (CI):1.43,6.01, p=0.001) and 5-years overall survival (OS) rates (OR=5.53, 95% (CI): 2.48,12.31, p<0.0001).
Conclusion
HIF-1α is associated with the malignant degree, FIGO stage, histological grade, lymph node metastasis, 5-years survival rate and recurrence rate of gynecological cancer. It may play an important role in clinical treatment and prognostic evaluation.
Introduction
Solid tumors outgrow their own vasculature beyond the size of several cubic millimeters, resulting in hypoxia. HIF-1 regulates cellular oxygen homeostasis, and plays a key role in hypoxic conditions that occur during tumor angiogenesis, invasion and metastasis [1, 2]. HIF-1 is a heterodimeric transcription factor that consists of α and β subunits. The β subunit is constitutively expressed, while the expression of HIF-1α is regulated by the oxygen level [3]. Under normoxic conditions, HIF-1α would be degraded due to targeted ubiquitination and degradation by the proteasome. This process is mediated by direct binding of von Hippel—Lindau tumor suppressor protein (pVHL), a component of the E3 ubiquitin—protein ligase complex, with the minimal N-terminal transactivation domain (N-TAD) located within the oxygen-dependent degradation domain of HIF-1α. On the contrary, in hypoxic conditions, the degradation of HIF-1α is suppressed and the expression of HIF-1α would increase. Over-expression of HIF-1α has been reported in many types of malignancies, including lung, prostate, breast, colon and rectum carcinoma, and in both regional and distant metastases, implying that HIF-1α may play a vital role in tumor progression [4–6].
Gynecological malignancies, including cancers of endometrium, cervix, ovary, vulva and vagina, account for 11.7% of all new cancers in women. The American Cancer Society estimates that 94,990 women will have been diagnosed with, and 28,790 women will have died of, cancer of the female genital tract in 2014 in the USA [7]. Thus, it is important to understand the mechanisms of carcinogenesis and progression in gynecological cancer. HIF-1α is a key cellular survival protein during hypoxia, and is associated with tumor progression and metastasis in various solid tumors. In gynecological malignancies, Birner et al. [8] suggested that HIF-1α was a facilitator of premalignant progression. Acs et al. [9] and Birner et al. [10] found a consistent correlation between tumor stage and HIF-1α expression. Moreover, Seeber et al. [11], Bachtiary et al. [12] and Shimogai et al. [13] proposed HIF-1α as a predictor of poor prognosis and response to therapy. However, results of studies on HIF-1α in gynecological cancer are not always consistent. We carried out the first meta-analysis to assess the potential association between HIF-1α and the clinicopathological parameters of gynecological cancer. Cancers of the vulva and vagina are relatively rare. No study on HIF-1α and the clinicopathological characteristics of these malignancies has been published. Cancers of endometrium, cervix and ovary were included as subgroups in the final analysis.
Materials and Methods
Search strategy
We conducted the literature searches and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (S1 PRISMA Checklist). The electronic databases including Cochrane Library, PUBMED, Web of Knowledge and clinical trial registries, were used for systematic literature searches. Eligibility was restricted to studies published from inception to October 2014 with abstract or full text available. No language restrictions were made. We employed “hypoxia- inducible factor”, “HIF-1α”, or “HIF-1”, concatenated with “gynecological”, “endometrial”, “cervical”, “ovarian”, “vulva”, “vagina” and “tumor”, “cancer”, “carcinoma”, or “malignancy” as search terms. A comprehensive search of reference lists of all review articles and original studies retrieved by this method was performed to identify additional reports.
Criteria for inclusion and exclusion
The inclusion criteria for primary studies were as follows: (1) primary gynecological cancer should be pathologically proven; and (2) HIF-1α expression should be detected with immunohistochemistry (IHC); and (3) the association between clinicopathologic variables and HIF-1α expression should be described; or (4) provides information on survival data; and (5) laboratory methodology of IHC: (5.1) the staining of protein should be described (nuclear, cytoplasm); and (5.2) tissue sample conservation (fixation in formalin, alcohol or paraffin); and (5.3) description of the revelation test procedure of the biological factors with the first antibody type, clone identification, second antibody type, reaction characteristics, coloration method and epitope unmasking method; and (5.4) description of the negative and positive control; and (5.5) definition of the level of positivity of the test; or (5.6) the pathologist evaluating the IHC outcome was double-blind (or random) to patient clinicopathologic data and outcome. When studies were retrospective, the pathologist blinding was simple-blind.
Exclusion criteria for primary studies were as follows: (1) review, abstract, case report, animal or cell studies; or (2) not possible to extract the exact data (the association between clinicopathologic variables and HIF-1α expression); or (3) patients received chemotherapy, radiotherapy, targeted therapy before operation; and (4) laboratory methodology of IHC: (4.1) the study design was not defined; or (4.2) was unclear and no detailed description of standard laboratory methodology about IHC; or (4.3) the pathologist blinding was unblinded.
Review procedure and data extraction
Titles and abstracts were studied to assess inclusion criteria and examined independently for eligibility by two reviewers (Y. Jin and H. Wang). Disagreements were resolved by consulting a third reviewer (Y. Wang). The study characteristics were recorded as follows: (1) the first author, the nationality of included patients, article publication year; (2) the number of patients, cancer cases, borderline cases and controls for positive HIF-1α expression (HIF-1α expression score ≥ +), which was measured by semi-quantitatively assessing the percentage of tumor cells expressing HIF-1α, intensity of cell staining and extent of staining; (3) the number of test cases (FIGO III–IV stage, lymph nodes metastasis) and control cases (FIGO I–II, no lymph nodes metastasis) for positive HIF-1α expression; (4) the number of test cases (Grade 3 or Grade 2) and control cases (Grade 1); (5) the hazard ratio of 5-year disease free survival (DFS) and OS.
Quality assessments
Newcastle-Ottawa Scale (NOS) was used to assess the methodological quality of the included case-control studies. A study can be awarded 1 point for each numbered item in nine of NOS. Studies with scores of 0–4 are considered as low-quality, while 5–9 as high-quality.
Statistical analysis
We estimated the odds ratio (OR) for clinicopathologic variables (FIGO III–IV vs. FIGO I–II; lymph nodes metastasis vs. no lymph nodes metastasis; Grade 3 or Grade 2 vs. Grade 1), 5-year DFS and 5-year overall survival (OS). Statistical heterogeneity assumption among studies was checked using the X2-based Q-test. When I 2 was less than 50%, pooled odds ratios, relative risk and 95% confidence intervals (CIs) were calculated using Mantel-Haenszel method with fixed effect models. Whereas significant heterogeneity among the studies was detected (I 2>50%), a random-effect model was adopted. If necessary, a sensitive analysis was also performed to evaluate the influence of individual studies on the final effect. All p-values were two-sided. A p-value <0.05 was considered significant. All the statistical analyses were performed using RevMan 5.0 software (The Cochrane Collaboration, Oxford, United Kingdom).
Results
Description and quality assessments of included studies
The bibliographical search yielded a total of 698 studies and full text or abstract was obtained for 91 studies. Thirty-two of these studies did not meet the inclusion criteria: four studies referred to a duplicate dataset, twenty-three studies did not present exact data to extract, and five was animal studies. Finally, fifty-nine independent studies [2, 8–65] were included in the final review. The processes of study selection were summarized in the flow diagram (Fig 1). The main characteristics of the eligible studies were shown in Table 1, and the quality assessments of the included studies were summarized in S1 Table.
Fig 1. Flowchart of study selection.
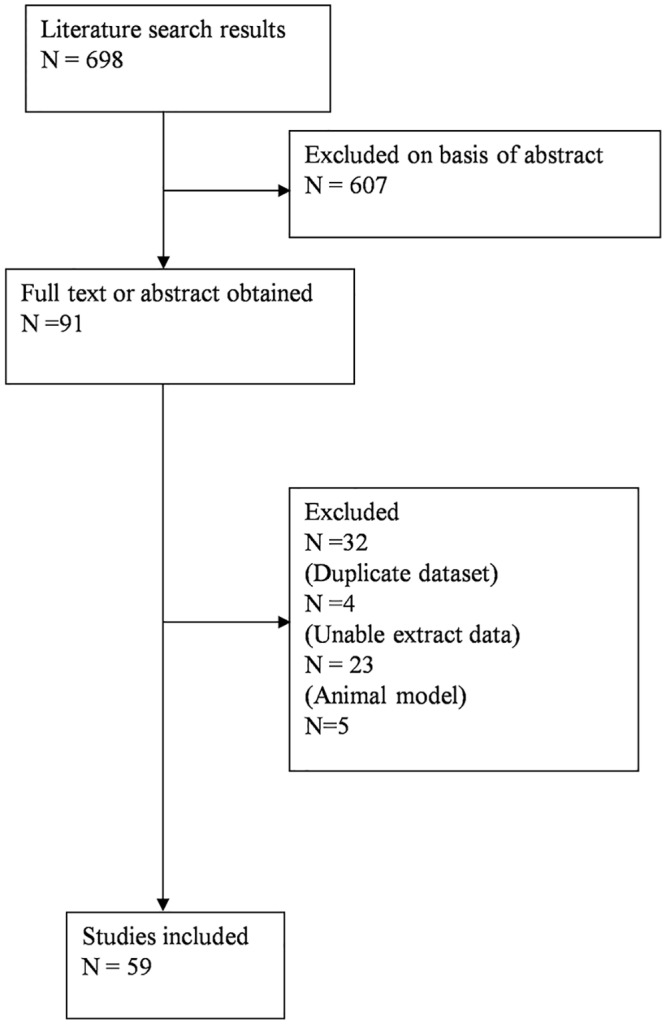
Sixty independent studies were included in the final review.
Table 1. Characteristics of studies included in this meta-analysis.
| Author | Number of patients | Year (country) | HIF-1α positive(negative) | Pathological type | Histological type | FIGO stage | Histological grade | Lymph node metastasis | 5-years overall survival rate | 5-years disease free survival rate |
|---|---|---|---|---|---|---|---|---|---|---|
| Ovarian cancer | (cancer/borderline/ benign) | (serous/clear cell/others) | (I–II/III–IV) | (G1/G2/G3) | (yes/no) | (<5/≥5) | (<5/≥5) | |||
| Daponte14 | 120 | 2008(Greece) | 61(59) | 78/22/20 | - | - | - | - | - | - |
| Shimogai13 | 66 | 2008 (Japan) | 11(55) | 66/-/- | 48/5/13 | 22/44 | - | 25/41 | 24/42 | 11/55 |
| Yu15 | 117 | 2012 (China) | 59(58) | 87/-/30 | 75/12* | 45/44 | - | 42/45 | 53/34 | - |
| Birner10 | 172 | 2001(Austria) | 116(56) | 102/50/20 | 64/8/30 | - | - | - | - | - |
| Osada16 | 107 | 2007 (Japan) | 82(25) | 72/17/18 | - | 48/24 | 32/30/10 | - | - | - |
| Shen17 | 63 | 2013 (China) | 55(8) | 63/-/- | - | 44/19 | 19/17/16 | - | - | - |
| Su18 | 81 | 2011 (China) | 40(41) | 35/22/24 | - | 13/22 | 4/17/14 | - | - | - |
| Yu19 | 30 | 2009 (China) | 26(4) | 30/-/- | 18/2/10 | 12/18 | 10/10/8 | - | - | - |
| Liu20 | 171 | 2012 (China) | 80(91) | 96/-/45 | 45/8/43 | 30/66 | 24/40/32 | - | - | - |
| Chen21 | 62 | 2011 (China) | 29(33) | 62/-/- | 40/22* | 26/36 | 25/37 △ | 36/26 | 44/18 | - |
| Fu22 | 119 | 2008 (China) | 70(49) | 101/-/- | 51/9/41 | 53/48 | - | - | - | - |
| Guo23 | 108 | 2010 (China) | 39(66) | 58/-/30 | - | 20/38 | 18/28/12 | 27/31 | - | - |
| Naka26 | 52 | 2007 (Japan) | 36(16) | 52/-/- | 29/9/14 | -/52 | 19/14/10 | - | - | - |
| Ji25 | 116 | 2013 (China) | 70(46) | 41/20/27 | - | 20/21 | - | 27/14 | - | - |
| Nakayama26 | 60 | 2002 (Japan) | 30(30) | 60/-/- | 29/17/14 # | 23/37 | 17/16/22 | - | - | - |
| Iida27 | 102 | 2008 (Japan) | 91(11) | 39/32/31 | - | - | - | - | - | - |
| Chen28 | 164 | 2012 (China) | 62(102) | 124/-/- | 80/44 ▲ | 53/71 | 49/75 △ | 50/74 | - | - |
| Li29 | 141 | 2011(China) | 66(75) | 60/21/30 | 40/20* | 19/41 | 23/37 | 36/24 | - | - |
| Wong30 | 53 | 2003(USA) | 22(31) | 37/-/16 | 29/2/6 | -/37 | - | - | - | - |
| Luo31 | 308 | 2005(China) | 208(100) | 238/19/38 | 148/20/70 | 77/161 | 53/101/84 | - | - | - |
| Wang32 | 145 | 2008(China) | 86(79) | 112/9/18 | 58/33/31 # | 46/76 | 24/48/38 | - | - | - |
| Tong34 | 31 | 2008(China) | 26(5) | 31/-/- | 31/-/- | - | - | 21/10 | - | 21/10 |
| Li33 | 73 | 2009(China) | 35(38) | 37/19/- | - | 13/24 | 12/25 $ | 27/10 | - | - |
| Miyazawa35 | 36 | 2009(Japan) | 21(2) | 23/2/11 | 5/7/11 | - | - | - | - | - |
| Yasuda36 | 74 | 2008(Japan) | 69(5) | 74/-/- | 21/18/35 | - | - | - | - | - |
| Cervical cancer | (cancer/CIN/normal) | (squamous/ others) | (I–II/III–IV) | (G1/G2/G3) | (yes/no) | (<5/≥5) | (<5/≥5) | |||
| Cheng37 | 158 | 2013(China) | 63(35) | 98/32/28 | 98/- | 57/41 @ | 42/35/21 | 39/59 | - | - |
| Kim38 | 745 | 2013(Korea) | 60(91) | 179/209/357 | 144/35 | 174/5 | - | - | 17/134 | 31/120 |
| Huang39 | 74 | 2014(China) | 39(35) | 74/-/- | 58/16 | 35/39 $ | 38/36 △ | 17/57 | - | - |
| Dellas40 | 44 | 2008(Germany) | 32(12) | 44/-/- | - | 9/35 | - | - | 19/25 | - |
| Birner8 | 106 | 2000(Austria) | 20(71) | 91/10/5 | - | 91/- | - | - | 17/74 | 28/63 |
| Bachtiary12 | 67 | 2003(Austria) | 32(35) | 67/-/- | 59/8 | 40/27 | 7/34/17 | 21/46 | - | - |
| Li41 | 120 | 2010(China) | 90(30) | 40/40/40 | 40/- | 40/- | 10/21/9 | 10/30 | - | - |
| Guo42 | 189 | 2008(China) | 93(96) | 79/90/20 | 79/- | 54/25 | 17/36/26 | - | - | - |
| Liu43 | 93 | 2008(China) | 26(19) | 45/28/20 | 45/- | 45/- | 29/16 △ | - | - | - |
| Zhang44 | 54 | 2009(China) | 28(26) | 34/10/10 | 23/11 | 34/- | 13/21 $ | 19/15 | - | - |
| Acs45 | 170 | 2003(USA) | 143(27) | 15/70/85 | 15/- | 15/- | - | - | - | - |
| Hutchison46 | 99 | 2004(United Kingdom) | 68(31) | 99/-/- | - | 57/42 | 17/57/14 | - | - | - |
| No47 | 116 | 2009(Korea) | 40(76) | 36/39/41 | - | - | 11/25 | - | - | |
| Ishikawa48 | 38 | 2004(Japan) | 20(18) | 38/-/- | 38/- | -/38 | - | - | - | 17/21 |
| Haugland49 | 101 | 2002(Canada) | 23(22) | 45/-/- | 33/12 | 30/15 | - | 11/34 | - | - |
| Burri50 | 91 | 2003(Switzerland) | 46(32) | 78/-/- | 63/15 | 9/43/26& | - | 30/47 | - | - |
| Markowska51 | 106 | 2007(Poland) | 81(25) | 106/-/- | 106/- | - | 29/46/31 | - | - | - |
| Endometrial cancer | (cancer/borderline/ normal) | (type 1/ type 2) | (I–II/III–IV) | (G1/G2/G3) | (yes/no) | (<5/≥5) | (<5/≥5) | |||
| Ozbudak52 | 100 | 2008(Turkey) | 45(55) | 100/-/- | 100/- | 69/31 | 60/25/15 | - | - | - |
| Feng53 | 187 | 2013(China) | 100(87) | 124/28/35 | 124/- | 101/23 | 57/41/26 | 31/93 | - | - |
| Espinosa54 | 64 | 2010(Italy) | 17(32) | 64/-/- | 64/- | 24/25 | 14/22/28 | - | - | - |
| Seeber69 | 108 | 2010(Netherlands) | 54(39) | 93/-/- | 75/18 | 75/18 | 28/47/18 | - | - | 18/72 |
| Pijnenborg55 | 65 | 2007(Netherlands) | 14(51) | 65/-/- | 65/- | 60/5 | 20/29/16 | - | - | 40/25 |
| Acs9 | 166 | 2004(USA) | 79(28) | 107/-/59 | 74/33 | 65/42 | 36/20/51 | - | - | - |
| Pansare56 | 149 | 2007(USA) | 54(90) | 149/-/- | 80/41 | 114/30 | 42/66 $ | - | - | - |
| Horrée57 | 79 | 2007(Netherlands) | 48(31) | 39/23/17 | 39/- | 23/16 | 6/21/12 | - | - | - |
| Koda58 | 85 | 2007(Poland) | 55(30) | 60/-/25 | - | 29/31 | 8/44/8 | - | - | - |
| Aybatli59 | 94 | 2011(Turkey) | 28(66) | 94/-/- | 76/18 | 64/30 | 36/30/28 | 34/60 | - | 9/85 |
| Yeramian60 | 93 | 2011(Spain and USA) | 26(55) | 93/-/- | 93/- | - | 26/35/21 | - | - | 9/72 |
| Li61 | 54 | 2008(China) | 20(34) | 42/-/12 | 36/6 | 21/21 | 8/34 $ | 32/10 | - | - |
| Zhai62 | 62 | 2007(China) | 25(37) | 42/-/20 | 42/- | 28/14 | 25/17 △ | 16/26 | - | - |
| Pan63 | 93 | 2011(China) | 51(42) | 52/23/18 | 52/- | 32/20 | 17/17/18 | 11/41 | - | - |
| Song64 | 40 | 2009(China) | 26(14) | 30/10/- | 20/10 | 27/3 | - | - | - | - |
| Sivridis2 | 106 | 2002(Greece) | 40(41) | 81/-/25 | 81/- | 81/- | 50/31 $ | - | 10/71 | - |
| Wang65 | 125 | 2010(China) | 65(33) | 105/-/20 | 105/- | 92/13 | 53/40/12 | - | 12/86 | - |
*: serous/mucinous;
#: serous/mucinous/others;
▲: serous/others;
△: G1-G2/G3;
$: G1/G2-G3;
@: Ia1-IIa/IIb-IIIb; $: Ia2-Ib1/Ib2-IIb; &: Ib-IIa/IIb-IIIa/IIIb-IVa
HIF-1α expression and pathological variables
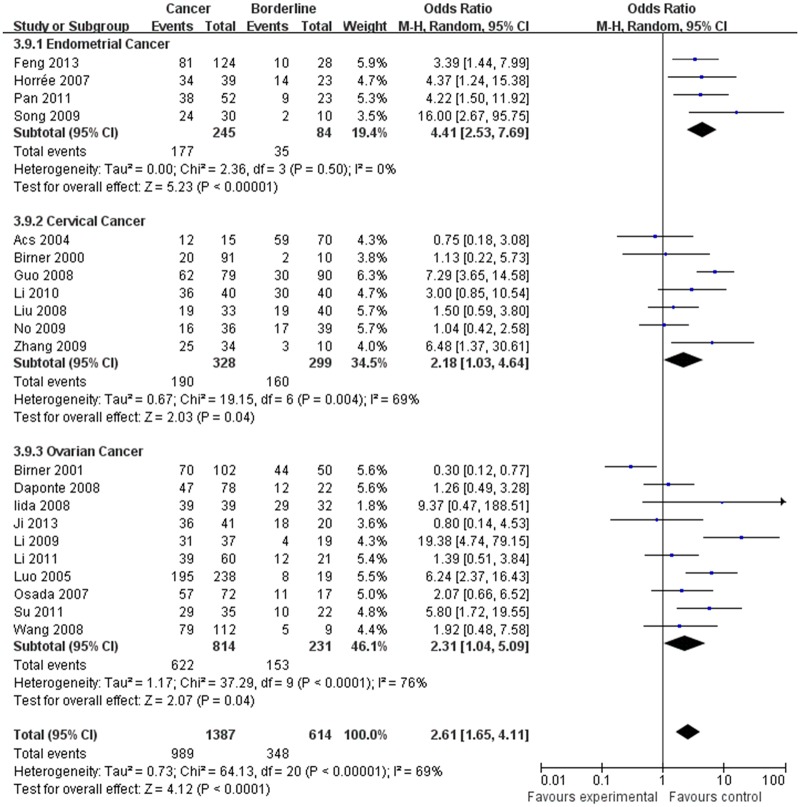
All 59 studies including 6612 patients explored the association between HIF-1α expression and clinicopathological variables of gynecological cancer. We performed pooled analyses with available data on the association between HIF-1α expression and pathological type, FIGO stage, histological type, and lymph node metastasis. Table 2 summarized the evaluations of association between HIF-1α expression and clinicopathological variables of gynecological cancer.
Table 2. Quantitative analyses of HIF-1α expression and clinicopathological variables of gynecological cancer.
| Variables | Number of patients | Test of association | Test of heterogeneity | Meta-analysis model | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Z test | p value | Q | p value | I 2 (%) | |||
| Pathological type | ||||||||
| Cancer vs Borderline | ||||||||
| Endometrial cancer | 212 | 4.45[2.57,7.71] | 5.33 | <0.00001 | 2.36 | 0.50 | 0 | Fixed |
| Cervical cancer | 328 | 2.36[1.04,5.38] | 2.05 | 0.04 | 18.09 | 0.003 | 72 | Random |
| Ovarian cancer | 1045 | 2.31[1.04,5.09] | 2.07 | 0.04 | 63.13 | <0.0001 | 76 | Random |
| Total | 1900 | 2.70[1.69,4.31] | 4.15 | <0.0001 | 63.13 | <0.0001 | 70 | Random |
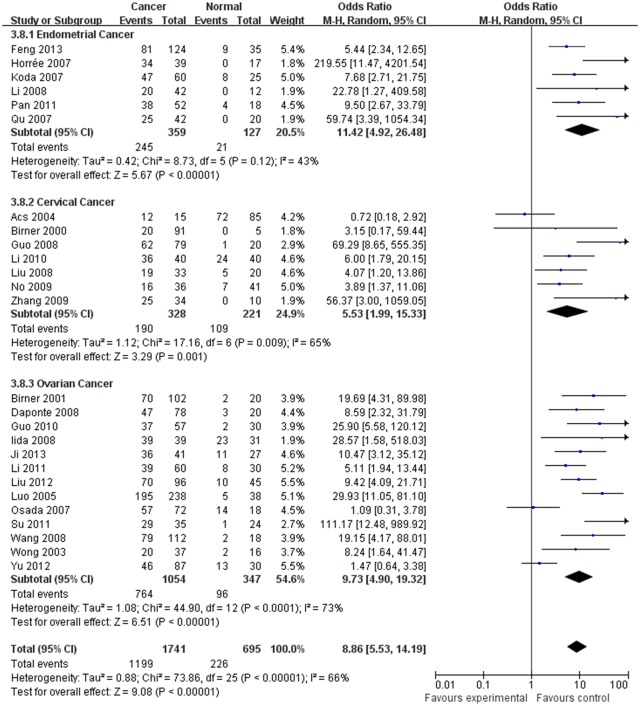
| Cancer vs Normal | ||||||||
| Endometrial cancer | 486 | 11.03[6.55,18.58] | 9.02 | <0.00001 | 8.73 | 0.12 | 43 | Fixed |
| Cervical cancer | 484 | 8.17[2.80,23.85] | 3.85 | 0.0001 | 21.59 | 0.003 | 68 | Random |
| Ovarian cancer | 1401 | 9.73[4.90,19.32] | 6.51 | <0.00001 | 44.90 | <0.0001 | 73 | Random |
| Total | 2371 | 9.59[5.97,15.39] | 9.36 | <0.00001 | 76.80 | <0.0001 | 66 | Random |
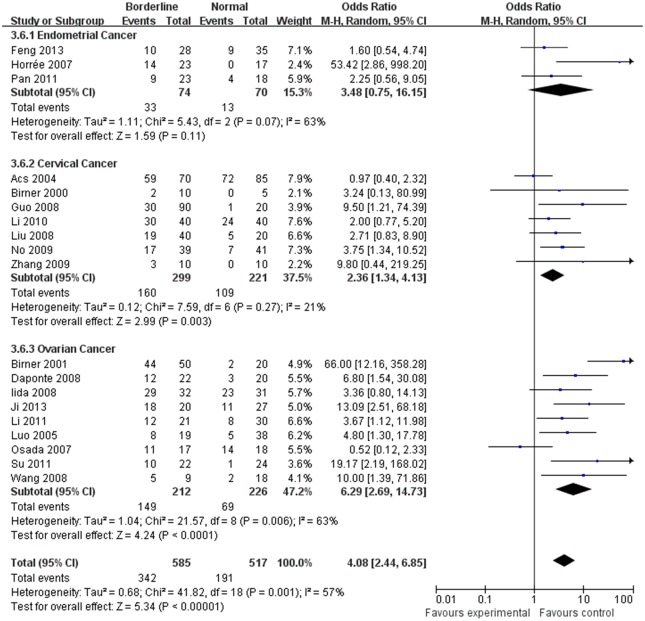
| Borderline vs Normal | ||||||||
| Endometrial cancer | 144 | 3.48[0.75,16.15] | 1.59 | 0.11 | 5.43 | 0.07 | 63 | Random |
| Cervical cancer | 520 | 2.40[1.52,3.78] | 3.78 | 0.0002 | 7.59 | 0.27 | 21 | Fixed |
| Ovarian cancer | 438 | 6.29[2.69,14.73] | 4.24 | <0.0001 | 21.57 | 0.0006 | 63 | Random |
| Total | 1087 | 4.13[2.43,7.02] | 5.24 | <0.00001 | 41.82 | 0.0007 | 59 | Random |
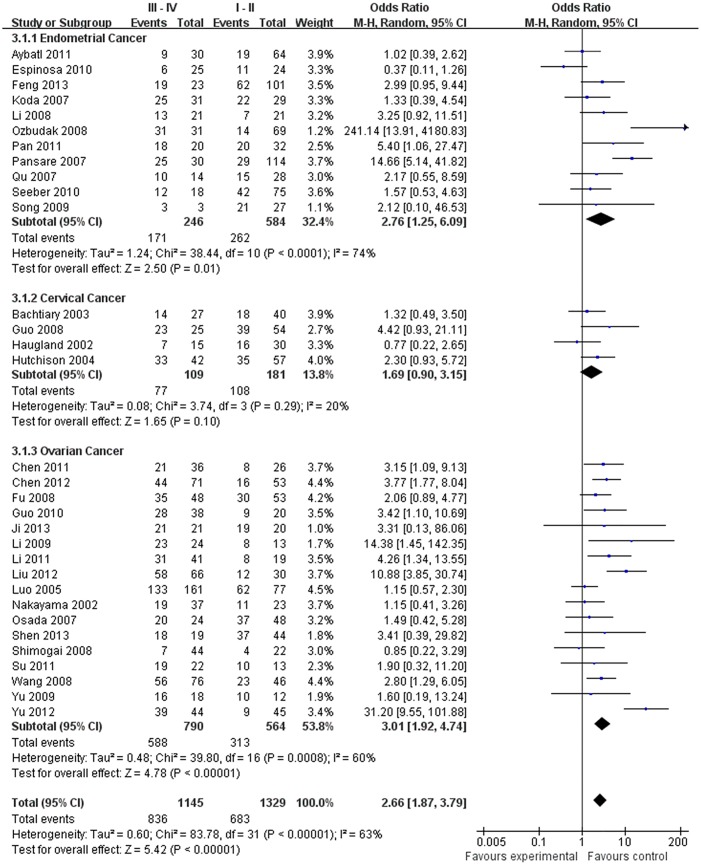
| FIGO stage | ||||||||
| Endometrial cancer | 830 | 2.76[1.25,6.09] | 2.50 | 0.01 | 38.44 | <0.0001 | 74 | Random |
| Cervical cancer | 290 | 1.76[1.03,2.99] | 2.08 | 0.04 | 3.74 | 0.29 | 20 | Fixed |
| Ovarian cancer | 1354 | 3.01[1.92,4.74] | 4.78 | <0.00001 | 39.80 | 0.0008 | 60 | Random |
| Total | 2474 | 2.66[1.87,3.79] | 5.42 | <0.00001 | 83.78 | <0.0001 | 63 | Random |
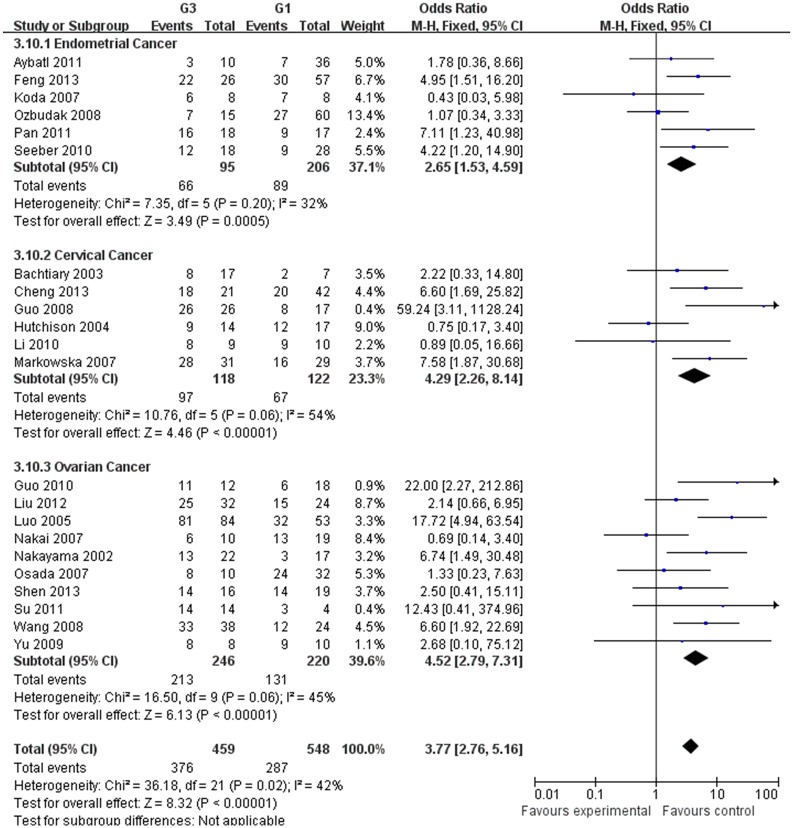
| Histological type | ||||||||
| G3 vs G1 | ||||||||
| Endometrial cancer | 301 | 2.65[1.53,4.59] | 3.49 | 0.0005 | 7.35 | 0.20 | 32 | Fixed |
| Cervical cancer | 240 | 4.29[2.26,8.14] | 4.46 | <0.00001 | 10.76 | 0.06 | 54 | Fixed |
| Ovarian cancer | 466 | 4.52[2.79,7.31] | 6.13 | <0.00001 | 16.50 | 0.06 | 45 | Fixed |
| Total | 1007 | 3.77[2.76,5.16] | 8.32 | <0.00001 | 36.18 | 0.02 | 42 | Fixed |
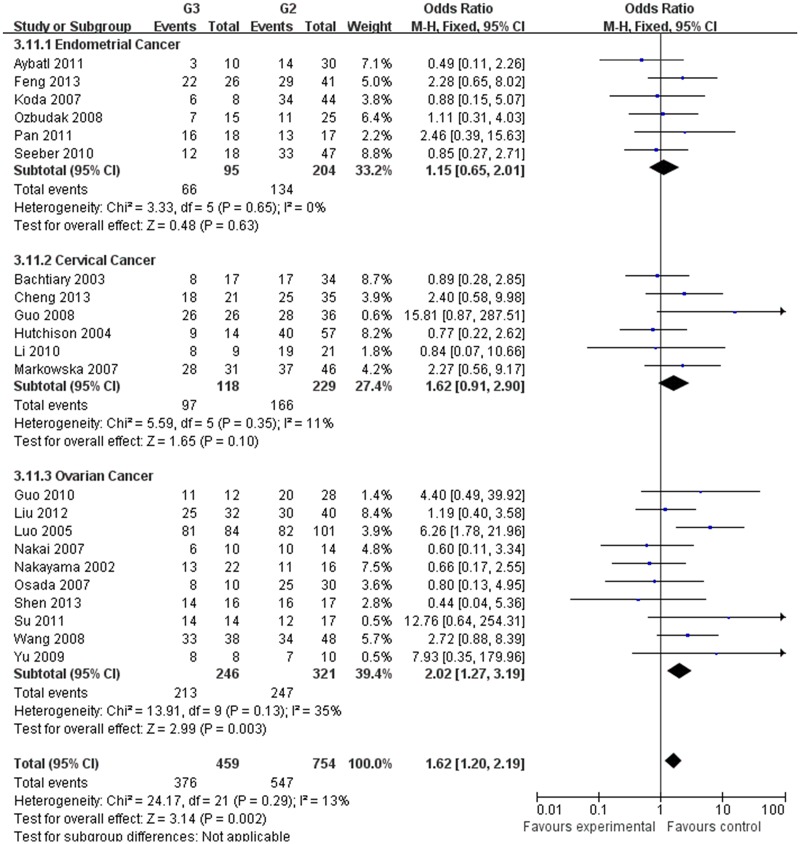
| G3 vs G2 | ||||||||
| Endometrial cancer | 299 | 1.15[0.65,2.01] | 0.48 | 0.63 | 3.33 | 0.65 | 0 | Fixed |
| Cervical cancer | 347 | 1.62[0.91,2.90] | 1.65 | 0.10 | 5.59 | 0.35 | 11 | Fixed |
| Ovarian cancer | 567 | 2.02[1.27,3.19] | 2.99 | 0.003 | 13.91 | 0.13 | 35 | Fixed |
| Total | 1213 | 1.62[1.20,2.19] | 3.14 | 0.002 | 24.17 | 0.29 | 13 | Fixed |
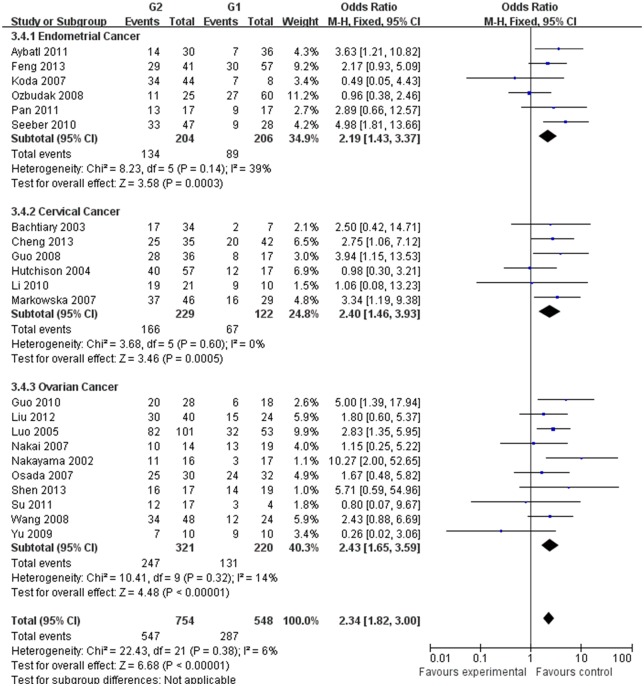
| G2 vs G1 | ||||||||
| Endometrial cancer | 410 | 2.19[1.43,3.37] | 3.58 | 0.0003 | 8.23 | 0.14 | 39 | Fixed |
| Cervical cancer | 351 | 2.40[1.46,3.93] | 3.46 | 0.0005 | 3.68 | 0.60 | 0 | Fixed |
| Ovarian cancer | 541 | 2.43[1.65,3.59] | 4.48 | <0.00001 | 10.41 | 0.32 | 14 | Fixed |
| Total | 1302 | 2.34[1.82,3.00] | 6.68 | <0.00001 | 22.43 | 0.38 | 6 | Fixed |
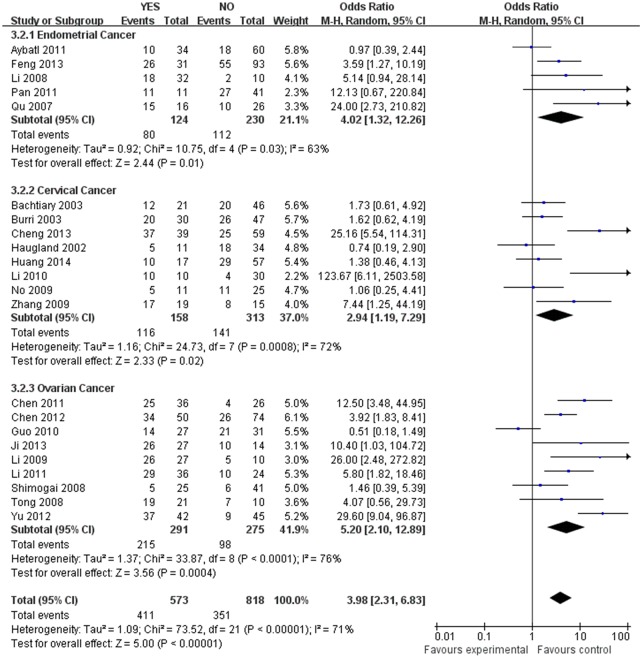
| Lymph node metastasis | ||||||||
| Endometrial cancer | 454 | 4.02[1.32,12.26] | 2.44 | 0.01 | 10.75 | 0.03 | 63 | Random |
| Cervical cancer | 471 | 2.94[1.19,7329] | 2.33 | 0.02 | 24.73 | 0.0008 | 72 | Random |
| Ovarian cancer | 566 | 5.20[2.10,12.89] | 3.56 | 0.0004 | 33.87 | <0.0001 | 76 | Random |
| Total | 1391 | 3.98[2.10,12.89] | 5.00 | <0.0001 | 3.98 | <0.0001 | 71 | Random |
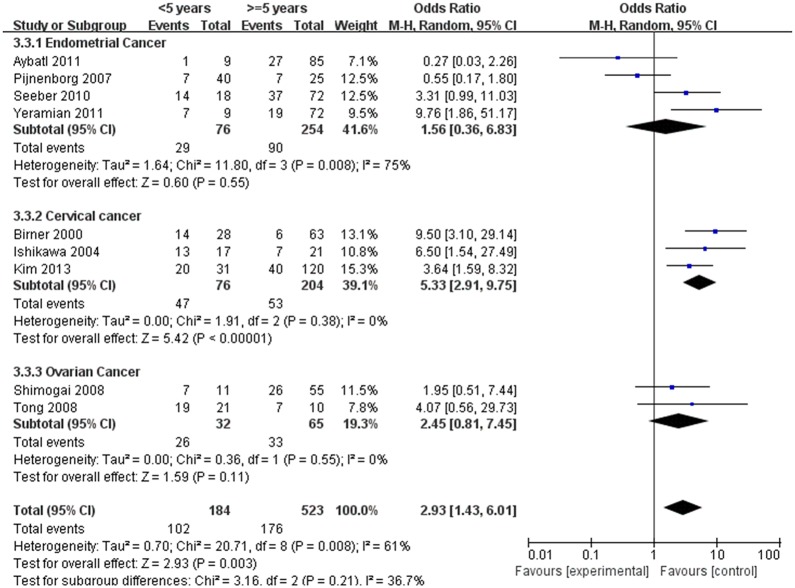
| 5-years desease free survival rate | ||||||||
| Endometrial cancer | 330 | 1.56[0.36,6.83] | 0.60 | 0.55 | 11.80 | 0.008 | 75 | Random |
| Cervical cancer | 280 | 5.28[2.90,9.63] | 5.43 | <0.00001 | 1.91 | 0.38 | 0 | Fixed |
| Ovarian cancer | 97 | 2.42[0.80,7.36] | 1.56 | 0.12 | 0.36 | 0.55 | 0 | Fixed |
| Total | 707 | 2.93[1.43,6.01] | 2.93 | 0.003 | 20.71 | 0.008 | 61 | Random |
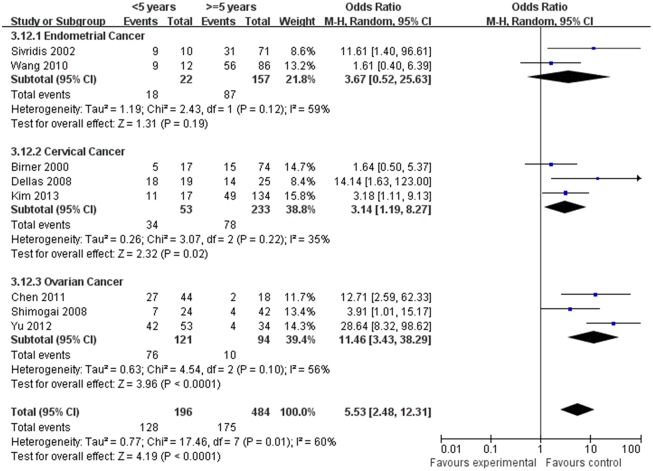
| 5-years overall survival rate | ||||||||
| Endometrial cancer | 179 | 3.67[0.52,25.63] | 1.31 | 0.19 | 2.43 | 0.12 | 59 | Random |
| Cervical cancer | 286 | 3.28[1.63,6.60] | 3.34 | 0.008 | 3.07 | 0.22 | 35 | Fixed |
| Ovarian cancer | 215 | 11.46[3.43,38.29] | 3.96 | <0.0001 | 4.54 | 0.10 | 56 | Random |
| Total | 680 | 5.53[2.48,12.31] | 4.19 | <0.0001 | 17.46 | 0.01 | 60 | Random |
The estimated pooled OR for all studies showed a significantly increased risk of malignant progression (cancer vs. borderline: OR, 2.70; 95% CI, 1.69–4.31, cancer vs. normal: OR, 9.59; 95% CI, 5.97–15.39, borderline vs. normal: OR, 4.13; 95% CI, 2.43–7.02, Figs 2–4, all p<0.05), higher FIGO stage (III–IV vs. I–II: OR, 2.66; 95% CI, 1.87–3.79, Fig 5, p<0.05), higher grade type (Grade 3 vs. Grade 1: OR, 3.77; 95% CI, 2.76–5.16, Grade 3 vs. Grade 2: OR, 1.62; 95% CI, 1.20–2.19, Grade 2 vs. Grade 1: OR, 2.34; 95% CI, 1.82–3.00, Figs 6–8, all p<0.05) and lymph node metastasis (yes vs. no: OR, 3.98; 95% CI, 2.10–12.89, Fig 9, p<0.05) in patients with positive HIF-1α expression. To explore potential sources of heterogeneity, we conducted subgroup analyses considering tumor types of gynecological cancer including endometrial, cervical and ovarian cancer. Almost all subgroup analyses maintained the positive association except the analysis of endometrial (borderline vs. normal: OR, 3.48; 95% CI, 0.75–16.15, Fig 4, p = 0.11, Grade 3 vs. Grade 2: OR, 1.15; 95% CI, 0.65–2.01, Fig 7, p = 0.63.) and cervical cancer (Grade 3 vs. Grade 2: OR, 1.62; 95% CI, 0.91–2.90, Fig 3, p = 0.10).
Fig 2. Forest plot of the expression of HIF-1α in cancer versus that in borderline tissue. (I 2 = 69%).
Fig 4. Forest plot of the expression of HIF-1α in borderline versus that in nomal tissue. (I 2 = 57%).
Fig 5. Forest plot of association between HIF-1α expression and FIGO stage. (I 2 = 63%).
Fig 6. Forest plot of the expression of HIF-1α in Grade 3 tissue versus that in Grade 1 tissue. (I 2 = 42%).
Fig 8. Forest plot of the expression of HIF-1α in Grade 2 tissue versus that in Grade 1 tissue. (I 2 = 6%).
Fig 9. Forest plot of association between HIF-1α expression and lymph node metastasis. (I 2 = 71%).
Fig 7. Forest plot of the expression of HIF-1α in Grade 3 tissue versus that in Grade 2 tissue. (I 2 = 13%).
Fig 3. Forest plot of the expression of HIF-1α in cancer versus that in nomal tissue. (I 2 = 66%).
HIF-1α expression and 5-year DFS rate, 5-year OS rate
The estimated pooled OR for 14 studies on the prognostic value of HIF-1α expression showed the positive expression of HIF-1α were associated with lower 5-year DFS and OS rate (<5 years vs. ≥5 years, Figs 10 and 11, p<0.05), the OR (95% CI) was 2.93(1.43,6.01), 5.53(2.48,12.31), respectively. To explore potential sources of heterogeneity, we conducted subgroup analyses. However, the subgroup of endometrial (DFS: OR, 1.56; 95% CI, 0.36–6.83, Fig 10, p = 0.55, OS: OR, 3.67; 95% CI, 0.52–25.63, Fig 11, p = 0.19) and ovarian cancer (DFS: OR, 2.42; 95% CI, 0.80–7.36, Fig 11, p = 0.12) did not maintain the positive association.
Fig 10. Forest plot of association between HIF-1α expression and 5-years desease free survival rate. (I 2 = 61%).
Fig 11. Forest plot of association between HIF-1α expression and 5-years overall survival rate. (I 2 = 60%).
Sensitivity analysis
Sensitivity analysis was performed to explore the influence of an individual study on the pooled results by repeating the meta-analysis while omitting some obviously different studies at the time. Statistically similar results were obtained by this procedure, indicating the stability of this meat-analysis (data not shown).
Discussion
HIF-1α is a key transcription factor that regulates cellular reaction to hypoxia. It is over-expressed in many types of malignancies in response to low oxygen concentration [66], and plays a key role in hypoxic conditions that occur during tumor angiogenesis, invasion and metastasis [67, 68]. In gynecological cancer, HIF-1α has been suggested as an adverse prognostic factor, but conflicting findings do exist [69]. Thus, pooled analysis was performed with available data on the association between HIF-1α expression and clinicopathological variables.
We demonstrated that the expression of HIF-1α in normal tissue was lower than that in borderline or cancer tissue in gynecological cancer, which is in agreement with previous findings from different studies [2, 8, 9, 16, 27, 30, 52, 57, 70]. HIF-1α may be a facilitator of premalignant progression in gynecological cancer. This positive association maintained in most subgroup analyses except in the “borderline vs. normal” of endometrial cancer. This inconsistence may result from a relatively small number of included studies (only three studies were in the subgroup analysis).
Clinicopathologicfeatures including pathological type, tumor stage, and lymph node metastasis are the major facts related to cancer-related prognosis. In our meta-analysis, higher HIF-1α expression was found to be associated with increased risk of lymph node metastasis, higher FIGO stage, higher histological grade, and lower 5-year OS and DFS rate. These findings revealed that HIF-1α could be considered as a hallmark of tumour progression, and a prognostic factor for gynecological cancer. To reveal the mechanisms, several included studies of this meta-analysis reported that HIF-1α is related to many critical aspects of gynecological cancer biology. HIF-1α synthesis could be increased by several growth factors, cytokines and other signaling molecules responsible for stimulating phosphatidylinositol 3-kinase (PI3K) or mitogen-activated protein kinase (MAPK) pathways [38]. The regulated markers of HIF-1α, such as glucose transporter type 1 (GLUT1), carbonic anhydrase 9 (CA9) and c-Met, have been found to be highly associated with poor prognosis in various cancers [38]. HIF-1α also regulates many cancer signaling pathways, including PI3K/AKT/mTOR, Notch, and Myc, to mediate tumor proliferation, invasion and migration [2, 8, 9, 16, 27, 30, 52, 57, 70].
However, the association between HIF-1α and the clinicopathologic features was not observed in subgroup analyses of “Grade 3 vs. Grade 2” in endometrial and cervical cancers. When stratified by cancer type, results of survival analysis were not statistically significant in the “endometrial and ovarian cancer” subgroup. We suggested that besides the heterogeneity of included studies, other factors related to clinicopathologic features of gynecological cancer might contribute to this inconsistence. For example, type I endometrial cancer is often characterized by mutations in tumor suppressor PTEN, while type II endometrial cancer generally contains the mutation of another tumor suppressor p53 [71–74]. In cervical cancer, the overexpression of human papillomavirus (HPV) and the loss of p53 promote tumor invasion and metastasis [75]. Thus, further studies included both HIF-1α and other factors are warranted to validate our findings, and to unravel the mechanism of carcinogenesis and progression in gynecological cancer.
Some limitations should be acknowledged. First, immunohistochemistry was a semiquantitative method, and this may affect the precision of the result. In this meta-analysis, no subgroup survival analysis was performed for different histological subtypes. Differences in primary antibodies, immunohistochemistry staining protocols, evaluation standards, and cut-off values for high HIF-1α expression might contribute to heterogeneity. However, this meta-analysis pooled series of studies and had higher statistical power to make up for this disadvantage to some extent. Further multicenter researches using standardized and quantitative methods are encouraged. Second, this meta-analysis included studies published in between 2001 and 2014. During those 13 years, improved surgical techniques and better perioperative care were developed at more specialized centers. The time-varying therapeutic regimen would be the major source of heterogeneity in cancer-related prognosis. For example, in the survival analysis of the “endometrial and ovarian cancer” subgroup, three studies reported postoperative adjuvant chemotherapy, fourteen studies reported postoperative adjuvant radiotherapy, while others did not provide any information about postoperative adjuvant therapy. Thus, the results of the prognosis analyses should be interpreted with caution. Third, more than half of included studies in this meta-analysis are from Asia. Because of this population bias, our results might not fully reveal the association of HIF-1α and clinicopathological characteristics of patients all over the world. Therefore, patients from a variety of countries should be studied to improve the reliability of our analysis in the near future.
Conclusions
Despite the limitations of this meta-analysis, we confirmed that HIF-1α is emerging as an important factor in the carcinogenesis of gynecological cancer. HIF-1α is associated with the malignantdegree, FIGO stage, histological grade, lymph node metastasis, 5-years survival rate and recurrence rate of gynecological cancer. We expect that HIF-1α may serve as a reliable tool for early and accurate prediction of cancer and may be a potential therapeutic target for gynecological cancer.
Supporting Information
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, et al. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97(6):1573–81. Epub 2003/03/11. 10.1002/cncr.11246 . [DOI] [PubMed] [Google Scholar]
- 2. Sivridis E, Giatromanolaki A, Gatter KC, Harris AL, Koukourakis MI, Tumor, et al. Association of hypoxia-inducible factors 1alpha and 2alpha with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer. 2002;95(5):1055–63. 10.1002/cncr.10774 . [DOI] [PubMed] [Google Scholar]
- 3. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(12):5510–4. Epub 1995/06/06. ; PubMed Central PMCID: PMCPmc41725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frank B, Hoffmeister M, Klopp N, Illig T, Chang-Claude J, Brenner H. Single nucleotide polymorphisms in Wnt signaling and cell death pathway genes and susceptibility to colorectal cancer. Carcinogenesis. 2010;31(8):1381–6. Epub 2010/04/21. 10.1093/carcin/bgq082 . [DOI] [PubMed] [Google Scholar]
- 5. Konac E, Onen HI, Metindir J, Alp E, Biri AA, Ekmekci A. An investigation of relationships between hypoxia-inducible factor-1 alpha gene polymorphisms and ovarian, cervical and endometrial cancers. Cancer detection and prevention. 2007;31(2):102–9. Epub 2007/04/10. 10.1016/j.cdp.2007.01.001 . [DOI] [PubMed] [Google Scholar]
- 6. Kim YH, Park IA, Park WY, Kim JW, Kim SC, Park NH, et al. Hypoxia-inducible factor 1alpha polymorphisms and early-stage cervical cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2011;21(1):2–7. Epub 2011/02/19. 10.1097/IGC.0b013e318204f6e6 . [DOI] [PubMed] [Google Scholar]
- 7. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. Epub 2014/01/09. 10.3322/caac.21208 . [DOI] [PubMed] [Google Scholar]
- 8. Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer research. 2000;60(17):4693–6. Epub 2000/09/15. . [PubMed] [Google Scholar]
- 9. Acs G, Xu X, Chu C, Acs P, Verma A. Prognostic significance of erythropoietin expression in human endometrial carcinoma. Cancer. 2004;100(11):2376–86. Epub 2004/05/26. 10.1002/cncr.20244 . [DOI] [PubMed] [Google Scholar]
- 10. Birner P, Schindl M, Obermair A, Breitenecker G, Oberhuber G. Expression of hypoxia-inducible factor 1alpha in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2001;7(6):1661–8. Epub 2001/06/19. . [PubMed] [Google Scholar]
- 11. Seeber LM, Horree N, van der Groep P, van der Wall E, Verheijen RH, van Diest PJ. Necrosis related HIF-1alpha expression predicts prognosis in patients with endometrioid endometrial carcinoma. BMC cancer. 2010;10:307 10.1186/1471-2407-10-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bachtiary B, Schindl M, Potter R, Dreier B, Knocke TH, Hainfellner JA, et al. Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9(6):2234–40. . [PubMed] [Google Scholar]
- 13. Shimogai R, Kigawa J, Itamochi H, Iba T, Kanamori Y, Oishi T, et al. Expression of hypoxia-inducible factor 1alpha gene affects the outcome in patients with ovarian cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2008;18(3):499–505. Epub 2008/05/15. 10.1111/j.1525-1438.2007.01055.x . [DOI] [PubMed] [Google Scholar]
- 14. Daponte A, Ioannou M, Mylonis I, Simos G, Minas M, Messinis IE, et al. Prognostic significance of Hypoxia-Inducible Factor 1 alpha(HIF-1 alpha) expression in serous ovarian cancer: an immunohistochemical study. BMC cancer. 2008;8:335 Epub 2008/11/19. 10.1186/1471-2407-8-335 ; PubMed Central PMCID: PMCPmc2651893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu L, Wu S, Zhou L, Song W. Correlation between bacterial L-form infection, expression of HIF-1alpha/MMP-9 and vasculogenic mimicry in epithelial ovarian cancer. Sheng li xue bao: [Acta physiologica Sinica]. 2012;64(6):657–65. Epub 2012/12/22. . [PubMed] [Google Scholar]
- 16. Osada R, Horiuchi A, Kikuchi N, Yoshida J, Hayashi A, Ota M, et al. Expression of hypoxia-inducible factor 1alpha, hypoxia-inducible factor 2alpha, and von Hippel-Lindau protein in epithelial ovarian neoplasms and allelic loss of von Hippel-Lindau gene: nuclear expression of hypoxia-inducible factor 1alpha is an independent prognostic factor in ovarian carcinoma. Human pathology. 2007;38(9):1310–20. Epub 2007/06/09. 10.1016/j.humpath.2007.02.010 . [DOI] [PubMed] [Google Scholar]
- 17. Shen Y, Zhang XY, Shi YQ, Wang Y, Guo DH. Significance of Glut-1, HIF-1 and P53 expression in ovarian cancer. Journal of Tianjin Medical University. 2013;(05):383–6, 437. [Google Scholar]
- 18. Su M, Zhang Y, Huang H. Expression of HIF-1alpha and MMP-2 in vasculogenic mimicry of ovarian cancer. Current Advances In Obstetrics and Gynecology. 2011;20(2):83–7. . [Google Scholar]
- 19. Yu J, Huang YC, Zhang LJ, Lu YB. Expression and significance of hypoxia- inducible factor-1α and glucose transporter protein- 1 in epithelial ovarian tumor. Chinese Clinical Oncology. 2009;(03):207–11. [Google Scholar]
- 20. Liu LX, Liu GM, Sun YH, He XP. Analysis of the Relationship between HIF-1а gene Expression and Drug Resistance of Chemotherapy in Ovarian Cancer. Progress in Modern Biomedicine. 2012;(02):276–9. [Google Scholar]
- 21. Chen Y, Zhang L, Liu X. The expression and clinical significance of semaphorin4D, hypoxia-inducible factor-1α and vascular endothelial growth factor in ovarian epithelial cancer. Chinese Journal of Obstetrics and Gynecology. 2011;46(10):786–8. . [Google Scholar]
- 22. Fu L, Feng W, Zhao YH, Wang AH, Wang BB, Yang LX, et al. Expression and significance of hypoxia inducible factor-la in ovarian epithelial cancer Chin J Lab Diagn. 2008;(11):1373–5. [Google Scholar]
- 23. Guo LL, Liu ZQ, Zhang LP, Guo GL. Expression and clinical significance of human leucocyte antigen protein G and hypoxia inducible factor- lα in epithelial ovarian carcinoma. Journal of Hainan Medical University. 2010;(04):421–4, 8. [Google Scholar]
- 24. Nakai H, Watanabe Y, Ueda H, Hoshiai H. Hypoxia inducible factor 1-alpha expression as a factor predictive of efficacy of taxane/platinum chemotherapy in advanced primary epithelial ovarian cancer. Cancer letters. 2007;251(1):164–7. Epub 2007/01/12. 10.1016/j.canlet.2006.11.017 . [DOI] [PubMed] [Google Scholar]
- 25. Ji F, Wang Y, Qiu L, Li S, Zhu J, Liang Z, et al. Hypoxia inducible factor 1alpha-mediated LOX expression correlates with migration and invasion in epithelial ovarian cancer. International journal of oncology. 2013;42(5):1578–88. 10.3892/ijo.2013.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakayama K, Kanzak A, Hata K, Katabuchi H, Okamura H, Miyazaki K, et al. Hypoxia-inducible factor 1 alpha (HIF-1 alpha) gene expression in human ovarian carcinoma. Cancer letters. 2002;176(2):215–23. Epub 2002/01/24. . [DOI] [PubMed] [Google Scholar]
- 27. Iida T, Yasuda M, Miyazawa M, Fujita M, Osamura RY, Hirasawa T, et al. Hypoxic status in ovarian serous and mucinous tumors: relationship between histological characteristics and HIF-1alpha/GLUT-1 expression. Archives of gynecology and obstetrics. 2008;277(6):539–46. 10.1007/s00404-007-0500-8 . [DOI] [PubMed] [Google Scholar]
- 28. Chen Y, Zhang L, Pan Y, Ren XB, Hao Q. Over-Expression of Semaphorin4D, Hypoxia-Inducible Factor-1 alpha and Vascular Endothelial Growth Factor Is Related to Poor Prognosis in Ovarian Epithelial Cancer. Int J Mol Sci. 2012;13(10):13264–74. 10.3390/ijms131013264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li GL, Wang Z, Han RF. Expression of Mitogen Activated Protein Kinase p38 and Hypoxia- inducible Factor la in Epithefial Ovarian Cancer. Clinical Medical Engineering. 2011;(04):507–9. [Google Scholar]
- 30. Wong C, Wellman TL, Lounsbury KM. VEGF and HIF-1alpha expression are increased in advanced stages of epithelial ovarian cancer. Gynecologic oncology. 2003;91(3):513–7. Epub 2003/12/17. . [DOI] [PubMed] [Google Scholar]
- 31. Luo J, Peng Z, Yang K, Wang H, Yang H, Dong D, et al. Relation between the expression of hypoxia inducible factor-1alpha and angiogenesis in ovarian cancer using tissue microarray. Zhonghua fu chan ke za zhi. 2005;40(1):38–41. Epub 2005/03/19. . [PubMed] [Google Scholar]
- 32. Wang XC, Li XY, Li SQ. Expression of HIF- 1A and VEGF and Their Relationship in Ovarian Neoplasms. Chin J Misdiagn. 2008;(20):4795–7. [Google Scholar]
- 33. Li SY, Yang WW, Wen QT, Xu L, Meng HM, Chen M, et al. Expression and significance of HIF-1alpha and PTEN in epithelial ovarian carcinoma and their correlation. Chinese Journal of Clinical and Experimental Pathology. 2009;25(4):361–3. . [Google Scholar]
- 34. Tong XG, Xu HM, Liu YP, Hou KZ. Role of Hypoxia-inducible Factor-1alpha(HIF-1alpha) and Angiogenic Factors in Peritoneal Metastases and Prognosis of Advanced Serous Ovarian Cancer. Chinese Journal of Clinical Oncology. 2008;35(5):272–6. . [Google Scholar]
- 35. Miyazawa M, Yasuda M, Fujita M, Hirasawa T, Kajiwara H, Hirabayashi K, et al. Association of hypoxia-inducible factor-1 expression with histology in epithelial ovarian tumors: a quantitative analysis of HIF-1. Archives of gynecology and obstetrics. 2009;279(6):789–96. Epub 2008/10/22. 10.1007/s00404-008-0816-z . [DOI] [PubMed] [Google Scholar]
- 36. Yasuda M, Miyazawa M, Fujita M, Kajiwara H, Iida T, Hirasawa T, et al. Expression of hypoxia inducible factor-1alpha (HIF-1alpha) and glucose transporter-1 (GLUT-1) in ovarian adenocarcinomas: difference in hypoxic status depending on histological character. Oncology reports. 2008;19(1):111–6. Epub 2007/12/22. . [PubMed] [Google Scholar]
- 37. Cheng Y, Chen G, Hong L, Zhou L, Hu M, Li B, et al. How does hypoxia inducible factor-1alpha participate in enhancing the glycolysis activity in cervical cancer? Annals of diagnostic pathology. 2013;17(3):305–11. 10.1016/j.anndiagpath.2012.12.002 . [DOI] [PubMed] [Google Scholar]
- 38. Kim BW, Cho H, Chung JY, Conway C, Ylaya K, Kim JH, et al. Prognostic assessment of hypoxia and metabolic markers in cervical cancer using automated digital image analysis of immunohistochemistry. Journal of translational medicine. 2013;11:185 10.1186/1479-5876-11-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang M, Chen Q, Xiao J, Yao T, Bian L, Liu C, et al. Overexpression of hypoxia-inducible factor-1alpha is a predictor of poor prognosis in cervical cancer: a clinicopathologic study and a meta-analysis. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2014;24(6):1054–64. Epub 2014/07/01. 10.1097/igc.0000000000000162 . [DOI] [PubMed] [Google Scholar]
- 40. Dellas K, Bache M, Pigorsch SU, Taubert H, Kappler M, Holzapfel D, et al. Prognostic impact of HIF-1alpha expression in patients with definitive radiotherapy for cervical cancer. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al]. 2008;184(3):169–74. 10.1007/s00066-008-1764-z . [DOI] [PubMed] [Google Scholar]
- 41. Li N, Sun Y, Yu Q. Expression of HIF-1α and E2F6 protein in cervical carcinoma tissues. Chinese Journal of Diagnostic Pathology. 2010;17(3):209–11. [Google Scholar]
- 42. Guo K-j, Xue H, Zhang Y. Expression and Significance of HIF-lα and VEGF in Progression of Cervical Cancer. Journal of China Medical University. 2008;37(2):237–9. [Google Scholar]
- 43. Liu M, Sun Y, Han SP, Zhao WB, Zheng CX, WU J. Expression and Role of HIF-1α and VEGF in Carcinogenesis and Progression of Cervical Squamous Cell Carcinoma. Chinese Journal of Clinical Oncology. 2008;35(16):925–8. [Google Scholar]
- 44. Zhang LJ, Zhang SL, Lu YM. The Expression of hypoxia Inducible Factor-1α and Glucose Transporter-1 in Cervical Cancer and Their Associations with Carcinogenesis. Journal of China Medical University. 2009;38(2):136–8. [Google Scholar]
- 45. Acs G, Zhang PJ, McGrath CM, Acs P, McBroom J, Mohyeldin A, et al. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression. The American journal of pathology. 2003;162(6):1789–806. 10.1016/S0002-9440(10)64314-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hutchison GJ, Valentine HR, Loncaster JA, Davidson SE, Hunter RD, Roberts SA, et al. Hypoxia-inducible factor 1alpha expression as an intrinsic marker of hypoxia: correlation with tumor oxygen, pimonidazole measurements, and outcome in locally advanced carcinoma of the cervix. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10(24):8405–12. Epub 2004/12/30. 10.1158/1078-0432.ccr-03-0135 . [DOI] [PubMed] [Google Scholar]
- 47. No JH, Jo H, Kim SH, Park IA, Kang D, Han SS, et al. Expression of vascular endothelial growth factor and hypoxia inducible factor-1alpha in cervical neoplasia. Annals of the New York Academy of Sciences. 2009;1171:105–10. 10.1111/j.1749-6632.2009.04891.x . [DOI] [PubMed] [Google Scholar]
- 48. Ishikawa H, Sakurai H, Hasegawa M, Mitsuhashi N, Takahashi M, Masuda N, et al. Expression of hypoxic-inducible factor 1alpha predicts metastasis-free survival after radiation therapy alone in stage IIIB cervical squamous cell carcinoma. International journal of radiation oncology, biology, physics. 2004;60(2):513–21. 10.1016/j.ijrobp.2004.03.025 . [DOI] [PubMed] [Google Scholar]
- 49. Haugland HK, Vukovic V, Pintilie M, Fyles AW, Milosevic M, Hill RP, et al. Expression of hypoxia-inducible factor-1alpha in cervical carcinomas: correlation with tumor oxygenation. International journal of radiation oncology, biology, physics. 2002;53(4):854–61. . [DOI] [PubMed] [Google Scholar]
- 50. Burri P, Djonov V, Aebersold DM, Lindel K, Studer U, Altermatt HJ, et al. Significant correlation of hypoxia-inducible factor-1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. International journal of radiation oncology, biology, physics. 2003;56(2):494–501. Epub 2003/05/10. . [DOI] [PubMed] [Google Scholar]
- 51. Markowska J, Grabowski JP, Tomaszewska K, Kojs Z, Pudelek J, Skrzypczak M, et al. Significance of hypoxia in uterine cervical cancer. Multicentre study. European journal of gynaecological oncology. 2007;28(5):386–8. . [PubMed] [Google Scholar]
- 52. Ozbudak IH, Karaveli S, Simsek T, Erdogan G, Pestereli E. Neoangiogenesis and expression of hypoxia-inducible factor 1alpha, vascular endothelial growth factor, and glucose transporter-1 in endometrioid type endometrium adenocarcinomas. Gynecologic oncology. 2008;108(3):603–8. 10.1016/j.ygyno.2007.11.028 . [DOI] [PubMed] [Google Scholar]
- 53. Feng Z, Gan H, Cai Z, Li N, Yang Z, Lu G, et al. Aberrant expression of hypoxia-inducible factor 1alpha, TWIST and E-cadherin is associated with aggressive tumor phenotypes in endometrioid endometrial carcinoma. Japanese journal of clinical oncology. 2013;43(4):396–403. 10.1093/jjco/hys237 . [DOI] [PubMed] [Google Scholar]
- 54. Espinosa I, Jose Carnicer M, Catasus L, Canet B, D'Angelo E, Zannoni GF, et al. Myometrial invasion and lymph node metastasis in endometrioid carcinomas: tumor-associated macrophages, microvessel density, and HIF1A have a crucial role. The American journal of surgical pathology. 2010;34(11):1708–14. Epub 2010/10/22. 10.1097/PAS.0b013e3181f32168 . [DOI] [PubMed] [Google Scholar]
- 55. Pijnenborg JM, Wijnakker M, Hagelstein J, Delvoux B, Groothuis PG. Hypoxia contributes to development of recurrent endometrial carcinoma. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2007;17(4):897–904. 10.1111/j.1525-1438.2007.00893.x . [DOI] [PubMed] [Google Scholar]
- 56. Pansare V, Munkarah AR, Schimp V, Haitham Arabi M, Saed GM, Morris RT, et al. Increased expression of hypoxia-inducible factor 1alpha in type I and type II endometrial carcinomas. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2007;20(1):35–43. 10.1038/modpathol.3800718 . [DOI] [PubMed] [Google Scholar]
- 57. Horree N, van Diest PJ, van der Groep P, Sie-Go DM, Heintz AP. Hypoxia and angiogenesis in endometrioid endometrial carcinogenesis. Cellular oncology: the official journal of the International Society for Cellular Oncology. 2007;29(3):219–27. Epub 2007/04/25. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Koda M, Sulkowska M, Wincewicz A, Kanczuga-Koda L, Musiatowicz B, Szymanska M, et al. Expression of leptin, leptin receptor, and hypoxia-inducible factor 1 alpha in human endometrial cancer. Annals of the New York Academy of Sciences. 2007;1095:90–8. 10.1196/annals.1397.013 . [DOI] [PubMed] [Google Scholar]
- 59. Aybatli A, Sayin C, Kaplan PB, Varol F, Altaner S, Sut N. The investigation of tumoral angiogenesis with HIF-1 alpha and microvessel density in women with endometrium cancer. Journal of the Turkish German Gynecological Association. 2012;13(1):37–44. 10.5152/jtgga.2012.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yeramian A, Santacana M, Sorolla A, Llobet D, Encinas M, Velasco A, et al. Nuclear factor-kappaB2/p100 promotes endometrial carcinoma cell survival under hypoxia in a HIF-1alpha independent manner. Laboratory investigation; a journal of technical methods and pathology. 2011;91(6):859–71. 10.1038/labinvest.2011.58 . [DOI] [PubMed] [Google Scholar]
- 61. Li XL, Zhong XJ, Deng YL. P38MAPK regulation of HIF-1 alpha on the growth of endometrial carcinoma metastasis impact. China Journal of Modern Medicine. 2008;18(19):2802–4. [Google Scholar]
- 62. Zhai L, Liu YD, Dai M. Relationship between expressions of PTEN and HIF-1α in adenoendometrial carcinoma. Journal of Harbin Medical University. 2007;41(3):247–9. [Google Scholar]
- 63. Pan X, Yang Q, Sun Z-b. Expression of HIF-1α, MMP-2 in endometrial carcinoma and its clinical significance. Chinese Journal of Practical Gynecology and Obstetrics. 2011;27(1):48–50. [Google Scholar]
- 64. Song Q, Xie YG, Shi GS, Zhang JG, Huang H, Xiao J. Correlational research between gene expression of HIF-1α,VEGF and color Doppler ultrasound appearance in endometrial cancer. Chin J Med Imaging Technol. 2009;25(5):870–3. [Google Scholar]
- 65. Wang J, Liu ZC, Ma ZH, Wang M, Zhang SL. The Effect of VEGF, HIF-1α,Vasohibin in Tumor Angiogenesis of Endometrioid Adenocarcinoma and its Relation to Prognosis. Chines Journal of Histochemistry and Cytochemistry. 2010;19(1):43–8. [Google Scholar]
- 66. Tanimoto K, Yoshiga K, Eguchi H, Kaneyasu M, Ukon K, Kumazaki T, et al. Hypoxia-inducible factor-1alpha polymorphisms associated with enhanced transactivation capacity, implying clinical significance. Carcinogenesis. 2003;24(11):1779–83. Epub 2003/08/16. 10.1093/carcin/bgg132 . [DOI] [PubMed] [Google Scholar]
- 67. Shieh TM, Chang KW, Tu HF, Shih YH, Ko SY, Chen YC, et al. Association between the polymorphisms in exon 12 of hypoxia-inducible factor-1alpha and the clinicopathological features of oral squamous cell carcinoma. Oral oncology. 2010;46(9):e47–53. Epub 2010/07/27. 10.1016/j.oraloncology.2010.04.009 . [DOI] [PubMed] [Google Scholar]
- 68. Munoz-Guerra MF, Fernandez-Contreras ME, Moreno AL, Martin ID, Herraez B, Gamallo C. Polymorphisms in the hypoxia inducible factor 1-alpha and the impact on the prognosis of early stages of oral cancer. Annals of surgical oncology. 2009;16(8):2351–8. Epub 2009/05/19. 10.1245/s10434-009-0503-8 . [DOI] [PubMed] [Google Scholar]
- 69. Seeber LM, Horree N, Vooijs MA, Heintz AP, van der Wall E, Verheijen RH, et al. The role of hypoxia inducible factor-1alpha in gynecological cancer. Critical reviews in oncology/hematology. 2011;78(3):173–84. Epub 2010/07/16. 10.1016/j.critrevonc.2010.05.003 . [DOI] [PubMed] [Google Scholar]
- 70. Lee S, Garner EI, Welch WR, Berkowitz RS, Mok SC. Over-expression of hypoxia-inducible factor 1 alpha in ovarian clear cell carcinoma. Gynecologic oncology. 2007;106(2):311–7. Epub 2007/05/29. 10.1016/j.ygyno.2007.03.041 ; PubMed Central PMCID: PMCPmc1995602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer research. 1999;59(22):5830–5. Epub 1999/12/03. . [PubMed] [Google Scholar]
- 72. Risinger JI, Hayes AK, Berchuck A, Barrett JC. PTEN/MMAC1 mutations in endometrial cancers. Cancer research. 1997;57(21):4736–8. Epub 1997/11/14. . [PubMed] [Google Scholar]
- 73. Kong D, Suzuki A, Zou TT, Sakurada A, Kemp LW, Wakatsuki S, et al. PTEN1 is frequently mutated in primary endometrial carcinomas. Nature genetics. 1997;17(2):143–4. Epub 1997/11/05. 10.1038/ng1097-143 . [DOI] [PubMed] [Google Scholar]
- 74. Cohn DE, Basil JB, Venegoni AR, Mutch DG, Rader JS, Herzog TJ, et al. Absence of PTEN repeat tract mutation in endometrial cancers with microsatellite instability. Gynecologic oncology. 2000;79(1):101–6. Epub 2000/09/28. 10.1006/gyno.2000.5900 . [DOI] [PubMed] [Google Scholar]
- 75. Tang X, Zhang Q, Nishitani J, Brown J, Shi S, Le AD. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(9):2568–76. Epub 2007/05/03. 10.1158/1078-0432.ccr-06-2704 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.