Abstract
Background
Antiretroviral treatments decrease HIV mother-to-child transmission through pre/post exposure prophylaxis and reduction of maternal viral load. We modeled in-utero and intra-partum HIV transmissions to investigate the preventive role of various antiretroviral treatments interventions.
Methods
We analysed data from 3,759 women-infant pairs enrolled in 3 randomized clinical trials evaluating (1) zidovudine monotherapy, (2) zidovudine plus perinatal single-dose nevirapine or (3) zidovudine plus lopinavir/ritonavir for the prevention of mother-to-child transmission of HIV in Thailand. All infants were formula-fed. Non-linear mixed effect modeling was used to express the viral load evolution under antiretroviral treatments and the probability of transmission.
Results
Median viral load was 4 log10 copies/mL (Interquartile range: 3.36–4.56) before antiretroviral treatments initiation. An Emax model described the viral load time-course during pregnancy. Half of the maximum effect of zidovudine (28% decrease) and lopinavir/ritonavir (72% decrease) were achieved after 98 and 12 days, respectively. Adjusted on viral load at baseline (Odds ratio = 1.50 [95% confidence interval: 1.34, 1.68] per log10 copies/mL increment), antiretroviral treatments duration (OR = 0.80 [0.75, 0.84] per week increment) but not the nature of antiretroviral treatments were associated with in-utero transmission. Adjusted on gestational age at delivery (<37 weeks, OR = 2.37 [1.37, 4.10]), baseline CD4 (Odds ratio = 0.79 [0.72, 0.88] per 100 cells/mm3 increment) and predicted viral load at delivery (OR = 1.47 [1.25, 1.64] per log10 copies/mL increment), single-dose nevirapine considerably reduced intra-partum transmission (OR = 0.32 [0.2, 0.51]).
Conclusion
These models determined the respective contributions of various antiretroviral strategies on prevention of mother-to-child transmission. This can help predict the efficacy of new antiretroviral treatments and/or prevention of mother-to-child transmission strategies particularly for women with no or late antenatal care who are at high risk of transmitting HIV to their offspring.
Trial Registration
This analysis is based on secondary data obtained from three clinical trials. ClinicalTrials.gov. NCT00386230, NCT00398684, NCT00409591.
Introduction
Mother-to-child transmission (MTCT) of HIV can occur during pregnancy (in-utero), labor/delivery (intra-partum) or breastfeeding (post-partum). In the absence of antiretroviral treatment (ART), the transmission rate is 10% during pregnancy, 15% during labor and delivery and 10% during breastfeeding [1]. Viral load (VL) is the main predictor of MTCT [2]. Over the last two decades, studies have demonstrated that antiretroviral treatment during gestation, intra-partum, and breastfeeding dramatically reduces MTCT [3–6]. ART reduces MTCT through two complementary mechanisms: ART can reduce viral load and decrease the infant exposure to maternal viruses. Antiretrovirals can also cross the placenta and provide pre/post-exposure prophylaxis to the fetus and infant [7,8]. The 2013 World Health Organization (WHO) consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection [9] recommend that all HIV infected pregnant and breastfeeding women should initiate antiretroviral therapy as early as possible in pregnancy and maintain it at least for as long as the child is exposed to HIV.
Due to the high efficacy of current strategies leading to transmission rates as low as 2% or less, the evaluation of new drugs or drug combinations or new strategies for prevention of mother to child transmission (PMTCT) of HIV requires very large sample sizes to demonstrate efficacy improvements or non-inferiority. Modelling the efficacy of ARTs on MTCT, taking into account known risk factors, becomes increasingly important to gain prior information and optimize clinical trial design.
The objective of this work was to model in-utero and intra-partum HIV transmissions and, after adjusting for known risk factors, to investigate the role of various antiretroviral drug interventions for the PMTCT.
Material and Methods
Patients
Data were collected from pregnant women and infants who participated in three perinatal HIV prevention trials in Thailand.
PHPT-1
(NCT00386230)[4], carried out between 1996 and 2000, was a randomized, double-blind equivalence trial which compared the efficacy of zidovudine (ZDV) starting at 28 weeks' gestation plus 6 weeks of treatment in infants (the reference, “long-long” regimen) versus zidovudine starting at 35 weeks' gestation, with 3 days of zidovudine in infants (“short-short” regimen), and long-short and short-long regimens.
PHPT-2
(NCT00398684) [10], carried out between 2000 and 2004, was a randomized, double-blind trial of three treatment regimens, which evaluated the efficacy of single dose nevirapine (sdNVP) in mother during labor and in neonates or in mother only in addition to zidovudine during the third trimester of pregnancy and at least one week in children. Women enrolled in two PHPT-2 sub-studies (i) an open-label study for women who presented after 28 weeks gestation and (ii) a nevirapine pharmacokinetic study[11] were also included.
PHPT-5 first phase
(NCT00409591) [12], carried out between 2008 and 2010, was a randomized, 3-arm, double-blind trial. The three ARV strategies initiated during the third trimester were (i) maternal ZDV plus sdNVP at onset of labour and two infant NVP doses (at birth and 48 hours of life), (ii) maternal ZDV and two infant NVP doses, (iii) Maternal ZDV plus lopinavir/ritonavir (LPV/r), with no maternal or infant NVP.
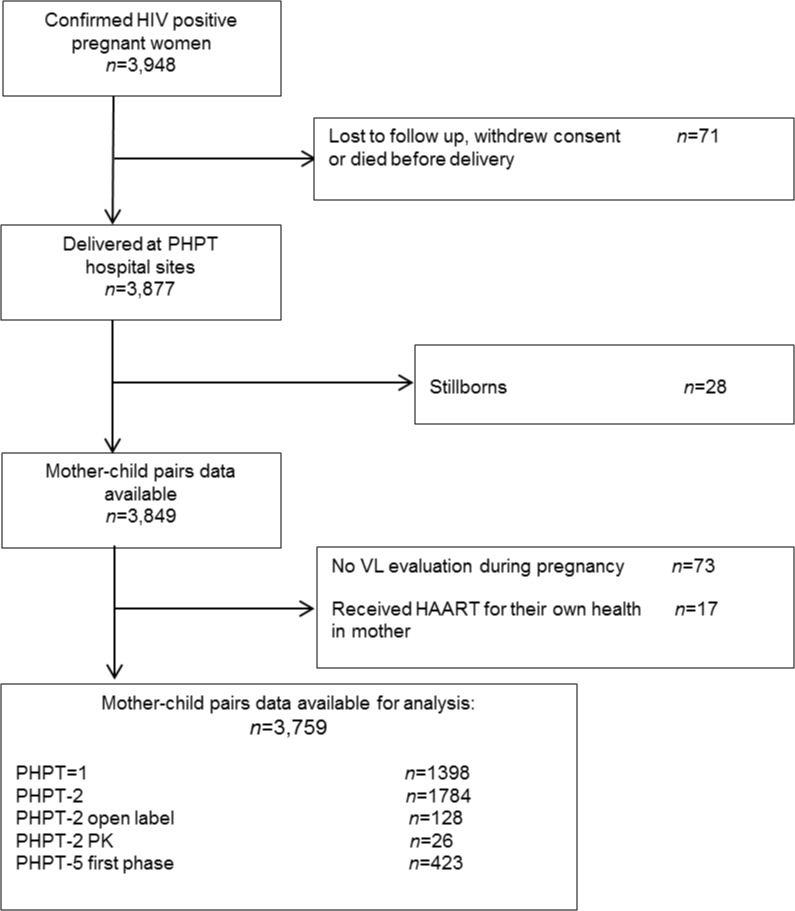
CD4 cell count and viral load were performed before starting antiretrovirals and during pregnancy. Viral loads were repeated at variable times during pregnancy and at delivery. All infants were formula fed. Of the 3,948 confirmed HIV positive pregnant women, 71 were lost to follow up, withdrew consent or died before delivery. Therefore, 3,877 women delivered at the PHPT hospital sites. After exclusion of 28 mothers of a stillborn child, 73 women who had no VL evaluation and 17 women who were receiving HAART for their own health, the aggregated dataset included 3,759 women with at least one VL sample during pregnancy.
Maternal plasma HIV-1 RNA levels
In all studies the maternal HIV-RNA measurement was planned prior to antiretroviral prophylaxis/treatment initiation to assess risk factors of transmission. In PHPT-1 and PHPT-2, VL samples were primarily collected for measurements at entry and delivery, while for PHPT-5, VL was measured monthly to assess HIV-RNA kinetics on antiretroviral drugs.
Plasma VL was assessed at the central PHPT laboratory in Chiang Mai University. Samples from PHPT-1 and PHPT-2 studies were tested using Cobas Amplicor HIV-1 Monitor kit version 1.5 (Roche Molecular Systems, USA) with a limit of quantification of 400 copies/mL; and samples from PHPT-5 first phase using the Abbott m2000 RealTime© HIV-1 assay (limit of quantification 40 copies/mL).
HIV status in infants and timing of transmission
To determine HIV infection status in infants, peripheral blood was drawn and spotted onto filter papers, dried and stored at -20°C before shipment to a central laboratory. Each of the PHPT samples were collected at birth, 6 weeks, 4 and 6 months. PHPT-5 samples were collected at birth, 7–10 days, 1, 2, 4 and 6 months of age.
In the original trials, infants were considered confirmed HIV-infected if samples obtained on two separate occasions were found positive by HIV-1 DNA PCR and confirmed HIV-uninfected if samples obtained on two separate occasions after one month of age were negative [13]. When only one DNA PCR was available and positive, infants were considered unconfirmed HIV-infected. When only one DNA PCR was available after the 1st week of life and negative, infants were considered unconfirmed HIV-uninfected. When only one DNA PCR was available within the 1st week of life and negative, infants were considered as indeterminate [13]. In the present analysis, unconfirmed HIV infected infants were considered HIV-infected and unconfirmed HIV-uninfected infants were considered HIV-uninfected, while indeterminate infants were excluded.
Infants with a positive DNA PCR result during the first week of life were considered to be infected during pregnancy (“in-utero” transmission); infants with negative HIV-DNA PCR results during the first week of life but with a subsequent positive result were considered to be infected during labor or delivery (“intra-partum” transmission)[13]. Twins were considered a single entity and discordant twins were counted as one infected infant.
Ethics
Each of the PHPT perinatal study protocols and their amendments, as well as the use of data for this analysis received ethical clearance from the Thai Ministry of Public Health, the Harvard School of Public Health and Chiang Mai University Faculty of Medical Associated Sciences Ethics Committees. The consent procedures were reviewed and approved by the ethics committees. Before enrollment, all women provided written informed consent for their participation and that of their infants.
Modeling of viral load time course during pregnancy
An Emax model was chosen since it is based upon pharmacological principles, i.e. the theory of drug action mediated by ligand-receptor interaction which translates in an hyperbolic equation. Because ZDV and LPV/r have 2 distinct sites and mechanisms of action, the effects were considered to be additive [14]. A proportional effect Emax model [14] was applied to describe the viral load at time T during pregnancy (VL, expressed in log values). The model took into account VL before treatment initiation (VL0) and the duration and nature of the 2 ARTs including ZDV and LPV/r (S1 Dataset) was composed as follows.
Model parameters were
VL 0: VL before treatment
E MAX: Treatment maximum effect
γ: Hill coefficient for treatment effect
T 50: Treatment duration to reach half of E MAX
Since ZDV and LPV/r have distinct sites and mechanisms of action, the effects of ZDV and LPV/r administered in combination were assumed to be additive [14]. Because the inhibition cannot exceed 100%, E MAX,LPV was deduced from E MAX,ZDV by
Where
E MAX,ZDV: ZDV maximum effect
E MAX,LPV: LPV/r maximum effect
Interindividual variability was modeled using an exponential error model, with η i being the interindividual random effect with mean 0 and variance ω 2.
Modeling of in-utero and intra-partum transmissions
In-utero and intra-partum transmissions were treated as independent outcomes. Logistic regression models with random effect (η) were developed to predict in-utero and intra-partum transmission according to relevant risk factors. For each odds ratio, point estimate and 95% confidence intervals are provided.
We investigated known risk factors for mother-to-child transmission of HIV [2,15,16]. In-utero transmission was assumed to depend on VL0 (log10copies/mL), drug(s) treatment duration(s) in weeks, CD4 count before treatment (CD4BASELINE) and gestational age (GA) at treatment initiation and at delivery (S2 Dataset). The risk of intra-partum transmission was dependent on VL at delivery (VLDELIVERY), itself predicted by the VL time-course model, perinatal NVP (sdNVP administered at onset of labor or during the first hours of life or both), maternal ZDV loading dose during labor, delivery mode, premature labor (Gestational age (GA) <37 weeks) and ART(s) administered to infants during the first weeks of life (S3 dataset).
Data analysis
VL time-courses and MTCT events were analysed using a non-linear mixed effect modeling approach. Parameters were estimated by computing the maximum likelihood estimators without any approximation of the model (no linearization) using the stochastic approximation expectation maximization (SAEM) algorithm combined to a Markov Chain Monte Carlo (MCMC) procedure. Data were analysed using MONOLIX (version 4.1.2, http://www.lixoft.com/) [17,18]. VL counts were log transformed and residual variability was described by an additive error, whereas an exponential model was used for between-subject variabilities (η). Data below the limit of quantification were left-censored [19]. The effect of a covariate on a structural parameter was retained if it produced a decrease in the Bayesian Information Criterion (BIC) compared to the baseline model i.e. the covariate-free model. A smaller BIC value signifies a model that better fits the data [20].
The logistic model for MTCT events analysis was written in a MLXTRAN script file (S1 MLXTRAN scripts); the random effect η was assumed to be normally distributed. In the univariate analyses, variables that both decreased BIC and had acceptable relative standard error (RSE<50%) were considered as significant factors to be included in the multivariable analyses. In the multivariable analysis, these variables were added one by one considering the largest drops in the BIC value to define the final model.
Visual predictive check (VPC) evaluation
Simulated VL time-courses were compared with the observed data to evaluate the performance of the model. The vector of model parameters from 400 replicates of the database was simulated. The 5th, 50th and 95th percentiles of the simulated dependent variables at each time were then overlaid on the observed data. The proportion of observed MTCT with their confidence intervals were plotted as a function of significant predictors. The 5th, 50th and 95th percentiles of the model predictions were simultaneously plotted. Visual inspection was used to confirm that the observed proportions were included in the limits defined by the percentiles curves. The residual sum of square (RSS) was provided in addition to graphical check.
Results
Characteristics of the study population
A total of 3,759 HIV-infected pregnant women enrolled in the PMTCT studies from 1996 to 2010 were included (Fig 1). Table 1 presents the baseline and delivery characteristics of the women included in the analysis and the treatments they received.
Fig 1. Population disposition.
Table 1. Characteristics of pregnant women and ARTs for perinatal HIV prevention according to study.
| Variable | PHPT-1 | PHPT-2 | PHPT-2 OPEN Label | PHPT-2 PK | PHPT-5 | Total |
|---|---|---|---|---|---|---|
| Number of women with at least 1 VL sample | 1,398 | 1,784 | 128 | 26 | 423 | 3,759 |
| Characteristics of pregnant women | ||||||
| Gestational age at enrollment (weeks) | ||||||
| Median | 28.0 | 31.0 | 38.4 | 33.9 | 29.0 | 29.6 |
| Interquartile range | 27.7 to 28.2 | 30.1 to 33.1 | 36.5 to 40.0 | 31.3 to 37.3 | 28.0 to 30.0 | 28.0 to 31.6 |
| CD4 at enrollment (cell count/ mm3) | ||||||
| Median | 360 | 376 | 368 | 454 | 458 | 385 |
| Interquartile range | 240 to 500 | 247 to 528 | 243 to 542 | 270 to 582 | 368 to 576 | 260 to 526 |
| Gestational age at delivery (weeks) | ||||||
| Median | 39.0 | 38.6 | 38.6 | 39.4 | 38.7 | 38.7 |
| Interquartile range | 38.0 to 40.0 | 37.9 to 39.6 | 37.0 to 40.0 | 38.1 to 40.6 | 37.8 to 39.7 | 37.9 to 39.7 |
| Type of delivery | ||||||
| 1) emergency C/section | 141 (10%) | 265 (15%) | 21 (16%) | 7 (27%) | 0 (0%) | 434 (11%) |
| 2) planned C/section | 111 (8%) | 105 (6%) | 9 (7%) | 3 (11%) | 59 (14%) | 287 (8%) |
| 3) vaginal delivery | 1,146 (82%) | 1,414 (79%) | 98 (77%) | 16 (62%) | 364 (86%) | 3,038 (81%) |
| Maternal treatment | ||||||
| ZDV during pregnancy | 1,387 (>99%) | 1,784 (100%) | 73 (57%) | 23 (88%) | 423 (100%) | 3,690 (98%) |
| ZDV duration (days) | ||||||
| Median | 57 | 67 | 0 | 52 | 71 | 65 |
| Interquartile range | 30 to 79 | 52 to 77 | 0 to 11.5 | 30 to 61 | 60 to 78 | 39 to 77 |
| LPV during pregnancy | - | - | - | - | 145 (4%) | 145 (4%) |
| LPV duration (days) | ||||||
| Median | - | - | - | - | 69 | 69 |
| Interquartile range | - | - | - | - | 55 to 77 | 55 to 77 |
| NVP at onset of labor | - | 1407 (79%) | 110 (86%) | 26 (100%) | 133 (31%) | 1,676 (45%) |
| ZDV loading dose at onset of labor | 1,336 (96%) | 1,777 (>99%) | 119 (93%) | 26 (100%) | 410 (97%) | 3,668 (98%) |
| Infant treatment | ||||||
| ZDV prophylaxis | 1,381 (99%) | 1,779 (>99%) | 127 (99%) | 26 (100%) | 420 (99%) | 3,733 (99%) |
| ZDV duration (days) | ||||||
| Median | 41 | 11 | 44 | 7 | 7 | 11 |
| Interquartile range | 3 to 42 | 10 to 14 | 41 to 48 | 6 to 7 | 7 to 7 | 7 to 41 |
| Postnatal NVP (infants) | - | 729 (41%) | 134 (97%) | 26 (100%) | 275 (65%) | 1,154 (31%) |
| Perinatal NVPa | - | 1,424 (80%) | 126 (98%) | 26 (100%) | 276 b (65%) | 1,852 (49%) |
a sdNVP either in the woman at onset of labor, in the infant or in both
b only in women who did not receive LPV/r
The HIV status of the infants were as follows: 174 (5%) confirmed HIV-infected, 3,411 (91%) confirmed HIV-uninfected, 9 (<1%) unconfirmed HIV-infected, 113 (3%) unconfirmed HIV-uninfected and 52 (1%) indeterminate. According to the definition for this analysis, there were 183 HIV transmissions and 52 indeterminate infants were excluded from the transmission analysis. Among the infected infants, there were 80 in-utero and 103 intra-partum transmissions.
ARTs during pregnancy
Of the 3,759 mother-infant pairs analysed, 1,751 (47%) received mother-infant ZDV monotherapy and 1,851 (49%) mother-infant ZDV plus perinatal sdNVP. In addition 145 (4%) mothers received ZDV plus LPV/r during the third trimester, without perinatal sdNVP.
VL time-course modeling
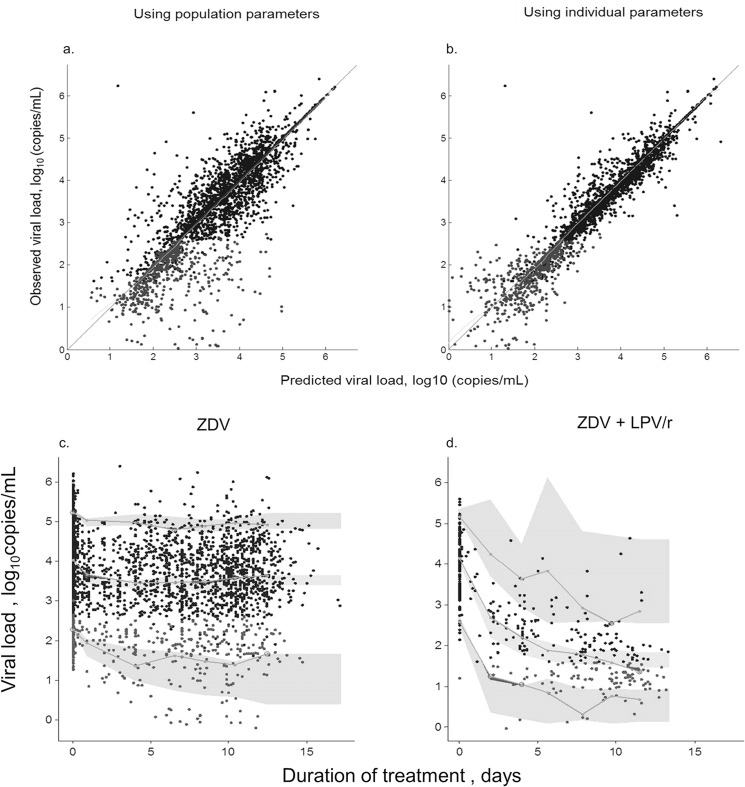
A total of 5,576 VL measurements in 3,759 subjects were available for modeling (median 1 measurement per patient, range 1 to 6). Sixty five percent of the women had only 1 measurement (all but 5% of these at ART initiation), and 35% had at ≥2 measurements. Median VL was 4 log10 copies/mL (IQR: 3.36–4.56) before ART initiation and 3.51 log10copies/ mL (IQR: 2.89–3.34) at delivery. VL at any time point during pregnancy was dependent on baseline VL and ZDV and LPV/r treatment durations and was well described by a combined Emax model. The Hill coefficient for ZDV effect (γZDV) γ ZDV was close to 1 and thus was fixed to 1. The η parameters for γZDV, LPV/r duration to reach half of E MAX,LPV (T 50,LPV) and the Hill coefficient for LPV/r (γLPV) γ ZDV were not statistically significant. Removing them from the model did not alter the quality of the fit or further decreased the BIC value (final model, BIC = 2113.33). None of the other covariates, including CD4 cell count and GA at baseline, had a significant effect on model parameters. All parameters were well estimated with RSE below 30% (Table 2). The model estimated that half of the maximum effect of ZDV (28% VL decrease from baseline) and LPV/r (72% decrease) were observed after 98 and 12 days respectively. Using the population parameter, the RSS was 747.39 while it was 369.20 when using individual parameter. The observed vs. model-predicted plots are shown in Fig 2A and 2B (top). The visual predictive checks are shown in Fig 2C and 2D (bottom).
Table 2. Population parameter estimates of HIV time-course model for 3,759 HIV-1-infected mothers enrolled in the PHPT-1, PHPT-2, and PHPT-5 studies.
| Parameters | Estimate | SE a | RSE b (%) |
|---|---|---|---|
| Structural model | |||
| T 50,ZDV | 98.3 | 12 | 12 |
| γZDV | 1 (fixed) | - | - |
| T 50,LPV | 11.6 | 3.3 | 29 |
| γLPV | 0.28 | 0.049 | 18 |
| E MAX,ZDV | 0.285 | 0.016 | 6 |
| E MAX,LPV | 0.715 | - | - |
| Statistical model | |||
| 2.34 | 0.15 | 6 | |
| 0.852 | 0.043 | 5 | |
| σ VL c | 0.197 | 0.0028 | 1 |
a SE, standard error of estimate
b RSE%, relative standard error (standard error of estimate / estimate*100)
c σ VL, residual (square roots of variances)
Fig 2. Diagnostic plots for viral load time-course model.
Top: 2a and 2b: Observed versus model predicted viral load values (expressed as log10 copies) of the population and individual predictions respectively. Solid black circles, measure values; grey symbols, simulation of below the limit of quantification data. Line, identity line. Bottom: Visual predictive check plots. (2c) Women receiving only zidovudine (ZDV); (2d) women receiving zidovudine plus lopinavir/ritonavir (ZDV+LPV/r).The lines denote the median, 5th and 95th percentiles for the observed data. The grey areas stand for the 95% confidence intervals of the median, 5th and 95th model prediction percentiles.
MTCT modeling
The MTCT models were built step by step from the basic model (without explanatory variable). Viral load at delivery (VLDELIVERY) was estimated through the VL time course final model using individual parameters.
In-utero transmission. Upon univariate analysis, CD4 count, gestational age, VL before treatment initiation and ZDV duration caused a drop in the BIC, indicating significant effects of these variables. In the multivariable analysis, only ZDV duration and VL before treatment initiation remained independently associated with in-utero transmission (Table 3). LPV/r duration and baseline CD4 count were not significantly associated with in-utero transmission.
Table 3. The univariate and multivariable analyses of the HIV in-utero model using data from 3,707 HIV-1-infected mothers enrolled in the PHPT-1, PHPT-2, and PHPT-5 studies.
| Predictors | Logit coefficient(95%CI) | Odds ratio(95%CI) | RSE (%) a | BIC b |
|---|---|---|---|---|
| Univariate analysis | ||||
| Baseline model | -3.71 (-3.89, -3.53) | - | 2 | 791.42 |
| ZDV duration (weeks) | -0.09 (-0.15, -0.04) | 0.91 (0.86, 0.96) | 29 | 772.04 |
| VL before treatment initiation (log10 copies/mL) | 0.23 (0.158, 0.31) | 1.26 (1.17, 1.36) | 17 | 778.06 |
| CD4 (per 100 cell count) | -0.13 (-0.22, -0.04) | 0.88 (0.80, 0.96) | 34 | 784.04 |
| Gestational age at baseline (days) | 0.003 (0.001, 0.005) | 1.003 (1.001, 1.005) | 33 | 794.26 |
| Multivariable analysis | ||||
| Model 1 | 740.13 | |||
| VL | 0.43 (0.29, 0.58) | 1.54 (1.34, 1.78) | 17 | |
| ZDV duration | -0.21 (-0.27, -0.15) | 0.81 (0.77, 0.86) | 15 | |
| CD4 | -0.19 (-0.30, -0.08) | 0.83 (0.74, 0.92) | 30 | |
| Model 2 | 746.69 | |||
| VL before treatment | 0.44 (0.22, 0.66) | 1.55 (1.25, 1.93) | 26 | |
| ZDV duration | -0.22 (-0.28, -0.16) | 0.80 (0.76, 0.85) | 13 | |
| GA at baseline | 3.44e-007 (-0.004, 0.004) | 1.00 (0.99, 1.004) | 6.25e+005 | |
| Final model c | 735.96 | |||
| VL before treatment | 0.41 (0.30, 0.52) | 1.50 (1.34, 1.68) | 14 | |
| Duration of ZDV | -0.23 (-0.28,-0.17) | 0.80 (0.75, 0.84) | 13 |
a RSE%, relative standard error (standard error of estimate / estimate*100)
b Bayesian Information Criterion
c Random effect of individuals: η~N(0,0.5272)
The final model was:
where ZDVDURATION is the ZDV duration during pregnancy (weeks).
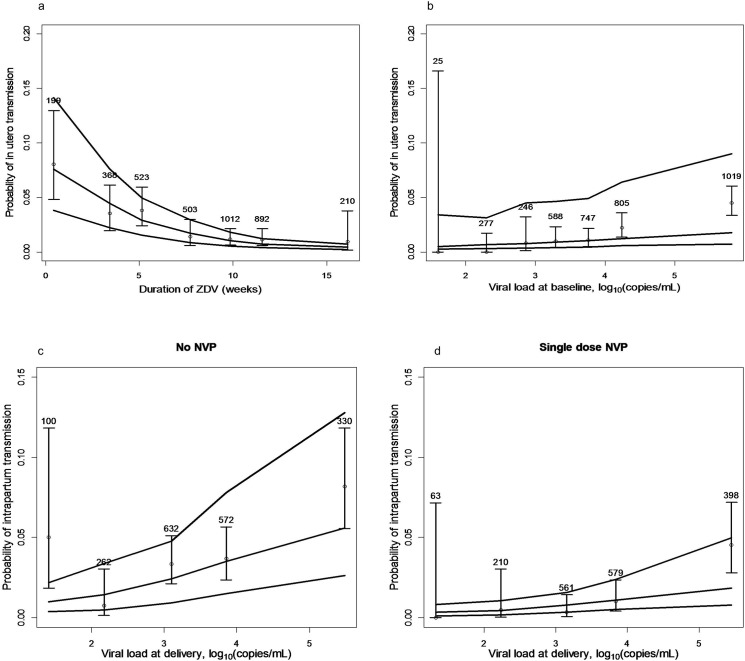
The probability of in-utero HIV transmission as a function of zidovudine duration is shown in Fig 3A, and that of in-utero HIV transmission as a function of viral load at baseline in Fig 3B.
Fig 3. Transmission probabilities according to zidovudine duration, viral load at baseline/delivery, and nevirapine intake.
A. Probability of in-utero HIV transmission as a function of zidovudine duration; B. Probability of in-utero HIV transmission as a function of viral load at baseline. The lines denote the median, 5th and 95thpercentiles of the model predictions. The open circles stand for the observed mean proportion of transmission, the solid vertical segments denote the corresponding 95% confidence intervals (numbers at top of each segment stand for the number of women in each time interval or VL interval). C. Probability of intra-partum HIV mother-to-child transmission as a function of viral load at delivery without single dose nevirapine; D. Probability of intra-partum HIV mother-to-child transmission as a function of viral load at delivery with single dose nevirapine.
Intra-partum transmission. Perinatal sdNVP, duration of ZDV, premature labor (GA at delivery<37 weeks)viral load at delivery and CD4 cell count were significantly associated with intra-partum transmission in the univariate analyses, while mode of delivery, ZDV loading dose and infants ZDV prophylaxis were not.
In the multivariable analysis, final model included VL at delivery, perinatal sdNVP administration, CD4 cell count and premature labor (Table 4). The duration of ZDV was no longer significant when other factors were included.
Table 4. The univariate and multivariable analyses of the HIV intra-partum transmission model using data from 3,707 HIV-1-infected mothers enrolled in the PHPT-1, PHPT-2, and PHPT-5 studies.
| Predictors | Logit coefficient(95%CI) | Odds ratio(95%CI) | RSE (%) a | BIC b |
|---|---|---|---|---|
| Univariate analysis | ||||
| Baseline model | -3.29 (-3.43, -3.15) | - | 2 | 968.77 |
| ZDV duration (weeks) | -0.049 (-0.09, -0.01) | 0.95 (0.91, 0.99) | 46 | 956.07 |
| VL at delivery (log10 copies/mL) | 0.17 (0.08, 0.26) | 1.18 (1.08, 1.30) | 26 | 953.04 |
| CD4 (per 100 cell count) | -0.18 (-0.27, -0.09) | 0.83 (0.77, 0.91) | 25 | 942.79 |
| Premature labor | 0.81 (0.30,1.32) | 2.25 (1.35, 3.75) | 32 | 957.50 |
| Perinatal NVP | -1.10 (-1.53, -0.67) | 0.33 (0.22, 0.51) | 20 | 943.94 |
| Multivariable analysis | ||||
| Model 1 | 926.61 | |||
| Perinatal NVP | -0.97 (-1.44, -0.50) | 0.38 (0.24, 0.61) | 25 | |
| CD4 | -0.22 (-0.33, -0.12) | 0.80 (0.72, 0.88) | 23 | |
| Model 2 | 916.58 | |||
| Perinatal NVP | -1.13 (-1.58, -0.68) | 0.32 (0.21, 0.51) | 21 | |
| CD4 | -0.23 (-0.33, -0.12) | 0.80 (0.72, 0.88) | 16 | |
| VL at delivery | 0.42 (0.29, 0.55) | 1.52 (1.34, 1.74) | 23 | |
| Model 3 | 922.80 | |||
| Perinatal NVP | -1.15 (-1.62, -0.68) | 0.32 (0.20, 0.51) | 21 | |
| CD4 | -0.153 (-0.25, -0.05) | 0.86 (0.78, 0.95) | 33 | |
| VL at delivery | 0.634 (0.51, 0.76) | 1.88 (1.67, 2.13) | 10 | |
| ZDV duration | 0.0001 (-0.0072, 0.0073) | 1.00 (0.99, 1.01)) | 3.67e+004 | |
| Final model c | 916.61 | |||
| Perinatal NVP | -1.13 (-1.58, -0.68) | 0.32 (0.21, 0.51) | 21 | |
| CD4 | -0.23 (-0.34, -0.13) | 0.79 (0.72, 0.88) | 22 | |
| VL at delivery | 0.36 (0.23, 0.50) | 1.44 (1.26, 1.64) | 19 | |
| Premature labor | 0.86 (0.31, 1.41) | 2.37 (1.37, 4.10) | 32 |
aRSE%, relative standard error (standard error of estimate / estimate*100)
b Bayesian Information Criterion
c Random effect of individuals: η~N(0,0.732)
The final model was:
where 1 CD4BASELINE unit is 100 cells /mm3, NVP and GADELIVERY are binary (YES = 1 or NO = 0)
Fig 3C and 3D show the probability of intra-partum HIV transmission as a function of viral load at delivery without and with single dose nevirapine, respectively.”
Discussion
The VL time-course model during pregnancy developed as a function of the type of treatment administered, its duration and the VL level at baseline, provided a good prediction of the VL level at delivery. This predicted VL could be used in the MTCT models. VL at treatment initiation and treatment duration were the main determinants of in-utero transmission, regardless of the ARV regimens used. High VL at delivery, absence of perinatal sdNVP and premature labor were associated with intra-partum transmission.
As previously shown in PACTG 076 [21], although ZDV monotherapy had only a slight effect on maternal VL (only 0.43 Log decrease in this analysis), it was very effective in reducing in-utero transmission. This is consistent with the accepted concept that ZDV, which cross the placenta freely, exerts its prophylactic effect largely through pre-post exposure prophylaxis [21]. In the late 1990, it was hypothesized that in-utero transmission would occur late in pregnancy [22]. This justified for the launch of several short ZDV course trials in developing countries [23–25]. However, this was not confirmed by the PHPT-1 trial where the rates of in-utero transmission were 1.6 versus 5.1% with long and short ZDV treatments, respectively [4,15]. This supports the WHO PMTCT 2013 guidelines recommending ART initiation as early as possible during pregnancy [9]. Although ZDV plus LPV/r was much more effective than ZDV alone in reducing VL, adding LPV/r to ZDV did not decrease further in-utero transmission [26].
Although it had no effect on in-utero transmission, the addition of LPV/r, which has limited perfusion through the placenta, had as expected a major effect on the VL at delivery (mean reduction, 2.18 log10copies/mL), and thus a major effect on intra-partum transmission. More importantly, after adjusting for all factors associated with transmission, perinatal sdNVP, in the mother only, the mother and her infant, or the infant only, markedly reduced the risk of intra-partum transmission at all viral load levels (Fig 3C and 3D).
Modeling of VL during LPV/r plus ZDV treatment (Fig 2D) showed that with a treatment duration less than 8–10 weeks before delivery, VL at delivery remained detectable in a large proportion of women. Accordingly, when mothers start HAART late in pregnancy, it is advisable to intensify the ART regimen by providing sdNVP to both mother and infant and a brief course of combined ART to the newborn in order to reduce the probability of intra-partum transmission [27–28]. Interestingly, in the presence of perinatal sdNVP, intra-partum transmission was only weakly associated with VL at delivery, indicating the potent pre/post exposure prophylactic effect of this drug.
Prematurity has been found to be associated with perinatal transmission in several studies [15,16,29]. It has been debated whether prematurity was a consequence of in-utero transmission or if premature infants were more vulnerable to HIV infection [30]. The fact that in this study prematurity was associated with intra-partum but not with in-utero transmission supports a higher vulnerability of premature children.
Several studies reported that planned caesarean section (CS) [31,32] reduced the risk of intra-partum transmission in particular when VL at delivery is high but, in this study, the percentage of women with planned C-section was too low (8%) to assess this factor.
This study has several limitations. In our definition of HIV status of the infants, we considered as infected or uninfected, infants with unconfirmed HIV status and excluded those who were indeterminate. However, a sensitivity analysis restricted to infants with confirmed HIV status provided very close results (data not shown). Datasets from other trials with different antiretroviral prophylaxis regimens could have been incorporated into the model but this would have perhaps offset the advantages of using data from trials performed in the same setting by the same team. Also, all subjects were from Thailand although there is no indication that risks of transmission and intervention effectiveness differ across ethnic groups.
In conclusion, our models provide insights on the respective roles of pre-exposure prophylaxis and maternal viral load reduction in preventing mother-to-child transmission according to the preventive strategy. With the regimens considered in this analysis, while the preventive effect of ART during pregnancy was essentially driven by transplacental pre/post exposure prophylaxis, both viral load reduction by the time of delivery and infant prophylaxis were important in preventing intra-partum transmission. Given the high efficacy of current interventions, clinical trials to test the efficacy of new antiretrovirals or PMTCT strategies have become more and more difficult to implement. A Bayesian approach with data from previous clinical trials could reduce sample sizes and help optimize trial design.
Supporting Information
(CSV)
(CSV)
(CSV)
(DOC)
Acknowledgments
We would like to thank all members of the hospital teams, and the women and children who participated in the PHPT perinatal studies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The clinical trials containing data used in this analysis were funded by the National Institutes of Health (PHPT-1: 5 R01 HD 33326; PHPT-2: NICHD R01 HD 39615; PHPT-5 First Phase, NICHD R01HD052461, R01HD056953, USA) and the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (PHPT-2: ANRS 1208). Patumrat Sripan received a scholarship from the French Embassy in Thailand and a Science Achievement Scholarship of Thailand for her PhD. Co-author Billy Amzal is employed by LASER Analytica. LASER Analytica provided support in the form of salary for author BA, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘author contributions’ section.
References
- 1. De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 2. Mofenson LM, Lambert JS, Stiehm ER, Bethel J, Meyer WA 3rd, Whitehouse J, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341: 385–393. 10.1056/NEJM199908053410601 [DOI] [PubMed] [Google Scholar]
- 3. Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331: 1173–1180. 10.1056/NEJM199411033311801 [DOI] [PubMed] [Google Scholar]
- 4. Lallemant M, Jourdain G, Le Coeur S, Kim S, Koetsawang S, Comeau AM, et al. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. N Engl J Med. 2000;343: 982–991. 10.1056/NEJM200010053431401 [DOI] [PubMed] [Google Scholar]
- 5. Mandelbrot L, Landreau-Mascaro A, Rekacewicz C, Berrebi A, Bénifla JL, Burgard M, et al. Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. JAMA. 2001;285: 2083–2093. [DOI] [PubMed] [Google Scholar]
- 6. Kesho Bora Study Group, de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11: 171–180. 10.1016/S1473-3099(10)70288-7 [DOI] [PubMed] [Google Scholar]
- 7. Chung MH, Kiarie JN, Richardson BA, Lehman DA, Overbaugh J, Njiri F, et al. Independent effects of nevirapine prophylaxis and HIV-1 RNA suppression in breast milk on early perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2007;46: 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362: 2282–2294. 10.1056/NEJMoa0907736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO | Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. In: WHO [Internet]. [cited 30 Jul 2014]. Available: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/.
- 10. Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, Koetsawang S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351: 217–228. 10.1056/NEJMoa033500 [DOI] [PubMed] [Google Scholar]
- 11. Cressey TR, Jourdain G, Lallemant MJ, Kunkeaw S, Jackson JB, Musoke P, et al. Persistence of nevirapine exposure during the postpartum period after intrapartum single-dose nevirapine in addition to zidovudine prophylaxis for the prevention of mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2005;38: 283–288. [PubMed] [Google Scholar]
- 12.Lallemant M, Jourdain G, Ngo-Giang-Huong N, Le Coeur S, Cressey TR, Puangsombat A, et al. Maternal and infant nevirapine versus infant only nevirapine, or maternal lopinavir/ritonavir in addition to standard zidovudine prophylaxis to prevent perinatal HIV in Thailand. 18th Conference on Retroviruses and Opportunistic Infections, Boston, USA,; 27 February-2 March.
- 13. Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992;327: 1246–1247. 10.1056/NEJM199210223271718 [DOI] [PubMed] [Google Scholar]
- 14. Holford NH, Sheiner LB. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet. 1981;6: 429–453. [DOI] [PubMed] [Google Scholar]
- 15. Jourdain G, Mary J-Y, Coeur SL, Ngo-Giang-Huong N, Yuthavisuthi P, Limtrakul A, et al. Risk factors for in utero or intrapartum mother-to-child transmission of human immunodeficiency virus type 1 in Thailand. J Infect Dis. 2007;196: 1629–1636. 10.1086/522009 [DOI] [PubMed] [Google Scholar]
- 16. Magder LS, Mofenson L, Paul ME, Zorrilla CD, Blattner WA, Tuomala RE, et al. Risk factors for in utero and intrapartum transmission of HIV. J Acquir Immune Defic Syndr. 2005;38: 87–95. [DOI] [PubMed] [Google Scholar]
- 17. Savic RM, Mentré F, Lavielle M. Implementation and evaluation of the SAEM algorithm for longitudinal ordered categorical data with an illustration in pharmacokinetics-pharmacodynamics. AAPS J. 2011;13: 44–53. 10.1208/s12248-010-9238-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Computational Statistics & Data Analysis. 2005;49: 1020–1038. 10.1016/j.csda.2004.07.002 [DOI] [Google Scholar]
- 19. Samson A, Lavielle M, Mentré F. Extension of the SAEM algorithm to left-censored data in nonlinear mixed-effects model: Application to HIV dynamics model. Computational Statistics & Data Analysis. 2006;51: 1562–1574. 10.1016/j.csda.2006.05.007 [DOI] [Google Scholar]
- 20. Raftery AE. Bayesian model selection in social research Sociological methodology. Cambridge, MA: Blackwell; 1995. pp. 111–196. [Google Scholar]
- 21. Sperling RS, Shapiro DE, Coombs RW, Todd JA, Herman SA, McSherry GD, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1996;335: 1621–1629. 10.1056/NEJM199611283352201 [DOI] [PubMed] [Google Scholar]
- 22. Rouzioux C, Costagliola D, Burgard M, Blanche S, Mayaux MJ, Griscelli C, et al. Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. The HIV Infection in Newborns French Collaborative Study Group. Am J Epidemiol. 1995;142: 1330–1337. [DOI] [PubMed] [Google Scholar]
- 23. Dabis F, Msellati P, Meda N, Welffens-Ekra C, You B, Manigart O, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d’Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mère-Enfant. Lancet. 1999;353: 786–792. [DOI] [PubMed] [Google Scholar]
- 24. Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353: 773–780. [DOI] [PubMed] [Google Scholar]
- 25. Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d’Ivoire: a randomised trial. Lancet. 1999;353: 781–785. 10.1016/S0140-6736(98)10412-9 [DOI] [PubMed] [Google Scholar]
- 26. Dryden-Peterson S, Jayeoba O, Hughes MD, Jibril H, Keapoletswe K, Tlale J, et al. Highly active antiretroviral therapy versus zidovudine for prevention of mother-to-child transmission in a programmatic setting, Botswana. J Acquir Immune Defic Syndr. 2011;58: 353–357. 10.1097/QAI.0b013e31822d4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Phanuphak N, Lolekha R. Thai national guidelines for the prevention of motherto-child transmission of HIV: March 2010. Asian Biomed. 2011;4: 529 10.5372/abm.v4i4.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor GP, Clayden P, Dhar J, Gandhi K, Gilleece Y, Harding K, et al. British HIV Association guidelines for the management of HIV infection in pregnant women 2012. HIV Med. 2012;13 Suppl 2: 87–157. 10.1111/j.1468-1293.2012.01030_2.x [DOI] [PubMed] [Google Scholar]
- 29. Kuhn L, Abrams EJ, Matheson PB, Thomas PA, Lambert G, Bamji M, et al. Timing of maternal-infant HIV transmission: associations between intrapartum factors and early polymerase chain reaction results. New York City Perinatal HIV Transmission Collaborative Study Group. AIDS. 1997;11: 429–435. [DOI] [PubMed] [Google Scholar]
- 30. Kuhn L, Steketee RW, Weedon J, Abrams EJ, Lambert G, Bamji M, et al. Distinct risk factors for intrauterine and intrapartum human immunodeficiency virus transmission and consequences for disease progression in infected children. Perinatal AIDS Collaborative Transmission Study. J Infect Dis. 1999;179: 52–58. 10.1086/314551 [DOI] [PubMed] [Google Scholar]
- 31. The International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340: 977–987. 10.1056/NEJM199904013401301 [DOI] [PubMed] [Google Scholar]
- 32. European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353: 1035–1039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(CSV)
(CSV)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.