Abstract
Developing multicolor upconversion nanoparticles (UCNPs) with the capability of regulating their emission wavelengths in the UV to visible range in response to external stimuli can offer more dynamic platforms for applications in high resolution bio-imaging, multicolor barcoding and driving multiple important photochemical reactions, such as photoswitching. In this communication, we have rationally designed single crystal core-shell structured UCNPs which are capable of orthogonal UV and visible emissions in response to two distinct NIR excitations at 808 and 980 nm. The orthogonal excitation-emission properties of such UCNPs, as well as their ability to utilize low power excitation, which attenuates any local heating from the lasers, endows the UCNPs with great potential for applications in materials and biological settings. As a proof of concept, the use of this UCNP for the efficient regulation of the two-way photoswitching of spiropyran by using dual wavelengths of NIR irradiation has been demonstrated.
Keywords: upconversion nanoparticles, core-shell nanoparticles, two-way photoswitching, dual NIR excitations
Lanthanide-doped upconversion nanoparticles (UCNPs) have recently gained much attention due to their unique capability of upconverting low energy near-infrared (NIR) light to high energy ultraviolet (UV) and visible light.[1] Combined with other excellent photo-physical properties, including long emission lifetimes, narrow emission band-widths, and high photostability, UCNPs have shown widespread application in fields ranging from bio-imaging and sensors to photovoltaics and solid-state devices.[2] One of the critical requirements for harvesting their full potential is to develop UNCPs with emission profiles specifically tuned towards their target applications. This includes synthesizing UCNPs with strong UV emission to trigger chemical reactions such as the photocleavage of photolabile groups,[3] and highly visible emitting UCNPs for nanomedicine-based applications such as photodynamic therapy (PDT),[4] as well as NIR emitting UCNPs for in vivo bio-imaging.[5] Among the developed various UCNPs with tailored emission profiles, multicolor UCNPs capable of regulating their emission wavelengths from the UV to visible range in response to external stimuli are garnering much interest recently, as they can offer more dynamic platforms for applications in high resolution bio-imaging, multicolor encoding and photoswitching.[6] However, there are few reports on such multicolor UCNPs. Of them, one typical example is the excitation-responsive UCNP, whose emission can be modulated between spectrally pure visible light and mixed UV/Vis emissions by changing the power density of 980 nm NIR excitation.[6b–d] This unique property of the UNCPs was then successfully applied toward driving important chemical reactions and their subsequent applications such as the two-way photoswitching of dithienylethene,[6b] the reversible control over the reflection of liquid crystals[6c] and modulating the biocatalytic activity of bacteria[6d]. However, the use of high power 980 nm NIR light in such UCNP systems, although advantageous, has been shown to cause severe local heating, which has detrimental effects on both solid-state devices and biological systems.[7] Moreover, the spectrally mixed UV/Vis emission of these UCNPs compromises the photoswitching system’s ability to reliably encode and transmit information in a spatiotemporally controlled manner. Thus, to overcome these limitations, there is a clear need to develop multicolor UCNPs capable of selectively generating spectrally resolved emissions in the UV and visible regions using external stimuli with negligible heating effects.
We herein describe the design and synthesis of a novel core-shell structured β-NaYF4:Nd3+/Yb3+/Tm3+@NaYF4:Nd3+@NaYF4@NaYF4:Yb3+/Er3+ UCNP (Tm@Er) possessing dual NIR excitations (808 and 980 nm) and the corresponding orthogonal emissions in the UV (347–475 nm)/visible (545 nm) range by using low power density excitation for minimal heating effects (Scheme 1). The unique photo-physical properties of these UCNPs represent a critical advance in many applications involving the construction of optical storage devices for information storage and transmission, developing advanced drug delivery systems which are capable of the sequential delivery of therapeutics, and multicolor bio-imaging and sensing. As a proof-concept experiment, we demonstrate the highly efficient two way photoswitching of spiropyran regulated by a single type of UCNP with dual NIR excitations.
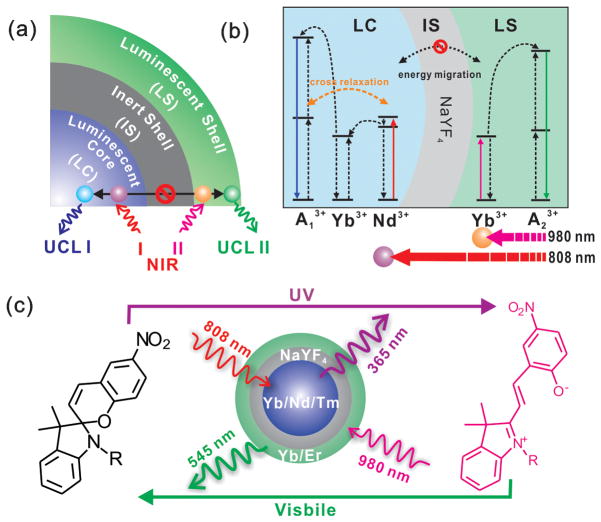
SCHEME 1.
(a) General structural design of the core-shell structured UCNPs. (b) Schematic illustration of the composition of the core-shell UCNPs and the simplified energy level diagram for the photon upconversion under NIR excitations. Yb3+ was selectively co-doped with and without Nd3+ into the LC and LS as a sensitizer, to endow the LC and LS with an excitation wavelength of 808 nm and 980 nm, respectively. Doping of Nd3+ also leads to enhanced cross-relaxation between Nd3+ and local activators, which quenches the UCL of the LC and thus endows the LC with a selective excitation of 808 nm with high power density. This structural design leads to orthogonal emissions in the luminescent core-shell under dual NIR excitations. (c) Direct and indirect two-way photoswitching of spiropyran by using UV/visible light and using Tm@Er UCNPs with dual NIR excitations.
The general structure of the UCNP contains a core and multiple shells, including a luminescent core (LC), an internal photon inert shell (IS) and an outer luminescent shell (LS) (Scheme 1a). In our developed UCNPs, the LC and LS contain different sensitizers and activators to generate orthogonal excitation and emission properties and the IS prevents energy migration between the two luminescent layers. Following this general design, NaYF4 was chosen as the host matrix because of its low lattice phonon energies and high upconversion efficiency.[1d] We then doped the LS with Yb3+ as a sensitizer, while co-doping the LC with both Nd3+ and Yb3+ (Scheme 1b). This specific arrangement of sensitizers affords an excitation of 980 nm to the UCNP LS, while the LC is responsive to both 980 nm and 808 nm excitations due to the separate main absorption peaks of Yb3+ and Nd3+ which locate at 980 and 808 nm, respectively.[8] It should be noted that Nd3+ cannot transfer its excitation energy to activators directly. As such, it requires the use of Yb3+ as a co-sensitizer, acting as an energy bridge.[8f–h] However, the presence of Nd3+ in the LC not only imbues the LC with 808 nm excitation, but also significantly decreases the upconversion luminescence (UCL) of the LC due to the enhanced cross-relaxation between Nd3+ and locally positioned activators (quenching effect of Nd3+,[8h] for more details see supporting information, Figure S1). As a result, the Nd3+ doped LC becomes selectively excited underhigh power 808 nm excitation, with no emission observed from low power 980 nm excitation. Additionally, a layer of NaYF4, without any dopant, was grown in between the LC and LS, as it can serve as a photon inert matrix which prevents energy migration between the LC and LS, which preserves their individual and spectrally pure excitation-emission properties.[9] As such, UCNPs with individual and orthogonal core-shell excitation-emission properties will be achieved, where the LS and LC will have individual emissions corresponding to individual excitations at 980 nm and 808 nm, respectively. Finally, Tm3+ and Er3+ were selected as activators of UCL due to their spectroscopic characteristics in the UV and visible region, respectively. In this work, two types of UCNPs were synthesized, henceforth abbreviated as Tm@Er UCNPs and Er@Tm UCNPs. Tm@Er UCNPs have Tm3+ doped in the LC and Er3+ doped in the LS, displaying UV-blue emission from the LC under 808 nm excitation and green emission from the LS under 980nm excitation. Er@Tm UCNPs have a reversed core-shell composition with regards to activator doping and therefore, reversed emissions. This difference in their emissive properties demonstrates the ability to orthogonally and selectively tune individual emissions based on the choice of activators of UCL (more details see main text discussion).
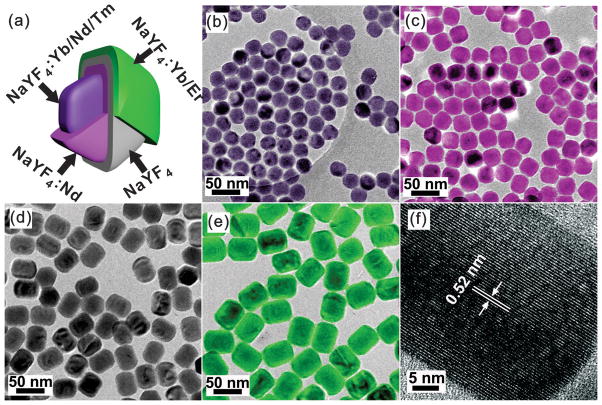
We first synthesized the Tm@Er core-shell UCNPs (Figure 1a). The UV-blue LC with 808 nm excitation was synthesized according to reported methods.[8g, h] It is comprised of a core (Figure 1b) doped with Yb3+, Nd3+, and Tm3+, as well as a sensitized shell (Figure 1c) doped with Nd3+ in high concentration. This core-shell structure minimizes the cross-relaxation between Nd3+ and the local activators (Tm3+) in the core due to the low Nd3+ dopant concentration (1 mol%), but maximizes the absorption of 808 nm excitation in the shell due to the high Nd3+ concentration (20%), endowing the nanoparticles with 808 nm excitation at low power density which minimizes local heating effects. As explained previously, to block the energy migration between the LC and LS, a layer of photon-inert NaYF4 was further deposited via epitaxial growth (Figure 1d). Finally, a green colored LS, composed of NaYF4:Yb/Er was deposited, led to a nanoparticle with a size of 41 nm × 52 nm (width × length, Figure. 1e). The shape evolution phenomena during the shell growth of the nanoparticles were ascribed to the kinetically favored anisotropic shell growth during the coating process, which was also observed by other groups.[8i, 10] The High-resolution TEM (HRTEM) image shown in Figure 1f reveals the (100) crystallographic planes of the Tm@Er UCNPs and demonstrates that the as-synthesized multishell UCNPs were single hexagonal phase crystals. Additionally, the energy-dispersive X-ray spectroscopy (EDX) spectrum indicates the presence of the elements Na, F, Y, Yb, Nd, Er, and Tm, which confirms the composition of the nanoparticles (see Supporting Information, Figure S2).
FIGURE 1.
(a) Composition of each layer of Tm@Er UCNPs. The false color of each layer represents the corresponding nanoparticles shown in b–e. (b–e) Low resolution TEM images of Tm@Er UCNPs constructed by epitaxial layer by layer growth. (f) High resolution TEM characterization of a single Tm@Er UCNP. The lattice extending, without interruption, across the particle is indicative of its single crystallinity.
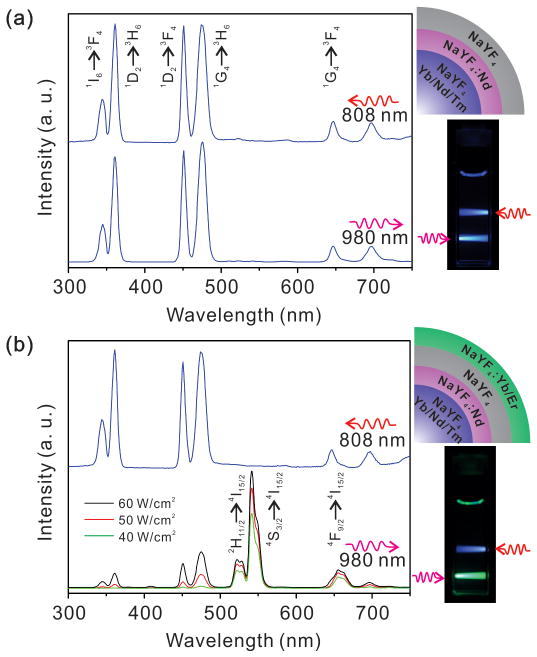
Next, we studied the upconversion profile of the as synthesized UCNPs under different wavelength laser excitations. As shown in Figure 2a, the LC of the Tm@Er UCNPs displays the characteristic emission peaks of Tm3+ in the UV-blue region when excited using 808 and 980 nm lasers, due to the presence of Nd3+ and Yb3+ in the luminescent core. Accordingly, a hexane solution of these core nanoparticles showed a deep blue colored band under 808 nm and 980 nm laser irradiation, which is easily seen by the naked eye (Figure 2a, right panel). For Tm@Er, only the characteristic emission peaks of Tm3+ can be seen under 808 nm irradiation (Figure 2b, up curve), demonstrating the selective excitation of LC by 808 nm excitation. In contrast, the UCNPs display strong characteristic emissions from Er3+ but relatively weak emissions from Tm3+ under 980 nm excitation with a power density of 60 W·cm−2 (Figure 2b, bottom curve), indicating that both the LC and LS are excited as both of them use Yb3+ as sensitizer. It should be noted that the relatively weaker emissions of Tm3+ in LC than that of Er3+ in LS may be due to i) the quenching effect of Nd3+ in LC,[8h] and ii) the non-linear excitation nature of UCNPs whereby the UV emissions from Tm3+ show a stronger dependence on excitation density than the visible emissions of Er3+ (a 4 photon vs 3 photon process).[6b] Consequently, decreasing the excitation power density eliminated the Tm3+ emissions more quickly than those of Er3+, and excitations of 40 W·cm−2 or lower led to the selective emissions of Er3+ in the LS only (Figure 2b, bottom curve). This result demonstrates that the emission of Tm@Er under 980 nm excitation can be easily modulated by regulating the excitation power/wavelength, and that the LS can be selectively excited by utilizing a low excitation power density due to the cooperative effect of the Nd3+ doping, the photon inert shell and the nonlinear excitation properties of UCNPs,[6b, 6c, 11] as represented (Scheme 1b). Accordingly, a hexane solution of these nanoparticles displays two different colored bands, blue and green, under 808 nm and 980 nm excitation, respectively, with excitation power densities of 40 W·cm−2 or lower, revealing the orthogonal excitation-emission property of the Tm@Er UNCPs. Control experiments demonstrate that UCNPs with the same core-shell structure, but without the critical internal photon inert shell, display strong energy migration and do not possess separate core shell excitation-emission properties (Figure S3).
FIGURE 2.
Upconversion luminescence profiles of the luminescent core with photon inert shell (a) and the Tm@Er UCNPs (b) under different wavelength excitations and varying power densities. The structures of the corresponding nanoparticles are illustrated in the right panel. The photographs show the UCNPs in hexane solution (1 wt%) under irradiation from two laser beams with wavelengths of 808 nm and 980 nm. The power density for 808 nm and 980 nm lasers is 60 W·cm−2.
To further demonstrate the rationale behind the structure of the proposed UCNPs, another type of UCNP, Er@Tm was synthesized based on the inverse composition of the activators Er3+ and Tm3+ (Figure S4). In contrast to Tm@Er UNCPs, the Er@Tm UNCPs show incomplete separation of the core-shell excitations and emissions. Accordingly, 980 nm laser excitation stimulated not only UV-blue emissions from Tm3+ in the LS, but also green emissions from Er3+ in the LC (Figure S4f), indicating a simultaneous excitation of the luminescent core and shell by 980 nm irradiation at high power density. The relatively weaker emission from Er3+ in the LC is mainly due to the quenching effect of Nd3+ on locally placed activators. Decreasing the excitation power density can minimize the emission from Er3+, but it will also result in decrease in UV emission from Tm3+ (Figure S5). These results strongly suggest the rational design of the Tm@Er UCNPs, which have orthogonal core-shell excitation-emission properties at distinct wavelengths to yield spectrally pure UV-blue and green emissions. Furthermore, since 808 nm and low-power density 980 nm NIR light both minimize any local heating from the lasers (Figure S6), these UCNPs have unique advantages for applications in materials and biological studies, such as for driving important photoreactions with high spatiotemporal control and reversibly modulating the structure and properties of molecules such as photoswitches, especially for advancing multicolor UCNP based photoswitching systems which require distinct excitation-emission peaks, high photostability, and minimal photo-inducible damage.
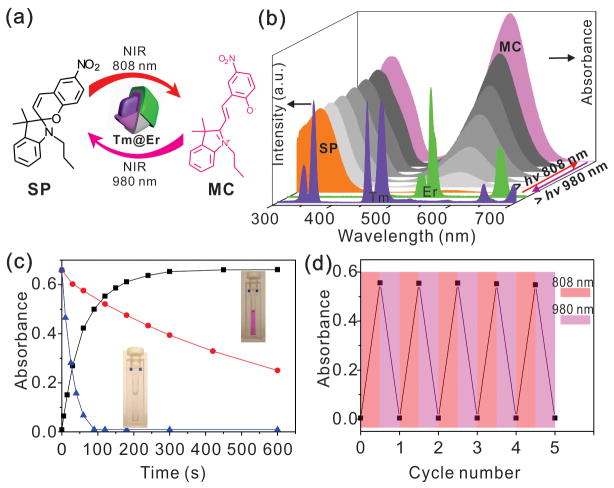
UCNP based photoswitching systems, in which UCNPs serve as an antenna and deliver high energy UV-Vis light through the upconversion of low energy NIR light, are capable of the spatiotemporal and reversible modulation of the structure and properties of molecules.[6b, 6c, 6g, 11] Such systems can overcome the limitations of traditional UV-Vis light-based photoswitching, which include photobleaching, toxicity, and poor penetration through biological tissues. Spiropyran is one of the most well studied photoswitchable molecules known to date and is widely used in the development of photoswitches, molecular machines and sensors.[12] Biomolecules such as nucleic acids, enzymes, cellular receptors, and ion channels have been functionalized with SP to remotely and orthogonally regulate cellular behavior including gene transcription, enzyme activity, and flux through ion channels with light.[13] As illustrated in Scheme 1c, the photoisomerizations between spiropyran (SP) and merocyanine (MC) can be regulated by UV and visible light.[12a] Importantly, the maximum absorption peaks of SP and MC that locate at 342 nm and 560 nm, overlap well with the UV emission from the Tm@Er UCNPs under 808 nm excitation and the green emission of our Tm@Er UCNPs under 980 nm excitation, respectively (Figure 3b). As such, we sought to utilize this well-characterized photoswitch in our system to demonstrate the NIR light-based two-way photoswitching ability of the synthesized Tm@Er UCNPs (Figure 3a).
FIGURE 3.
(a) Schematic illustration of two-way photoswitching of SP by using Tm@Er UCNPs with dual NIR excitations. (b) Tm3+ and Er3+ emissions from Tm@Er nanoparticles under 808 nm and 980 nm excitations, respectively, which overlap well with the absorption peaks of SP and MC. The evolution of the UV/Vis absorption spectrum of the photoisomerization of SP and MC under 808 nm and 980 nm light irradiation in the presence of Tm@Er UCNPs. (c) Kinetic monitoring of the photoswitching reaction of SP and MC under 808 nm and 980 nm light irradiation with Tm@Er UCNPs by using the characteristic absorbance of MC at 560 nm. The red dotted line shows the kinetics of the reaction of MC to SP in dark conditions. (d) Dual NIR light-driven photoswitching of SP over many cycles in THF/methanol (9/1, v/v) solution with 5 wt% of Tm@Er UCNPs and 10 μM of SP by monitoring the absorbance of MC at 560 nm.
The photoswitching of SP by Tm@Er UCNPs was carried out using a simple mixture of SP and Tm@Er (more details see Figure S7). As shown in Figure 3b, irradiation of the colorless solution of SP and UCNPs with an 808 nm laser leads to the photo-isomerization of SP to MC, resulting in a bright pink solution which is characterized by a red shift in the UV peak as well as a dramatic enhancement in the absorption centered at 560 nm. These results indicate that the ring-opening reaction of the SP to MC was effectively driven by 808 nm excitation. Based on the kinetics monitoring of the photoswitching reaction using the characteristic absorption of MC at 560 nm (Figure 3c), 92 % of the photostationary state can be obtained within 120 seconds with 808 nm excitation with a power density of 40 W·cm−2. Thereafter, the NIR light driven photoisomerization of MC to SP was performed by irradiating the solution with a 980 nm laser. The solution quickly goes back to colorless and the absorption spectrum shows a blue shift in the UV absorption band and a decrease in intensity at 560 nm (Figure 3b). The kinetics of this photo reaction under 980 nm irradiation with Tm@Er UCNPs is much faster than those of the dark condition (Figure 3c). After 980 nm irradiation for 90 seconds with a power density of 15 W·cm−2 the absorption peak of MC no longer exists, indicating a complete photoisomerization reaction of SP to MC. These results demonstrate the successful two-way photoswitching of spiropyran, which is effectively driven using Tm@Er UNCPs with dual NIR excitations at low power density.
We further tested the integrity of SP photoswitching by using Tm@Er UCNPs with low power density 808 nm and 980 nm laser excitations, shown in Figure 3d. A schematic for the “remote control” two-way photoswitching of SP and the Tm@Er UNCPs by using two NIR lasers is shown in Figure 3a. It is worth noting that there is more than 90% retention of SP after 5 cycles of photoswitching, indicating the robustness and reversibility of our UNCP based photoswitching system which is mainly due to the attenuation of detrimental heating effects and photobleaching by using NIR light with low excitation power density. Finally, we demonstrated the construction of mesoporous silica coated Tm@Er (UCNP@MSN), which allow the UCNPs to be easily dispersed in aqueous solutions and undergo facile surface chemistry for further functionalization and application (Figure S8).
In conclusion, we have successfully demonstrated the rational design and preparation of a new type of UCNP, which is capable of producing orthogonal and spectrally pure emissions from core-shell NIR excitations at low power density. In particular, the incorporation of two separate excitation triggers allows for a higher degree of control than achievable using previous systems. In contrast to previously reported photoswitch systems, our system excludes the necessity of high energy UV/Vis, as well as high power density NIR light, which attenuates any system damage from multi-photon absorption and local heating. Taken together, these results demonstrate that both our novel designed UCNP and UCNP-SP based NIR-driven two-way photoswitching platform has great implications for applications in materials science and biology studies.
Supplementary Material
Acknowledgments
We would like to acknowledge the kind support from Prof. Riman for fluorescent lifetime measurements. K.-B. Lee acknowledges financial support from the NIH Director’s Innovator Award [1DP20D006462-01], National Institute of Biomedical Imaging and Bioengineering of the NIH [1R21NS085569-01], the N.J. Commission on Spinal Cord grant [09-3085-SCR-E-0], and the Rutgers Faculty Research Grant Program.
References
- 1.a) Auzel F. Chem Rev. 2004;104:139. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]; b) Wu SW, Han G, Milliron DJ, Aloni S, Altoe V, Talapin DV, Cohen BE, Schuck PJ. P Natl Acad Sci USA. 2009;106:10917. doi: 10.1073/pnas.0904792106. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang F, Han Y, Lim CS, Lu Y, Wang J, Xu J, Chen H, Zhang C, Hong M, Liu X. Nature. 2010;463:1061. doi: 10.1038/nature08777. [DOI] [PubMed] [Google Scholar]; d) Wang F, Deng R, Wang J, Wang QX, Han Y, Zhu H, Chen X, Liu X. Nat Mater. 2011;10:968. doi: 10.1038/nmat3149. [DOI] [PubMed] [Google Scholar]; e) Wang F, Liu X. Chem Soc Rev. 2009;38:976. doi: 10.1039/b809132n. [DOI] [PubMed] [Google Scholar]; f) Wang J, Deng R, MacDonald MA, Chen B, Yuan J, Wang F, Chi D, Hor TSA, Zhang P, Liu G, Han Y, Liu X. Nat Mater. 2014;13:157. doi: 10.1038/nmat3804. [DOI] [PubMed] [Google Scholar]; g) Haase M, Schafer H. Angew Chem Int Ed. 2011;50:5808. doi: 10.1002/anie.201005159. [DOI] [PubMed] [Google Scholar]
- 2.a) Li LL, Wu P, Hwang K, Lu Y. J Am Chem Soc. 2013;135:2411. doi: 10.1021/ja310432u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang Y, Zheng F, Yang T, Zhou W, Liu Y, Man N, Zhang L, Jin N, Dou Q, Zhang Y, Li Z, Wen LP. Nat Mater. 2012;11:817. doi: 10.1038/nmat3363. [DOI] [PubMed] [Google Scholar]; c) Deng R, Xie X, Vendrell M, Chang Y, Liu X. J Am Chem Soc. 2011;133:20168. doi: 10.1021/ja2100774. [DOI] [PubMed] [Google Scholar]; d) Gorris H, Ali R, Saleh SM, Wolfbeis OS. Adv Mater. 2011;23:1652. doi: 10.1002/adma.201004697. [DOI] [PubMed] [Google Scholar]; e) Nam SH, Bae YM, Park YI, Kim JH, Kim HM, Choi JS, Lee KT, Hyeon T, Suh YD. Angew Chem Int Ed. 2011;50:6093. doi: 10.1002/anie.201007979. [DOI] [PubMed] [Google Scholar]; f) Huang X, Han S, Huang W, Liu X. Chem Soc Rev. 2013;42:173. doi: 10.1039/c2cs35288e. [DOI] [PubMed] [Google Scholar]; g) Gorris HH, Wolfbeis OS. Angew Chem Int Ed. 2013;52:3584. doi: 10.1002/anie.201208196. [DOI] [PubMed] [Google Scholar]; h) Sun L, Wang Y, Yan C. Acc Chem Res. 2014;47:1001. doi: 10.1021/ar400218t. [DOI] [PubMed] [Google Scholar]; i) Yang Y, Shao Q, Deng R, Wang C, Teng X, Cheng K, Cheng Z, Huang L, Liu Z, Liu X, Xing B. Angew Chem Int Ed. 2012;51:3125. doi: 10.1002/anie.201107919. [DOI] [PubMed] [Google Scholar]; j) Gu Z, Yan L, Tian G, Li S, Chai Z, Zhao Y. Adv Mater. 2013;25:3758. doi: 10.1002/adma.201301197. [DOI] [PubMed] [Google Scholar]; k) Liu Y, Zhou S, Tu D, Chen Z, Huang M, Zhu H, Ma E, Chen X. J Am Chem Soc. 2012;134:15083. doi: 10.1021/ja306066a. [DOI] [PubMed] [Google Scholar]; l) Wang M, Mi CC, Wang WX, Liu CH, Wu YF, Xu ZR, Mao CB, Xu SK. ACS Nano. 2009;3:1580. doi: 10.1021/nn900491j. [DOI] [PubMed] [Google Scholar]; m) Liu Q, Sun Y, Yang T, Feng W, Li C, Li F. J Am Chem Soc. 2011;133:17122. doi: 10.1021/ja207078s. [DOI] [PubMed] [Google Scholar]; n) Yang J, Shen D, Li X, Li W, Fang Y, Wei Y, Yao C, Tu B, Zhang F, Zhao D. Chem –Eur J. 2012;18:13642. doi: 10.1002/chem.201202336. [DOI] [PubMed] [Google Scholar]
- 3.a) Li W, Wang J, Ren J, Qu X. J Am Chem Soc. 2014;136:2248. doi: 10.1021/ja412364m. [DOI] [PubMed] [Google Scholar]; b) Yan B, Boyer JC, Branda NR, Zhao Y. J Am Chem Soc. 2011;133:19714. doi: 10.1021/ja209793b. [DOI] [PubMed] [Google Scholar]; c) Shen J, Chen G, Ohulchanskyy TY, Kesseli SJ, Buchholz S, Li Z, Prasad PN, Han G. Small. 2013;9:3213. doi: 10.1002/smll.201300234. [DOI] [PubMed] [Google Scholar]; d) Carling CJ, Nourmohammadian F, Boyer JC, Branda NR. Angew Chem Int Ed. 2010;49:3782. doi: 10.1002/anie.201000611. [DOI] [PubMed] [Google Scholar]
- 4.a) Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y. Nat Med. 2012;18:1580. doi: 10.1038/nm.2933. [DOI] [PubMed] [Google Scholar]; b) Cui S, Yin D, Chen Y, Di Y, Chen H, Ma Y, Achilefu S, Gu Y. ACS Nano. 2013;7:676. doi: 10.1021/nn304872n. [DOI] [PubMed] [Google Scholar]; c) Qiao XF, Zhou JC, Xiao JW, Wang YF, Sun LD, Yan CH. Nanoscale. 2012;4:4611. doi: 10.1039/c2nr30938f. [DOI] [PubMed] [Google Scholar]; d) Wang C, Cheng L, Liu YM, Wang XJ, Ma XX, Deng ZY, Li YG, Liu Z. Adv Funct Mater. 2013;23:3077. [Google Scholar]
- 5.a) Chen G, Ohulchanskyy TY, Kumar R, Agren H, Prasad PN. ACS Nano. 2010;4:3163. doi: 10.1021/nn100457j. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dong NN, Pedroni M, Piccinelli F, Conti G, Sbarbati A, Ramirez-Hernandez JE, Maestro LM, Iglesias-de la Cruz MC, Sanz-Rodriguez F, Juarranz A, Chen F, Vetrone F, Capobianco JA, Sole JG, Bettinelli M, Jaque D, Speghini A. ACS Nano. 2011;5:8665. doi: 10.1021/nn202490m. [DOI] [PubMed] [Google Scholar]
- 6.a) Boyer JC, Carling CJ, Chua SY, Wilson D, Johnsen B, Baillie D, Branda NR. Chem –Eur J. 2012;18:3122. doi: 10.1002/chem.201103767. [DOI] [PubMed] [Google Scholar]; b) Boyer JC, Carling CJ, Gates BD, Branda NR. J Am Chem Soc. 2010;132:15766. doi: 10.1021/ja107184z. [DOI] [PubMed] [Google Scholar]; c) Wang L, Dong H, Li Y, Xue C, Sun LD, Yan CH, Li Q. J Am Chem Soc. 2014;136:4480. doi: 10.1021/ja500933h. [DOI] [PubMed] [Google Scholar]; d) Chen Z, Zhou L, Bing W, Zhang Z, Li Z, Ren J, Qu X. J Am Chem Soc. 2014;136:7498. doi: 10.1021/ja503123m. [DOI] [PubMed] [Google Scholar]; e) Zhang F, Shi Q, Zhang Y, Shi Y, Ding K, Zhao D, Stucky GD. Adv Mater. 2011;23:3775. doi: 10.1002/adma.201101868. [DOI] [PubMed] [Google Scholar]; f) Zhang C, Xu CH, Sun LD, Yan CH. Chem -Asian J. 2012;7:2225. doi: 10.1002/asia.201200446. [DOI] [PubMed] [Google Scholar]; g) Zhang BF, Frigoli M, Angiuli F, Vetrone F, Capobianco JA. Chem Commun. 2012;48:7244. doi: 10.1039/c2cc33052k. [DOI] [PubMed] [Google Scholar]
- 7.a) Zhan Q, Qian J, Liang H, Somesfalean G, Wang D, He S, Zhang Z, Andersson-Engels S. ACS Nano. 2011;5:3744. doi: 10.1021/nn200110j. [DOI] [PubMed] [Google Scholar]; b) Jiang Z, Xu M, Li F, Yu Y. J Am Chem Soc. 2013;135:16446. doi: 10.1021/ja406020r. [DOI] [PubMed] [Google Scholar]
- 8.a) Qiu J, Kawamoto Y. J Appl Phys. 2002;91:954. [Google Scholar]; b) Courrol LC, Ranieri IM, Tarelho LVG, Baldochi SL, Gomes L, Vieira ND. J Appl Phys. 2005;98:113504. [Google Scholar]; c) Huang YL, Jang KH, Seo HJ, Jang KW. J Appl Phys. 2006;100:83513. [Google Scholar]; d) Koepke C, Wisniewski K, Sikorski L, Piatkowski D, Kowalska K, Naftaly M. Opt Mater. 2006;28:129. [Google Scholar]; e) Lupei A, Lupei V, Gheorghe C, Ikesue A, Osiac E. Opt Mater. 2009;31:744. [Google Scholar]; f) Wang YF, Liu GY, Sun LD, Xiao JW, Zhou JC, Yan CH. ACS Nano. 2013;7:7200. doi: 10.1021/nn402601d. [DOI] [PubMed] [Google Scholar]; g) Xie X, Gao N, Deng R, Sun Q, Xu Q, Liu X. J Am Chem Soc. 2013;135:12608. doi: 10.1021/ja4075002. [DOI] [PubMed] [Google Scholar]; h) Shen J, Chen GY, Vu AM, Fan W, Bilsel OS, Chang CC, Han G. Adv Opt Mater. 2013;1:644. [Google Scholar]; i) Wen H, Zhu H, Chen X, Hung TF, Wang B, Zhu G, Yu SF, Wang F. Angew Chem Int Ed. 2013;52:13419. doi: 10.1002/anie.201306811. [DOI] [PubMed] [Google Scholar]
- 9.Su Q, Han S, Xie X, Zhu H, Chen H, Chen C, Liu R, Chen X, Wang F, Liu X. J Am Chem Soc. 2012;134:20849. doi: 10.1021/ja3111048. [DOI] [PubMed] [Google Scholar]
- 10.Abel KA, Boyer JC, Andrei CM, van Veggel FCJM. J Phys Chem Lett. 2011;2:185. [Google Scholar]; b) Zhang C, Lee JY. ACS Nano. 2013;7:4393. doi: 10.1021/nn4009214. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Bu W, Pan L, Shi J. Angew Chem Int Ed. 2013;52:4375. doi: 10.1002/anie.201300183. [DOI] [PubMed] [Google Scholar]
- 12.a) Klajn R. Chem Soc Rev. 2014;43:148. doi: 10.1039/c3cs60181a. [DOI] [PubMed] [Google Scholar]; b) Minkin VI. Chem Rev. 2004;104:2751. doi: 10.1021/cr020088u. [DOI] [PubMed] [Google Scholar]
- 13.a) Sakata T, Yan YL, Marriott G. P Natl Acad Sci USA. 2005;102:4759. doi: 10.1073/pnas.0405265102. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ito Y, Sugimura N, Kwon OH, Imanishi Y. Nat Biotechnol. 1999;17:73. doi: 10.1038/5250. [DOI] [PubMed] [Google Scholar]; c) Beyer C, Wagenknecht HA. Synlett. 2010:1371. [Google Scholar]; d) Kocer A, Walko M, Meijberg W, Feringa BL. Science. 2005;309:755. doi: 10.1126/science.1114760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.