Abstract
The evaluation of candidate optical contrast agents for brain tumor delineation in ex vivo models may not accurately predict their activity in vivo. This study describes an in vivo model system designed to assess optical contrast agents for brain tumor delineation. The brain tumor window (BTW) model was created by performing biparietal craniectomies on 8-week-old Sprague-Dawley rats, injecting 9L glioma cells into the cortex and bonding a cover slip to the cranial defect with cyanoacrylate glue. Tumor growth was followed serially and occurred in an exponential fashion. Once tumors on the cortical surface achieved a 1 mm radius, intravenous contrast agents were injected while the appearance of the cortical surface was recorded. Computerized image analysis was used to quantitatively evaluate visible differences between tumor and normal brain. Tumor margins became readily apparent following contrast administration in the BTW model. Based on red component intensity, tumor delineation improved fourfold at 50 min post-contrast administration in the BTW model (P<0.002). In summary, window placement overlying an implanted glioma is technically possible and well tolerated in the rat. The BTW model is a valid system for assessing the in vivo activity of optical contrast agents.
Keywords: Brain tumor, Cranial window, Intraoperative imaging
Increasingly robust evidence suggests that the extent of surgical resection correlates with patient outcome for all types of brain tumors [1]. Stereotactic navigation, intraoperative ultrasound and intraoperative MRI have been developed been developed to improve the extent of resection. However, these technologies are limited by generating data that physically separated from the operative field, requiring the surgeon to correlate an image with the reality of the appearance of the operative field. To bridge the gap between diagnostic images and the operating field, investigators have long proposed the use of dyes to optically delineate tumor margins [2–9].
Experimental evaluation of tumor-delineating dyes has been carried out exclusively in ex vivo models. However, due to the visual differences between perfused and non-perfused tissue, we suggest that the properties of candidate optical contrast agents could be best characterized using in vivo, rather than ex vivo models. Therefore, we aimed to create an animal model to allow dynamic, in vivo visualization of the tumor brain interface. We describe a combination of the conventional 9L implanted glioma model with the chronic closed cranial window model to create the brain tumor window (BTW) model, a new system for evaluating the visual appearance of experimental brain tumors in vivo.
Materials and Methods
The 9L gliosarcoma cell line was cultured under standard cell culture conditions in RPMI media with 10% fetal bovine serum (InVitrogen, Carlsbad CA).
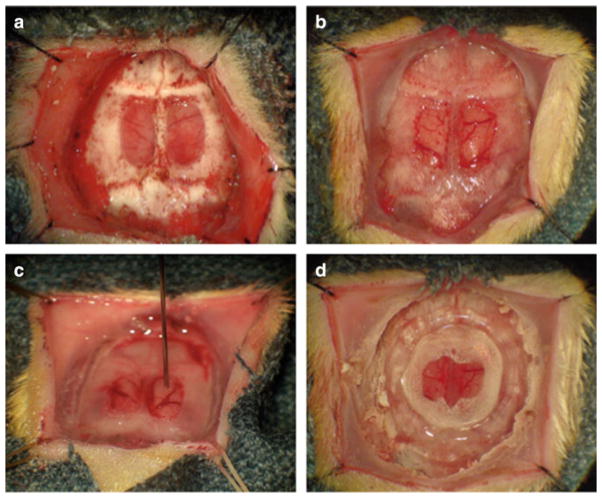
Approval from the University Committee on Use and Care of Animals at the University of Michigan was obtained prior to all experiments. Sprague-Dawley rats weighing 250–350 g were premedicated with buprenorphine 0.1 mg/kg and anesthetized with inhaled isoflurane delivered through a 16 gauge endotracheal tube. Vancomycin (25 mg/kg) was administered intraperitoneally prior to incision. After fixation in a stereotactic head holder, a midline incision was carried out over the skull from the frontal region to the occipito-cervical junction. Sutures were placed 2 mm from the apex of the superior and inferior apices of the incision and placed on tension to retract the scalp (Fig. 1a). The periosteum was detached from the skull through blunt dissection and removed using bipolar electrocautery. The periosteum, in continuity with fascial layers overlying the muscles of mastication, was removed to expose a sagitally-oriented ridge of bone. The bregma and lambda were identified as landmarks for placement of a craniectomy (Fig. 1a). Bleeding during exposure was controlled with bone wax, bipolar electrocautery and retraction. A high-speed drill was used to perform a nearly full thickness craniectomy of the medial aspect of the parietal bones bilaterally leaving an egg-shell thickness of bone overlying the dura (Fig. 1a). Minimal drilling was carried out of the bone overlying the superior sagittal sinus. The residual layer of bone was removed with a fine forceps and curved Penfield dissector. A linear rent in the dura in the coronal plane, towards the caudal end of the craniectomy was created using a bent 30-gauge needle. The caudal-most dura was then incised in a radial fashion along the base of the cranial defect and reflected cranially so that it could be removed in a single flap. Care was taken to avoid disrupting the large cortical veins entering the superior sagittal sinus in the midline which are continuous with the dura that must be removed medially. A dissecting forcep with curved tines was helpful in dissecting the dura without causing damage to the underlying structures. Once the dura was removed (Fig. 1b), the brain was irrigated with sterile normal saline. 105 9L cells were injected at a depth of 1mm into the right or left frontal portion of the exposed brain in an area lacking large blood vessels (Fig. 1c). A thin round glass microscope cover slip (10 mm diameter) was placed over the cranial defect (Fig. 1d). The edges of the cover slip were covered with two layers of cyanoacrylate glue (Fig. 1d) and a custom cut plastic ring was sown to the scalp with 3–0 nylon suture to enable continuous in vivo visualization of the implanted window (data not shown).
Fig. 1.
Essential stages in the creation of a brain tumor window model: After reflecting the scalp edges laterally, a nearly full thickness craniectomy is performed, sparing the bone over the superior sagittal sinus (a). Once the remaining bone overlying the dura has been removed, a small durotomy at the base of the craniectomy is created to create a larger linear dural rent which is used to peel the dura forward, ideally in a single flap. Once the dural flap has been removed, the shiny arachnoidal surface of the brain is appreciated (b). Under microscopic guidance, a 10 μL, 26 gauge Hamilton syringe mounted to a steretactic injector is lowered through the meninges, 1 mm deep into an avasacular region of cortex (c). After injection, the surface of the brain is irrigated to remove residual tumor cells. A 10 mm glass cover slip is carefully placed on the surface of the brain and a confluent circumferential layer of cyanoacrylate glue, overlapping the edge of the coverslip and the craniectomy margin is applied (d). Glue is usually dry within 45 min at which time a plastic ring stenting the scalp open for continuous observation is applied
Animals were weighed and monitored daily. The surface of the cortex was inspected for signs of tumor growth in the region of the injection. Signs of tumor growth observed include hypervascularity, pinkish color change in the region of tumor injection and elevated cell mass at the site of injection. The size of the cortical region showing signs of tumor growth was measured serially to generate a tumor growth curve. Animals were imaged by MRI using previously published protocols to confirm the presence of tumors [10].
When the radius of tumors reached 1 mm, and the tumors were clearly visible adjacent to normal brain tissue, they were used for evaluation of optical contrast agents. A cut-down was performed to establish femoral venous access. PE50 surgical tubing was placed (0.25″ inner diameter, Dow Corning, Midland, Michigan) into the right femoral vein. Animals were then placed into a stereotactic head holder and continuous video recording with a high-definition camcorder under visible was then initiated. Coomassie blue (CB), an optical contrast agent was administered intravenously over 5 min using a Medfusion 3500 syringe pump (Medex, Dublin, OH) infusion syringe pump.
Video collected during the experiment was examined qualitatively to evaluate the difference in appearance between normal cortex and tumor tissue. Still images were generated from the video and analyzed colorimetrically with Image J as previously described to quantify the degree of color change in the tumor [11]. The difference in red hue was found to be the best method to reflect the visual difference between tumor and normal brain.
Brain tissue was examined ex vivo following each experiment on both a macroscopic and microscopic level. At the conclusion of each experiment, the brain was removed and photographed digitally on a standard white background. Gross coronal sections were photographed digitally then immersed in 4% paraformaldehyde for 15 min followed by 40% sucrose solution for 24 h. The sections were frozen in Tissue-Tek O.C.T. Compound (Sakura Finetek, The Netherlands). Routine hematoxylin and eosin staining was performed on 15 mm coronal sections created with a cryotome.
Results
Technique Development
The BTW model was refined using 50 animals. 49/50 animals demonstrated a 2–3 day period of approximately 7% body weight loss, but did not show any behavioral abnormalities. Among the complications encountered in the initial model development were, failure to thrive (resulting in death on the 2nd post-operative day), infection underlying the window (8/50), no tumor growth (6/50), diffuse tumor growth (3/50) and extensive hemorrhage under the window (3/50). One animal failed to thrive, possibly due to stroke. All other animals returned to their preoperative weight, baseline neurological status and to the expected rate of growth for rats of their age by the 5th post-operative day.
Tumor growth was evaluated serially whenever possible. The progression of tumor growth observed is demonstrated in Fig. 2. Assuming a spherical growth pattern, tumor growth followed an exponential curve (data not shown). Roughly spherical tumor geometry was confirmed by MRI (data not shown). On average, tumor became visible after four days and achieved a radius of 1 mm by 12.8 days. Tumor tissue appeared slightly redder than the normal cortical surface and has vascular markings that are distinct from normal cortex.
Fig. 2.
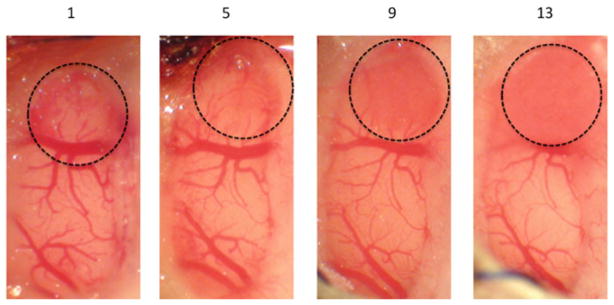
Progression of tumor growth in the brain tumor window model. Tumor expands radially and can be tracked following implantation. A representative brain tumor window model is shown 1, 5, 9, and 13 days post-implantation
BTW Model Characterization
After the BTW model could be reliably and consistently created, 6 BTW animals possessing optimal imaging characteristics were used to evaluate the ability of contrast agents to delineate neoplastic tissue in the BTW model. Prior to contrast administration, it was difficult to visibly delineate the margins or implanted tumor in comparison to adjacent normal cortex (Fig. 3). Following contrast administration, there was a marked improvement in our ability to distinguish tumor from normal brain (Fig. 3). The calculated difference in red hue, reflecting the degree of color difference between tumor and normal brain, improved from 47.3 pre-contrast to 141.6 post-contrast (P<0.0002). Maximal delineation was achieved within 50 min of contrast administration and persisted throughout the entire 2-h experiment.
Fig. 3.
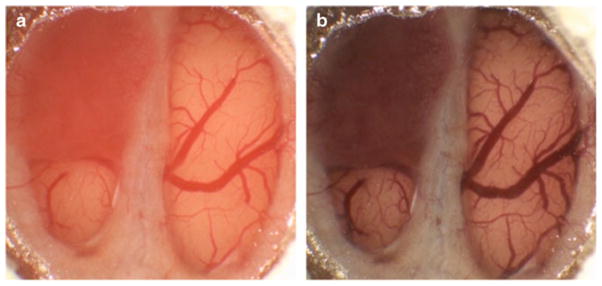
Images of a brain tumor window model 12-days post-implantation before (a) and 50 min after Coomassie blue administration (b). Note the relatively subtle difference in the color of the tumor prior to contrast administration and the clear difference between the appearance of tumor and normal brain post-contrast
Whole brains harvested from animals following experiments were evaluated histologically. Grossly, there was a spherical superficial tissue mass at the site of the injection. Microscopic examination of specimens confirmed evidence of a spherical implanted glioma adjacent to normal cortex. Glioma tissue appeared hypercellular in comparison to normal brain. The nuclear to cytoplasmic ratio in the neoplastic appearing tissue was much greater than that of cells identified in the normal cortical tissue. The nuclei of the implanted tumor appeared highly polymorphic. Mitotic figures were easily identified. Fronds of tumor cells could be observed infiltrating into normal cortex adjacent to tumor (data not shown).
Delineation of tumor was compared in the BTW and the conventional implanted 9L glioma model (data not shown). The conventional ex vivo model overestimated the degree of delineation by CB (P<0.03). There was a sharp change in red hue at the visually apparent and MRI-defined tumor margin in both the BTW and conventional models.
Discussion
Achieving gross total resection is a key component of current treatment of gliomas [1]. Visible contrast agents such as fluorescein, [7] indocyanine-green [2] and bromophenol blue [6] hold promise in assisting in the delineation of neoplastic tissue within the operative field [3]. Fluorescent contrast agents such as, 5-aminolevulinic acid [8] and 5-aminofluorescein labeled albumin [12] have been demonstrated to be useful in delineating glioma tissue intra-operatively with fluorescent microscopy. We have recently suggested that dye-loaded nanoparticles may hold promise as visible contrast agents for brain tumor delineation [13].
We felt that the best way to evaluate candidate contrast agents was through a model system that would recreate the common difficulty surgeons face in distinguishing glioma from normal brain. We combined a common model for studying gliomas (9L gliosarcoma) with one primarily used to study the cerebral vasculature (cranial window model) to create the brain tumor window model. The initial complications encountered in the development of the model (superficial cortical hemorrhage, infection, failure or tumor growth, excessive tumor growth and stroke) subsided with experience. The result of our efforts was a model that allows direct observation of a proliferating tumor in vivo with subtly visible margins. From a qualitative and quantitative perspective tumor margins in the BTW model are dramatically clearer following the administration of contrast agents.
Our data suggests that conventional ex vivo 9L glioma models may overestimate the degree of delineation afforded by candidate contrast agents. We feel this observation arises from the lack of perfusion in ex vivo specimens. Both models have tumor margins that are well delineated by contrast and correspond to MRI-defined tumor margins. Since human tumors margins are rarely as regular as those of implanted gliomas, the utility of the BTW model might be improved if it were performed with a more infiltrative type of tumor.
In addition, there are a number of potential applications of the brain tumor window model that may not relate to studying visible contrast agents. First, the BTW model might be used for evaluating fluorescent and near-IR agents for tumor delineation. Since the BTW allows direct observation of tumor tissue in situ, it is possible that it could be used to track the response of tumor to anti-cancer therapies. In summary, while we have demonstrated the utility of the BTW model for evaluating visible contrast agents, it is possible that the BTW model may assist be useful in a variety of in vivo glioma studies.
Footnotes
Conflict of interest statement
We declare that we have no conflict of interest.
Disclosures
This work was supported by grants from the National Institute of Biomedical Imaging and Bioengineering (1R01EB007977-01, to RK), the National Cancer Institute (1R21CA125297-01A1, to RK, and 1F32CA126295-01A1, to DAO), and the 2007 CNS Basic/Translational Resident Research Fellowship (to DAO). The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Daniel A. Orringer, Department of Neurosurgery, University of Michigan Health System, 1500 E. Medical Center Drive, Ann Arbor, MI 48109-5338, USA
Thomas Chen, University of Michigan, Ann Arbor, MI USA.
Dah-Luen Huang, Department of Neurosurgery, University of Michigan Health System, 1500 E. Medical Center Drive, Ann Arbor, MI 48109-5338, USA.
Martin Philbert, Department of Toxicology, University of Michigan, Ann Arbor, MI USA.
Raoul Kopelman, Department of Chemistry, University of Michigan, Ann Arbor, MI USA.
Oren Sagher, Email: osagher@umich.edu, Department of Neurosurgery, University of Michigan Health System, 1500 E. Medical Center Drive, Ann Arbor, MI 48109-5338, USA.
References
- 1.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–764. doi: 10.1227/01.neu.0000318159.21731.cf. discussion 264–266. [DOI] [PubMed] [Google Scholar]
- 2.Britz GW, Ghatan S, Spence AM, Berger MS. Intracarotid RMP-7 enhanced indocyanine green staining of tumors in a rat glioma model. J Neurooncol. 2002;56:227–232. doi: 10.1023/a:1015035213228. [DOI] [PubMed] [Google Scholar]
- 3.Hansen DA, Spence AM, Carski T, Berger MS. Indocyanine green (ICG) staining and demarcation of tumor margins in a rat glioma model. Surg Neurol. 1993;40:451–456. doi: 10.1016/0090-3019(93)90046-4. [DOI] [PubMed] [Google Scholar]
- 4.Moore GE. Fluorescein as an agent in the differentiation of normal and malignant tissues. Science. 1947;106:130–131. doi: 10.1126/science.106.2745.130-a. [DOI] [PubMed] [Google Scholar]
- 5.Moore GE, Peyton WT, et al. The clinical use of fluorescein in neurosurgery; the localization of brain tumors. J Neurosurg. 1948;5:392–398. doi: 10.3171/jns.1948.5.4.0392. [DOI] [PubMed] [Google Scholar]
- 6.Ozawa T, Britz GW, Kinder DH, Spence AM, VandenBerg S, Lamborn KR, Deen DF, Berger MS. Bromophenol blue staining of tumors in a rat glioma model. Neurosurgery. 2005;57:1041–1047. doi: 10.1227/01.neu.0000180036.42193.f6. discussion 1041–1047. [DOI] [PubMed] [Google Scholar]
- 7.Shinoda J, Yano H, Yoshimura S, Okumura A, Kaku Y, Iwama T, Sakai N. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J Neurosurg. 2003;99:597–603. doi: 10.3171/jns.2003.99.3.0597. [DOI] [PubMed] [Google Scholar]
- 8.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 9.Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, Kwok D, Munoz NM, Sze RW, Grady WM, Greenberg NM, Ellenbogen RG, Olson JM. Tumor paint: a chlorotoxin: Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 10.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YE, Woolliscroft MJ, Sugai JV, Johnson TD, Philbert MA, Kopelman R, Rehemtulla A, Ross BD. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin Cancer Res. 2006;12:6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 11.Orringer DA, Koo YE, Chen T, Kim G, Hah HJ, Xu H, Wang S, Keep R, Philbert MA, Kopelman R, Sagher O. In vitro characterization of a targeted, dye-loaded nanodevice for intraoperative tumor delineation. Neurosurgery. 2009;64:965–971. doi: 10.1227/01.NEU.0000344150.81021.AA. discussion 971–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremer P, Mahmoudreza F, Ding R, Pritsch M, Zoubaa S, Frei E. Intraoperative fluorescence staining of malignant brain tumors using 5-aminofluorescein-labeled albumin. Neurosurgery. 2009;64:53–60. doi: 10.1227/01.NEU.0000335787.17029.67. discussion 60–61. [DOI] [PubMed] [Google Scholar]
- 13.Orringer DA, Koo YE, Chen T, Kopelman R, Sagher O, Philbert MA. Small solutions for big problems: the application of nanoparticles to brain tumor diagnosis and therapy. Clin Pharmacol Ther. 2009;85:531–534. doi: 10.1038/clpt.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]