Abstract
Background
Tricuspid regurgitation (TR) and right ventricular (RV) dysfunction adversely affect outcomes in patients with heart failure or mitral valve disease, but their impact on outcomes in patients with aortic stenosis (AS) treated with transcatheter aortic valve replacement (TAVR) has not been well characterized.
Methods and Results
Among 542 patients with symptomatic AS treated in the PARTNER II trial (inoperable cohort) with a SAPIEN or SAPIEN XT valve via a transfemoral approach, baseline TR severity, right atrial (RA) and RV size, and RV function were evaluated by echocardiography according to established guidelines. One-year mortality was 16.9%, 17.2%, 32.6%, and 61.1% for patients with no/trace (n=167), mild (n=205), moderate (n=117), and severe (n=18) TR, respectively (p<0.001). Increasing severity of RV dysfunction as well as RA and RV enlargement were also associated with increased mortality (p<0.001). After multivariable adjustment, severe TR (HR 3.20, 95% CI 1.50–6.82, p=0.003) and moderate TR (HR 1.60, 95% CI 1.02–2.52, p=0.042) remained associated with increased mortality as did RA and RV enlargement, but not RV dysfunction. There was an interaction between TR and mitral regurgitation severity (p=0.04); the increased hazard of death associated with moderate/severe TR only occurred in those with no/trace/mild mitral regurgitation.
Conclusions
In inoperable patients treated with TAVR, moderate or severe TR and right heart enlargement are independently associated with increased 1-year mortality, however the association between moderate or severe TR and an increased hazard of death was only found in those with minimal MR at baseline. These findings may improve our assessment of anticipated benefit from TAVR and support the need for future studies on TR and the right heart, including whether concomitant treatment of TR in operable but high risk patients with AS is warranted.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01314313.
Keywords: aortic valve stenosis, echocardiography, heart failure, transcatheter aortic valve replacement, tricuspid regurgitation
Coexisting mitral regurgitation (MR) is commonly present in patients with aortic stenosis (AS) referred for transcatheter aortic valve replacement (TAVR).1–3 Although there is conflicting data, significant MR appears to be associated with worse clinical outcomes after TAVR and its presence may influence whether a concomitant mitral procedure should be performed to maximize clinical benefit.1–3 In contrast, few studies have evaluated the effect of significant tricuspid regurgitation (TR) on outcomes in this patient population.4 Related to this, right ventricular (RV) dysfunction adversely affects survival in patients with non-AS heart failure.5,6 However, the effect of right heart size and function has not been evaluated in patients undergoing TAVR. Accordingly, we evaluated the effect of significant TR and right heart enlargement and dysfunction on mortality in the inoperable cohort of the PARTNER (Placement of Aortic Transcatheter Valves) II trial.
Methods
Study population
The design, inclusion and exclusion criteria, and primary results of the inoperable cohort (Cohort B) of the PARTNER II randomized clinical trial have been reported.7 The trial was designed to compare the safety and effectiveness of the new lower-profile Edwards SAPIEN XT transcatheter heart valve to the SAPIEN valve (approved by the U.S. Food and Drug Administration). The complete inclusion and exclusion criteria are provided in the data supplement. These patients had severe AS with an aortic valve area (AVA) <0.8 cm2 (or indexed AVA <0.5 cm2/m2) and either resting or inducible mean gradient >40 mmHg or peak jet velocity >4 m/s. They were symptomatic from AS (NYHA functional class ≥2) and had prohibitive surgical risk as defined by a risk of death or serious irreversible morbidity exceeding 50% by 30 days after conventional aortic valve surgery as assessed by the heart team. Patients were eligible for the trial if they had suitable transfemoral access and, once enrolled, were randomized to therapy with the Edwards SAPIEN or SAPIEN XT transcatheter heart valve. For this analysis, we included only patients who received treatment with TAVR (the “as treated” population). Among these patients, 99.3% (538 out of 542) completed 1-year follow-up in the trial. Clinical characteristics were reported by the enrolling sites. Frailty was determined by an integrated evaluation of hand grip strength, 5-meter walking speed, serum albumin level, and an assessment of independence in performing activities of daily living.8,9 The study protocol was approved by the institutional review board at each enrolling site and all patients provided written informed consent.
Echocardiography and hemodynamics
An independent core laboratory analyzed all echocardiograms. The severity of AS was determined by measuring mean and peak gradients across the valve using the modified Bernoulli equation and by calculating AVA using the continuity equation. Measurements of left ventricular (LV) chamber dimensions, ejection fraction, LV mass, and left atrial volume were made as recommended by the American Society of Echocardiography (ASE).10,11 Stroke volume was calculated as the LV outflow tract area multiplied by the pulsed wave Doppler LV outflow tract velocity-time integral. The severity of aortic and mitral regurgitation were determined by an integrative method that includes quantitative and qualitative criteria.12 TR severity was determined by an integrative, semi-quantitative approach as recommended by the ASE, including assessment of tricuspid valve morphology, right atrial (RA) and RV size, inferior vena cava size, jet area, vena contracta width, proximal isovelocity surface area radius, jet density and contour, and hepatic vein flow.12 RA area was measured in the 4 chamber view.13 When images were adequate, RV size was determined by measuring the basal and mid RV diameter and base-to-apex length in the 4 chamber view and categorized as normal or mildly, moderately, or severely dilated according to ASE guidelines.10,13 The three measurements were utilized to determine the appropriate category, usually characterized by two out of the three measurements falling in a particular category. RV function was determined by measuring systolic and diastolic area and RV fractional area change when feasible (or by visual estimate) and categorized as normal or mild, moderate, or severe dysfunction according to ASE criteria.10,13 TR jet velocity and estimated right ventricular systolic pressure were measured as recommended.13 Per protocol, echocardiograms were obtained at baseline (within 45 days of TAVR), and post-TAVR at discharge (or 7 days), 30 days, and 1 year. The baseline (pre-procedure) echocardiogram was utilized in all of our survival analyses. A pulmonary artery catheter was routinely used during the TAVR cases to obtain invasive hemodynamic measurements before balloon aortic valvuloplasty and after transcatheter valve placement. The measurements reported are those obtained prior to balloon aortic valvuloplasty.
Statistical analysis
Continuous variables were summarized as mean±SD and evaluated using an overall F-test from an analysis of variance. Categorical variables were compared with the chi-square or Fisher exact test as appropriate. The primary endpoint for this analysis was all-cause death up to 1 year. Time-to-event curves for 1-year survival, based on all available follow-up data, were performed with the use of Kaplan-Meier estimates and were compared between groups with the use of the log-rank test. Cox proportional hazards models were used to calculate hazard ratios and to test for interactions between TR severity (moderate/severe versus none/trace/mild) and age, sex, renal function, LV ejection fraction, MR severity, RV size, RV function, RA size, and pulmonary artery pressure). Multivariable Cox proportional hazards models adjusted for baseline characteristics with a univariable association (p<0.10) with 1-year mortality including age, sex, body mass index, STS score, prior myocardial infarction, prior coronary artery bypass grafting surgery, frailty, permanent pacemaker, atrial arrhythmia, aortic valve mean gradient, LV ejection fraction, and mitral regurgitation. These multivariable analyses evaluated the adjusted relationship between TR severity, RA and RV size, and RV function (each individually) and mortality. They also evaluated the adjusted relationship between TR severity paired with RA size, RV size, or RV function and mortality. To evaluate change in TR severity over time in those with moderate or severe TR at baseline, we reported the number of patients with no/trace/mild, moderate, and severe TR at 30 days and 1 year among patients who survived 1 year with echocardiograms available for analysis at baseline, 30 days, and 1 year. We reported similar data among those with no/trace/mild TR at baseline. To explore the relationship between an improvement in pre-procedure moderate or severe TR and clinical outcomes, we compared 30 day to 1 year mortality in patients that improved from moderate to severe TR at baseline to no/trace/mild TR at 30 days versus those who continued to have moderate or severe TR at 30 days. All statistical analyses were performed with SAS software, version 9.2.
Results
Patient population
Among the 553 patients enrolled in the PARTNER II trial Cohort B, 542 were treated with TAVR (SAPIEN [n=263], SAPIEN XT [n=279]). The clinical and echocardiographic characteristics of patients according to the severity of TR are shown in Table 1. Patients with worse TR were older, had a lower body mass index, higher STS score, and greater prevalence of atrial arrhythmia and frailty, but there were no differences in sex, severe heart failure symptoms, coronary disease, or lung disease. Patients with more significant TR had a lower LV ejection fraction and stroke volume index, larger left atrial size, and greater prevalence of moderate/severe mitral regurgitation. Worse TR was also associated with a larger RA and RV, worse RV function, and higher RV systolic pressures.
Table 1.
Baseline characteristics according to tricuspid regurgitation severity
| n* (507) |
No/trace/mild TR (n=372) |
Moderate TR (n=117) |
Severe TR (n=18) |
p-value | |
|---|---|---|---|---|---|
| Clinical | |||||
| Age | 507 | 84±8 | 86±10 | 88±5 | 0.01 |
| Female | 507 | 51 | 48 | 39 | 0.55 |
| Body mass index (kg/m2) | 505 | 28.3 ± 6.9 | 26.7 ± 6.2 | 24.4 ± 5.4 | 0.008 |
| STS Score | 507 | 10.1 ± 5.3 | 11.5 ± 6.0 | 12.7 ± 5.7 | 0.01 |
| Hypertension | 507 | 90 | 91 | 94 | 0.84 |
| Diabetes mellitus | 507 | 37 | 33 | 11 | 0.06 |
| NYHA class 4 | 507 | 48 | 48 | 44 | 0.96 |
| Coronary disease | 507 | 67 | 62 | 72 | 0.59 |
| Prior myocardial infarction | 507 | 21 | 19 | 6 | 0.28 |
| Prior coronary artery bypass surgery | 507 | 26 | 26 | 28 | 0.98 |
| Peripheral vascular disease | 507 | 29 | 29 | 33 | 0.93 |
| Atrial arrhythmia | 507 | 31 | 50 | 72 | <0.001 |
| Permanent pacemaker | 507 | 15 | 33 | 11 | <0.001 |
| Renal disease (creatinine ≥2) | 507 | 9 | 16 | 17 | 0.09 |
| Liver disease | 507 | 4.6 | 6.0 | 0 | 0.52 |
| Chronic obstructive lung disease | 507 | 28 | 25 | 33 | 0.69 |
| Oxygen dependent | 507 | 14 | 17 | 17 | 0.64 |
| Frailty | 507 | 55 | 68 | 67 | 0.04 |
| Echocardiography | |||||
| LV ejection fraction (%) | 507 | 53 ± 12 | 49 ± 14 | 50 ± 13 | 0.02 |
| LVMi (g/m2) | 487 | 130 ± 34 | 132 ± 36 | 131 ± 32 | 0.90 |
| Stroke volume index (ml/m2) | 493 | 37 ± 10 | 32 ± 10 | 30 ± 6 | <0.001 |
| LV end-diastolic dimension (cm) | 487 | 4.4 ± 0.7 | 4.4 ± 0.8 | 4.5 ± 0.6 | 0.83 |
| LV end-systolic dimension (cm) | 486 | 3.21 ± 0.88 | 3.31 ± 0.96 (113) | 3.26 ± 0.94 (18) | 0.56 |
| LA volume index (mL/m2) | 392 | 43 ± 15 | 49 ± 18 | 62 ± 31 | <0.001 |
| AVA index (cm2/m2) | 492 | 0.35 ± 0.09 | 0.32 ± 0.10 | 0.33 ± 0.08 | 0.003 |
| AV mean gradient (mmHg) | 505 | 46 ± 13 | 45 ± 16 | 38 ± 11 | 0.07 |
| AV peak gradient (mmHg) | 505 | 80 ± 22 | 78 ± 26 | 68 ± 17 | 0.08 |
| Moderate/severe total AR (%) | 504 | 14 | 19 | 22 | 0.38 |
| Moderate/severe MR (%) | 502 | 21 | 49 | 67 | < 0.001 |
| RA area index (cm2/m2) | 458 | 9.09 ± 2.52 | 11.60 ± 3.68 | 16.40 ± 6.65 | < 0.001 |
| RV dilation (any) | 476 | 15 | 49 | 56 | <0.001 |
| RV dilation (mild/mod/sev) | 476 | 12/3/0 | 30/15/5 | 28/28/0 | <0.001 |
| RV dysfunction (any) | 471 | 24 | 54 | 50 | <0.001 |
| RV dysfunction (mild/mod/sev) | 471 | 18/6/0 | 37/15/2 | 22/17/11 | <0.001 |
| TR jet velocity (m/s) | 422 | 2.8 ± 0.5 | 3.3 ± 0.5 | 3.1 ± 0.6 | <0.001 |
| RV systolic pressure (mmHg) | 370 | 38 ± 13 | 52 ± 16 | 50 ± 16 | <0.001 |
| Hemodynamics (pre-BAV) | |||||
| RA (mmHg) | 419 | 10 (7,14) | 10 (6,16) | 9 (5,14) | 0.91 |
| PASP (mmHg) | 439 | 40 (32,52) | 44 (35,58) | 43 (30,52) | 0.09 |
| mPAP (mmHg) | 415 | 27 (20,35) | 31 (23,39) | 28 (21,37) | 0.08 |
| Wedge (mean) (mmHg) | 280 | 18 (13,22) | 18 (12,25) | 18 (13,24) | 0.79 |
| LVEDP (mmHg) | 400 | 15 (9,22) | 14 (8,21) | 11 (6,17) | 0.17 |
| Aortic (systolic) (mmHg) | 476 | 121 (108,137) | 117 (102,133) | 119 (96,135) | 0.19 |
| Cardiac index (ml/m2) | 408 | 2.1 (1.7,2.6) | 1.9 (1.5,2.3) | 1.9 (1.7,2.2) | 0.004 |
Data shown as % or mean ± SD.
n is the number of subjects with information reported on the particular variable from among the 507 patients with severity of tricuspid regurgitation reported on the baseline echocardiogram.
Abbreviations: TR, tricuspid regurgitation; STS, Society of Thoracic Surgeons; NYHA, New York Heart Association; LV, left ventricular; LVMi, LV mass index; AVA, aortic valve area; AR, aortic regurgitation; MR, mitral regurgitation; RA, right atrial; RV, right ventricular; PASP, pulmonary artery systolic pressure; mPAP, mean pulmonary artery pressure; LVEDP, LV end-diastolic pressure.
Mortality based on tricuspid regurgitation severity and the right heart
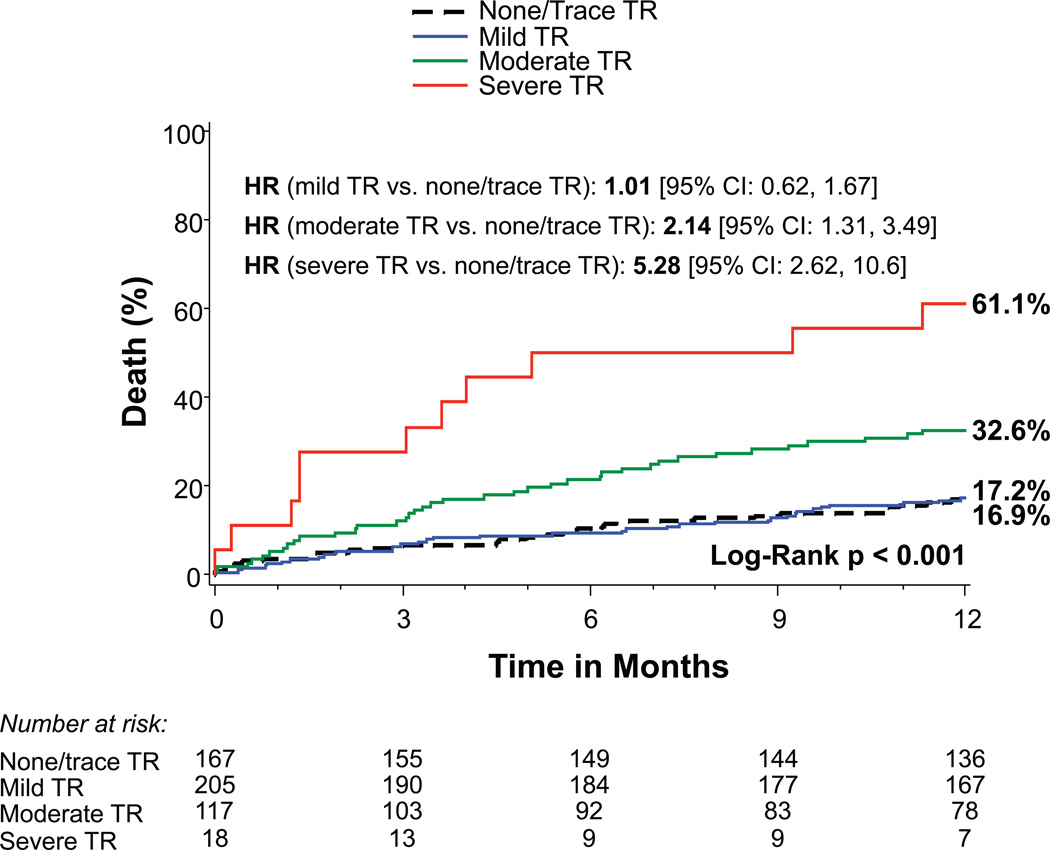
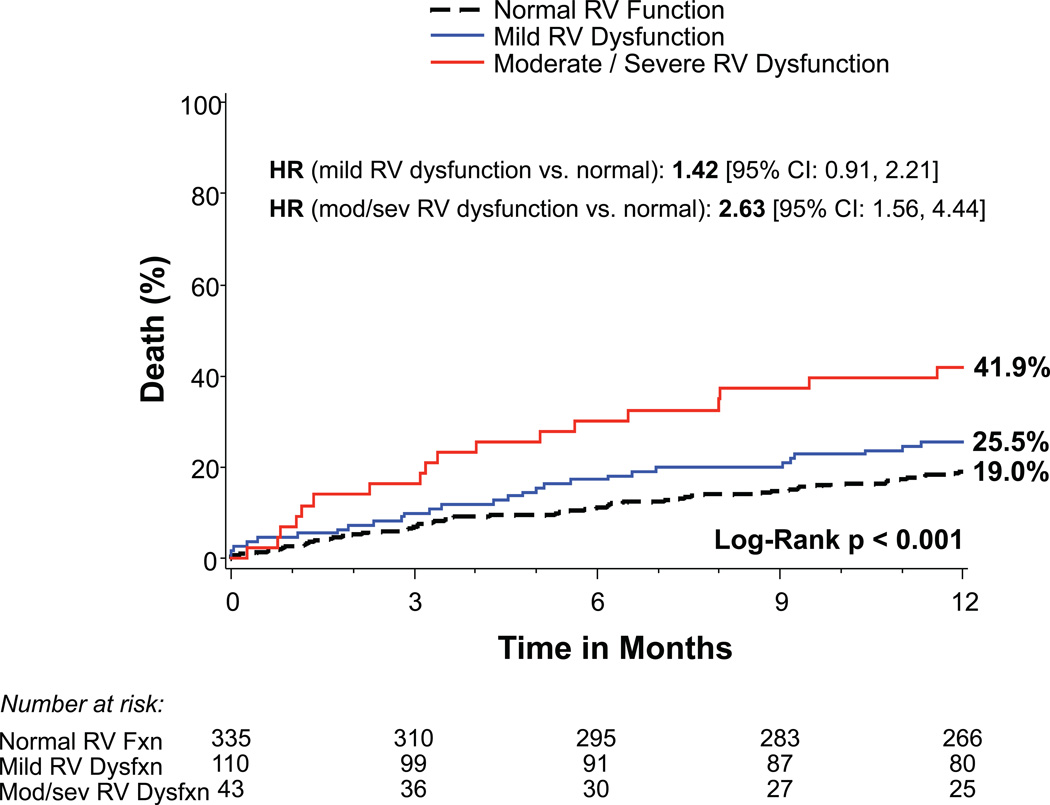
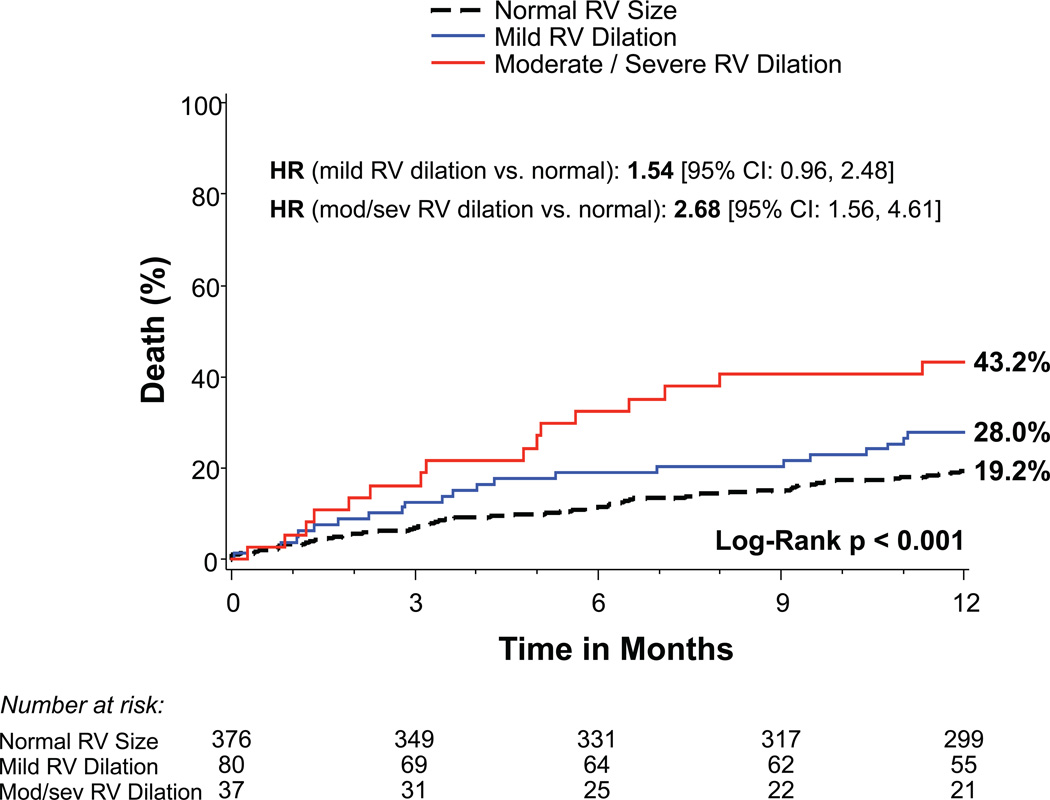
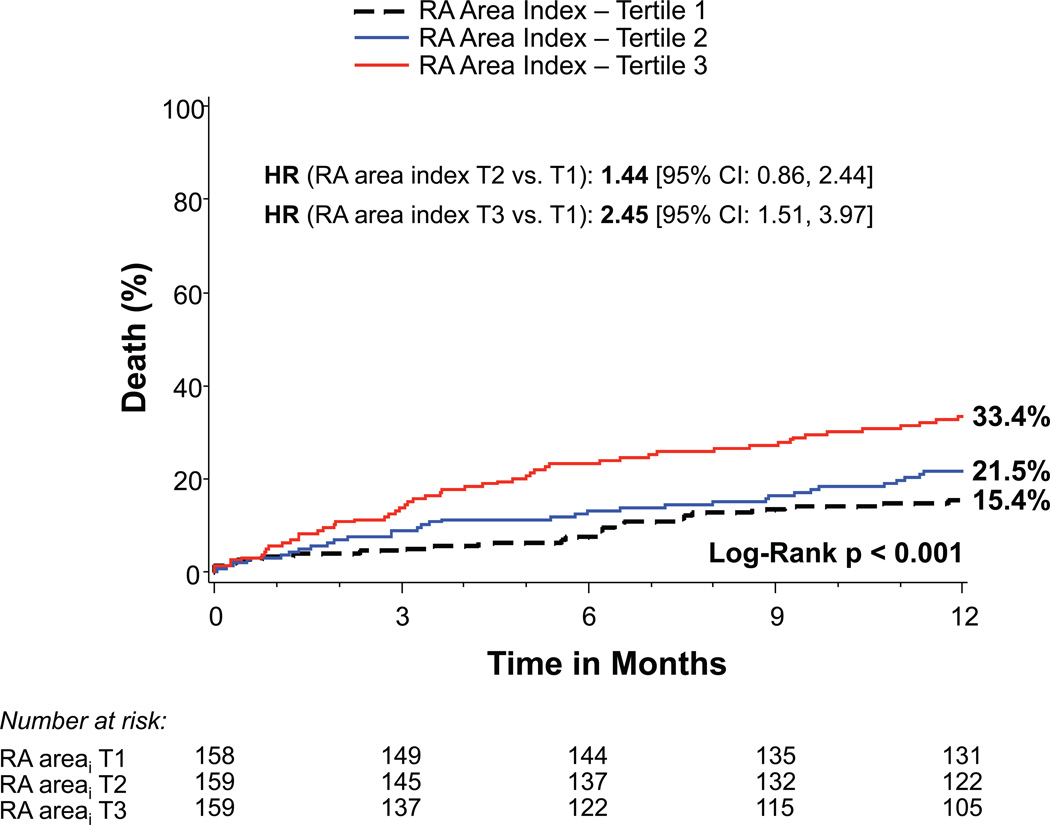
Patients with more significant TR prior to TAVR had increased 1-year mortality: 32.6% for moderate TR and 61.1% for severe TR compared to 16.9% and 17.2% for those with no/trace or mild TR, respectively (p<0.001) (Figure 1A). Increasing severity of RV dysfunction was also associated with mortality (p<0.001) as were RA and RV enlargement (p<0.001) (Figures 1B–D). Of note, tricuspid valve regurgitation velocity and estimated right ventricular systolic pressure were not significantly associated with 1-year mortality in univariable analysis (p=0.22 and p=0.07, respectively). After multivariable adjustment, severe TR (HR 3.20, 95% CI 1.50–6.82, p=0.003) and moderate TR (HR 1.60, 95% CI 1.02–2.52, p=0.042) remained significantly associated with increased mortality (Table 2). RA and RV enlargement were also associated with increased mortality after adjustment, including a greater than 2-fold increase in the hazard of death for moderate or severe RV dilation (HR 2.20, 95% CI 1.24–3.90, p=0.007). After multivariable adjustment, the association between RV dysfunction and increased mortality was no longer significant (Table 2).
Figure 1. Time-to-event curves for 1-year death from any cause.
One-year time-to-event curves are shown for death from any cause in the as-treated population of the PARTNER II trial inoperable cohort according to tricuspid regurgitation (TR) severity (A), right ventricular (RV) function (B), RV size (C), and right atrial (RA) index (D). The event rates were calculated with the use of Kaplan-Meier methods and compared with the use of the log-rank test. Abbreviations: HR, hazard ratio; refer to Table 1.
Table 2.
Multivariable analysis for 1-year all-cause death.
| Variable | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Tricuspid regurgitation | ||
| TR severity (moderate/severe vs. no/trace/mild) | 1.76 (1.14, 2.70) | 0.01 |
| TR severity (no/trace/mild referent) | --- | --- |
| Moderate TR | 1.60 (1.02, 2.52) | 0.042 |
| Severe TR | 3.20 (1.50, 6.82) | 0.003 |
| Right ventricular function | ||
| RV dysfunction (any) vs. normal | 1.19 (0.77, 1.85) | 0.44 |
| RV function (normal is referent) | --- | --- |
| Mild RV dysfunction | 1.06 (0.65, 1.73) | 0.81 |
| Moderate or severe RV dysfunction | 1.62 (0.87, 3.01) | 0.13 |
| Right ventricular size | ||
| RV dilation (any) vs. normal | 1.70 (1.11, 2.59) | 0.01 |
| RV dilation (normal is referent) | ||
| Mild RV dilation | 1.47 (0.89, 2.42) | 0.13 |
| Moderate or severe RV dilation | 2.20 (1.24, 3.90) | 0.007 |
| Right atrial index | ||
| RA area index (per 1 cm2/m2 increase) | 1.07 (1.02, 1.12) | 0.008 |
| RA area index ≥median vs. <median | 1.55 (0.98, 2.43) | 0.058 |
| RA area index ≥ vs. < optimal cut-point* | 1.59 (1.01, 2.51) | 0.046 |
Multivariable Cox proportional hazards models evaluating the association between tricuspid regurgitation (TR) severity, right atrial (RA) and right ventricular (RV) size, and RV function and 1-year all-cause death, adjusted for age, sex, body mass index, STS score, prior infarct, prior CABG, frailty, permanent pacemaker, atrial arrhythmia, aortic valve mean gradient, left ventricular ejection fraction, and mitral regurgitation.
RA area index optimal cut-point: 9.16 cm2/m2
Abbreviations: Refer to Table 1.
Because they did not have a univariable association with mortality and because they were missing in 17% (tricuspid valve regurgitation velocity) and 27% (estimated right ventricular systolic pressure) of subjects with TR severity assessed due to inadequate images of the TR jet or inferior vena cava, we did not include an estimate of pulmonary pressures in our primary multivariable models. To further evaluate the association between TR severity and mortality, we forced various measurements of pulmonary artery pressures (echocardiographic and invasive hemodynamics) into the TR models from Table 2 (Supplemental Table 1). The association between moderate/severe TR and an increased hazard of mortality after TAVR was similar to our primary findings. Additionally, we adjusted for the severity of paravalvular aortic regurgitation and indices of pulmonary artery pressures on the discharge echocardiogram (Supplemental Table 1). Although the association between moderate/severe TR and increased mortality was modestly attenuated in these models, the point estimates are similar and these models included substantially fewer patients and deaths than the primary models.
When TR was combined with each of these right heart parameters in multivariable analyses, moderate/severe TR generally remained significantly associated with increased mortality. The relationship between RV dilation and mortality was of borderline significance after adjustment for TR severity (p=0.058), whereas RV dysfunction and RA enlargement were not associated with mortality after adjustment for TR severity (Table 3). When moderate/severe TR occurred with any degree of RV dilation or an increased RA size, those patients had an adjusted hazard of death greater than 2 compared to patients with no/mild TR and a normal RV size or smaller RA size (Table 3).
Table 3.
Multivariable analysis of tricuspid regurgitation combined with right heart size and function.
| Variable | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Right ventricular function and tricuspid regurgitation | ||
| Model 1 | ||
| TR severity (moderate/severe vs. no/trace/mild) | 1.73 (1.09, 2.75) | 0.02 |
| RV dysfunction (any) vs. normal | 1.11 (0.70, 1.75) | 0.67 |
| Model 2 | ||
| Groups (referent: no/trace/mild TR and normal RV function) | --- | --- |
| RV dysfunction (any) and no/trace/mild TR | 1.21 (0.65, 2.23) | 0.55 |
| Normal RV function and mod/severe TR | 1.88 (1.03, 3.44) | 0.040 |
| RV dysfunction (any) and mod/severe TR | 1.90 (1.07, 3.37) | 0.03 |
| Right ventricular size and tricuspid regurgitation | ||
| Model 1 | ||
| TR severity (moderate/severe vs. no/trace/mild) | 1.60 (1.00, 2.55) | 0.048 |
| RV dilation (any) vs. normal | 1.54 (0.99, 2.41) | 0.058 |
| Model 2 | ||
| Groups (referent: no/trace/mild TR and normal RV size) | ||
| RV dilation (any) and no/trace/mild TR | 1.42 (0.72, 2.83) | 0.31 |
| Normal RV size and mod/severe TR | 1.52 (0.85, 2.70) | 0.15 |
| RV dilation (any) and mod/severe TR | 2.49 (1.46, 4.25) | <0.001 |
| Right atrial index and tricuspid regurgitation | ||
| Model 1 | ||
| TR severity (moderate/severe vs. no/trace/mild) | 1.57 (0.98, 2.49) | 0.058 |
| RA area index ≥ vs. < optimal cut-point* | 1.39 (0.86, 2.26) | 0.18 |
| Model 2 | ||
| Groups (referent: no/trace/mild TR and RA area index <cut-point) | --- | --- |
| RA area index (≥ optimal cut-point) and no/trace/mild TR | 1.56 (0.90, 2.70) | 0.11 |
| RA area index < optimal cut-point and mod/severe TR | 2.10 (0.92, 4.78) | 0.08 |
| RA area index (≥ optimal cut-point) and mod/severe TR | 2.18 (1.21, 3.94) | 0.009 |
Multivariable Cox proportional hazards models evaluating the association between tricuspid regurgitation (TR) severity (moderate/severe vs. no/trace/mild) combined with right heart size and function and 1-year all-cause death. All results are adjusted for age, sex, body mass index, STS score, prior infarct, prior CABG, frailty, permanent pacemaker, atrial arrhythmia, aortic valve mean gradient, left ventricular ejection fraction, and mitral regurgitation. For each set of variables (RV function + TR, RV size + TR, and RA area index + TR), there are 2 models shows. In each case, model 1 includes TR severity and the right heart variable (RV function, RV size, or RA area index) as individual variables in the model, adjusted for the variables listed above. Model 2 combines TR severity and the right heart variable to create 4 groups as described, adjusted for the same variables.
RA area index optimal cut-point: 9.16 cm2/m2
Abbreviations: Refer to Table 1.
Among patients with moderate/severe TR at baseline and a 30 day assessment of TR severity (n=111), those with improvement to no/trace/mild TR at 30 days (n=34) had a lower rate of death between 30 days and 1 year (20.6%) than those (n=77) with persistent moderate/severe TR (30%), although patient numbers and events were limited and this was not statistically significant (p=0.30). Regardless of baseline TR severity, moderate/severe TR compared to no/trace/mild TR at 30 days was associated with an increased hazard of 30 day to 1 year mortality (unadjusted HR 2.39, 95% CI 1.54–3.73) and there was no interaction between 30 day TR severity and pulmonary pressures with respect to 30 day to 1 year mortality (Supplemental Figure 1).
Tricuspid and mitral regurgitation
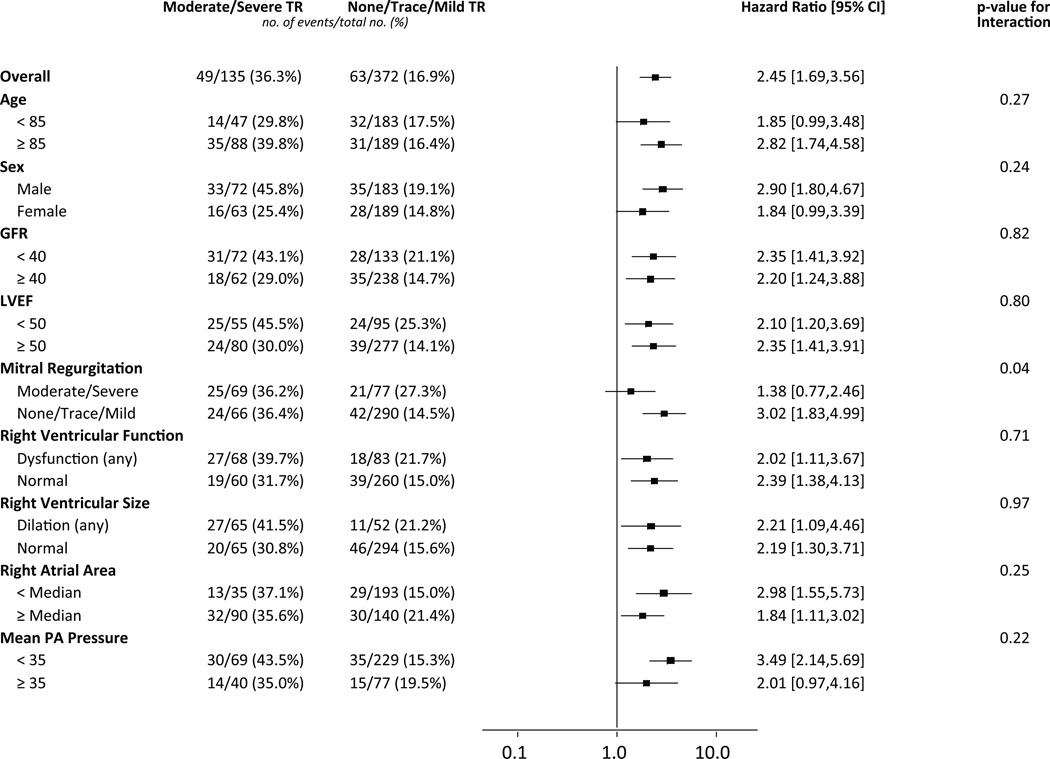
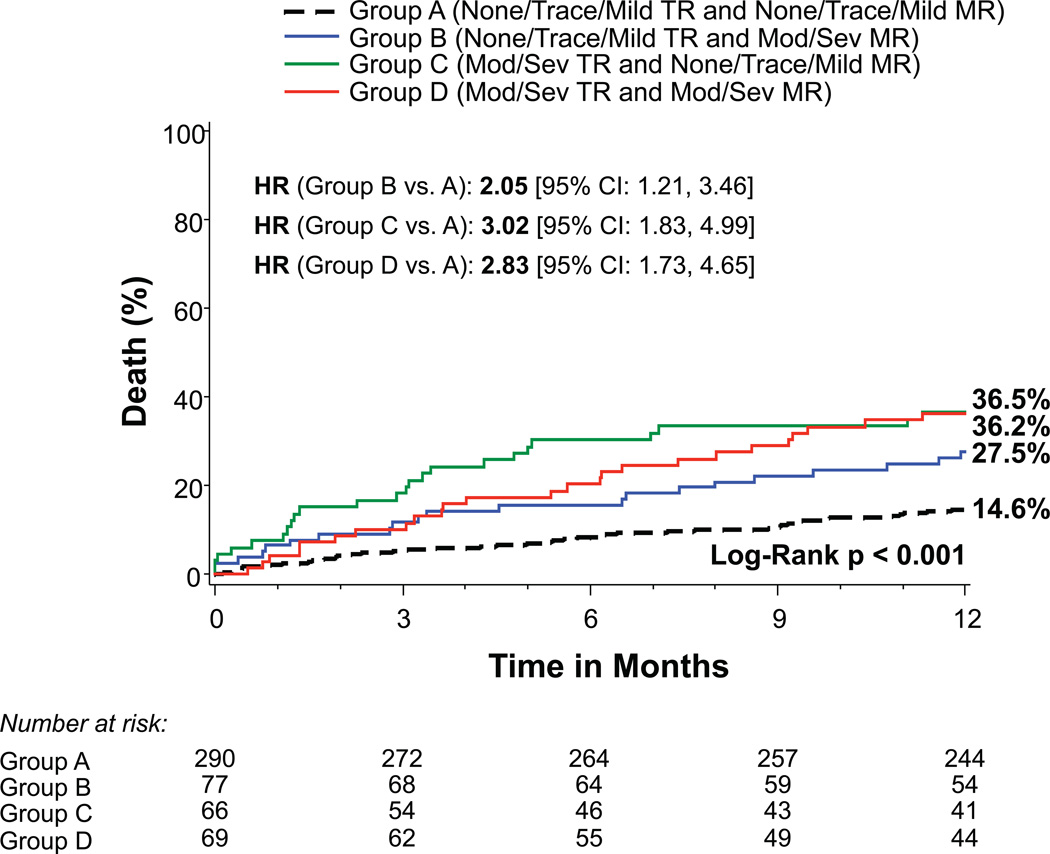
We evaluated whether the association between moderate/severe TR and increased mortality was consistent across several sub-groups (Figure 2). There was a significant interaction between TR and MR severity (interaction p=0.04). In patients with no/trace/mild MR, the presence of moderate/severe TR was associated with an increased unadjusted 1-year mortality (unadjusted HR 3.02, 95% CI 1.83–4.99), whereas it was not in those with concomitant moderate/severe MR (unadjusted HR 1.38, 95% CI 0.77–2.46) (Figure 2). Patients with moderate/severe TR and no/trace/mild MR had a 1-year mortality of 36.5% compared to 14.6% in patients with no/trace/mild TR and MR (Figure 3), which in adjusted analyses was associated with an increased hazard of death in the first year after TAVR (adjusted HR 2.55, 95% CI 1.50–4.32, p<0.001) (Table 4). Among patients with moderate/severe MR, moderate/severe TR was not associated with an increased hazard of death compared to no/trace/mild TR in adjusted analyses (adjusted HR 1.08, 95% CI 0.58–1.98).
Figure 2. Effect of clinical and echocardiographic factors on the association between tricuspid regurgitation severity and 1-year death from any cause.
Cox proportional hazards models were used to evaluate the hazard ratios for 1-year death from any cause for patients with moderate/severe versus no/trace/mild tricuspid regurgitation (TR) in the clinical and echocardiographic sub-groups shown. Abbreviations: GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; PA, pulmonary artery.
Figure 3. Time-to-event curves for 1-year death from any cause according to mitral and tricuspid regurgitation severity.
One-year time-to-event curves are shown for death from any cause in the as-treated population of the PARTNER II trial inoperable cohort for patients with no/trace/mild TR and no/trace/mild MR (Group A), no/trace/mild TR and moderate/severe MR (Group B), moderate/severe TR and no/trace/mild MR (Group C), and moderate/severe TR and moderate/severe MR (Group D). The event rates were calculated with the use of Kaplan-Meier methods and compared with the use of the log-rank test.
Table 4.
Multivariable analysis for groups based on the severity of tricuspid and mitral regurgitation.
| Tricuspid and Mitral Regurgitation Groups | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Groups (referent: no/trace/mild TR and no/trace/mild MR) | --- | --- |
| No/trace/mild TR and moderate/severe MR | 1.60 (0.93, 2.77) | 0.09 |
| Moderate/severe TR and no/trace/mild MR | 2.55 (1.50, 4.32) | <0.001 |
| Moderate/severe TR and moderate/severe MR | 1.73 (1.00, 2.97) | 0.049 |
Multivariable Cox proportional hazards model evaluating the association between tricuspid regurgitation (TR) severity (moderate/severe vs. no/trace/mild) combined with mitral regurgitation (TR) severity (moderate/severe vs. no/trace/mild) and 1-year all-cause death, adjusted for age, sex, body mass index, STS score, prior infarct, prior CABG, frailty, permanent pacemaker, atrial arrhythmia, aortic valve mean gradient, and left ventricular ejection fraction.
Abbreviations: Refer to Table 1.
Change in tricuspid regurgitation and right ventricular function after TAVR
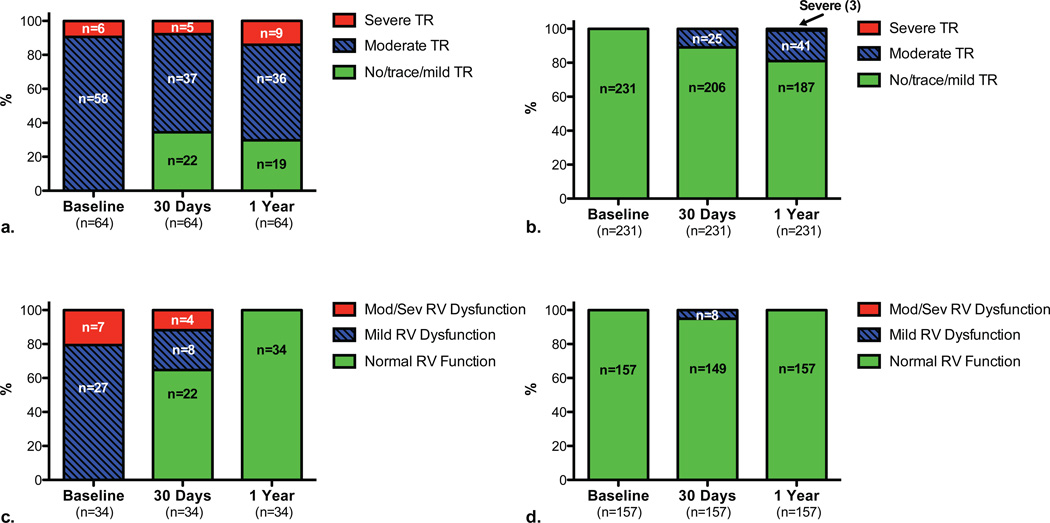
Among 1-year survivors with moderate or severe TR at baseline, approximately one-third had improvement to no/trace/mild TR at 30-day and 1-year follow-up (Figure 4a). In contrast, among 1-year survivors with no/trace/mild TR at baseline, a smaller percentage had progression to moderate or severe TR at 30-day and 1-year follow-up (Figure 4b). Comparable data is shown for RV function over time in Figures 4c and 4d.
Figure 4. Change in tricuspid regurgitation severity and right ventricular function over time after transcatheter aortic valve replacement.
Among patients who survived 1 year after transcatheter aortic valve replacement and had TR assessed at baseline, 30 days, and 1 year, the number and percentages of patients with no/trace/mild, moderate, and severe TR are shown at baseline, 30 days, and 1 year for those with moderate or severe TR at baseline (a) or no/trace/mild TR at baseline (b). Among patients who survived 1 year after transcatheter aortic valve replacement and had RV function assessed at baseline, 30 days, and 1 year, the number and percentages of patients with normal RV function, mild RV dysfunction, and moderate/severe RV dysfunction are shown at baseline, 30 days, and 1 year for those with any RV dysfunction at baseline (c) or normal RV function at baseline (d).
Discussion
In patients with prohibitive surgical risk treated with TAVR in the PARTNER II trial, we found that moderate or severe TR and right heart enlargement and dysfunction are associated with increased mortality during the first year after the procedure. Severe TR was associated with a 3-fold increase in the hazard of 1-year mortality after multivariable adjustment. Similarly, after adjustment, larger RA size and RV dilation were associated with increased mortality, whereas RV dysfunction was not. Interestingly, the relationship between moderate/severe TR and increased 1-year mortality differed based on the severity of MR at baseline in that moderate/severe TR was associated with a 3-fold higher hazard of death in those with minimal MR, but was not associated with increased mortality in those with concomitant moderate or severe MR. Collectively, these findings demonstrate the importance of right-sided pathology in outcomes for patients undergoing TAVR and may have implications for treatment decisions, including assessment of anticipated benefit from TAVR and whether concomitant surgical treatment of TR should be considered in selected operable patients.
Recently, Barbanti et al. reported that while moderate/severe TR was associated with worse survival in patients undergoing TAVR, this association was no longer significant after multivariable adjustment.4 When comparing their study to the present one, there are some notable differences. They looked at all patients treated with TAVR via femoral and alternative access routes at a single center, whereas we evaluated patients treated only via a transfemoral approach within the context of a multicenter trial who were considered “inoperable.” In the Barbanti study compared to the PARTNER II inoperable cohort, there were differences in the prevalence of moderate/severe TR (15.2% versus 26.6%, respectively) and moderate/severe MR (40.1% versus 29.1%, respectively), which could have influenced the findings. Just as there have been conflicting results regarding the effect of significant MR on outcomes after TAVR,1–3 perhaps the effect of significant TR differs based on a variety of factors that remain to be elucidated. Our results, however, are consistent with studies in patients undergoing mitral valve surgery and an unselected population of patients undergoing an echocardiogram demonstrating the adverse effect of significant TR on mortality.14–16 Beyond providing another evaluation of the effect of significant TR on outcomes after TAVR, we extend the findings of Barbanti et al. by providing new insights on the effects of right heart dilation and dysfunction.
We did not observe an association between pulmonary hypertension and mortality, while several other studies have demonstrated an association between increased pulmonary pressures and increased mortality after AVR.17–21 However, in some of these studies the association between pulmonary hypertension and increased mortality was quite modest.22 For example, Lucon et al. recently reported that in the large FRANCE 2 registry mild to moderate pulmonary hypertension was not associated with 1-year mortality. While severe pulmonary hypertension was associated with increased mortality, this association was quite modest (HR 1.33, 95% CI 1.01–1.75). The median pulmonary artery systolic pressure was minimally different between survivors and non-survivors (45 vs. 43 mmHg). Other studies in this patient population have not identified pulmonary hypertension as a risk factor for mortality.4 As such, we believe our finding that pulmonary pressures are not associated with 1-year mortality in the PARTNER 2B study is not an anomaly. We believe this lack of an association between estimates of pulmonary artery pressure and mortality in this cohort may be explained by several factors. First, the relationship between pulmonary artery pressure and mortality may not be linear and may be more clearly observed when grouping patients based on the severity of pulmonary hypertension. Second, perhaps in this relatively sicker and inoperable cohort of patients the relationship between pulmonary hypertension and mortality is diluted by the numerous comorbidities and other factors that are driving mortality. Third, as RV function begins to worsen (RV dysfunction was present in 32% of this cohort), the pulmonary pressures may begin to drop as disease progression advances. This would confound a clear association between higher pulmonary pressures and increased mortality.
The Right Heart and Outcomes after TAVR
Several studies have demonstrated the independent and additive adverse effect of RV dysfunction on clinical outcomes in patients with left-sided heart failure.5,6,23 Small studies have evaluated the effect on peri-operative outcomes of RV dysfunction in patients with mitral or aortic valve disease,24 but the effect of RV dysfunction on longer-term outcomes after valve replacement, particularly TAVR, has not been studied. RV function may be abnormal in patients with AS due to 1) pressure overload from increased left-sided filling pressures and pulmonary artery pressures transmitted to the right side; 2) volume overload from fluid retention or concomitant TR; 3) myocardial ischemia from coronary disease; or 4) ventricular interdependence due to septal dysfunction. We found that while increasing severity of RV dysfunction was associated with worse clinical outcomes, this relationship was no longer significant after multivariable adjustment. However, more quantitative or sensitive measures of RV function than those obtained in this study may identify a stronger association. In contrast, we found that RV dilation was independently associated with mortality in patients treated with TAVR, which is analogous to and consistent with several studies that have identified LV remodeling/size as a better predictor of outcomes than LV function (e.g., ejection fraction) in patients with left-sided heart failure.25,26 Perhaps RV size better reflects the chronicity and severity of pressure or volume overload on the RV than ventricular function. While most studies evaluating the RV in patients with left-sided heart failure have examined the prognostic significance of RV dysfunction, others have demonstrated an independent association between RV enlargement and mortality in patients with dilated cardiomyopathy.27
Tricuspid and Mitral Regurgitation
We found that the increased hazard of moderate/severe TR in patients treated with TAVR differed based on the severity of concomitant MR. Why might moderate/severe TR increase the hazard of death in those with no/trace/mild MR, but not in those with moderate/severe MR? It is noteworthy that patients with no/trace/mild TR and MR had a low 1-year mortality at 14.6%, demonstrating an excellent outcome for patients with isolated AS treated with TAVR. Compared to this group of patients, those with moderate/severe TR had an adjusted hazard of death 2.5-fold higher. Among patients with moderate/severe MR, those with concomitant moderate/severe TR had a higher 1-year mortality compared to those with no/trace/mild TR (36.2% vs. 27.5%, respectively), but the increased hazard of death associated with moderate/severe TR was not significant among these patients in unadjusted or adjusted analyses. To the extent that moderate/severe TR is influenced by the presence of moderate/severe MR (perhaps due to increased pulmonary venous and arterial pressures from significant MR exacerbating pressure overload and dilation of the RV), the MR may get somewhat better after TAVR, thereby reducing pressure overload on the RV and facilitating improvement in TR. In contrast, if moderate/severe TR is not influenced by the presence of MR (because it is absent or minimal), then TAVR will not lead to any reduction of MR that might help reduce concomitant TR. Further studies are needed to clarify this relationship and its clinical implications; our data would suggest that it may be all the more important to consider a concomitant tricuspid valve procedure in those patients with minimal MR.
Clinical Implications
At a time when the utilization of TAVR is rapidly expanding and extending into lower risk patients, the issue of concomitant mitral and tricuspid valve regurgitation, their effect on clinical outcomes, and how to best treat the patient with multiple valve lesions will require further investigation and careful discussions in the context of a heart valve team. Our findings demonstrate that significant TR portends a worse prognosis after TAVR in extreme risk patients and suggest that it should be considered in a comparable light to concomitant significant MR when making treatment decisions in patients considered for TAVR. While the patients in the current study were considered prohibitive risk for surgery such that a concomitant tricuspid valve procedure was not really a viable option, our findings raise important questions about how significant TR (and perhaps tricuspid annular dilation) ought to factor into treatment decisions (TAVR versus surgical valve replacement) for patients with more modest surgical risk. It may be that in some patients, the addition of a tricuspid repair to surgical aortic valve replacement may lead to better outcomes than TAVR accompanied by no treatment of TR or later surgical repair. Treatment guidelines for intermediate and high risk patients with severe AS and concomitant significant TR or MR will require additional studies aimed at clarifying these issues. Beyond the question of whether and when concomitant TR should be corrected, our findings show that in patients at prohibitive surgical risk, the presence of severe TR portends a very poor prognosis even if the AS is treated with TAVR, which may shift the risk/benefit ratio toward not performing TAVR.
Limitations
Our findings apply to patients at prohibitive risk for conventional surgery who were treated with TAVR via a transfemoral approach. Further studies are needed to evaluate whether significant TR and right heart dilation and dysfunction adversely affect prognosis after TAVR in patients at intermediate to high risk and those treated via alternative access approaches. Given the small number of patients with severe TR, the hazard of mortality associated with severe TR should be confirmed in future studies. Quantitative measurements of TR severity were not systematically recorded by the core laboratory. The percentage of patients for whom RV function was evaluated by visual estimate versus RV fractional area change is not known. RV function was not evaluated with longitudinal strain or tricuspid annular velocity or displacement, which may have provided a more detailed, quantitative assessment and identified a stronger association between abnormal RV function and mortality. Moreover, due to the complex geometry of the RV, 3-dimensional echocardiography or magnetic resonance imaging may provide additional insights and better characterize the relationship between RV size/structure and outcomes than two-dimensional echocardiography as used in this study. For the analyses that included pulmonary hypertension, we did not have information to reliably classify the type or group of pulmonary hypertension. There was no adjustment made for multiple comparisons, so borderline significant p-values should be interpreted with caution. Finally, although it was likely functional in most cases, we lack a characterization of TR as primary versus secondary/functional.
Conclusion
In the prohibitive risk arm of the PARTNER II trial, moderate or severe TR occurred in 27% of patients and was associated with increased mortality in unadjusted and adjusted analyses, including a 3-fold increase in the adjusted hazard of death among those with severe TR. The increased hazard of death associated with moderate/severe TR was observed in those with no/trace/mild MR, but not in those with concomitant moderate/severe MR. RA and RV dilation were also associated with increased mortality, whereas RV dysfunction was not after multivariable adjustment. Further studies are needed to clarify these relationships and their implications for treatment decisions.
Supplementary Material
Acknowledgments
The authors thank Maria Alu and Girma M. Ayele, PhD for their assistance in the preparation of the manuscript.
Sources of Funding: The PARTNER trial was funded by Edwards Lifesciences and the protocol was designed collaboratively by the Sponsor and the Steering Committee. The present analysis was carried out by academic investigators through the PARTNER Publications Office with no direct involvement of the sponsor in the analysis, drafting of the manuscript, or the decision to publish. Dr. Lindman is supported by K23 HL116660 from the NIH.
Dr. Lindman is a site co-investigator for the PARTNER Trial. Dr. Lerakis has received consulting fees/honoraria from Edwards Lifesciences. Dr. Mack is an unpaid member of the PARTNER Trial Executive Committee. Dr. Suri’s institution, the Mayo Clinic, has received aortic valve replacement trial funding to the Division of Cardiovascular Surgery from Edwards Lifesciences, St. Jude Medical and Sorin Medical, and he is a national PI for the PERCEVAL Trial (Sorin Medical), on the Steering Committee for the Portico Trial (St. Jude Medical), and co-investigator for the PARTNER II (Edwards Lifesciences) and COAPT (Abbott) Trials. Dr. Thourani is an unpaid member of the PARTNER Trial Steering Committee, the national co-PI of the PARTNER 2 SAPIEN 3 trial, and is a consultant for and has research grants with Edwards Lifesciences, Sorin Medical, St. Jude Medical, Boston Scientific, and Medtronic. Dr. Babaliaros has received consulting fees/honoraria from DirectFlow Medical. Dr. Whisenant has received consulting fees/honoraria from Edwards Lifesciences. Dr. Miller is supported by an R01 research grant from the NHLBI #HL67025, is an unpaid member of the PARTNER Trial Executive Committee, and has received consulting fees/honoraria from Abbott Vascular, St. Jude Medical, and Medtronic. Dr. Tuzcu is an unpaid member of the PARTNER Trial Executive Committee. Dr. Svensson is an unpaid member of the PARTNER Trial Executive Committee, holds equity in Cardiosolutions and ValvXchange, and has Intellectual Property Rights/Royalties from Posthorax. Dr. Leon is an unpaid member of the PARTNER Trial Executive Committee.
Footnotes
Disclosures: The other authors report no potential conflicts of interest.
References
- 1.Toggweiler S, Boone RH, Rodes-Cabau J, Humphries KH, Lee M, Nombela-Franco L, Bagur R, Willson AB, Binder RK, Gurvitch R, Grewal J, Moss R, Munt B, Thompson CR, Freeman M, Ye J, Cheung A, Dumont E, Wood DA, Webb JG. Transcatheter aortic valve replacement: outcomes of patients with moderate or severe mitral regurgitation. J Am Coll Cardiol. 2012;59:2068–2074. doi: 10.1016/j.jacc.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Barbanti M, Webb JG, Hahn RT, Feldman T, Boone RH, Smith CR, Kodali S, Zajarias A, Thompson CR, Green P, Babaliaros V, Makkar RR, Szeto WY, Douglas PS, McAndrew T, Hueter I, Miller DC, Leon MB Placement of Aortic Transcatheter Valve Trial I. Impact of Preoperative Moderate/Severe Mitral Regurgitation on 2-Year Outcome After Transcatheter and Surgical Aortic Valve Replacement: Insight From the Placement of Aortic Transcatheter Valve (PARTNER) Trial Cohort A. Circulation. 2013;128:2776–2784. doi: 10.1161/CIRCULATIONAHA.113.003885. [DOI] [PubMed] [Google Scholar]
- 3.Bedogni F, Latib A, De Marco F, Agnifili M, Oreglia J, Pizzocri S, Latini RA, Lanotte S, Petronio AS, De Carlo M, Ettori F, Fiorina C, Poli A, Cirri S, De Servi S, Ramondo A, Tarantini G, Marzocchi A, Fiorilli R, Klugmann S, Ussia GP, Tamburino C, Maisano F, Brambilla N, Colombo A, Testa L. Interplay Between Mitral Regurgitation and Transcatheter Aortic Valve Replacement With the CoreValve Revalving System: A Multicenter Registry. Circulation. 2013;128:2145–2153. doi: 10.1161/CIRCULATIONAHA.113.001822. [DOI] [PubMed] [Google Scholar]
- 4.Barbanti M, Binder RK, Dvir D, Tan J, Freeman M, Thompson CR, Cheung A, Wood DA, Leipsic J, Webb JG. Prevalence and impact of preoperative moderate/severe tricuspid regurgitation on patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2015;85:677–684. doi: 10.1002/ccd.25512. [DOI] [PubMed] [Google Scholar]
- 5.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 6.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 7.Leon MB. A Randomized Evaluation of the SAPIEN XT Transcatheter Valve System in Patients with Aortic Stenosis Who Are Not Candidates for Surgery: PARTNER II, Inoperable Cohort. Presented at: ACC.13 (62nd Annual Scientific Sessions of the American College of Cardiology); March 10, 2013; San Francisco, CA. http://www.tctmd.com/show.aspx?id=398&ref_id=118126. [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 12.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 13.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Calafiore AM, Gallina S, Iaco AL, Contini M, Bivona A, Gagliardi M, Bosco P, Di Mauro M. Mitral valve surgery for functional mitral regurgitation: should moderate-or-more tricuspid regurgitation be treated? a propensity score analysis. Ann Thorac Surg. 2009;87:698–703. doi: 10.1016/j.athoracsur.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Di Mauro M, Bivona A, Iaco AL, Contini M, Gagliardi M, Varone E, Gallina S, Calafiore AM. Mitral valve surgery for functional mitral regurgitation: prognostic role of tricuspid regurgitation. Eur J Cardiothorac Surg. 2009;35:635–639. doi: 10.1016/j.ejcts.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 17.Melby SJ, Moon MR, Lindman BR, Bailey MS, Hill LL, Damiano RJ., Jr Impact of pulmonary hypertension on outcomes after aortic valve replacement for aortic valve stenosis. J Thorac Cardiovasc Surg. 2011;141:1424–1430. doi: 10.1016/j.jtcvs.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochelliere R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Dor I, Goldstein SA, Pichard AD, Satler LF, Maluenda G, Li Y, Syed AI, Gonzalez MA, Gaglia MA, Jr, Wakabayashi K, Delhaye C, Belle L, Wang Z, Collins SD, Torguson R, Okubagzi P, Aderotoye A, Xue Z, Suddath WO, Kent KM, Epstein SE, Lindsay J, Waksman R. Clinical profile, prognostic implication, and response to treatment of pulmonary hypertension in patients with severe aortic stenosis. Am J Cardiol. 2011;107:1046–1051. doi: 10.1016/j.amjcard.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Malouf JF, Enriquez-Sarano M, Pellikka PA, Oh JK, Bailey KR, Chandrasekaran K, Mullany CJ, Tajik AJ. Severe pulmonary hypertension in patients with severe aortic valve stenosis: clinical profile and prognostic implications. J Am Coll Cardiol. 2002;40:789–795. doi: 10.1016/s0735-1097(02)02002-8. [DOI] [PubMed] [Google Scholar]
- 21.Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A, Poli A, Antoniucci D, Napodano M, De Carlo M, Fiorina C, Ussia GP. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 22.Lucon A, Oger E, Bedossa M, Boulmier D, Verhoye JP, Eltchaninoff H, Iung B, Leguerrier A, Laskar M, Leprince P, Gilard M, Le Breton H. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: study from the FRANCE 2 registry. Circ Cardiovasc Interv. 2014;7:240–247. doi: 10.1161/CIRCINTERVENTIONS.113.000482. [DOI] [PubMed] [Google Scholar]
- 23.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 24.Haddad F, Denault AY, Couture P, Cartier R, Pellerin M, Levesque S, Lambert J, Tardif JC. Right ventricular myocardial performance index predicts perioperative mortality or circulatory failure in high-risk valvular surgery. J Am Soc Echocardiogr. 2007;20:1065–1072. doi: 10.1016/j.echo.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 26.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 27.Sun JP, James KB, Yang XS, Solankhi N, Shah MS, Arheart KL, Thomas JD, Stewart WJ. Comparison of mortality rates and progression of left ventricular dysfunction in patients with idiopathic dilated cardiomyopathy and dilated versus nondilated right ventricular cavities. Am J Cardiol. 1997;80:1583–1587. doi: 10.1016/s0002-9149(97)00780-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.