Abstract
The endothelium plays a crucial role in maintaining vascular homeostasis by producing several vasodilating factors, including nitric oxide (NO), prostacyclin (PGI2), and endothelium-dependent hyperpolarisation (EDH); however, the balance between endothelial relaxing and contracting factors is disrupted in disease states such as diabetes mellitus and hypertension. Most reported studies of endothelial dysfunction in diabetes focused on the actions of NO; however, there is accumulating evidence demonstrating that in addition to NO, PGI2 and EDH are likely to contribute to the vasodilatation of blood vessels. EDH plays an important role as a regulator of vascular tone and reactivity in resistance and conduit arteries of animal models and humans. PGI2 only plays a minimal role in endothelium-dependent vasodilatation but may serve as an important compensatory mechanism in conditions in which NO and EDH activities are decreased. Further studies are needed to determine the exact roles of EDH and PGI2 in the development of endothelial dysfunction and clinical vasculopathy in humans with type 1 and type 2 diabetes.
Keywords: diabetes mellitus, endothelium-dependent hyperpolarisation, endothelium, potassium channels, prostacyclin
Introduction
Diabetes mellitus (DM) is a growing public health concern with increasing prevalence worldwide. It was estimated that 285 million (6.4%) people had DM in 2010, and it was projected that diabetes would affect 439 million (7.7%) adults by 2030 (1). In Malaysia, 8.3% of the adult population was diagnosed with diabetes in 1996, and this figure had increased to 11.6% in 2006 (2). DM is a major contributor to cardiovascular complications. It is associated with significant mortality and morbidity due to diabetes related micro- and macro-vascular complications.
Endothelial cells play crucial roles in regulating vascular tone by releasing vasodilator substances, including nitric oxide (NO), prostacyclin (PGI2), and endothelium-dependent hyperpolarisation (EDH). Impairment in the synthesis of these substances may lead to endothelial dysfunction (3) and progression of vascular disease. Impaired endothelium-dependent vasodilatation is seen in both conduit and resistance arteries from different animal models and in humans with diabetes (4–6). Most studies of endothelial dysfunction in diabetes have focused on the actions of NO; however, blocking NO synthesis with nitric oxide synthase (NOS) inhibitors does not always prevent endothelium-dependent vasodilatation (4,7–9). Thus, other endothelium-derived relaxing factors (EDRF) also contribute to endothelium-dependent vasodilatations. Accumulating evidence demonstrates that PGI2 and EDH contribute to the vasodilatation of blood vessels.
Endothelium-Dependent Hyperpolarisation (EDH)
Endothelium-dependent vasodilatation in response to various neurohumoural mediators [eg., bradykinin (BK) and acetylcholine (ACh)] and also physical stimuli (eg., shear stress) are attributed to the release of NO and/or PGI2 (10); however, in blood vessels from different species, responses cannot be totally explained by the release of these two mediators only. In the presence of cyclooxygenases (COX) and NOS inhibitors, stimulation of the vascular endothelium is still able to elicit vasodilatation in various vascular preparations. The vasodilatation observed is associated with the hyperpolarisation of smooth muscle that has been attributed to EDH (4).
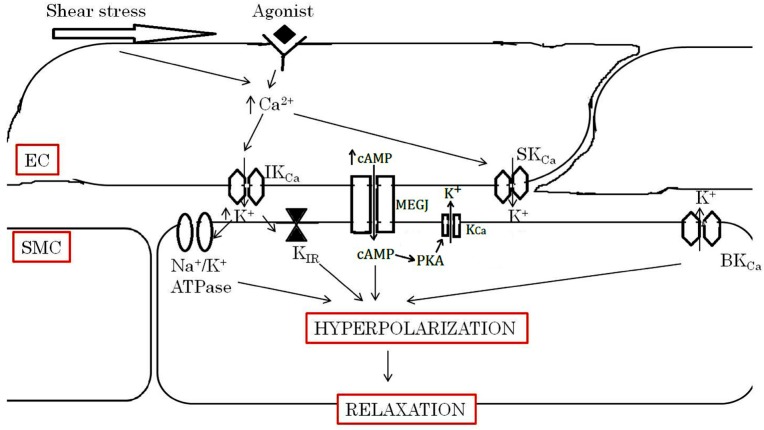
EDH causes the relaxation of the vascular smooth muscle by hyperpolarising its cell membrane and closing the voltage-operated calcium channels, which results in a reduced intracellular free calcium level (11). EDH-type responses are attributed to a number of mechanisms whose contribution varies within and between the vascular beds and species studied. Candidates for EDH include cytochrome P450 metabolites of arachidonic acid, 15-epoxyeicosatrienoic acid (EET) (12), and hydrogen peroxide (H2O2) (13) in coronary arteries, EET in subcutaneous arteries (14), potassium ion in renal arteries (15), EET in internal mammary arteries (16), and H2O2 in mesenteric arteries (17). EDH-type responses in human blood vessels are associated with the activation of various types of potassium channels, including the IKCa and SKCa channels in the endothelium in particular (11). Recent evidence suggests that C-type natriuretic peptide also serves as an EDH mediator via activation of the inwardly rectifier K+ channel and the adenosine triphosphate-dependent K+ channels (KATP) on smooth muscles and subsequent hyperpolarisation (10,18–20). There is also evidence that supports the role of myoendothelial gap junctions (MEGJ) as the route for EDH-mediated responses in vascular smooth muscles (Figure 1) (21).
Figure 1:
Simplified figure for EDH-type responses. The activation of endothelial muscarinic receptors by ACh and shear stress exerted by the flowing blood increase endothelial intracellular calcium concentrations and activate SKCa and IKCa, which are located in endothelial cells. Increased intracellular calcium causes endothelial hyperpolarisation. The hyperpolarisation can be transferred from endothelial cells to the underlying smooth muscle through MEGJ. cAMP formed in EC may diffuse to underlying SMC via MEGJ, leading to hyperpolarisation mainly through PKA-dependent activation K+ channels. cAMP may also facilitate the spread of currents through MEGJ, enabling the hyperpolarisation of regions electrically distant from the endothelium. In addition, the accumulation of potassium ions in the intracellular space activates Na+/K+ ATPase and KIR channels on smooth muscle cells and causes hyperpolarisation, which lead to vasodilatation.
Role of EDH-Mediated Responses in Diabetes
Although there is evidence to suggest that EDH-mediated responses become altered in diseases, such as diabetes and hypertension, and aging, the exact nature of its alteration in diabetes remains to be elucidated. In vascular beds of various animal models and in humans with type 1 and type 2 diabetes, endothelium-dependent vasodilatations have been reported to be reduced, unaltered, or enhanced. The references for the alterations are explained in the following paragraphs.
Animal model: Type 1 diabetes
As endocrine disorders, type 1 and type 2 diabetes represent quite complex diseases in which different bodily systems are involved. The main characteristic of type-1 diabetes is an autoimmune destruction of the pancreatic cells, leading to deficiency in insulin production. Type 1 diabetes model is commonly developed using the chemical ablation of the pancreatic beta cells using streptozotocin (STZ) (22).
Decreased EDH-Mediated Responses
A reduction in the contribution of EDH-mediated responses has been reported in macroand microvasculature of STZ-induced diabetic models (4,6,23). EDH-mediated vasodilatation produced by ACh diminished in retinal arterioles (24), mesenteric (4,6) and carotid arteries of STZ-induced diabetic rats (25), and in mesenteric arteries of diabetic mice (26,27).
An impaired EDH-mediated response may arise from several mechanisms. This includes abnormalities in the adenosine 3’,5’-cyclic monophosphate cyclic (cAMP) signaling pathway and protein kinase (PKA) activity (28,29) and an increased synthesis of lysophosphatidylcholine (LPC)-induced inhibition of EDH activity (26).
In vascular smooth muscles, vasomotor tone is, in part, reliant on gap junction permeability to metabolites and solutes, such as cAMP (30). cAMP formed in endothelial cells may diffuse to underlying smooth muscle via MEGJ, leading to hyperpolarisation mainly through PKA-dependent activation K+ channels. It may also facilitate the spread of the current through gap junctions, enabling the transmission of EDH-mediated responses to regions electrically distant from the endothelium via EC-EC gap junctions (21). It has been reported that the cAMP level was reduced in mesenteric arteries of diabetic rats, resulting from the increase in phosphodiesterase (PDE) activity (6). Inhibition of PDE activity enhances EDH-mediated vasodilatations in mesenteric arteries in both control and diabetic rats (6,29). Hence, it is possible that the impairment of EDH-mediated responses observed in the mesenteric arteries of diabetic rats occurred due to the reduction in cAMP concentration (6). It has also been reported that the impairment of cAMP-mediated vasodilatation seen in the diabetic state may be partly due to the lack of PKA activity (29); however, the vasodilatation of retinal arterioles to an adenylyl cyclase activator, forskolin, was not reduced in STZ-induced diabetic rats (24). Thus, although the decrease in the vascular responses of retinal arterioles seems to be attributed to an impairment in EDH-mediated vasodilatation, diminished cAMP activity is unlikely to play a role in the impairment of vasodilatation in the retinal arterioles of diabetic rats (24).
Impaired EDH-mediated vasodilatation in mesenteric arteries in STZ-induced diabetic mice may be due to the increase in plasma low density lipoprotein cholesterol (LDL-C) and/or lysophosphatidylcholine (LPC)-induced inhibition of EDH production (26). In STZ-induced diabetic animals, the following sequence of events may occur: (a) STZ-induced diabetes augments plasma LDL-C (31) and reduces vascular superoxide dismutase (SOD) content and activity (26); (b) the reduced SOD activity causes an accumulation of superoxide anions; and (c) the accumulated superoxide anions may oxidize LDL-C (32) to form LPC. LPC, a major phospholipid component (40–50%) of oxidised LDL, is transferred to the endothelial surface to inhibit EDH-mediated vasodilatation (33). LPC inhibits EDH-mediated relaxation in diabetes presumably via a decreased release of calcium from the endoplasmic reticulum within the endothelium and/or a decreased influx of calcium concentration into the endothelium (26). Indeed, an increased cytosolic calcium concentration within endothelial cells is a key step in the synthesis and release of NO as well as EDH (10). In endothelial cells, an agonist binds to a cell surface receptor, leading to the hydrolysis of phosphatidylinositol 4,5-bisphosphate to produce inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol. IP3 serves as a mediator of calcium release from the endoplasmic reticulum. It has been demonstrated that LPC activates protein kinase C (PKC), which exerts a negative feedback control on the surface receptor-coupled IP3 formation and the subsequent mobilization of calcium from intracellular stores (34). Another possibility in which LPC may cause an impairment of EDH-mediated responses in mice mesenteric arteries is via generation of superoxide anions (26). Recent evidence suggests that radical scavengers improve the impaired EDH-mediated responses seen in animal model of diabetes (35). The precise role of reactive oxygen species (ROS) on EDH-mediated responses under diabetic conditions remains unclear. The possible mechanisms by which ROS induces the impairment of EDH-mediated responses are alterations in EDH synthesis and/or release, gap junction integrity, and K+ channels modulation (26). It is difficult to identify the exact mechanism from the currently available evidence.
Increased EDH-Mediated Responses
EDH-mediated responses may serve as compensatory mechanisms when NO-mediated vasodilatation is compromised in diseases such as diabetes (36). This context was supported by observations that the contribution of EDH-mediated vasodilatations were increased in the aorta (37,38) and the mesenteric artery (39) of STZ-induced diabetic animals. It has been shown that superoxide production was increased in the aorta in diabetic rats, and this may contribute to the impairment in NO-mediated vasodilatation. Some evidence from experimental animals reported that endothelial NO dampens EDH-mediated responses under physiological conditions and that the latter becomes more apparent when the production of NO is curtailed (40,41). It has been demonstrated that small doses of the NO donor, sodium nitroprusside, inhibited EDH-mediated vasodilatation in dog coronary microcirculation (41). In addition, it has also been suggested that endotoxin shock, characterised by a large amount of NO production, resulted in the inhibition of cytochrome P450 enzyme activity (42).
In summary, studies in animal models of type 1 diabetes showed both decreased and increased EDH-mediated responses. The reason for this discrepancy may be due to differences in the strain of rats used in those studies as well as a variation in methodology between laboratories. For example, decreased EDH-mediated responses were shown in blood vessels of Wistar rats (23,25), while Sprague Dawley rats showed increased EDH-mediated responses (37,38). In addition, the differences may also be due to the duration of diabetes. A longer duration of diabetes (12 weeks or more) (39) showed increased EDH-mediated responses compared with shorter durations of diabetes (10 weeks) (4) (Table 1).
Table 1:
EDH-mediated responses and diabetes
| Species | Model/Duration of diabetes | Blood vessels | EDH responses | Mechanisms |
|---|---|---|---|---|
| Type 1 diabetes | ||||
| SD rat | STZ/10 w | Mesenteric (4) | Decrease | Did not involve a decrease in channel expression, which may be due to a disruption of the downstream pathways of IKCa and SKCa channels |
| Wistar rat | STZ/12 w | Mesenteric (6) | Decrease | Reduction in cAMP |
| Wistar rat | STZ/8 w | Retinal arteriole (24) | Decrease | Not determined |
| Wistar rat | STZ/10 w | Carotid (25) | Decrease | May be due to changes to ATP-sensitive K+ channels on smooth muscles |
| Mice | STZ/10 w | Mesenteric (26, 27) | Decrease | May be due to LPC-induced inhibition of EDH |
| Mice | STZ/17-18 w | Thoracic aorta (37) | Increase | Not determined |
| SD rat | STZ/8 w | Thoracic aorta (38) | Increase | Not determined |
| SD rat | STZ/12 w | Femoral and mesenteric (39) | Increase | Not determined |
| Human | Pregnant women | Subcutaneous (7) | Preserved | - |
| Type 2 diabetes | ||||
| Rat | OLEFT | Mesenteric (43) | Decrease | Defect cAMP/PKA signalling and endothelial K+ channel |
| Zucker Diabetic | Mesenteric (44) | Decrease | - | |
| Fatty rat | Sciatic nerve epineural arterioles (47, 48) | |||
| Goto-Kakizaki rat | Mesenteric (45) | Decrease | May be due to changes to ATP-sensitive K+ channels on smooth muscles | |
| Mice | db/db | mice Coronory (46) | Decrease | - |
| Mice | db/db | mice Mesenteric (49) | Increase | - |
| Rabbit | Alloxan | Renal (50) | Preserved | - |
Animal models: Type 2 diabetes
The type 2 diabetes model is characterised by insulin resistance and the inability of the pancreatic beta cells to compensate. Because type 2 diabetes is linked with obesity, many animal models with type 2 diabetes are obese. These models include the db/db mouse, the Zucker Diabetic Fatty rat, and the Otsuka Long-Evans Tokushima Fat rat (OLEFT); however, not all type-2 diabetes animals are obese. Thus, a few models of type 2 diabetes have been developed in lean animals. An example of this model is the Goto Kakizaki rat, which is characterised by glucose intolerance and defective glucose-induced insulin secretion (22).
Decreased EDH-Mediated Responses
An impaired EDH-mediated vasodilatation of mesenteric vascular beds have been demonstrated in different diabetic models of type-2 diabetes, such as OLEFT rats (43), Zucker Diabetic Fatty rats (44), Goto-Kakizaki rats (45), and coronary arterioles of db/db (diabetic) mice (46). One laboratory reported that ACh-induced EDH-mediated vasodilatation was impaired in the sciatic nerve epineural arterioles from the type-2 diabetic Zucker Diabetic Fatty rats (47,48).
In OLEFT rats, the impairment of EDH-mediated vasodilatation in the mesenteric vascular bed was attributable not only to a defect in the cAMP/PKA signaling activities but also to defects in the activation of the endothelial K+ channels (43). In the small mesenteric arteries from Zucker Diabetic Fatty rats, although no reduction in the gene expression of IKCa and SKCa was observed, SKCa dependent EDH-mediated vasodilatations were impaired (44). Indeed, defective EDH-mediated vasodilatations can be expected to be caused by any significant loss of these hyperpolarizing K+ channels from the endothelium. In mesenteric arteries of Goto-Kakizaki rats, it has been reported that both the EDH-mediated responses and the endothelium-independent vasodilatation to the KATP channel opener levcromakalim were impaired (45). This finding provides evidence that the smooth muscle responsiveness to KATP channel openers is impaired in the mesenteric arteries of type 2 diabetes.
It has been demonstrated that endothelium-mediated vasodilatations were predominantly NO-dependent in coronary arterioles from a wild type mice (46); however, in db/db mice, NO-mediated vasodilatations were reduced, supporting the view that EDH-mediated responses play a pivotal role in maintaining the coronary blood flow when the availability of NO is reduced in diabetic mice. Three mechanisms involving K+, EETs, and/or H2O2 are reported to be involved in the EDH-mediated responses in normal coronary circulation (46). The impairment of H2O2 responses and the abnormalities in K+ channels may possibly be mechanisms in the reduction of EDH-mediated vasodilatations in db/db mice; however EETs is unlikely to play a role. The impaired vasodilatation can be restored by the administration of the neutralizing antibody interleukin-6 (IL-6), indicating that IL-6 plays a potentially pivotal role in EDH-dependent endothelial dysfunction in type 2 diabetes (46).
Increased EDH-Mediated Responses
Increased EDH-mediated responses have been shown in the small mesenteric arteries of db/db mice (49). In this study, BK-induced EDH-mediated vasodilatation in the mesenteric arteries of diabetic mice were regulated through cytochrome P450, which activated the high conductance Ca2+-activated K+ channels (BKCa) and led to hyperpolarisation and vasodilatation; however, ACh-induced vasodilatations involved neither cytochrome P450 product nor H2O2, suggesting that a K+-dependent EDH-mediated response may play a role in ACh-induced endothelium-dependent responses in the small mesenteric arteries of diabetic mice (49).
Preserved EDH-Mediated Responses
In isolated renal arteries of type 2 diabetic rabbits, the contribution of EDH to ACh-induced vasodilatations were not affected under diabetic conditions (50). The EDH component was found to act mainly through BKCa channels under normal and diabetic conditions, while the KATP channels were involved in mediating the ACh vasodilator response only in normal preparations (50).
In summary, the animal models of type 2 diabetes have reported decreased, increased, and also preserved EDH-mediated responses. The reason for this discrepancy may be because of the differences in the species used in the studies and/or variable methodology. For example, decreased EDH-mediated responses were shown in the blood vessels of rat models (43–45), while mice (49) and rabbit (50) models showed increased and preserved EDH-mediated responses, respectively. In addition, the difference may also be due to the age of the animals used. For example, db/db mice aged between 24–30 weeks showed decreased EDH-mediated responses (46), whereas db/db at a younger age (16 weeks) showed increased EDH-mediated responses (49) (Table 1).
EDH-mediated responses in human studies
The existence and importance of EDH have been demonstrated in various human vascular beds. It was reported that EDH-mediated vasodilatations were observed in healthy human coronary arteries (13), subcutaneous arteries (14), renal arteries (15), internal mammary arteries (16), and mesenteric arteries (17). In addition, a few studies have suggested that EDH plays a primary role in agonist-induced vasodilatation in healthy human forearm blood flow (51–54).
Only a few reports on the EDH-mediated response in diabetic patients have been made. In small subcutaneous arteries from pregnant women with well-controlled pre-existing type 1 diabetes, EDH-mediated responses appear to play a dominant role in the vasodilatation. In fact, the vascular function is not altered by the presence of diabetes in pregnancy (7). It has been shown that BK-induced endothelial-dependent vasodilatations in both subcutaneous and mesenteric arteries were mediated entirely by EDH (55). An acute incubation with a high glucose concentration (20 mM) impaired BK-induced vasodilatations of subcutaneous arteries but enhanced vasodilatation in mesenteric arteries, whereas ACh-induced vasodilatation in both blood vessels increased (55). This suggests that a short period of high glucose concentration exerts a variable influence on the endothelial function in human isolated blood vessels that is dependent on the agonist used and the vessel studied.
Prostacyclin
PGI2 is a major prostanoid produced by endothelial cells in vitro. PGI2 stimulates the prostacyclin receptor (IP receptor) in the vascular smooth muscle, producing relaxation under physiological conditions (56); however, the contribution of PGI2 to endothelium-dependent vasodilatation has increased in conditions in which other pathways leading to vasodilatation are inhibited.
Role of Prostacyclin in Diabetes
Previous studies on the role of PGI2 in endothelium-dependent vasodilatations in diabetes have yielded conflicting results. A few studies demonstrated that PGI2 was involved in the preservation of endothelium-dependent vasodilatations in diabetes (37,57,58), while one study showed a contrasting finding (4). Again, this may be related to a difference in the study design. It has been demonstrated that ACh-induced, endothelium-dependent vasodilatations in the renal vascular arteries are partly regulated by PGI2 in STZ-induced diabetic rats but not in control rats (57). PGI2 has also been reported to contribute to increased ACh-induced, endothelium-dependent vasodilatation in the aorta of early stage STZ-induced diabetic mice (37). An impairment of ACh-induced vasodilatations in the aorta in diabetes rats can be restored by oral treatment with the PGI2 analogue, beraprost sodium (58). In the mesenteric vascular bed of STZ-induced diabetic mice, endothelial dysfunction was prevented by a complementary up-regulation of COX-2 expression and activity (59). This suggests that PGI2 signaling may be increased as a way of partially or completely compensating for the decrease in NO- or EDH-mediated responses.
In contrast, endothelium-dependent vasodilatations in the mesenteric arteries of either the control or the STZ-induced diabetic rats were not affected by COX inhibition with indomethacin. This suggests that there was no contribution of PGI2 to ACh-induced, endothelium-dependent vasodilatations in the mesenteric arteries of diabetic rats (4).
The differences between the findings from the studies by Shen et al. (2003) and Leo et al. (2011) may be related to the differences in animal species. For example, the diabetic mice showed a contribution of PGI2 in their endothelium-dependent vasodilatations (37), whereas diabetic rats showed no contribution of PGI2 (4). In addition, the discrepancy may also be due to differences in animal strains. For example, the Wistar rats (57) showed a contribution of PGI2 in endothelium-dependent vasodilatations, while the Sprague Dawley rats showed no contribution of PGI2 (4).
PGI2-mediated relaxation in humans with diabetes has so far been poorly investigated. PGI2 production has been shown to be impaired in the cultured umbilical vein endothelial cells of patients with type 1 diabetes 60). The absence of relaxation in response to PGI2 has been attributed to an early dysfunction of IP receptors in the vascular smooth muscle in the disease state (61). It has been demonstrated that there was no change in the expression of the IP receptor protein in diabetic patients compared with healthy controls subjects; however, that result did not rule out differences in the responsiveness of the receptors to the action of PGI2 at the level of the microcirculation (62). In contrast, in human diabetes, COX-2-derived PGI2 can play a compensatory role for the decreased NO bioavailability in forearm blood flow (63) as well as in coronary arterioles (64).
Conclusions
Firstly, in addition to NO, EDH plays an important role as a regulator of vascular tone and reactivity. The endothelium-dependent vasodilatations observed in the resistance vessels of animal models and humans are largely attributed to EDH. In type 1 and type 2 diabetes, the EDH-mediated responses may be increased, decreased, or preserved. The nature and mechanism of the EDH action can differ between vascular beds, animal species and strains, and disease duration to account for this apparent variability.
Secondly, although PGI2 only plays a minimal role in endothelium-dependent vasodilatations, it can exert important compensatory mechanisms in conditions in which NO and EDH activities are decreased, such as in the diabetic state.
Further investigations are important to determine the exact roles of EDH and PGI2 in the development of endothelial dysfunctions in humans with type 1 and type 2 diabetes. Understanding the pathophysiology of the disordered endothelium-dependent vasodilatations in diabetes can help in determining which therapeutic agents to use in order to restore the endothelial functions and to reduce the vascular complications of diabetes.
Acknowledgments
None.
Footnotes
Conflict of interest
None.
Funds
We thank Malaysian Ministry of Higher Education for providing a scholarship to Siti Safiah M. We also thank USM Postgraduate Research Grant Scheme (1001/PPSP/8145001) and USM Research University Grant (1001/PPSP/812085).
Authors’ Contributions
Conception and design, drafting of the article, critical revision of the article for the important intellectual content, final approval of the article: SSM, AHGR
Administrative, technical or logistic support: AHGR
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. doi: 10.1016/j.diabres.2009.10.007 . [DOI] [PubMed] [Google Scholar]
- 2.Letchuman GR, Wan Nazaimoon WM, Wan Mohamad WB, Chandran LR, Tee GH, Jamaiyah H, et al. Prevalence of diabetes in the Malaysian National Health Morbidity Survey III 2006. Med J Malaysia. 2010;65(3):180–186. [PubMed] [Google Scholar]
- 3.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196(2):193–222. doi: 10.1111/j.1748-1716.2009.01964.x. doi: 10.1111/j.1748-1716.2009.01964.x . [DOI] [PubMed] [Google Scholar]
- 4.Leo CH, Hart JL, Woodman OL. Impairment of both nitric oxide-mediated and EDHF-type relaxation in small mesenteric arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol. 2011;162(2):365–377. doi: 10.1111/j.1476-5381.2010.01023.x. doi: 10.1111/j.1476-5381.2010.01023.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto T, Kakami M, Noguchi E, Kobayashi T, Kamata K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;293(3):H1480–H1490. doi: 10.1152/ajpheart.00229.2007. doi: 10.1152/ajpheart.00229.2007 . [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto T, Kobayashi T, Kamata K. Alterations in EDHF-type relaxation and phosphodiesterase activity in mesenteric arteries from diabetic rats. Am J Physiol Heart Circ Physiol. 2003;285(1):H283–291. doi: 10.1152/ajpheart.00954.2002. doi: 10.1152/ajpheart.00954.2002 . [DOI] [PubMed] [Google Scholar]
- 7.Ang C, Hillier C, Johnston F, Cameron A, Greer I, Lumsden MA. Endothelial function is preserved in pregnant women with well-controlled type 1 diabetes. BJOG. 2002;109(6):699–707. doi: 10.1111/j.1471-0528.2002.01353.x. doi: 10.1111/j.1471-0528.2002.01353.x . [DOI] [PubMed] [Google Scholar]
- 8.Taddei S, Ghiadoni L, Virdis A, Buralli S, Salvetti A. Vasodilation to bradykinin is mediated by an ouabain-sensitive pathway as a compensatory mechanism for impaired nitric oxide availability in essential hypertensive patients. Circulation. 1999;100(13):1400–1405. doi: 10.1161/01.cir.100.13.1400. doi: 10.1161/01.CIR.100.13.1400 . [DOI] [PubMed] [Google Scholar]
- 9.Sainsbury CA, Coleman J, Brady AJ, Connell JM, Hillier C, Petrie JR. Endothelium-dependent relaxation is resistant to inhibition of nitric oxide synthesis, but sensitive to blockade of calciumactivated potassium channels in essential hypertension. J Hum Hypertens. 2007;21(10):808–814. doi: 10.1038/sj.jhh.1002226. doi: 10.1038/sj.jhh.1002226 . [DOI] [PubMed] [Google Scholar]
- 10.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23(8):374–380. doi: 10.1016/s0165-6147(02)02050-3. doi: 10.1016/S0165-6147(02)02050-3 . [DOI] [PubMed] [Google Scholar]
- 11.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26(6):1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. doi: 10.1161/01. ATV. 0000217611. 81085.c5 . [DOI] [PubMed] [Google Scholar]
- 12.Miura H, Liu Y, Gutterman DD. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channels. Circulation. 1999;99(24):3132–3138. doi: 10.1161/01.cir.99.24.3132. doi: 10.1161/01.CIR.99.24.3132 . [DOI] [PubMed] [Google Scholar]
- 13.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flowinduced dilation of human coronary arterioles. Circ Res. 2003;92(2):e31–40. doi: 10.1161/01.res.0000054200.44505.ab. doi: 10.1161/01.RES.0000054200.44505.AB . [DOI] [PubMed] [Google Scholar]
- 14.Coats P, Johnston F, MacDonald J, McMurray JJ, Hillier C. Endothelium-derived hyperpolarizing factor: identification and mechanisms of action in human subcutaneous resistance arteries. Circulation. 2001;103(12):1702–1708. doi: 10.1161/01.cir.103.12.1702. doi: 10.1161/01.CIR.103.12.1792 . [DOI] [PubMed] [Google Scholar]
- 15.Bussemaker E, Popp R, Binder J, Busse R, Fleming I. Characterization of the endothelium-derived hyperpolarizing factor (EDHF) response in the human interlobar artery. Kidney Int. 2003;63(5):1749–1755. doi: 10.1046/j.1523-1755.2003.00910.x. doi: 10.1046/j.1523-1755.2003.00910.x . [DOI] [PubMed] [Google Scholar]
- 16.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, et al. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107(5):769–776. doi: 10.1161/01.cir.0000047278.28407.c2. doi: 10.1161/01.CIR.0000047278.28407.C2 . [DOI] [PubMed] [Google Scholar]
- 17.Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun. 2002;290(3):909–913. doi: 10.1006/bbrc.2001.6278. doi: 10.1006/bbrc.2001.6278 . [DOI] [PubMed] [Google Scholar]
- 18.Ahluwalia A, Hobbs AJ. Endothelium-derived C-type natriuretic peptide: more than just a hyperpolarizing factor. Trends Pharmacol Sci. 2005;26(3):162–167. doi: 10.1016/j.tips.2005.01.005. doi: 10.1016/j.tips.2005.01.005 . [DOI] [PubMed] [Google Scholar]
- 19.Feletou M, Vanhoutte PM. EDHF: new therapeutic targets? Pharmacol Res. 2004;49(6):565–580. doi: 10.1016/j.phrs.2003.10.017. doi: 10.1016/j.phrs.2003.10.017 . [DOI] [PubMed] [Google Scholar]
- 20.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA. 2003;100(3):1426–1431. doi: 10.1073/pnas.0336365100. doi: 10.1073/pnas.0336365100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141(6):881–903. doi: 10.1038/sj.bjp.0705698. doi: 10.1038/sj.bjp.0705698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King AJF. The use of animal models in diabetes research. Br J Pharmacol. 2012;166(3):877–894. doi: 10.1111/j.1476-5381.2012.01911.x. doi: 10.1111/j.1476-5381.2012.01911.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wigg SJ, Tare M, Tonta MA, O'Brien RC, Meredith IT, Parkington HC. Comparison of effects of diabetes mellitus on an EDHF-dependent and an EDH-Findependent artery. Am J Physiol Heart Circ Physiol. 2001;281(1):H232–240. doi: 10.1152/ajpheart.2001.281.1.H232. [DOI] [PubMed] [Google Scholar]
- 24.Nakazawa T, Kaneko Y, Mori A, Saito M, Sakamoto K, Nakahara T, et al. Attenuation of nitric oxide- and prostaglandin-independent vasodilation of retinal arterioles induced by acetylcholine in streptozotocintreated rats. Vascul Pharmacol. 2007;46(3):153–159. doi: 10.1016/j.vph.2006.09.002. doi: 10.1016/j.vph.2006.09.002 . [DOI] [PubMed] [Google Scholar]
- 25.Kamata K, Ohuchi K, Kirisawa H. Altered endotheliumdependent and -independent hyperpolarization and endothelium-dependent relaxation in carotid arteries isolated from streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 2000;361(1):52–59. doi: 10.1007/s002100000248. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Miyamori K, Kobayashi T, Kamata K. Specific impairment of endothelium-derived hyperpolarizing factor-type relaxation in mesenteric arteries from streptozotocin-induced diabetic mice. Vascul Pharmacol. 2006;44(6):450–460. doi: 10.1016/j.vph.2006.02.007. doi: 10.1016/j.vph.2006.02.007 . [DOI] [PubMed] [Google Scholar]
- 27.Morikawa K, Matoba T, Kubota H, Hatanaka M, Fujiki T, Takahashi S, et al. Influence of diabetes mellitus, hypercholesterolemia, and their combination on EDHF-mediated responses in mice. J Cardiovasc Pharmacol. 2005;45(5):485–490. doi: 10.1097/01.fjc.0000159657.93922.cb. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto T, Kobayashi T, Kamata K. Phosphodiesterases in the vascular system. J Smooth Muscle Res. 2003;39(4):67–86. doi: 10.1540/jsmr.39.67. doi: http://dx.doi.org/10.1540/jsmr.39.67 . [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto T, Wakabayashi K, Kobayashi T, Kamata K. Diabetes-related changes in cAMP-dependent protein kinase activity and decrease in relaxation response in rat mesenteric artery. Am J Physiol Heart Circ Physiol. 2004;287(3):H1064–1071. doi: 10.1152/ajpheart.00069.2004. doi: 10.1152/ ajpheart.00069.2004 . [DOI] [PubMed] [Google Scholar]
- 30.Melman A, Rehman J. Pathophysiology of erectile dysfunction. Mol Urol. 1999;3(2):87–102. [PubMed] [Google Scholar]
- 31.Makino A, Ohuchi K, Kamata K. Mechanisms underlying the attenuation of endothelium-dependent vasodilatation in the mesenteric arterial bed of the streptozotocin-induced diabetic rat. Br J Pharmacol. 2000;130(3):549–556. doi: 10.1038/sj.bjp.0703354. doi: 10.1038/sj.bjp.0703354 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi T, Kamata K. Relationship among cholesterol, superoxide anion and endotheliumdependent relaxation in diabetic rats. Eur J Pharmacol. 1999;367(2–3):213–222. doi: 10.1016/s0014-2999(98)00971-6. doi: 10.1016/S0014-2999(98)00971-6 . [DOI] [PubMed] [Google Scholar]
- 33.McIntyre TM, Zimmerman GA, Prescott SM. Biologically Active Oxidized Phospholipids. J Biol Chem. 1999;274(36):25189–25192. doi: 10.1074/jbc.274.36.25189. doi: 10.1074/jbc.274.36.25189 . [DOI] [PubMed] [Google Scholar]
- 34.Kugiyama K, Ohgushi M, Sugiyama S, Murohara T, Fukunaga K, Miyamoto E, Yasue H. Lysophosphatidylchioline inhibits surface receptormediated intracellular signals in endothelial cells by a pathway involving protein kinase C activation. Circ Res. 1992;71(6):1422–1428. doi: 10.1161/01.res.71.6.1422. doi: 10.1161/01.RES.71.6.1422 . [DOI] [PubMed] [Google Scholar]
- 35.Ozkan MH, Uma S. Inhibition of acetylcholineinduced EDHF response by elevated glucose in rat mesenteric artery. Life Sci. 2005;78(1):14–21. doi: 10.1016/j.lfs.2005.02.036. doi: 10.1016/j.lfs.2005.02.036 . [DOI] [PubMed] [Google Scholar]
- 36.Luksha L, Agewall S, Kublickiene K. Endotheliumderived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis. 2009;202(2):330–344. doi: 10.1016/j.atherosclerosis.2008.06.008. doi: 10.1016/j.atherosclerosis.2008.06.008 . [DOI] [PubMed] [Google Scholar]
- 37.Shen B, Ye CL, Ye KH, Liu JJ. Mechanism underlying enhanced endothelium-dependent vasodilatation in thoracic aorta of early stage streptozotocin-induced diabetic mice. Acta Pharmacol Sin. 2003;24(5):422–428. [PubMed] [Google Scholar]
- 38.Malakul W, Thirawarapan S, Suvitayavat W, Woodman OL. Type 1 diabetes and hypercholesterolaemia reveal the contribution of endothelium-derived hyperpolarizing factor to endothelium-dependent relaxation of the rat aorta. Clin Exp Pharmacol Physiol. 2008;35(2):192–200. doi: 10.1111/j.1440-1681.2007.04811.x. doi: 10.1111/j.1440-1681.2007.04811.x . [DOI] [PubMed] [Google Scholar]
- 39.Shi Y, Ku DD, Man RY, Vanhoutte PM. Augmented endothelium-derived hyperpolarizing factormediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. J Pharmacol Exp Ther. 2006;318(1):276–281. doi: 10.1124/jpet.105.099739. doi: 10.1124/jpet.105.099739 . [DOI] [PubMed] [Google Scholar]
- 40.Thollon C, Fournet-Bourguignon MP, Saboureau D, Lesage L, Reure H, Vanhoutte PM, Vilaine JP. Consequences of reduced production of NO on vascular reactivity of porcine coronary arteries after angioplasty: importance of EDHF. Br J Pharmacol. 2002;136(8):1153–1161. doi: 10.1038/sj.bjp.0704828. doi: 10.1038/sj.bjp.0704828 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilatation in vivo. Am J Physiol Heart Circ Physiol. 2000;279(2):H459–465. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- 42.Chamulitrat W, Jordan SJ, Mason RP. Nitric oxide production during endotoxic shock in carbon tetrachloride-treated rats. Mol Pharmacol. 1994;46(2):391–397. [PubMed] [Google Scholar]
- 43.Matsumoto T, Kobayashi T, Kamata K. Mechanisms underlying the impaired EDHF-type relaxation response in mesenteric arteries from Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Eur J Pharmacol. 2006;538(1-3):132–140. doi: 10.1016/j.ejphar.2006.04.006. doi: 10.1016/j.ejphar.2006.04.006 . [DOI] [PubMed] [Google Scholar]
- 44.Burnham MP, Johnson IT, Weston AH. Impaired small-conductance Ca2+-activated K+ channeldependent EDHF responses in Type II diabetic ZDF rats. Br J Pharmacol. 2006;148(4):434–441. doi: 10.1038/sj.bjp.0706748. doi: 10.1038/sj.bjp.0706748 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oniki H, Fujii K, Kansui Y, Goto K, Iida M. Effects of angiotensin II receptor antagonist on impaired endothelium-dependent and endothelium-independent relaxations in type II diabetic rats. J Hypertens. 2006;24(2):331–338. doi: 10.1097/01.hjh.0000200518.34980.cc. doi: 10.3109/10641963.2012.702829 . [DOI] [PubMed] [Google Scholar]
- 46.Park Y, Capobianco S, Gao X, Falck JR, Dellsperger KC, Zhang C. Role of EDHF in type 2 diabetes-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;295(5):H1982–1988. doi: 10.1152/ajpheart.01261.2007. doi: 10.1152/ajpheart.01261.2007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA. Changes in endoneurial blood flow, motor nerve conduction velocity and vascular relaxation of epineurial arterioles of the sciatic nerve in ZDF–obese diabetic rats. Diabetes Metab Res Rev. 2002;18(1):49–56. doi: 10.1002/dmrr.257. doi: 10.1002/dmrr.257 . [DOI] [PubMed] [Google Scholar]
- 48.Coppey LJ, Gellett JS, Yorek MA. Mediation of vascular relaxation in epineurial arterioles of the sciatic nerve: effect of diabetes in type 1 and type 2 diabetic rat models. Endothelium. 2003;10(2):89–94. doi: 10.1080/10623320303366. doi: 10.1080/10623320303366 . [DOI] [PubMed] [Google Scholar]
- 49.Pannirselvam M, Ding H, Anderson TJ, Triggle CR. Pharmacological characteristics of endotheliumderived hyperpolarizing factor-mediated relaxation of small mesenteric arteries from db/db mice. Eur J Pharmacol. 2006;551(1–3):98–107. doi: 10.1016/j.ejphar.2006.08.086. doi: 10.1111/j.1476-5381.2010.01023.x . [DOI] [PubMed] [Google Scholar]
- 50.Yousif MH, Cherian A, Oriowo MA. Endothelium-dependent relaxation in isolated renal arteries of diabetic rabbits. Auton Autacoid Pharmacol. 2002;22(2):73–82. doi: 10.1046/j.1474-8673.2002.00244.x. doi: 10.1046/j.1474-8673.2002.00244.x . [DOI] [PubMed] [Google Scholar]
- 51.Inokuchi K, Hirooka Y, Shimokawa H, Sakai K, Kishi T, Ito K, et al. Role of endothelium-derived hyperpolarizing factor in human forearm circulation. Hypertension. 2003;42(5):919–924. doi: 10.1161/01.HYP.0000097548.92665.16. doi: 10.1161/01.HYP.0000097548.92665.16 . [DOI] [PubMed] [Google Scholar]
- 52.Honing ML, Smits P, Morrison PJ, Rabelink TJ. Bradykinin-induced vasodilation of human forearm resistance vessels is primarily mediated by endothelium-dependent hyperpolarization. Hypertension. 2000;35(6):1314–1318. doi: 10.1161/01.hyp.35.6.1314. doi: 10.1161/01.HYP.35.6.1314 . [DOI] [PubMed] [Google Scholar]
- 53.Katz SD, Krum H. Acetylcholine-mediated vasodilation in the forearm circulation of patients with heart failure: indirect evidence for the role of endothelium-derived hyperpolarizing factor. Am J Cardiol. 2001;87(9):1089–1092. doi: 10.1016/s0002-9149(01)01466-7. doi: 10.1016/S0002-9149 (01)01466-7 . [DOI] [PubMed] [Google Scholar]
- 54.Halcox JP, Narayanan S, Cramer-Joyce L, Mincemoyer R, Quyyumi AA. Characterization of endo-theliumderived hyperpolarizing factor in the human forearm microcirculation. Am J Physiol Heart Circ Physiol. 2001;280(6):H2470–2477. doi: 10.1152/ajpheart.2001.280.6.H2470. [DOI] [PubMed] [Google Scholar]
- 55.MacKenzie A, Cooper EJ, Dowell FJ. Differential effects of glucose on agonist-induced relaxations in human mesenteric and subcutaneous arteries. Br J Pharmacol. 2008;153(3):480–487. doi: 10.1038/sj.bjp.0707592. doi: 10.1038/sj.bjp.0707592 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linton MF, Fazio S. Cyclooxygenase products and atherosclerosis. Drug Discov Today Ther Strateg. 2008;5(1):25–36. doi: 10.1016/j.ddstr.2008.05.006. doi: 10.1016/j.ddstr.2008.05.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamata K, Hosokawa M, Matsumoto T, Kobayashi T. Acetylcholine-induced vasodilation in the perfused kidney of the streptozotocin-induced diabetic rat: role of prostacyclin. J Smooth Muscle Res. 2006;42(5):159–170. doi: 10.1540/jsmr.42.159. doi: http://dx.doi.org/10.1540/jsmr.42.159 . [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto K, Morishita R, Tomita N, Moriguchi A, Yamasaki K, Aoki M, et al. Impaired endothelial dysfunction in diabetes mellitus rats was restored by oral administration of prostaglandin I2 analogue. J Endocrinol. 2002;175(1):217–223. doi: 10.1677/joe.0.1750217. doi: 10.1677/joe.0.1751217 . [DOI] [PubMed] [Google Scholar]
- 59.Nacci C, Tarquinio M, De Benedictis L, Mauro A, Zigrino A, Carratu MR, et al. Endothelial dysfunction in mice with streptozotocin-induced type 1 diabetes is opposed by compensatory overexpression of cyclooxygenase-2 in the vasculature. Endocrinology. 2009;150(2):849–861. doi: 10.1210/en.2008-1069. doi: 10.1210/en.2008-1069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolego C, Buccellati C, Radaelli T, Cetin I, Puglisi L, Folco G, et al. eNOS, COX-2, and prostacyclin production are impaired in endothelial cells from diabetics. Biochem Biophys Res Commun. 2006;339(1):188–190. doi: 10.1016/j.bbrc.2005.11.017. doi: 10.1016/j.bbrc.2005.11.017 . [DOI] [PubMed] [Google Scholar]
- 61.Feletou M, Huang Y, Vanhoutte PM. Endothelium–mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol. 2011;164(3):894–912. doi: 10.1111/j.1476-5381.2011.01276.x. doi: 10.1111/j.1476-5381.2011.01276.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mokhtar SS, Vanhoutte PM, Leung SW, Yusof MI, Sulaiman WA, Saad AZ, et al. Reduced expression of prostacyclin synthase and nitric oxide synthase in subcutaneous arteries of type 2 diabetic patients. Tohoku J Exp Med. 2013;231(3):217–222. doi: 10.1620/tjem.231.217. doi: http://dx.doi.org/10.1620/tjem.231.217 . [DOI] [PubMed] [Google Scholar]
- 63.Meeking DR, Browne DL, Allard S, Munday J, Chowienczyck PJ, Shaw KM, et al. Effects of cyclo-oxygenase inhibition on vasodilatory response to acetylcholine in patients with type 1 diabetes and nondiabetic subjects. Diabetes Care. 2000;23(12):1840–1843. doi: 10.2337/diacare.23.12.1840. doi: 10.2337/diacare.23.12.1840 . [DOI] [PubMed] [Google Scholar]
- 64.Szerafin T, Erdei N, Fulop T, Pasztor ET, Edes I, Koller A, et al. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ Res. 2006;99(5):e12–17. doi: 10.1161/01.RES.0000241051.83067.62. doi: 10.1161/01.RES.0000241051.83067.62 . [DOI] [PubMed] [Google Scholar]