Abstract
Background: The dorsal raphe nucleus (DRN) influences a wide range of behavioral and reward function. In this study, we evaluated electrical stimulation and inactivation of DRN on morphine conditioned place preference (CPP).
Methods: The rats were anesthetised (n = 7 for each group) and the electrode and cannula were implanted into the DRN by stereotaxic instrument. Electrical stimulation (100μA) and reversible inactivation by lidocaine were induced into DRN and then morphine-induced CPP was investigated.
Results: The stimulation of DRN in combination with effective dose of morphine showed a significant decrease only on expression phases 20s (SD 33.7) when compared with morphine group 119.85s (SD 23.7) (One way ANOVA, Tukey’s; P = 0.036). Also, this stimulation in combination with ineffective dose of morphine showed a significant increase only on acquisition phases 67.5s (SD 41.2) of CPP compared with morphine group -46s (SD 18.51) (P = 0.034). Also, there were not significant differences in inactivation of DRN by lidocaine on different phase of CPP (P = 0.091)
Conclusion: It is possible that electrical stimulation of the DRN with changes in concentration of serotonin or involving other transmitters such as glutamate and gamma amino butyric acid (GABA) would be involved to these changes of CPP.
Keywords: conditioned place, electrical stimulation, morphine, dorsal raphe nucleus, Lidocaine, rat
Introduction
It has been shown that dorsal raphe nucleus (DRN) has an important role to control and modulate many behaviors (1). Reported that serotonin releases from this nucleus which is related to reward behaviors (1–3), as well as other functions, for example the rhythm of sleep–wake (4,5), appetite (6), locomotion (7), emotion and social behavior (8,9) and learning and memory (10). Nevertheless, the mechanism of the serotonin system in the cognitive and motivational behavior has not been cleared yet. Electrophysiological investigations of the raphe nuclei have been concentrated mostly on motor behavior and rhythm of sleep–wake (10). Moreover, some evidence report that decrease in serotonergic neurotransmission involves independency and opioid tolerance (11). Opioids are produced endogenously and their receptors are recognized in the periaqueductal gray matter and the DRN (12). Opioids enhance extracellular serotonin in some area in the brain which are innervated by the DRN (13). According to previous report acute morphine administration increased serotonin turnover in the mammalian brain, but the increase in turnover was attenuated after chronic morphine administration (11). In addition, it has been shown that the 5-hydroxytryptamine (5-HT) is involved in the mechanisms, related to the withdrawal syndrome behavior associated with naloxone induced withdrawal in rat (14). Some evidence report that a selective lesion of 5-HT neurotransmissions in the dorsal raphe nucleus does not modify affective behavior but instead, 5-HT seem to control the activities associated to the creation of object memory (15).
Other studies showed that the lesion of the DRN has no effect on passive avoidance retention (16). There is no document to show the role of the DRN in different phase of conditioned place preference (CPP). The CPP has developed as a routine experimental protocol to measure the rewarding consequences of drug abuse (17,18). Various rewards, as sweetened solutions, drugs abused and eating pleasant food-stuff by humans, electrical brain stimulation, have been demonstrated to induce CPP (19). Using of opioid in other animals such as rabbits and monkeys should be induced CPP (18,20,21). Administration of opiates, similar other drug abuse, will induce inclinations for conditioning of separate environments (22). Previous studies are indicated that pharmacological inhibition and electrolytic lesions of the DRN prevent stressor potentiation of morphine CPP in rats (23). In addition, withdrawal of syndrome signs were decreased by application of electrical stimulation in DRN, in comparison with morphine groups (14). The goal of this study was to investigate the effect of electrical stimulation (100 μA) and reversible inactivation by lidocaine on DRN in combination with effective and non-effective doses of morphine (2.5 and 0.5 mg/kg) respectively on different phase of morphine-induced CPP were investigated.
Materials and Methods
Animals
Wistar rats with male gender (Isfahan University, Isfahan, Iran) weighing 200–250 g, were used in this study. Rats were maintained in animal house at 12 h light – 12 h dark normal cycle with water and food available at all times. The laboratory temperature was maintained at 22–25 °C. For at least 10 days prior to surgery, all rats were allowed to adapt to the laboratory environment. In each group of experiments seven rats were used.
Surgery
All rats were anesthetised with chloral hydrate injected intraperitoneally (400 mg/kg) and after shaving their heads were located in a stereotaxic instrument, then were implanted a cannula (22 G) or stimulating electrode into the DRN. Coordinates of the point is (AP) –7.92 mm; (ML) 0.2 mm; (DV) 6.4 mm relative to bregmae (24). Finally, the cannula and stimulating electrode were anchored to the skull by dental cement. In order to protect from infection, Penicillin (0.2 ml i.p) was administered immediately after the surgery. Subsequent surgery, each rat was alone kept in animal house for 72 h.
Micro injection method
Initially, the rats were kept in hand and the injection needle (30 G) which was related to the Hamilton syringe through a short polyethylene tube in the cannula was placed. Then 0.5 μL of 2% lidocaine hydrochloride (Bayer) was injected for 60 sec (25).
Drugs
During the experiment, morphine sulfate daily by dissolving in saline 0.9% are prepared for injection (subcutaneously).
Apparatus
The CPP apparatus consisted of three chambers (A, B, and C). Two chambers (A and B) are the same size but in different colors. The walls and floor of the A chamber is a black and white while the walls and floor of the B chamber is white. The C chamber was smaller and by Guillotine door is connected to A and B chambers.
Behavioral procedure
The Behavioral procedure of CPP is done on five continuous days and has three distinct phases (22–26,27).
Pre-test
In the pre-test (day 1) each rat is placed in C chamber while the middle door was open and the rat is allowed to move freely for 15 minutes in all chambers. The time spent in each chamber (A and B) was recorded by the apparatus.
Conditioning
This phase consisted of 3 days (from day 2 to day 4). This stage consisted of six sessions (3 saline and 3 morphine) and each session lasts 45 minutes. Guillotine door is closed and daily injection is performed in two stages with a 6 h interval. In this case, in the morning of the second day (8 am) after subcutaneous injection of morphine, rats were confined to one chamber of the apparatus for 45 minutes. In the evening with an interval of 6 h (2 pm) after injection of saline instead of morphine rats were confined to in other side of the apparatus for 45 minutes. On the day 3, morphine, and saline injections were contrary to the day 2. On the day 4, morphine and saline injection were same as the day 2 (22–26).
Test
This phase includes the day 5. At this phase Guillotine door is open as the first day and the rats can freely move in all chambers for 15 minutes and the spent of time in the chamber of rats received morphine are recorded. The several of preference were computed as the difference (in second) between the times spent in morphine receiving chamber on the test day and the first day.
Experimental design
One week after surgery, rats were randomly assigned to 15 groups (n = 7 in each) as follows:
1. Group 1: Saline
2. Group 2: Saline+ stimulation (100 μA; Acquisition
3. Group 3: Saline+ stimulation (100 μA; Expression)
4. Group 4: Saline+ Lidocaine (0.5 μL; Acquisition)
5. Group 5: Saline+ Lidocaine (0.5 μL; Expression)
6. Group 6: Morphine (0.5 mg/kg, Sc)
7. Group 7: Morphine (2.5 mg/kg, Sc)
8. Group 8: Morphine+ Stimulation (0.5 mg/kg+ Sc+ 100 μA; Acquisition)
9. Group 9: Morphine+ Stimulation (0.5 mg/kg+ Sc+ 100 μA; Expression)
10. Group 10: Morphine+ Stimulation (2.5 mg/kg+ Sc+ 100 μA; Acquisition)
11. Group 11: Morphine+ Stimulation (2.5 mg/kg+ Sc+ 100 μA; Expression)
12. Group 12: Morphine+ Lidocaine (0.5 mg/kg+ Sc+ 0.5 μL; Acquisition)
13. Group 13: Morphine+ Lidocaine (0.5 mg/kg+ Sc+ 0.5 μL; Expression)
14. Group 14: Morphine+ Lidocaine (2.5 mg/kg+ Sc+ 0.5 μL; Acquisition)
15. Group 15: Morphine+ Lidocaine (2.5 mg/kg+ Sc+ 0.5 μL; Expression)
Determine effect and non-effect dose of morphine
In order to determine effective and non-effective dose of morphine, different doses (0.5, 2.5, 5, 7.5, and 10 mg/kg) were used. Saline and morphine were injected (Sc) during 3 days of conditioning phase and the spent of time in the morphine chamber on day 5 minus that the spent of time in this chamber in the day 1 was computed to assess induced of CPP.
The method of electrical stimulation
To electrical stimulation, the current intensity (100 μA) with a constant frequency of (25 Hz) was used (22). Each animal was stimulated for 10 minutes (Stimulator Isolator A36O, WPI, USA) and morphine (effective and ineffective doses) was administered after 15 minutes (22). Electrical stimulation for the acquisition group during the conditioning stage and for expression group during the test stage was applied.
Histology
At the end of experiments, the rats were sacrificed and with 0.9% saline followed by 10% formalin were perfused and their brains were removed carefully then before slices were placed in 10% formalin for 72 hours. In order to evaluate the place of the stimulating electrode and cannula in the DRN, sections were examined (Figure 1) (24).
Figure 1:

Location of electrode (a) and cannula (b) in the DRN of rats used in the stimulation studies and reversible inactivation after using lidocaine. The location of stimulating electrodes and cannula in the DRN are shown by arrows.
Statistical analysis
All results are indicated as mean (SD). In order to analyse data, one - way ANOVA following Tukey post-test was used. Calculations were performed using the SPSS statistical software 21.
Results
The results showed that there was a significant (One way ANOVA, Tukey’s: P = 0.006) enhancement only in dose of (2.5 mg/kg) of morphine 178.4 s (SD 18.4) comparative with control group 57.1 s (SD 8.8). Therefore, in this study 0.5 mg/kg of morphine as ineffective dose and 2.5 mg/kg of morphine as effective dose was used. The results demonstrated that morphine response on CPP was not dose dependent (Figure 2). The stimulation of the DRN (current intensity; 100μA) with effective dose of morphine did not show significant differences on acquisition phase (P = 0.250) whereas there was significant decrease 20 s (SD 33.7) on expression phase compared to morphine group 119.85 s (SD 23.7) (P = 0.036) on CPP paradigm (Figure 2 and 4). Also this current intensity (100 μA) with ineffective dose of morphine showed a significant increase on acquisition phase 67.5 s (SD 41.2) of CPP compared to morphine group –46 s (SD 18.51) (P = 0.034) but did not show significant differences on expression phase (P = 0.280) compared to morphine group on CPP paradigm (Figure 3 and 4). In addition results indicated that there was not significant differences (P = 0.091) in reversible inactivation of the DRN after using lidocaine on different phases of CPP by effect and non-effect dose of morphine compared to morphine group on CPP paradigm but there was significant differences on acquisition phase in sal + lidocaine group 54.6 s (SD 35) compared to morphine group –46 s (SD 18.51) on CPP (*P = 0.046) and Comparative with the mor+lidocaine group –31.3 s (SD 3.02) (#P = 0.035) (Figure 5 and 6).
Figure 2:
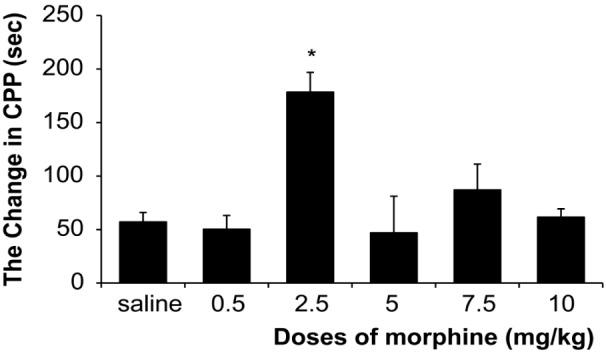
The effects of various doses of morphine administration on CPP for determining the effect and non-effect doses of morphine, (One way ANOVA, Tukey’s: *P = 0.034) comparative with the saline group.
Figure 3:
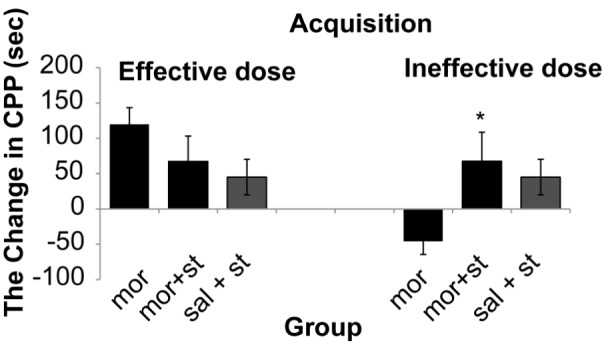
Electrical stimulation with current intensity (100 μA) of dorsal raphe nucleus with effect and non-effect doses of morphine on acquisition of conditioned place preference, (One way ANOVA, Tukey’s: *P = 0.041) comparative with the morphine group. Abbreviation: mor = morphine; st = stimulation; sal = saline.
Figure 4:
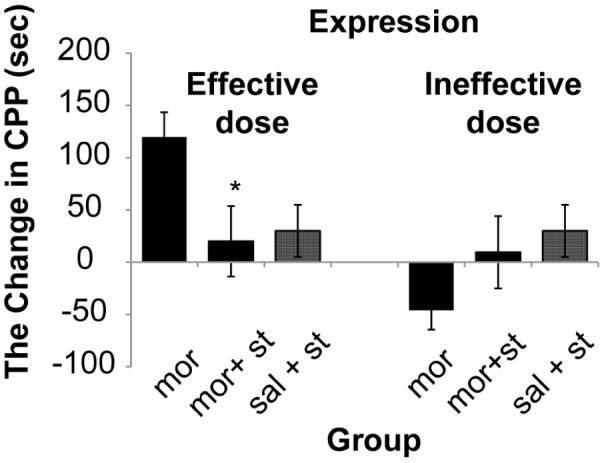
Electrical stimulation with current intensity (100 μA) of dorsal raphe nucleus with effect and non-effect doses of morphine on expression of conditioned place preference, (One way ANOVA, Tukey’s: *P = 0.036) Comparative with the morphine group. Abbreviation: mor = morphine; st = stimulation; sal = saline.
Figure 5:
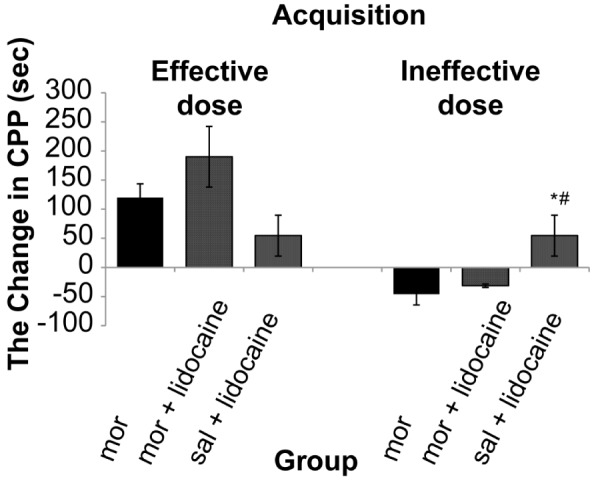
Reversible inactivation of DRN by lidocaine with effect and non-effect doses of morphine on acquisition of conditioned place preference, (One way ANOVA, Tukey’s: *P = 0.032) comparative with the morphine group and (One way ANOVA, Tukey’s: #P = 0.043) comparative with the mor+lidocaine group. Abbreviation: mor = morphine; sal = saline.
Figure 6:
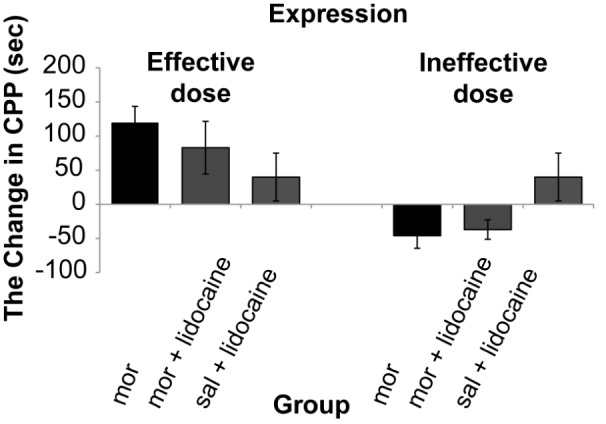
Reversible inactivation of DRN by lidocaine with effect and non-effect doses of morphine on expression of conditioned place preference, (One way ANOVA, Tukey’s: P = 0.250). Abbreviation: mor = morphine; sal = saline.
Our data also indicated that electrical stimulation and reversible inactivation of DRN did not have a significant effect (P = 0.250) on the locomotors activities (Figure 7 and 8).
Figure 7:
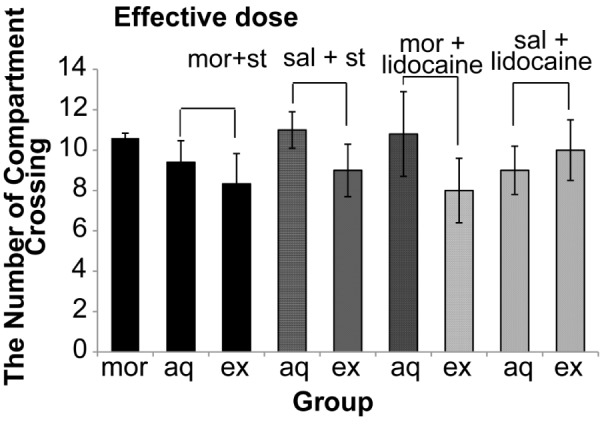
The effect of electrical activation and reversible inactivation of DRN with effective doses of morphine on motor activity in CPP (One way ANOVA, Tukey’s: P = 0.074). Abbreviation: mor = morphine; st = stimulation; sal = saline; aq = acquisition; ex = expression.
Figure 8:
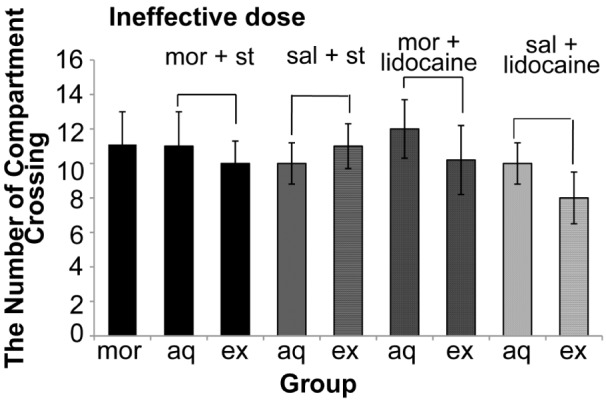
The effect of electrical activation and reversible inactivation of DRN with non-effective doses of morphine on motor activity CPP (One way ANOVA, Tukey’s: P = 0.092). Abbreviation: mor = morphine; st = stimulation; sal = saline; aq = acquisition; ex = expression.
Discussion
Morphine is one of the most frequently used pain-relieving drugs to acute pains, but the euphoria effect of this opioid produce a difficulty in therapeutic strategies as drug abuse (21,28). On the other hand, CPP has become one of the acceptable animal models to evaluate the rewarding properties of drug abuse and other neurotransmitters (17,18). Our results showed that there was a significant enhancement only in dose of (2.5 mg/kg) comparative with control group. There were significant differences on expression phases compared to morphine group on CPP paradigm. Also, this current intensity (100 μA) with ineffective dose of morphine showed different responses on acquisition phases of CPP.
Several researches have showed the mechanism of neurobiology on rewarding properties of opiate using the Conditioned Place Preference model, somewhat fewer investigation has been done to examine the effects of the DRN stimulation or lesion on morphine-induced CPP. Drug addiction is also known to be associated with dysfunction of motivational systems and memory in rats (29). Some of the investigators indicated the effect of chemical stimulation or electrical on different sites of the central nervous system and its influence on animal’s behaviors (30,31). Data showed the administration of opiate induced CPP (18,20,21). In addition, morphine-induced CPP was not dose dependent (Figure 2). Several studies demonstrated that administration of opiates raises the desire for opioid in drug-free addicts and may restore drug seeking actions after long periods of extinction in opiate-experienced animals (29,32). Consistent with these behavioral data, other studies demonstrated morphine induced pleasure, which is associated to the location where in these actions happened (27,33). Our results indicated that the stimulation of the DRN with high current intensity (100 μA) in grouping with non-effect dose of morphine can induce acquisition phase of CPP by morphine (Figure 2 and 4), while high current intensity the DRN stimulation (100 μA) in grouping with affect dose of morphine could destroy CPP induced by morphine (Figure 3). It demonstrated that there were not significant differences in the DRN reversible inactivation after injection of lidocaine in different phase of CPP by effect of morphine on CPP paradigm (Figure 2, 3, and 4). Since, our data indicated that electrical activation of the DRN with intensity 100 μA reinforces ineffective induced CPP by morphine. This consequence may be because of an enhancement in the affect signal or adequate reaction to the rewarding stimuli, which memory forms and reinforces learning in the conditioning procedure. It is notable that the DRN projects to areas involved in facilitating drug reward such as the medial prefrontal cortex and the shell of the nucleus accumbens (23). In addition, it has been reported that, increase in 5-HT above normal levels within the medial prefrontal cortex and nucleus accumbens increase dopamine efflux in these areas (34). On the other hand, it is reported that the serotonin neuronal part of dorsal raphe nucleus could control the ventral tegmental area (VTA) a dopamine (DA) projection to the NAc (35). Electrical stimulation also leads to an increase in dopamine neurotransmission (36). So it is possible that the DRN electrical stimulation resulted in elevation levels of serotonin in the nucleus accumbens, followed the release of dopamine, is increased and will lead to increase memory formation and reinforce learning. Electrical activation of the DRN with high intensities may increase morphine-induced CPP due to increasing the craving for opiates in drug addicts. Conversely our data revealed that electrical stimulation of the DRN with high intensity blocks effective induced CPP by morphine in expression phase. It may be due to a decrease in the insufficient response to the rewarding stimulator reward signal, which damage memory formation and learning in the conditioning procedure. Therefore learning insufficiency, that damages conditioning procedure, might be destroy induced CPP by morphine (31). Parallel to these findings, it was proposed that chronic high-frequency stimulation suppress morphine reinforcement (37). Furthermore, some of the researches obtained different results after special effects of electrical activation on CPP (27,37). In harmony with these data, other investigates indicated that peripheral electrical activation suppressed both the reinstatement of extinguished CPP and the expression of CPP induced by morphine (32).
Conclusions
In summary according to the role of the DRN in learning and memory it is possible that electrical stimulation of this nucleus leads to reduction in the reward signal or inadequate response to the rewarding stimuli, which impair learning and memory formation in the conditioning process, is responsible for these changes in CPP.
Acknowledgments
The authors would like to thank Dr Ali Nasimi and Dr Maryam Radahmadi for their valuable assistance. Conduction of the present research was made possible through the supports received from Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Conflict of interest
None.
Funds
I confirm that I have mentioned all organisations that funded my research in the acknowledgements section of my submission where appropriate.
Authors’ Contributions
Conception and design, critical revision of the article for the important intellectual content: AAP, HA
Drafting of the article: GRG, AAP, HA
Administrative, technical or logistic support: GRG, SK, MAN
Collection and assembly of data: GRG
References
- 1.Nakamura K. The role of the dorsal raphé nucleus in reward-seeking behavior. Front Integr Neurosci. 2013;7:760. doi: 10.3389/fnint.2013.00060. doi: 10.3389/fnint.2013.00060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doya K. Metalearning and neuromodulation. Neural Netw. 2002;15(4–6):495–506. doi: 10.1016/s0893-6080(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 3.Schweighofer N, Tanaka SC, Doya K. Serotonin and the evaluation of future rewards: theory, experiments, and possible neural mechanisms. Ann N Y Acad Sci. 2007;1104:289–300. doi: 10.1196/annals.1390.011. [DOI] [PubMed] [Google Scholar]
- 4.Dugovic C. Role of serotonin in sleep mechanisms. Rev Neurol (Paris) 2001;157(11 Pt 2):S16–19. doi: 10.1111/j.1749-6632.1990.tb16907.x . [PubMed] [Google Scholar]
- 5.Guzman-Marin R, Alam MN, Szymusiak R, Drucker-Colin R, Gong H, McGinty D. Discharge modulation of rat dorsal raphe neurons during sleep and waking: effects of preoptic/basal forebrain warming. Brain Res. 2000;875(1–2):23–34. doi: 10.1016/s0006-8993(00)02561-0. doi: 10.1016/S0006-8993(00)02561-0 . [DOI] [PubMed] [Google Scholar]
- 6.Curzon G. Serotonin and appetite. Ann N Y Acad Sci. 1990;600:521–530; discussion 530-531. doi: 10.1111/j.1749-6632.1990.tb16907.x. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci. 1993;16(9):346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- 8.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289(5479):591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 9.Graeff FG. Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev. 2004;28(3):239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28(20):5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao R, Ma Z, Auerbach SB. Alteration in regulation of serotonin release in rat dorsal raphe nucleus after prolonged exposure to morphine. J Pharmacol Exp Ther. 1998;286(1):481–488. [PubMed] [Google Scholar]
- 12.Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol. 1999;405(4):450–471. doi: 10.1002/(SICI)1096-9861(19990322)405:4<450::AID-CNE2>3.0.CO;2-# . [PubMed] [Google Scholar]
- 13.Tao R, Auerbach S. Involvement of the dorsal raphe but not median raphe nucleus in morphine-induced increases in serotonin release in the rat forebrain. Neuroscience. 1995;68(2):553–561. doi: 10.1016/0306-4522(95)00154-b. doi: 10.1016/0306-4522(95)00154-B . [DOI] [PubMed] [Google Scholar]
- 14.Alaei H, Pourshanazari AA, Rafati A. Electrical stimulation of nucleus raphe dorsalis changes morphine self-administration and withdrawal symptoms in rats. Pathophysiology. 2002;9(1):1–5. doi: 10.1016/s0928-4680(02)00050-0. doi: 10.1016/S0928-4680(02)00050-0 . [DOI] [PubMed] [Google Scholar]
- 15.Lieben CK, Steinbusch HW, Blokland A. 5, 7-DHT lesion of the dorsal raphe nuclei impairs object recognition but not affective behavior and corticosterone response to stressor in the rat. Behav Brain Res. 2006;168(2):197–207. doi: 10.1016/j.bbr.2005.11.003. doi: 10.1016/j.bbr.2005.11.003 . [DOI] [PubMed] [Google Scholar]
- 16.Sarihi A, Motamedi F, Naghdi N, Rashidy-Pour A. Lidocaine reversible inactivation of the median raphe nucleus has no effect on reference memory but enhances working memory versions of the Morris water maze task. Behav Brain Res. 2000;114(1–2):1–9. doi: 10.1016/s0166-4328(00)00176-5. doi: 10.1016/S0166-4328(00)00176-5 . [DOI] [PubMed] [Google Scholar]
- 17.Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci. 1997;111(6):1324–1334. doi: 10.1037//0735-7044.111.6.1324. doi: 10.1037/0735-7044.111.6.1324 . [DOI] [PubMed] [Google Scholar]
- 18.Rezayof A, Nazari-Serenjeh F, Zarrindast MR, Sepehri H, Delphi L. Morphine-induced place preference: involvement of cholinergic receptors of the ventral tegmental area. Eur J Pharmacol. 2007;562(1–2):92–102. doi: 10.1016/j.ejphar.2007.01.081. doi: 10.1016/j.ejphar.2007.01.081 . [DOI] [PubMed] [Google Scholar]
- 19.Liang J, Ping XJ, Li YJ, Ma YY, Wu LZ, Han JS, et al. Morphine-induced conditioned place preference in rats is inhibited by electroacupuncture at 2 Hz: role of enkephalin in the nucleus accumbens. Neuropharmacology. 2010;58(1):233–240. doi: 10.1016/j.neuropharm.2009.07.007. doi: 10.1016/j.neuropharm.2009.07.007 . [DOI] [PubMed] [Google Scholar]
- 20.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. doi: 10.1016/j.neuropharm.2008.07.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. doi: 10.1038/npp.2009.110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kargari A, Ramshini E, Alaei H, Sedighi M, Oryan S. Different current intensities electrical stimulation of prelimbic cortex of mPFC produces different effects on morphine-induced conditioned place preference in rats. Behav Brain Res. 2012;231(1):187–192. doi: 10.1016/j.bbr.2012.03.016. doi: 10.1016/j.bbr.2012.03.016 . [DOI] [PubMed] [Google Scholar]
- 23.Will MJ, Der-Avakian A, Bland ST, Grahn RE, Hammack SE, Sparks PD, et al. Electrolytic lesions and pharmacological inhibition of the dorsal raphe nucleus prevent stressor potentiation of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2004;171(2):191–198. doi: 10.1007/s00213-003-1572-1. doi: 0.1007/s00213-003-1572-1 . [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. Access Online via Elsevier; 2006. The rat brain in stereotaxic coordinates: hard cover edition. [Google Scholar]
- 25.Sarihi A, Yazdi M, Heshmatian B, Salehi I, Behzadi G, Naghdi N, et al. The Effects of Lidocaine Reversible Inactivation of the Dorsal Raphe Nucleus on Passive Avoidance Learning in Rats. Basic Clinical Neuroscience. 2011;2(4):27–35. [Google Scholar]
- 26.Hao Y, Yang J, Sun J, Qi J, Dong Y, Wu CF. Lesions of the medial prefrontal cortex prevent the acquisition but not reinstatement of morphine-induced conditioned place preference in mice. Neurosci Lett. 2008;433(1):48–53. doi: 10.1016/j.neulet.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 27.You ZB, Tzschentke TM, Brodin E, Wise RA. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an in vivo microdialysis study in freely moving rats. J Neurosci. 1998;18(16):6492–6500. doi: 10.1523/JNEUROSCI.18-16-06492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. European Neuropsychopharmacology. 2007;17(6):377–393. doi: 10.1016/j.euroneuro.2006.10.006. doi: http://dx.doi.org/10.1016/j.euroneuro.2006.10.006 . [DOI] [PubMed] [Google Scholar]
- 29.Niu H, Zheng Y, Huma T, Rizak JD, Li L, Wang G, et al. Lesion of olfactory epithelium attenuates expression of morphine-induced behavioral sensitization and reinstatement of drug-primed conditioned place preference in mice. Pharmacol Biochemis Behav. 2013;103(3):526–534. doi: 10.1016/j.pbb.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Hao Y, Yang JY, Guo M, Wu CF, Wu MF. Morphine decreases extracellular levels of glutamate in the anterior cingulate cortex: an in vivo microdialysis study in freely moving rats. Brain research. 2005;1040(1–2):191–196. doi: 10.1016/j.brainres.2005.01.072. doi: 10.1016/j.brainres.2005.01.072 . [DOI] [PubMed] [Google Scholar]
- 31.Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci. 1995;15(5):3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi XD, Wang GB, Ma Yy, Ren W, Luo F, Cui CL, et al. Repeated peripheral electrical stimulations suppress both morphine-induced CPP and reinstatement of extinguished CPP in rats: accelerated expression of PPE and PPD mRNA in NAc implicated. Brain Res Mol Brain Res. 2004;130(1–2):124–133. doi: 10.1016/j.molbrainres.2004.07.016. doi: 10.1016/j.molbrainres.2004.07.016 . [DOI] [PubMed] [Google Scholar]
- 33.Tzschentke T. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol. 2001;63(3):241–320. doi: 10.1016/s0301-0082(00)00033-2. doi: 10.1016/S0301-0082(00)00033-2 . [DOI] [PubMed] [Google Scholar]
- 34.Zangen A, Nakash R, Overstreet DH, Yadid G. Association between depressive behavior and absence of serotonin–dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl) 2001;155(4):434–439. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimoto K, McBride WJ. Regulation of nucleus accumbens dopamine release by the dorsal raphe nucleus in the rat. Neurochem Res. 1992;17(5):401–407. doi: 10.1007/BF00969884. [DOI] [PubMed] [Google Scholar]
- 36.Tao R, Auerbach SB. GABAergic and glutamatergic afferents in the dorsal raphe nucleus mediate morphine-induced increases in serotonin efflux in the rat central nervous system. J Pharmacology Exp Therapeutics. 2002;303(2):704–710. doi: 10.1124/jpet.102.038133. [DOI] [PubMed] [Google Scholar]
- 37.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21(3):467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]


