Abstract
The goal of PCORnet is to create a community of research that includes patients, clinicians, and health care delivery systems to improve the nation's ability to conduct comparative-effectiveness research.
In 2013, the Patient Centered Outcomes Research Institute (PCORI) committed $93.5 million to develop a new large national network infrastructure for community-based observational and interventional studies that can be generalized to many populations. Designated PCORnet, the network was created for three primary reasons.1 First, physicians, patients, and health care systems need more and better evidence about the outcomes of different treatment options. Second, although the United States spent approximately 17.2% of its gross national product on health care in the year 2012, totaling $8,915 per person, there remain substantial disparities in health care between populations within the United States and between the United States and other countries, all of which spend less per citizen2,3 on health care. Third, current research infrastructures and methods have generated remarkable advances but also have substantial limitations, including: relatively long time periods between the initiation of project conception and completion, with further substantial delay in the implementation of results; high costs, often because new infrastructures for patient identification, recruitment, and follow-up are created de novo and then dismantled at the conclusion of each project; a paucity of patient-centered research, resulting in studies that do not address questions and outcomes of interest to patients; and a lack of generalizability, given research populations and settings often differ from those in the communities where most people live and receive their care.
The primary goal of PCORnet is to create a community of research that includes patients, clinicians and health care delivery systems to improve the nation's ability to conduct comparative-effectiveness research. The initiative will develop a large network representative of the underlying population, which: integrates data systems and patients; actively engages patients in the research process; is efficient, rapid, and timely in its conduct of observational studies; and is capable of efficiently completing large clinical trials. The network will perform these tasks within a wide range of health care systems that can automate identification, recruitment, and follow-up of patients and rapidly implement findings in practice.
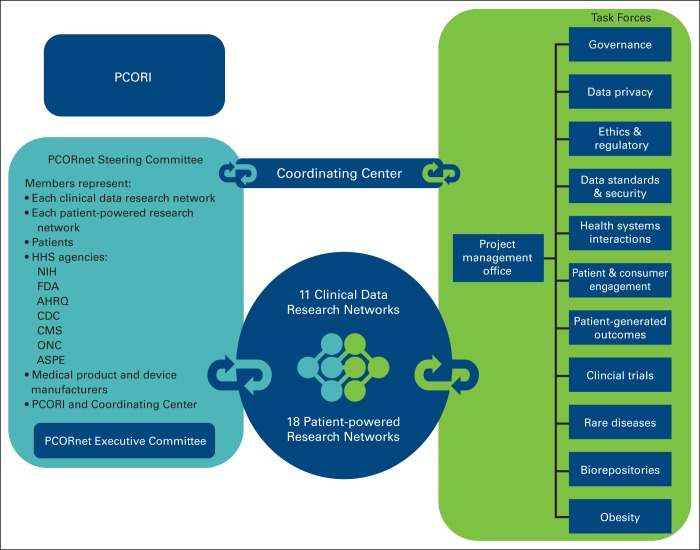
In December 2013, the PCORI Board of Governors approved funding of the PCORnet infrastructure. The organizational structure included awards to 11 clinical data research networks (CDRNs) and 18 patient-powered research networks (PPRNs). It also started developing task forces covering topics from patient privacy to disease-specific working groups and developed a diverse group of stakeholders throughout the United States that include funding agencies, PCORI, and patients (Figure 1).
Figure 1.
PCORnet organizational structure. AHRQ, Agency for Healthcare Research and Quality; ASPE, Assistant Secretary for Planning and Evaluation; CDC, Centers for Disease Control; CMS, Centers for Medicare and Medicaid Services; FDA, Food and Drug Administration; HHS, Health and Human Services; NIH, National Institutes of Health; ONC, Office of the National Coordinator for Health Information Technology; PCORI, Patient Centered Outcomes Research Institute.
The PPRNs consist of patient-led organizations that typically focus on a single condition or disease; examples include inflammatory bowel disease, heart disease, and multiple sclerosis.4 These networks each have an active, involved patient population of at least 50 patients for rare diseases or at least 50,000 patients for common conditions. The PPRNs are required to involve patients in the governance of their networks, create standardized databases amenable to sharing with other PCORnet members, and be able to respond to questions regarding potential research studies.5
The CDRNs are embedded in different types of health delivery systems.4,5 Each network must create a research-ready data set of at least 1 million patients, with comprehensive, longitudinal data on patterns and results of treatment. Each will also develop an infrastructure, integrated into the health care operations of the system, that can conduct multisite clinical trials.5 Each system must be secure to minimize the risk of inadvertent disclosure of information on individual patients, and it must demonstrate the ability to engage the patients, clinicians, and clinical leaders of the health care system in the development and ongoing management of the network. Data set utility is enhanced through the use of the PCORnet Common Data Model, which standardizes the domains and variable definitions. PCORI anticipates that interested researchers outside the networks will be able to send simple queries to the PCORnet Coordinating Center to characterize patient subgroups and provide information about the feasibility of envisioned projects. In addition, each network will characterize and survey three cohorts of patients: one with a common condition, one with a rare condition, and one focused on obesity.
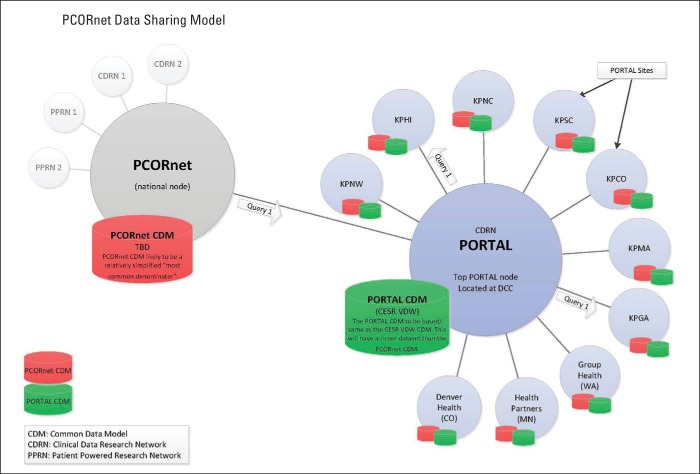
The Kaiser Permanente and Strategic Partners Patient Outcomes Research to Advance Learning (PORTAL) Network is an example of a CDRN; it includes a colorectal cancer cohort (common disease), a severe congenital heart disease cohort (rare disease), and an obesity cohort (Appendix Figure A1, online only). PORTAL includes 10 integrated health care systems with approximately 12 million members (approximately one of every 30 citizens in United States). It uses a pre-existing data infrastructure developed by the HMO Research Network and the National Cancer Institute Cancer Research Network, known as the Virtual Data Warehouse (VDW).6,7 The VDW maps heterogeneous source data into standardized variables organized in identical data structures. These data are derived from electronic medical records and other electronic data systems (eg, enrollment, demographics) at each member institution. The VDW is virtual in that the data reside behind the firewall of each institution until assembled into a specific analytic data set. A unique advantage of the VDW is that data sets can be developed across multiple participating research sites using a single computer program.
PORTAL provides one example of how PCORnet can engage patients, providers, researchers, and health system leaders. It includes patients with the selected conditions as well as members of patient advocacy groups, such as Fight Colorectal Cancer, the Adult Congenital Heart Association, and the African American Health Coalition. The patients provide information regarding issues that are most important to them, feedback on research plans, and suggestions on survey design.8 Meetings with health care system leaders, including the directors of quality departments for member health care systems, provide insights into priorities of the health care systems, their methods for implementing new quality initiatives, and the resources available. These resources can then be aligned to facilitate large-scale research projects and improve care delivery.
PCORnet is in its initial developmental phase; networks are currently assembling data sets, identifying patient cohorts, and initiating surveys. The current PCORnet Common Data Model includes multiple domains (demographics, diagnoses, procedures, encounters), and future iterations will expand these domains. Examples of feasible studies include large pragmatic trials and observational studies that use existing data systems to efficiently identify eligible patients and capture follow-up events. PCORI has approved the first PCORnet trial, a comparison of optimal aspirin dosing for secondary prevention of cardiovascular events.9 A sample feasible observational study within PCORnet is an evaluation of differences in quality metrics and outcomes between individuals or systems, such as variations in the performance of diagnostic tests or differences in outcomes after medical procedures.10 A study such as this has already been completed within one of the PCORnet sites, Kaiser Permanente Northern California, using the VDW and other data elements developed within the National Cancer Institute Population-Based Research to Optimize Screening Through Personalized Regimens (PROSPR) initiative. This study evaluated how often physicians who perform colonoscopy detect precancerous polyps, known as the adenoma detection rate, and then contrasted these rates with patients' outcomes. The study found that even among physicians who met existing national guidelines for adenoma detection, higher levels of adenoma detection were associated with a substantially lower future risk of colorectal cancer and death resulting from colorectal cancer.11
In summary, PCORnet is a substantial new investment in the nation's research infrastructure. It uses a novel structure that promises to break down traditional impediments to research by integrating patients, researchers, and health care delivery leaders into all phases of the research process. This structure seeks to not only develop research questions that are important to patients and clinicians but also provide the data platform to answer those questions. Furthermore, its organizational structure enhances its potential for rapidly disseminating findings and evaluating implementation. Ideally, this will accelerate the dissemination of important research findings into clinical practice, while improving health outcomes and reducing health care costs.
Acknowledgment
The data infrastructure for PCORnet is supported by Contract No. CDRN-1306-04681 from the Patient Outcomes Research Institute. The infrastructure within the Kaiser Permanente and Strategic Partners Clinical Data Research Network builds upon data structures that receive ongoing support from the National Cancer Institute Cancer (NCI) Research Network (Grant No. U24 CA171524) and the Kaiser Permanente Center for Effectiveness and Safety Research. D.A.C. is also supported by the NCI PROSPR initiative (Grant No. U54 CA163262), which provided data for this presentation. We thank all members of the PCORnet and PORTAL collaborations, beyond the listed authors.
Appendix
Figure A1.
Organization of Kaiser Permanente and Strategic Partners Patient Outcomes Research to Advance Learning (PORTAL) Network and PCORnet data infrastructures. CESR, Center for Effectiveness and Safety Research; DCC, Data Coordinating Center; KPCO, Kaiser Permanente Colorado; KPGA, Kaiser Permanente Georgia; KPHI, Kaiser Permanente Hawaii; KPMA, Kaiser Permanente Mid-Atlantic; KPNC, Kaiser Permanente Northern California; KPNW, Kaiser Permanente Northwest; KPSC, Kaiser Permanente Southern California; TBD, to be determined; VDW, Virtual Data Warehouse.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: All authors
Collection and assembly of data: Douglas A. Corley
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Building Data Infrastructure to Evaluate and Improve Quality: PCORnet
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Douglas A. Corley
Employment: Permanente Medical Group
Stock or Other Ownership: Permanente Medical Group
Research Funding: Permanente Medical Group
Travel, Accommodations, Expenses: Permanente Medical Group
Heather Spencer Feigelson
No relationship to disclose
Tracy A. Lieu
Employment: Permanente Medical Group
Leadership: Permanente Medical Group
Travel, Accommodations, Expenses: Permanente Medical Group
Elizabeth A. McGlynn
Employment: Southern California Permanente Medical Group
References
- 1.Patient-Centered Outcomes Research Institute. The National Patient-Centered Clinical Research Network. http://www.pcornet.org/
- 2.Centers for Medicare and Medicaid Services. National health expenditures 2012 highlights. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html.
- 3.Davis K, Stremikis K, Squires D, et al. Mirror, mirror on the wall, 2014 update: How the U.S. health care system compares internationally. http://www.commonwealthfund.org/publications/fund-reports/2014/jun/mirror-mirror.
- 4.Patient-Centered Outcomes Research Institute. Clinical data and patient-powered research networks: Awarded projects. http://www.pcori.org/node/4505.
- 5.National Patient-Centered Clinical Research Network. About PCORnet. http://www.pcornet.org/about-pcornet/
- 6.Ross TR, Ng D, Brown JS, et al. The HMO research network virtual data warehouse: A public data model to support collaboration. http://repository.academyhealth.org/egems/vol2/iss1/2. [DOI] [PMC free article] [PubMed]
- 7.National Cancer Institute. Cancer Research Network: Informatics and research tools. http://crn.cancer.gov/resources/informatics.html.
- 8.Fight Colorectal Cancer. http://fightcolorectalcancer.org/
- 9.Patient-Centered Outcomes Research Institute. Optimal aspirin dose for patients with coronary artery disease approved as topic for first PCORnet research trial. http://www.pcori.org/content/optimal-aspirin-dose-patients-coronary-artery-disease-approved-topic-first-pcornet-research.
- 10.Corley DA, Jensen CD, Marks AR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: Implications for screening and quality programs. Clin Gastroenterol Hepatol. 2013;11:172–180. doi: 10.1016/j.cgh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:2541. doi: 10.1056/NEJMc1405329. [DOI] [PubMed] [Google Scholar]