The authors did not see evidence that multidisciplinary teams (MDTs) affect patient survival or cost of care, or studies of how or which MDT processes and structures were associated with success.
Abstract
Purpose:
The management of cancer varies across its type, stage, and natural history. This necessitates involvement of a variety of individuals and groups across a number of provider types. Evidence from other fields suggests that a team-based approach helps organize and optimize tasks that involve individuals and groups, but team effectiveness has not been fully evaluated in oncology-related care.
Methods:
We undertook a systematic review of literature published between 2009 and 2014 to identify studies of all teams with clear membership, a comparator group, and patient-level metrics of cancer care. When those teams included two or more people with specialty training relevant to the care of patients with cancer, we called them multidisciplinary care teams (MDTs). After reviews and exclusions, 16 studies were thoroughly evaluated: two addressing screening and diagnosis, 11 addressing treatment, two addressing palliative care, and one addressing end-of-life care. The studies included a variety of end points (eg, adherence to quality indicators, patient satisfaction with care, mortality).
Results:
Teams for screening and its follow-up improved screening use and reduced time to follow-up colonoscopy after an abnormal screen. Discussion of cases within MDTs improved the planning of therapy, adherence to recommended preoperative assessment, pain control, and adherence to medications. We did not see convincing evidence that MDTs affect patient survival or cost of care, or studies of how or which MDT processes and structures were associated with success.
Conclusion:
Further research should focus on the association between team processes and structures, efficiency in delivery of care, and mortality.
Introduction
Teams and teamwork have a long rhetorical history in medical literature because they are an intuitive solution to the information and technical burdens of medical care.1 Teams are defined as two or more people who interact dynamically, interdependently, and adaptively to achieve a common goal.1–3 During the treatment phase, a cancer care team is commonly identified as a multidisciplinary care team (MDT) and typically includes clinicians with radiologic, pathologic, surgical, therapeutic radiation and/or oncologic medical and nursing knowledge.4 The clinicians involved in MDTs include nurses, nurse practitioners, physicians, laboratory or radiation therapy technicians, pharmacists, social workers, and other relevant staff. Other groups with specialty training that is not exclusive to oncology, such as primary care, palliative care, and hospice care also contribute to the care of people with cancer. This review addresses all teams involved in cancer care across the cancer care continuum from diagnosis through the end of life.5
A growing body of literature demonstrates that team-based care and efforts to optimize teamwork can reduce mortality; improve hospital management of medications; and improve outpatient management of diabetes, depression, and other medical conditions.6–8 The demand for teams has grown in parallel with health care reform and the public's increasing expectations for improved quality and value in health care. The Affordable Care Act (ACA) incents teamwork and care coordination by encouraging establishment of the Patient Centered Medical Home and Accountable Care Organization.9 ASCO identified MDTs of appropriately skilled personnel as a cornerstone of quality care.9–11 At the same time, our understanding of molecular drivers of cancer and therapeutics targeting genomic alterations demand supplementary technical expertise in the context of an impending workforce shortage in oncology.12 Understanding how teams affect cancer care delivery will help determine the role teams may play in addressing these challenges to the health care system.
Despite incentives and interest in teams, we know less than expected about what makes teamwork effective in cancer care practice.1,3,7,13 What we know about teams in medical practice comes primarily from work in the elderly, and specific surgical and chronic care conditions.1,7,13 MDTs are one example of work explicitly examining cancer care teams, and these teams have grown from a single-point-in-time consultation to the requirement that 80% of patients presented in these MDTs be followed prospectively.14,15 In 2010, two reviews noted that little had been published on the relationship between MDT composition, function, and effectiveness in cancer care delivery.16,14 By that time, most studies of cancer MDTs focused on their composition and processes, though one randomized trial showed their effectiveness in improving the quality of life for people with advanced disease.13,16 A more recent prospective survey evaluated improvements in their functioning with time.4 This article will review the effectiveness of all teams involved in cancer care, including MDTs, since 2010. The goal of the article is to discuss how to apply evidence on teams in clinical practice and identify what is needed in future research on teams.
Methods
A literature search was conducted across multiple medical, health, and social science databases (PubMed, Scopus, ABI/INFORM Complete, Embase) for peer-reviewed articles evaluating team-based approaches to adult or pediatric cancer care published from August 26, 2009, to August 26, 2014. We chose papers published after the 2010 reviews of MDTs and focused on major facets of team effectiveness, including teamwork processes, quality of care, and patient outcomes in cancer care. Articles were selected if they (1) clearly defined team membership, (2) included a comparison group, (3) included at least one patient outcome (eg, use of guideline-consistent therapy, pain control, patient satisfaction, quality of life, disease-free survival), and (4) were reported in English.
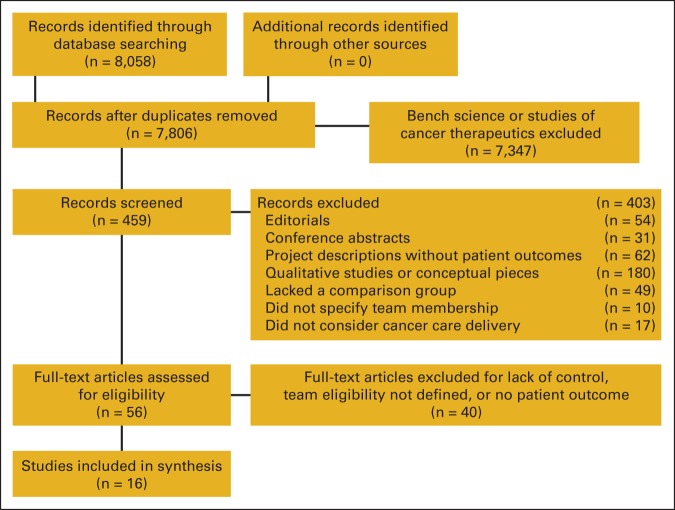
After the searches were conducted, we reviewed the titles and abstracts to eliminate irrelevant citations. The exact words used in the search process and steps for excluding and including citations are further documented in the Appendix (online only). As noted in Figure 1, the search resulted in identifying 7,806 citations of which 7,347 were eliminated on the basis of titles and abstracts reflecting bench science, or abstracts without an associated article. The remaining 459 abstracts were examined more closely. We eliminated 403 because they were editorials or letters (n = 54), conference abstracts (n = 31), project descriptions without patient outcomes (n = 62), qualitative studies or conceptual pieces (n = 180), because they lacked a comparison group (n = 49), did not specify the team membership (n = 10), or did not consider cancer care delivery (n = 17). That left 56 records that were candidates for the study based on descriptions in abstracts. Four team members each individually coded half of the remaining 56 full-text articles, resulting in each article being coded twice. Differences in codes were reconciled across coding pairs. Coders first assessed whether each article met the study's eligibility criteria. During this assessment, 40 articles were excluded from analysis because they had one or more of the following characteristics: no description of who was on the team, no comparison groups, no patient outcomes, or no reference to the term “team” in the article title or abstract. That left 16 articles that met all inclusion criteria (Table 1).
Figure 1.
Literature inclusion and exclusion.
Table 1.
16 Studies of Teams Involved in Cancer Care
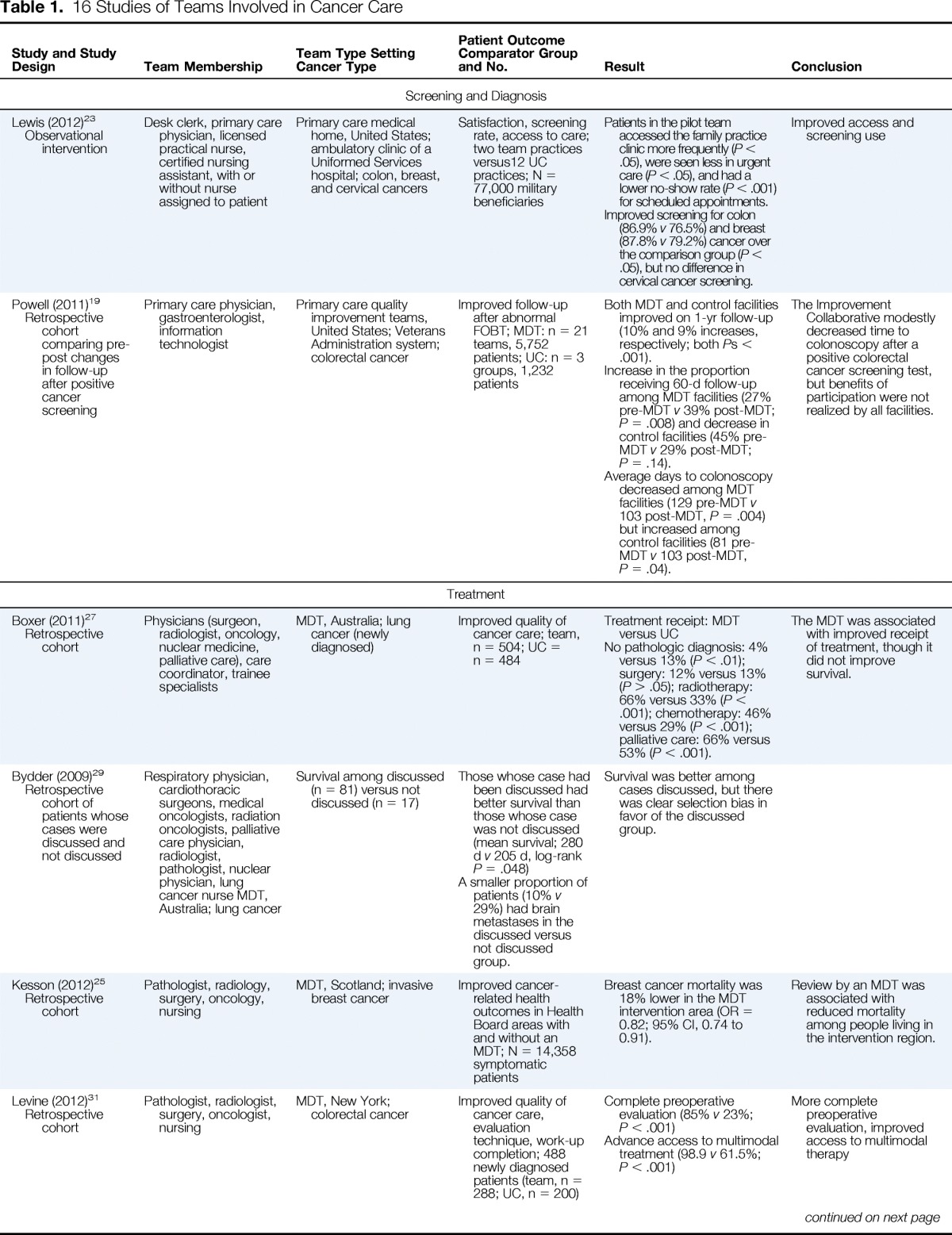
| Study and Study Design | Team Membership | Team Type Setting Cancer Type | Patient Outcome Comparator Group and No. | Result | Conclusion |
|---|---|---|---|---|---|
| Screening and Diagnosis | |||||
| Lewis (2012)23 Observational intervention |
Desk clerk, primary care physician, licensed practical nurse, certified nursing assistant, with or without nurse assigned to patient | Primary care medical home, United States; ambulatory clinic of a Uniformed Services hospital; colon, breast, and cervical cancers | Satisfaction, screening rate, access to care; two team practices versus12 UC practices; N = 77,000 military beneficiaries | Patients in the pilot team accessed the family practice clinic more frequently (P < .05), were seen less in urgent care (P < .05), and had a lower no-show rate (P < .001) for scheduled appointments. Improved screening for colon (86.9% v 76.5%) and breast (87.8% v 79.2%) cancer over the comparison group (P < .05), but no difference in cervical cancer screening. |
Improved access and screening use |
| Powell (2011)19 Retrospective cohort comparing pre-post changes in follow-up after positive cancer screening |
Primary care physician, gastroenterologist, information technologist | Primary care quality improvement teams, United States; Veterans Administration system; colorectal cancer | Improved follow-up after abnormal FOBT; MDT: n = 21 teams, 5,752 patients; UC: n = 3 groups, 1,232 patients | Both MDT and control facilities improved on 1-yr follow-up (10% and 9% increases, respectively; both Ps < .001). Increase in the proportion receiving 60-d follow-up among MDT facilities (27% pre-MDT v 39% post-MDT; P = .008) and decrease in control facilities (45% pre-MDT v 29% post-MDT; P = .14). Average days to colonoscopy decreased among MDT facilities (129 pre-MDT v 103 post-MDT, P = .004) but increased among control facilities (81 pre-MDT v 103 post-MDT, P = .04). |
The Improvement Collaborative modestly decreased time to colonoscopy after a positive colorectal cancer screening test, but benefits of participation were not realized by all facilities. |
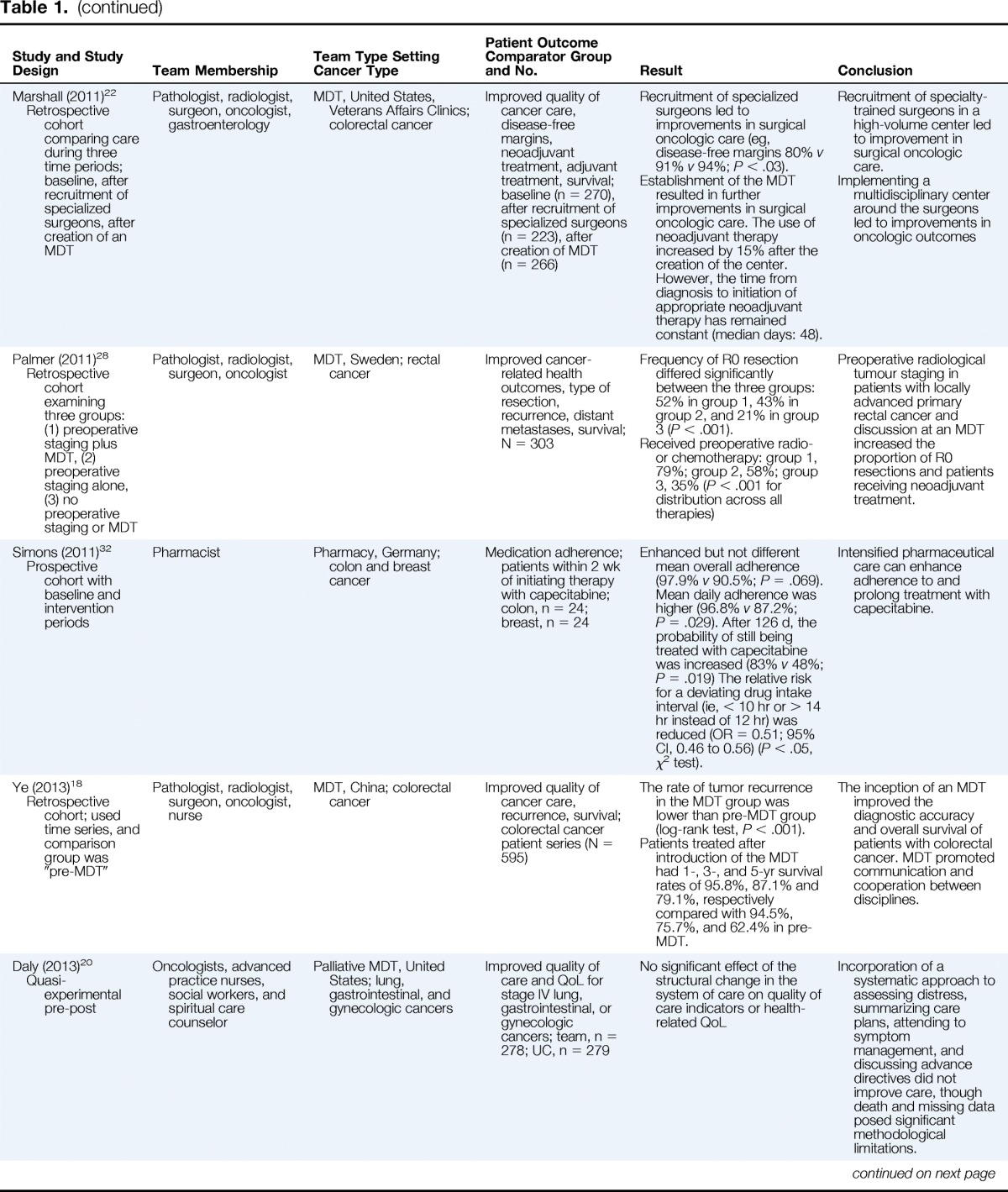
| Treatment | |||||
| Boxer (2011)27 Retrospective cohort |
Physicians (surgeon, radiologist, oncology, nuclear medicine, palliative care), care coordinator, trainee specialists | MDT, Australia; lung cancer (newly diagnosed) | Improved quality of cancer care; team, n = 504; UC = n = 484 | Treatment receipt: MDT versus UC No pathologic diagnosis: 4% versus 13% (P < .01); surgery: 12% versus 13% (P > .05); radiotherapy: 66% versus 33% (P < .001); chemotherapy: 46% versus 29% (P < .001); palliative care: 66% versus 53% (P < .001). |
The MDT was associated with improved receipt of treatment, though it did not improve survival. |
| Bydder (2009)29 Retrospective cohort of patients whose cases were discussed and not discussed |
Respiratory physician, cardiothoracic surgeons, medical oncologists, radiation oncologists, palliative care physician, radiologist, pathologist, nuclear physician, lung cancer nurse MDT, Australia; lung cancer | Survival among discussed (n = 81) versus not discussed (n = 17) | Those whose case had been discussed had better survival than those whose case was not discussed (mean survival; 280 d v 205 d, log-rank P = .048) A smaller proportion of patients (10% v 29%) had brain metastases in the discussed versus not discussed group. |
Survival was better among cases discussed, but there was clear selection bias in favor of the discussed group. | |
| Kesson (2012)25 Retrospective cohort |
Pathologist, radiology, surgery, oncology, nursing | MDT, Scotland; invasive breast cancer | Improved cancer-related health outcomes in Health Board areas with and without an MDT; N = 14,358 symptomatic patients | Breast cancer mortality was 18% lower in the MDT intervention area (OR = 0.82; 95% CI, 0.74 to 0.91). | Review by an MDT was associated with reduced mortality among people living in the intervention region. |
| Levine (2012)31 Retrospective cohort |
Pathologist, radiologist, surgery, oncologist, nursing | MDT, New York; colorectal cancer | Improved quality of cancer care, evaluation technique, work-up completion; 488 newly diagnosed patients (team, n = 288; UC, n = 200) | Complete preoperative evaluation (85% v 23%; P < .001) Advance access to multimodal treatment (98.9 v 61.5%; P < .001) |
More complete preoperative evaluation, improved access to multimodal therapy |
| Marshall (2011)22 Retrospective cohort comparing care during three time periods; baseline, after recruitment of specialized surgeons, after creation of an MDT |
Pathologist, radiologist, surgeon, oncologist, gastroenterology | MDT, United States, Veterans Affairs Clinics; colorectal cancer | Improved quality of cancer care, disease-free margins, neoadjuvant treatment, adjuvant treatment, survival; baseline (n = 270), after recruitment of specialized surgeons (n = 223), after creation of MDT (n = 266) | Recruitment of specialized surgeons led to improvements in surgical oncologic care (eg, disease-free margins 80% v 91% v 94%; P < .03). Establishment of the MDT resulted in further improvements in surgical oncologic care. The use of neoadjuvant therapy increased by 15% after the creation of the center. However, the time from diagnosis to initiation of appropriate neoadjuvant therapy has remained constant (median days: 48). |
Recruitment of specialty-trained surgeons in a high-volume center led to improvement in surgical oncologic care. Implementing a multidisciplinary center around the surgeons led to improvements in oncologic outcomes |
| Palmer (2011)28 Retrospective cohort examining three groups: (1) preoperative staging plus MDT, (2) preoperative staging alone, (3) no preoperative staging or MDT |
Pathologist, radiologist, surgeon, oncologist | MDT, Sweden; rectal cancer | Improved cancer-related health outcomes, type of resection, recurrence, distant metastases, survival; N = 303 | Frequency of R0 resection differed significantly between the three groups: 52% in group 1, 43% in group 2, and 21% in group 3 (P < .001). Received preoperative radio- or chemotherapy: group 1, 79%; group 2, 58%; group 3, 35% (P < .001 for distribution across all therapies) |
Preoperative radiological tumour staging in patients with locally advanced primary rectal cancer and discussion at an MDT increased the proportion of R0 resections and patients receiving neoadjuvant treatment. |
| Simons (2011)32 Prospective cohort with baseline and intervention periods |
Pharmacist | Pharmacy, Germany; colon and breast cancer | Medication adherence; patients within 2 wk of initiating therapy with capecitabine; colon, n = 24; breast, n = 24 | Enhanced but not different mean overall adherence (97.9% v 90.5%; P = .069). Mean daily adherence was higher (96.8% v 87.2%; P = .029). After 126 d, the probability of still being treated with capecitabine was increased (83% v 48%; P = .019) The relative risk for a deviating drug intake interval (ie, < 10 hr or > 14 hr instead of 12 hr) was reduced (OR = 0.51; 95% CI, 0.46 to 0.56) (P < .05, χ2 test). | Intensified pharmaceutical care can enhance adherence to and prolong treatment with capecitabine. |
| Ye (2013)18 Retrospective cohort; used time series, and comparison group was “pre-MDT” |
Pathologist, radiologist, surgeon, oncologist, nurse | MDT, China; colorectal cancer | Improved quality of cancer care, recurrence, survival; colorectal cancer patient series (N = 595) | The rate of tumor recurrence in the MDT group was lower than pre-MDT group (log-rank test, P < .001). Patients treated after introduction of the MDT had 1-, 3-, and 5-yr survival rates of 95.8%, 87.1% and 79.1%, respectively compared with 94.5%, 75.7%, and 62.4% in pre-MDT. |
The inception of an MDT improved the diagnostic accuracy and overall survival of patients with colorectal cancer. MDT promoted communication and cooperation between disciplines. |
| Daly (2013)20 Quasi-experimental pre-post |
Oncologists, advanced practice nurses, social workers, and spiritual care counselor | Palliative MDT, United States; lung, gastrointestinal, and gynecologic cancers | Improved quality of care and QoL for stage IV lung, gastrointestinal, or gynecologic cancers; team, n = 278; UC, n = 279 | No significant effect of the structural change in the system of care on quality of care indicators or health-related QoL | Incorporation of a systematic approach to assessing distress, summarizing care plans, attending to symptom management, and discussing advance directives did not improve care, though death and missing data posed significant methodological limitations. |
| Kelly (2013)17 Retrospective cohort with pre- versus post-MDT comparison |
Otolaryngologist, radiation oncologist, medical oncologist | Multidisciplinary, Australia; head and neck cancer | Improved clinical quality indicators of care; pre, n = 48; post, n = 65 | MDT had higher rates of dental assessment (59% v 22%; P < .001), nutritional assessment (57% v 39%; P = .015), PET staging (41% v 2%, P < .001), chemo-radiotherapy for locally advanced disease (66% v 16%, P < .001) and adjuvant chemoradiotherapy for high-risk disease (49% v 16%; P < .001). Interval between surgery and radiotherapy was shorter in the post-MDT group (P = .009), as was the mean length of hospitalization (P = .002). |
MDTs improve the likelihood of guideline consistent care for patients with head and neck cancer, including improved dental and nutritional assessment, cancer staging, time to treatment, and post-operative treatment. |
| Wille-Jorgensen (2013)21 Retrospective cohort comparing 3-yr pre-MDT with 2-yr post-MDT |
Surgeons, oncologists, radiologists, pathologists, and clinical physiologists | MDT, Denmark; colorectal cancer | Patient survival and preoperative staging; pre, n = 467; post, n = 344 | Staged preoperatively: pre 51% versus post 87% (P < .001) MRI performed: pre 21% versus post 72% (P < .001) Postoperative mortality within 30 d: pre 9% versus post 5% (P = .007) |
Use of MDTs led to increased preoperative staging and higher rates of MRI scans. Also, post-operative mortality was lower in patients treated by the MDT. |
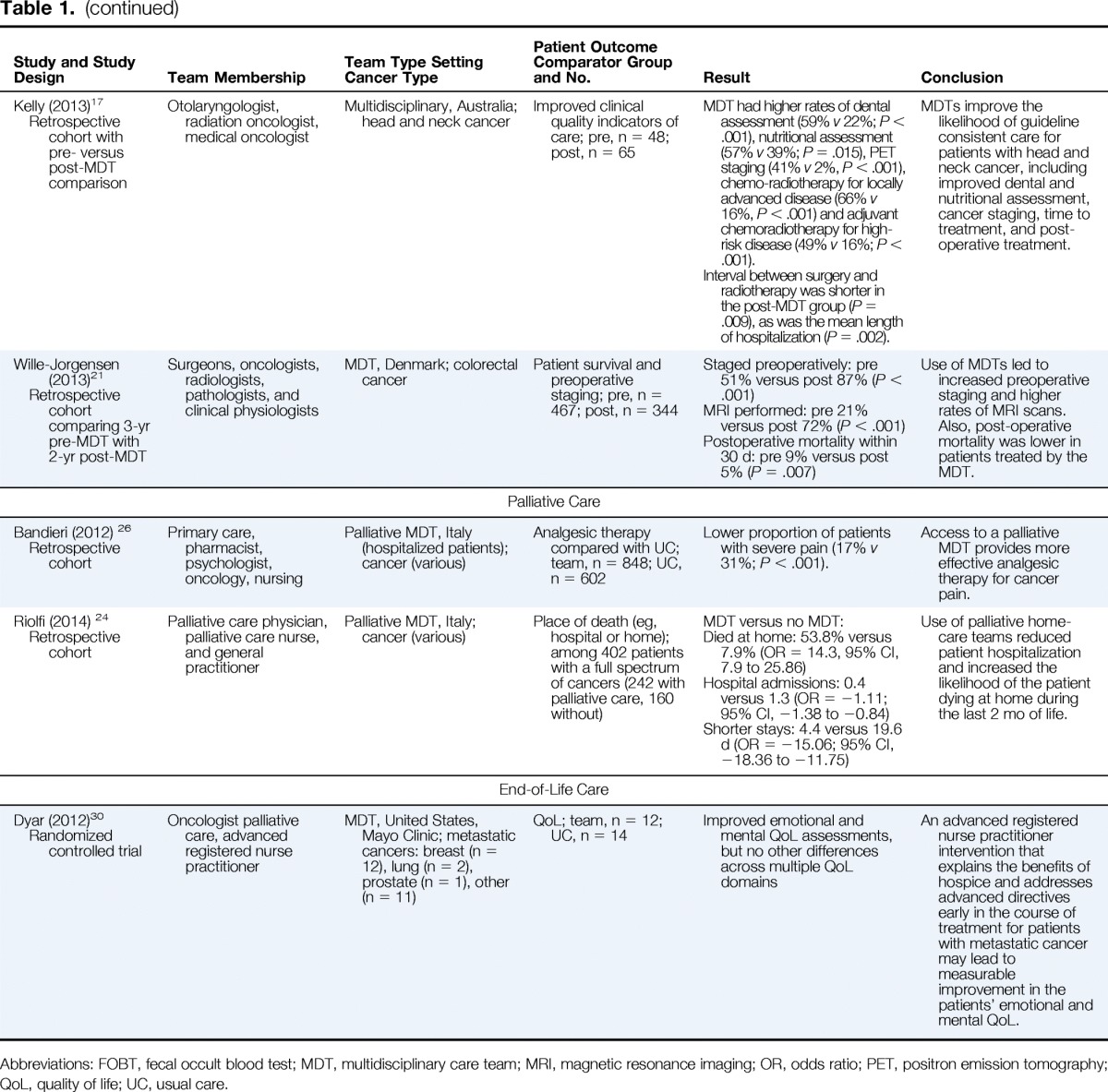
| Palliative Care | |||||
| Bandieri (2012) 26 Retrospective cohort |
Primary care, pharmacist, psychologist, oncology, nursing | Palliative MDT, Italy (hospitalized patients); cancer (various) | Analgesic therapy compared with UC; team, n = 848; UC, n = 602 | Lower proportion of patients with severe pain (17% v 31%; P < .001). | Access to a palliative MDT provides more effective analgesic therapy for cancer pain. |
| Riolfi (2014) 24 Retrospective cohort |
Palliative care physician, palliative care nurse, and general practitioner | Palliative MDT, Italy; cancer (various) | Place of death (eg, hospital or home); among 402 patients with a full spectrum of cancers (242 with palliative care, 160 without) | MDT versus no MDT: Died at home: 53.8% versus 7.9% (OR = 14.3, 95% CI, 7.9 to 25.86) Hospital admissions: 0.4 versus 1.3 (OR = −1.11; 95% CI, −1.38 to −0.84) Shorter stays: 4.4 versus 19.6 d (OR = −15.06; 95% CI, −18.36 to −11.75) |
Use of palliative home-care teams reduced patient hospitalization and increased the likelihood of the patient dying at home during the last 2 mo of life. |
| End-of-Life Care | |||||
| Dyar (2012)30 Randomized controlled trial |
Oncologist palliative care, advanced registered nurse practitioner | MDT, United States, Mayo Clinic; metastatic cancers: breast (n = 12), lung (n = 2), prostate (n = 1), other (n = 11) | QoL; team, n = 12; UC, n = 14 | Improved emotional and mental QoL assessments, but no other differences across multiple QoL domains | An advanced registered nurse practitioner intervention that explains the benefits of hospice and addresses advanced directives early in the course of treatment for patients with metastatic cancer may lead to measurable improvement in the patients' emotional and mental QoL. |
Abbreviations: FOBT, fecal occult blood test; MDT, multidisciplinary care team; MRI, magnetic resonance imaging; OR, odds ratio; PET, positron emission tomography; QoL, quality of life; UC, usual care.
Results
Table 1 shows the results of the review of teams across the cancer care continuum. Two studies of teams addressed screening and diagnosis, 11 addressed MDTs during active treatment, two addressed palliative care, and one addressed care at the end of life. The studies examined a variety of end points, including adherence to quality indicators (n = 10),17–19,22,23,26–28,31,32 patient-centered outcomes including satisfaction with care experience (n = 1), quality of life (n = 2),20,30 and mortality (n = 3).21,25,29 Four studies used time series designs to evaluate care before and after implementation of an MDT.17–20 Most had some contemporaneous comparison group, including one MDT study that suffered from selection bias.21–29 There is one randomized controlled trial30 and one observational intervention23 with a pre-post design. Ten studies occurred outside the United States, and six occurred within the United States.
Team membership varied from primary care physician–led teams that included license practical nurses, nursing assistants, and a desk clerk (n = 2), to MDTs (n = 13) whose membership varied across studies (oncology, pathology, radiology, surgery, nursing), or MDTs with the addition of expertise specific to the interests of the study (eg, pharmacists; n = 1).
The use of teams for screening and follow-up to screening results in increased guideline-compliant follow-up and improved timeliness of follow-up.17,19,23 Discussion of cases within MDTs appears to positively affect the planning of therapy, leading to improved adherence to recommended preoperative assessment.27,28,31 Adherence to capecitabine and pain medications is also reported to be improved with MDTs.26,32 All studies showed improvement in one or more of the patient metrics of care. Finally, palliative care teams reduced hospitalizations and improved quality of life at the end of life.24,26,30
Discussion
The rationale for team-based cancer care is strong. The information needed to attend to individual patients varies by cancer type, stage, and place in the cancer care continuum where individuals are seeking care: asymptomatic or symptomatic, at increased risk due to genetics or behavior, diagnosed, under treatment, seeking palliation, or at the end of life. Each type of care along the continuum has a different purpose and requires a different knowledge base, so the group of clinicians involved varies and may expand or contract over time.5 As a result, people seeking screening and cancer care expect to be cared for by groups of individuals and many expect these groups to communicate, coordinate, and cooperate effectively. Patients and their loved ones increasingly expect the providers involved in their care to function as expert teams.
These 16 studies suggest that using team-based approaches across the care continuum may offer promising pathways to improve quality, access, and patient-centered outcomes. Although the majority of studies to date have focused on evaluating the effects of MDTs during active treatment, a small number of studies also evaluated the impact of team-based approaches to screening/diagnosis and palliative or end-of-life care. We found more studies of multidisciplinary and palliative care; however, they were excluded because they did not include comparator groups or patient-centered outcomes. This suggests the need for more comparative studies of cancer care teams in the future and recognition of the important but rudimentary nature of current knowledge about cancer care teams.
Our findings provide several important messages for clinical practice. First, the use of teams for screening and follow-up to screening results in improvements in guideline-compliant follow-up and timeliness of follow-up. Second, current evidence empirically demonstrates that discussing cases within MDTs positively affects therapy planning and implementation, leading to improved adherence to recommended preoperative assessment. Third, pain control and adherence to an oral medication were also reported to be improved with MDTs. Though these findings are based on observational comparisons and time-series analysis, they are consistent with expectations for MDTs.
In this review, we did not see convincing evidence linking MDTs to patient survival. The one study that examined survival suffered from selection bias that would invalidate the comparison.29 Furthermore, included studies did not meaningfully evaluate teamwork processes used by MDTs (ie, how team members communicated, cooperated, and coordinated their work around a given patient or panel of patients) that could provide insight about which characteristics are associated with success. Although there have been mixed-methods studies examining some of these processes, often these studies lack a meaningful comparator or fail to report outcome indices, two key inclusion criteria for the current review.4,33–36 We need to understand how team composition, organization, documentation, and follow-up affect longitudinal care. As greater emphasis is placed on the value of care, we also need to understand how these characteristics affect the trade-off between the costs and effectiveness of care delivery. For example, a survey of 173 MDT members participating in teams focused on urologic cancer care found that 68% reported that participating in MDT activities improved efficiency in clinical decision making, discussions with patients, specialty referrals, and documentation.37 However, variability among MDT members in their sense of preparation and participation in MDT discussions has also been identified.38,39 Additionally, some work has begun to characterize MDTs in practice and evaluate their improvement over time.4 However, as was noted several years ago, more must be done to evaluate the effects of MDTs on short-term patient morbidity and long-term outcomes.14
Implications for Practice
Despite limited evidence in oncology, cancer care clinicians can use what has been learned from work in business, the military, aviation and other areas of health care.40–42 For example we know that providers can use several techniques to improve their teamwork: briefings or planning sessions at the start of each day; debriefings or postcase discussions; closed-loop communication techniques; and efforts to clarify roles, responsibilities, and interdependencies both among team members in one's own practice and with other practices involved in care of their patient.8
Work in other fields has also identified eight hallmarks (“Cs”) of effective teams, four of which describe characteristics of successful teams: (1) negotiating and developing shared goals for mutually shared patients or patient populations, sharing unique information proactively, working jointly to make sense of available information (communication); (2) demonstrating explicit commitment to working collaboratively (cooperation); (3) orchestrating explicit coordination of activities and identifying cues or triggers indicating that key steps have been completed or are in progress (coordination); and (4) developing trust and the desire to work together in the future (cohesion).41,43 An additional three Cs that help teams address these first four are (5) belief that the team as a unit can accomplish shared goals (collective efficacy), (6) shared description and understanding of the team characteristics (collective identity), and (7) the development of the ability to recognize the pertinent cues of team members and use the teams collective resources (cognition).41 Finally, the eighth C is a need for coaching, since 58% of people who are trained in teamwork require additional reinforcement and deliberate practice to sustain the changes in their performance.44 These eight characteristics highlight the complexity of teams and teamwork and further suggest that it is not enough to invoke teams; they need training.
The best teams in sports rely on coaching and devote considerable time to practice. The requirements are no different in medicine.8,45 It takes time to develop an effective team that is persistent and has support for its work.46 In the face of this complexity, it is some relief that there are ways to develop team skills.40 TeamSTEPPS is a training program that has been shown to reduce mistakes in medical practice, improve performance in surgery and obstetric units, and improve diabetic care.8 Larger effect sizes for team work were reported for bundled team-training interventions that included tools and organizational changes to support sustainment and transfer of teamwork competencies into daily practice.8 Though the Agency for Healthcare Research and Quality, which developed TeamSTEPPS, has not created a module for cancer care, the strategies and practices would likely translate to oncology specialty care.
Future Directions
Building on the conceptualization of teams in other disciplines, we can develop a better understanding of teams in medicine. The broad evidence base examining teams in health care and other contexts suggests that realizing the full return on investment of teams for both patients and providers calls for more than simply encouraging clinicians to adopt optimal teamwork habits in daily practice. Teams develop cognitive, motivational, and behavioral processes that influence their effectiveness.48 For example teams can develop shared culture and climate, shared mental maps of their work (shared mental models), and an understanding of who knows what (transactive memory) so they can efficiently perform their part of the work each time a similar task emerges; they develop a sense of cohesion, trust, efficacy, and potency that motivates their work, and they develop behavioral norms.48 We need studies that evaluate cancer care teams for these qualities and show their importance to cancer care delivery.
Such a study becomes even more complex as MDTs expand to include members working virtually, combine members across different teams, and require collaboration between entire teams across different organizations or care systems. We currently have relatively limited direct evidence about how these factors influence MDT effectiveness in the cancer care context. These are areas ripe for investigation. Understanding and testing how various inputs, processes, and contextual factors influence MDT outcomes is critical for understanding how to best structure and invest in creating effective team-based approaches to care. It is necessary if we are to achieve the vision of simultaneously improving care quality, patient outcomes, and reducing costs while using our increasingly limited cancer care workforce resources most efficiently. Continuing to build the evidence linking different team structures, processes, and contextual influences to MDT effectiveness is also important for understanding how MDTs might offer avenues for efficiency gains that are critical for giving clinicians breathing room to find the joy and meaning in their daily work that drew them into their profession in the first place.
The practical implications of clinicians working effectively as teams are more important than ever. As health care providers have tried to actively implement team-based care in patient-centered medical homes, they have recognized this complexity and reported that achieving a functional team is unlikely to occur without coaching and organizational support.46 Concerns about the cost and quality of health care delivery are prompting consumers, payers, and policymakers to demand enhanced value of health care services. The three studies we found in palliative care suggested the potential for fewer hospitalizations and better quality of life at the end of life. More widespread adoption of team-based care strategies in the presence of advanced cancer has the potential to contribute to increased value of cancer care services.
Despite encouraging findings in these 16 studies of teams, there are some limitations and new questions. There is only one randomized trial among the studies, and many are time-series analyses that do not account for contemporaneous changes that might explain differences in staging practices, adherence to treatment guidelines, or survival. Future work needs to account for contemporary trends in care and look more closely at how we conceive teams, measure their critical functions in cancer care and facilitate their work. Though the studies did not measure effect of teams on provider satisfaction, evidence that oncologists are experiencing significant feelings of burnout prompt examination of whether teams may improve mental and emotional well-being of clinicians involved. Additionally, despite our best efforts to be comprehensive, it is possible that relevant articles may not have been picked up by our search methods, and that negative studies have not been reported. Finally, our review is limited to published peer-reviewed literature. It is plausible that studies demonstrating no statistically or clinically meaningful effects have lower probability of appearing in peer-reviewed outlets compared with studies that do report significant differences or effects.
Conclusion
Overall, our findings suggest limited, though promising, evidence concerning the effectiveness of MDTs for cancer care to date. Though there is evidence that MDTs improved treatment planning, there is little evidence about how teams achieve that end, and limited evidence that MDTs affect the long-term survival of patients with cancer. The evidence regarding teamwork in cancer care is therefore rudimentary, although it points to the potential to improve cancer care value and health outcomes. We need more research on how cancer care teams function and affect long-term outcomes, and we need a better understanding of how lessons about teams in other settings applies to teams in cancer care. We begin to explore the application of principles to teams in cancer care in an associated article.49 National Cancer Institute (NCI) and ASCO are using this evidence review and accompanying diagnostic case manuscript to launch an initiative to facilitate team researchers and clinicians' collaboration to bring what is known about teams into cancer care delivery. Information about the NCI-ASCO Teams in Cancer Care Delivery Initiative is available on the ASCO Web site at www.ASCO.org/teams.
Acknowledgment
Supported in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E (H.M.E.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Appendix
The following words were used to search each database listed below and duplicates were then removed from the combined pool of identified records.
PubMed
(((teams[All Fields] OR teamwork[All Fields]) OR multidisciplinary cooperation[All Fields]) OR (“cooperative behavior”[MeSH Terms] OR (“cooperative”[All Fields] AND “behavior”[All Fields]) OR “cooperative behavior”[All Fields] OR “collaboration”[All Fields])) AND “neoplasms”[MeSH Terms] AND ((Clinical Trial[ptyp] OR Comparative Study[ptyp]) AND “2009/08/27”[PDat] : “2014/08/25”[PDat] AND “humans”[MeSH Terms])
Results: 270
Embase
‘cancer’/exp OR ‘cancer’ AND teams OR ‘teamwork’/exp OR teamwork OR ‘multidisciplinary cooperation’ OR collaboration ('comparative study'/de OR ‘human’/de OR ‘randomized controlled trial’/de) AND ‘neoplasm’/de AND (2009:py OR 2010:py OR 2011:py OR 2012:py OR 2013:py OR 2014:py)
Scopus
(TITLE-ABS-KEY(cancer) AND TITLE-ABS-KEY(teams) OR TITLE-ABS-KEY(teamwork) OR TITLE-ABS-KEY(“multidisciplinary cooperation”) OR TITLE-ABS-KEY(collaboration)) AND SUBJAREA(mult OR medi OR nurs OR vete OR dent OR heal OR mult OR arts OR busi OR deci OR econ OR psyc OR soci) AND PUBYEAR more than 2008 AND (LIMIT-TO(LANGUAGE, “English”)) AND (EXCLUDE(SUBJAREA, “BIOC”)) AND (EXCLUDE(SUBJAREA, “PHAR”) OR EXCLUDE(SUBJAREA, “IMMU”))
ABI/INFORM Complete
ab(cancer) AND ab((teams OR teamwork)) OR ab((“multidisciplinary cooperation” OR collaboration)) AND (randomized control OR comparative)
Date: From August 2009 to August 2014; Source type: Scholarly Journals; Document type: Article: Language: English; peer reviewed
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: Stephen H. Taplin, Sallie Weaver, Eduardo Salas, Veronica Chollette, Michael P. Kosty
Administrative support: Stephen H. Taplin, Veronica Chollette, Suanna S. Bruinooge
Provision of study materials or patients: Stephen H. Taplin
Collection and assembly of data: Stephen H. Taplin, Veronica Chollette, Heather M. Edwards
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Reviewing Cancer Care Team Effectiveness
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Stephen H. Taplin
No relationship to disclose
Sallie Weaver
No relationship to disclose
Eduardo Salas
No relationship to disclose
Veronica Chollette
Employment: Healthcare at Home
Leadership: Healthcare at Home
Stock or Other Ownership: Healthcare at Home
Heather M. Edwards
Employment: Leidos Biomedical
Suanna S. Bruinooge
No relationship to disclose
Michael P. Kosty
Speakers' Bureau: Astellas Pharma, Genentech/Roche, Sanofi, Lilly, Bayer
Research Funding: Genentech/Roche (Inst), Merck Serono (Inst)
References
- 1.Poole M, Real K. Groups and teams in health care: Communication and effectiveness. In: Thompson T, Dorsey A, Miller K, et al., editors. Handbook of Health Communication. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. pp. 369–402. [Google Scholar]
- 2.Mathieu J, Maynard MT, Rapp T, et al. Team effectiveness 1997-2007: A review of recent advancements and a glimpse into the future. J Manage. 2008;34:410–476. [Google Scholar]
- 3.Cohen SG, Bailey DE. What makes teams work: Group effectiveness research from the shop floor to the executive suite. J Manage. 1997;23:239–289. [Google Scholar]
- 4.Friedman EL, Chawla N, Morris PT, et al. Assessing the development of multidisciplinary care: Experience of the National Cancer Institute Community Cancer Centers Program. J Oncol Pract. 2014;10:e36–e43. doi: 10.1200/JOP.2014.001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taplin SH, Rodgers AB. Toward improving the quality of cancer care: Addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010:3–10. doi: 10.1093/jncimonographs/lgq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West MA, Guthrie JP, Dawson JF, et al. Reducing patient mortality in hospitals: The role of human resource management. J Organiz Behav. 2006;27:983–1002. [Google Scholar]
- 7.Bosch M, Faber MJ, Cruijsberg J, et al. Review article: Effectiveness of patient care teams and the role of clinical expertise and coordination: A literature review. Med Care Res Rev. 2009;66:5S–35S. doi: 10.1177/1077558709343295. [DOI] [PubMed] [Google Scholar]
- 8.Weaver SJ, Dy SM, Rosen MA. Team-training in healthcare: A narrative synthesis of the literature. BMJ Qual Saf. 2014;23:359–372. doi: 10.1136/bmjqs-2013-001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Public Law 111-148. Patient Protection and Affordable Care Act. www.hhs.gov/healthcare/facts/timeline/timeline-text.html#2012.
- 10.American Society of Clinical Oncology, European Society for Medical Oncology. ASCO-ESMO consensus statement on quality cancer care. Ann Oncol. 2006;17:1063–1064. doi: 10.1093/annonc/mdl152. [DOI] [PubMed] [Google Scholar]
- 11.King HB, Battles J, Baker DP, et al. TeamSTEPPS: Team strategies and tools to enhance performance and patient safety. In: Henriksen K, Battles JB, Keyes MA, et al., editors. Advances in Patient Safety: New Directions and Alternative Approaches (Vol. 3: Performance and Tools) Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 12.Yang W, Williams JH, Hogan PF, et al. Projected supply of and demand for oncologists and radiation oncologists through 2025: An aging, better-insured population will result in shortage. J Oncol Pract. 2014;10:39–45. doi: 10.1200/JOP.2013.001319. [DOI] [PubMed] [Google Scholar]
- 13.Lemieux-Charles L, McGuire WL. What do we know about health care team effectiveness? A review of the literature. Med Care Res Rev. 2006;63:263–300. doi: 10.1177/1077558706287003. [DOI] [PubMed] [Google Scholar]
- 14.Fennell M, Prabhu Das I, Clauser S, et al. The organization of multidisciplinary care teams: Modeling internal and external influences on cancer care quality. J Natl Cancer Inst Monogr. 2010;40:72–80. doi: 10.1093/jncimonographs/lgq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Surgeons, Commission on Cancer. Cancer Program Standards 2012: Ensuring Patient-Centered Care. Chicago, IL: American College of Surgeons; 2012. [Google Scholar]
- 16.Taylor C, Munro AJ, Glynne-Jones R, et al. Multidisciplinary team working in cancer: What is the evidence? BMJ. 2010;340:c951. doi: 10.1136/bmj.c951. [DOI] [PubMed] [Google Scholar]
- 17.Kelly SL, Jackson JE, Hickey BE, et al. Multidisciplinary clinic care improves adherence to best practice in head and neck cancer. Am J Otolaryngol. 2013;34:57–60. doi: 10.1016/j.amjoto.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Ye YJ, Shen ZL, Sun XT, et al. Impact of multidisciplinary team working on the management of colorectal cancer. Chin Med J (Engl) 2012;125:172–177. [PubMed] [Google Scholar]
- 19.Powell AA, Nugent S, Ordin DL, et al. Evaluation of a VHA collaborative to improve follow-up after a positive colorectal cancer screening test. Med Care. 2011;49:897–903. doi: 10.1097/MLR.0b013e3182204944. [DOI] [PubMed] [Google Scholar]
- 20.Daly BJ, Douglas SL, Gunzler D, et al. Clinical trial of a supportive care team for patients with advanced cancer. J Pain Symptom Manage. 2013;46:775. doi: 10.1016/j.jpainsymman.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wille-Jørgensen P, Sparre P, Glenthøj A, et al. Result of the implementation of multidisciplinary teams in rectal cancer. Colorectal Dis. 2013;15:410–413. doi: 10.1111/codi.12013. [DOI] [PubMed] [Google Scholar]
- 22.Marshall CL, Balentine CJ, Robinson CN, et al. A multidisciplinary cancer center maximizes surgeons' impact. J Surg Res. 2011;171:15–22. doi: 10.1016/j.jss.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Lewis PC, Holcomb B. A model for patient-centered Army primary care. Mil Med. 2012;177:1502–1507. doi: 10.7205/milmed-d-12-00076. [DOI] [PubMed] [Google Scholar]
- 24.Riolfi M, Buja A, Zanardo C, et al. Effectiveness of palliative home-care services in reducing hospital admissions and determinants of hospitalization for terminally ill patients followed up by a palliative home-care team: A retrospective cohort study. Palliat Med. 2014;28:403–411. doi: 10.1177/0269216313517283. [DOI] [PubMed] [Google Scholar]
- 25.Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: Retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344:e2718. doi: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandieri E, Sichetti D, Romero M, et al. Impact of early access to a palliative/supportive care intervention on pain management in patients with cancer. Ann Oncol. 2012;23:2016–2020. doi: 10.1093/annonc/mds103. [DOI] [PubMed] [Google Scholar]
- 27.Boxer MM, Vinod SK, Shafiq J, et al. Do multidisciplinary team meetings make a difference in the management of lung cancer? Cancer. 2011;117:5112–120. doi: 10.1002/cncr.26149. [DOI] [PubMed] [Google Scholar]
- 28.Palmer G, Martling A, Cedermark B, et al. Preoperative tumour staging with multidisciplinary team assessment improves the outcome in locally advanced primary rectal cancer. Colorectal Dis. 2011;13:1361–1369. doi: 10.1111/j.1463-1318.2010.02460.x. [DOI] [PubMed] [Google Scholar]
- 29.Bydder S, Nowak A, Marion K, et al. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39:838–841. doi: 10.1111/j.1445-5994.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 30.Dyar S, Lesperance M, Shannon R, et al. A nurse practitioner directed intervention improves the quality of life of patients with metastatic cancer: Results of a randomized pilot study. J Palliat Med. 2012;15:890–895. doi: 10.1089/jpm.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine RA, Chawla B, Bergeron S, et al. Multidisciplinary management of colorectal cancer enhances access to multimodal therapy and compliance with National Comprehensive Cancer Network (NCCN) guidelines. Int J Colorectal Dis. 2012;27:1531–158. doi: 10.1007/s00384-012-1501-z. [DOI] [PubMed] [Google Scholar]
- 32.Simons S, Ringsdorf S, Braun M, et al. Enhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical care. Support Care Cancer. 2011;19:1009–1018. doi: 10.1007/s00520-010-0927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunnell CA, Weingart SN, Swanson S, et al. Models of multidisciplinary cancer care: Physician and patient perceptions in a comprehensive cancer center. J Oncol Pract. 2010;6:283–288. doi: 10.1200/JOP.2010.000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reference deleted.
- 35.Lamb BW, Brown KF, Nagpal K, et al. Quality of care management decisions by multidisciplinary cancer teams: A systematic review. Ann Surg Oncol. 2011;18:2116–2125. doi: 10.1245/s10434-011-1675-6. [DOI] [PubMed] [Google Scholar]
- 36.Lamb BW, Sevdalis N, Taylor C, et al. Multidisciplinary team working across different tumour types: Analysis of a national survey. Ann Oncol. 2012;23:1293–1300. doi: 10.1093/annonc/mdr453. [DOI] [PubMed] [Google Scholar]
- 37.Lamb BW, Jalil RT, Sevdalis N, et al. Strategies to improve the efficiency and utility of multidisciplinary team meetings in urology cancer care: A survey study. BMC Health Serv Res. 2014;14:377. doi: 10.1186/1472-6963-14-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jalil R, Lamb B, Russ S, et al. The cancer multi-disciplinary team from the coordinators' perspective: Results from a national survey in the UK. BMC Health Serv Res. 2012;12:457. doi: 10.1186/1472-6963-12-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamb BW, Taylor C, Lamb JN, et al. Facilitators and barriers to teamworking and patient centeredness in multidisciplinary cancer teams: Findings of a national study. Ann Surg Oncol. 2013;20:1408–1416. doi: 10.1245/s10434-012-2676-9. [DOI] [PubMed] [Google Scholar]
- 40.Salas E, Wilson KA, Murphy CE, et al. Communicating, coordinating, and cooperating when lives depend on it: Tips for teamwork. Jt Comm J Qual Patient Saf. 2008;34:333–341. doi: 10.1016/s1553-7250(08)34042-2. [DOI] [PubMed] [Google Scholar]
- 41.Weaver SJ, Feitosa J, Salas E, et al. The theoretical drivers and models of team performance and effectiveness for patient safety. In: Salas E, Frush K, editors. Improving Patient Safety Through Teamwork and Team Training. New York, NY: Oxford University Press; 2012. pp. 3–79. [Google Scholar]
- 42.Frush K, Maynard L, Koeble C, et al. Regulating and monitoring teamwork and training in healthcare: Issues and challenges. In: Salas E, Frush K, editors. Improving Patient Safety Through Teamwork and Team Training. New York, NY: Oxford University Press; 2012. pp. 94–104. [Google Scholar]
- 43.Salas E, Shuffler ML, Thayer AL, et al. Understanding and improving teamwork in organizations: a scientifically based practical guide. Hum Resource Manage. [epub ahead of print on October 29, 2014] [Google Scholar]
- 44.King HB, Harden SW. Sustainment of teamwork. In: Salas E, Frush K, editors. Improving Patient Safety Through Teamwork and Team Training. New York, NY: Oxford University Press; 2013. pp. 188–197. [Google Scholar]
- 45.Salas E, Rosen MA. Building high reliability teams: Progress and some reflections on teamwork training. BMJ Qual Saf. 2013;22:369–373. doi: 10.1136/bmjqs-2013-002015. [DOI] [PubMed] [Google Scholar]
- 46.Crabtree BF, Nutting PA, Miller WL, et al. Summary of the National Demonstration Project and recommendations for the patient-centered medical home. Ann Fam Med. 2010;8(suppl 1):S80–90. S92. doi: 10.1370/afm.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reference deleted.
- 48.Kozlowski SWJ, Ilgen DR. Enhancing the effectiveness of work groups and teams. Psychol Sci. 2006;7:77–124. doi: 10.1111/j.1529-1006.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- 49.Taplin SH, Weaver S, Chollette V, et al. Teams and teamwork during a cancer diagnosis. Interdependency within and between teams. J Oncol Pract. 2015;11:231–238. doi: 10.1200/JOP.2014.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]