Among patients with lung or colorectal cancer, frequent physician tumor board engagement was associated with patient clinical trial participation and higher rates of curative-intent surgery for stage I to II NSCLC but not with overall survival.
Abstract
Purpose:
Multidisciplinary tumor board meetings are common in cancer care, but limited evidence is available about their benefits. We assessed the associations of tumor board participation and structure with care delivery and patient outcomes.
Methods:
As part of the CanCORS study, we surveyed 1,601 oncologists and surgeons about participation in tumor boards and specific tumor board features. Among 4,620 patients with lung or colorectal cancer diagnosed from 2003 to 2005 and seen by 1,198 of these physicians, we assessed associations of tumor board participation with patient survival, clinical trial enrollment, guideline-recommended care, and patient-reported quality, adjusting for patient and physician characteristics.
Results:
Weekly physician tumor board participation (v participation less often or never) was not associated with patient survival, although in exploratory subgroup analyses, weekly participation was associated with lower mortality for extensive-stage small-cell lung cancer and stage IV colorectal cancer. Patients treated by the 54% of physicians participating in tumor boards weekly (v less often or never) were more likely to enroll onto clinical trials (odds ratio [OR], 1.6; 95% CI, 1.1 to 2.2). Patients with stage I to II non–small-cell lung cancer (NSCLC) whose physicians participated in tumor boards weekly were more likely to undergo curative-intent surgery (OR, 2.9; 95% CI, 1.3 to 6.8), although those with stage I to II NSCLC whose physicians' meetings reviewed > one cancer site were less likely to undergo curative-intent surgery (OR, 0.1; 95% CI, 0.03 to 0.4).
Conclusion:
Among patients with lung or colorectal cancer, frequent physician tumor board engagement was associated with patient clinical trial participation and higher rates of curative-intent surgery for stage I to II NSCLC but not with overall survival.
Introduction
Tumor board conferences allow cancer care providers to discuss patient cases in multidisciplinary settings, and they also serve educational functions.1,2 These meetings are cornerstones of the American College of Surgeons Commission on Cancer accreditation program.3 Tumor boards have been prevalent for years.4 Nevertheless, participation patterns vary; for example, one study of breast cancer providers found that high-volume medical oncologists participated in tumor board meetings more frequently than low-volume surgeons.5
Few studies have investigated the nature of tumor board discussions or their impact on patient care and outcomes. One survey of Oregon hospitals found that tumor board recommendations were generally implemented.6 Two single-institution studies reviewed records of patients discussed at tumor boards and found that the meetings changed surgical recommendations for more than half of patients with breast cancer7 and overall treatment recommendations in 23.6% of patient cases of pancreatic cancer.8 In one Veterans Affairs (VA) medical center, for patient cases of rectal cancer presented at tumor boards, guideline-recommended therapy was more likely to be administered.9 However, a recent national study of VA medical centers found few associations between tumor boards and either patient survival or process measures, such as adjuvant chemotherapy for stage III colon cancer and surgery for stage I to II non–small-cell lung cancer (NSCLC).10,11 The study ascertained the presence of general or cancer-specific tumor boards at each center, but information about tumor board meeting structure was not available.
In this study, we used data from the CanCORS (Cancer Care Outcomes Research and Surveillance)12 study to characterize tumor board participation and features of tumor board meetings among physicians caring for a large, population- and health system–based cohort of patients with lung or colorectal cancer. We assessed associations between physician or practice traits and tumor board characteristics. We also examined associations between tumor boards and patient overall survival, clinical trial enrollment, delivery of guideline-recommended treatment, patient-reported quality of care, and patient ratings of health care team communication.
Methods
Study Design
CanCORS is a prospective observational study assessing care patterns and outcomes for patients with lung or colorectal cancer diagnosed between 2003 and 2005.12 Patients lived within one of five geographic regions (northern California, Los Angeles County, North Carolina, Iowa, or Alabama) or received care in one of five health maintenance organizations or 15 VA centers.13 The CanCORS cohort is representative of US patients with lung or colorectal cancer.13 Baseline patient surveys were administered to participants approximately 3 to 6 months after diagnosis. Patients who were unable to complete a full survey were offered a brief version; surrogates completed surveys for patients who were deceased by the time of the baseline survey or too ill to participate. A total of 9,732 patients had baseline surveys available (American Association of Public Opinion Research cooperation rate14 was 58.9% for patients with lung cancer and 61.0% for patients with colorectal cancer).13 Follow-up surveys were administered to patients or their surrogates approximately 14 months after diagnosis for those alive at the baseline survey (response rate, 81%). Medical record abstraction was performed through 15 months after diagnosis. Medical records were available for 77% of the overall cohort (separate consent was required for medical record review). Vital status was last updated in 2012 for 77% of patients; other CanCORS sites had complete survival information through 2010 or 2011. We excluded 603 patients with unknown cancer stage. The study was approved by human subjects committees of all participating institutions. All patients (or patient surrogates) provided verbal or written consent for participation.
In the baseline survey, patients identified physicians playing key roles in their care. We then surveyed these physicians (participation rate of 61% among physicians with known contact information).15 Of 4,326 physician respondents, we restricted the sample to 1,648 surgeons, medical oncologists, or radiation oncologists (other physicians were not asked about tumor board participation) and excluded 47 who did not answer the question about participation in tumor board meetings, for a final cohort of 1,601 physicians.
We merged physician and patient data, assigning one key physician to each patient. Where available, we linked to the physician described by patients as the most important in helping them make treatment decisions (38% of patient–physician links). Otherwise, we followed an algorithm according to specialty (Appendix Table A1, online only), because the impact of specialist tumor board participation may vary by disease type and stage. Patients with small-cell lung cancer (SCLC) were categorized as having limited-stage (stage I to III) versus extensive-stage disease (stage IV). We excluded 26 patient–physician links in which thoracic surgeons were linked to patients with colorectal cancer, or surgical oncologists or colorectal surgeons to patients with lung cancer. Physicians could be linked to multiple patients. We thus identified 4,620 patients linked to 1,198 physicians. For survival analyses, we excluded 37 patients enrolled through the Harvard Pilgrim Health Care CanCORS site, for whom vital status data were unavailable, leaving 4,593 patients linked to 1,184 physicians.
Outcome Variables
Physicians were asked whether they participated in tumor boards. Those who did were asked how often they participated and about features of their tumor boards, including whether the meetings served treatment planning functions, evaluated treatment decisions, reviewed only challenging patient cases, reviewed multiple tumor sites (v single site), or served as teaching sessions only (without review of specific patient cases).
For analyses of associations between physician tumor board participation and cancer care, outcome variables included: overall survival; patient participation in a clinical trial by 15 months after diagnosis, measured via baseline survey, follow-up survey, and medical record review16; delivery of three guideline-recommended therapies (surgery for stage I to II NSCLC, adjuvant chemotherapy for stage III colon cancer, and neoadjuvant or adjuvant chemoradiotherapy therapy for stage II to III rectal cancer)17–19; patient-reported overall care quality; and patient ratings of health care team communication.
Surgery for stage I to II NSCLC was defined as curative-intent surgery (ie, pneumonectomy, lobectomy, or wedge resection) within 6 months of diagnosis, ascertained via medical record review. Adjuvant chemotherapy for stage III colon cancer was defined as documentation of chemotherapy within 180 days of surgery or patient-reported chemotherapy after cancer surgery on the baseline survey conducted 3 to 6 months after diagnosis. Neoadjuvant or adjuvant chemoradiotherapy for stage II to III rectal cancer was defined as documentation of treatment within 180 days of surgery or patient-reported treatment or planned treatment on the baseline survey 3 to 6 months after diagnosis.
Patient ratings of cancer care quality were assessed using a 5-point Likert scale (excellent, very good, good, fair, poor); for analysis, we divided responses into excellent versus all other responses. Ratings of health care team communication were based on six questions derived from the Consumer Assessment of Healthcare Providers and Systems survey, as described previously.20,21 For this analysis, we calculated the mean of the individual scores from these questions (each ranging from 0 to 3) and assessed whether patients reported a top score (51% of ratings) versus any other score. We excluded 709 patients (15%) who responded to < four of the six questions; results were similar in sensitivity analyses that included all patients, using multiply imputed data for missing responses (data not shown).
Independent Variables
Our independent variables included physician age, sex, specialty, practice setting, teaching role, number of patients with lung or colorectal cancer seen each month, National Cancer Institute cancer center affiliation, and Community Clinical Oncology Program affiliation. We also assessed patient age at diagnosis, sex, race/ethnicity, marital status, educational attainment, income, survey type (patient v surrogate survey), receipt of surgery or planned surgery, receipt of chemotherapy, receipt of radiation therapy, number of self-reported comorbid conditions,22 cancer type, and stage.23 Physician and patient variables were categorized as listed in Table 1.
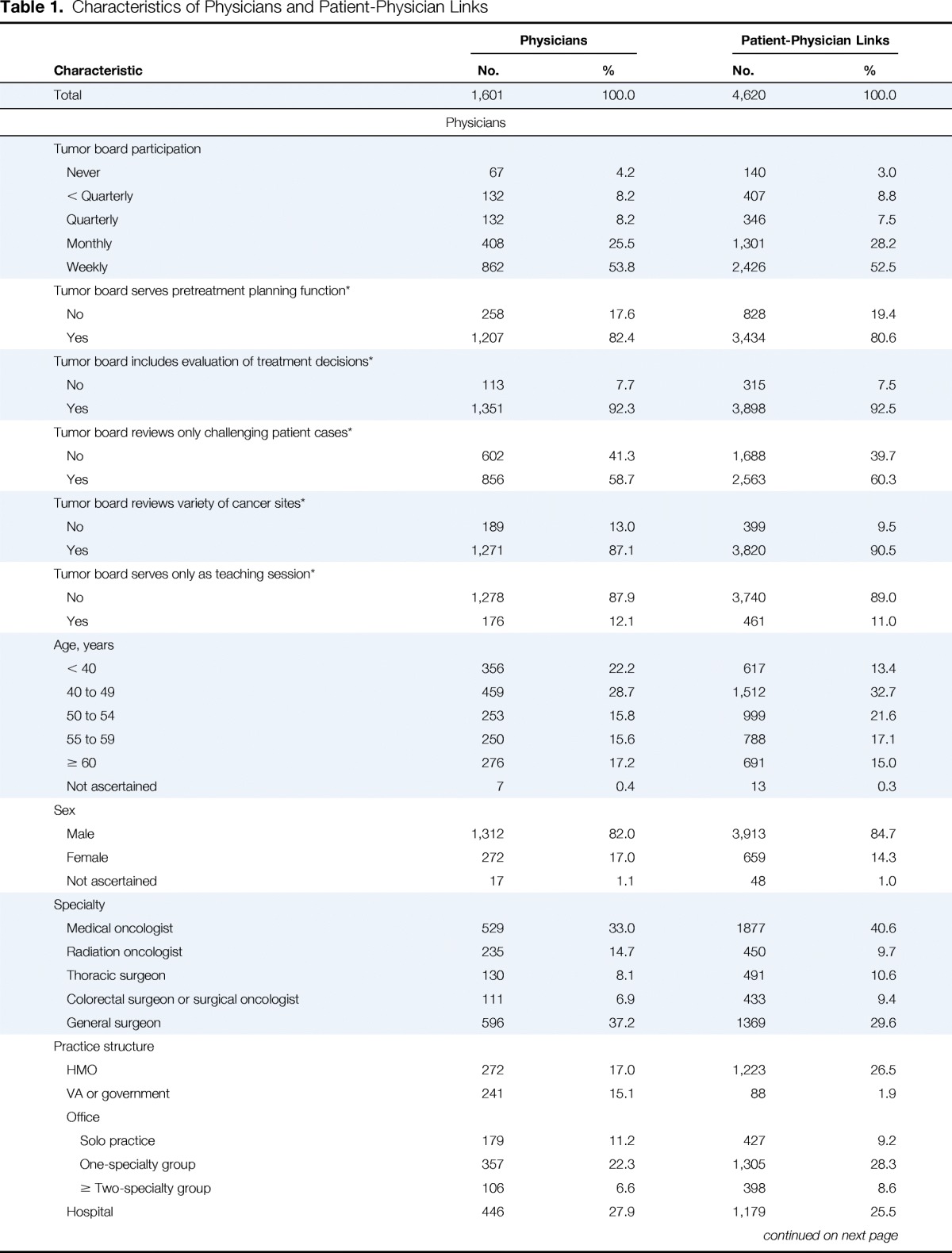
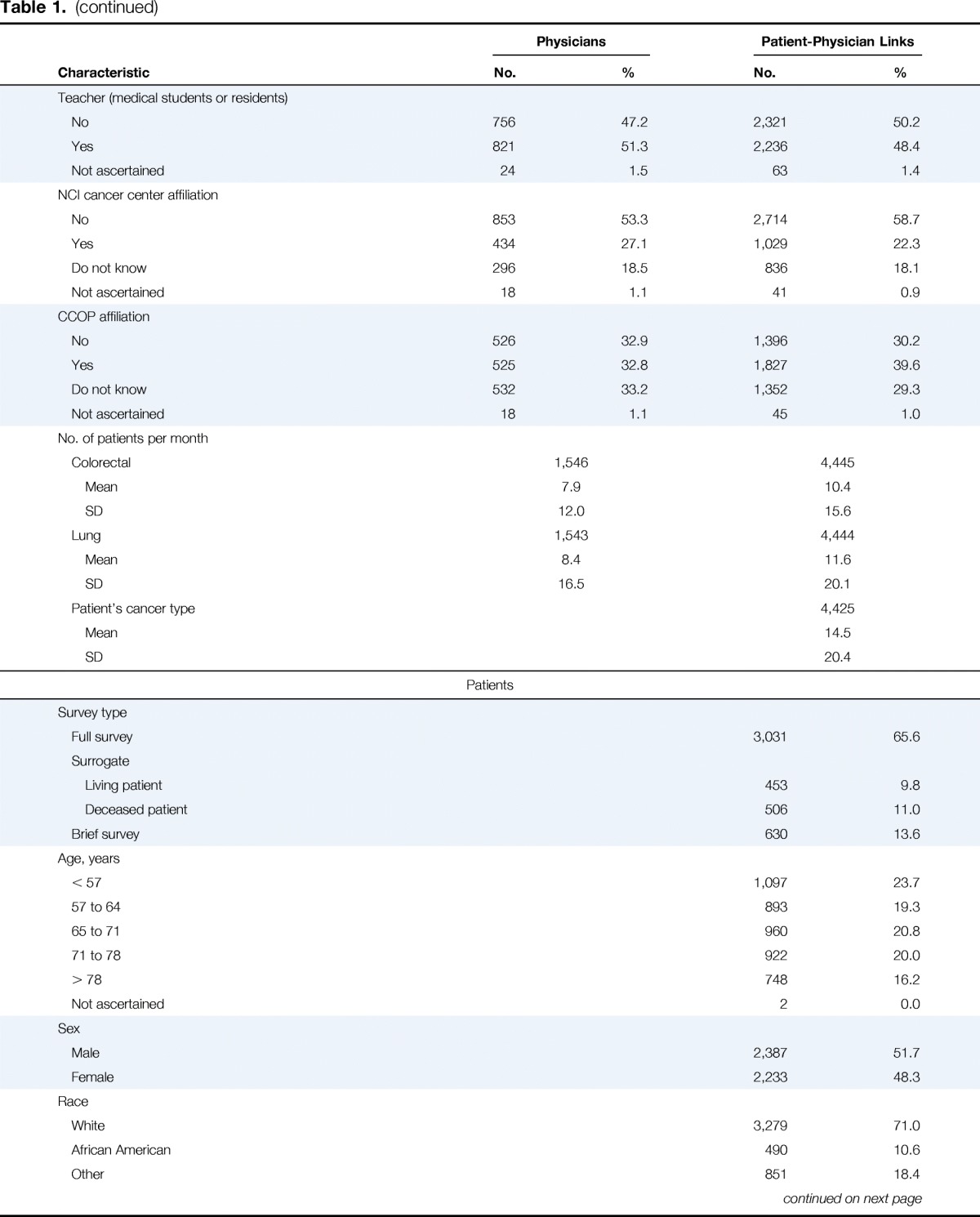
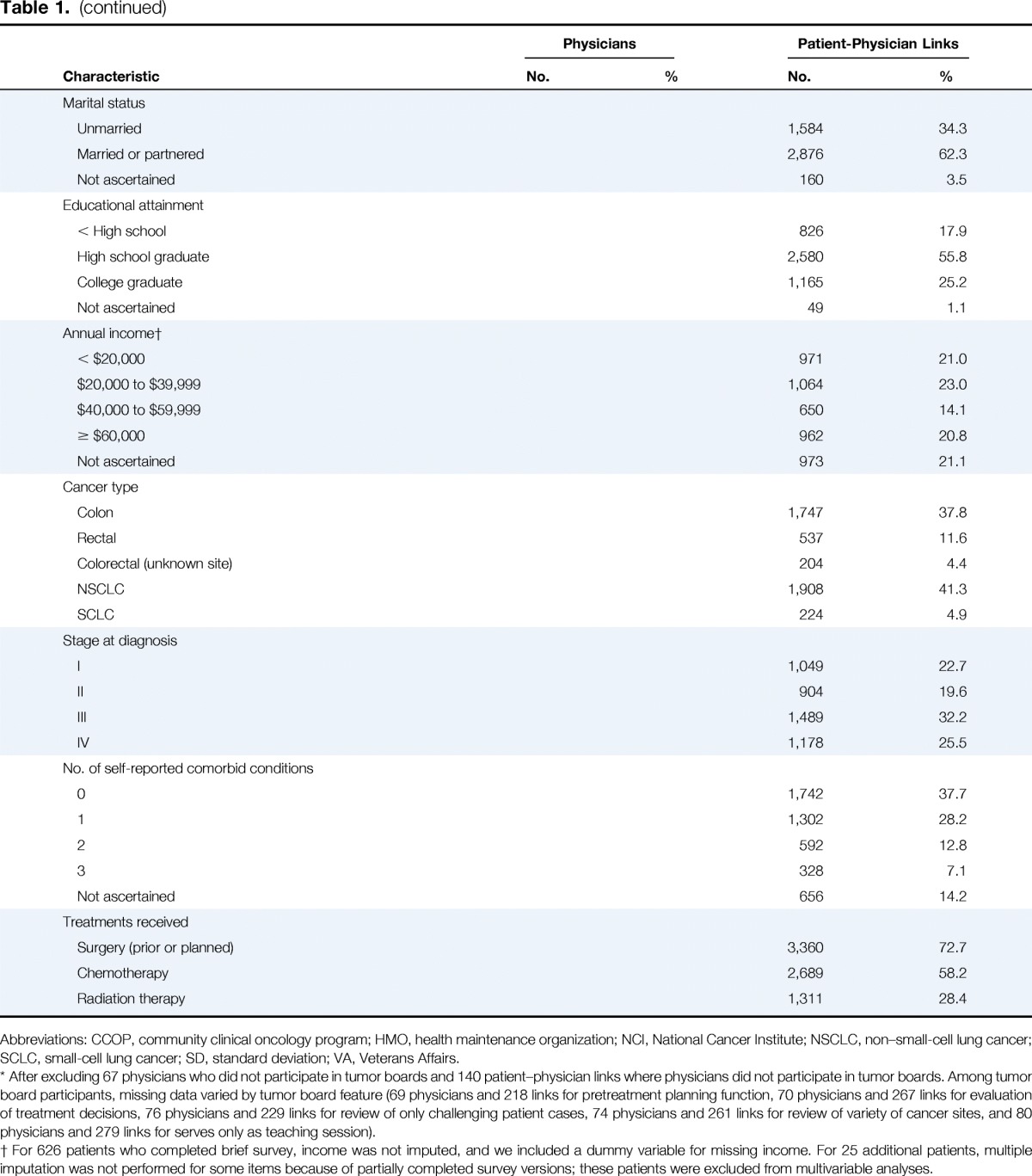
Table 1.
Characteristics of Physicians and Patient-Physician Links
| Characteristic | Physicians |
Patient-Physician Links |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Total | 1,601 | 100.0 | 4,620 | 100.0 |
| Physicians | ||||
| Tumor board participation | ||||
| Never | 67 | 4.2 | 140 | 3.0 |
| < Quarterly | 132 | 8.2 | 407 | 8.8 |
| Quarterly | 132 | 8.2 | 346 | 7.5 |
| Monthly | 408 | 25.5 | 1,301 | 28.2 |
| Weekly | 862 | 53.8 | 2,426 | 52.5 |
| Tumor board serves pretreatment planning function* | ||||
| No | 258 | 17.6 | 828 | 19.4 |
| Yes | 1,207 | 82.4 | 3,434 | 80.6 |
| Tumor board includes evaluation of treatment decisions* | ||||
| No | 113 | 7.7 | 315 | 7.5 |
| Yes | 1,351 | 92.3 | 3,898 | 92.5 |
| Tumor board reviews only challenging patient cases* | ||||
| No | 602 | 41.3 | 1,688 | 39.7 |
| Yes | 856 | 58.7 | 2,563 | 60.3 |
| Tumor board reviews variety of cancer sites* | ||||
| No | 189 | 13.0 | 399 | 9.5 |
| Yes | 1,271 | 87.1 | 3,820 | 90.5 |
| Tumor board serves only as teaching session* | ||||
| No | 1,278 | 87.9 | 3,740 | 89.0 |
| Yes | 176 | 12.1 | 461 | 11.0 |
| Age, years | ||||
| < 40 | 356 | 22.2 | 617 | 13.4 |
| 40 to 49 | 459 | 28.7 | 1,512 | 32.7 |
| 50 to 54 | 253 | 15.8 | 999 | 21.6 |
| 55 to 59 | 250 | 15.6 | 788 | 17.1 |
| ≥ 60 | 276 | 17.2 | 691 | 15.0 |
| Not ascertained | 7 | 0.4 | 13 | 0.3 |
| Sex | ||||
| Male | 1,312 | 82.0 | 3,913 | 84.7 |
| Female | 272 | 17.0 | 659 | 14.3 |
| Not ascertained | 17 | 1.1 | 48 | 1.0 |
| Specialty | ||||
| Medical oncologist | 529 | 33.0 | 1877 | 40.6 |
| Radiation oncologist | 235 | 14.7 | 450 | 9.7 |
| Thoracic surgeon | 130 | 8.1 | 491 | 10.6 |
| Colorectal surgeon or surgical oncologist | 111 | 6.9 | 433 | 9.4 |
| General surgeon | 596 | 37.2 | 1369 | 29.6 |
| Practice structure | ||||
| HMO | 272 | 17.0 | 1,223 | 26.5 |
| VA or government | 241 | 15.1 | 88 | 1.9 |
| Office | ||||
| Solo practice | 179 | 11.2 | 427 | 9.2 |
| One-specialty group | 357 | 22.3 | 1,305 | 28.3 |
| ≥ Two-specialty group | 106 | 6.6 | 398 | 8.6 |
| Hospital | 446 | 27.9 | 1,179 | 25.5 |
| Teacher (medical students or residents) | ||||
| No | 756 | 47.2 | 2,321 | 50.2 |
| Yes | 821 | 51.3 | 2,236 | 48.4 |
| Not ascertained | 24 | 1.5 | 63 | 1.4 |
| NCI cancer center affiliation | ||||
| No | 853 | 53.3 | 2,714 | 58.7 |
| Yes | 434 | 27.1 | 1,029 | 22.3 |
| Do not know | 296 | 18.5 | 836 | 18.1 |
| Not ascertained | 18 | 1.1 | 41 | 0.9 |
| CCOP affiliation | ||||
| No | 526 | 32.9 | 1,396 | 30.2 |
| Yes | 525 | 32.8 | 1,827 | 39.6 |
| Do not know | 532 | 33.2 | 1,352 | 29.3 |
| Not ascertained | 18 | 1.1 | 45 | 1.0 |
| No. of patients per month | ||||
| Colorectal | 1,546 | 4,445 | ||
| Mean | 7.9 | 10.4 | ||
| SD | 12.0 | 15.6 | ||
| Lung | 1,543 | 4,444 | ||
| Mean | 8.4 | 11.6 | ||
| SD | 16.5 | 20.1 | ||
| Patient's cancer type | 4,425 | |||
| Mean | 14.5 | |||
| SD | 20.4 | |||
| Patients | ||||
| Survey type | ||||
| Full survey | 3,031 | 65.6 | ||
| Surrogate | ||||
| Living patient | 453 | 9.8 | ||
| Deceased patient | 506 | 11.0 | ||
| Brief survey | 630 | 13.6 | ||
| Age, years | ||||
| < 57 | 1,097 | 23.7 | ||
| 57 to 64 | 893 | 19.3 | ||
| 65 to 71 | 960 | 20.8 | ||
| 71 to 78 | 922 | 20.0 | ||
| > 78 | 748 | 16.2 | ||
| Not ascertained | 2 | 0.0 | ||
| Sex | ||||
| Male | 2,387 | 51.7 | ||
| Female | 2,233 | 48.3 | ||
| Race | ||||
| White | 3,279 | 71.0 | ||
| African American | 490 | 10.6 | ||
| Other | 851 | 18.4 | ||
| Marital status | ||||
| Unmarried | 1,584 | 34.3 | ||
| Married or partnered | 2,876 | 62.3 | ||
| Not ascertained | 160 | 3.5 | ||
| Educational attainment | ||||
| < High school | 826 | 17.9 | ||
| High school graduate | 2,580 | 55.8 | ||
| College graduate | 1,165 | 25.2 | ||
| Not ascertained | 49 | 1.1 | ||
| Annual income† | ||||
| < $20,000 | 971 | 21.0 | ||
| $20,000 to $39,999 | 1,064 | 23.0 | ||
| $40,000 to $59,999 | 650 | 14.1 | ||
| ≥ $60,000 | 962 | 20.8 | ||
| Not ascertained | 973 | 21.1 | ||
| Cancer type | ||||
| Colon | 1,747 | 37.8 | ||
| Rectal | 537 | 11.6 | ||
| Colorectal (unknown site) | 204 | 4.4 | ||
| NSCLC | 1,908 | 41.3 | ||
| SCLC | 224 | 4.9 | ||
| Stage at diagnosis | ||||
| I | 1,049 | 22.7 | ||
| II | 904 | 19.6 | ||
| III | 1,489 | 32.2 | ||
| IV | 1,178 | 25.5 | ||
| No. of self-reported comorbid conditions | ||||
| 0 | 1,742 | 37.7 | ||
| 1 | 1,302 | 28.2 | ||
| 2 | 592 | 12.8 | ||
| 3 | 328 | 7.1 | ||
| Not ascertained | 656 | 14.2 | ||
| Treatments received | ||||
| Surgery (prior or planned) | 3,360 | 72.7 | ||
| Chemotherapy | 2,689 | 58.2 | ||
| Radiation therapy | 1,311 | 28.4 | ||
Abbreviations: CCOP, community clinical oncology program; HMO, health maintenance organization; NCI, National Cancer Institute; NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer; SD, standard deviation; VA, Veterans Affairs.
After excluding 67 physicians who did not participate in tumor boards and 140 patient–physician links where physicians did not participate in tumor boards. Among tumor board participants, missing data varied by tumor board feature (69 physicians and 218 links for pretreatment planning function, 70 physicians and 267 links for evaluation of treatment decisions, 76 physicians and 229 links for review of only challenging patient cases, 74 physicians and 261 links for review of variety of cancer sites, and 80 physicians and 279 links for serves only as teaching session).
For 626 patients who completed brief survey, income was not imputed, and we included a dummy variable for missing income. For 25 additional patients, multiple imputation was not performed for some items because of partially completed survey versions; these patients were excluded from multivariable analyses.
Statistical Analyses
Item nonresponse for physician and patient surveys was < 10%, except for patient questions not included in the brief survey version, including income (21% missing) and number of comorbid conditions (14% missing). For multivariable analyses, multiple imputation was used to impute missing data.24 For survival analyses, we used Cox proportional hazards models, stratified by cancer type and American Joint Committee on Cancer stage. Additionally, because the impact of tumor board participation on patient outcome may vary according to cancer type and stage, we conducted subgroup analyses in which these associations were tested separately within each stratum. For analyses of clinical trial participation, treatments delivered, and ratings of care quality and care team communication, we used multivariable logistic regression, adjusting for physician and patient characteristics as listed in Tables 2 and 3.
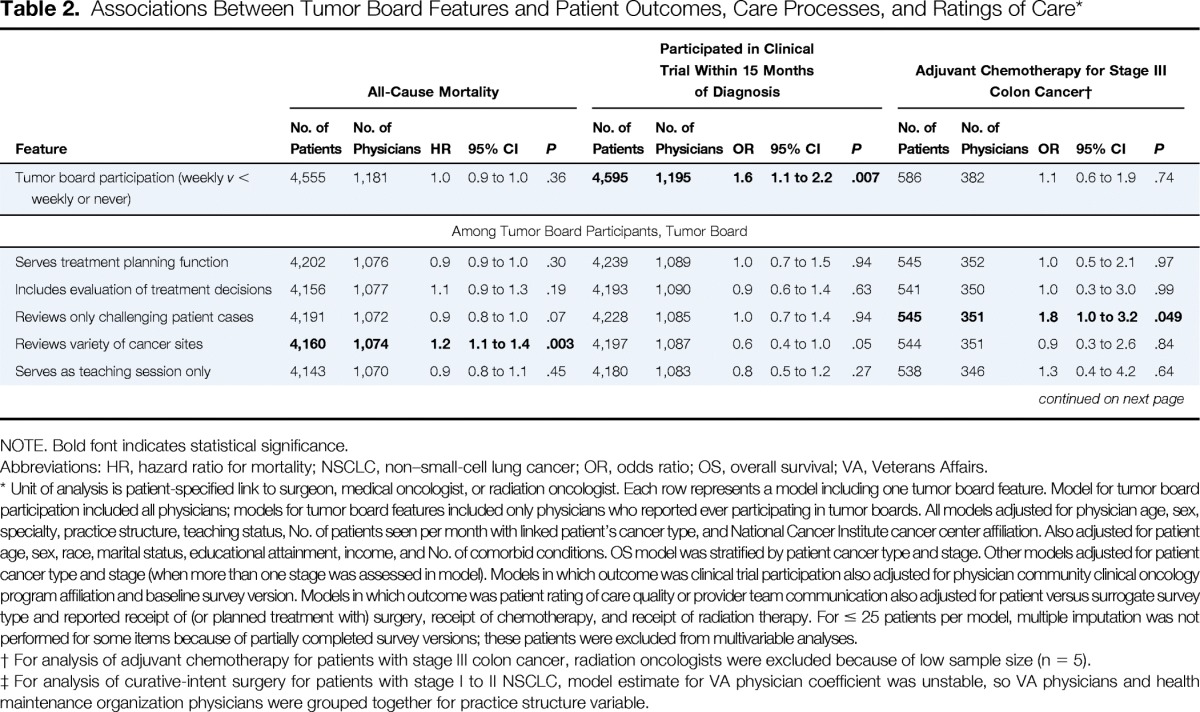
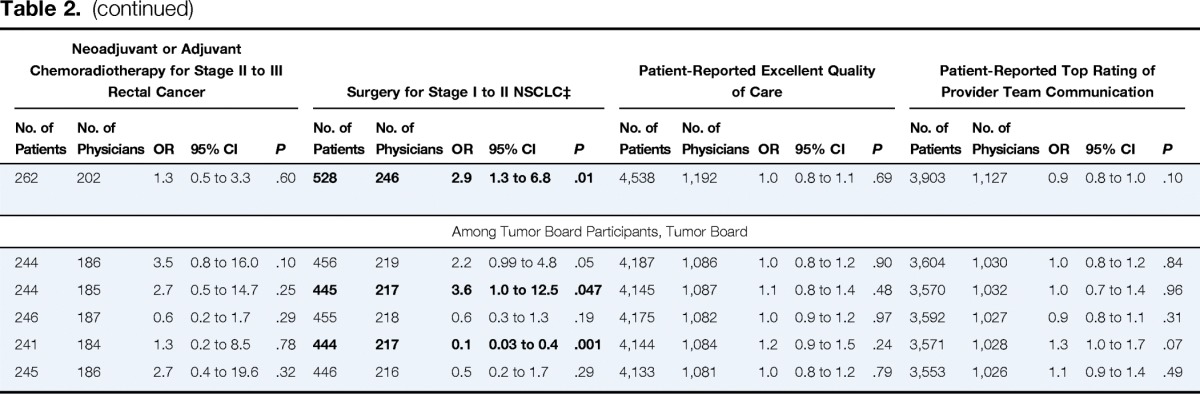
Table 2.
Associations Between Tumor Board Features and Patient Outcomes, Care Processes, and Ratings of Care*
| Feature | All-Cause Mortality |
Participated in Clinical Trial Within 15 Months of Diagnosis |
Adjuvant Chemotherapy for Stage III Colon Cancer† |
Neoadjuvant or Adjuvant Chemoradiotherapy for Stage II to III Rectal Cancer |
Surgery for Stage I to II NSCLC‡ |
Patient-Reported Excellent Quality of Care |
Patient-Reported Top Rating of Provider Team Communication |
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | No. of Physicians | HR | 95% CI | P | No. of Patients | No. of Physicians | OR | 95% CI | P | No. of Patients | No. of Physicians | OR | 95% CI | P | No. of Patients | No. of Physicians | OR | 95% CI | P | No. of Patients | No. of Physicians | OR | 95% CI | P | No. of Patients | No. of Physicians | OR | 95% CI | P | No. of Patients | No. of Physicians | OR | 95% CI | P | |
| Tumor board participation (weekly v < weekly or never) | 4,555 | 1,181 | 1.0 | 0.9 to 1.0 | .36 | 4,595 | 1,195 | 1.6 | 1.1 to 2.2 | .007 | 586 | 382 | 1.1 | 0.6 to 1.9 | .74 | 262 | 202 | 1.3 | 0.5 to 3.3 | .60 | 528 | 246 | 2.9 | 1.3 to 6.8 | .01 | 4,538 | 1,192 | 1.0 | 0.8 to 1.1 | .69 | 3,903 | 1,127 | 0.9 | 0.8 to 1.0 | .10 |
| Among Tumor Board Participants, Tumor Board | |||||||||||||||||||||||||||||||||||
| Serves treatment planning function | 4,202 | 1,076 | 0.9 | 0.9 to 1.0 | .30 | 4,239 | 1,089 | 1.0 | 0.7 to 1.5 | .94 | 545 | 352 | 1.0 | 0.5 to 2.1 | .97 | 244 | 186 | 3.5 | 0.8 to 16.0 | .10 | 456 | 219 | 2.2 | 0.99 to 4.8 | .05 | 4,187 | 1,086 | 1.0 | 0.8 to 1.2 | .90 | 3,604 | 1,030 | 1.0 | 0.8 to 1.2 | .84 |
| Includes evaluation of treatment decisions | 4,156 | 1,077 | 1.1 | 0.9 to 1.3 | .19 | 4,193 | 1,090 | 0.9 | 0.6 to 1.4 | .63 | 541 | 350 | 1.0 | 0.3 to 3.0 | .99 | 244 | 185 | 2.7 | 0.5 to 14.7 | .25 | 445 | 217 | 3.6 | 1.0 to 12.5 | .047 | 4,145 | 1,087 | 1.1 | 0.8 to 1.4 | .48 | 3,570 | 1,032 | 1.0 | 0.7 to 1.4 | .96 |
| Reviews only challenging patient cases | 4,191 | 1,072 | 0.9 | 0.8 to 1.0 | .07 | 4,228 | 1,085 | 1.0 | 0.7 to 1.4 | .94 | 545 | 351 | 1.8 | 1.0 to 3.2 | .049 | 246 | 187 | 0.6 | 0.2 to 1.7 | .29 | 455 | 218 | 0.6 | 0.3 to 1.3 | .19 | 4,175 | 1,082 | 1.0 | 0.9 to 1.2 | .97 | 3,592 | 1,027 | 0.9 | 0.8 to 1.1 | .31 |
| Reviews variety of cancer sites | 4,160 | 1,074 | 1.2 | 1.1 to 1.4 | .003 | 4,197 | 1,087 | 0.6 | 0.4 to 1.0 | .05 | 544 | 351 | 0.9 | 0.3 to 2.6 | .84 | 241 | 184 | 1.3 | 0.2 to 8.5 | .78 | 444 | 217 | 0.1 | 0.03 to 0.4 | .001 | 4,144 | 1,084 | 1.2 | 0.9 to 1.5 | .24 | 3,571 | 1,028 | 1.3 | 1.0 to 1.7 | .07 |
| Serves as teaching session only | 4,143 | 1,070 | 0.9 | 0.8 to 1.1 | .45 | 4,180 | 1,083 | 0.8 | 0.5 to 1.2 | .27 | 538 | 346 | 1.3 | 0.4 to 4.2 | .64 | 245 | 186 | 2.7 | 0.4 to 19.6 | .32 | 446 | 216 | 0.5 | 0.2 to 1.7 | .29 | 4,133 | 1,081 | 1.0 | 0.8 to 1.2 | .79 | 3,553 | 1,026 | 1.1 | 0.9 to 1.4 | .49 |
NOTE. Bold font indicates statistical significance.
Abbreviations: HR, hazard ratio for mortality; NSCLC, non–small-cell lung cancer; OR, odds ratio; OS, overall survival; VA, Veterans Affairs.
Unit of analysis is patient-specified link to surgeon, medical oncologist, or radiation oncologist. Each row represents a model including one tumor board feature. Model for tumor board participation included all physicians; models for tumor board features included only physicians who reported ever participating in tumor boards. All models adjusted for physician age, sex, specialty, practice structure, teaching status, No. of patients seen per month with linked patient's cancer type, and National Cancer Institute cancer center affiliation. Also adjusted for patient age, sex, race, marital status, educational attainment, income, and No. of comorbid conditions. OS model was stratified by patient cancer type and stage. Other models adjusted for patient cancer type and stage (when more than one stage was assessed in model). Models in which outcome was clinical trial participation also adjusted for physician community clinical oncology program affiliation and baseline survey version. Models in which outcome was patient rating of care quality or provider team communication also adjusted for patient versus surrogate survey type and reported receipt of (or planned treatment with) surgery, receipt of chemotherapy, and receipt of radiation therapy. For ≤ 25 patients per model, multiple imputation was not performed for some items because of partially completed survey versions; these patients were excluded from multivariable analyses.
For analysis of adjuvant chemotherapy for patients with stage III colon cancer, radiation oncologists were excluded because of low sample size (n = 5).
For analysis of curative-intent surgery for patients with stage I to II NSCLC, model estimate for VA physician coefficient was unstable, so VA physicians and health maintenance organization physicians were grouped together for practice structure variable.
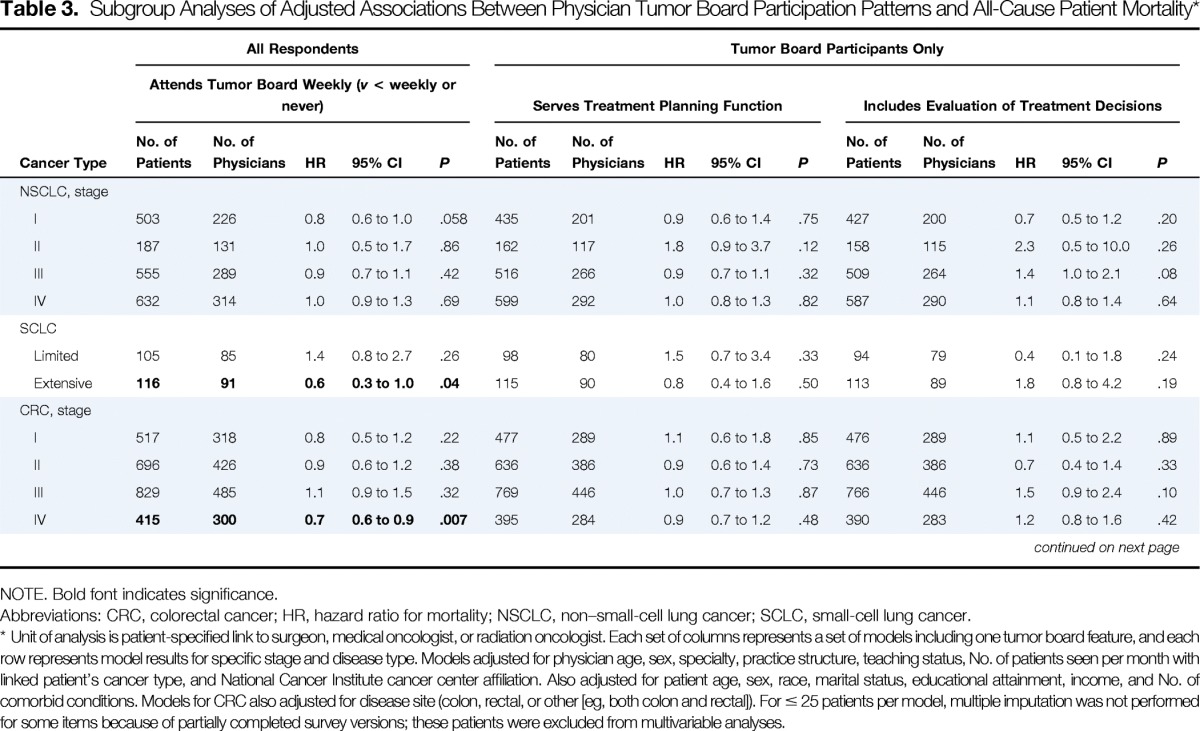
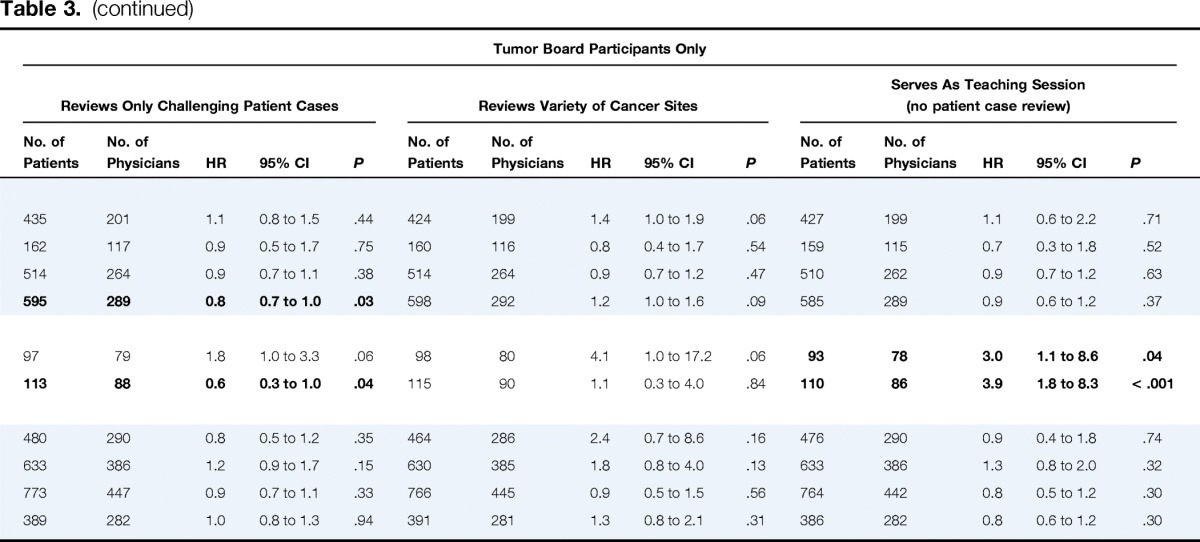
Table 3.
Subgroup Analyses of Adjusted Associations Between Physician Tumor Board Participation Patterns and All-Cause Patient Mortality*
| Cancer Type | All Respondents |
Tumor Board Participants Only |
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attends Tumor Board Weekly (v < weekly or never) |
Serves Treatment Planning Function |
Includes Evaluation of Treatment Decisions |
Reviews Only Challenging Patient Cases |
Reviews Variety of Cancer Sites |
Serves As Teaching Session (no patient case review) |
|||||||||||||||||||||||||
| No. of Patients | No. of Physicians | HR | 95% CI | P | No. of Patients | No. of Physicians | HR | 95% CI | P | No. of Patients | No. of Physicians | HR | 95% CI | P | No. of Patients | No. of Physicians | HR | 95% CI | P | No. of Patients | No. of Physicians | HR | 95% CI | P | No. of Patients | No. of Physicians | HR | 95% CI | P | |
| NSCLC, stage | ||||||||||||||||||||||||||||||
| I | 503 | 226 | 0.8 | 0.6 to 1.0 | .058 | 435 | 201 | 0.9 | 0.6 to 1.4 | .75 | 427 | 200 | 0.7 | 0.5 to 1.2 | .20 | 435 | 201 | 1.1 | 0.8 to 1.5 | .44 | 424 | 199 | 1.4 | 1.0 to 1.9 | .06 | 427 | 199 | 1.1 | 0.6 to 2.2 | .71 |
| II | 187 | 131 | 1.0 | 0.5 to 1.7 | .86 | 162 | 117 | 1.8 | 0.9 to 3.7 | .12 | 158 | 115 | 2.3 | 0.5 to 10.0 | .26 | 162 | 117 | 0.9 | 0.5 to 1.7 | .75 | 160 | 116 | 0.8 | 0.4 to 1.7 | .54 | 159 | 115 | 0.7 | 0.3 to 1.8 | .52 |
| III | 555 | 289 | 0.9 | 0.7 to 1.1 | .42 | 516 | 266 | 0.9 | 0.7 to 1.1 | .32 | 509 | 264 | 1.4 | 1.0 to 2.1 | .08 | 514 | 264 | 0.9 | 0.7 to 1.1 | .38 | 514 | 264 | 0.9 | 0.7 to 1.2 | .47 | 510 | 262 | 0.9 | 0.7 to 1.2 | .63 |
| IV | 632 | 314 | 1.0 | 0.9 to 1.3 | .69 | 599 | 292 | 1.0 | 0.8 to 1.3 | .82 | 587 | 290 | 1.1 | 0.8 to 1.4 | .64 | 595 | 289 | 0.8 | 0.7 to 1.0 | .03 | 598 | 292 | 1.2 | 1.0 to 1.6 | .09 | 585 | 289 | 0.9 | 0.6 to 1.2 | .37 |
| SCLC | ||||||||||||||||||||||||||||||
| Limited | 105 | 85 | 1.4 | 0.8 to 2.7 | .26 | 98 | 80 | 1.5 | 0.7 to 3.4 | .33 | 94 | 79 | 0.4 | 0.1 to 1.8 | .24 | 97 | 79 | 1.8 | 1.0 to 3.3 | .06 | 98 | 80 | 4.1 | 1.0 to 17.2 | .06 | 93 | 78 | 3.0 | 1.1 to 8.6 | .04 |
| Extensive | 116 | 91 | 0.6 | 0.3 to 1.0 | .04 | 115 | 90 | 0.8 | 0.4 to 1.6 | .50 | 113 | 89 | 1.8 | 0.8 to 4.2 | .19 | 113 | 88 | 0.6 | 0.3 to 1.0 | .04 | 115 | 90 | 1.1 | 0.3 to 4.0 | .84 | 110 | 86 | 3.9 | 1.8 to 8.3 | < .001 |
| CRC, stage | ||||||||||||||||||||||||||||||
| I | 517 | 318 | 0.8 | 0.5 to 1.2 | .22 | 477 | 289 | 1.1 | 0.6 to 1.8 | .85 | 476 | 289 | 1.1 | 0.5 to 2.2 | .89 | 480 | 290 | 0.8 | 0.5 to 1.2 | .35 | 464 | 286 | 2.4 | 0.7 to 8.6 | .16 | 476 | 290 | 0.9 | 0.4 to 1.8 | .74 |
| II | 696 | 426 | 0.9 | 0.6 to 1.2 | .38 | 636 | 386 | 0.9 | 0.6 to 1.4 | .73 | 636 | 386 | 0.7 | 0.4 to 1.4 | .33 | 633 | 386 | 1.2 | 0.9 to 1.7 | .15 | 630 | 385 | 1.8 | 0.8 to 4.0 | .13 | 633 | 386 | 1.3 | 0.8 to 2.0 | .32 |
| III | 829 | 485 | 1.1 | 0.9 to 1.5 | .32 | 769 | 446 | 1.0 | 0.7 to 1.3 | .87 | 766 | 446 | 1.5 | 0.9 to 2.4 | .10 | 773 | 447 | 0.9 | 0.7 to 1.1 | .33 | 766 | 445 | 0.9 | 0.5 to 1.5 | .56 | 764 | 442 | 0.8 | 0.5 to 1.2 | .30 |
| IV | 415 | 300 | 0.7 | 0.6 to 0.9 | .007 | 395 | 284 | 0.9 | 0.7 to 1.2 | .48 | 390 | 283 | 1.2 | 0.8 to 1.6 | .42 | 389 | 282 | 1.0 | 0.8 to 1.3 | .94 | 391 | 281 | 1.3 | 0.8 to 2.1 | .31 | 386 | 282 | 0.8 | 0.6 to 1.2 | .30 |
NOTE. Bold font indicates significance.
Abbreviations: CRC, colorectal cancer; HR, hazard ratio for mortality; NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
Unit of analysis is patient-specified link to surgeon, medical oncologist, or radiation oncologist. Each set of columns represents a set of models including one tumor board feature, and each row represents model results for specific stage and disease type. Models adjusted for physician age, sex, specialty, practice structure, teaching status, No. of patients seen per month with linked patient's cancer type, and National Cancer Institute cancer center affiliation. Also adjusted for patient age, sex, race, marital status, educational attainment, income, and No. of comorbid conditions. Models for CRC also adjusted for disease site (colon, rectal, or other [eg, both colon and rectal]). For ≤ 25 patients per model, multiple imputation was not performed for some items because of partially completed survey versions; these patients were excluded from multivariable analyses.
We used robust SE estimates to account for repeated measures among physicians. Significance tests were two sided. Analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC) and STATA software (version 13; STATA, College Station, TX).
Results
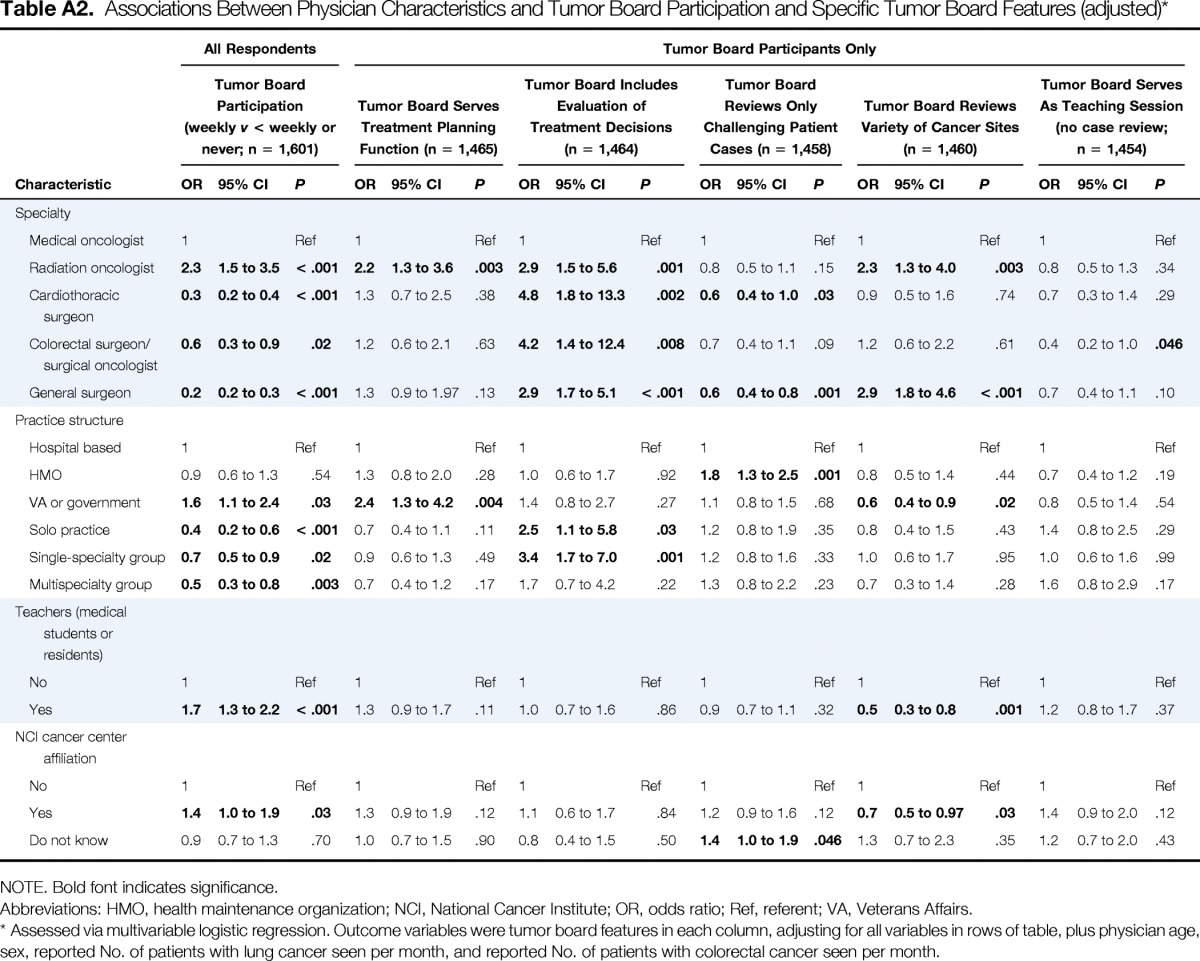
Physician and patient characteristics are listed in Table 1. Overall, 53.8% of physicians reported participating in tumor boards weekly, 42.0% less frequently, and 4.2% not at all. Among tumor board participants, a pretreatment planning function was described by 82.4% of physicians, evaluation of treatment decisions by 92.3%, review only of challenging patient cases by 58.7%, review of a variety of cancer sites by 87.1%, and an educational function without review of participant cases by 12.1%. After adjustment for all physician characteristics included in Table 1, radiation oncologists were more likely than medical oncologists to participate weekly versus less than weekly or never (odds ratio [OR] 2.3; 95% CI, 1.5 to 3.5); cardiothoracic surgeons, general surgeons, and surgical oncologists or colorectal surgeons were less likely than medical oncologists to participate weekly (all P ≤ .02; Appendix Table A2, online only).
Weekly tumor board participation was not associated with overall survival (hazard ratio [HR] for all-cause mortality, 1.0; 95% CI, 0.9 to 1.0), although patients of physicians whose tumor boards reviewed a variety of cancer sites (v only one) had slightly higher mortality (HR, 1.2; 95% CI, 1.1 to 1.4). No other tumor board features were associated with patient survival (Table 2). Overall, 7% of patients had evidence of enrollment onto clinical trials by 15 months after diagnosis. In adjusted analyses, patients whose physicians participated in tumor boards weekly (v less than weekly or never) were more likely to report enrollment onto trials (OR, 1.6; 95% CI, 1.1 to 2.2).
Rates of guideline-recommended treatments were high; 79% of patients with stage III colon cancer received adjuvant chemotherapy, 83% of those with stage II to III rectal cancer received neoadjuvant or adjuvant chemoradiotherapy, and 85% of patients with stage I to II NSCLC underwent curative-intent surgery. In adjusted analyses, patients with stage I to II NSCLC whose physicians attended tumor boards weekly were more likely to undergo curative-intent surgery (OR, 2.9; 95% CI, 1.3 to 6.8); those whose physicians' tumor boards included evaluation of prior treatment decisions were also slightly more likely to undergo curative-intent surgery (P = .047), and those whose physicians' tumor boards reviewed a variety of cancer sites were less likely (OR, 0.1; 95% CI, 0.03 to 0.4; Table 2). Patients with stage III colon cancer whose physicians' tumor boards reviewed only challenging patient cases were slightly more likely to receive adjuvant chemotherapy (P = .049). Receipt of chemoradiotherapy for stage II to III rectal cancer was not associated with tumor board participation or features.
Just over half (53%) of patients rated overall care quality as excellent, and 51% provided top ratings for communication within their health care teams. There were no significant associations between tumor board features and patient ratings of care quality or health care team communication (Table 2).
In exploratory subgroup analyses, patients with extensive-stage SCLC whose physicians participated in tumor boards weekly had lower mortality (HR, 0.6; 95% CI, 0.3 to 1.0; P = .04; Table 3), as did patients with stage IV colorectal cancer (HR, 0.7; 95% CI, 0.6 to 0.9; P = .007). Patients with stage IV NSCLC or extensive-stage SCLC whose physicians' tumor boards reviewed only challenging patient cases had lower mortality, but for patients with SCLC, mortality was higher when physicians' tumor boards served only as teaching sessions (all P ≤ .04).
Discussion
Tumor boards are common, but little evidence is available about associations between tumor board participation, tumor board features, and individual patient care patterns or outcomes. We did not find strong evidence of an association between tumor board participation factors and overall survival. This may reflect the possibility that only a relatively small proportion of patients benefit most from specific multidisciplinary discussions. Indeed, our failure to find evidence that weekly tumor boards improved survival in the overall cohort should not be interpreted as evidence against a benefit for some patients. Our primary analysis of overall survival within the full cohort was statistically conservative and did not allow for heterogeneity of the effect of tumor boards across cancer types and stages of disease; however, in exploratory subgroup analyses, frequent tumor board participation was associated with improved survival among patients with extensive-stage SCLC or stage IV colorectal cancer, which may represent a focus for future research.
We also observed few associations between physician tumor board participation and care delivery or subjective patient outcomes. Tumor boards may be most beneficial for complex patient cases or unusual clinical scenarios, whereas interventions such as curative-intent surgery for early-stage NSCLC or adjuvant chemotherapy for stage III colon cancer are standard therapies for these conditions.17,18 Nevertheless, even for patients with stage II to III rectal cancer, for which standard therapy includes both chemotherapy and radiation therapy,19 tumor board participation was not associated with delivery of guideline-recommended therapy, in contrast to one prior analysis.9 The lack of associations between tumor board participation and subjective patient reports of care quality or provider team communication was somewhat surprising, but this may be related to the behind-the-scenes nature of tumor boards, of which patients may not be fully aware.
We found that weekly tumor board participation was associated with a greater likelihood that patients discussed clinical trial participation or enrolled onto trials. The rate of clinical trial participation in adult cancer populations has historically been ≤ 5%,16,25,26 yet focused multidisciplinary team training can increase awareness, clarity, and enthusiasm regarding clinical trial enrollment.27 Our findings suggest that physician tumor board engagement may be associated with improved patient clinical trial participation rates. We adjusted for physician characteristics such as practice structure, clinical volume, specialty, and National Cancer Institute cancer center affiliation, but we still cannot exclude the possibility that this finding was in part a result of residual confounding by other institutional characteristics. Nevertheless, this association is clinically plausible and may inform future efforts to optimize clinical trial accrual.
Strengths of our analysis included its representative,13 population- and health system–based cohort and survey data from treating physicians. To our knowledge, this is the only large multicenter study to have linked tumor board participation patterns among individual physicians to clinical care delivery and outcomes. Nevertheless, there are limitations. We attempted to survey all physicians named by patients as most important in determining their treatment plans, but not all physicians responded, and when the most important physician's survey was not available, we linked patients to physicians with whom they had discussed particular treatments. Thus, nonresponse bias could have affected our findings. Additionally, tumor board attendance records were not available in this study, so we relied on physician self-report regarding both frequency of attendance and features of tumor boards, which could not be independently validated. We also could not assess whether specific patient cases were discussed at a tumor board; however, for tumor boards that reviewed only a subset of patient cases, differential selection of more complex or borderline clinical patient cases for presentation would likely have made it difficult to assess the impact of a multidisciplinary meeting, even after adjustment for cancer type, stage, and comorbidity. Still, this limitation precluded assessment of other potentially important outcomes that could be affected by multidisciplinary collaboration, such as timeliness or cost of care. We examined care for patients whose stage and cancer type had been determined, which did not allow assessment of the important function of tumor boards in reviewing pathology and correctly classifying type and stage of disease. Finally, we did not adjust P values for multiple comparisons.
In conclusion, we found little evidence of an impact of physician tumor board engagement on patient survival, patient-reported quality, or ratings of communication. Nevertheless, higher clinical trial participation rates among patients whose physicians participated actively in tumor boards could represent a focus for future efforts to optimize trial accrual. Patients with stage I to II NSCLC whose physicians participated actively were more likely to undergo curative-intent surgery. Further research into the effects of tumor boards should focus on those aspects of tumor board meetings most likely to benefit patients with complex disease.
Acknowledgment
The CanCORS (Cancer Care Outcomes Research and Surveillance) Consortium was supported by Grant No. U01CA093344 from the National Cancer Institute (NCI) to the Statistical Coordinating Center; by grants to the NCI-supported primary data collection and research centers (Grants No. U01CA093332 to Dana-Farber Cancer Institute/Cancer Research Network, No. U01CA093324 to Harvard Medical School/Northern California Cancer Center, No. U01CA093348 to RAND/University of California Los Angeles, No. U01CA093329 to University of Alabama at Birmingham, No. U01CA093339 to University of Iowa, and No. U01CA093326 to University of North Carolina); by Department of Veterans Affairs (VA) Grant No. CRS-02-164 to the Durham VA Medical Center; and by NCI Grant No. 1R01CA164021-01A1 (N.L.K., M.B.L.). We thank Clifford Y. Ko, MD, MS, for helpful feedback on an early draft of this article.
Appendix
Table A1.
Algorithm for Assignment of Physicians to Patients When More Than One Physician Linked to Given Patient
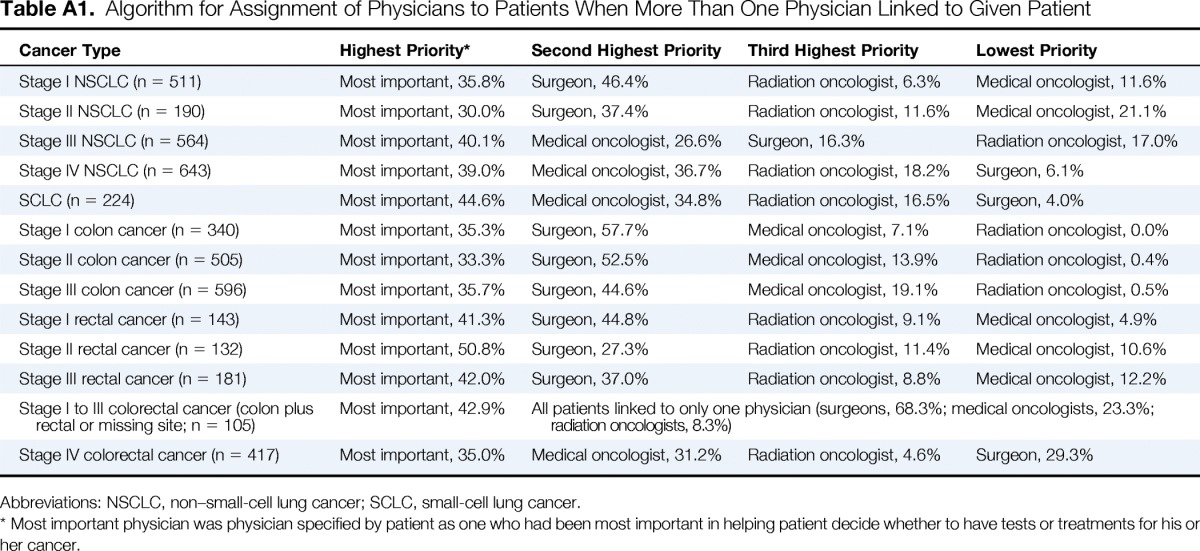
| Cancer Type | Highest Priority* | Second Highest Priority | Third Highest Priority | Lowest Priority |
|---|---|---|---|---|
| Stage I NSCLC (n = 511) | Most important, 35.8% | Surgeon, 46.4% | Radiation oncologist, 6.3% | Medical oncologist, 11.6% |
| Stage II NSCLC (n = 190) | Most important, 30.0% | Surgeon, 37.4% | Radiation oncologist, 11.6% | Medical oncologist, 21.1% |
| Stage III NSCLC (n = 564) | Most important, 40.1% | Medical oncologist, 26.6% | Surgeon, 16.3% | Radiation oncologist, 17.0% |
| Stage IV NSCLC (n = 643) | Most important, 39.0% | Medical oncologist, 36.7% | Radiation oncologist, 18.2% | Surgeon, 6.1% |
| SCLC (n = 224) | Most important, 44.6% | Medical oncologist, 34.8% | Radiation oncologist, 16.5% | Surgeon, 4.0% |
| Stage I colon cancer (n = 340) | Most important, 35.3% | Surgeon, 57.7% | Medical oncologist, 7.1% | Radiation oncologist, 0.0% |
| Stage II colon cancer (n = 505) | Most important, 33.3% | Surgeon, 52.5% | Medical oncologist, 13.9% | Radiation oncologist, 0.4% |
| Stage III colon cancer (n = 596) | Most important, 35.7% | Surgeon, 44.6% | Medical oncologist, 19.1% | Radiation oncologist, 0.5% |
| Stage I rectal cancer (n = 143) | Most important, 41.3% | Surgeon, 44.8% | Radiation oncologist, 9.1% | Medical oncologist, 4.9% |
| Stage II rectal cancer (n = 132) | Most important, 50.8% | Surgeon, 27.3% | Radiation oncologist, 11.4% | Medical oncologist, 10.6% |
| Stage III rectal cancer (n = 181) | Most important, 42.0% | Surgeon, 37.0% | Radiation oncologist, 8.8% | Medical oncologist, 12.2% |
| Stage I to III colorectal cancer (colon plus rectal or missing site; n = 105) | Most important, 42.9% | All patients linked to only one physician (surgeons, 68.3%; medical oncologists, 23.3%; radiation oncologists, 8.3%) | ||
| Stage IV colorectal cancer (n = 417) | Most important, 35.0% | Medical oncologist, 31.2% | Radiation oncologist, 4.6% | Surgeon, 29.3% |
Abbreviations: NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
Most important physician was physician specified by patient as one who had been most important in helping patient decide whether to have tests or treatments for his or her cancer.
Table A2.
Associations Between Physician Characteristics and Tumor Board Participation and Specific Tumor Board Features (adjusted)*
| Characteristic | All Respondents |
Tumor Board Participants Only |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Board Participation (weekly v < weekly or never; n = 1,601) |
Tumor Board Serves Treatment Planning Function (n = 1,465) |
Tumor Board Includes Evaluation of Treatment Decisions (n = 1,464) |
Tumor Board Reviews Only Challenging Patient Cases (n = 1,458) |
Tumor Board Reviews Variety of Cancer Sites (n = 1,460) |
Tumor Board Serves As Teaching Session (no case review; n = 1,454) |
|||||||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Specialty | ||||||||||||||||||
| Medical oncologist | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | ||||||
| Radiation oncologist | 2.3 | 1.5 to 3.5 | < .001 | 2.2 | 1.3 to 3.6 | .003 | 2.9 | 1.5 to 5.6 | .001 | 0.8 | 0.5 to 1.1 | .15 | 2.3 | 1.3 to 4.0 | .003 | 0.8 | 0.5 to 1.3 | .34 |
| Cardiothoracic surgeon | 0.3 | 0.2 to 0.4 | < .001 | 1.3 | 0.7 to 2.5 | .38 | 4.8 | 1.8 to 13.3 | .002 | 0.6 | 0.4 to 1.0 | .03 | 0.9 | 0.5 to 1.6 | .74 | 0.7 | 0.3 to 1.4 | .29 |
| Colorectal surgeon/surgical oncologist | 0.6 | 0.3 to 0.9 | .02 | 1.2 | 0.6 to 2.1 | .63 | 4.2 | 1.4 to 12.4 | .008 | 0.7 | 0.4 to 1.1 | .09 | 1.2 | 0.6 to 2.2 | .61 | 0.4 | 0.2 to 1.0 | .046 |
| General surgeon | 0.2 | 0.2 to 0.3 | < .001 | 1.3 | 0.9 to 1.97 | .13 | 2.9 | 1.7 to 5.1 | < .001 | 0.6 | 0.4 to 0.8 | .001 | 2.9 | 1.8 to 4.6 | < .001 | 0.7 | 0.4 to 1.1 | .10 |
| Practice structure | ||||||||||||||||||
| Hospital based | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | ||||||
| HMO | 0.9 | 0.6 to 1.3 | .54 | 1.3 | 0.8 to 2.0 | .28 | 1.0 | 0.6 to 1.7 | .92 | 1.8 | 1.3 to 2.5 | .001 | 0.8 | 0.5 to 1.4 | .44 | 0.7 | 0.4 to 1.2 | .19 |
| VA or government | 1.6 | 1.1 to 2.4 | .03 | 2.4 | 1.3 to 4.2 | .004 | 1.4 | 0.8 to 2.7 | .27 | 1.1 | 0.8 to 1.5 | .68 | 0.6 | 0.4 to 0.9 | .02 | 0.8 | 0.5 to 1.4 | .54 |
| Solo practice | 0.4 | 0.2 to 0.6 | < .001 | 0.7 | 0.4 to 1.1 | .11 | 2.5 | 1.1 to 5.8 | .03 | 1.2 | 0.8 to 1.9 | .35 | 0.8 | 0.4 to 1.5 | .43 | 1.4 | 0.8 to 2.5 | .29 |
| Single-specialty group | 0.7 | 0.5 to 0.9 | .02 | 0.9 | 0.6 to 1.3 | .49 | 3.4 | 1.7 to 7.0 | .001 | 1.2 | 0.8 to 1.6 | .33 | 1.0 | 0.6 to 1.7 | .95 | 1.0 | 0.6 to 1.6 | .99 |
| Multispecialty group | 0.5 | 0.3 to 0.8 | .003 | 0.7 | 0.4 to 1.2 | .17 | 1.7 | 0.7 to 4.2 | .22 | 1.3 | 0.8 to 2.2 | .23 | 0.7 | 0.3 to 1.4 | .28 | 1.6 | 0.8 to 2.9 | .17 |
| Teachers (medical students or residents) | ||||||||||||||||||
| No | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | ||||||
| Yes | 1.7 | 1.3 to 2.2 | < .001 | 1.3 | 0.9 to 1.7 | .11 | 1.0 | 0.7 to 1.6 | .86 | 0.9 | 0.7 to 1.1 | .32 | 0.5 | 0.3 to 0.8 | .001 | 1.2 | 0.8 to 1.7 | .37 |
| NCI cancer center affiliation | ||||||||||||||||||
| No | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | ||||||
| Yes | 1.4 | 1.0 to 1.9 | .03 | 1.3 | 0.9 to 1.9 | .12 | 1.1 | 0.6 to 1.7 | .84 | 1.2 | 0.9 to 1.6 | .12 | 0.7 | 0.5 to 0.97 | .03 | 1.4 | 0.9 to 2.0 | .12 |
| Do not know | 0.9 | 0.7 to 1.3 | .70 | 1.0 | 0.7 to 1.5 | .90 | 0.8 | 0.4 to 1.5 | .50 | 1.4 | 1.0 to 1.9 | .046 | 1.3 | 0.7 to 2.3 | .35 | 1.2 | 0.7 to 2.0 | .43 |
NOTE. Bold font indicates significance.
Abbreviations: HMO, health maintenance organization; NCI, National Cancer Institute; OR, odds ratio; Ref, referent; VA, Veterans Affairs.
Assessed via multivariable logistic regression. Outcome variables were tumor board features in each column, adjusting for all variables in rows of table, plus physician age, sex, reported No. of patients with lung cancer seen per month, and reported No. of patients with colorectal cancer seen per month.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: Kenneth L. Kehl, Mary Beth Landrum, Katherine L. Kahn, Nancy L. Keating
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tumor Board Participation Among Physicians Caring for Patients With Lung or Colorectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Kenneth L. Kehl
No relationship to disclose
Mary Beth Landrum
Honoraria: Aetna (I), VHA (I)
Research Funding: Pfizer (Inst)
Katherine L. Kahn
No relationship to disclose
Stacy W. Gray
No relationship to disclose
Aileen B. Chen
No relationship to disclose
Nancy L. Keating
No relationship to disclose
References
- 1.Gross GE. The role of the tumor board in a community hospital. CA Cancer J Clin. 1987;37:88–92. doi: 10.3322/canjclin.37.2.88. [DOI] [PubMed] [Google Scholar]
- 2.Gatcliffe TA, Coleman RL. Tumor board: More than treatment planning—A 1-year prospective survey. J Cancer Educ. 2008;23:235–237. doi: 10.1080/08858190802189014. [DOI] [PubMed] [Google Scholar]
- 3.American College of Surgeons. Cancer Program Standards 2012: Ensuring Patient-Centered Care. https://www.facs.org/∼/media/files/quality%20programs/cancer/coc/programstandards2012.ashx.
- 4.Henson DE, Frelick RW, Ford LG, et al. Results of a national survey of characteristics of hospital tumor conferences. Surg Gynecol Obstet. 1990;170:1–6. [PubMed] [Google Scholar]
- 5.Scher KS, Tisnado DM, Rose DE, et al. Physician and practice characteristics influencing tumor board attendance: Results from the provider survey of the Los Angeles Women's Health Study. J Oncol Pract. 2011;7:103–110. doi: 10.1200/JOP.2010.000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petty JK, Vetto JT. Beyond doughnuts: Tumor board recommendations influence patient care. J Cancer Educ. 2002;17:97–100. doi: 10.1080/08858190209528807. [DOI] [PubMed] [Google Scholar]
- 7.Newman EA, Guest AB, Helvie MA, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer. 2006;107:2346–2351. doi: 10.1002/cncr.22266. [DOI] [PubMed] [Google Scholar]
- 8.Pawlik TM, Laheru D, Hruban RH, et al. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–2088. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham NS, Gossey JT, Davila JA, et al. Receipt of recommended therapy by patients with advanced colorectal cancer. Am J Gastroenterol. 2006;101:1320–1328. doi: 10.1111/j.1572-0241.2006.00545.x. [DOI] [PubMed] [Google Scholar]
- 10.Keating NL, Landrum MB, Lamont EB, et al. Tumor boards and the quality of cancer care. J Natl Cancer Inst. 2013;105:113–121. doi: 10.1093/jnci/djs502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blayney DW. Tumor boards (team huddles) aren't enough to reach the goal. J Natl Cancer Inst. 2013;105:82–84. doi: 10.1093/jnci/djs523. [DOI] [PubMed] [Google Scholar]
- 12.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Catalano PJ, Ayanian JZ, Weeks JC, et al. Representativeness of participants in the cancer care outcomes research and surveillance consortium relative to the surveillance, epidemiology, and end results program. Med Care. 2013;51:e9–e15. doi: 10.1097/MLR.0b013e318222a711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. ed 5. Lenexa, KS: American Association for Public Opinion Research; 2008. [Google Scholar]
- 15.Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant chemotherapy for stage III colon cancer: Do physicians agree about the importance of patient age and comorbidity? J Clin Oncol. 2008;26:2532–2537. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 16.Fouad MN, Lee JY, Catalano PJ, et al. Enrollment of patients with lung and colorectal cancers onto clinical trials. J Oncol Pract. 2013;9:e40–e47. doi: 10.1200/JOP.2012.000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Non-small cell lung cancer (version 3.2014) http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 18.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Colon cancer (version 3.2014) http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 19.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Rectal cancer (version 3.2014) http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 20.Ayanian JZ, Zaslavsky AM, Arora NK, et al. Patients' experiences with care for lung cancer and colorectal cancer: Findings from the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2010;28:4154–4161. doi: 10.1200/JCO.2009.27.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hays RD, Shaul JA, Williams VS, et al. Psychometric properties of the CAHPS 1.0 survey measures: Consumer Assessment of Health Plans Study. Med Care. 1999;37(suppl):MS22–MS31. doi: 10.1097/00005650-199903001-00003. [DOI] [PubMed] [Google Scholar]
- 22.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Greene FL, Page DL, Fleming ID, editors. AJCC Cancer Staging Manual. ed 6. New York, NY: Springer; 2002. [Google Scholar]
- 24.He Y, Zaslavsky AM, Landrum MB, et al. Multiple imputation in a large-scale complex survey: A practical guide. Stat Methods Med Res. 2010;19:653–670. doi: 10.1177/0962280208101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehl KL, Arora NK, Schrag D, et al. Discussions about clinical trials among patients with newly diagnosed lung and colorectal cancer. J Natl Cancer Inst. 2014;106:1–9. doi: 10.1093/jnci/dju216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute of Medicine. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 27.Fallowfield L, Langridge C, Jenkins V. Communication skills training for breast cancer teams talking about trials. Breast. 2014;23:193–197. doi: 10.1016/j.breast.2013.11.009. [DOI] [PubMed] [Google Scholar]


