Although pneumothorax was frequent in outpatients after IGTTNB, rates of hospitalization and chest tube placement were low. As screening for lung cancer increases, the authors anticipate an increased need for IGTTNB.
Abstract
Purpose:
Image-guided transthoracic needle biopsy (IGTTNB) is an important tool in the diagnosis of patients with cancer. Common complications include pneumothorax and chest tube placement, with rates ranging from 6% to 57%. We performed a population-based study to determine patterns of use, complications, and costs associated with IGTTNB.
Methods:
The Premier Perspective database was used to identify patients with cancer with ≥ one claim for IGTTNB from 2006 to 2012. Patients were stratified on the basis of inpatient versus outpatient setting. Pneumothorax was defined by a new claim within 1 month of IGTTNB; hospitalization and chest tube placement rates were analyzed. Multivariable analysis was used to identify factors associated with pneumothorax.
Results:
We Identified 79,518 patients with cancer who underwent IGTTNB: 42,955 (54.0%) outpatients and 36,563 (46.0%) inpatients. Of patients who underwent outpatient IGTTNB, 5,261 (12.2%) developed a pneumothorax. Of those, 1,006 (19.1%, 2.3% of total) were hospitalized, and 180 (3.4%, 0.42% of total) required chest tubes. Pneumothorax after outpatient IGTTNB was associated with number of comorbidities, rural site, hospital bed size of more than 600, and biopsy of parenchymal as opposed to pleural lesions. Of patients who underwent inpatient IGTTNB, 7,830 (21.4%) developed a pneumothorax, and 2,894 (36.0%, 7.9% of total) required chest tube. Over time, total IGTTNB volume increased by 40.6%, and mean outpatient cost per procedure increased by 24.4%.
Conclusion:
While pneumothorax was frequent in outpatients, rates of hospitalization and chest tube placement were low. As screening for lung cancer increases, we anticipate an increased need for IGTNBB. Patients can be reassured by the low rate of serious complications.
Introduction
Image-guided transthoracic needle biopsy (IGTTNB) is a common procedure used to diagnose the nature of pulmonary nodules.1,2 IGTTNB can be performed with a variety of guidance modalities, including fluoroscopy, ultrasound, and computed tomography (CT).3–8 CT-guided biopsies have become the most popular imaging modality in recent years, replacing fluoroscopy.2,7,9 IGTTNB also serves as an alternative to open lung biopsy, which is associated with significant morbidity including respiratory failure, postoperative bleeding, infection, continuous air leakage, and cardiac complications, and requires hospital admission.10 In a recent single-institution study that evaluated the costs and complications of 149 patients who underwent video-assisted thoracic surgery (VATS), a type of open lung biopsy, after a diagnosis of lung cancer, the median hospital length of stay (LOS) was 4 days, with a 30-day mortality rate of 0.7%, a morbidity rate of 37.6%, and a mean hospital cost of $18,637 per patient.11
IGTTNB is considered to be a safe procedure; however, complications that include pneumothorax, hemorrhage, and systemic air embolism can occur.2,3 Previously published reports have shown a wide variation in complication rates, with rates of pneumothorax that vary from 8.2 to 56.6% and rates of chest tube placement that vary from 5.8 to 53.2% among patients who developed a pneumothorax.6,12–21 Predictors of pneumothorax development from previous studies include age, location of pulmonary nodules, baseline pulmonary function, needle size and trajectory angle, and operator experience.6,9,12–16,19–21 However, many of these previous studies of IGTTNB have had a small sample size and evaluated cases from a single, and often high-volume, institution. In previous studies, the median sample size was 150 patients (range, 50 to 1,033) and often included a mix of both inpatients and outpatients.6,9,13–21
The objectives of this study were to evaluate trends and safety of IGTTNB in patients with an underlying cancer diagnosis using a large national hospital database.
Methods
Data Source
Data from the Perspective database (Premier, Charlotte, NC), a voluntary, fee-supported database originally developed to measure resource use and quality of care, were used. Perspective contains a representative sample of more than 500 acute-care hospitals throughout the United States that contribute data on inpatient hospital admissions and outpatient procedures, including patient demographics, disease characteristics, and procedures performed; the database also collects information about all billed services rendered during a patient's hospital stay. Hospitals within this database are predominantly small to midsize (78% with < 400 beds, 15% with 400 to 600 beds, and 7% with > 600 beds), geographically diverse (13% located in the Northeast, 25% in the Midwest, 42% in the South, 20% in the West), nonteaching facilities (74% nonteaching) that serve a largely urban population (74% urban location). Data in Perspective undergo a rigorous quality control process and have been used in several outcome studies.22–28
Patient Selection
We analyzed patients with a cancer diagnosis (International Classification of Diseases, Ninth Revision [ICD-9] codes 140 to 208) who had one or more transthoracic biopsy procedures (ICD-9 codes 34.24, 33.26, or text code for percutaneous biopsy of thorax, lung, pleura, and mediastinum) from 2006 to 2012. Patients were stratified on the basis of their biopsy setting (inpatient v outpatient).
Clinical and Demographic Characteristics
Demographic data analyzed included age, race (white, black, or other), marital status (married, single, unknown), insurance status (Medicare, Medicaid, commercial, uninsured, or unknown), and year of admission. Tumor characteristics included tumor site (lung [ICD-9 code 162 only], nonlung [ICD-9 codes 140 to 161 or 163 to 208], or combination [ICD-9 codes 162 plus 140 to 161 or 163 to 208]) and site of biopsy (parenchymal or pleural). The hospitals at which patients were treated were characterized by location (urban, rural), teaching status (yes, no), size (< 400 beds, 400 to 600 beds, > 600 beds) and region of the country (northeast, midwest, south and west). Risk adjustment for comorbid conditions was performed using the Elixhauser comorbidity score.29,30
Main Outcome Measures
The primary end point of the study was development of pneumothorax (ICD-9 code 512) within 1 month after IGTTNB. Secondary outcomes included hospitalization within 1 month of pneumothorax development and chest tube placement during the same hospitalization as pneumothorax (Current Procedural Terminology [CPT] codes 32019, 32020 from 2006 to 2008 and 32550, 32551 from 2008-2012). Use of claims data to identify procedures, interventions, and postprocedural complications is a common methodology in studies using administrative data. Use of ICD-9 and CPT codes to identify interventions and postprocedural complications has a positive predictive value of approximately 75% to 100% (using the SEER-Medicare database).31 A 1-month time frame was chosen to capture the majority of postprocedural complications, as the Perspective database reports diagnoses and procedure dates in a month/year format rather than actual date of occurrence. In addition, LOS and average hospitalization cost after a pneumothorax were compared between those who received their biopsy in the outpatient setting, those who received their biopsy in the inpatient setting and remained hospitalized for pneumothorax, and those who received their biopsy in the inpatient setting and were rehospitalized after a pneumothorax diagnosis. Other factors evaluated were frequency of type of IGTTNB by imaging modality as assessed by ICD-9 and CPT codes; number of claims for IGTTNB by year; mean outpatient cost of IGTTNB by year; total cost of IGTTNB by year (calculated by multiplying total number of claims by mean outpatient cost for IGTTNB); and rates of other complications including air embolism, hemorrhage; and hemoptysis determined by ICD-9 codes. Costs were captured as actual patient costs, consisting of direct patients costs that included supplies, labor, and equipment, plus fixed costs such as overhead.
Statistical Analysis
The exposure variable was IGTTNB. The outcome variables were development of pneumothorax within 1 month of IGTTNB, hospitalization within 1 month of IGTTNB, chest tube placement after pneumothorax development, hospital LOS, and hospital costs. Covariates were included that are known to be related to quality of care or were significant in an unadjusted analysis. Models included age, race, marital status, insurance status, tumor and biopsy site, hospital location, hospital teaching status, hospital bed size, hospital region, and patient comorbidity score. Frequency distributions between categorical variables were compared using the χ2 test. We used multivariable logistic regression models that included patient, hospital, and tumor characteristics to determine factors associated with pneumothorax after IGTTNB. In addition, we evaluated factors associated with complications from a pneumothorax after IGTTNB. Results are reported with odds ratios (ORs) and 95% CIs. Totals costs were bundled by admission in order to determine costs related to IGTTNB procedures, and no other aspects of the hospital admission, only outpatient procedures, were considered. To calculate total costs of IGTTNB, total claims for IGTTNB (both inpatient and outpatient) were multiplied by outpatient mean cost by year. All analyses were performed with SAS version 9.2 (SAS Institute, Inc, Cary, NC).
Results
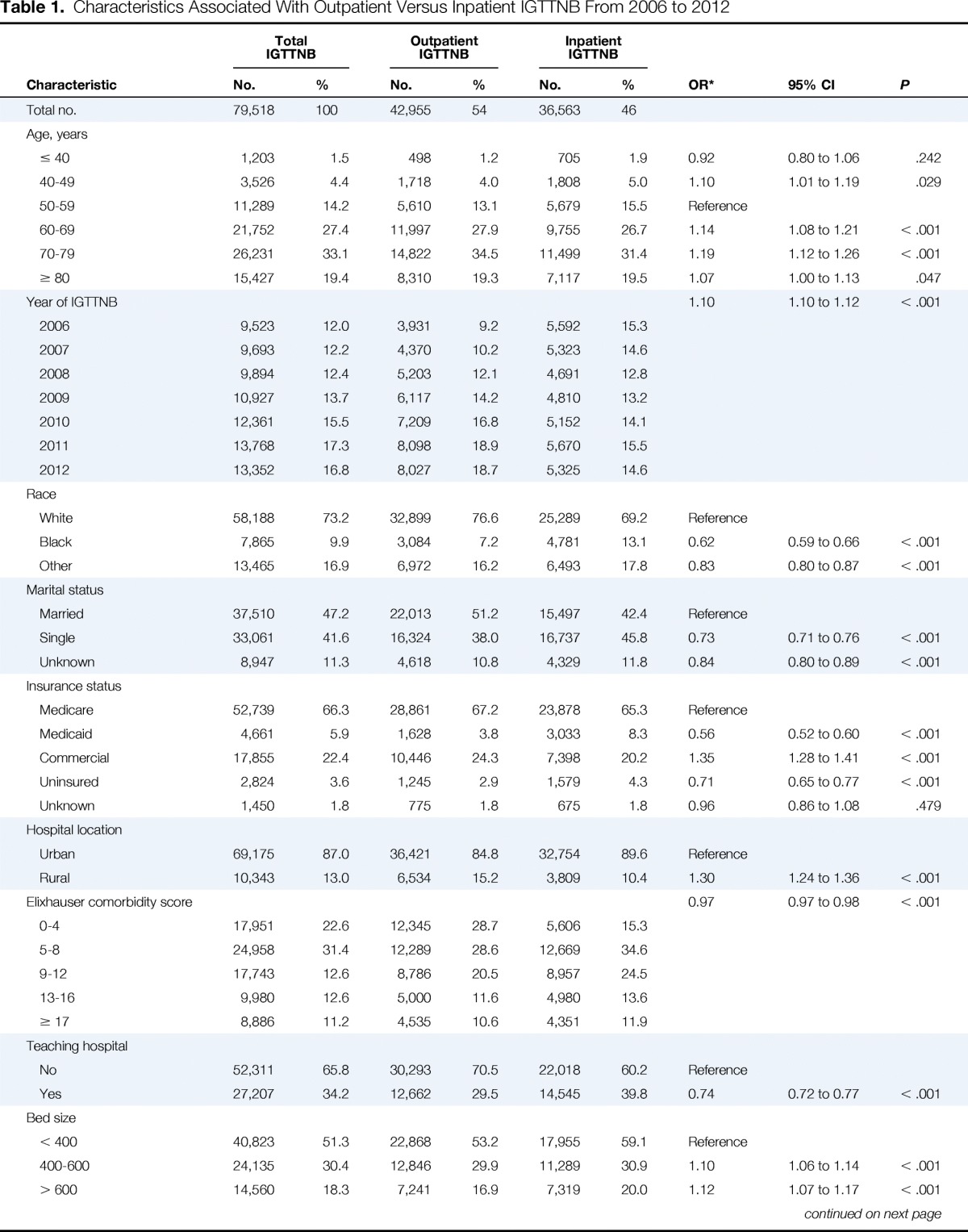
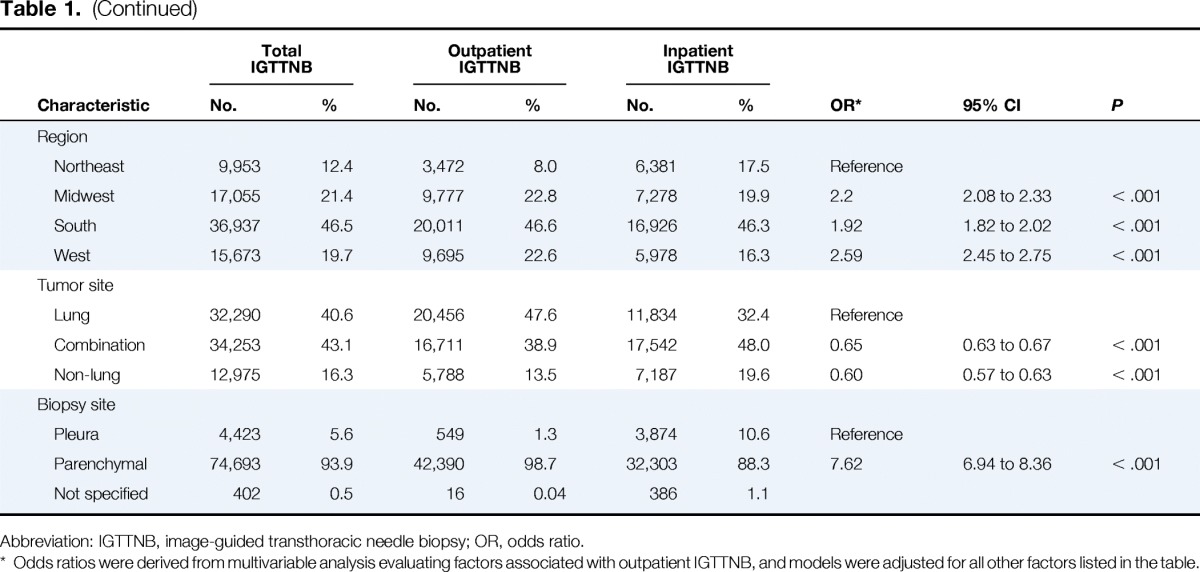
We identified 79,518 people with a diagnosis of cancer who underwent IGTTNB, and of these, 42,955 (54.0%) underwent IGTTNB as an outpatient and 36,563 (46.0%) underwent IGTTNB as an inpatient. Nineteen thousand eight hundred sixty patients (25.0%) had a single cancer diagnosis (77.9% with lung cancer, 5.8% with lymphoma, 1.3% with breast cancer, 15.0% with other cancers) and 59,658 (75%) had more than one cancer diagnosis (68.3% lung cancer, 2.7% lymphoma, 1.6% breast cancer, 1.0% colon cancer, and 26.4% with other cancers). Clinical and demographic characteristics of the entire cohort and subcohorts are displayed in Table 1. CT scan was used in 96.4% of cases, ultrasound in 2.89% of cases, fluoroscopy in 0.67% of cases, and x-ray in 0.02% of cases.
Table 1.
Characteristics Associated With Outpatient Versus Inpatient IGTTNB From 2006 to 2012
| Characteristic | Total IGTTNB |
Outpatient IGTTNB |
Inpatient IGTTNB |
OR* | 95% CI | P | |||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||||
| Total no. | 79,518 | 100 | 42,955 | 54 | 36,563 | 46 | |||
| Age, years | |||||||||
| ≤ 40 | 1,203 | 1.5 | 498 | 1.2 | 705 | 1.9 | 0.92 | 0.80 to 1.06 | .242 |
| 40-49 | 3,526 | 4.4 | 1,718 | 4.0 | 1,808 | 5.0 | 1.10 | 1.01 to 1.19 | .029 |
| 50-59 | 11,289 | 14.2 | 5,610 | 13.1 | 5,679 | 15.5 | Reference | ||
| 60-69 | 21,752 | 27.4 | 11,997 | 27.9 | 9,755 | 26.7 | 1.14 | 1.08 to 1.21 | < .001 |
| 70-79 | 26,231 | 33.1 | 14,822 | 34.5 | 11,499 | 31.4 | 1.19 | 1.12 to 1.26 | < .001 |
| ≥ 80 | 15,427 | 19.4 | 8,310 | 19.3 | 7,117 | 19.5 | 1.07 | 1.00 to 1.13 | .047 |
| Year of IGTTNB | 1.10 | 1.10 to 1.12 | < .001 | ||||||
| 2006 | 9,523 | 12.0 | 3,931 | 9.2 | 5,592 | 15.3 | |||
| 2007 | 9,693 | 12.2 | 4,370 | 10.2 | 5,323 | 14.6 | |||
| 2008 | 9,894 | 12.4 | 5,203 | 12.1 | 4,691 | 12.8 | |||
| 2009 | 10,927 | 13.7 | 6,117 | 14.2 | 4,810 | 13.2 | |||
| 2010 | 12,361 | 15.5 | 7,209 | 16.8 | 5,152 | 14.1 | |||
| 2011 | 13,768 | 17.3 | 8,098 | 18.9 | 5,670 | 15.5 | |||
| 2012 | 13,352 | 16.8 | 8,027 | 18.7 | 5,325 | 14.6 | |||
| Race | |||||||||
| White | 58,188 | 73.2 | 32,899 | 76.6 | 25,289 | 69.2 | Reference | ||
| Black | 7,865 | 9.9 | 3,084 | 7.2 | 4,781 | 13.1 | 0.62 | 0.59 to 0.66 | < .001 |
| Other | 13,465 | 16.9 | 6,972 | 16.2 | 6,493 | 17.8 | 0.83 | 0.80 to 0.87 | < .001 |
| Marital status | |||||||||
| Married | 37,510 | 47.2 | 22,013 | 51.2 | 15,497 | 42.4 | Reference | ||
| Single | 33,061 | 41.6 | 16,324 | 38.0 | 16,737 | 45.8 | 0.73 | 0.71 to 0.76 | < .001 |
| Unknown | 8,947 | 11.3 | 4,618 | 10.8 | 4,329 | 11.8 | 0.84 | 0.80 to 0.89 | < .001 |
| Insurance status | |||||||||
| Medicare | 52,739 | 66.3 | 28,861 | 67.2 | 23,878 | 65.3 | Reference | ||
| Medicaid | 4,661 | 5.9 | 1,628 | 3.8 | 3,033 | 8.3 | 0.56 | 0.52 to 0.60 | < .001 |
| Commercial | 17,855 | 22.4 | 10,446 | 24.3 | 7,398 | 20.2 | 1.35 | 1.28 to 1.41 | < .001 |
| Uninsured | 2,824 | 3.6 | 1,245 | 2.9 | 1,579 | 4.3 | 0.71 | 0.65 to 0.77 | < .001 |
| Unknown | 1,450 | 1.8 | 775 | 1.8 | 675 | 1.8 | 0.96 | 0.86 to 1.08 | .479 |
| Hospital location | |||||||||
| Urban | 69,175 | 87.0 | 36,421 | 84.8 | 32,754 | 89.6 | Reference | ||
| Rural | 10,343 | 13.0 | 6,534 | 15.2 | 3,809 | 10.4 | 1.30 | 1.24 to 1.36 | < .001 |
| Elixhauser comorbidity score | 0.97 | 0.97 to 0.98 | < .001 | ||||||
| 0-4 | 17,951 | 22.6 | 12,345 | 28.7 | 5,606 | 15.3 | |||
| 5-8 | 24,958 | 31.4 | 12,289 | 28.6 | 12,669 | 34.6 | |||
| 9-12 | 17,743 | 12.6 | 8,786 | 20.5 | 8,957 | 24.5 | |||
| 13-16 | 9,980 | 12.6 | 5,000 | 11.6 | 4,980 | 13.6 | |||
| ≥ 17 | 8,886 | 11.2 | 4,535 | 10.6 | 4,351 | 11.9 | |||
| Teaching hospital | |||||||||
| No | 52,311 | 65.8 | 30,293 | 70.5 | 22,018 | 60.2 | Reference | ||
| Yes | 27,207 | 34.2 | 12,662 | 29.5 | 14,545 | 39.8 | 0.74 | 0.72 to 0.77 | < .001 |
| Bed size | |||||||||
| < 400 | 40,823 | 51.3 | 22,868 | 53.2 | 17,955 | 59.1 | Reference | ||
| 400-600 | 24,135 | 30.4 | 12,846 | 29.9 | 11,289 | 30.9 | 1.10 | 1.06 to 1.14 | < .001 |
| > 600 | 14,560 | 18.3 | 7,241 | 16.9 | 7,319 | 20.0 | 1.12 | 1.07 to 1.17 | < .001 |
| Region | |||||||||
| Northeast | 9,953 | 12.4 | 3,472 | 8.0 | 6,381 | 17.5 | Reference | ||
| Midwest | 17,055 | 21.4 | 9,777 | 22.8 | 7,278 | 19.9 | 2.2 | 2.08 to 2.33 | < .001 |
| South | 36,937 | 46.5 | 20,011 | 46.6 | 16,926 | 46.3 | 1.92 | 1.82 to 2.02 | < .001 |
| West | 15,673 | 19.7 | 9,695 | 22.6 | 5,978 | 16.3 | 2.59 | 2.45 to 2.75 | < .001 |
| Tumor site | |||||||||
| Lung | 32,290 | 40.6 | 20,456 | 47.6 | 11,834 | 32.4 | Reference | ||
| Combination | 34,253 | 43.1 | 16,711 | 38.9 | 17,542 | 48.0 | 0.65 | 0.63 to 0.67 | < .001 |
| Non-lung | 12,975 | 16.3 | 5,788 | 13.5 | 7,187 | 19.6 | 0.60 | 0.57 to 0.63 | < .001 |
| Biopsy site | |||||||||
| Pleura | 4,423 | 5.6 | 549 | 1.3 | 3,874 | 10.6 | Reference | ||
| Parenchymal | 74,693 | 93.9 | 42,390 | 98.7 | 32,303 | 88.3 | 7.62 | 6.94 to 8.36 | < .001 |
| Not specified | 402 | 0.5 | 16 | 0.04 | 386 | 1.1 | |||
Abbreviation: IGTTNB, image-guided transthoracic needle biopsy; OR, odds ratio.
Odds ratios were derived from multivariable analysis evaluating factors associated with outpatient IGTTNB, and models were adjusted for all other factors listed in the table.
In a multivariable model (Table 1), compared with patients who underwent IGTTNB as an inpatient, outpatients were more likely to have advanced age, age 60 to 69 (OR = 1.14; 95% CI, 1.08 to 1.21), age 70 to 79 (OR = 1.19; 95% CI, 1.12 to 1.26), and age ≥ 80 (OR = 1.07, 95% CI, 1.00 to 1.13) compared with those age 50 to 69; more likely to have commercial insurance (OR = 1.35; 95% CI, 1.28 to 1.41) compared with Medicare insurance; more likely to have IGTTNB at a rural location (OR = 1.30; 95% CI, 1.24 to 1.36); and more likely to undergo parenchymal biopsy (OR = 7.62; 95% CI, 6.94 to 8.36) compared with pleural biopsy. Patients who underwent outpatient IGTTNB were less likely to be of black race (OR = 0.62; 95% CI, 0.59 to 0.66) compared with white race, to have single marital status (OR = 0.73; 95% CI, 0.71 to 0.76), have Medicaid insurance (OR = 0.56; 95% CI, 0.52 to 0.60) or be uninsured (OR = 0.71; 95% CI, 0.65 to 0.77) compared with Medicare insurance, and have a combination of lung and other cancer (OR = 0.65; 95% CI, 0.63 to 0.67) or nonlung cancer diagnosis (OR = 0.60; 95% CI, 0.57 to 0.63) compared with those with only lung cancer.
Of the entire cohort, 13,091 (16.5%) developed a pneumothorax within 1 month of IGTTNB; 3,074 of these patients (23.5% with pneumothorax, 3.9% of the total) had a chest tube placed. There were seven patients (0.01%) who developed an air embolism, 197 patients (0.2%) with hemorrhage, and 1,852 patients with hemoptysis (2.3%) within 1 month of IGTTNB (Figure 1).
Figure 1.
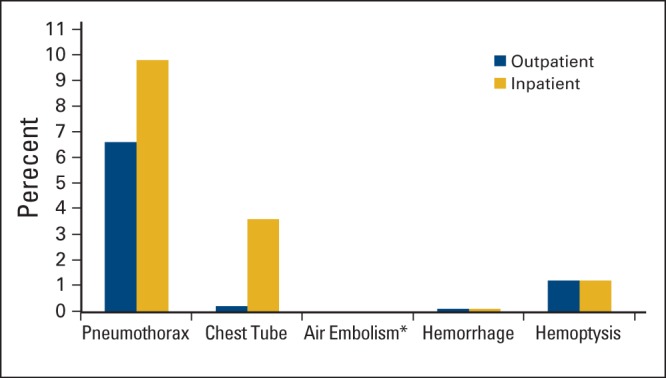
Complication rates in patients with cancer undergoing image-guided transthoracic needle biopsy from 2006 to 2012 (N = 79,518). (*) < 0.01%.
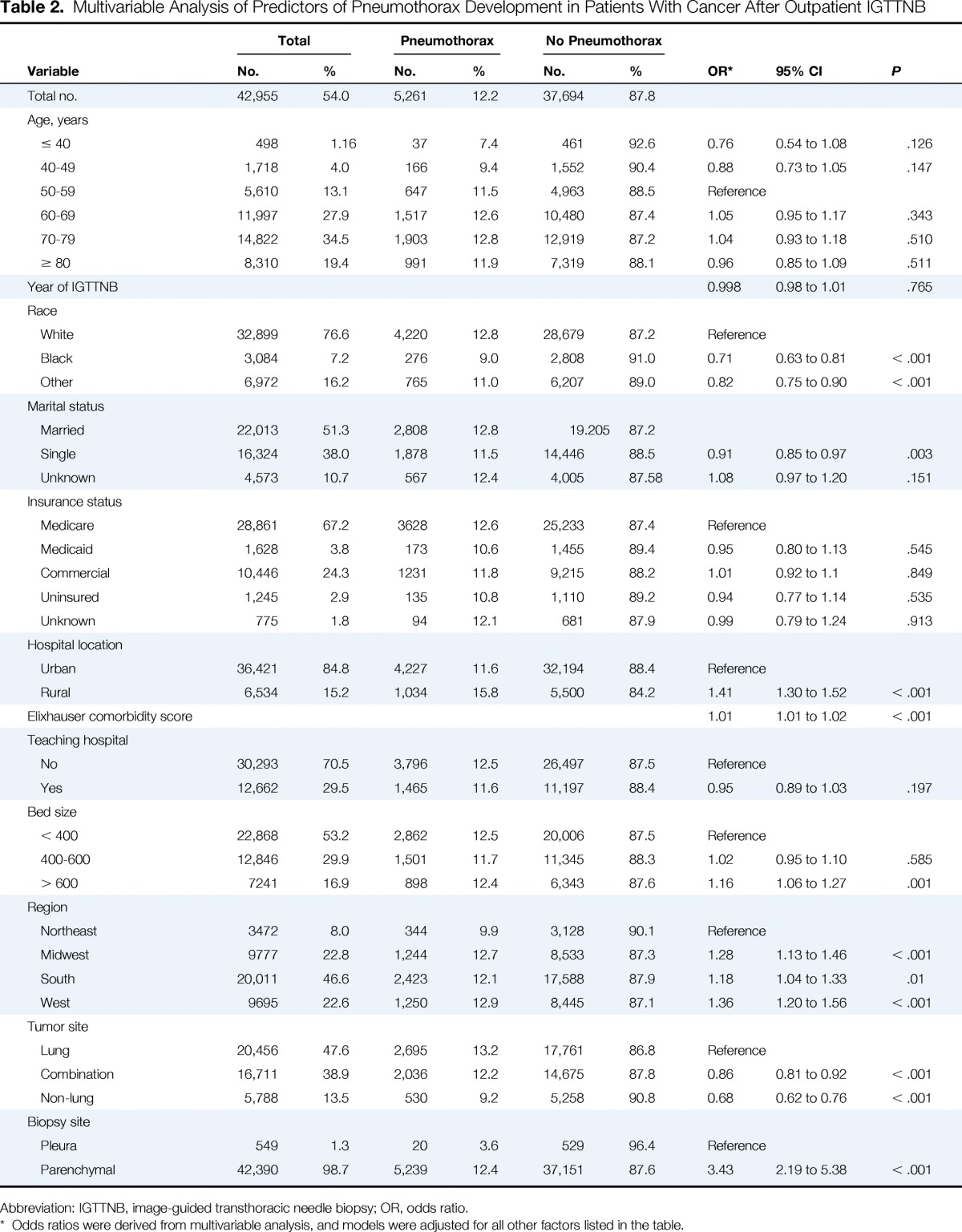
Of the 42,955 patients who underwent IGTTNB as an outpatient, 5,261 (12.2%) developed a pneumothorax within 1 month of their procedure; 1,006 of these patients (19.1% with pneumothorax, 2.3% of the total) were hospitalized, and 180 (17.9% of those hospitalized, 3.4% with pneumothorax, and 0.42% of the total) had a chest tube placed. In a multivariable model of patients who underwent IGTTNB as an outpatient (Table 2), pneumothorax development was less likely in patients who were black (OR = 0.71, 95% CI, 0.63 to 0.81) compared to those who were white, had a diagnosis of combination of lung and other cancer (OR = 0.86; 95% CI, 0.81 to 0.92) or a diagnosis of nonlung cancer (OR = 0.68; 95% CI, 0.62 to 0.76) compared to those with only lung cancer. Pneumothorax development was significantly more likely to occur in patients at rural sites (OR = 1.41; 95% CI, 1.30 to 1.52), at hospitals more than 600 beds (OR = 1.16; 95% CI, 1.06 to 1.27), and parenchymal location of biopsy (OR = 3.43; 95% CI, 2.19 to 5.38) compared with pleural location of biopsy.
Table 2.
Multivariable Analysis of Predictors of Pneumothorax Development in Patients With Cancer After Outpatient IGTTNB
| Variable | Total |
Pneumothorax |
No Pneumothorax |
OR* | 95% CI | P | |||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||||
| Total no. | 42,955 | 54.0 | 5,261 | 12.2 | 37,694 | 87.8 | |||
| Age, years | |||||||||
| ≤ 40 | 498 | 1.16 | 37 | 7.4 | 461 | 92.6 | 0.76 | 0.54 to 1.08 | .126 |
| 40-49 | 1,718 | 4.0 | 166 | 9.4 | 1,552 | 90.4 | 0.88 | 0.73 to 1.05 | .147 |
| 50-59 | 5,610 | 13.1 | 647 | 11.5 | 4,963 | 88.5 | Reference | ||
| 60-69 | 11,997 | 27.9 | 1,517 | 12.6 | 10,480 | 87.4 | 1.05 | 0.95 to 1.17 | .343 |
| 70-79 | 14,822 | 34.5 | 1,903 | 12.8 | 12,919 | 87.2 | 1.04 | 0.93 to 1.18 | .510 |
| ≥ 80 | 8,310 | 19.4 | 991 | 11.9 | 7,319 | 88.1 | 0.96 | 0.85 to 1.09 | .511 |
| Year of IGTTNB | 0.998 | 0.98 to 1.01 | .765 | ||||||
| Race | |||||||||
| White | 32,899 | 76.6 | 4,220 | 12.8 | 28,679 | 87.2 | Reference | ||
| Black | 3,084 | 7.2 | 276 | 9.0 | 2,808 | 91.0 | 0.71 | 0.63 to 0.81 | < .001 |
| Other | 6,972 | 16.2 | 765 | 11.0 | 6,207 | 89.0 | 0.82 | 0.75 to 0.90 | < .001 |
| Marital status | |||||||||
| Married | 22,013 | 51.3 | 2,808 | 12.8 | 19.205 | 87.2 | |||
| Single | 16,324 | 38.0 | 1,878 | 11.5 | 14,446 | 88.5 | 0.91 | 0.85 to 0.97 | .003 |
| Unknown | 4,573 | 10.7 | 567 | 12.4 | 4,005 | 87.58 | 1.08 | 0.97 to 1.20 | .151 |
| Insurance status | |||||||||
| Medicare | 28,861 | 67.2 | 3628 | 12.6 | 25,233 | 87.4 | Reference | ||
| Medicaid | 1,628 | 3.8 | 173 | 10.6 | 1,455 | 89.4 | 0.95 | 0.80 to 1.13 | .545 |
| Commercial | 10,446 | 24.3 | 1231 | 11.8 | 9,215 | 88.2 | 1.01 | 0.92 to 1.1 | .849 |
| Uninsured | 1,245 | 2.9 | 135 | 10.8 | 1,110 | 89.2 | 0.94 | 0.77 to 1.14 | .535 |
| Unknown | 775 | 1.8 | 94 | 12.1 | 681 | 87.9 | 0.99 | 0.79 to 1.24 | .913 |
| Hospital location | |||||||||
| Urban | 36,421 | 84.8 | 4,227 | 11.6 | 32,194 | 88.4 | Reference | ||
| Rural | 6,534 | 15.2 | 1,034 | 15.8 | 5,500 | 84.2 | 1.41 | 1.30 to 1.52 | < .001 |
| Elixhauser comorbidity score | 1.01 | 1.01 to 1.02 | < .001 | ||||||
| Teaching hospital | |||||||||
| No | 30,293 | 70.5 | 3,796 | 12.5 | 26,497 | 87.5 | Reference | ||
| Yes | 12,662 | 29.5 | 1,465 | 11.6 | 11,197 | 88.4 | 0.95 | 0.89 to 1.03 | .197 |
| Bed size | |||||||||
| < 400 | 22,868 | 53.2 | 2,862 | 12.5 | 20,006 | 87.5 | Reference | ||
| 400-600 | 12,846 | 29.9 | 1,501 | 11.7 | 11,345 | 88.3 | 1.02 | 0.95 to 1.10 | .585 |
| > 600 | 7241 | 16.9 | 898 | 12.4 | 6,343 | 87.6 | 1.16 | 1.06 to 1.27 | .001 |
| Region | |||||||||
| Northeast | 3472 | 8.0 | 344 | 9.9 | 3,128 | 90.1 | Reference | ||
| Midwest | 9777 | 22.8 | 1,244 | 12.7 | 8,533 | 87.3 | 1.28 | 1.13 to 1.46 | < .001 |
| South | 20,011 | 46.6 | 2,423 | 12.1 | 17,588 | 87.9 | 1.18 | 1.04 to 1.33 | .01 |
| West | 9695 | 22.6 | 1,250 | 12.9 | 8,445 | 87.1 | 1.36 | 1.20 to 1.56 | < .001 |
| Tumor site | |||||||||
| Lung | 20,456 | 47.6 | 2,695 | 13.2 | 17,761 | 86.8 | Reference | ||
| Combination | 16,711 | 38.9 | 2,036 | 12.2 | 14,675 | 87.8 | 0.86 | 0.81 to 0.92 | < .001 |
| Non-lung | 5,788 | 13.5 | 530 | 9.2 | 5,258 | 90.8 | 0.68 | 0.62 to 0.76 | < .001 |
| Biopsy site | |||||||||
| Pleura | 549 | 1.3 | 20 | 3.6 | 529 | 96.4 | Reference | ||
| Parenchymal | 42,390 | 98.7 | 5,239 | 12.4 | 37,151 | 87.6 | 3.43 | 2.19 to 5.38 | < .001 |
Abbreviation: IGTTNB, image-guided transthoracic needle biopsy; OR, odds ratio.
Odds ratios were derived from multivariable analysis, and models were adjusted for all other factors listed in the table.
Of the 36,563 patients who underwent IGTTNB as an inpatient, 7,830 (21.4%) developed a pneumothorax within 1 month. Of these, 7,409 (94.6% with pneumothorax) were hospitalized under the same admission for IGTTNB, 335 (4.3%) were hospitalized under a separate admission from IGTTNB, and 86 (1.1%) were treated as outpatients. Two thousand eight hundred ninety-four (36.0% with pneumothorax and 7.9% of total) had a chest tube placed (Figure 1C).
Patients hospitalized within 1 month of outpatient IGTTNB for pneumothorax had a median LOS of 6 days (IQR, 4 to 9 days), and a median cost of $9,942 (IQR, $4,819 to $22,145). Patients who developed pneumothorax during the same admission as inpatient IGTTNB had a median LOS of 6 days (IQR, 3 to 11 days), and median cost of $8,908 (IQR, $4,440 to $18,279). Patients hospitalized within 1 month of inpatient IGTTNB for pneumothorax had a median LOS of 7 days (IQR, 4 to 12 days), and median cost of $15,569 (IQR, $6,785 to $30,352).
Over time, total IGTTNB claims increased by 40.6%, from 22,756 in 2006 to 31,998 in 2012. Mean outpatient IGTNNB cost per procedure increased by 24.4%, from $1,243 in 2006 to $1,545 in 2012. Total IGTTNB costs increased by 74.9%, from $28,276,933 in 2006 to $49,447,945 in 2012 (Figure 2).
Figure 2.
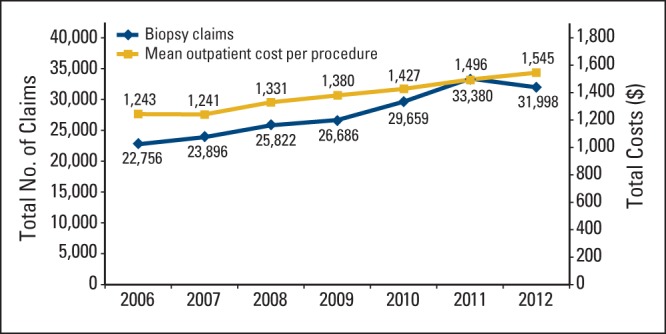
Trends in use and cost of image-guided transthoracic needle biopsy in patients with cancer from 2006 to 2012.
Discussion
We found that IGTTNB use has increased substantially over time in patients with cancer. Unlike prior studies that found pneumothorax rates of up to 56%, in this large national hospital database, we found pneumothorax rates of 12.2% of the total outpatient population. Reassuringly, only 2.3% were hospitalized within 1 month after the procedure, and only 0.42% required chest tube placement. Rates of pneumothorax and chest tube placement were substantially lower in patients who underwent outpatient versus inpatient IGTTNB, which likely represents selection of healthier patients who underwent outpatient IGTTNB. For patients who underwent outpatient IGTTNB and were subsequently hospitalized after a pneumothorax, median LOS was short. Volume of IGTTNB increased by 40.6% from 2006 to 2012; however, the mean outpatient cost per procedure of IGTTNB increased minimally ($303) in the same time frame.
Compared with previously reported studies, rates of pneumothorax and chest tube placement were lower. Pneumothorax rates previously reported ranged from 8.2%14 to 56.6%,18 rates of subsequent chest tube placement ranged from 5.8%13 to 44.4%17 of patients who developed a pneumothorax and 1.0%14 to 18.5%6 of the total population undergoing IGTTNB. Predictors of pneumothorax development from previous studies included biopsy location,6,13,15,16,19 needle size,13,21 and pulmonary function.16,19 Our study included data from more than 500 hospitals representing 79,518 patients, whereas previous studies were single-institution analyses. We found that parenchymal biopsies were associated with higher rates of pneumothorax development, which was also noted by Larscheidet al in their study,6 which demonstrated a four-fold increased risk of pneumothorax after parenchymal biopsy.
Patients who underwent outpatient IGTTNB at hospitals located outside metropolitan areas had an increased risk of postprocedure pneumothorax (OR = 1.41). Approximately 20% of the US population lives in rural areas.32 Markin et al33 recently examined trends in oncologic surgery performed in rural hospitals from 1998 to 2009 and found that at these institutions, the proportion of oncologic surgeries has decreased from 12% in 1998% to 6% in 2009. Multivariable analysis demonstrated that patients who underwent complex cancer surgery had worse inpatient mortality at rural hospitals compared with urban hospitals (OR = 2.10; 95% CI, 1.67 to 2.64). It is not surprising that outpatient radiologic procedures follow the same trends.
Interestingly, unlike other studies that have found increased complications among racial minority patients,34–37 we found that black patients were less likely to develop pneumothorax compared with white patients. It is possible that selection bias for the procedure may contribute to this finding. A recent population-based study of 17,378 patients with locally advanced non–small-cell lung cancer found in multivariable analysis that black patients were less likely to undergo surgical resection compared with white patients (OR = 0.57; 95% CI, 0.51 to 0.65), although 5-year overall survival was significantly higher in the group that received surgery (29% v 6.8%; P < .001). Of patients who did have surgery, there was a trend toward increased mortality in black patients compared with white patients (OR = 1.10; 95% CI, 1.00 to 1.22).38
In our cohort, patients with lung cancer had higher rates of complications compared with patients with other cancer diagnoses. Patients with lung cancer have a greater likelihood of underlying pulmonary disease and decreased pulmonary reserve. It is estimated that 50% to 90% of patients with lung cancer also carry a diagnosis for chronic obstructive pulmonary disease (COPD). A matched cohort analysis found that smokers diagnosed with lung cancer were 6 times more likely to have COPD compared with smokers without lung cancer, along with a statistically significant decrease in FEV1, FEV1% predicted, and FEV1/FVC.39 Another study of patients with early-stage non–small-cell lung cancer found that COPD was independently predictive of worse outcomes, including 5-year overall survival and disease-free survival.40 A single-institution retrospective review of outcomes of 704 patients undergoing VATS found a low postoperative death rate at 1.3%; however, all occurred in patients with primary lung cancer, and of those patients, 55% had underlying interstitial lung disease.41
Although our study benefits from the inclusion of a large cohort of patients with cancer who underwent IGTNNB, we recognize several important limitations. Given the inherent differences in the inpatient and the outpatient population, including increased comorbidities and complexities that may have independently accounted for a proportion of complications not directly related to IGTTNB, we were unable to compare predictors of pneumothorax between patients who had outpatient IGTTNB and those who had inpatient IGTTNB. The Perspective database contains a large sample of patients throughout the United States; however, the database has a relatively high proportion of patients treated at small to midsize, nonteaching, urban, and Southern facilities. As a result, our findings may not be generalizable to the entire US population. The Perspective database also does not include data regarding household income, education level, cancer stage, and distance from household to hospital, which may influence likelihood of undergoing a IGTNNB. Perspectives reports dates of procedures and complications in a month/year format, lacking the ability to provide specific dates of post procedural complications like pneumothorax or chest tube placement, so it is unknown the exact timing of postprocedural complications. Although we defined complications as claims within 1 month, we may have included patients with complications from other causes. The use of administrative data also limited our ability to obtain details of underlying cancer diagnosis, including tumor characteristics and stage; in the combination group, we were unable to determine how many patients had a nonlung cancer with lung metastases versus two separate primary cancer diagnoses (including one lung cancer).
In conclusion, in a large hospital-based sample, we have found that the use of IGTTNB has increased substantially over the past decade. Although the rate of pneumothorax was less frequent than previously reported, we still found that approximately 12% of outpatient procedures were followed by a pneumothorax. However, reassuringly, hospitalization or chest tube placement resulting from this procedure was uncommon. Despite an increase in total volume, the price per procedure has only increased modestly over time. With new guidelines from the US Preventative Services Task Force recommending annual low-dose CT screening for lung cancer in a select population,42 we anticipate an increased need for minimally invasive diagnostic modalities for pulmonary nodules. Patients undergoing IGTNBB can be reassured by the low rate of serious complications from this procedure.
Acknowledgment
Supported by National Cancer Institute Fellowship R25 CA094061-12 (M.K.A.), a Young Investigator Award from the ASCO Conquer Cancer Foundation (M.K.A.), and by National Cancer Institute Grants No. NCI R01 CA166084 (D.L.H.) and NCI R01CA169121 (J.D.W.).
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: Melissa K. Accordino, Jason D. Wright, Dawn L. Hershman
Financial support: Dawn L. Hershman
Administrative support: Jason D. Wright, Dawn L. Hershman
Collection and assembly of data: Melissa K. Accordino, Dawn L. Hershman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Trends in Use and Safety of Image-Guided Transthoracic Needle Biopsies in Patients With Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Melissa K. Accordino
No relationship to disclose
Jason D. Wright
Research Funding: Genentech (Inst)
Donna Buono
No relationship to disclose
Alfred I. Neugut
Consulting or Advisory Role: Executive Health Exams, Intl, Pfizer
Dawn L. Hershman
No relationship to disclose
References
- 1.Sinner WN. Pulmonary neoplasms diagnosed with transthoracic needle biopsy. Cancer. 1979;43:1533–1540. doi: 10.1002/1097-0142(197904)43:4<1533::aid-cncr2820430447>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Klein JS, Zarka MA. Transthoracic needle biopsy: An overview. J Thoracic Imaging. 1997;12:232–249. doi: 10.1097/00005382-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Berquist TH, Bailey PB, Cortese DA, et al. Transthoracic needle biopsy: Accuracy and complications in relation to location and type of lesion. Mayo Clinic Proc. 1980;55:475–481. [PubMed] [Google Scholar]
- 4.Layfield LJ, Coogan A, Johnston WW, et al. Transthoracic fine needle aspiration biopsy. Sensitivity in relation to guidance technique and lesion size and location. Acta Cytologica. 1996;40:687–690. doi: 10.1159/000333940. [DOI] [PubMed] [Google Scholar]
- 5.Yuan A, Yang PC, Chang DB, et al. Ultrasound-guided aspiration biopsy of small peripheral pulmonary nodules. Chest. 1992;4:926–930. doi: 10.1378/chest.101.4.926. [DOI] [PubMed] [Google Scholar]
- 6.Larscheid RC, Thorpe PE, Scott WJ. Percutaneous transthoracic needle aspiration biopsy: A comprehensive review of its current role in the diagnosis and treatment of lung tumors. Chest. 1998;114:704–709. doi: 10.1378/chest.114.3.704. [DOI] [PubMed] [Google Scholar]
- 7.Haaga JR, Alfidi RJ. Precise biopsy localization by computer tomography. Radiology. 1976;118:603–607. doi: 10.1148/118.3.603. [DOI] [PubMed] [Google Scholar]
- 8.vanSonnenberg E, Casola G, Ho M, et al. Difficult thoracic lesions: CT-guided biopsy experience in 150 cases. Radiology. 1988;167:457–461. doi: 10.1148/radiology.167.2.3357956. [DOI] [PubMed] [Google Scholar]
- 9.Hirose T, Mori K, Machida S, et al. Computed tomographic fluoroscopy-guided transthoracic needle biopsy for diagnosis of pulmonary nodules. Japan J Clin Oncol. 2000;30:259–262. doi: 10.1093/jjco/hyd070. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Zhang J, Cheng Z, et al. Factors affecting major morbidity after video-assisted thoracic surgery for lung cancer. J Surg Res. 2014 Jul 28; doi: 10.1016/j.jss.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 11.Medbery RL, Perez SD, Force SD, et al. Video-assisted thoracic surgery lobectomy cost variability: Implications for a bundled payment era. Ann Thorac Surg. 2014;97:1686–1692. doi: 10.1016/j.athoracsur.2014.01.021. discussion 1692-1683. [DOI] [PubMed] [Google Scholar]
- 12.Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: A survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59:60–64. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: Multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126:748–754. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 14.Arslan S, Yilmaz A, Bayramgürler B, Uzman O, et al. CT-guided transthoracic fine needle aspiration of pulmonary lesions: Accuracy and complications in 294 patients. Med Sci Monit. 2002;8:CR493–CR497. [PubMed] [Google Scholar]
- 15.Hiraki T, Mimura H, Gobara H, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: Retrospective analysis of the procedures conducted over a 9-year period. AJR Am J Roentgenol. 2010;194:809–814. doi: 10.2214/AJR.09.3224. [DOI] [PubMed] [Google Scholar]
- 16.Kazerooni EA, Lim FT, Mikhail A, et al. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology. 1996;198:371–375. doi: 10.1148/radiology.198.2.8596834. [DOI] [PubMed] [Google Scholar]
- 17.Lima CD, Nunes RA, Saito EH, et al. Results and complications of CT-guided transthoracic fine-needle aspiration biopsy of pulmonary lesions. J Bras Pneumol. 2011;37:209–216. doi: 10.1590/s1806-37132011000200011. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura M, Yoshizako T, Koyama S, et al. Risk factors influencing chest tube placement among patients with pneumothorax because of CT-guided needle biopsy of the lung. J Med Imaging Radiat Oncol. 2011;55:474–478. doi: 10.1111/j.1754-9485.2011.02283.x. [DOI] [PubMed] [Google Scholar]
- 19.Saji H, Nakamura H, Tsuchida T, et al. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: The angle of the needle trajectory is a novel predictor. Chest. 2002;121:1521–1526. doi: 10.1378/chest.121.5.1521. [DOI] [PubMed] [Google Scholar]
- 20.Yamagami T, Nakamura T, Iida S, et al. Management of pneumothorax after percutaneous CT-guided lung biopsy. Chest. 2002;121:1159–1164. doi: 10.1378/chest.121.4.1159. [DOI] [PubMed] [Google Scholar]
- 21.Geraghty PR, Kee ST, McFarlane G, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: Needle size and pneumothorax rate. Radiology. 2003;229:475–481. doi: 10.1148/radiol.2291020499. [DOI] [PubMed] [Google Scholar]
- 22.Lagu T, Rothberg MB, Nathanson BH, et al. The relationship between hospital spending and mortality in patients with sepsis. Arch Intern Med. 2011;171:292–299. doi: 10.1001/archinternmed.2011.12. [DOI] [PubMed] [Google Scholar]
- 23.Lindenauer PK, Pekow PS, Lahti MC, et al. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303:2359–2367. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 24.Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. New Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 25.Rothberg MB, Pekow PS, Lahti M, et al. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 26.Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: Analysis of hospital records. Ann Intern Med. 2011;154:516–522. doi: 10.7326/0003-4819-154-8-201104190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright JD, Neugut AI, Ananth CV, et al. Deviations from guideline-based therapy for febrile neutropenia in cancer patients and their effect on outcomes. JAMA. 2013;173:559–568. doi: 10.1001/jamainternmed.2013.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hershman DL, Richards CA, Kalinsky K, et al. Influence of health insurance, hospital factors and physician volume on receipt of immediate post-mastectomy reconstruction in women with invasive and non-invasive breast cancer. Breast Cancer Res Treat. 2012;136:535–545. doi: 10.1007/s10549-012-2273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 31.Potosky AL, Warren JL, Riedel ER, et al. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40(suppl 8):62–68. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Census Bureau: GCT-P1. Urban/rural and metropolitan/nonmetropolitan population. 2000. Census 2000 summary file 1. Washington DUCB. http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_SF1_GCTP1.US26&prodType=table.
- 33.Markin A, Habermann EB, Chow CJ, et al. Rurality and cancer surgery in the United States. Am J Surg. 2012;204:569–573. doi: 10.1016/j.amjsurg.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Zwintscher NP, Steele SR, Martin MJ, et al. The effect of race on outcomes for appendicitis in children: A nationwide analysis. Am J Surg. 2014;207:748–753. doi: 10.1016/j.amjsurg.2013.12.020. discussion 753. [DOI] [PubMed] [Google Scholar]
- 35.Causey MW, McVay D, Hatch Q, et al. The impact of race on outcomes following emergency surgery: An American College of Surgeons National Surgical Quality Improvement Program assessment. Am J Surg. 2013;206:172–179. doi: 10.1016/j.amjsurg.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Martin CT, Pugely AJ, Gao Y, et al. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: A review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95:e98. doi: 10.2106/JBJS.L.01440. 91-10. [DOI] [PubMed] [Google Scholar]
- 37.Masoomi H, Kang CY, Chen A, et al. Predictive factors of in-hospital mortality in colon and rectal surgery. J Am Coll Surg. 2012;215:255–261. doi: 10.1016/j.jamcollsurg.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Speicher PJ, Englum BR, Ganapathi AM, et al. Outcomes after treatment of 17 378 patients with locally advanced (T3N0-2) non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014 Jul 8; doi: 10.1093/ejcts/ezu270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Resp J. 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 40.Zhai R, Yu X, Shafer A, Wain JC, Christiani DC. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest. 2014;145:346–353. doi: 10.1378/chest.13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: Single institutional experience with 704 cases. Ann Thorac Surg. 2010;89:S2118–S2122. doi: 10.1016/j.athoracsur.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 42.Moyer VA, U.S. Preventive Services Task Force: Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]


