Specialist collaboration is associated with lower mortality without increased cost for patients with stage III colon cancer.
Abstract
Purpose:
Collaboration between specialists is essential for achieving high-value care in patients with complex cancer needs. We explore how collaboration between oncologists and surgeons affects mortality and cost for patients requiring multispecialty cancer care.
Patients and Methods:
This was a retrospective cohort study of patients with stage III colon cancer from SEER-Medicare diagnosed between 2000 and 2009. Patients were assigned to a primary treating surgeon and oncologist. Collaboration between surgeon and oncologist was measured as the number of patients shared between them; this has been shown to reflect advice seeking and referral relationships between physicians. Outcomes included hazards for all-cause mortality, subhazards for colon cancer–specific mortality, and cost of care at 12 months.
Results:
A total of 9,329 patients received care from 3,623 different surgeons and 2,319 medical oncologists, representing 6,827 unique surgeon–medical oncologist pairs. As the number of patients shared between specialists increased from to one to five (25th to 75th percentile), patients experienced an approximately 20% improved survival benefit from all-cause and colon cancer–specific mortalities. Specifically, for each additional patient shared between oncologist and surgeon, all-cause mortality improved by 5% (hazard ratio, 0.95; 95%CI, 0.92 to 0.97), and colon cancer–specific mortality improved by 5% (subhazard ratio, 0.95; 95% CI, 0.91 to 0.97). There was no association with cost.
Conclusion:
Specialist collaboration is associated with lower mortality without increased cost among patients with stage III colon cancer. Facilitating formal and informal collaboration between specialists may be an important strategy for improving the care of patients with complex cancers.
Introduction
With cancer burden rising and the complexity of treatment growing, cost of cancer care has outpaced that of medical care overall and is projected to reach $158 billion by 2020.1,2 Furthermore, quality and outcomes across the cancer continuum remain suboptimal.3 Consequently, clinicians and policymakers are focused on delivering high-value care—that is, care that maximizes patient outcomes while containing cumulative cost.4,5
Coordination of cancer care has received significant attention as a strategy to promote high-value care.6 Cancer treatment frequently requires multiple modalities across several care settings, delivered by a variety of health professionals and over an extended period of time. Without appropriate coordination, patients experience worse access to treatment, poorer outcomes, and higher costs.7–12 The Institute of Medicine has identified the “poorly coordinated delivery” of cancer care as a “priority area for improvement,”6(p10) and many current health care reforms seek to improve coordination of care through building integrated networks of providers to deliver complex care.13
One aspect of care coordination that has rarely been studied is collaboration between cancer specialists.14 Collaboration reflects the extent to which specialists work together to achieve optimal outcomes for a given patient and may result from better interpersonal information exchange and reflect longstanding relationships between specialists.15–19
We focus on the potential collaboration between surgeons and medical oncologists for stage III colon cancer. Colorectal cancer is the third leading cause of cancer mortality and the second most expensive cancer in the United States.1 Stage III colon cancer requires timely surgery and adjuvant chemotherapy to improve survival.20 Because this involves coordination between specialists, patients with stage III colon cancer are vulnerable to poor coordination; many patients do not receive guideline-concordant care, and disparities exist.20
We used administrative claims data to explore whether patients who receive care from surgeons and medical oncologists who potentially collaborate more frequently with each other have improved overall survival, colon cancer–specific survival, and lower 12-month cost of care. We operationalized collaboration between surgeons and oncologists as the number of patients they shared (ie, when both providers bill for medical services for the same patient), because this has been shown to reflect advice seeking and referral relationships between physicians.21–24
Patients and Methods
Study Population
Patients with colon cancer diagnosed between 2000 and 2009 were identified using SEER-Medicare data, a population-based cancer registry (ie, SEER) linked to claims from Medicare. SEER encompasses 17 sites and approximately 28% of the US population; Medicare claims have been linked to approximately 93% of the Medicare patients in SEER.25
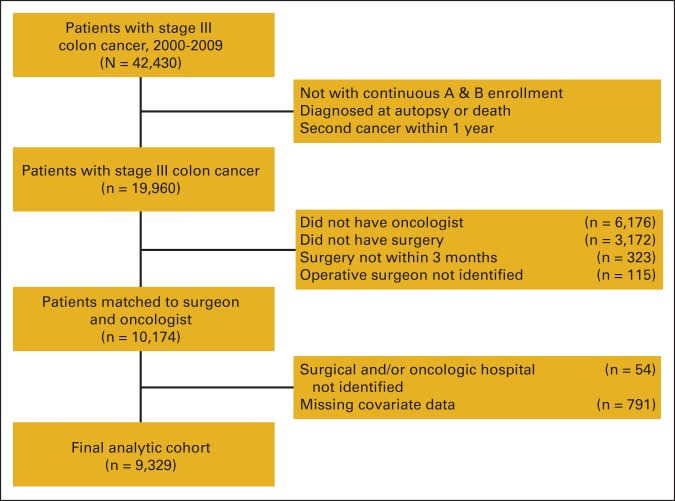
Patients eligible for inclusion included those with stage III colon cancer (not rectal cancer) with continuous Part A and B Medicare coverage during the 12 months before and 12 months after their diagnosis date. We excluded patients age < 66 years who were enrolled in a health maintenance organization during the 2-year interval, diagnosed at autopsy or death, or diagnosed with a second cancer within 12 months of the colon cancer diagnosis (Appendix Figure A1, online only). We excluded patients who could not be assigned to an oncologist (n = 6,176), who did not undergo any surgery (n = 3,127) or underwent surgery beyond 3 months of diagnosis (n = 323), who could not be assigned to a surgical and/or oncologic hospital (n = 54), and whose operative surgeon could not be identified (n = 115). Because investigation of missingness revealed the missing data mechanism likely to be completely random, we excluded those who had missing covariate information (n = 791).26 The final analytic cohort included 9,329 patients.
Measures
Outcomes.
All-cause mortality with survival time calculated from date of colon cancer diagnosis to Medicare date of death (or censor date of December 31, 2011) was the primary outcome. Colon cancer–specific mortality was a secondary outcome; the censor date was December 31, 2009, because cause of death was not available thereafter. Total cost of care at 12 months after diagnosis was calculated as the total reimbursement made on patient claims using the Medicare Provider Analysis and Review File, Carrier Claims, and the Outpatient Statistical Analysis File.27
Patient sharing between medical oncologist and surgeon.
To generate more stable estimates of shared patients, we used all patients with colon cancer diagnosed between 2000 and 2009 in SEER-Medicare files, regardless of stage, who could be assigned to both a surgeon and medical oncologist. Patients were assigned to the surgeon who performed their definitive colon cancer surgery based on billing codes. For the 420 patients (4.5%) who underwent more than one surgery, assignment of surgical care was based on the first operation.28,29 Regarding medical oncologic care, for the 653 patients (7%) who received care from > one oncologist, we assigned patients to the medical oncologist who billed for the plurality of their visits—that is, the single medical oncologist who billed for the greatest number of visits—in the year after diagnosis.30,31 We used specialty codes (available in Carrier File) 83 and 90 to identify medical oncologists and 2, 28, and 91 to identify surgeons. A total of 31,310 patients with colon cancer were assigned to both a surgeon and medical oncologist. On the basis of these physician assignments for patients, we counted the number of patients shared by each unique oncologist–surgeon pair for each calendar year. Because of the non-normal distribution of shared patients, the number of shared patients between a pair of providers was top coded at eight patients per year, representing the 99th percentile of the distribution.
Patient-level covariates.
Covariates included age, sex, self-reported Medicare race (black, white, or other), census tract median household income (in quartiles), year of diagnosis, Charlson comorbidity score in the 12 months before diagnosis, urban or rural residence, and SEER site. Cancer characteristics included tumor grade, adequate lymph node resection during surgery (≥ 12 lymph nodes), and, for patients diagnosed from 2004 onward, cancer substage.32
Physician-level covariates.
Yearly surgical volume was tabulated using the total number of all patients with colon cancer on whom surgeons operated in a given year,29 modeled in quartiles (< two, two, three to four, > four patient cases per year). Similarly, the yearly panel size of all patients with colon cancer attributed to each medical oncologist was modeled in quartiles (< two, two to three, four to five, > five patient cases per year).
Hospital-level covariates.
Surgical hospital was defined as the location of the surgical procedure. Because a large portion of medical oncologic care is delivered in the outpatient setting, we used the approach of Bynum et al33 to assign patients to the hospital where their medical oncologist billed for their inpatient care; oncologists who did not bill for any inpatient claims were assigned to the hospital where the plurality of their patients were admitted in a given year. Hospital characteristics from the SEER-Medicare Hospital File included National Cancer Institute–recognized status, academic hospital status (whether teaching hospital or affiliated with one), and for-profit status (government or voluntary nonprofit v for-profit). We determined the volume of patients with colon cancer (all stages) who underwent colon resection (as identified by International Classification of Diseases [ninth revision] codes) and received medical oncologic care at hospitals between 2000 and 2009.34,35 Hospital volume was analyzed in quartiles, with the following cutoffs: volume of patients with colon cancer receiving surgical care at the surgical hospital (< 112, 112 to 198, 199 to 312, > 312 patient cases) and volume of patients with colon cancer receiving oncologic care at the oncologic hospital (< 130, 130 to 210, 211 to 320, > 320 patient cases).
Statistical Analyses
We used Cox proportional hazards to model all-cause mortality and Fine and Gray's method for competing risk regression to model mortality resulting from colon cancer, where death resulting from other causes was a competing risk.36 To assess the proportional hazards assumption, we used the Grambsch and Therneau test of nonzero slope.37
Total cost of care accrued at 12 months was modeled with generalized linear models.38 Modified Park test determined the distribution and link functions39 used for modeling our data: gamma variance distribution and log link for nonextreme costs (bottom 95% of patient costs) and inverse Gaussian variance distribution and log link for extreme costs (top 5%). All US dollar values were inflated to 2009 using annual gross domestic product price indices.40
For all models, we used multivariable regression to adjust for patient, physician, and hospital covariates. Variance inflation factors were examined to check for multicollinearity between provider and hospital characteristics. We corrected for clustering within each medical–surgical hospital pair in which the patient received care using generalized estimating equations for cost models and robust variance estimation for survival analysis.
We performed sensitivity analyses to ensure robustness of findings. First, we modeled all analyses varying the way patient sharing was operationalized: as continuous, without top coding, and as binary (upper v lower three quartiles). Second, although previous studies have used quartiles to adjust for provider patient volumes on cancer outcomes,29,41 to ensure our findings for patient sharing did not result from residual confounding from yearly surgical volume and oncologist panel size, we adjusted for these as continuous variables. Third, we modeled all analyses controlling for substage in the subcohort of patients diagnosed from 2004 onward (when this information became available). Fourth, we modeled total cost of care at 6 months and costs at both 6 and 12 months only among those surviving to each time. Fifth, we tested for potential interactions between patient sharing and race, median census tract income, urban or rural residence, and comorbidity index. Sixth, we included patients who had missing information for covariates, applying multiple imputation to model missing values using all other available patient information. Finally, because collaboration may be more challenging across hospital systems, we estimated effects for patient sharing after controlling for whether patients received their surgical and medical oncologic care at a single hospital or different hospitals as well as considering this construct as an effect modifier. Data were analyzed used STATA IC software (version 12.1; STATA, College Station, TX). Our study received approval from the Johns Hopkins University School of Medicine Institutional Review Board.
Results
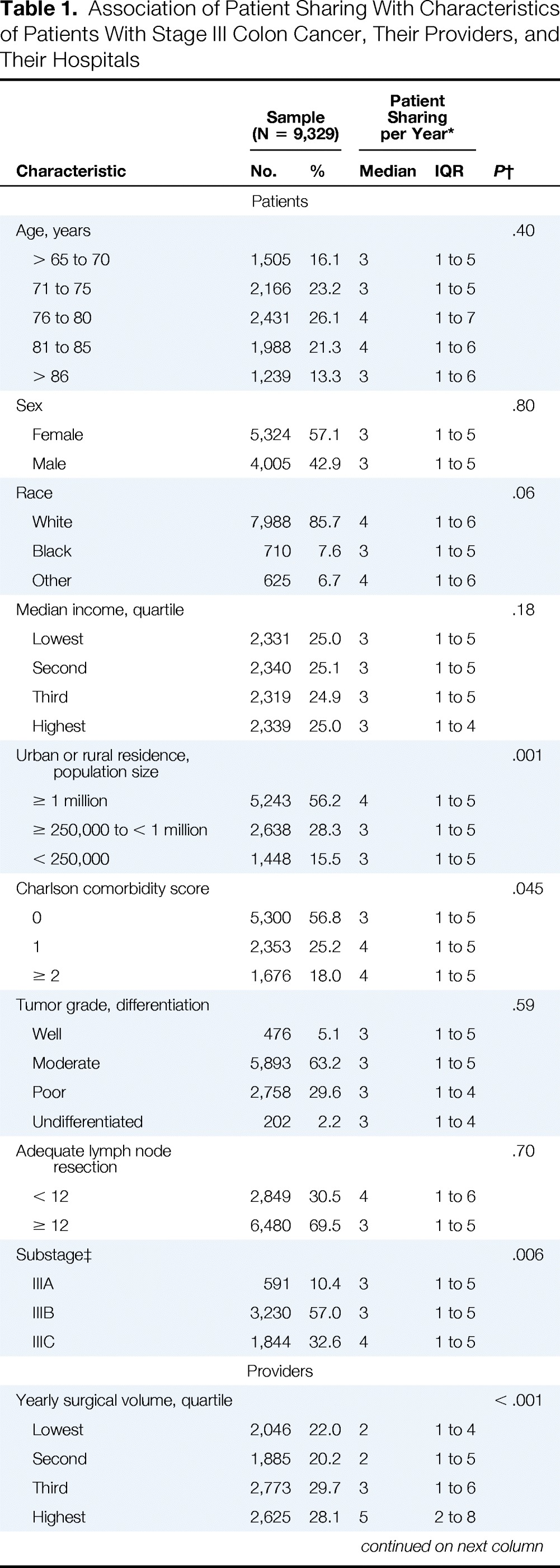
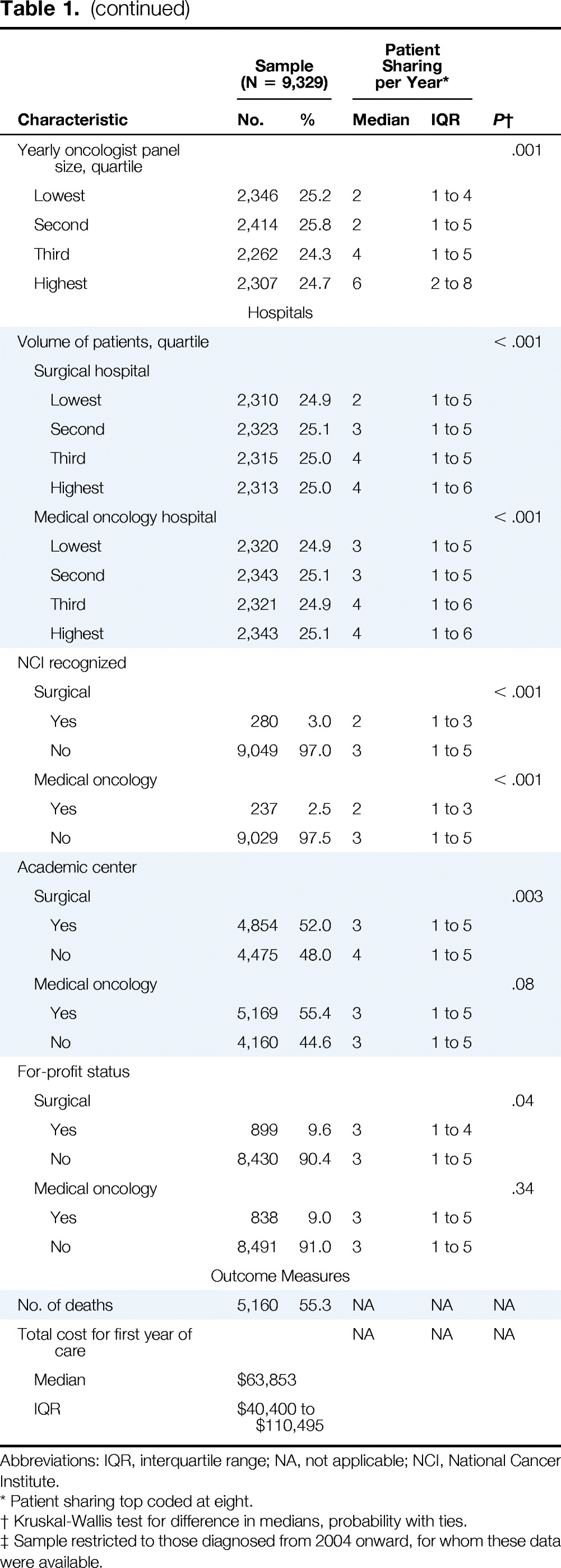
Table 1 summarizes characteristics of patients with stage III colon cancer in our analytic sample. There were 9,329 individuals assigned to 3,623 different surgeons and 2,319 medical oncologists, representing 6,827 unique surgeon–medical oncologist pairs. Their physicians shared a median of three patients with colon cancer per year (interquartile range, one to five); 28% of patients were treated by a surgeon–medical oncologist pair who shared no other patients in that diagnosis year (ie, patient sharing between these physicians was limited to only one patient with colon cancer in our study). Rural patients had lower numbers of shared patients between physicians than their urban counterparts.
Table 1.
Association of Patient Sharing With Characteristics of Patients With Stage III Colon Cancer, Their Providers, and Their Hospitals
| Characteristic | Sample (N = 9,329) |
Patient Sharing per Year* |
P† | ||
|---|---|---|---|---|---|
| No. | % | Median | IQR | ||
| Patients | |||||
| Age, years | .40 | ||||
| > 65 to 70 | 1,505 | 16.1 | 3 | 1 to 5 | |
| 71 to 75 | 2,166 | 23.2 | 3 | 1 to 5 | |
| 76 to 80 | 2,431 | 26.1 | 4 | 1 to 7 | |
| 81 to 85 | 1,988 | 21.3 | 4 | 1 to 6 | |
| > 86 | 1,239 | 13.3 | 3 | 1 to 6 | |
| Sex | .80 | ||||
| Female | 5,324 | 57.1 | 3 | 1 to 5 | |
| Male | 4,005 | 42.9 | 3 | 1 to 5 | |
| Race | .06 | ||||
| White | 7,988 | 85.7 | 4 | 1 to 6 | |
| Black | 710 | 7.6 | 3 | 1 to 5 | |
| Other | 625 | 6.7 | 4 | 1 to 6 | |
| Median income, quartile | .18 | ||||
| Lowest | 2,331 | 25.0 | 3 | 1 to 5 | |
| Second | 2,340 | 25.1 | 3 | 1 to 5 | |
| Third | 2,319 | 24.9 | 3 | 1 to 5 | |
| Highest | 2,339 | 25.0 | 3 | 1 to 4 | |
| Urban or rural residence, population size | .001 | ||||
| ≥ 1 million | 5,243 | 56.2 | 4 | 1 to 5 | |
| ≥ 250,000 to < 1 million | 2,638 | 28.3 | 3 | 1 to 5 | |
| < 250,000 | 1,448 | 15.5 | 3 | 1 to 5 | |
| Charlson comorbidity score | .045 | ||||
| 0 | 5,300 | 56.8 | 3 | 1 to 5 | |
| 1 | 2,353 | 25.2 | 4 | 1 to 5 | |
| ≥ 2 | 1,676 | 18.0 | 4 | 1 to 5 | |
| Tumor grade, differentiation | .59 | ||||
| Well | 476 | 5.1 | 3 | 1 to 5 | |
| Moderate | 5,893 | 63.2 | 3 | 1 to 5 | |
| Poor | 2,758 | 29.6 | 3 | 1 to 4 | |
| Undifferentiated | 202 | 2.2 | 3 | 1 to 4 | |
| Adequate lymph node resection | .70 | ||||
| < 12 | 2,849 | 30.5 | 4 | 1 to 6 | |
| ≥ 12 | 6,480 | 69.5 | 3 | 1 to 5 | |
| Substage‡ | .006 | ||||
| IIIA | 591 | 10.4 | 3 | 1 to 5 | |
| IIIB | 3,230 | 57.0 | 3 | 1 to 5 | |
| IIIC | 1,844 | 32.6 | 4 | 1 to 5 | |
| Providers | |||||
| Yearly surgical volume, quartile | < .001 | ||||
| Lowest | 2,046 | 22.0 | 2 | 1 to 4 | |
| Second | 1,885 | 20.2 | 2 | 1 to 5 | |
| Third | 2,773 | 29.7 | 3 | 1 to 6 | |
| Highest | 2,625 | 28.1 | 5 | 2 to 8 | |
| Yearly oncologist panel size, quartile | .001 | ||||
| Lowest | 2,346 | 25.2 | 2 | 1 to 4 | |
| Second | 2,414 | 25.8 | 2 | 1 to 5 | |
| Third | 2,262 | 24.3 | 4 | 1 to 5 | |
| Highest | 2,307 | 24.7 | 6 | 2 to 8 | |
| Hospitals | |||||
| Volume of patients, quartile | < .001 | ||||
| Surgical hospital | |||||
| Lowest | 2,310 | 24.9 | 2 | 1 to 5 | |
| Second | 2,323 | 25.1 | 3 | 1 to 5 | |
| Third | 2,315 | 25.0 | 4 | 1 to 5 | |
| Highest | 2,313 | 25.0 | 4 | 1 to 6 | |
| Medical oncology hospital | < .001 | ||||
| Lowest | 2,320 | 24.9 | 3 | 1 to 5 | |
| Second | 2,343 | 25.1 | 3 | 1 to 5 | |
| Third | 2,321 | 24.9 | 4 | 1 to 6 | |
| Highest | 2,343 | 25.1 | 4 | 1 to 6 | |
| NCI recognized | |||||
| Surgical | < .001 | ||||
| Yes | 280 | 3.0 | 2 | 1 to 3 | |
| No | 9,049 | 97.0 | 3 | 1 to 5 | |
| Medical oncology | < .001 | ||||
| Yes | 237 | 2.5 | 2 | 1 to 3 | |
| No | 9,029 | 97.5 | 3 | 1 to 5 | |
| Academic center | |||||
| Surgical | .003 | ||||
| Yes | 4,854 | 52.0 | 3 | 1 to 5 | |
| No | 4,475 | 48.0 | 4 | 1 to 5 | |
| Medical oncology | .08 | ||||
| Yes | 5,169 | 55.4 | 3 | 1 to 5 | |
| No | 4,160 | 44.6 | 3 | 1 to 5 | |
| For-profit status | |||||
| Surgical | .04 | ||||
| Yes | 899 | 9.6 | 3 | 1 to 4 | |
| No | 8,430 | 90.4 | 3 | 1 to 5 | |
| Medical oncology | .34 | ||||
| Yes | 838 | 9.0 | 3 | 1 to 5 | |
| No | 8,491 | 91.0 | 3 | 1 to 5 | |
| Outcome Measures | |||||
| No. of deaths | 5,160 | 55.3 | NA | NA | NA |
| Total cost for first year of care | NA | NA | NA | ||
| Median | $63,853 | ||||
| IQR | $40,400 to $110,495 | ||||
Abbreviations: IQR, interquartile range; NA, not applicable; NCI, National Cancer Institute.
Patient sharing top coded at eight.
Kruskal-Wallis test for difference in medians, probability with ties.
Sample restricted to those diagnosed from 2004 onward, for whom these data were available.
Overall, 5,160 patients (55.3%) died during the 12-year observation period (median and total time at risk, 3.6 and 39,448 years, respectively); median survival time was 5.3 years. Cause of death data were available up to 2009; during this 10-year period (median and total time at risk, 2.6 and 30,617 years, respectively), 2,537 patients (27%) died as a result of colon cancer; more than 75% of deaths at 5.3 years (median survival time for cohort) resulted from colon cancer. Median unadjusted cost of care at 12 months was $63,853 (interquartile range, $40,400 to $110,495).
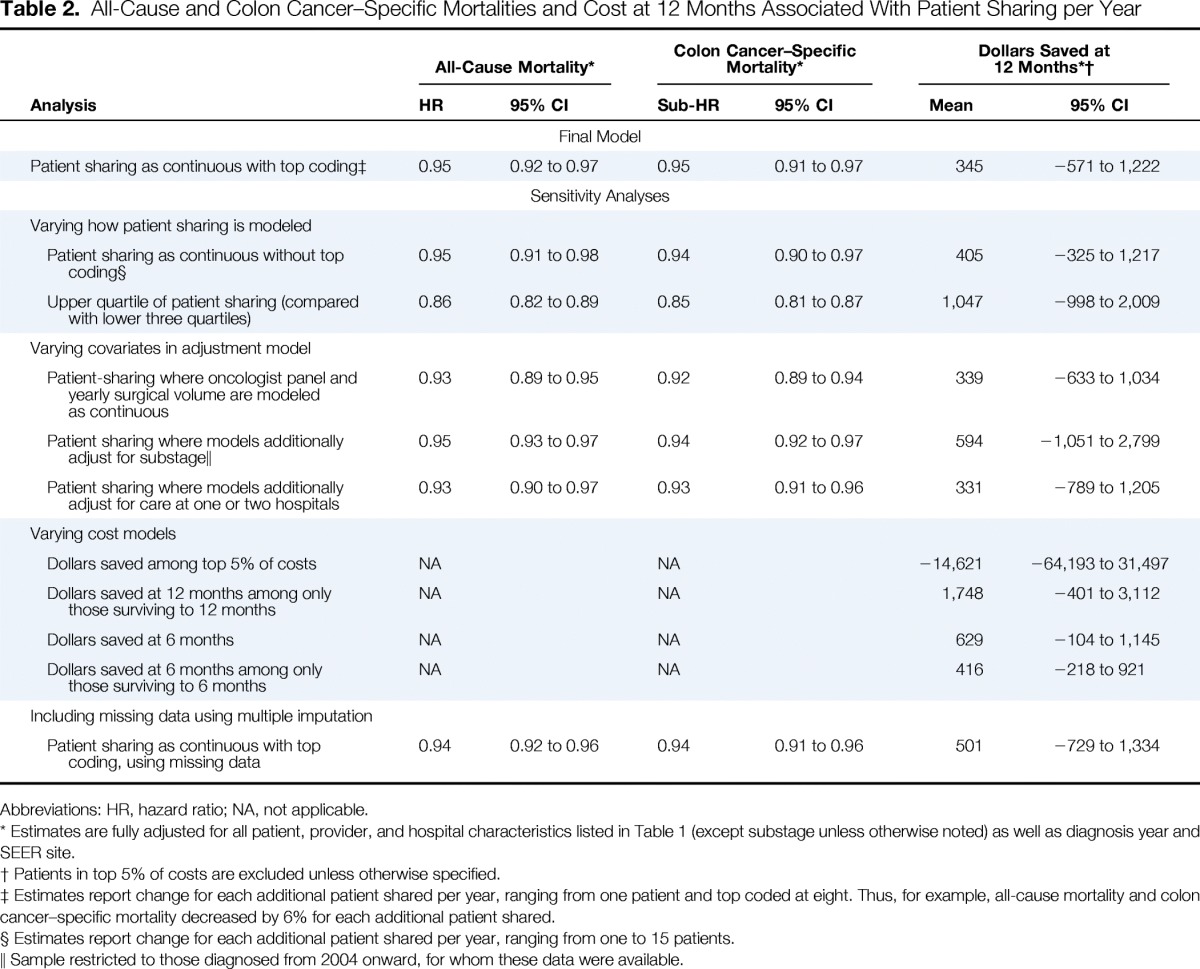
Figure 1 shows unadjusted Kaplan-Meier curves for all-cause and colon cancer–specific mortalities for patients in the 25th (one patient shared), 50th (three patients), and 75th percentiles (five patients) of patient sharing; there is a statistically significant improvement in all-cause and colon cancer–specific survival with increasing patient sharing. Table 2 summarizes the fully adjusted regression models for all-cause mortality, colon cancer–specific mortality, and total cost of care at 12 months. In fully adjusted analyses, for each additional patient shared per year between oncologist and surgeon, all-cause mortality improved by 5% (hazard ratio, 0.95; 95% CI, 0.92 to 0.97). A similar survival benefit was observed with colon cancer–specific mortality, with a 5% improvement in colon cancer–specific survival for every each additional patient shared (subhazard ratio, 0.95; 95% CI, 0.91 to 0.97). Patient sharing between physicians was not associated with cost of care.
Figure 1.
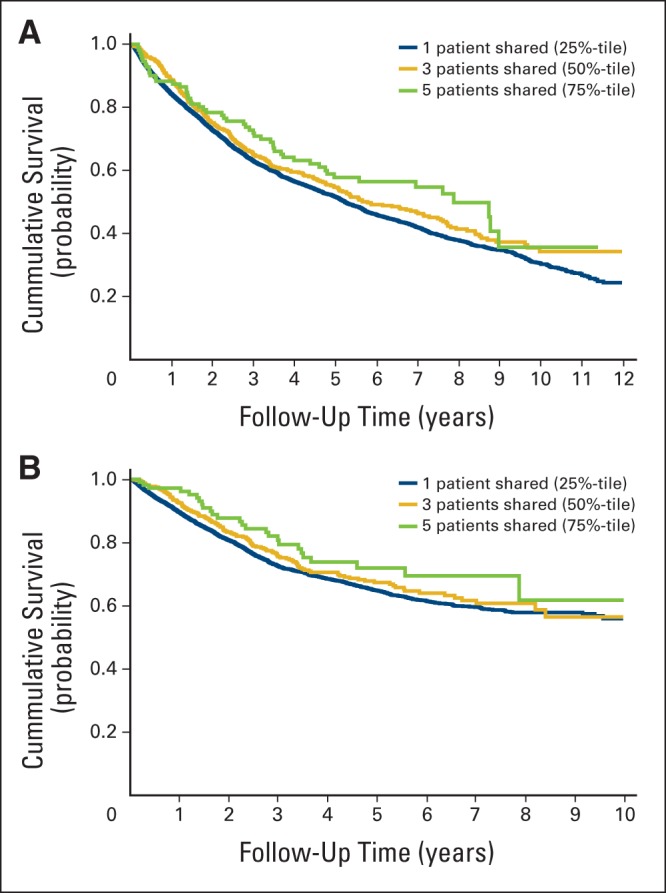
Kaplan-Meier survival curves from date of colon cancer diagnosis for (A) all-cause mortality and (B) colon cancer–specific mortality.
Table 2.
All-Cause and Colon Cancer–Specific Mortalities and Cost at 12 Months Associated With Patient Sharing per Year
| Analysis | All-Cause Mortality* |
Colon Cancer–Specific Mortality* |
Dollars Saved at 12 Months*† |
|||
|---|---|---|---|---|---|---|
| HR | 95% CI | Sub-HR | 95% CI | Mean | 95% CI | |
| Final Model | ||||||
| Patient sharing as continuous with top coding‡ | 0.95 | 0.92 to 0.97 | 0.95 | 0.91 to 0.97 | 345 | −571 to 1,222 |
| Sensitivity Analyses | ||||||
| Varying how patient sharing is modeled | ||||||
| Patient sharing as continuous without top coding§ | 0.95 | 0.91 to 0.98 | 0.94 | 0.90 to 0.97 | 405 | −325 to 1,217 |
| Upper quartile of patient sharing (compared with lower three quartiles) | 0.86 | 0.82 to 0.89 | 0.85 | 0.81 to 0.87 | 1,047 | −998 to 2,009 |
| Varying covariates in adjustment model | ||||||
| Patient-sharing where oncologist panel and yearly surgical volume are modeled as continuous | 0.93 | 0.89 to 0.95 | 0.92 | 0.89 to 0.94 | 339 | −633 to 1,034 |
| Patient sharing where models additionally adjust for substage‖ | 0.95 | 0.93 to 0.97 | 0.94 | 0.92 to 0.97 | 594 | −1,051 to 2,799 |
| Patient sharing where models additionally adjust for care at one or two hospitals | 0.93 | 0.90 to 0.97 | 0.93 | 0.91 to 0.96 | 331 | −789 to 1,205 |
| Varying cost models | ||||||
| Dollars saved among top 5% of costs | NA | NA | −14,621 | −64,193 to 31,497 | ||
| Dollars saved at 12 months among only those surviving to 12 months | NA | NA | 1,748 | −401 to 3,112 | ||
| Dollars saved at 6 months | NA | NA | 629 | −104 to 1,145 | ||
| Dollars saved at 6 months among only those surviving to 6 months | NA | NA | 416 | −218 to 921 | ||
| Including missing data using multiple imputation | ||||||
| Patient sharing as continuous with top coding, using missing data | 0.94 | 0.92 to 0.96 | 0.94 | 0.91 to 0.96 | 501 | −729 to 1,334 |
Abbreviations: HR, hazard ratio; NA, not applicable.
Estimates are fully adjusted for all patient, provider, and hospital characteristics listed in Table 1 (except substage unless otherwise noted) as well as diagnosis year and SEER site.
Patients in top 5% of costs are excluded unless otherwise specified.
Estimates report change for each additional patient shared per year, ranging from one patient and top coded at eight. Thus, for example, all-cause mortality and colon cancer–specific mortality decreased by 6% for each additional patient shared.
Estimates report change for each additional patient shared per year, ranging from one to 15 patients.
Sample restricted to those diagnosed from 2004 onward, for whom these data were available.
Sensitivity analyses are summarized in Table 2. The significant association between number of shared patients per year and survival persisted when we varied how we modeled patient sharing. Accounting for whether a patient received surgical and medical oncologic care from providers of the same hospital (v two different hospitals) did not alter our findings. No prespecified tests of interaction were statistically significant.
Discussion
We found that potential collaboration between a patient's treating surgeon and oncologist is associated with improved patient survival. For each patient shared per year between specialists, we observed an associated 5% survival benefit. As the number of patients shared increased from to one to five (25th to 75th percentile), this translated into an approximately 20% improved survival benefit from all-cause and colon cancer–specific mortalities. In contrast, patients' cost of care at 12 months did not vary by the number of patients shared between physicians. These results raise important considerations, which may inform strategies for improving the value of care for patients with colon cancer.
Previous studies have demonstrated that a surgeon's operative volume and hospital surgical volume affect outcomes of patients with colon cancer.29,34,35,42–44 We found that the volume of patients shared between a patient's surgeon and oncologist also played a role in patient outcomes. Our study suggests that patients with stage III colon cancer treated by physicians who share many patients experience a survival benefit over their counterparts treated by physicians who share fewer patients.
As a measure of potential physician interaction,21 patient sharing may capture both formal and informal opportunities for meaningful collaboration between two providers, which has been shown to improve outcomes. Formal mechanisms that promote collaboration may include working in the same practice, sharing electronic medical records, and using electronic referrals. Adoption of shared electronic medical records by health systems may be associated with small but significant changes in mortality and cost45,46; however, these associations are mixed.47
Even after accounting for whether specialists worked in the same or different hospitals, patient sharing remained associated with lower mortality, which may suggest that additional informal mechanisms are important. A recent meta-analysis on physician collaboration found that improving interactive communication (two-way communication between providers in form of e-mail exchanges, telephone conversations, and so on) “offers an equal if not better return on investment than many clinical interventions.”48(p253) Similarly, shared care, which refers to joint participation (and information exchange) between physicians in the planning of patient care, has been shown in some settings to improve cancer outcomes.49–52 Providers may feel an increased sense of professional accountability to deliver timely, appropriate care when working with colleagues with whom they frequently collaborate.53 These formal and informal mechanisms may improve coordination of care, resulting in timely transition to adjuvant chemotherapy, earlier detection and management of both postsurgical and chemotherapy-related complications, and decreased treatment-related errors, thereby improving survival.
It is noteworthy that a substantial minority of patients with colon cancer (> 25%) in our study received care from physicians who did not otherwise share fee-for-service Medicare patients in a given year. We were unable to determine the reasons for this. However, for high-risk cancer surgeries, policy efforts to promote care from high-volume surgeons may have unintended consequences for collaboration.29,34,35,42–44 Although high-volume surgeons may be more likely to communicate with local oncologists in decisions about ongoing care,54 little is known about how geography affects collaboration. Rural residents with cancer may be at highest risk for receiving care across geographic divides, and the danger of poor collaboration may be further heightened by an oncologic workforce in these communities that is understaffed and experiences high turnover rates.55–59
Although prior literature has demonstrated associations between higher patient sharing and lower cost among patients with chronic illnesses and in cancer survivorship care,23,60 we did not find a statistically significant cost savings associated with patient sharing among the patients with stage III colon cancer in our study. There are considerable costs associated with guideline-concordant treatment for stage III colon cancer, including initiation of adjuvant chemotherapy within 4 months of diagnosis.20 Therefore, improved survival that is cost neutral may reflect high-value resource use among collaborating physicians rather than wasteful care that may occur more often in the care of patients with physicians who collaborate less.
There are limitations to our study. First, there were a substantial number of patients in our cohort who could not be assigned to a medical oncologist; these findings are consistent with other population-based studies, which have found that 30% to 50% of patients with stage III colon cancer do not receive chemotherapy.61–63 Understanding the reasons why patients do not receive timely referral to a medical oncologist and adjuvant treatment, and if this is related to patient sharing, is an important area of future study. Furthermore, because of concerns regarding completeness of chemotherapy claims after changes in billing codes during the study period, we were unable to examine whether timely administration of chemotherapy potentially explained improved survival among patients receiving care from high–patient-sharing physicians. Second, to ensure high patient sharing was not simply a marker of two skilled physicians who shared many overlapping patients because of the high volume of patients each saw, we included several volume measures in our model and ensured our findings were robust to several ways of operationalizing volume. Our volume measures were based solely on Medicare data; however, volume measures constructed using Medicare data are highly correlated with those constructed from all-payer data.64,65 Third, we used total cost of care because of the potential difficulties in trying to specify solely cancer-related costs. Finally, because of the sample of patients in SEER-Medicare data, this study focused on patients in fee-for-service Medicare and may not be generalizable to younger patients or those in preferred provider organizations or health maintenance organizations or with other types of insurance.
Notwithstanding these limitations, our work underscores the importance of care-delivery strategies that encourage regular collaboration between physicians who care for patients with complex illnesses. In this setting, formal and informal systems may facilitate meaningful interaction between providers who regularly share patients, improving survival of those with cancer without increasing cost. It remains uncertain whether the implementation of policy creating integrated delivery systems, such as accountable care organizations and bundled payments, will encourage referrals within a limited pool of specialist providers, thereby providing the opportunity for providers to work together more frequently. Development of strategies intended to improve the care of those with complex cancers may benefit from supporting collaboration between specialists.
Acknowledgment
Supported by Career Development Award No. K07CA151910 from the National Cancer Institute (NCI) and Office of Behavioral and Social Science Research (C.E.P.); by Grant No. U54CA153710 from the NCI Center to Reduce Cancer Health Disparities' Community Networks Program to the Johns Hopkins Center to Reduce Cancer Disparities; by the Maryland Cigarette Restitution Fund; and by National Heart Lung Blood Institute Training Grant No. 5T32HL007180-38.
Appendix
Figure A1.
Flow chart of inclusions and exclusions for analytic cohort.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: All authors
Financial support: Tanvir Hussain, Craig E. Pollack
Administrative support: Craig E. Pollack
Collection and assembly of data: Tanvir Hussain, Hsien-Yen Chang, Craig E. Pollack
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Collaboration Between Surgeons and Medical Oncologists and Outcomes for Patients With Stage III Colon Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Tanvir Hussain
No relationship to disclose
Hsien-Yen Chang
No relationship to disclose
Christine M. Veenstra
No relationship to disclose
Craig E. Pollack
Stock or Other Ownership: Amgen, Danaher, Dow Chemical Company, Ecolab, Gilead Sciences, General Electric, Qiagen, Cerner, Illumina, sanofi-aventis, Stericycle, Thermo Fisher Scientific
References
- 1.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Clinical Oncology. The state of cancer care in America, 2014: A report by the American Society of Clinical Oncology. J Oncol Pract. 2014;10:119–142. doi: 10.1200/JOP.2014.001386. [DOI] [PubMed] [Google Scholar]
- 4.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 5.Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: The cost of cancer care. J Clin Oncol. 2009;27:3868–3874. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 6.Levit LA, Balogh E, Nass SJ, et al. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 7.Crossing the Quality Chasm. A New Health System for the 21st Century. http://www.nap.edu/openbook.php?record_id=10027. [PubMed]
- 8.Davis K. Understanding International Experience with Care Coordination: A Comparison of Performance. Philadelphia, PA: ABIM Foundation; 2007. [Google Scholar]
- 9.World Health Organization. The World Health Report 2000: Health Systems, Improving Performance. http://www.who.int/whr/2000/en/
- 10.World Health Organization. Everybody's Business: Strengthening Health Systems to Improve Health Outcomes—WHO's Framework for Action, 2007. http://www.who.int/healthsystems/strategy/everybodys_business.pdf.
- 11.Bodenheimer T. Coordinating Care: A Perilous Journey Through the Health Care System. Philadelphia, PA: ABIM Foundation; 2007. [DOI] [PubMed] [Google Scholar]
- 12.Forster AJ, Murff HJ, Peterson JF, et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 13.Fisher ES, McClellan MB, Safran DG. Building the path to accountable care. N Engl J Med. 2011;365:2445–2447. doi: 10.1056/NEJMp1112442. [DOI] [PubMed] [Google Scholar]
- 14.Veenstra CM, Epstein AJ, Liao K, et al. The effect of care setting in the delivery of high-value colon cancer care. Cancer. 2014;120:3237–3244. doi: 10.1002/cncr.28874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saultz JW. Defining and measuring interpersonal continuity of care. Ann Fam Med. 2003;1:134–143. doi: 10.1370/afm.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagin CM. Collaboration between nurses and physicians: No longer a choice. Acad Med. 1992;67:295–303. doi: 10.1097/00001888-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 17.O'Daniel M, Rosenstein AR. Professional communication and team collaboration. In: Hughes R, editor. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 18.Baggs JG, Schmitt MH. Collaboration between nurses and physicians. Image J Nurs Sch. 1988;20:145–149. doi: 10.1111/j.1547-5069.1988.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 19.Christensen C, Larson JR., Jr Collaborative medical decision making. Med Decis Making. 1993;13:339–346. doi: 10.1177/0272989X9301300410. [DOI] [PubMed] [Google Scholar]
- 20.Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network quality measures. J Clin Oncol. 2008;26:3631–3637. doi: 10.1200/JCO.2008.16.5068. [DOI] [PubMed] [Google Scholar]
- 21.Barnett ML, Landon BE, O'Malley AJ, et al. Mapping physician networks with self-reported and administrative data. Health Serv Res. 2011;46:1592–1609. doi: 10.1111/j.1475-6773.2011.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnett ML, Christakis NA, O'Malley J, et al. Physician patient-sharing networks and the cost and intensity of care in US hospitals. Med Care. 2012;50:152–160. doi: 10.1097/MLR.0b013e31822dcef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollack CE, Weissman GE, Lemke KW, et al. Patient sharing among physicians and costs of care: A network analytic approach to care coordination using claims data. J Gen Intern Med. 2013;28:459–465. doi: 10.1007/s11606-012-2104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luke DA, Harris JK. Network analysis in public health: History, methods, and applications. Annu Rev Public Health. 2007;28:69–93. doi: 10.1146/annurev.publhealth.28.021406.144132. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute. Healthcare Delivery Research: SEER-Medicare linked database. http://healthcaredelivery.cancer.gov/seermedicare/
- 26.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Medicare and Medicaid Services. Bundled Payments for Care Improvement (BPCI) initiative. http://innovation.cms.gov/initiatives/bundled-payments/
- 28.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236:583–592. doi: 10.1097/00000658-200211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrag D, Panageas KS, Riedel E, et al. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. J Surg Oncol. 2003;83:68–78. doi: 10.1002/jso.10244. discussion 78-79. [DOI] [PubMed] [Google Scholar]
- 30.Veenstra CM, Epstein AJ, Liao K, et al. The effect of care setting in delivery of high value colon cancer care. Cancer. 2014;120:3237–3244. doi: 10.1002/cncr.28874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pham HH, Schrag D, O'Malley AS, et al. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356:1130–1139. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 32.Quah HM, Chou JF, Gonen M, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. 2008;51:503–507. doi: 10.1007/s10350-008-9246-z. [DOI] [PubMed] [Google Scholar]
- 33.Bynum JP, Bernal-Delgado E, Gottlieb D, et al. Assigning ambulatory patients and their physicians to hospitals: A method for obtaining population-based provider performance measurements. Health Serv Res. 2007;42:45–62. doi: 10.1111/j.1475-6773.2006.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 35.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 36.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 37.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 38.Moran JL, Solomon PJ, Peisach AR, et al. New models for old questions: Generalized linear models for cost prediction. J Eval Clin Pract. 2007;13:381–389. doi: 10.1111/j.1365-2753.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- 39.Manning WG, Mullahy J. Estimating log models: To transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 40.Agency for Healthcare Research and Quality. Using appropriate price indices for analyses of health care expenditures or income across multiple years. http://meps.ahrq.gov/about_meps/Price_Index.shtml.
- 41.Schrag D, Bach PB, Dahlman C, et al. Identifying and measuring hospital characteristics using the SEER-Medicare data and other claims-based sources. Med Care. 2002;40(suppl):IV-96–IV-103. doi: 10.1097/00005650-200208001-00013. [DOI] [PubMed] [Google Scholar]
- 42.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 43.Stitzenberg KB, Meropol NJ. Trends in centralization of cancer surgery. Ann Surg Oncol. 2010;17:2824–2831. doi: 10.1245/s10434-010-1159-0. [DOI] [PubMed] [Google Scholar]
- 44.Earle CC, Neumann PJ, Gelber RD, et al. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–1792. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, Kuo YF, Goodwin JS. The effect of electronic medical record adoption on outcomes in US hospitals. BMC Health Serv Res. 2013;13:39. doi: 10.1186/1472-6963-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amarasingham R, Plantinga L, Diener-West M, et al. Clinical information technologies and inpatient outcomes: A multiple hospital study. Arch Intern Med. 2009;169:108–114. doi: 10.1001/archinternmed.2008.520. [DOI] [PubMed] [Google Scholar]
- 47.DesRoches CM, Campbell EG, Vogeli C, et al. Electronic health records' limited successes suggest more targeted uses. Health Aff (Millwood) 2010;29:639–646. doi: 10.1377/hlthaff.2009.1086. [DOI] [PubMed] [Google Scholar]
- 48.Foy R, Hempel S, Rubenstein L, et al. Meta-analysis: Effect of interactive communication between collaborating primary care physicians and specialists. Ann Intern Med. 2010;152:247–258. doi: 10.7326/0003-4819-152-4-201002160-00010. [DOI] [PubMed] [Google Scholar]
- 49.Hickman M, Drummond N, Grimshaw J. A taxonomy of shared care for chronic disease. J Public Health Med. 1994;16:447–454. doi: 10.1093/oxfordjournals.pubmed.a043026. [DOI] [PubMed] [Google Scholar]
- 50.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117–5124. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 51.Smith SM, Allwright S, O'Dowd T. Effectiveness of shared care across the interface between primary and specialty care in chronic disease management. Cochrane Database Syst Rev. 2007;3:CD004910. doi: 10.1002/14651858.CD004910.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Aubin M, Giguère A, Martin M, et al. Interventions to improve continuity of care in the follow-up of patients with cancer. Cochrane Database Syst Rev. 2012;7:CD007672. doi: 10.1002/14651858.CD007672.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miles PV, Conway PH, Pawlson LG. Physician professionalism and accountability: The role of collaborative improvement networks. Pediatrics. 2013;131(suppl 4):S204–S209. doi: 10.1542/peds.2012-3786G. [DOI] [PubMed] [Google Scholar]
- 54.Rogers SO, Jr, Ayanian JZ, Ko CY, et al. Surgeons' volume of colorectal cancer procedures and collaborative decision-making about adjuvant therapies. Ann Surg. 2009;250:895–900. doi: 10.1097/SLA.0b013e3181afe0c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aboagye JK, Kaiser HE, Hayanga AJ. Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: A physician workforce issue. JAMA Surg. doi: 10.1001/jamasurg.2013.5062. [epub ahead of print on April 16, 2014] [DOI] [PubMed] [Google Scholar]
- 56.Onega T, Duell EJ, Shi X, et al. Geographic access to cancer care in the U.S. Cancer. 2008;112:909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 57.Casey MM, Thiede Call K, Klingner JM. Are rural residents less likely to obtain recommended preventive healthcare services? Am J Prev Med. 2001;21:182–188. doi: 10.1016/s0749-3797(01)00349-x. [DOI] [PubMed] [Google Scholar]
- 58.Sankaranarayanan J, Watanabe-Galloway S, Sun J, et al. Age and rural residence effects on accessing colorectal cancer treatments: A registry study. Am J Manag Care. 2010;16:265–273. [PubMed] [Google Scholar]
- 59.Hayanga AJ, Waljee AK, Kaiser HE, et al. Racial clustering and access to colorectal surgeons, gastroenterologists, and radiation oncologists by African Americans and Asian Americans in the United States: A county-level data analysis. Arch Surg. 2009;144:532–535. doi: 10.1001/archsurg.2009.68. [DOI] [PubMed] [Google Scholar]
- 60.Pollack CE, Frick KD, Herbert RJ, et al. It's who you know: Patient-sharing, quality, and costs of cancer survivorship care. J Cancer Surviv. 2014;8:156–166. doi: 10.1007/s11764-014-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phelip JM, Molinié F, Delafosse P, et al. A population-based study of adjuvant chemotherapy for stage-II and -III colon cancers. Gastroenterol Clin Biol. 2010;34:144–149. doi: 10.1016/j.gcb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 62.Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93:850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 63.Sundararajan V, Grann VR, Jacobson JS, et al. Variations in the use of adjuvant chemotherapy for node-positive colon cancer in the elderly: A population-based study. Cancer J. 2001;7:213–218. [PubMed] [Google Scholar]
- 64.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 65.Chowdhury MM, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg. 2007;94:145–161. doi: 10.1002/bjs.5714. [DOI] [PubMed] [Google Scholar]