Several months before the compliance deadline, fewer than half of applicant institutions had begun distress screening. Adding implementation strategies to mandated quality care standards may reduce uncertainty on how to comply.
Abstract
Purpose:
The American College of Surgeons Commission on Cancer (CoC) has set psychosocial distress screening as a new patient care standard to be met by 2015. The standard requires CoC-accredited cancer centers to integrate and monitor distress screening and, when needed, refer patients to psychosocial health care services. We describe the uptake of distress screening reported by applicants to a distress screening cancer education program and the degree of and barriers to implementation of distress screening programs reported by selected participants.
Materials and Methods:
This cross-sectional study collected quantitative data online from applicants to the program between August 1 and November 15, 2013, described by frequencies, percentages, and measures of central tendency, and qualitative data in person from accepted participants on February 13, 2014, analyzed using an integrated approach to open-ended data.
Results:
Applications were received from 70 institutions, 29 of which had started distress screening. Seven of 18 selected applicant institutions had not begun screening patients for distress. Analysis of qualitative data showed that all participants needed to create buy-in among key cancer center staff, including oncologists; to decide how to conduct screening in their institution in a way that complied with the standard; and to pilot test screening before large-scale rollout.
Conclusion:
Fourteen months before the compliance deadline, fewer than half of applicant institutions had begun distress screening. Adding implementation strategies to mandated quality care standards may reduce uncertainty about how to comply. Support from key staff members such as oncologists may increase uptake of distress screening.
Introduction
Since 1997, the National Comprehensive Cancer Network clinical practice guidelines on oncology distress management1 have called for routine psychosocial distress screening of patients with cancer. Although high levels of psychosocial distress have been found in patients with cancer,2,3 without distress screening, primary oncologists are unlikely to identify patients with clinically significant distress.4 In 2008, the Institute of Medicine found ample evidence to support a mandate that routine distress management be instituted across care sites.5 On the strength of the Institute of Medicine report, the American College of Surgeons Commission on Cancer (CoC) issued a new psychosocial distress screening standard to which CoC-accredited cancer centers must adhere by 2015.6 Standard 3.2 on psychosocial distress screening in the CoC “Cancer Program Standards 2012: Ensuring Patient-Centered Care” states: “The cancer committee develops and implements a process to integrate and monitor on-site psychosocial distress screening and referral for the provision of psychosocial care.”6p77
Time is short for meeting the CoC accreditation standard, but uptake of routine psychosocial distress screening has been slow and incomplete.7,8 Two issues affect this uptake. First, cancer care professionals need to be trained on how to conduct routine psychosocial distress screening, and second, they need a system for implementing psychosocial distress screening programs. To address both issues, we developed a cancer education program to train cancer care professionals to conduct psychosocial distress screening and to support them over 2 years in the implementation and maintenance of psychosocial distress screening programs. The purpose of this article is to describe the uptake of distress screening reported by applicants to the cancer education program and to describe the degree of, barriers to, and goals for implementation of distress screening programs reported by selected participants at the start of their participation in the program.
Materials and Methods
This cross-sectional, descriptive study is part of a larger cancer education program that uses the RE-AIM (Reach Effectiveness Adoption Implementation Maintenance) framework to assess the effect of the education program on the reach, effectiveness, adoption, implementation, and maintenance of psychosocial distress screening programs at participants' cancer centers.9 Announcements about the program appeared online (http://apos-society.org/screening/) and in trade periodicals.10
Two individuals involved in psychosocial care completed a single application for their cancer setting. Each application required letters of support from two cancer center administrators. Applications were received online between August 1 and November 15, 2013.
Data collected on applications included: type of institution; type of cancer center; numbers of patients with cancer treated at the institution; race and ethnicity of population of patients with cancer served; whether the institution has a psychosocial oncology program; whether the cancer center screens patients for distress and, if so, to what degree; and profession of the two individuals applying from each institution. These data were described by frequencies, percentages, and measures of central tendency. Data were stored in Microsoft Access 7 (http://products.office.com/en-us/access) and imported to SPSS (version 20; SPSS, Chicago, IL) for analysis. A four-member expert panel reviewed the applications and selected 18 cancer centers (36 individuals) to participate in the first cohort of the 2-year training program. Selections were based on administrators' and applicants' demonstration of commitment to the 2-year cancer education program and on assembling a cohort of participants from institutions of varying sizes and types, from different geographic regions, and with different levels of experience in psychosocial distress screening. Applicants were not excluded based on professional discipline.
Selected participants met on February 13, 2014, for an in-person workshop. The curriculum was taught by expert faculty and focused on the history of psychosocial distress screening, the five-step distress management process, distress screening tools, and communication and implementation strategies. The five-step distress management process consists of the following: (1) a brief screening to identify patients with distress; (2) clinical assessment of patients who screen positive for distress; (3) referral to psychosocial health care resources, if needed; (4) follow-up with primary oncology team and family/primary caregivers; and (5) documentation of steps one to four in the medical record and auditing of medical records for quality improvement.1
At the end of the workshop, participants decided on short- and long-term implementation goals for implementing psychosocial distress screening in their practice environment. They wrote these goals down on a data collection form developed by an author (M.G.) that allowed for a short paragraph of free text for each goal. Participants were instructed that goals were to be timed and measurable. These goals were analyzed using the integrated approach to analyzing open-ended data described by Bradley et al.11 The process included immersion in the details of the data and, using predetermined codes that reflected the five-step distress screening process, identification of important patterns and themes. Identified themes were compared. Similar themes were combined, and definitions for the categories of themes were developed and refined. Coding of transcripts was performed independently three authors (M.L., E.E., R.M.), discrepancies were discussed, and final coding was agreed on by all authors. Themes were analyzed by institution size and type, whether the institution had a psychosocial oncology program, professional disciplines, and institutional level of implementation. Representative participant quotes were identified according to institutional level of implementation: (1) no experience with distress screening, (2) experience with distress screening in one clinic or patient population, or (3) experience with distress screening in ≥ two clinics or patient populations. Transcripts of themes were stored in Microsoft Access 7 and imported into Microsoft Word 7 (http://products.office.com/en-us/Word) for analysis.
Results
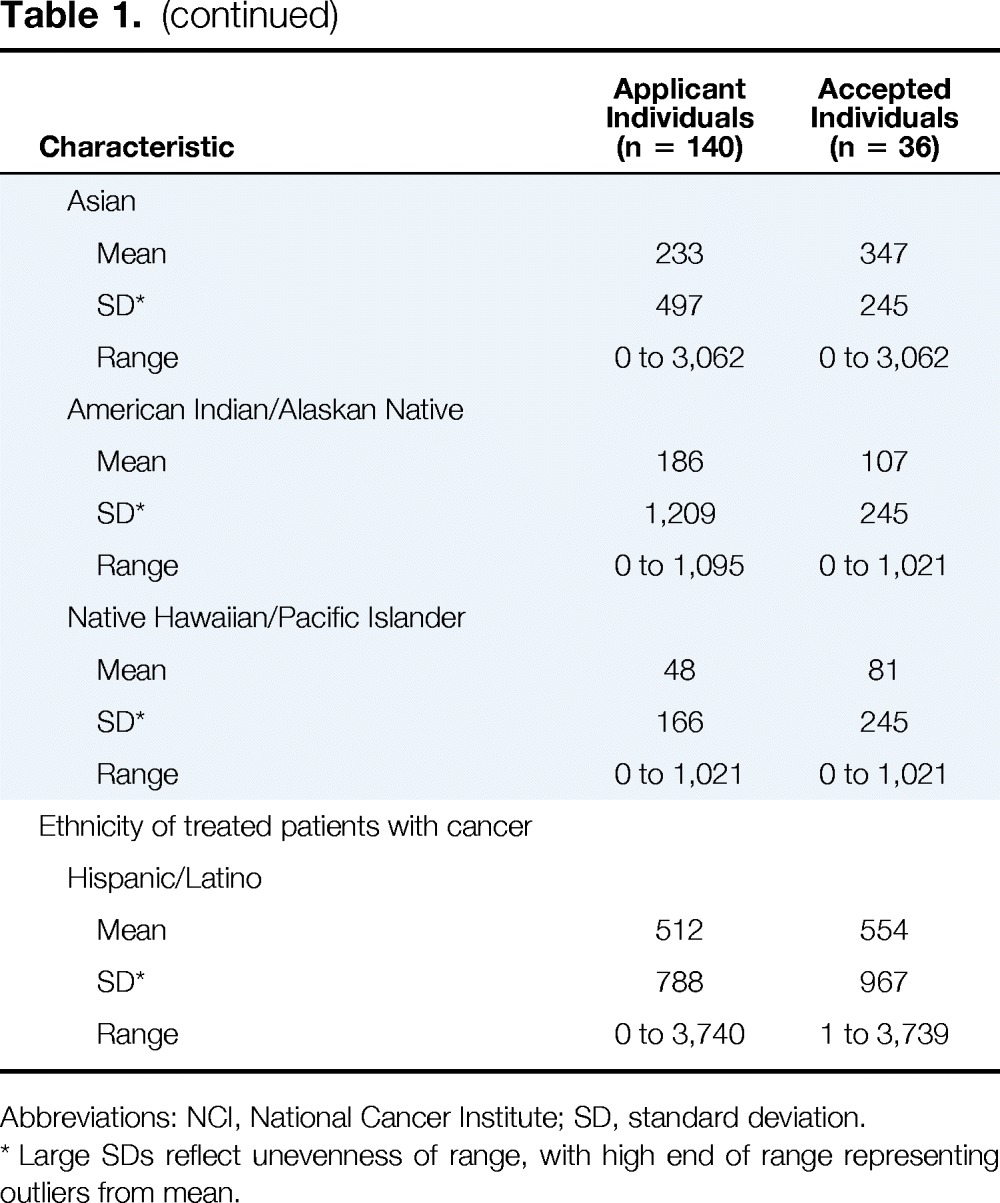
Applications were received from 70 institutions across the United States (Table 1). The majority (96%) of applications were from not-for-profit institutions. Twenty-five applications (36%) were received from institutions that categorized themselves as community cancer centers, and 24 (34%) were from institutions that were National Cancer Institute (NCI) –designated cancer centers. Thirty-seven applicant institutions (53%) had a psychosocial oncology program, but only 29 (41%) had started screening. The majority (49%) of the individuals who submitted an institutional application were social workers, followed by nurses (32%). Applications were received from all regions of the United States.
Table 1.
Characteristics of Applicant and Accepted Institutions
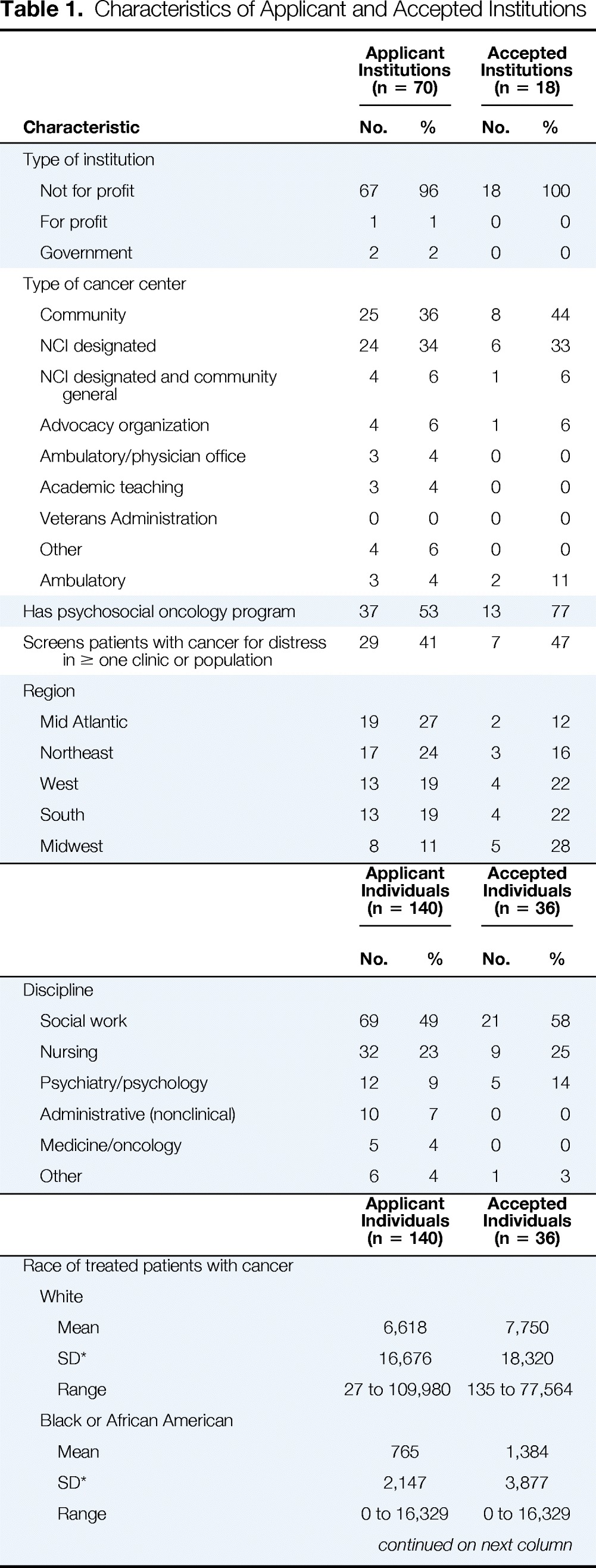
| Characteristic | Applicant Institutions (n = 70) |
Accepted Institutions (n = 18) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Type of institution | ||||
| Not for profit | 67 | 96 | 18 | 100 |
| For profit | 1 | 1 | 0 | 0 |
| Government | 2 | 2 | 0 | 0 |
| Type of cancer center | ||||
| Community | 25 | 36 | 8 | 44 |
| NCI designated | 24 | 34 | 6 | 33 |
| NCI designated and community general | 4 | 6 | 1 | 6 |
| Advocacy organization | 4 | 6 | 1 | 6 |
| Ambulatory/physician office | 3 | 4 | 0 | 0 |
| Academic teaching | 3 | 4 | 0 | 0 |
| Veterans Administration | 0 | 0 | 0 | 0 |
| Other | 4 | 6 | 0 | 0 |
| Ambulatory | 3 | 4 | 2 | 11 |
| Has psychosocial oncology program | 37 | 53 | 13 | 77 |
| Screens patients with cancer for distress in ≥ one clinic or population | 29 | 41 | 7 | 47 |
| Region | ||||
| Mid Atlantic | 19 | 27 | 2 | 12 |
| Northeast | 17 | 24 | 3 | 16 |
| West | 13 | 19 | 4 | 22 |
| South | 13 | 19 | 4 | 22 |
| Midwest | 8 | 11 | 5 | 28 |
| Applicant Individuals (n = 140) |
Accepted Individuals (n = 36) |
|||
|---|---|---|---|---|
| No. | % | No. | % | |
| Discipline | ||||
| Social work | 69 | 49 | 21 | 58 |
| Nursing | 32 | 23 | 9 | 25 |
| Psychiatry/psychology | 12 | 9 | 5 | 14 |
| Administrative (nonclinical) | 10 | 7 | 0 | 0 |
| Medicine/oncology | 5 | 4 | 0 | 0 |
| Other | 6 | 4 | 1 | 3 |
| Applicant Individuals (n = 140) | Accepted Individuals (n = 36) | |
|---|---|---|
| Race of treated patients with cancer | ||
| White | ||
| Mean | 6,618 | 7,750 |
| SD* | 16,676 | 18,320 |
| Range | 27 to 109,980 | 135 to 77,564 |
| Black or African American | ||
| Mean | 765 | 1,384 |
| SD* | 2,147 | 3,877 |
| Range | 0 to 16,329 | 0 to 16,329 |
| Characteristic | Applicant Individuals (n = 140) | Accepted Individuals (n = 36) |
|---|---|---|
| Asian | ||
| Mean | 233 | 347 |
| SD* | 497 | 245 |
| Range | 0 to 3,062 | 0 to 3,062 |
| American Indian/Alaskan Native | ||
| Mean | 186 | 107 |
| SD* | 1,209 | 245 |
| Range | 0 to 1,095 | 0 to 1,021 |
| Native Hawaiian/Pacific Islander | ||
| Mean | 48 | 81 |
| SD* | 166 | 245 |
| Range | 0 to 1,021 | 0 to 1,021 |
| Ethnicity of treated patients with cancer | ||
| Hispanic/Latino | ||
| Mean | 512 | 554 |
| SD* | 788 | 967 |
| Range | 0 to 3,740 | 1 to 3,739 |
Abbreviations: NCI, National Cancer Institute; SD, standard deviation.
Large SDs reflect unevenness of range, with high end of range representing outliers from mean.
The characteristics of applicant institutions accepted to enroll in the cancer education program (Figure 1) closely mirror those of received applications (Table 1). Seven (41.2%) accepted applicant institutions were community cancer centers, and six (35%) were NCI-designated cancer centers. Seven (46.7%) of the selected applicant institutions had not begun screening patients for distress. One accepted dyad included a medical oncologist who left his position before the cancer education program; the other dyad member and institution administrators replaced the medical oncologist with an advanced practice nurse.
Figure 1.
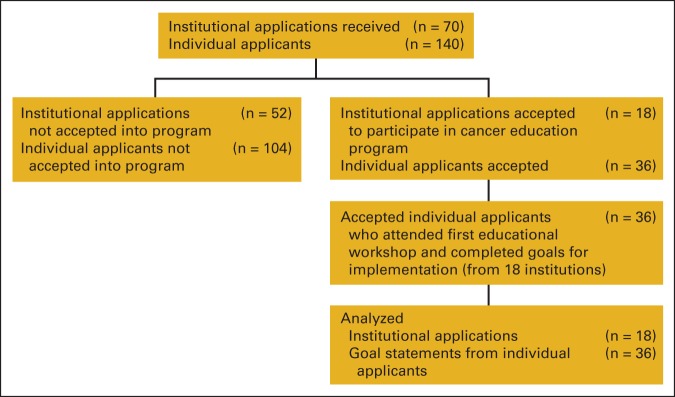
Study flow chart.
Analysis of the goals of participant institutions for implementation revealed three themes among all participant institutions: creating buy-in, deciding on and developing specifics, and pilot testing and beginning. Representative quotes differ depending on level of implementation at the participant institution. Analysis did not reveal differences in themes by institution size or type, whether the institution had a psychosocial oncology program, or professional disciplines of dyadic members; however, themes differed depending on level of implementation. Description of major themes and representative quotes by level of implementation are listed in Table 2.
Table 2.
Themes and Representative Quotes of Participants' Goals for Implementation of Distress Screening
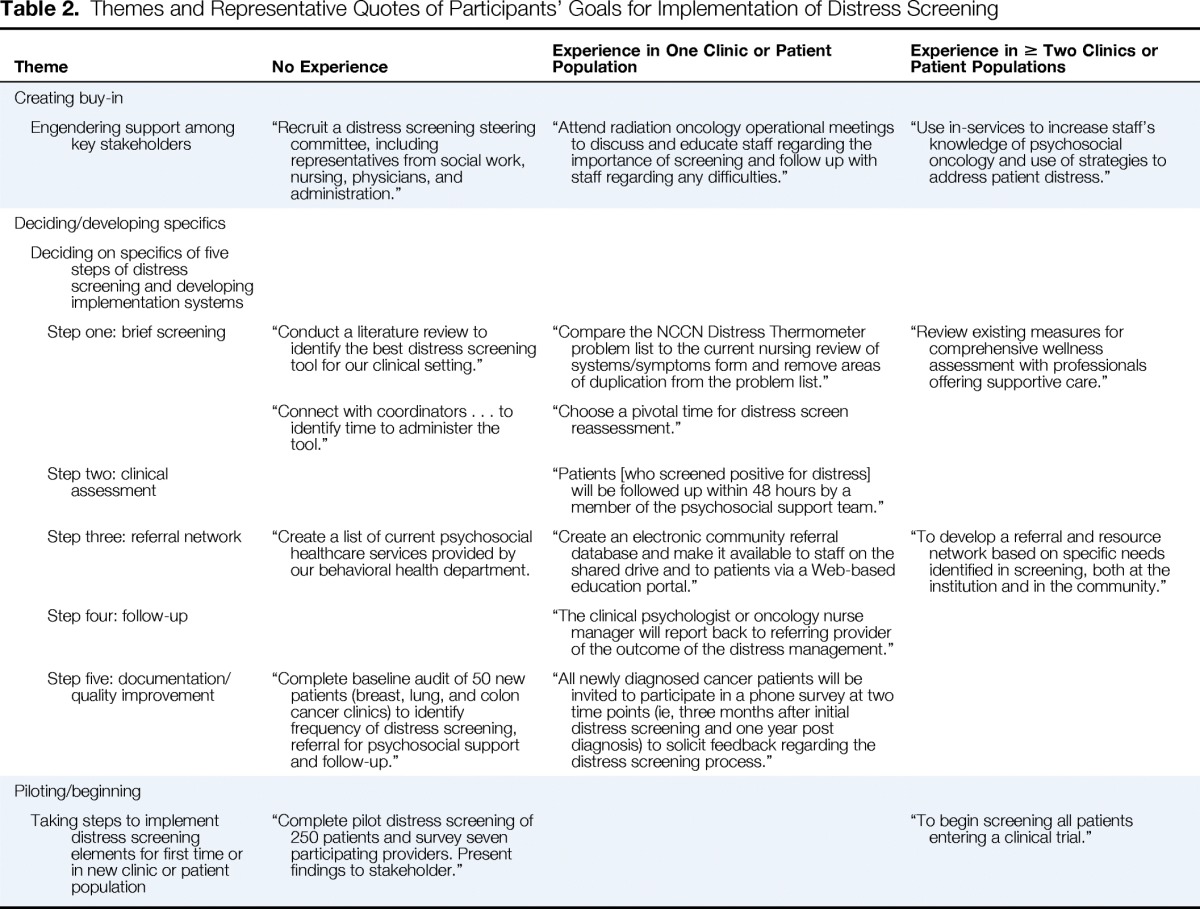
| Theme | No Experience | Experience in One Clinic or Patient Population | Experience in ≥ Two Clinics or Patient Populations |
|---|---|---|---|
| Creating buy-in | |||
| Engendering support among key stakeholders | “Recruit a distress screening steering committee, including representatives from social work, nursing, physicians, and administration.” | “Attend radiation oncology operational meetings to discuss and educate staff regarding the importance of screening and follow up with staff regarding any difficulties.” | “Use in-services to increase staff's knowledge of psychosocial oncology and use of strategies to address patient distress.” |
| Deciding/developing specifics | |||
| Deciding on specifics of five steps of distress screening and developing implementation systems | |||
| Step one: brief screening | “Conduct a literature review to identify the best distress screening tool for our clinical setting.” | “Compare the NCCN Distress Thermometer problem list to the current nursing review of systems/symptoms form and remove areas of duplication from the problem list.” | “Review existing measures for comprehensive wellness assessment with professionals offering supportive care.” |
| “Connect with coordinators … to identify time to administer the tool.” | “Choose a pivotal time for distress screen reassessment.” | ||
| Step two: clinical assessment | “Patients [who screened positive for distress] will be followed up within 48 hours by a member of the psychosocial support team.” | ||
| Step three: referral network | “Create a list of current psychosocial healthcare services provided by our behavioral health department. | “Create an electronic community referral database and make it available to staff on the shared drive and to patients via a Web-based education portal.” | “To develop a referral and resource network based on specific needs identified in screening, both at the institution and in the community.” |
| Step four: follow-up | “The clinical psychologist or oncology nurse manager will report back to referring provider of the outcome of the distress management.” | ||
| Step five: documentation/quality improvement | “Complete baseline audit of 50 new patients (breast, lung, and colon cancer clinics) to identify frequency of distress screening, referral for psychosocial support and follow-up.” | “All newly diagnosed cancer patients will be invited to participate in a phone survey at two time points (ie, three months after initial distress screening and one year post diagnosis) to solicit feedback regarding the distress screening process.” | |
| Piloting/beginning | |||
| Taking steps to implement distress screening elements for first time or in new clinic or patient population | “Complete pilot distress screening of 250 patients and survey seven participating providers. Present findings to stakeholder.” | “To begin screening all patients entering a clinical trial.” |
Creating Buy-In
Regardless of the level of implementation of distress screening, participant institutions expressed the need to engender support among key stakeholders. These stakeholders included administrators and patient care professionals. Some participant institutions with no distress screening experience planned to create buy-in by developing multidisciplinary steering committees or task forces to plan implementation. Members of the multidisciplinary committee would have some so-called skin in the game and thus be champions for distress screening within their respective disciplines. Participant institutions that were screening in at least one clinic or patient population planned to create buy-in in clinics where they wished to expand by attending clinic meetings and educating clinic staff, particularly nurses, to allay fears of increased workload from screening. Participant institutions with more distress screening experience planned to partner with cancer nurses by offering in-service training to increase their knowledge and distress management skills.
Deciding and Developing
Institutions, regardless of prior experience with distress screening, had to make implementation decisions about the five-step distress screening process, either for initial implementation or for scaling up of existing distress screening programs.
Step one: brief screening.
Participants had two decisions to make regarding the first step in the distress screening process: which brief screening tool to use and when to conduct the brief screening.
Regardless of level of implementation, participants were not settled on a distress screening tool, and their considerations focused on the fit of the tool with the institutional environment. For example, participants from a large academic cancer center that had not yet begun to screen patients for distress indicated that they would use a literature search to identify the best screening tool for their setting. Participating institutions that came with distress screening experience were all using the National Comprehensive Cancer Network Distress Thermometer (DT), but not all were using the problem list that accompanies the DT. For example, some institutions found that the problem list overlapped with other reviews of symptoms that cancer care professionals (mostly nurses) were already taking. The goals of these institutions focused on adapting the DT problem list to institutional needs.
It was unclear to participants with no or some distress screening experience which visit is a pivotal visit, for both the initial screen, which is required by the CoC standard, and for reassessment screens. A strategy for identifying good times to screen patients for distress was to include patient care staff in the decision-making process, because patient care staff “knew when patients would be most responsive to distress screening” and when screening would work best in clinic flow. Participating institutions with more experience did not mention timing of screening.
Step two: clinical assessment.
Only participating institutions with some screening experience mentioned clinical assessment. Individuals from one such institution identified the goal of having a member of the psychosocial team assess patients within 48 hours of a positive screen.
Step three: referral.
Participating institutions from all levels of experience identified developing referral networks as a task they had to complete. A large nonacademic cancer center with no distress screening experience had to identify psychosocial health care services provided within the institution itself. Participating institutions with more experience had to develop referral networks of community-based psychosocial health care services and develop processes for disseminating these networks to patient care staff.
Step four: follow-up.
Only one participating institution identified the need to follow up with the primary oncology team on patient outcomes vis-á-vis distress management.
Step five: documentation and quality improvement.
Only participating institutions with no or little experience with screening indicated the importance of documenting the process in patients' medical records and auditing for documentation of, or for patient satisfaction with, distress management.
Pilot Testing and Beginning
Participating institutions at both ends of the experience spectrum said they would implement distress screening for the first time in a new clinic or patient population. For those with no experience, this took the form of pilot testing distress screening and reporting the results of the pilot to key stakeholders. For those with more experience, this took the form of rolling out the distress screening process in a clinic or patient population they had yet to screen.
Discussion
On the basis of the strength of the evidence, the CoC6 has mandated distress screening and psychosocial referral as a standard that accredited cancer centers must meet by 2015. This approaching deadline notwithstanding, only 53% of applicants to our cancer education program in 2013 had a psychosocial oncology program, and only 41% had begun distress screening at all. Size (large or small), geographic location, type of cancer center (community or NCI designated), and whether the institution had a psychosocial oncology program had little bearing on whether applicant cancer centers had started the process of implementing distress screening. Such low uptake of distress screening only 2 years before the CoC standard becomes mandated suggests that cancer centers need guidance concerning the elements involved in meeting the standard and support at all stages of implementation.
New standards can be seen as hurdles for cancer centers at all stages of implementation. For example, cancer centers may believe that adopting a new standard will require more resources, which may not be forthcoming in an era of financial constraints. Clinic staff may think that adopting a new standard will slow down the flow of their clinics; patient care staff, either from lack of knowledge or from the belief that the standard is superfluous, may not see the need for the new standard. It was clear across all participating institutions, regardless of the level of experience with distress screening, that buy-in needed to be created among key stakeholders—from administrators to patient care staff.
It was also apparent that participating institutions were unclear about specific elements of the new distress screening standard. For example, which screening tool to use, how to adapt it to the clinic environment, and what is considered a pivotal visit perplexed participants. Surprisingly, few institutions mentioned the need to determine how to integrate clinical assessment of patients who screened positive for distress. This may be a result of level of implementation. Institutions with no experience first had to determine which tool to use and when to screen before they decided who would assess patients who screened positive for psychosocial distress. Institutions with more experience had already adopted a screening tool, but they remained uncertain about when to screen and which clinician would clinically assess patients with positive screens. Few institutions mentioned the need to follow up with patients' primary care team on distress management, even though follow-up is key.12,13
The linear approach of requiring distress screening in everyday practice begins with the standard itself rather than the end goals of adoption, implementation, and maintenance of distress screening programs. This approach creates uncertainty about the relevance of the five-step distress screening process to local institutional environments and results in slow uptake.
Uptake of the distress screening standard may be improved, however, if it were accompanied with an implementation framework such as the evidence integration triangle (EIT; Figure 2)14–16 and consensus-based recommendations.17 The EIT would help cancer centers to understand the implementation process, including which key stakeholders may need to be included in the process. Enumerating the evidence for distress screening as part of the standard itself could be used to convince key stakeholders of improvements in quality of care achieved through implementing the standard. Giving evidence-based examples of elements of the five-step distress screening process, such as identifying evidence-based screening tools and how to conduct clinical assessments of patients with positive screens, may enable cancer centers to identify screening tools adaptable to their practice environments and the best clinicians to assess patients with positive screens.15 Finally, the EIT would give examples of practical progress or quality improvement measures by which to judge implementation and efficacy of distress screening.18
Figure 2.
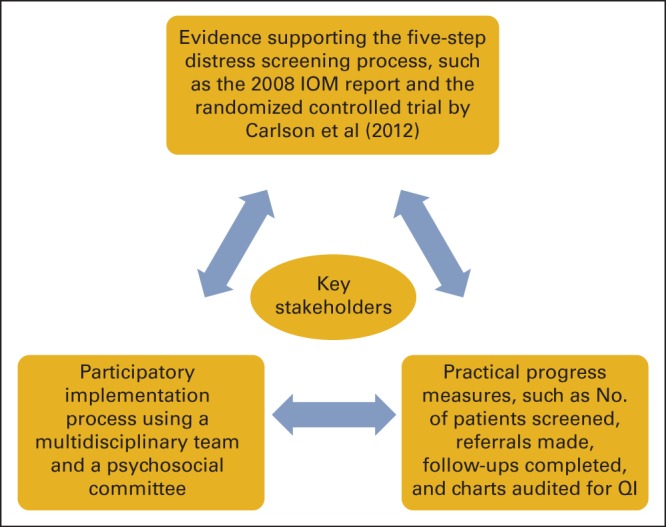
Example of evidence integration triangle for distress screening implementation. Data adapted.14 IOM, Institute of Medicine; QI, quality improvement.
Nearly three quarters (72%) of individuals submitting applications on behalf of their institutions were social workers and nurses. No oncologic surgeon was part of an application dyad. Yet surgeons may be the first clinicians to inform patients of a cancer diagnosis, information that begins a period of approximately 100 days known as existential plight,19–21 during which patients ask questions about life and death. These questions may be distressing to patients, who thus may need psychosocial care. Distress screening programs, if they are to be successful, need the support of the entire cancer care team, which includes oncologic surgeons, to identify patients who may need psychosocial care, even near the time of diagnosis.22
Our findings may not fully represent the state of distress screening implementation across the country, given possible selection bias resulting from our data being drawn from applications to a cancer education program on implementation and maintenance of distress screening programs. The small sample also limits our study. However, the diverse size, type, and location of the cancer centers represented in our applicant pool and in our participating cancer centers do present a snapshot of uptake of distress screening across the United States.
In conclusion, the results of this cross-sectional study of applicants to a cancer education program focused on psychosocial distress screening programs suggest that cancer centers need guidance in developing the five-step distress screening process and support during the rollout of their distress screening programs. Fewer than half of applicants had not begun screening patients for distress in their cancer centers, and only slightly more than half had a psychosocial oncology program. Analysis of selected participants' goals for implementation suggests that participants were not familiar with all five steps of the distress screening process (eg, which distress screening tool to use, when to screen, which clinician to conduct screening, and which clinician to evaluate results). Implementation strategies and consensus-based recommendations may elucidate the five-step distress screening process and reduce uncertainty about the elements involved in each step. Support from oncologists would strengthen implementation of distress screening programs.
Acknowledgment
Supported by National Cancer Institute Award No. R25CA177553-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: All authors
Collection and assembly of data: Mark Lazenby, Elizabeth Ercolano, Ruth McCorkle
Data analysis and interpretation: Mark Lazenby, Elizabeth Ercolano, Ruth McCorkle
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Supporting Commission on Cancer–Mandated Psychosocial Distress Screening With Implementation Strategies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Mark Lazenby
No relationship to disclose
Elizabeth Ercolano
No relationship to disclose
Marcia Grant
No relationship to disclose
Jimmie C. Holland
No relationship to disclose
Paul B. Jacobsen
Consulting or Advisory Role: Onyx Pharmaceuticals, Philips Healthcare
Research Funding: Pfizer, On Q Health
Travel, Accommodations, Expenses: Onyx Pharmaceuticals
Ruth McCorkle
No relationship to disclose
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Distress Management (version 3.2013) www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PMC free article] [PubMed]
- 2.Zabora J, Brintzenhofeszoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Carlson L, Groff S, Maciejewski O, et al. Screening for distress in lung and breast cancer outpatients: A randomized controlled trial. J Clin Oncol. 2010;28:4884–4891. doi: 10.1200/JCO.2009.27.3698. [DOI] [PubMed] [Google Scholar]
- 4.Pirl WF, Muriel A, Hwang V, et al. Screening for psychosocial distress: A national survey of oncologists. J Support Oncol. 2007;5:499–504. [PubMed] [Google Scholar]
- 5.Institute of Medicine. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 6.American College of Surgeons Commission on Cancer. Cancer Program Standards 2012 (version 1.2): Ensuring Patient-Centered Care. http://facs.org/cancer/coc/programstandards2012.html.
- 7.Donovan K, Jacobsen P. Progress in the implementation of NCCN guidelines for distress management by member institutions. J Natl Compr Canc Netw. 2013;11:223–226. doi: 10.6004/jnccn.2013.0029. [DOI] [PubMed] [Google Scholar]
- 8.Carlson LE, Groff SL, Maciejewski O, et al. Screening for distress in lung and breast cancer outpatients: A randomized controlled trial. J Clin Oncol. 2010;28:4884–4891. doi: 10.1200/JCO.2009.27.3698. [DOI] [PubMed] [Google Scholar]
- 9.Glasgow R, Vinson C, Chambers D, et al. National Institutes of Health approaches to dissemination and implementation science: Current and future directions. Am J Pub Health. 2012;102:1274–1281. doi: 10.2105/AJPH.2012.300755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buxton D, Daugherty A, Kennedy V, et al. Distress screening for oncology patients: Practical steps for implementing a comprehensive distress screening program. Oncology Issues. 2014;2014:48–52. [Google Scholar]
- 11.Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: Developing taxonomy, themes, and theory. Health services research. 2007;42:1758–1772. doi: 10.1111/j.1475-6773.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katon W, Von Korff M, Lin E, et al. A randomized trial of psychiatric consultation with distressed high utilizers. Gen Hosp Psychiatry. 1992;14:86–98. doi: 10.1016/0163-8343(92)90033-7. [DOI] [PubMed] [Google Scholar]
- 13.Chubak J, Aiello Bowles EJ, Tuzzio L, et al. Perspectives of cancer survivors on the role of different healthcare providers in an integrated delivery system. J Cancer Surviv. 2014;8:229–238. doi: 10.1007/s11764-013-0335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glasgow R, Green L, Taylor M, et al. An evidence integration triangle for aligning science with policy and practice. Am J Prev Med. 2012;42:646–654. doi: 10.1016/j.amepre.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazenby M. The international endorsement of US distress screening and psychosocial guidelines in oncology: A model for dissemination. J Natl Compr Canc Netw. 2014;12:221–227. doi: 10.6004/jnccn.2014.0023. [DOI] [PubMed] [Google Scholar]
- 16.Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new american college of surgeons commission on cancer distress screening standard. J Natl Compr Canc Netw. 2013;11:214–221. doi: 10.6004/jnccn.2013.0028. [DOI] [PubMed] [Google Scholar]
- 17.Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: Report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120:2946–2954. doi: 10.1002/cncr.28750. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen PB, Kadlubek P. Changes over time in quality of psychosocial care: Results from the Quality Oncology Practice Initiative (QOPI) J Clin Oncol. 2010;28(suppl):447s. abstr 6000. [Google Scholar]
- 19.Weisman AD, Worden JW. The existential plight in cancer: Significance of the first 100 days. Int J Psychiatry Med. 1976;7:1–15. doi: 10.2190/uq2g-ugv1-3ppc-6387. [DOI] [PubMed] [Google Scholar]
- 20.McCorkle R, Quint-Benoliel J. Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med. 1983;17:431–438. doi: 10.1016/0277-9536(83)90348-9. [DOI] [PubMed] [Google Scholar]
- 21.Bai M, Lazenby M, Jeon S, et al. Exploring the relationship between spiritual well-being and quality of life among patients newly diagnosed with advanced cancer. Palliat Support Care. 2014;3:1–9. doi: 10.1017/S1478951514000820. [DOI] [PubMed] [Google Scholar]
- 22.Lazenby M, Dixon J, Bai M, et al. Comparing the Distress Thermometer (DT) with the Patient Health Questionnaire (PHQ)-2 for screening for possible cases of depression among patients newly diagnosed with advanced cancer. Palliat Support Care. 2014;12:63–68. doi: 10.1017/S1478951513000394. [DOI] [PubMed] [Google Scholar]


