Abstract
Background. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) is a well-characterized entity that may share clinical and morphological findings with other low-grade non-Hodgkin's lymphomas. Dissemination of MALT-type lymphoma to bone marrow and peripheral blood simultaneously with the presence of T-large granular cell leukemia (T-LGL) has rarely been reported. Case Presentation. This is the case of a 42-year-old male who presented with a gastric MALT-type lymphoma, disseminated to the bone marrow and the peripheral blood with high serum IgM levels and t(11;18)(q21;q21). The morphological, immunophenotypical and, immunohistochemical studies of the successive bone marrow and peripheral blood samples had revealed the coexistence of two distinct lymphoma cell populations: a B-cell, marginal zone type population expressing CD19, CD20, CD22, CD79b, IgM, and kappa light chain, and a T-large granular cell population, developed after treatment with rituximab expressing CD3, CD8, CD5, CD7, and CD45. Conclusion. Based on the analysis of this unusual case we performed an extensive review of the literature to elucidate the relationship between T-LGL and B-cell lymphomas and to emphasize the importance of paraprotein analysis at diagnosis of gastric MALT lymphoma.
1. Introduction
Mucosa-associated lymphoid tissue (MALT) lymphomas are extranodal B-cell marginal zone lymphomas that generally follow an indolent course. Fifty percent of all MALT lymphomas arise from the stomach and are commonly associated with Helicobacter pylori infection. Nongastric MALT lymphomas occur in the lung, salivary gland, skin, and other organs often associated with autoimmune disease. Bone marrow involvement has been reported in 23.5% to 37% of cases at presentation [1–3]. Leukemic dissemination has only sporadically been reported [4, 5].
Disseminated MALT lymphoma may mimic Waldenstrom macroglobulinemia by causing Waldenstrom syndrome. Monoclonal gammopathy (MG) was detected in 17.2% of cases with B-cell NHL [6] and 36% of cases with MALT-type lymphoma [7]. IgG was more frequent in cases with aggressive NHL, while IgM was more common in cases with low-grade NHL. It was usually associated with advanced disease, typically showing bone marrow and peripheral blood involvement (Table 1) [8–10]. However, Wöhrer et al. found that MG although a common phenomenon in MALT lymphoma was not correlated with clinical stage, genetic findings, H. pylori status, or response to treatment [7].
Table 1.
Reported cases of gastric MALT lymphoma with monoclonal gammopathy.
| Author/year | Age | Sex | H pylori | Dissemination | Serum Ig | Genetic findings | Bcl-2 | Reference |
|---|---|---|---|---|---|---|---|---|
| Levine et al./1989 | 54 | F | − | None | IgM, IgD, λ | T(11;18) | [16] | |
| Allez et al./1999 | 31 | M | − | BM | IgMκ | Tri 3 | [17] | |
| Griesser et al./1990 | 46 | F | NA | BM | IgMκ | NA | + | [5] |
| Leroux et al./1993 | 58 | M | − | GN | IgAλ | T(11;18) | [18] | |
| Hirase et al./2000 | 77 | M | − | BM, PB | IgMκ | T(11;18) | − | [8] |
| 57 | F | − | BM, PB | IgMλ | T(11;18) | − | ||
| Iwase et al./2000 | 80 | M | NA | BM | IgMκ | NK | [19] | |
| Okada et al./2001 | 77 | F | − | BM | IgMλ | NK | [20] | |
| Valdez et al./2001 | 79 | M | + | BM | IgMλ | NA | [9] | |
| Kunisaki et al./2003 | 66 | M | NA | BM, PB, PE | IgMκ | T(11;18) | [21] | |
| Wöhrer et al./2004 | 90 | M | + | None | IgGκ | NK | [22] | |
| 55 | M | + | BM, PB | IgAκ | T(11;18) | |||
| Ye et al./2004 | 78 | F | − | BM, PB | IgMλ | Bcl-10 | [23] | |
| Gimeno et al./2006 | 69 | F | + | None | IgMκ | NK | [24] | |
| Lantuejoul et al./2007 | 50 | F | + | BM, lung | Ig λ | NA | [25] | |
| Salle et al./2007 | 59 | M | NA | None | IgMκ | NA | [26] | |
| Ohno and Isoda/2008 | 55 | M | + | BM, PB | IgAκ | T(11;18) | [27] | |
| Almehmi and Fields/2009 | 66 | F | + | None | IgMκ | NK | + | [28] |
| Reitter et al./2010 | 35 | F | + | BM, PB | IgMλ | Tri 3q,18q | + | [4] |
| Hirota-Kawadobora et al./2012 | 70 | M | + | BM | IgM | NK | [29] | |
| Wu et al./2014 | 51 | M | NA | None | IgAλ | NA | [30] |
NA: not available; NK: normal karyotype; BM: bone marrow; PB: peripheral blood; PE: pleural effusion.
CD5 expression is rare in MALT lymphoma and is often associated with nongastric disease and an increased tendency to present with disseminated disease [11].
The detection of t(11;18)(q21;q21) is useful in disseminated cases. This translocation was predominantly found in gastric MALT lymphoma [12], associated with the resistance to H. pylori eradication therapy [13, 14] and associated with the development of H. pylori-independent gastric MALT lymphoma [15].
Concomitant or sequential occurrence of MALT lymphoma and other primary B-cell neoplasms has been reported [53]. Coexistence of B-cell and T-cell lymphomatous populations in the same patient has rarely been reported [54, 55]. However, association of T-cell leukemia and MALT lymphoma had not yet been described.
We describe in the present report a case of disseminated gastric MALT lymphoma, with t(11;18)(q21;q21), MG, resistance to H. pylori eradication, chemotherapy and immunotherapy, and subsequent appearance of a predominant T-cell large granular leukemia.
2. Case Report
A 42-year-old male/bricklayer was admitted in 2006 for high grade fever and dyspnea. He had a 2-month history of epigastric pain, peptic discomfort, and dizziness. Physical examination showed left pleural effusion, ascites, dehydration, and cachectic appearance. Laboratory tests revealed a hemoglobin level of 10.5 g/dL, a white blood cell count of 21.3 Giga/L with 80% neutrophils and 12% atypical lymphocytes, a total serum protein level of 101 g/L with hypoalbuminemia at 30 g/L (N: 40–47 g/L), an IgM level of 52 g/L (0.5–2.4 g/L), a kappa light chains level of 9.07 g/L (N: 2–4.4 g/L), a kappa/lambda ratio of 7.96 (N: 1.35–2.65), and a C reactive protein (CRP) of 365 mg/L (N: <5 mg/L); the Bence-Jones protein in urine was negative; the renal and liver function tests, LDH, and β2-microglobulin titers were normal. The serologic tests for EBV, CMV, and chronic viral hepatitis including HCV were unremarkable. The total body CT scan showed a left pleural effusion with lower lobe atelectasis, circumferential thickening of the gastric wall predominantly affecting the greater curvature. There was no hepatomegaly, splenomegaly, or brain lesion. A total skeletal survey showed no bone lesion. The bronchial endoscopy showed no tumor. Gastric endoscopy confirmed the presence of a huge tumor with surface ulceration at the greater curvature. Histological examination of biopsies revealed a typical lymphoepithelial lesion compatible with low-grade MALT-type lymphoma and positive Helicobacter pylori chronic gastritis. The immunostaining showed positive CD20, CD5, CD38, and κ-light chain stains but negative CD10, cyclin D1, and λ-light chain stains. The bone marrow aspirate and biopsy showed colonization with plasmacytoid cells and dense infiltrations by small lymphocytes extending to the paratrabecular zone. The karyotype study of the bone marrow aspirate revealed a typical, specific translocation of gastric MALT lymphoma, t(11;18)(q21;q21) in 30 out of 38 metaphases (Figure 3).
Figure 3.

The karyotype of the malignant bone marrow cells at presentation that shows the t(11;18)(q21;q21).
The cytological examination of the pleural and peritoneal fluids was negative. The histological examination of the pleural biopsy showed a nonspecific subacute suppurative pleuritis with no evidence of malignancy.
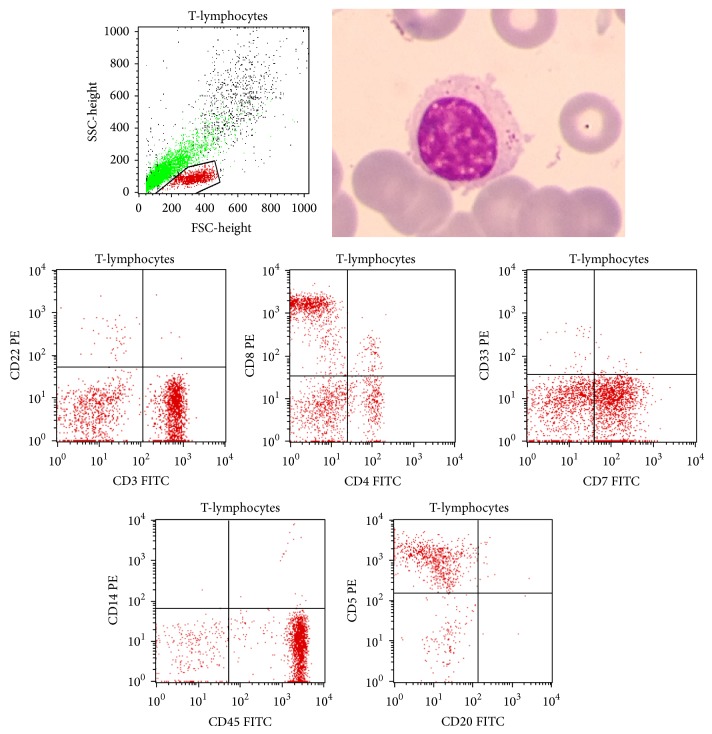
After adequate hydration and antibiotic therapy, the respiratory function and the white blood cell count returned to normal but with an atypical plasmacytoid lymphocytosis reaching 35%. The patient received 6 courses of cyclophosphamide, fludarabine, and rituximab and then 3 courses of cisplatin-based chemotherapy. The gastric lesion and the monoclonal paraproteinemia remained, however, unchanged. The cytological examination of the bone marrow aspirate revealed the presence of a predominant mature granular lymphocytosis associated with an atypical plasmacytoid lymphocytosis. The flow cytometry analysis of this aspirate identified two cell populations, one population of T-cells expressing mainly the CD8+/CD3+/CD5+/CD7+/CD45+ immunophenotype representing 40% of the examined cells (Figure 1) and a second population representing 20% of these cells and consisting of monoclonal B-cells expressing kappa light chain, IgM, CD19, CD79b, CD20, and CD22.
Figure 1.
A T-lymphocyte showing an abundant granular cytoplasm and selected gated dual parameter dot plots of T-cells displaying CD3, CD8, CD7, CD45, and CD5.
The patient was ultimately put on an expectant management option “watchful waiting.” The serum electrophoresis peak remained the same (Figure 4); the gastric lesion remained unchanged during four years. The patient died from an evolving pulmonary infection in 2013.
Figure 4.
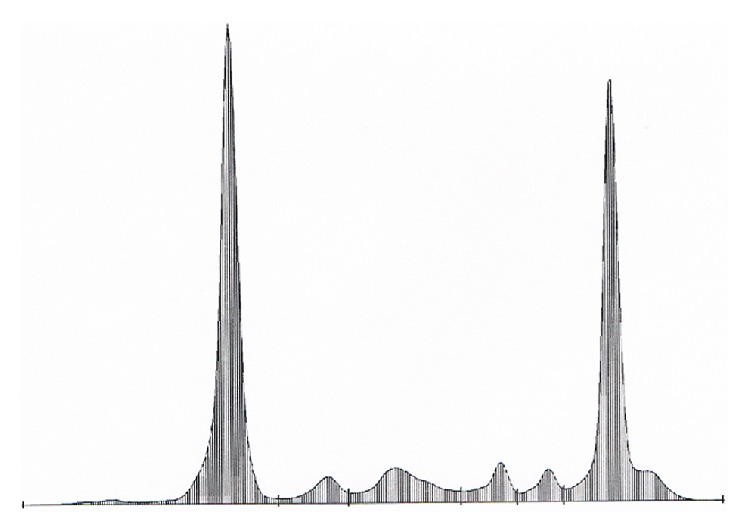
The serum protein electrophoresis graph that remained unchanged during the course of the disease.
Molecular study of T-cell receptor genes was attempted in postmortem using the paraffin-embedded bone marrow specimen that has failed to assess clonality because of the degraded DNA.
3. Discussion
The leukemic presentation, the refractoriness to chemoimmunotherapy, the IgM kappa production, and the presence of t(11;18)(q21;q21) characterize the clinical picture of our patient with gastric MALT lymphoma. Therefore, the marginal zone B-cell population invading the bone marrow and the peripheral blood has decreased, after treatment with rituximab, and a second malignant T-cell population emerged and became predominant.
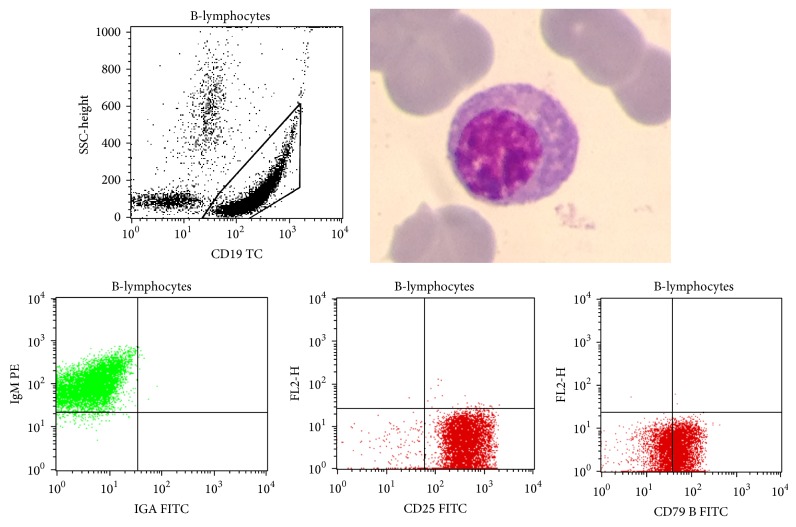
The tumor cells at the initial presentation had plasmacytic differentiation (Figure 2). The plasmacytic morphology may be found in 30% of extragastric MALT-type lymphoma [7] and only 10% of gastric MALT lymphoma [56, 57]. It is a rare finding in lymphomas with t(11;18)(q21;q21) which are mostly associated with monocytoid morphology [56].
Figure 2.
A B-lymphocyte showing a plasmacytic differentiation and selected gated dual parameter dot plots displaying CD19, IgM, CD25, and CD79a.
Leukemic dissemination has only sporadically been described in MALT lymphoma [4, 5]. It has been significantly related to bone marrow infiltration [58]. However, the presence of a serum monoclonal component has not been associated with the disseminated disease [6].
The translocation t(11;18)(q21;q21) is the most structural chromosomal abnormality found in MALT-type lymphoma occurring in about one-third of the cases, involving different sites mainly gastric ones, at any stage [58]. It has been shown that this translocation is a marker of resistance to H. pylori eradication and may indicate that it confers an independent growth advantage [59]. The trisomy of chromosome 3 represents the most frequent numerical abnormality in MALT lymphoma; however, it is not specific for this lymphoma subtype and has no prognostic significance although it has been associated with a plasmacytoid appearance of the leukemic lymphocytes and IgM hypergammaglobulinemia [60]. Although t(14;18)(q32;q21)/IGH-BCL2 is the genetic hallmark of follicular lymphoma, this reciprocal translocation, closely related to t(11;18), has been described in extranodal marginal zone lymphoma, mostly nongastric MALT lymphoma [58]. BCL10 nuclear expression is also closely related to the presence of the t(11;18) and found in disseminated gastric MALT lymphoma [13, 15].
Gastric MALT lymphoma with t(11;18) and extragastric MALT lymphoma with trisomy 18 are groups with the higher risk of dissemination [61].
CD5 expression is typically absent in MALT-type lymphoma; however, it is sometimes aberrantly coexpressed in nongastric, even localized disease [62] and associated with increased tendency to relapse, refractoriness to therapy, and dissemination to bone marrow [11, 63]. It has also been associated with monoclonal paraprotein production in some cases (Table 2).
Table 2.
Reported cases of nongastric MALT lymphoma with monoclonal gammopathy.
| Author/year | Age/sex | Primary MALT lymphoma | Chronic disease | Dissemination | Serum Ig | CD5 | Genetic findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Levine et al./1989 | 56/M | Eye | — | BM | IgMλ | − | T(11;18) | [16] |
| Ueda et al./1996 | 48/M | Liver | — | — | IgMκ | + | NA | [31] |
| Matsumoto et al./1996 | 74/F | Duodenum | — | — | IgAκ | − | NA | [32] |
| Nakata et al./1997 | 74/M | Eyes | — | — | IgMκ | − | NA | [33] |
| Mak et al./1998 | 62/M | Kidney | IgA NP | GI tract | IgMλ | − | NA | [34] |
| Sakai et al./2000 | 72 | Ileum and colon | ITP, AIH | − | IgGκ | − | NA | [35] |
| Valdez et al./2001 | 50/M | Nasopharynx | — | BM | IgMκ | − | NA | [9] |
| 40/M | Eye and lung | — | — | IgMκ | − | NA | ||
| 60/F | Salivary gland | Gougerot syndrome | BM | IgMκ | − | NA | ||
| 61/F | Lung | — | PE, skin, and pericardium | IgMκ | − | NA | ||
| 74/M | Eye and pharynx | — | — | IgM, IgAκ | − | NA | ||
| Nagakawa et al./2002 | 61/M | Lung | — | BM | IgM | NA | [36] | |
| Pachmann et al./2002 | 59/ | Salivary gland | — | BM, LN, kidneys, liver | IgGλ | − | NA | [37] |
| Stokes et al./2002 | 72/F | Kidney | MPGN | — | IgMκ | − | NA | [38] |
| Thieblemont et al./2002 | 60/F | Thyroid | Hashimoto | — | IgGκ | − | NA | [39] |
| Saito et al./2004 | 65/F | Small bowel | GN and ascariasis | Ascites | IgMκ | NA | T(11;18) | [40] |
| Takasaki et al./2005 | 84/M | Lung | — | BM and PE | IgM | − | T(11;18) | [41] |
| Dalle et al./2006 | 49/M | Skin | Schnitzler syndrome | BM | IgMκ | + | NA | [42] |
| Gomyo et al./2007 | 67/F | Pleura | — | — | IgM | − | T(14:18) | [43] |
| Schulze et al./2007 | 75/M | Lung | — | BM | IgMκ | − | T(11;18) | [44] |
| Ohno and Isoda/2008 | 77/M | Lung | — | BM and PB | IgMκ | − | T(11;18) | [27] |
| Murota et al./2009 | 73/F | Skin | Schnitzler syndrome | BM | IgMκ | − | NA | [45] |
| Mikolaenko and Listinsky/2009 | 75/F | Salivary gland | RA | BM and lung | IgMλ | + | NA | [46] |
| Peces et al./2010 | 77/M | Kidney | Barrett's esophagus | BM | IgMκ | − | NA | [47] |
| Mitchum et al./2010 | 46/M | Skin | — | BM | IgMκ | − | Bcl-2 | [48] |
| Ikuta et al./2010 | 54/F | Colon | — | — | IgMκ | − | NA | [49] |
| Kim et al./2011 | 66/M | Small bowel | — | BM | IgMλ | − | NA | [50] |
| Lacoste et al./2013 | 74/F | Skin | Angiomatosis | BM and PB | IgMκ | + | NA | [51] |
| Wu et al./2014 | 70/M | Lung | — | — | IgAκ | − | NA | [30] |
| Chi et al./2014 | 72/F | Kidney | CKD | BM and PB | IgMκ | − | NA | [52] |
NA: not available; NK: normal karyotype; PE: pleural effusion; BM: bone marrow; PB: peripheral blood; IgA NP: IgA nephropathy; ITP: idiopathic thrombocytopenic purpura; AIH:autoimmune hepatitis; MPGN: membranoproliferative glomerulonephritis; GN: glomerulonephritis; RA: rheumatoid arthritis; CKD: chronic kidney disease.
Positive expression of BCL2 has been associated with unfavorable survival in extranodal diffuse large B-cell lymphomas (DLBCL) and MALT lymphomas [64].
The frequency of H. pylori infection is higher in MALT lymphoma restricted to the stomach. Although the eradication of H. pylori may result in clinical and histological remission in 90% of patients, molecular evidence of persistent gastric MALT lymphoma may be found in 40% of these cases [65].
The curative potential of chemotherapy and immunotherapy is questionable [66]. Plasmacytic differentiation and monoclonal gammopathy do not influence the rate of disease progression. Rituximab has only moderate activity in terms of inducing objective responses in disseminated MALT lymphoma. However, long-term disease stabilization along with a symptomatic benefit has been seen in some patients [61]. Moreover rituximab could select latent clonal CD20− populations in some patients.
The morphology and the immunophenotype CD3+/CD5+/CD7+/CD45+/CD10− of the second malignant population in the bone marrow of the presented patient are consistent with the sequential occurrence of a T-large granular cell leukemia. The association of clonal T-LGL proliferations with clonal B-cell lymphoproliferative disorders, although rare, is now well recognized [54, 67]. T-LGL is a chronic and often indolent T-cell proliferation. The transformation of an indolent lymphoma to a more aggressive one of the same immunological origin is a well-recognized event. In a population-based series of unselected patients with multiple histology lymphomas, Tucci et al. [53] reported that the most frequent transformation from marginal zone lymphoma was to DLBCL. Reciprocally a sequential appearance of marginal zone lymphoma after treatment for DLBCL has also been observed which may have been unrecognized in the first diagnostic biopsy. Coexistence of B-cell and T-cell lymphoma populations in the bone marrow and peripheral blood of the same patient has rarely been reported. Synchronous clonal T-LGL has been reported in patients with splenic marginal zone lymphoma [54, 55].
Reported cases of gastric and nongastric MALT-type lymphoma with monoclonal gammopathy are summarized in Tables 1 and 2. Thymic MALT lymphoma seems to be clinicopathologically a distinctive form with prevalence in Asians, strong association with autoimmune disease, marked female predominance, frequent presence of epithelium-lined cysts, almost invariable presence of a neoplastic plasma cell component, expression of IgA phenotype, and absence of API2-MALT1 gene fusion [68, 69]. Cases of chronic autoimmune thyroiditis (Hashimoto's thyroiditis) have been reported in patients with MALT lymphoma. Most of these patients had tumors with plasmacytic differentiation and two of them presented with monoclonal gammopathy [70].
4. Conclusion
Leukemic dissemination and monoclonal macroglobulinemia have only sporadically been described in MALT-type lymphoma. Furthermore, subsequent development of T-cell LGL with simultaneous presence of two different lymphoma populations in the peripheral blood and the bone marrow remains an unusual event. Thus, pending additional data, we recommend including the paraprotein analysis and the flow cytometric studies in the pretherapeutic workup of patients with MALT lymphoma.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Thieblemont C. Clinical presentation and management of marginal zone lymphomas. Hematology/the Education Program of the American Society of Hematology. 2005;2005(1):307–313. doi: 10.1182/asheducation-2005.1.307. [DOI] [PubMed] [Google Scholar]
- 2.Sretenovic M., Colovic M., Jankovic G., et al. More than a third of non-gastric malt lymphomas are disseminated at diagnosis: a single center survey. European Journal of Haematology. 2009;82(5):373–380. doi: 10.1111/j.1600-0609.2009.01217.x. [DOI] [PubMed] [Google Scholar]
- 3.Papaxoinis G., Fountzilas G., Rontogianni D., et al. Low-grade mucosa-associated lymphoid tissue lymphoma: a retrospective analysis of 97 patients by the Hellenic Cooperative Oncology Group (HeCOG) Annals of Oncology. 2008;19(4):780–786. doi: 10.1093/annonc/mdm529. [DOI] [PubMed] [Google Scholar]
- 4.Reitter S., Neumeister P., Beham-Schmid C., et al. A case of generalized MALT lymphoma with IgM paraproteinemia and peripheral blood involvement. Annals of Hematology. 2010;89(2):213–214. doi: 10.1007/s00277-009-0787-6. [DOI] [PubMed] [Google Scholar]
- 5.Griesser H., Kaiser U., Augener W., Tiemann M., Lennert K. B-cell lymphoma of the mucosa-associated lymphatic tissue (malt) presenting with bone marrow and peripheral blood involvement. Leukemia Research. 1990;14(7):617–622. doi: 10.1016/0145-2126(90)90016-3. [DOI] [PubMed] [Google Scholar]
- 6.Economopoulos T., Papageorgiou S., Dimopoulos M. A., et al. Non-Hodgkin's Lymphomas in Greece according to the WHO classification of lymphoid neoplasms. A retrospective analysis of 810 cases. Acta Haematologica. 2005;113(2):97–103. doi: 10.1159/000083446. [DOI] [PubMed] [Google Scholar]
- 7.Wöhrer S., Streubel B., Bartsch R., Chott A., Raderer M. Monoclonal immunoglobulin production is a frequent event in patients with mucosa-associated lymphoid tissue lymphoma. Clinical Cancer Research. 2004;10(21):7179–7181. doi: 10.1158/1078-0432.CCR-04-0803. [DOI] [PubMed] [Google Scholar]
- 8.Hirase N., Yufu Y., Abe Y., et al. Primary macroglobulinemia with t(11;18)(q21;q21) Cancer Genetics and Cytogenetics. 2000;117(2):113–117. doi: 10.1016/s0165-4608(99)00156-9. [DOI] [PubMed] [Google Scholar]
- 9.Valdez R., Finn W. G., Ross C. W., Singleton T. P., Tworek J. A., Schnitzer B. Waldenström macroglobulinemia caused by extranodal marginal zone B-cell lymphoma: a report of six cases. American Journal of Clinical Pathology. 2001;116(5):683–690. doi: 10.1309/6wpx-66cm-kgrh-v4rw. [DOI] [PubMed] [Google Scholar]
- 10.Asatiani E., Cohen P., Ozdemirli M., Kessler C. M., Mavromatis B., Cheson B. D. Monoclonal gammopathy in extranodal marginal zone lymphoma (ENMZL) correlates with advanced disease and bone marrow involvement. American Journal of Hematology. 2004;77(2):144–146. doi: 10.1002/ajh.20157. [DOI] [PubMed] [Google Scholar]
- 11.Jaso J., Chen L., Li S., et al. CD5-positive mucosa-associated lymphoid tissue (MALT) lymphoma: a clinicopathologic study of 14 cases. Human Pathology. 2012;43(9):1436–1443. doi: 10.1016/j.humpath.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Rosenwald A., Ott G., Stilgenbauer S., et al. Exclusive detection of the t(11;18)(q21;q21) in extranodal marginal zone B cell lymphomas (MZBL) of MALT type in contrast to other MZBL and extranodal large B cell lymphomas. The American Journal of Pathology. 1999;155(6):1817–1821. doi: 10.1016/S0002-9440(10)65499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H., Ye H., RuskoneFourmestraux A., et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122(5):1286–1294. doi: 10.1053/gast.2002.33047. [DOI] [PubMed] [Google Scholar]
- 14.Lévy M., Copie-Bergman C., Molinier-Frenkel V., et al. Treatment of t(11;18)-positive gastric mucosa-associated lymphoid tissue lymphoma with rituximab and chlorambucil: clinical, histological, and molecular follow-up. Leukemia and Lymphoma. 2010;51(2):284–290. doi: 10.3109/10428190903431820. [DOI] [PubMed] [Google Scholar]
- 15.Ye H., Liu H., Raderer M., et al. High incidence of t(11;18)(q21;q21) in Helicobacter pylori-negative gastric MALT lymphoma. Blood. 2003;101(7):2547–2550. doi: 10.1182/blood-2002-10-3167. [DOI] [PubMed] [Google Scholar]
- 16.Levine E. G., Arthur D. C., Machnicki J., et al. Four new recurring translocations in non-Hodgkin lymphoma. Blood. 1989;74(5):1796–1800. [PubMed] [Google Scholar]
- 17.Allez M., Mariette X., Linares G., Bertheau P., Jian R., Brouet J.-C. Low-grade MALT lymphoma mimicking Waldenstrom's macroglobulinemia. Leukemia. 1999;13(3):484–485. doi: 10.1038/sj.leu.2401206. [DOI] [PubMed] [Google Scholar]
- 18.Leroux D., Seite P., Hillion J., et al. t(11;18)(q21;q21) may delineate a spectrum of diffuse small B-cell lymphoma with extranodal involvement. Genes Chromosomes and Cancer. 1993;7(1):54–56. doi: 10.1002/gcc.2870070109. [DOI] [PubMed] [Google Scholar]
- 19.Iwase S., Takahara S., Sekikawa T., et al. Disseminated MALT lymphoma associated with macroglobulinemia. Rinshō Ketsueki. 2000;41(11):1183–1188. [PubMed] [Google Scholar]
- 20.Okada Y., Mori H., Maeda T., Ito Y., Hasegawa M., Kageyama T. Autopsy case of lymphoplasmacytic lymphoma with a large submucosal tumor in the stomach. Pathology International. 2001;51(10):802–806. doi: 10.1046/j.1440-1827.2001.01274.x. [DOI] [PubMed] [Google Scholar]
- 21.Kunisaki Y., Muta T., Yamano Y., Kobayashi Y. Detection of two cell populations corresponding to distinct maturation stages in API-2/MLT-positive mucosa-associated lymphoid tissue lymphoma cells proliferating in pleural effusion. International Journal of Hematology. 2003;78(4):357–361. doi: 10.1007/bf02983562. [DOI] [PubMed] [Google Scholar]
- 22.Wöhrer S., Raderer M., Streubel B., Chott A., Drach J. Concomitant occurrence of MALT lymphoma and multiple myeloma. Annals of Hematology. 2004;83(9):600–603. doi: 10.1007/s00277-004-0870-y. [DOI] [PubMed] [Google Scholar]
- 23.Ye H., Chuang S. S., Dogan A., Isaacson P. G., Du M. Q. t(1;14) and t(11;18) in the differential diagnosis of Waldenström's macroglobulinemia. Modern Pathology. 2004;17(9):1150–1154. doi: 10.1038/modpathol.3800164. [DOI] [PubMed] [Google Scholar]
- 24.Gimeno E., Sorlí L., Serrano S., Besses C., Salar A. Monoclonal cryoglobulinemia: the first manifestation of gastric marginal B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) Leukemia Research. 2006;30(11):1465–1466. doi: 10.1016/j.leukres.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Lantuejoul S., Moulai N., Quetant S., et al. Unusual cystic presentation of pulmonary nodular amyloidosis associated with MALT-type lymphoma. European Respiratory Journal. 2007;30(3):589–592. doi: 10.1183/09031936.00136605. [DOI] [PubMed] [Google Scholar]
- 26.Salle V., Smail A., Joly J.-P., et al. Gastric MALT lymphoma presenting as Waldenström's macroglobulinemia without bone marrow involvement. Clinical Lymphoma and Myeloma. 2007;7(7):470–471. doi: 10.3816/CLM.2007.n.029. [DOI] [PubMed] [Google Scholar]
- 27.Ohno H., Isoda K. t(11;18)(q21;q21)-positive advanced-stage MALT lymphoma associated with monoclonal gammopathy: resistance to rituximab or rituximab-containing chemotherapy. Journal of Clinical and Experimental Hematopathology. 2008;48(2):47–54. doi: 10.3960/jslrt.48.47. [DOI] [PubMed] [Google Scholar]
- 28.Almehmi A., Fields T. A. Cryoglobulinemic glomerulopathy complicating helicobacter pylori-associated gastric mucosa-associated lymphoid tissue lymphoma. American Journal of Kidney Diseases. 2009;54(4):770–774. doi: 10.1053/j.ajkd.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Hirota-Kawadobora M., Matsuda K., Yamauchi K., et al. Waldenström macroglobulinemia transforming from t (11;18) (q21;q21) -negative gastric MALT lymphoma after systemic dissemination. Rinsho Byori. 2012;60(6):528–535. [PubMed] [Google Scholar]
- 30.Wu B., Chen P., Wang W., Li F., Zou S., Cheng Y. IgA monoclonal gammopathy accompanying extranodal B cell lymphomas. Annals of Hematology. 2014;93(3):521–522. doi: 10.1007/s00277-013-1825-y. [DOI] [PubMed] [Google Scholar]
- 31.Ueda G., Oka K., Matsumoto T., et al. Primary hepatic marginal zone B-cell lymphoma with mantle cell lymphoma phenotype. Virchows Archiv. 1996;428(4-5):311–314. doi: 10.1007/BF00196707. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto S., Kinoshita Y., Fukuda H., et al. ‘Mediterranean lymphoma’ treated with antibiotics. Internal Medicine. 1996;35(12):961–965. doi: 10.2169/internalmedicine.35.961. [DOI] [PubMed] [Google Scholar]
- 33.Nakata M., Matsuno Y., Takenaka T., et al. B-cell lymphoma accompanying monoclonal macroglobulinemia with features suggesting marginal zone B-cell lymphoma. International Journal of Hematology. 1997;65(4):405–411. doi: 10.1016/s0925-5710(96)00565-8. [DOI] [PubMed] [Google Scholar]
- 34.Mak S. K., Wong P.-N., Lo K.-Y., Wong A. K. M. Successful treatment of IgA nephropathy in association with low-grade B- Cell lymphoma of the mucosa-associated lymphoid tissue type. American Journal of Kidney Diseases. 1998;31(4):713–718. doi: 10.1053/ajkd.1998.v31.pm9531192. [DOI] [PubMed] [Google Scholar]
- 35.Sakai A., Katayama Y., Mizuno A., et al. MALT lymphoma producing IgG-kappa type M-protein. Rinsho Ketsueki. 2000;41(8):658–663. [PubMed] [Google Scholar]
- 36.Nagakawa H., Mikami M., Takahashi Y., Yatomi M., Takenouchi T. A case of fatal MALT lymphoma of pulmonary origin with systemic metastasis and manifesting macroglobulinemia. Nihon Kokyuki Gakkai Zasshi. 2002;40(4):299–303. [PubMed] [Google Scholar]
- 37.Pachmann S., Anderegg B., Müller-Höcker J., et al. Monoclonal gammopathy after low-grade MALT lymphoma: evidence for a second neoplasm. American Journal of Hematology. 2002;70(2):167–173. doi: 10.1002/ajh.10104. [DOI] [PubMed] [Google Scholar]
- 38.Stokes M. B., Wood B., Alpers C. E. Membranoproliferative glomerulonephritis associated with low-grade B cell lymphoma presenting in the kidney. Clinical Nephrology. 2002;57(4):303–309. doi: 10.5414/CNP57303. [DOI] [PubMed] [Google Scholar]
- 39.Thieblemont C., Mayer A., Dumontet C., et al. Primary thyroid lymphoma is a heterogeneous disease. The Journal of Clinical Endocrinology & Metabolism. 2002;87(1):105–111. doi: 10.1210/jc.87.1.105. [DOI] [PubMed] [Google Scholar]
- 40.Saito T., Tamaru J.-I., Kishi H., et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) arising in the small intestine with monoclonal cryoglobulinemia. Pathology International. 2004;54(9):712–718. doi: 10.1111/j.1440-1827.2004.01684.x. [DOI] [PubMed] [Google Scholar]
- 41.Takasaki H., Takabayashi M., Yamaji S., et al. Pulmonary MALT lymphoma with macroglobulinemia. Rinsho Ketsueki. 2005;46(2):144–146. [PubMed] [Google Scholar]
- 42.Dalle S., Balme B., Sebban C., Pariset C., Berger F., Thomas L. Schnitzler syndrome associated with systemic marginal zone B-cell lymphoma. British Journal of Dermatology. 2006;155(4):827–829. doi: 10.1111/j.1365-2133.2006.07417.x. [DOI] [PubMed] [Google Scholar]
- 43.Gomyo H., Kajimoto K., Maeda A., et al. t(14;18)(q32;q21)-bearing pleural MALT lymphoma with IgM paraproteinemia: value of detection of specific cytogenetic abnormalities in the differential diagnosis of MALT lymphoma and lymphoplasmacytic lymphoma. Hematology. 2007;12(4):315–318. doi: 10.1080/10245330701383866. [DOI] [PubMed] [Google Scholar]
- 44.Schulze S. M., Edavettal M. M., Fares L., II, Costic J., Moser R., Steeger J. Malt Lymphoma of the Lung Presenting with an igm Monoclonal Gammopathy. Surgical Rounds, May 2007.
- 45.Murota H., Shoda Y., Ishibashi T., Sugahara H., Matsumura I., Katayama I. Improvement of recurrent urticaria in a patient with Schnitzler syndrome associated with B-cell lymphoma with combination rituximab and radiotherapy. Journal of the American Academy of Dermatology. 2009;61(6):1070–1075. doi: 10.1016/j.jaad.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 46.Mikolaenko I., Listinsky C. M. Systemic CD5+ MALT lymphoma: presentation with Waldenstrom syndrome. Annals of Diagnostic Pathology. 2009;13(4):272–277. doi: 10.1016/j.anndiagpath.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Peces R., Vega-Cabrera C., Peces C., Pobes A., Fresno M. F. MALT B cell lymphoma with kidney damage and monoclonal gammopathy: a case study and literature review. Nefrologia. 2010;30(6):681–686. doi: 10.3265/nefrologia.pre2010.jun.10428. [DOI] [PubMed] [Google Scholar]
- 48.Mitchum M., Scorza M., Thomas B., Galeckas K. Cutaneous marginal zone B-cell lymphoma in a patient previously diagnosed with cutaneous Waldenström macroglobulinemia. Journal of the American Academy of Dermatology. 2010;63(2):e59–e61. doi: 10.1016/j.jaad.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Ikuta K., Fujiya M., Ueno N., et al. Atypical mucosa-associated lymphoid tissue lymphoma in the transverse colon associated with macroglobulinemia. Internal Medicine. 2010;49(7):677–682. doi: 10.2169/internalmedicine.49.3160. [DOI] [PubMed] [Google Scholar]
- 50.Kim D. Y., Kim Y.-S., Huh H. J., et al. A case of monoclonal gammopathy in extranodal marginal zone B-cell lymphoma of the small intestine. Korean Journal of Laboratory Medicine. 2011;31(1):18–21. doi: 10.3343/kjlm.2011.31.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lacoste C., Duong T. A., Dupuis J., et al. Leg ulcer associated with type I cryoglobulinaemia due to incipient B-cell lymphoma. Annales de Dermatologie et de Vénéréologie. 2013;140(5):367–372. doi: 10.1016/j.annder.2013.01.429. [DOI] [PubMed] [Google Scholar]
- 52.Chi P.-J., Pei S.-N., Huang T.-L., Huang S.-C., Ng H.-Y., Lee C.-T. Renal MALT lymphoma associated with Waldenström macroglobulinemia. Journal of the Formosan Medical Association. 2014;113(4):255–257. doi: 10.1016/j.jfma.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Tucci A., Motta M., Ungari M., et al. The development of more than one histologic type of lymphoma in the same patient is frequent and confers a worse prognosis. Haematologica. 2005;90(3):348–352. [PubMed] [Google Scholar]
- 54.Papadaki T., Stamatopoulos K., Kosmas C., Kapsimali V., Economopoulos T. A unique case of splenic marginal zone-cell lymphoma with synchronous clonal T-cell large granular lymphocyte proliferation: an immunologic, immunohistochemical and genotypic study. Leukemia Research. 2003;27(1):85–87. doi: 10.1016/s0145-2126(02)00128-5. [DOI] [PubMed] [Google Scholar]
- 55.Lima M., Gonçalves C., Marques L., et al. Association of CD4+/CD56+/CD57+/CD8+dim large granular lymphocytic leukemia, splenic B-cell lymphoma with circulating villous lymphocytes, and idiopathic erythrocytosis. Annals of Hematology. 2001;80(11):685–690. doi: 10.1007/s002770100369. [DOI] [PubMed] [Google Scholar]
- 56.Wang G., Auerbach A., Wei M., et al. t(11;18)(q21;q21) in extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue in stomach: a study of 48 cases. Modern Pathology. 2009;22(1):79–86. doi: 10.1038/modpathol.2008.155. [DOI] [PubMed] [Google Scholar]
- 57.Wöhrer S., Troch M., Streubel B., et al. Pathology and clinical course of MALT lymphoma with plasmacytic differentiation. Annals of Oncology. 2007;18(12):2020–2024. doi: 10.1093/annonc/mdm375. [DOI] [PubMed] [Google Scholar]
- 58.Dierlamm J. Genetic abnormalities in marginal zone B-cell lymphoma. Haematologica. 2003;88(1):8–12. [PubMed] [Google Scholar]
- 59.Liu H., Ye H., Ruskone-Fourmestraux A., et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122(5):1286–1294. doi: 10.1053/gast.2002.33047. [DOI] [PubMed] [Google Scholar]
- 60.Wong K. F., So C. C., Chan J. C. W., Kho B. C. S., Chan J. K. C. Gain of chromosome 3/3q in B-cell chronic lymphoproliferative disorder is associated with plasmacytoid differentiation with or without IgM overproduction. Cancer Genetics and Cytogenetics. 2002;136(1):82–85. doi: 10.1016/s0165-4608(02)00526-5. [DOI] [PubMed] [Google Scholar]
- 61.Raderer M., Wöhrer S., Streubel B., et al. Assessment of disease dissemination in gastric compared with extragastric mucosa-associated lymphoid tissue lymphoma using extensive staging: a single-center experience. Journal of Clinical Oncology. 2006;24(19):3136–3141. doi: 10.1200/jco.2006.06.0723. [DOI] [PubMed] [Google Scholar]
- 62.Ballesteros E., Osborne B. M., Matsushima A. Y. CD5+ low-grade marginal zone B-cell lymphomas with localized presentation. American Journal of Surgical Pathology. 1998;22(2):201–207. doi: 10.1097/00000478-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Ferry J. A., Yang W. I., Zukerberg L. R., Wotherspoon A. C., Arnold A., Harris N. L. CD5+ extranodal marginal zone B-cell (MALT) lymphoma. A low grade neoplasm with a propensity for bone marrow involvement and relapse. American Journal of Clinical Pathology. 1996;105(1):31–37. doi: 10.1093/ajcp/105.1.31. [DOI] [PubMed] [Google Scholar]
- 64.Chatzitolios A., Venizelos I., Tripsiannis G., Anastassopoulos G., Papadopoulos N. Prognostic significance of CD95, P53, and BCL2 expression in extranodal non-Hodgkin's lymphoma. Annals of Hematology. 2010;89(9):889–896. doi: 10.1007/s00277-010-0945-x. [DOI] [PubMed] [Google Scholar]
- 65.Ott M. M., Rosenwald A., Katzenberger T., et al. Marginal zone B-cell lymphomas (MZBL) arising at different sites represent different biological entities. Genes Chromosomes and Cancer. 2000;28(4):380–386. doi: 10.1002/1098-2264(200008)28:4lt;380::aid-gcc3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 66.Wöhrer S., Kiesewetter B., Fischbach J., et al. Retrospective comparison of the effectiveness of various treatment modalities of extragastric MALT lymphoma: a single-center analysis. Annals of Hematology. 2014;93(8):1287–1295. doi: 10.1007/s00277-014-2042-z. [DOI] [PubMed] [Google Scholar]
- 67.Papadaki T., Stamatopoulos K., Kosmas C., et al. Clonal T-large granular lymphocyte proliferations associated with clonal B cell lymphoproliferative disorders: report of eight cases. Leukemia. 2002;16(10):2167–2169. doi: 10.1038/sj.leu.2402643. [DOI] [PubMed] [Google Scholar]
- 68.Shimizu K., Yoshida J., Kakegawa S., et al. Primary thymic mucosa-associated lymphoid tissue lymphoma: diagnostic tips. Journal of Thoracic Oncology. 2010;5(1):117–121. doi: 10.1097/jto.0b013e3181c07df8. [DOI] [PubMed] [Google Scholar]
- 69.Inagaki H., Chan J. K. C., Ng J. W. M., et al. Primary thymic extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features. American Journal of Pathology. 2002;160(4):1435–1443. doi: 10.1016/S0002-9440(10)62569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Troch M., Woehrer S., Streubel B., et al. Chronic autoimmune thyroiditis (Hashimoto's thyroiditis) in patients with MALT lymphoma. Annals of Oncology. 2008;19(7):1336–1339. doi: 10.1093/annonc/mdn049. [DOI] [PubMed] [Google Scholar]