Abstract
In some group-living organisms, labor is divided among individuals. This allocation to particular tasks is frequently stable and predicted by individual physiology. Social insects are excellent model organisms in which to investigate the interplay between physiology and individual behavior, as division of labor is an important feature within colonies, and individual physiology varies among the highly related individuals of the colony. Previous studies have investigated what factors are important in determining how likely an individual is, compared to nest-mates, to perform certain tasks. One such task is foraging. Corpulence (i.e., percent lipid) has been shown to determine foraging propensity in honey bees and ants, with leaner individuals being more likely to be foragers. Is this a general trend across all social insects? Here we report data analyzing the individual physiology, specifically the percent lipid, of worker bumble bees (Bombus impatiens) from whom we also analyze behavioral task data. Bumble bees are also unusual among the social bees in that workers may vary widely in size. Surprisingly we find that, unlike other social insects, percent lipid is not associated with task propensity. Rather, body size closely predicts individual relative lipid stores, with smaller worker bees being allometrically fatter than larger worker bees.
Keywords: Lipid, Size polymorphism, Social insects, Bumble bees, Division of labor
Introduction
Foraging is arguably one of the riskiest behaviors that an animal may undertake (Porter and Jorgensen 1981; Jeanne 1986; Suhonen 1993; Pugesek 1995; Visscher and Dukas 1997; Kukuk et al. 1998). Searching is costly in terms of time investment and energy consumption. Leaving shelter to find food increases an animal’s exposure to predation (Alford 1975; Krebs and Davies 1997). Of course, food collection is necessary, and every solitary animal must engage in this activity. However, in group living organisms, members may specialize on particular tasks, a process known as division of labor. These individuals are therefore faced with a “decision” either to forage or to engage in some other perhaps safer job.
Bees, ants, and wasps are popular organisms in which to study foraging decisions, as division of labor is a key feature of social insect societies (Robinson 1992; Beshers and Fewell 2001). Many correlates in different social insect species have been identified as good markers for an individual’s propensity to forage. Previous work has identified the markers that play an important role in different organisms, such as age in ants, honey bees, bumble bees, and wasps (Hölldobler and Wilson 1990; Jeanne 1991; O’Donnell and Jeanne 1992; Robinson 1992; O’Donnell 1995; Yerushalmi et al. 2006); genes in honey bees (Ben-Shahar et al. 2002); physiology in ants and honey bees (Robinson 1987; Dornhaus and Powell 2010); ambient temperature in ants (Hölldobler and Wilson 1990); dominance status in ants (Powell and Tschinkel 1999); and body size in wasps and bumble bees (Wilson 1980; Hölldobler and Wilson 1990; O’Donnell and Jeanne 1995b; Goulson et al. 2002). One frequently identified factor is percent lipid, where a decrease in individual lipid stores precedes foraging in many social insects [wasps (O’Donnell and Jeanne 1995c; Markiewicz and O’Donnell 2001), honey bees (Toth et al. 2005; Toth and Robinson 2005), ants (Porter and Jorgensen 1981; Tschinkel 1987; Tschinkel 1998; Blanchard et al. 2000; Robinson et al. 2009a, b)]. It was hypothesized that keeping foragers (who are exposed to higher predation pressures) lean may be adaptive because they are often lost to the colony (i.e., a “disposable caste” (Porter and Jorgensen 1981; O’Donnell and Jeanne 1995a)), thus reducing total energy loss to the colony. Also, leaner foragers might be better at maneuvering in the field or lighter in flight (Porter and Jorgensen 1981; Robinson et al. 2009a). However, in both circumstances, worker foraging propensity remains individually plastic and frequently affected by a combination of the above factors (O’Donnell and Jeanne 1995c; Børgesen 2000; Markiewicz and O’Donnell 2001; Toth et al. 2005; Robinson et al. 2009b).
Whereas previous work in ants and honey bees has advanced our understanding of what plays a role in foraging propensity, less is known about the relative importance of the factors, many of which correlate in bumble bees (Bombus spp.). Bumble bee workers, although highly related, may display a 10-fold difference in mass that is continuous, normally distributed, and symmetric around a single mean (Goulson 2003; Couvillon and Dornhaus 2010; Couvillon et al. 2010). This degree of size variation is unique to Bombus among the eusocial bees and appears to arise from the interplay of the spatial organization of the nest, the workers, and the brood (Couvillon and Dornhaus 2009; Jandt and Dornhaus 2009). Larger workers develop and emerge from the nest center, which is also where the nurse worker bees tend to be found. Body size predicts worker task, where smaller bees are more likely to nurse and larger bees are more likely to forage (Goulson et al. 2002; Jandt and Dornhaus 2009); there may also be an influence of age on foraging propensity (Yerushalmi et al. 2006). However, overall the effect is weak, and the probability of task switching remains high (Jandt et al. 2009).
In a previous study, small bumble bee workers survived colony starvation significantly longer than their larger sisters (Couvillon and Dornhaus 2010). Why might this be? One hypothesis is that the smaller bumble bee workers may possess more stored nutritive resources, which may lead to the increased longevity during colony starvation. For example, smaller workers may possess higher lipid content per unit body mass than larger workers. In this type of allometry, although absolute lipid content may increase with body size, the mass specific lipid content (percent lipid) would be highest in small bees. However, even if that were the case, it is not clear whether it would be caused by body size per se, or a consequence of division of labor. Since (lack of) lipid stores is linked with increased foraging propensity in other social insects, and larger workers in bumble bees are more likely to forage (Goulson et al. 2002), larger workers may be leaner in preparation for or as consequence of their foraging task. It is therefore necessary to analyze separately the relative contribution of body size (measured here as body mass) and propensity to perform particular tasks to individual bumble bee physiology. In other words, is the amount of lipid stored more strongly predicted by body mass, task (foraging likelihood), or an interaction of the two?
This we test here. We measured and analyzed lipid content of all the workers in bumble bee colonies for whom we had also collected task data. Interestingly, we did find a wide variation among workers in the amount of stored lipids. However, individual percent lipid was not strongly associated with task repertoire, which was unexpected given the observed patterns in other insects. Instead, body mass strongly predicted relative lipid content (percent lipid), with smaller bees being allometrically more corpulent.
Materials and methods
Study organism and experimental set-up
We ordered two bumble bee colonies (B. impatiens; colonies A and B) from Koppert Biological Systems (Romulus, MI). Colonies were queenright with 15–20 workers at the start of the experiment and grew to over 100 workers by the end. Each colony was housed in a wooden nest box (22 × 22 × 11 cm) with screened ventilation holes and Plexiglass lid. In this way, the entire colony was visible, and an opening in the lid allowed for daily delivery of 1 rounded tsp of defrosted (fresh frozen) ground pollen. Nest boxes were connected by 30 cm long plastic tubing to a separate foraging arena (58 × 36 × 40 cm), where sugar solution (“BeeHappy”, Koppert) was provided in feeders ad libitum. Feline Pine © cat litter covered the nest box base, which helped remove moisture that can lead to fungal growth. Room temperature (25°C) was held constant. As reported in previous studies, bumble bees quickly adjust to overhead lights (12: 12 L:D) and behaved normally (Couvillon and Dornhaus 2009; Jandt and Dornhaus 2009). We tagged all workers with uniquely numbered plastic tags (‘Opalithplättchen’) at the start of the experiment. Additionally, throughout the course of the behavioral data collection, we tagged newly emerged bees (callows). Ultimately we tagged 116 bees from Colony A and 120 bees from Colony B. To allow the bees to acclimate to the laboratory, we waited 2 weeks after colony delivery to begin behavioral data collection. As bumble bees produce the range of sizes throughout the colony cycle, body size should not be confounded by worker age (Couvillon et al. 2010).
Assigning task repertoire for each bee
As it was necessary to identify the task repertoire of each bee, we used instantaneous scan sampling 3 days a week from 26 February, 2009–17 March, 2009 (Altmann 1974; Jandt et al. 2009). On these days, we located and observed all visible workers and recorded the task that each bee was performing. This took approximately 1 h per colony, and we identified 80–90% of the worker bees each day; only those bees observed on five or more days were included in the analyses. Tasks were divided into nursing and foraging categories. Nursing tasks can be subdivided into larval feeding, incubating, constructing honeypots, inspecting the nest surface, scraping wax from pupal clumps, or drinking honey (Cameron 1989). Foraging tasks can be subdivided into collecting nectar, collecting pollen, or probing honeypots inside the nest (Cameron 1989). We also recorded guarding, fanning, or inactivity, and more rare tasks like excavating and undertaking (Cameron 1989; Jandt et al. 2009).
We classified individuals as nurses, foragers, or “mixed”. Nurses were defined as individuals that spent ≥75% of observations performing nursing tasks; foragers were defined as individuals that spent ≥75% of observations performing foraging tasks. “Mixed” workers did not fall into either category. These criteria and analyses are common methods of assigning task repertoire (Cameron 1989; Jandt et al. 2009).
Extraction and quantification of lipids
After we collected the behavioral data, we discontinued pollen feeding for 2 days; this allowed for worker crops to be emptied of pollen, which is a rich source of fat. We froze the colonies and collected bees in individually labeled Eppendorf tubes. When we were ready for lipid extraction, we gently scraped off the number tags and quartered each bee along the mid-sagittal and transverse planes with a clean razor blade. We placed bees in batches of six on individual weigh boats in an oven to dry for 8 days at 60°C. We determined with a small batch of non-experimental bees that after 8 days, the mass of the bees no longer decreased, indicating that all the moisture had evaporated.
We determined our lipid extraction methodology from the literature (Folch et al. 1957; Bligh and Dyer 1959) and from discussion with a chemist (Prof. Leif Abrell) and insect physiologist (Prof. Goggy Davidowitz). First we recorded the dry mass (body mass) of each bee and placed it in a labeled Eppendorf tube and added under a chemical hood 700 µl of 2:1 DCM-MeOH (dichloromethane-methanol), carefully pipetting up and down to encourage mixing. Using a disposable pestle, we ground the bee for 60 s, capped the tubes, placed them in a rack, and secured the rack to a 0.4amp Vortexer, where they were shaken for 15 min. Next we spun the tubes (14K rpm) in a desktop centrifuge for 4 min, allowing the tubes to sit for an additional 4 min after the spin. At this stage, lipids are dissolved in the supernatant, whereas non-soluable components (e.g., exoskeleton) remained as a pellet at the bottom of the Eppendorf tube. We carefully drew off the supernatant, pipetting it into second clean, labeled, individually weighed Eppendorf tube. This we set aside.
Meanwhile, we resuspended the pellet in a second 700 µl of DCM-MeOH and repeated the process. The second drawn-off supernatant was combined with the first, and the pellet/first Eppendorf tube was discarded. We added 540 µl of 0.9% NaCl to the supernatant in the second Eppendorf tube to precipitate phase separation of aqueous and organic layer. Tubes were shaken for 30 s and allowed to sit in a rack at room temperature for 5 min. At that time, 2 layers were clearly visible–a top, aqueous layer, which may be discarded, and a heavier, organic bottom layer, which contained the lipids. We carefully siphoned off the top layer with a pipette, leaving the organic phase behind. The aqueous layer was discarded. The bottom organic layer was left in the uncapped Eppendorf tube under a hood for 24 h. After 24 h, the liquid solvent had all evaporated, leaving behind a lipid residue. The tubes were weighed, and the original mass of the individual tube was subtracted to obtain the total lipid mass. With this total lipid mass, we were able to calculate % lipid for each bee using the original dry mass (body mass).
Statistical analysis
All statistics were done using JMP ® v.8.0.2. We removed from the data set one bee from colony A and 14 bees from colony B because we had insufficient task data on those individuals. We ultimately analyzed 195 bees: 14 nurses (3 from A, 11 from B), 77 foragers (34 from A, 43 from B), and 104 ‘mixed’ workers (58 from A, 46 from B).
We used a Generalized Linear Model, blocking for colony-level effects, to determine whether percent lipid (arcsine transformed) could be predicted by task category (nurse, forager, or mixed), dry mass (body mass), or an interaction of the two. The analysis was done in this way because previous work has demonstrated that body size predicts foraging behavior/task; therefore, we thought it necessary to include body size as a factor in analyses.
Additionally, we analyzed the relationship between dry mass (body mass; mg) and total lipids (mg) by allometric regression. Allometry was tested by log-transforming the raw data (dry mass [mg] and total lipids [mg]) and running an OLS (Ordinary Least Squares) analysis on the data (Warton et al. 2006). In allometric analysis, the log-linearized slope of the regression between body mass and lipid content allows for comparison of relative lipid content as body size increases within a colony. A slope value of one, for instance, would indicate that a doubling of body size correlated with a doubling of lipid content, and slopes above or below one show that there is proportionally more or less lipid as worker bee size increases. If, for example, the slope is significantly less than 1, this indicates a negative allometry, where smaller bees possess proportionally more lipid for their dry mass.
Results
Body mass strongly predicts percent lipid
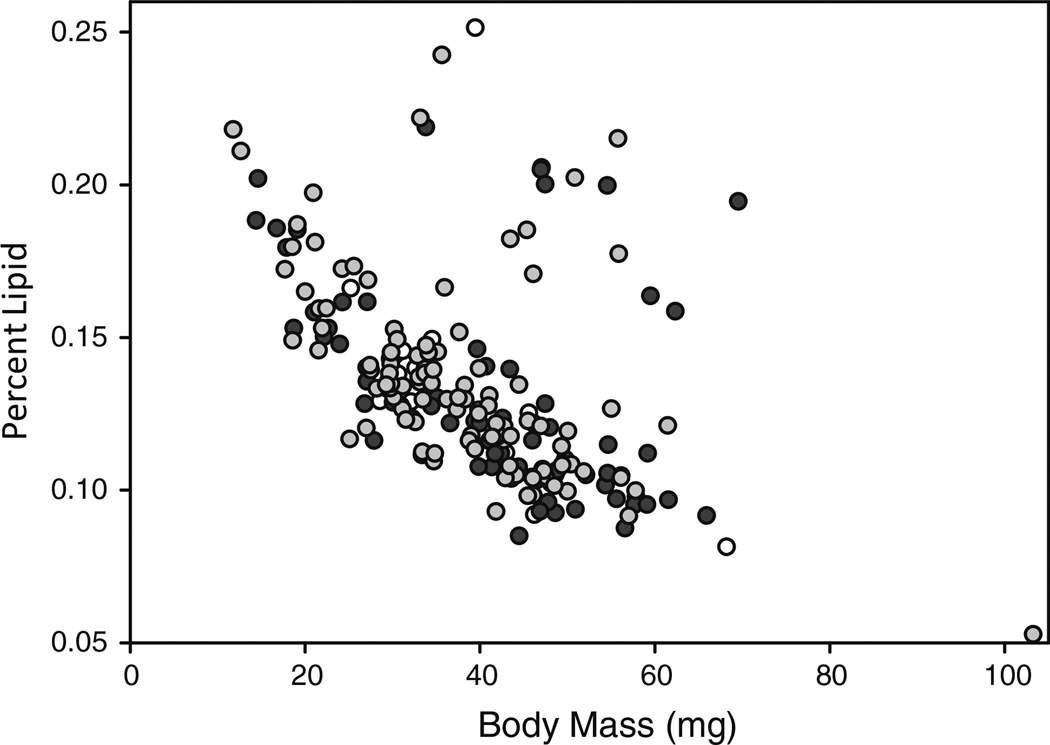
There was a significant relationship between percent lipid (measured as lipid/mass) and dry mass (body mass) of bees (χ2 = 28.00, df = 1, p < 0.001; Fig. 1). Given the direction of the relationship, smaller bees have greater percent lipid than larger bees (see Fig. 3). These results are consistent across colonies (χ2 = 0.55, df = 1, p = 0.46). Therefore, body size is a significant factor for predicting individual percent lipid.
Fig. 1.
Bumble bee worker body size, not task, is a significant predictor for individual percent lipid. Body mass is measured as dry mass (mg), and percent lipid is (Lipid (mg)/Body Mass (mg)) and is therefore corrected for body mass. Small bees have proportionally more lipids than their larger sisters, irrespective of task (white circles: nurses; black circles: foragers; grey circles: mixed behavior). There was no effect of colony, so data here are pooled for both Colonies A and B
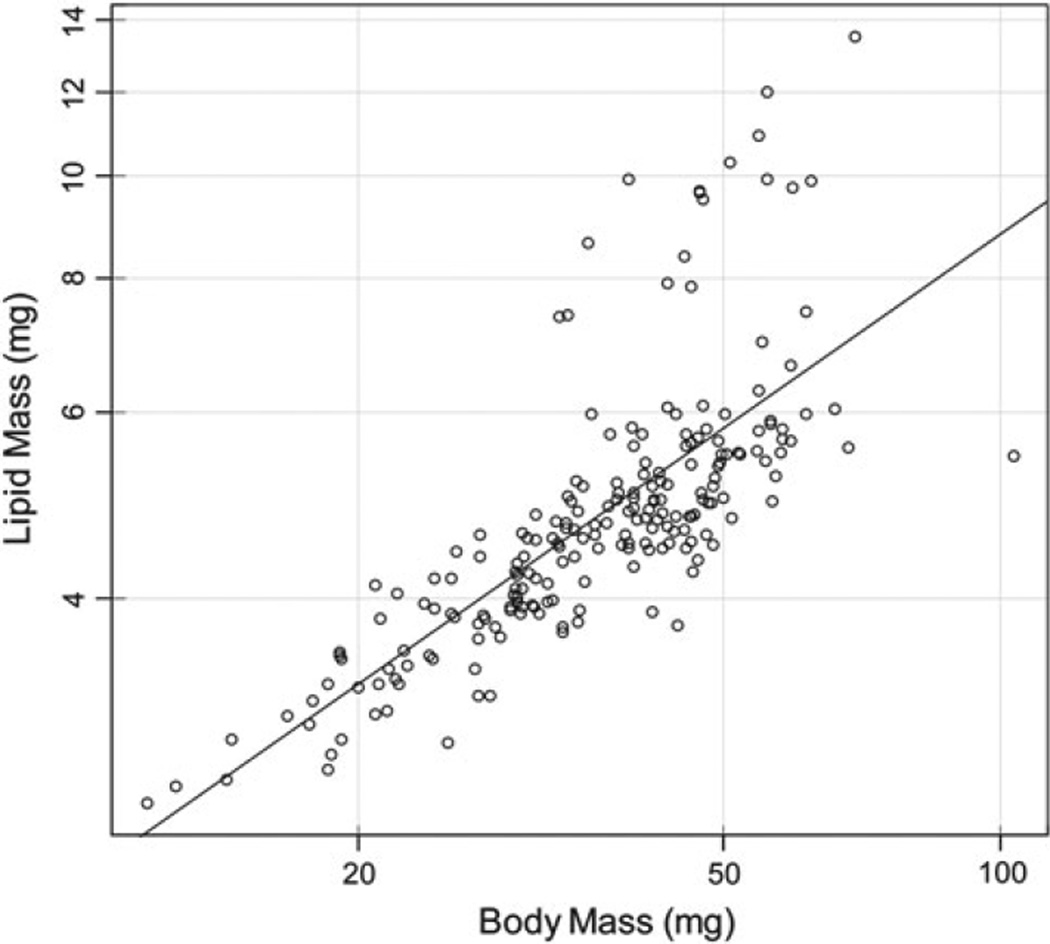
Fig. 3.
Total lipids (lipid mass (mg)) increases with increasing body size (body mass (mg)) (linear regression, dry mass (mg), R2 = 0.41, p = 0.001). However, the slope of the line (m = 0.61, CI = 0.53–0.68) is significantly less than 1 (F = 114.32, p < 0.001), indicating a negative allometry, where smaller bees possess proportionally more lipids
A small number (~16 individuals) of mostly larger bees with unusually high percent lipid clusters separately from the rest of the data (seen here and in Fig. 3). These individuals were not performing any specific subset of tasks, and so their function is unknown.
There is no effect either of task or task × mass interaction on percent lipid in bees
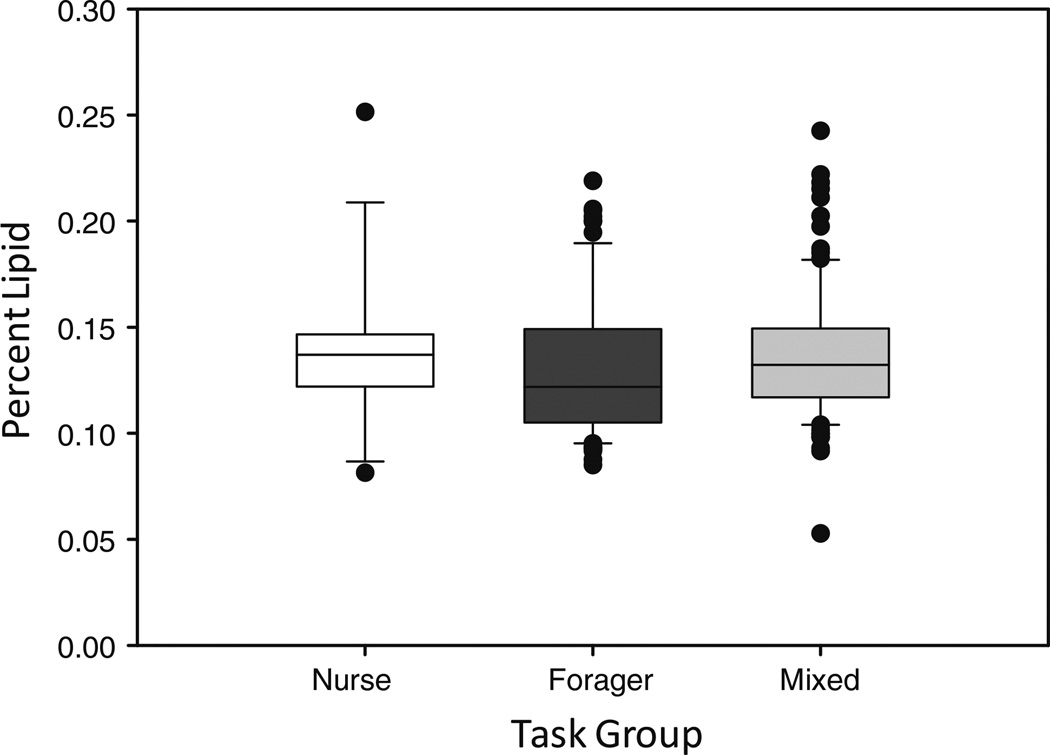
We found no evidence that task (nurse, forager, or mixed) could be used to predict percent lipid (χ2 = 0.20, df = 2, p = 0.90; Figs. 1, 2). The effect of the interaction between dry mass (body mass) and task on percent lipid was also highly non-significant (χ2 = 1.23, df = 2, p = 0.54). Therefore, task is not a significant factor for predicting individual percent lipid.
Fig. 2.
There was no difference in corpulence (percent lipid): (Lipid (mg)/Body Mass (mg)) between bumble bee worker nurses (n = 14), foragers (n = 77), or mixed (n = 104) (χ2 = 0.20, df = 2, p = 0.90). Additionally, there was no interaction effect of worker task and body size, as measured by dry mass, on percent lipid (χ2 = 1.23, df = 2, p = 0.54). Boxes represent medians (solid line) ± 25th percentile range. Whiskers denote 10th–90th percentiles, while dots denote outliers
When task (nursing or foraging) is measured as a continuous, quantitative variable (% time), we see no significant correlative relationship: individuals with disproportionately more lipids do not spend more time nursing (Generalized Linear Model, blocked for colony level effects: Factor Dry Mass: χ2 = 3.31, df = 1, p = 0.07; % Lipid (Arcsine transformed): χ2 = 0.50, df = 1, p = 0.48; Dry Mass × % Lipid Interaction: χ2 = 0.10, df = 1, p = 0.75). While larger bees were more likely to forage, there is no evidence to suggest that individuals with disproportionately less lipids spend more time foraging (Generalized Linear Model, blocked for colony level effects: Factor Dry Mass: χ2 = 4.25, df = 1, p = 0.04; % Lipid (Arcsine transformed): χ2 = 2.03, df = 1, p = 0.15; Dry Mass x % Lipid Interaction: χ2 < 0.01, df = 1, 0.95) (data not shown).
Total lipid amount does positively correlate with body mass; however, percent lipid allometrically decreases with increasing body mass
Total lipids positively correlates with body size (Linear Regression, dry mass (mg), R2 = 0.41, p = 0.001) (Fig. 3). Additionally, we found that when examining total lipids (mg) with body size (mg), the resultant slope was significantly different from 1, indicating allometry (R2 = 0.56; Log [Total lipids (mg)] = (0.61)(Log [Dry mass (mg)]−0.27); Fig. 3). Specifically, the slope (m = 0.61, CI = 0.53–0.68) was significantly <1 (F = 114.32; p < 0.001), indicating a negative allometry between total lipids and body mass. Smaller bees, although possessing less total lipids, are proportionally more corpulent.
Discussion
Here we show that in smaller worker bumble bees, a significantly higher proportion of body tissue consists of lipids, most likely fat storage, compared to larger worker bumble bees. Therefore, there is a mass-specific lipid content decrease with increasing size, perhaps as a consequence of another body part’s over-development. However, individual task performance did not significantly correlate with percent lipid. This is surprising, as a decrease in lipids in some other insects has been associated with that individual’s propensity to forage (O’Donnell and Jeanne 1995c; Toth and Robinson 2005; Robinson et al. 2009a).
In our experimental set-up, foragers were not required to fly to gather nectar. Instead, they crawled through 30 cm of tubing to an arena containing a feeder, therefore saving on energetically-costly flight. It is possible that if foraging activity were more strenuous, foragers would indeed lose more of their nutrient storage (O’Donnell and Jeanne 1995c). However, the relationship of lipid storage and foraging has in the literature been reported such that a decline in lipids usually precedes the onset of foraging, rather than being a consequence of it (O’Donnell and Jeanne 1995c; Toth and Robinson 2005; Robinson et al. 2009a). Indeed, foraging itself seems to have very little—if any—effect on the lipid stores in honey bee foragers (Toth and Robinson 2005), a result anticipated by evidence that Hymenopteran foragers exclusively oxidize carbohydrates during flight (Heinrich 1975; Rothe and Nachtigall 1989; Gäde and Auerswald 1998). In addition, the correlation of low lipid stores with foraging activity has also been reported in ants, which do not fly (Blanchard et al. 2000; Robinson et al. 2009a, b). Therefore, it is likely that even if bumble bees in our experiment had to fly to forage, we would not have found a relationship of foraging activity with lipid stores.
We did not control for the ages of analyzed workers. Previous work has demonstrated that all sizes of workers are produced throughout the colony cycle (Couvillon et al. 2010); we extracted lipids on the workers at the end of the behavioral observations, so all ages/sizes were present, and any effect of age would therefore be distributed throughout the population. It was also demonstrated in ants (Blanchard et al. 2000), wasps (O’Donnell and Jeanne 1995c), and honey bees (Toth and Robinson 2005) that age is not strongly associated with level of fat stores. However, not controlling for age means we are unable to rule out if the lipid content distribution we describe is present from birth or accumulates during adulthood. Still, we are confident at least that task does not explain differences in nutrient distribution. It would be an interesting follow-up to examine the percent lipid in newly emerged (callow) bees, whose body size and shape is fixed upon eclosion, as it is in all holometabolous insects.
Our criterion for assigning task data was strict, where an individual must be observed performing a task ≥75% of time to be considered in that category (e.g. nurse). This was necessary, as individual task switching is often observed (Jandt et al. 2009). Lowering the threshold for task assignment would likely not change the results, as it is clear from Fig. 2 that a range of body sizes and percent lipids is dispersed throughout all three task types.
In some other social insects, particularly certain ant species, individuals with high nutrient stores fulfill an additional adaptive purpose in a unique caste known as repletes, which are not found in bumble bees. These individuals carry a disproportionate amount of nutrients (i.e., carbohydrates, lipids, proteins) that are stored and available at the colony level (Wilson 1974; Porter and Tschinkel 1985; Tschinkel 1993, 1998; Hahn 2006). Repletes may not perform other tasks in the colony. Thus, it is interesting to consider that for repletes, lipid storage is a task in itself (Burgett and Young 1974; Lachaud et al. 1992). Therefore, future studies on lipid content in bumble bees should also analyze inactivity to determine if, for example, fatter individuals are more likely to not do more traditional tasks.
How might smaller workers have developed to possess disproportionately more lipids, irrespective of task? One hypothesis is that adult worker size and body composition (e.g. lipid content) are products of nutritional conditions experienced during the larval period. In insects generally, body size and content are highly influenced by both the amount and quality of food consumed (Raubenheimer and Simpson 1996, 1999; Ojeda-Avila et al. 2003; Shingleton et al. 2007). As with many social insects, larval nutrition is determined by the nursing worker adults within the colony, and adult size is directly correlated with the quantity of food received as a larva (Plowright and Jay 1968; Pendrel and Plowright 1981; Sutcliffe and Plowright 1988, 1990; Pereboom et al. 2003). Nurses feed 4–15 larvae in a single bout on a regurgitated mixture of nectar and pollen; these larvae are located throughout the nest from center to periphery (Katayama 1973; Michener 1974; Alford 1975; Ribeiro et al. 1999). A larva’s physical position in a colony, fixed at oviposition in bumble bees, will influence how much food she receives and, therefore her adult size and potentially lipid content. Larvae at the center of the nest are fed more often than those at the periphery and develop into larger bees (Couvillon and Dornhaus 2009) that also possess a smaller proportion of lipids comprising their body mass.
How, then, might food given more frequently to the larvae in the nest center result in a bigger/leaner bee, while the same food given less frequently to the larvae at the nest periphery result in a smaller/fatter bee? One potential explanation is that being fed less often or with smaller amounts of food per feeding-bout causes shifts in the relative allocation to storage during larval development, presumably at a cost to overall growth. As resources become scarce or diminished, organisms may store a greater proportion of their incoming resources in reserves (Raubenheimer and Simpson 1997; Raguso et al. 2007). For example, frequent feeding may down-regulate the amount of larval resources that are committed to storage, while growth processes in general are up-regulated, creating a larger though leaner adult. In contrast, longer periods between feeding bouts or general reduction in larval nutrition may cause the larvae to commit more nutritional resources to storage rather than growth, ultimately producing a smaller though more corpulent adult. Although hand-rearing bumble bee larva is difficult (Pereboom 1997), it would be illuminating to test directly if food quantity and feeding frequency affect size variation and individual body composition.
Why then would smaller bees develop proportionally higher lipid content? Previous work that found a strong effect of task on relative individual lipid content proposed non-mutually exclusive adaptive hypotheses, namely the idea of a “disposable caste” (Porter and Jorgensen 1981; O’Donnell and Jeanne 1995a; Woyciechowski and Kozlowski 1998; Tofilski 2002) or that leaner foragers are more manoeuvrable (Porter and Jorgensen 1981; Robinson et al. 2009a). However, we now know that task is not a strong factor in predicting percent lipid in bumble bees. The adaptive function of having smaller bees with proportionally more lipids remains unclear.
Smaller workers bumble bees are more robust to starvation compared to larger workers (Couvillon and Dornhaus 2010). Now we know the smaller workers are fatter, which may explain this result. It appears that the function of lipids in bumble bees is different from the role it plays in honey bee and ant foraging propensity. Therefore, these data contribute to our understanding of how social organization and physiology may interrelate differently across closely related taxa with similar social organizations. Just how physiological variation develops and why it evolves clearly requires further investigation.
Acknowledgments
We would like to thank both Leif Abrell and Goggy Davidowitz for their help with our lipid extraction methodology. Additionally, Goggy allowed us to use his lab space for the work. Thanks to LASI Data Club for helpful discussions. MJC was funded by an NIH PERT Postdoctoral Fellowship through the Center for Insect Science at the University of Arizona.
Contributor Information
Margaret J. Couvillon, Email: M.Couvillon@Sussex.ac.uk, Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, USA; Laboratory of Apiculture and Social Insects, School of Life Sciences, University of Sussex, Falmer, Brighton BN1 9QG, UK.
Jennifer M. Jandt, Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, USA
Jennifer Bonds, Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, USA.
Bryan R. Helm, Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, USA
Anna Dornhaus, Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, USA.
References
- Alford DV. Bumblebees. London: Davis-Poynter; 1975. [Google Scholar]
- Altmann J. Observational Study of Behavior: Sampling Methods. Behaviour. 1974;49:227–266. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y, Robichon A, Sokolowski MB, Robinson GE. Influence of gene action across different time scales on behavior. Science. 2002;296(5568):741–744. doi: 10.1126/science.1069911. [DOI] [PubMed] [Google Scholar]
- Beshers SN, Fewell JH. Models of division of labor in social insects. Annu Rev Entomol. 2001;46(1):413–440. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- Blanchard GB, Orledge GM, Reynolds SE, Franks NR. Division of labour and seasonality in the ant Leptothorax albipennis: worker corpulence and its influence on behaviour. Anim Behav. 2000;59(4):723–738. doi: 10.1006/anbe.1999.1374. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Physiol Pharm. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Børgesen LW. Nutritional function of replete workers in the pharaoh’s ant, Monomorium pharaonis. Insect Soc. 2000;47(2):141–146. [Google Scholar]
- Burgett DM, Young RG. Lipid storage by honey ant repletes. Ann Entomol Soc Am. 1974;67:743–744. [Google Scholar]
- Cameron SA. Temporal patterns of division of labor among workers in the primitively eusocial bumble bee, Bombus griseocollis (Hymenoptera: Apidae)) Ethology. 1989;80:137–151. [Google Scholar]
- Couvillon MJ, Dornhaus A. Location, location, location: larvae position inside the nest is correlated with adult body size in worker bumble bees (Bombus impatiens) P Roy Soc Lond B Bio. 2009;276:2411–2418. doi: 10.1098/rspb.2009.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvillon MJ, Dornhaus A. Small worker bumble bees (Bombus impatiens) are hardier against starvation than their larger sisters. Insect Soc. 2010;57:193–197. doi: 10.1007/s00040-010-0064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvillon MJ, Jandt J, Duong N, Dornhaus A. Ontogeny of worker body size distribution in bumble bee (Bombus impatiens) colonies. Ecol Entomol. 2010;35:424–435. doi: 10.1111/j.1365-2311.2010.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornhaus A, Powell S. Foraging and defence strategies. In: Lach L, Parr CL, Abbott KL, editors. Ant ecology. Oxford: Oxford University Press; 2010. p. 385. [Google Scholar]
- Folch J, Lees M, Sloane-Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:495–509. [PubMed] [Google Scholar]
- Gäde G, Auerswald L. Flight metabolism in carpenter bees and primary structure of their hypertrehalosaemic peptide. Exp Biol Online. 1998;3(6):1–11. [Google Scholar]
- Goulson D. Bumblebees—behaviour and ecology. Oxford: Oxford University Press; 2003. [Google Scholar]
- Goulson D, Peat J, Stout JC, Tucker J, Darvill B, Derwent LC, Hughes WOH. Can alloethism in workers of the bumblebee, Bombus terrestris, be explained in terms of foraging efficiency? Anim Behav. 2002;64:123–130. [Google Scholar]
- Hahn DA. Two closely related species of desert carpenter ant differ in individual-level allocation to fat storage. Physiol Biochem Zool. 2006;79:847–856. doi: 10.1086/505995. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Energetics of pollination. Annu Rev Ecol Syst. 1975;6:139–170. [Google Scholar]
- Hölldobler B, Wilson EO. The ants. Cambridge, MA: Belknap Press; 1990. [Google Scholar]
- Jandt J, Dornhaus A. Spatial organization and division of labor in the bumble bee, Bombus impatiens. Anim Behav. 2009;77:641–651. [Google Scholar]
- Jandt J, Huang E, Dornhaus A. Weak specialization of workers inside a bumble bee (Bombus impatiens) nest. Behav Ecol Sociobiol. 2009;63(12):1829–1836. [Google Scholar]
- Jeanne RL. The organization of work in Polybia occidentalis: costs and benefits of specialization in a social wasp. Behav Ecol Sociobiol. 1986;19(5):333–341. [Google Scholar]
- Jeanne RL. Polyethism. In: Ross KG, Matthews RW, editors. The social biology of wasps. Ithaca: Cornell University; 1991. [Google Scholar]
- Katayama E. Observations on the brood development in Bombus ignitus (Hymenoptera, Apidae) II. Brood development and feeding habits. Kontyû. 1973;41:203–216. [Google Scholar]
- Krebs JR, Davies NB. Behavioural ecology: an evolutionary approach. Oxford: Blackwell Scientific Publications; 1997. [Google Scholar]
- Kukuk PF, Ward SA, Jozwiak A. Mutualistic benefits generate an unequal distribution of risky activities among unrelated group members. Naturwissenschaften. 1998;85(9):445–449. [Google Scholar]
- Lachaud JP, Passera L, Grimal A, Detrain C, Beugnon G. Lipid storage by major workers and starvation reistance in the ant Pheidole pallidula (Hymenoptera, Formicidae) In: Billen J, editor. Biology and evolution of social insects. Leuven (Belgium): Leuven University Press; 1992. pp. 153–160. [Google Scholar]
- Markiewicz D, O’Donnell S. Social dominance, task performance and nutrition: implications for reproduction in eusocial wasps. J Comp Physiol A. 2001;187(5):327–333. doi: 10.1007/s003590100204. [DOI] [PubMed] [Google Scholar]
- Michener CD. The social behaviour of the bees. Cambridge, Mass: Harvard University Press; 1974. [Google Scholar]
- O’Donnell S, Jeanne RL. Implications of senescence patterns for the evolution of age polyethism in eusocial insects. Behav Ecol. 1995a;6:269–273. [Google Scholar]
- O’Donnell S, Jeanne RL. The roles of body size and dominance in division of labor among workers of the eusocial wasp Polybia occidentalis (Olivier) (Hymenoptera: Vespidae) J Kansas Entomol Soc. 1995b;68(1):43–50. [Google Scholar]
- O’Donnell S, Jeanne RL. Worker lipid stores decrease with outside-nest task performance in wasps: implications for the evolution of age polyethism. Cell Mol Life Sci. 1995c;51(7):749–752. [Google Scholar]
- O’Donnell S. Division of labor in post-emergence colonies of the primitively eusocial wasp Polistes instabilis de Saussure (Hymenoptera: Vespidae) Insect Soc. 1995;42(1):17–29. [Google Scholar]
- O’Donnell S, Jeanne RL. Lifelong patterns of forager behaviour in a tropical swarm-founding wasp: effects of specialization and activity level on longevity. Anim Behav. 1992;44(6):1021–1027. [Google Scholar]
- Ojeda-Avila T, Arthur Woods H, Raguso RA. Effects of dietary variation on growth, composition, and maturation of Manduca sexta (Sphingidae: Lepidoptera) J Insect Physiol. 2003;49(4):293–306. doi: 10.1016/s0022-1910(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Pendrel BA, Plowright RC. Larval feeding by adult bumble bee workers (Hymenoptera, Apidae) Behav Ecol Sociobiol. 1981;8(2):71–76. [Google Scholar]
- Pereboom JJM. while they banquet splendidly the future mother. Utrecht University; 1997. [Google Scholar]
- Pereboom JJM, Velthuis HHW, Duchateau MJ. The organisation of larval feeding in bumblebees (Hymenoptera, Apidae) and its significance to caste differentiation. Insect Soc. 2003;50(2):127–133. [Google Scholar]
- Plowright RC, Jay SC. Caste differentiation in bumblebees (Bombus latr - Hym) 1. Determination of female size. Insect Soc. 1968;15(2):171–192. [Google Scholar]
- Porter SD, Jorgensen CD. Foragers of the harvester ant, Pogonomyrmex owyheei: a disposable caste? Behav Ecol Sociobiol. 1981;9(4):247–256. [Google Scholar]
- Porter SD, Tschinkel WR. Fire ant polymorphism: the ergonomics of brood production. Behav Ecol Sociobiol. 1985;16:323–336. [Google Scholar]
- Powell S, Tschinkel WR. Ritualized conflict in Odontomachus brunneus and the generation of interaction-based task allocation: a new organizational mechanism in ants. Anim Behav. 1999;58:965–972. doi: 10.1006/anbe.1999.1238. [DOI] [PubMed] [Google Scholar]
- Pugesek BH. Offspring growth in the California gull: reproductive effort and parental experience hypotheses. Anim Behav. 1995;49:641–647. [Google Scholar]
- Raguso RA, Ojeda-Avila T, Desai S, Jurkiewicz MA, Arthur Woods H. The influence of larval diet on adult feeding behaviour in the tobacco hornworm moth, Manduca sexta. J Insect Physiol. 2007;53(9):923–932. doi: 10.1016/j.jinsphys.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson SJ. Meeting nutrient requirements: the roles of power and efficiency. Entomol Exp Appl. 1996;80(1):65–68. [Google Scholar]
- Raubenheimer D, Simpson SJ. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res Rev. 1997;10:151–179. doi: 10.1079/NRR19970009. [DOI] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson SJ. Integrating nutrition: a geometrical approach. Entomol Exp Appl. 1999;91(1):67–82. [Google Scholar]
- Ribeiro MF, Velthuis HHW, Duchateau MJ, van der Tweel I. Feeding frequency and caste differentiation in Bombus terrestris larvae. Insect Soc. 1999;46:306–314. [Google Scholar]
- Robinson GE. Regulation of honey bee age polyethism by juvenile hormone. Behav Ecol Sociobiol. 1987;20(5):329–338. [Google Scholar]
- Robinson GE. Regulation of division-of-labor in insect societies. Annu Rev Entomol. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- Robinson E, Richardson T, Sendova-Franks A, Feinerman O, Franks N. Radio tagging reveals the roles of corpulence, experience and social information in ant decision making. Behav Ecol Sociobiol. 2009a;63(5):627–636. [Google Scholar]
- Robinson EJH, Feinerman O, Franks NR. Flexible task allocation and the organization of work in ants. P Roy Soc Lond B Bio. 2009b;276(1677):4373–4380. doi: 10.1098/rspb.2009.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe U, Nachtigall W. Flight of the honey bee. J Comp Physiol A. 1989;158(6):739–749. [Google Scholar]
- Shingleton AW, Frankino WA, Flatt T, Nijhout HF, Emlen DJ. Size and shape: the developmental regulation of static allometry in insects. Bioessays. 2007;29(6):536–548. doi: 10.1002/bies.20584. [DOI] [PubMed] [Google Scholar]
- Suhonen J. Predation risk influences the use of foraging sites by tits. Ecology. 1993;74(4):1197–1203. [Google Scholar]
- Sutcliffe GH, Plowright RC. The effects of food supply on adult size in the bumble bee Bombus terricola Kirby (Hymenoptera, Apidae) Can Entomol. 1988;120(12):1051–1058. [Google Scholar]
- Sutcliffe GH, Plowright RC. The effects of pollen availability on development time in the bumble bee Bombus terricola K (Hymenoptera, Apidae) Can J Zool. 1990;68(6):1120–1123. [Google Scholar]
- Tofilski A. Influence of age polyethism on longevity of workers in social insects. Behav Ecol Sociobiol. 2002;51:234–237. [Google Scholar]
- Toth AL, Robinson GE. Worker nutrition and division of labour in honeybees. Anim Behav. 2005;69(2):427–435. [Google Scholar]
- Toth AL, Kantarovich S, Meisel AF, Robinson GE. Nutritional status influences socially regulated foraging ontogeny in honey bees. J Exp Biol. 2005;208(24):4641–4649. doi: 10.1242/jeb.01956. [DOI] [PubMed] [Google Scholar]
- Tschinkel W. Seasonal life history and nest architecture of a winter-active ant, Prenolepis imparis. Insect Soc. 1987;34(3):143–164. [Google Scholar]
- Tschinkel WR. Sociometry and sociogenesis of colonies of the fire ant Solenopsis invicta during one annual cycle. Ecol Monogr. 1993;63:425–457. [Google Scholar]
- Tschinkel WR. Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius: worker characteristics in relation to colony size and season. Insect Soc. 1998;45(4):385–410. [Google Scholar]
- Visscher PK, Dukas R. Suvivorship of foraging honey bees. Insect Soc. 1997;44:1–5. [Google Scholar]
- Warton DI, Wright IJ, Falster DS, Westoby M. Bivariate line-fitting methods for allometry. Biol Rev. 2006;81:259–291. doi: 10.1017/S1464793106007007. [DOI] [PubMed] [Google Scholar]
- Wilson EO. The soldier of the ant Camponotus (Colobopsis) fraxinicola as a trophic caste. Psyche. 1974;81:182–188. [Google Scholar]
- Wilson EO. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta) I. The overall pattern in A. sexdens. Behav Ecol Sociobiol. 1980;7:143–156. [Google Scholar]
- Woyciechowski M, Kozlowski J. Division of labor by division of risk according to worker life expectancy in the honey bee (Apis mellifera L.) Apidologie. 1998;29:191–205. [Google Scholar]
- Yerushalmi S, Bodenhaimer S, Bloch G. Developmentally determined attenuation in circadian rhythms links chronobiology to social organization in bees. J Exp Biol. 2006;209(6):1044–1051. doi: 10.1242/jeb.02125. [DOI] [PubMed] [Google Scholar]