Abstract
The unfolded protein response (UPR) remediates endoplasmic reticulum (ER) stress. IRE1, a component of the UPR, senses misfolded protein and cleaves XBP1 mRNA, which is ligated to code for the prosurvival transcription factor. IRE1 also cleaves other mRNAs preceding their degradation, termed regulated IRE1-dependent mRNA decay (RIDD). It has been reported that RIDD may be involved in cell viability under stress and therefore may contribute to cancer cell viability. To investigate RIDD targets that may have functional relevance in cell survival, we identified conserved RIDD targets containing stringent IRE1 RNase target sequences. Using a systematic bioinformatics approach with quantitative-PCR (qPCR) validation, we show that only BLOC1S1 is consistently a RIDD target in all systems tested. Using cancer cell lines, we show that BLOC1S1 is specifically cleaved by IRE1 at guanine 444, but only under conditions of IRE1 hyperactivation. BLOC1S1 cleavage is temporally separate from XBP1 splicing, occurring after depletion of unspliced XBP1. Expression of an uncleavable BLOC1S1 mutant or inhibition of RIDD using an IRE1 RNase inhibitor did not affect cellular recovery from acute ER stress. These data demonstrate that although hyperactivated IRE1 specifically cleaves BLOC1S1, this cleavage event and RIDD as a whole are dispensable for cell viability under acute stress.
INTRODUCTION
The unfolded protein response (UPR) controls cell survival under proteotoxic stress. A key regulator of signaling in the UPR is serine/threonine protein kinase/endoribonuclease IRE1, a transmembrane receptor within the endoplasmic reticulum (ER) with kinase and RNase activities. Accumulation of misfolded protein within the ER leads to oligomerization and phosphorylation of IRE1 and activation of its RNase activity (1, 2). The primary target of IRE1 RNase activity in humans is the mRNA for the transcription factor X-box binding protein 1 (XBP1). Full-length XBP1 mRNA (XBP1u) is cleaved by IRE1 within two stem-loop structures, and a 26-bp intron is removed (3). After removal of the intron, the mRNA is ligated to produce the mature spliced transcript variant of XBP1 mRNA (XBP1s). This splicing event introduces a frameshift to code for the full-length, active XBP1 protein (3). XBP1 activates expression of genes involved in adaptation to an increased protein load (4). Hyperactivated IRE1 has also been shown to target multiple mRNAs for degradation upon stress in an XBP1-independent manner. This phenomenon, known as regulated IRE1-dependent mRNA decay (RIDD), was originally shown in Drosophila cells, where a subset of genes were downregulated under stress in an IRE1-dependent but XBP1-independent manner (5). RIDD also occurs in the yeast species Candida glabrata (6) and Schizosaccharomyces pombe (6, 7) and in mammalian cells (8, 9). In addition to the data for cell lines, data from mice show that RIDD substrates are downregulated following dosing with tunicamycin (Tm) or in the presence of hyperactivated IRE1 due to XBP1 deletion (10, 11). It has been proposed that the specificity of the IRE1 RNase domain is reduced during RIDD, leading to the cleavage of substrates at multiple sites, often with little structural homology to XBP1u stem-loops (9–18). The physiological presence of RIDD in mammals is unclear. So far, it has only been proven to occur under chemically induced stress (8–11, 14, 17) or in an XBP1-depleted genetic background, which leads to hyperactivation of IRE1 (10, 11, 15, 17, 19, 20). Both pro-cell survival and proapoptotic roles for RIDD have been described (17, 21, 22).
Many cancer types rely on cellular pathways controlling adaptation and cell survival under ER stress (23). A particular example is multiple myeloma, a plasma cell malignancy that progresses from an asymptomatic stage, smoldering myeloma, through the clinical disease myeloma to plasma cell leukemia (24). It is characterized by the secretion of high levels of paraprotein, which leads to a reliance of myeloma cells on the UPR (25). This reliance is highlighted by the sensitivity of myeloma cells to the proteasome inhibitor bortezomib, which deregulates the UPR as part of its mechanism of action (26), as well as other agents that target chaperones such as Hsp90 (27). Due to its sensitivity to the deregulation of protein handling, we hypothesized that myeloma may also rely on RIDD for the control of cell viability under stress and therefore represents a good model in which to study the possible role of RIDD in cancer cell survival.
In this study, we investigated the occurrence and role of RIDD in myeloma cell survival under ER stress. We searched bioinformatically for mRNA transcripts containing stem-loops with high structural similarity to XBP1u cleavage sites that could be specific targets of IRE1 and may therefore have functional relevance. BLOC1S1 was the only RIDD target consistently identified in published microarray data and is also a specific RIDD target in myeloma. BLOC1S1 is specifically cleaved at guanine 444, but not at two other similar sequences without hairpin structures. Surprisingly, inhibition of BLOC1S1 cleavage, or RIDD as a whole, did not affect cell viability under acute ER stress.
MATERIALS AND METHODS
qPCR.
Total RNA was extracted from cells using an RNeasy minikit (Qiagen) according to the manufacturer's protocol. For cells that were transduced with exogenous BLOC1S1, mRNA was treated with DNase to eliminate possible contamination with genomic DNA or plasmid. cDNA was synthesized from 200 ng (or 1 μg [see Fig. 2A]) RNA using qScript cDNA Supermix (Quanta Biosciences) according to the manufacturer's protocol. The cDNA was diluted 1:20 (or 1:10 [see Fig. 2A]) in water, and then 7.5 μl was used per reaction for quantitative PCR (qPCR) in a 25-μl reaction volume using Power SYBR green master mix (Life Technologies) and an Applied Biosystems 7500 fast real-time PCR system. The qPCR cycling conditions were 15 s at 95°C and 1 min at 60°C. Expression of BLOC1S1 (NM_001487.3), CHOP (also known as DDIT-3) (NM_001195053.1), or qPCR tag (qTag) for exogenous BLOC1S1 was measured relative to GAPDH (NM_002046.5) by quantative PCR using the following PCR primers at 300 nM each, or 900 nM for CHOP reverse primer: BLOC1S1 forward primer, CCCAATTTGCCAAGCAGACA; BLOC1S1 reverse primer, CATCCCCAATTTCCTTGAGTGC; CHOP forward primer, TGGAAATGAAGAGGAAGAATCAAAA; CHOP reverse primer, CAGCCAAGCCAGAGAAGCA; GAPDH forward primer, GAAGGTGAAGGTCGGAGTC; GAPDH reverse primer, GAAGATGGTGATGGGATTTC; qTag forward primer, GAGGCTGACAAGCCTTGAATAA; qTag reverse primer, GAGTCAGGCGATACGTGG. Melting point analysis was performed and confirmed single products. Threshold cycle (CT) values for no-reverse transcriptase (no-RT) controls were >35, and there were at least 7 qPCR cycles between no-RT controls and the lowest-abundance samples. Analysis of qPCR data was performed using Applied Biosystems 7500 fast software and the ΔΔCT quantitation method. Statistical analysis was performed using GraphPad Prism software.
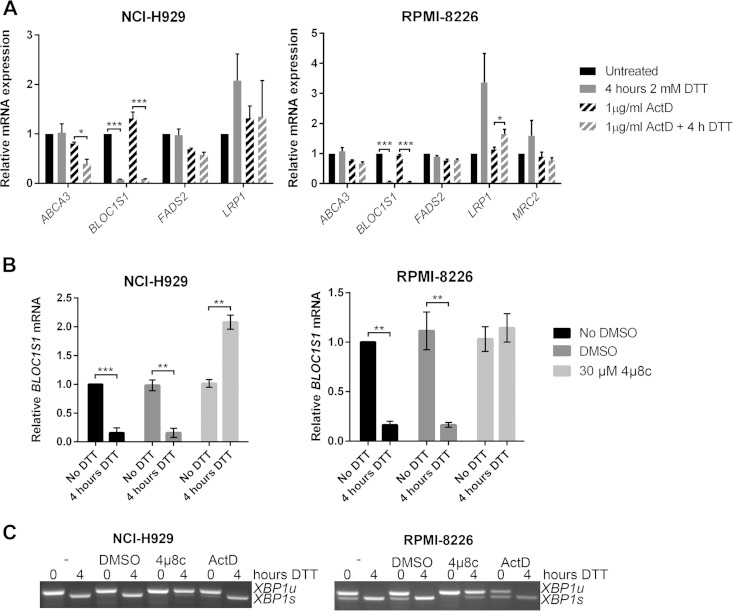
FIG 2.
BLOC1S1 is a RIDD target in myeloma cells. (A) TaqMan qPCR measuring expression of consistently identified RIDD targets containing a consensus IRE1 target sequence in stressed or unstressed myeloma cell lines with or without ActD. (B) Relative BLOC1S1 expression measured by SYBR green qPCR in the indicated myeloma cell lines treated with 2 mM DTT in the presence or absence of DMSO vehicle control or 4μ8c pretreatment. (C) Representative agarose gel electrophoresis of the RT-PCR product surrounding the XBP1 splice site in the samples used for panel B. The qPCR data are normalized to GAPDH mRNA and are expressed relative to untreated cells. (A and B) The data are means ± standard errors of the mean (SEM) from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; unpaired t test.
For TaqMan qPCR assays, 9 μl of a 1:20 (or 1:10 [see Fig. 2A]) cDNA dilution was used per reaction. The following TaqMan gene expression assays were used (Life Technologies) with TaqMan gene expression master mix (Life Technologies): GAPDH (glyceraldehyde-3-phosphate dehydrogenase), Hs02758991_g1; BLOC1S1, Hs00155241_m1; FADS2, Hs00188654_m1; MRC2, Hs00195862_m1; LRP1, Hs00233856_m1; ABCA3, Hs00975530_m1; and XBP1s, Hs03929085_g1; XBP1u, Hs02856596_m1.
Measurement of XBP1 splicing by agarose gel electrophoresis.
cDNA was synthesized from 200 ng RNA using qScript cDNA Supermix (Quanta Biosciences). The cDNA was diluted 1:20, and 5 μl was used per reaction for PCR in a 50-μl reaction volume using Platinum Taq polymerase (Life Technologies) according to the manufacturer's protocol. The primers used were XBP1 forward primer, CCTTGTAGTTGAGAACCAGG, and XBP1 reverse primer, GGGGCTTGGTATATATGTGG. The cycling conditions were 94°C for 2 min, followed by 32 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s; 6× loading buffer was added to the product, and electrophoresis was performed on 10 μl per sample in a 2% agarose gel containing GelRed nucleic acid stain (Biotium). The gels were imaged using a UVP Bio Doc-It system. Bands were quantified using ImageJ (NIH). Percent splicing was calculated as the amount of XBP1s as a percentage of total XBP1. Statistical analysis was performed using GraphPad Prism software.
Identification of mRNA transcripts containing consensus IRE1 target sequences.
Consensus IRE1 RNase target sequences were generated based on the conserved bases and characteristics of the XBP1u cleavage sites. This gave a list of 262,144 sequences conforming to the consensus. Human orthologues of Drosophila, mouse, and rat RIDD genes were found by querying the NCBI HomoloGene database. Human transcripts potentially regulated by RIDD were identified by aligning the IRE1 target sequences against the NCBI RefSeq database using the short-read aligner BWA (28), with no gaps and no mismatches allowed. Transcript names were extracted from the resulting SAM files using Perl scripts that also removed artifacts caused by BWA, allowing reverse-complement matches of the input sequences.
Cell culture and proteotoxic stress.
NCI-H929 and RPMI-8226 myeloma cells were obtained from the American Type Culture Collection (ATCC). HT-1080 cells were obtained from the European Collection of Cell Cultures (ECACC). All cell lines tested negative for mycoplasma by PCR. Cells were passaged in RPMI 1640 medium (NCI-H929 and RPMI-8226 cells) or minimal essential medium (HT-1080 cells) containing 10% fetal bovine serum and 100 mM GlutaMax. To induce proteotoxic stress, cells were seeded at a density of 5 × 105 cells/ml (NCI-H929 and RPMI-8226) or 4 × 104 cells per well in a 24-well format (HT-1080) and incubated for at least 6 h to equilibrate prior to treatment with 1 μg/ml or 10 μg/ml tunicamycin (Sigma) or 0.2 mM or 2 mM dithiothreitol (DTT) (Sigma). For washout experiments, cells were treated with 2 mM DTT for 90 or 60 min and then washed once and resuspended in fresh medium. For inhibition of IRE1 RNase activity, cells were treated with 30 μM 4μ8c (Merck Millipore; IRE1 inhibitor III, product 412512) dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO when added to cells was 0.1%. Actinomycin D (ActD) (Sigma) was used to inhibit transcription where indicated (see Fig. 2).
Cell lysis and Western blotting.
For Western blotting, cells were lysed in buffer containing 1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 10 mM Tris, pH 7.6, complete protease inhibitor (Roche), and PhosStop phosphatase inhibitor (Roche). Protein concentrations in supernatants were measured by bicinchoninic acid (BCA) assay (Thermo Scientific), and lysates were diluted to equal concentrations. Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). The membranes were blocked in 5% bovine serum albumin and then incubated with antibodies against phospho-IRE1 (serine 724; Abcam; ab124945), IRE1 (Cell Signaling Technology; 3294), XBP1 (BioLegend; 619502), or β-actin (Sigma), followed by appropriate horseradish peroxidase (HRP)-linked secondary antibodies. Blots were developed using ECL Plus reagent (GE Healthcare) and XAR film (Kodak).
In vitro RNA transcription and cleavage by IRE1.
His-tagged human G547-L977 IRE1 was expressed in Sf9 insect cells and purified as described previously (29).
RNA corresponding to cDNA sequences of full-length BLOC1S1 or a section of XBP1u from adenosine 266 uracil 602 [XBP1u(266–602)] was transcribed in vitro from pBluescript vectors using a Megascript T7 transcription kit (Life Technologies) according to the manufacturer's protocol.
BLOC1S1 (600 ng) or XBP1u(266-602) (375 ng) (approximately equal molar quantities) was incubated at 37°C for the times indicated in the figures and legends with G547-L977 IRE1 in buffer containing 40 mM HEPES, pH 7.4, 120 mM NaCl, 1.6 mM DTT, 0.8 mM EDTA, and 8% (wt/vol) glycerol. Sample loading buffer (1.5×; 95% formamide, 0.025% bromophenol blue, 0.025% xylene cyanol, 0.025% sodium dodecyl sulfate, 5 mM EDTA) was added to a 1× concentration to stop the reaction and heated to 95°C for 5 min before cooling on ice. Samples were separated by urea gel electrophoresis as described below.
A protocol for urea PAGE was adapted from that of Rio et al. (30). The gel contained 8 M urea (Sigma) and 6% acrylamide/bisacrylamide in TBE buffer (89 mM Tris, 89 mM boric acid, 2 mM EDTA). The gel was prerun for 30 to 60 min in 1× TBE at 180 V to remove excess persulfate, and the wells were rinsed with TBE before loading to remove excess urea. Samples were prepared in sample buffer (95% formamide, 0.025% bromophenol blue, 0.025% xylene cyanol, 0.025% sodium dodecyl sulfate, 5 mM EDTA) and heated to 95°C for 5 min before cooling on ice. The gel was run at 180 V until resolved.
The gel was then fixed in TBE plus 40% methanol and 10% acetic acid and then stained with SYBR gold (Life Technologies) in 1× TBE. RNA was imaged using a UV transilluminator BioDoc-It system (UVP, United Kingdom).
Cloning of BLOC1S1 and generation of a G444C mutant.
RNA was isolated from NCI-H929 cells using an RNeasy kit (Qiagen) and converted to cDNA using qScript (Quanta Biosciences). BLOC1S1 DNA was amplified using the following primers in a PCR using Platinum Taq (Life Technologies): forward, AGGAGATCTGCCGCCGCGATCGCACACAGCGGTCACGTGACATGG, and reverse, ACTCGAGAAACGGAGGCTTGTGTTTTATTCAAGG. The amplified product was purified on a ChargeSwitch DNA purification column (Life Technologies) and cloned into pCMV6-Entry (Origene) using AsiSI and XhoI restriction sites. The G444C mutation was introduced into BLOC1S1 in pCMV6 using a QuikChange Lightning kit (Agilent) according to the manufacturer's protocol with the following primers: sense, TACAAAGGGCAGCTCCAGTCTGCCCCTTC, and antisense, GAAGGGGCAGACTGGAGCTGCCCTTTGTA. G444C and wild-type (WT) BLOC1S1 were then transferred to the pRRLsin.EF1α.Neo vector (a gift from Eric So) by PCR and cloning using PmeI and SpeI restriction sites. A qPCR tag and poly(A) addition site were ligated to the 3′ end of the BLOC1S1 sequence in pRRLsin.EF1α.Neo using synthesized DNA oligomers (Sigma).
Lentiviral transduction of myeloma cell lines.
HEK293-T17 cells were transfected with the following plasmids in the indicated ratios using a calcium chloride transfection method: 16 μg pRRLsin.Neo plasmid, 20 μg pR8.74 (Addgene), and 5 μg pVSV-G (Addgene). The medium was changed after 24 h, and 1 μM sodium butyrate was added. Supernatants were collected 48 h and 72 h after transfection and combined. The supernatant was passed through a 0.45-μm PES filter (Millipore), and then virus was concentrated using Lenti-X concentrator (Clontech) according to the manufacturer's protocol. The purified virus was resuspended in 1 ml phosphate-buffered saline (PBS), and then 500 μl was added to 2.5 ml RPMI-8226 or NCI-H929 cells at a density of 105 cells/ml. The transduced cells were incubated for 72 h before the medium was replaced with fresh medium containing 1 mg/ml G418 (Invivogen) to select transformed cells. The cells were maintained in selection medium for 10 days before removing the G418. The cells were incubated without G418 for at least 72 h before performing downstream experiments.
WST-1 cell viability assays.
WST-1 assays were conducted in 96-well plates using WST-1 reagent (Roche) according to the manufacturer's protocol. For WST-1 assays using myeloma cell lines, cells were treated as described above and then diluted 1 in 5 after DTT washout to avoid saturation of the assay. For HT-1080 fibrosarcoma cells, cells were seeded at a density of 4 × 103 cells per well 24 h prior to treatment. After treatment, the DTT was washed off to prevent interference with the assay. Absorbance at 440 nm was measured using an Epoch plate reader (BioTek instruments). Absorbance at 630 nm was used as the reference wavelength and subtracted from the absorbance at 440 nm. Negative-control readings were subtracted from sample readings to remove background.
RESULTS
BLOC1S1 is the most consistently identified RIDD target.
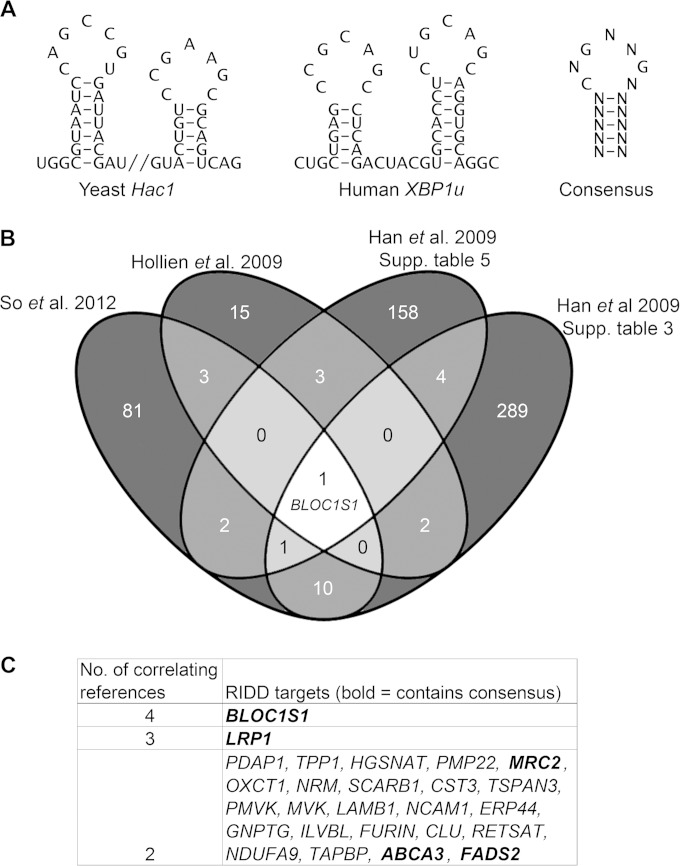
Several publications have identified potential RIDD targets in mammalian systems by microarray analysis (8, 9, 11). It is unclear which of these are directly cleaved by IRE1, as relatively few have been validated by other means. We hypothesized that RIDD targets containing stem-loops with the same secondary structure and conserved bases as human unspliced XBP1 (XBP1u) or yeast Hac1 stem-loops may be cleaved more readily than other RIDD targets and may thus be specific targets of IRE1. We therefore searched for transcripts containing sequences that may fold into these structures. Human XBP1u and yeast Hac1 stem-loops have conserved features that were used to define a consensus IRE1 target sequence. This is a 7-base sequence containing three conserved bases essential for cleavage (3) flanked by a hairpin of at least 5 bases (Fig. 1A). Every permutation of the consensus IRE1 target sequence was generated, and they were aligned against the NCBI RefSeq human transcript database, resulting in a list of 3,192 transcripts containing one or more consensus sequences, corresponding to 1,927 genes (see Table S1 in the supplemental material). Not all of these mRNAs would be cleaved by IRE1 in vivo, because mRNA cleavage by IRE1 may also require the mRNA to be targeted to the ER, as is the case for XBP1u (31). To identify targets most likely to be IRE1 substrates in cells, we cross-referenced the list of consensus-containing genes against published microarray data identifying transcripts degraded under ER stress in an IRE1-dependent manner in mammalian cells. Four sets of microarray data were included, and all the gene lists were converted to human orthologues before analysis (see Table S2 in the supplemental material) (8, 9, 11). A total of 56 potential RIDD targets with at least one XBP1-like stem-loop were identified (see Table S3 in the supplemental material).
FIG 1.
Identification of consistent RIDD substrates containing IRE1 consensus target sequences. (A) Sequences of stem-loops cleaved by IRE1 in Hac1 and XBP1u and the consensus structure. (B) Venn diagram showing numbers and overlap of RIDD targets identified in the indicated data sets (So et al. 2012, reference 10; Hollien et al. 2009, reference 8; Han et al. 2009, reference 9). (C) RIDD targets identified in more than one study. The boldface transcripts contain the consensus IRE1 target sequence.
In order to further increase stringency, we searched for the most consistently identified RIDD targets by comparing the lists of genes identified in each of the four microarrays. Twenty-six genes overlapped in at least two microarrays (Fig. 1B and C), five of which also contained a consensus IRE1 target sequence (Fig. 1C [boldface genes]; see Table S3 in the supplemental material). One of these, BLOC1S1, was the only gene that was correlated between all four studies (Fig. 1C).
We hypothesized that RIDD targets that are most consistently identified would be most likely to have functional relevance. In order to investigate the cleavage and functional relevance of these consistently identified RIDD targets, we used multiple myeloma as a model system, as it is particularly sensitive to treatments that perturb protein homeostasis. We first tested whether the five genes that were correlated in at least two studies and contained a consensus cleavage sequence were degraded under ER stress in myeloma cells. Myeloma cell lines, NCI-H929 and RPMI-8226, were treated with the reducing agent DTT for 4 h in the presence or absence of ActD to inhibit transcription, and transcript expression was measured by TaqMan qPCR. These conditions were selected because 2 mM DTT is a commonly used concentration of the stressor that robustly induces RIDD (5, 8, 32). Of the five genes tested, MRC2 gene expression was too low to be reliably measured (CT > 35) in NCI-H929 cells, and of the other four, only BLOC1S1 was reduced under ER stress in both cell lines (Fig. 2A). In NCI-H929 cells but not in RPMI-8226 cells, ABCA3 expression was significantly reduced when DTT was added in combination with ActD (Fig. 2A). This indicates that RIDD targets may be different among cell lines of the same disease origin and that BLOC1S1 is a universal RIDD target.
To confirm that BLOC1S1 degradation in myeloma cell lines is IRE1 dependent, NCI-H929 and RPMI-8226 myeloma cell lines were treated with DTT in the presence or absence of the IRE1 RNase inhibitor 4μ8c, and BLOC1S1 expression was measured by SYBR green qPCR. 4μ8c is a small-molecule inhibitor that covalently binds to IRE1 and blocks substrate access to the RNase active site (33). DTT-induced BLOC1S1 degradation was reversed by 4μ8c, showing that IRE1 RNase activity is required for cleavage. Interestingly, 4μ8c treatment alone did not increase BLOC1S1 expression (see Fig. 4B), suggesting that RIDD does not occur under basal conditions in these cell lines. We confirmed that 4μ8c, but not ActD, inhibited XBP1 splicing, as observed by agarose gel electrophoresis of the XBP1 PCR product surrounding the splice site (Fig. 2C).
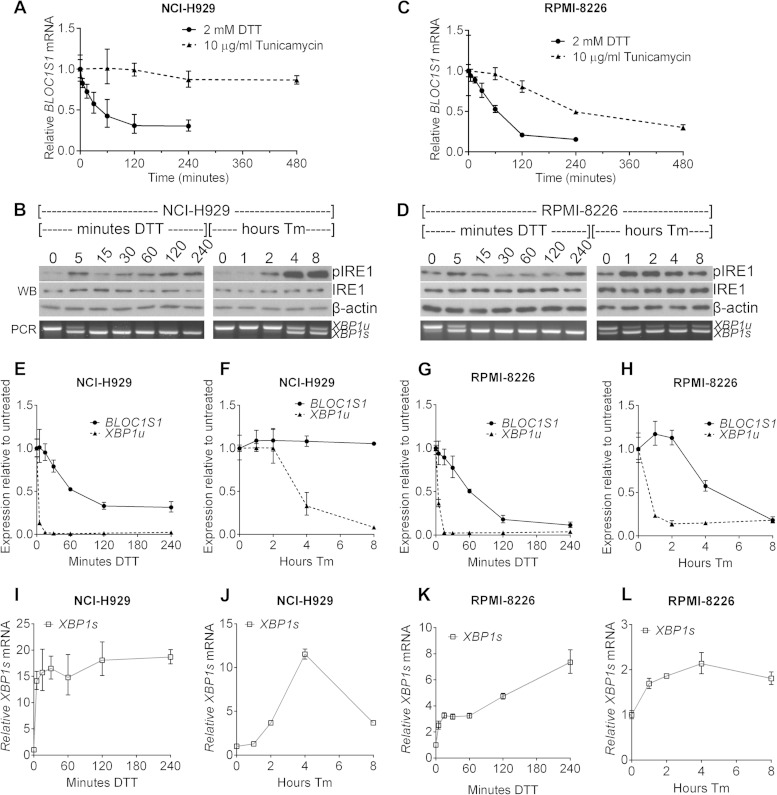
FIG 4.
BLOC1S1 degradation and XBP1 splicing are temporally separate. (A and C) Relative expression of BLOC1S1 in NCI-H929 or RPMI-8226 cells treated with the indicated stressors over time. BLOC1S1 mRNA was measured by SYBR green qPCR and normalized to the GAPDH control and is presented relative to time zero. (B and D) Western blots showing phospho-IRE1, IRE1, and β-actin in the cells treated for panel A. Also shown is agarose gel electrophoresis of the RT-PCR product surrounding the XBP1 splice site from the samples used in panel A. (E to L) TaqMan qPCR measurements of BLOC1S1, XBP1u, and XBP1s for cells treated as indicated. The data were normalized to the GAPDH control and are presented relative to untreated cells. The data are representative of two independent experiments, and error bars show SEM from three technical replicates.
Degradation of BLOC1S1 is temporally separate from XBP1 splicing.
Previous studies have observed RIDD predominantly under conditions of pharmacological ER stress or when XBP1u has been depleted, leading to IRE1 hyperactivation (8–11, 14, 15, 17, 19, 21). These stresses are likely to be stronger than those found under physiological circumstances. To test the conditions under which BLOC1S1 degradation occurs in myeloma, stressors that induce different amounts of ER stress were tested. The glycosylation inhibitor Tm is a relatively mild stressor compared to DTT and induces ER stress more slowly (34). NCI-H929 and RPMI-8226 cells were treated with two concentrations of Tm or DTT for 4 h, and expression of BLOC1S1 was measured by SYBR green qPCR. BLOC1S1 expression was strongly reduced in both cell lines when treated with 2 mM DTT and significantly reduced in RPMI-8226 cells but not in NCI-H929 cells after Tm treatment (Fig. 3A). Interestingly, in both cell lines, degradation of BLOC1S1 was observed only when most of the XBP1u substrate had been spliced (Fig. 3A to C).
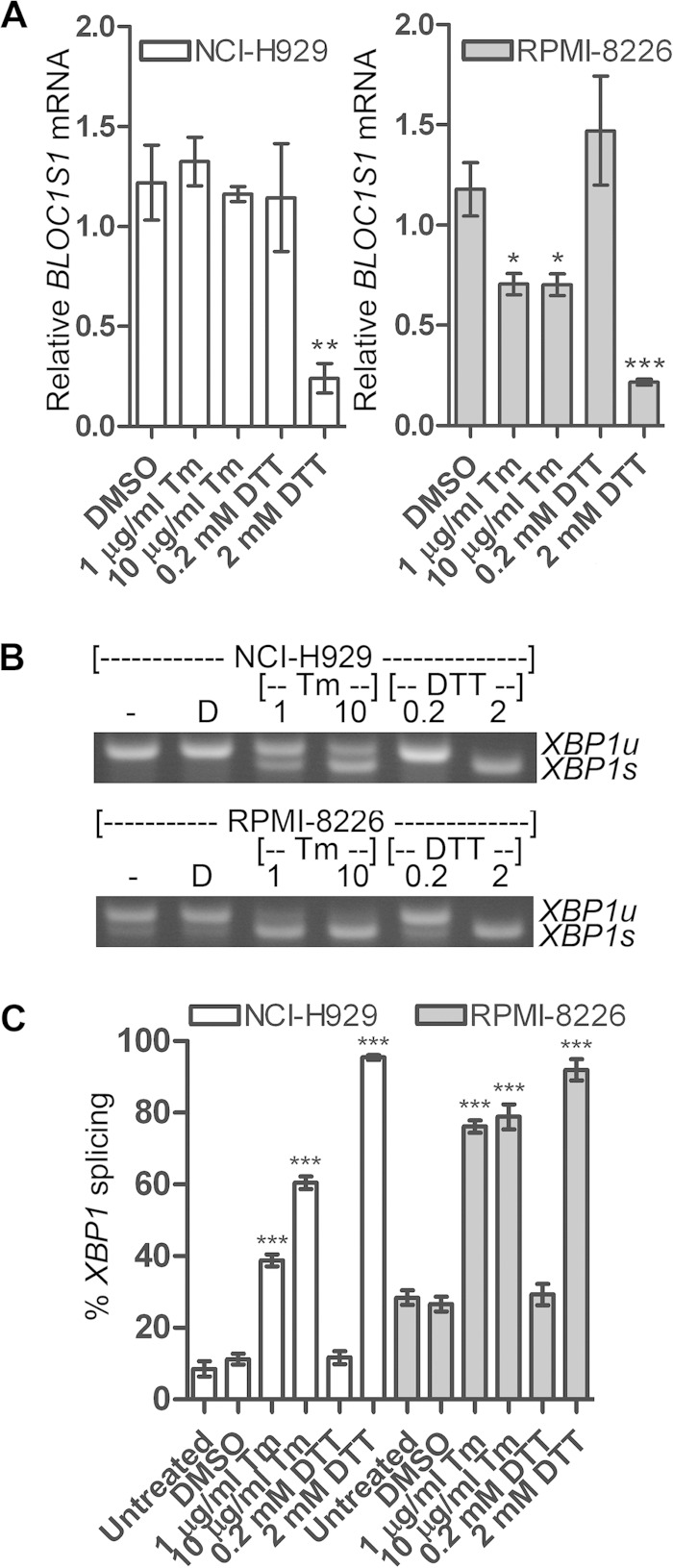
FIG 3.
Degradation of BLOC1S1 is stressor dependent. (A) Relative quantities of BLOC1S1 in NCI-H929 and RPMI-8226 cells treated with the indicated stressors for 4 h. The mRNA level was measured by SYBR green qPCR and normalized to GAPDH and is shown relative to untreated cells. The data are means ± SEM of three independent experiments. (B) Representative agarose gel electrophoresis of the RT-PCR product surrounding the XBP1 splice site in the samples used for panel A. (C) Quantification of percent XBP1 splicing from the gel images shown in panel B. The data are means ± SEM of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; unpaired t test compared to DMSO for tunicamycin and single-sample t test for DTT, where the vehicle was water.
These data suggest that RIDD of BLOC1S1 occurs only under extreme stress in myeloma cells when XBP1u has been depleted by splicing. Data from tunicamycin-treated NCI-H929 cells demonstrate that endogenous IRE1 can be activated to cleave XBP1u mRNA without activating RIDD.
To investigate the potential temporal difference between XBP1 splicing and RIDD of BLOC1S1, time courses of stressor treatment were carried out. NCI-H929 and RPMI-8226 cells were treated with 10 μg/ml Tm for 1 to 8 h or with 2 mM DTT for 5 to 240 min. DTT treatment was not extended because of toxicity. XBP1 splicing and BLOC1S1 mRNA levels were measured as before. It has previously been suggested that the IRE1 kinase domain determines whether the RNase domain conducts RIDD or XBP1 splicing (9). To detect kinase activation in myeloma, IRE1 kinase activity was assessed by Western blotting for autophosphorylation at serine 724.
As observed in Fig. 3, degradation of BLOC1S1 did not take place under tunicamycin treatment in NCI-H929 cells, despite the occurrence of XBP1 splicing (Fig. 4A and B). Under DTT treatment in both cell lines, and under tunicamycin treatment in RPMI-8226 cells, BLOC1S1 degradation took place, but at a slower pace than XBP1 splicing (Fig. 4A to D).
In both NCI-H929 and RPMI-8226 cells, tunicamycin-induced XBP1 splicing and IRE1 phosphorylation were temporally well correlated. Both occurred more rapidly in RPMI-8226 cells, by 1 h after tunicamycin addition, compared to NCI-H929 cells, where phosphorylation and XBP1 splicing increased after 4 h. Under DTT treatment, XBP1 splicing was more rapid than for tunicamycin treatment, with nearly complete splicing occurring by 15 min after treatment and remaining for the duration of the time course. This did not correlate well with IRE1 phosphorylation, which showed a biphasic response, increasing by 5 min after DTT treatment, subsiding by 30 min, and beginning to return by 4 h of DTT treatment in both cell lines (Fig. 4A to D). Phosphorylation of IRE1 was stronger under Tm treatment than with DTT (Fig. 4B and D). Weaker phosphorylation of IRE1 at serine 724 under DTT treatment than with tunicamycin treatment has previously been observed, but the reason for this is unclear (2). To ensure that the observed differences in BLOC1S1 degradation and XBP1 splicing were not attributable to differences in sensitivity of the respective assays, we measured BLOC1S1, XBP1u, and XBP1s by commercially available TaqMan qPCR assays. Direct comparison of BLOC1S1 and XBP1u using TaqMan assays showed the same temporal separation of RIDD and XBP1u cleavage that were observed by SYBR green and agarose gel electrophoresis (Fig. 4E to H). XBP1s increased with the same kinetics as XBP1u reduction, as expected (Fig. 4I to L). It should be noted that TaqMan qPCR detected a decrease in both XBP1u and XBP1s at 8 h of tunicamycin treatment in NCI-H929 cells that was not detected by the agarose gel electrophoresis (Fig. 4B, F, and J). However, the proportion of splicing was consistently correctly reported by the agarose gel electrophoresis assay, showing that it is an adequate method to measure IRE1 RNase activity (Fig. 4B and D to L). Measurements of BLOC1S1 by SYBR green and TaqMan qPCR were equivalent (Fig. 4A, C, and E to H).
Together, these results show that BLOC1S1 cleavage and degradation are temporally separate from XBP1 splicing and do not correlate with phosphorylation of IRE1 at S724. The data also show that IRE1 RNase activity can be assessed by agarose gel electrophoresis of the XBP1 PCR product and that measurements of BLOC1S1 by SYBR green and TaqMan qPCR are equivalent.
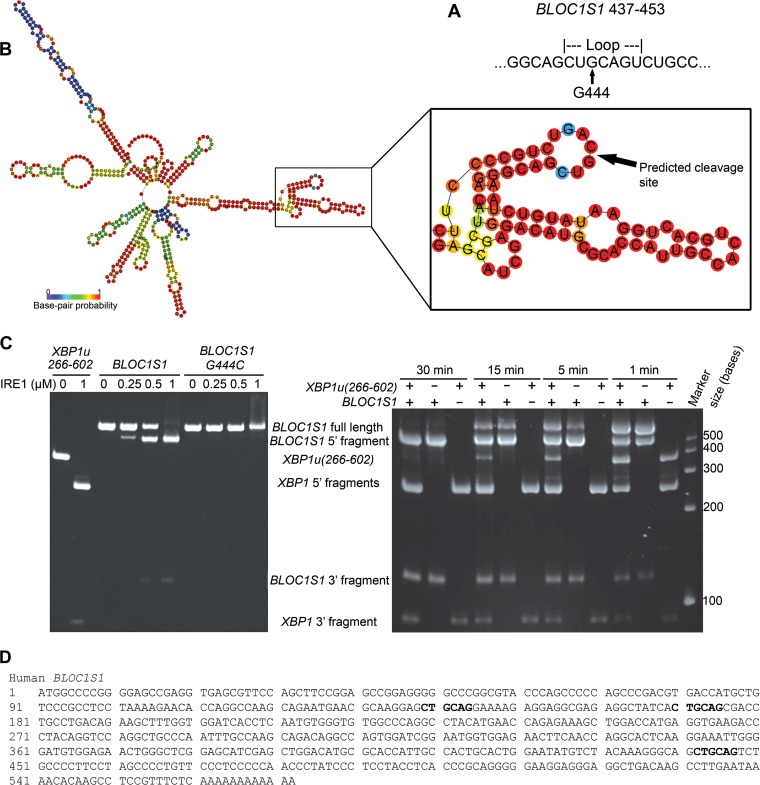
BLOC1S1 is specifically cleaved by IRE1 at guanine 444.
We hypothesized that BLOC1S1 would be cleaved at the XBP1-like stem-loop sequence we identified bioinformatically (see Table S3 in the supplemental material), and we would expect IRE1 to cleave this sequence at guanine 444 within the loop (Fig. 5A). To investigate this cleavage site, we first tested whether the secondary structure of BLOC1S1 would be predicted to form a stem-loop at G444 by folding full-length BLOC1S1 mRNA in silico using the RNAfold Web server (35). Indeed, the stem-loop surrounding G444 was predicted to fold with high probability (Fig. 5B). To investigate cleavage by IRE1 at this site, BLOC1S1 was cloned from myeloma cells, and a G444C mutation was introduced by site-directed mutagenesis. Guanine-to-cytosine mutation at the equivalent site in XBP1u is known to inhibit cleavage by IRE1 (3), and guanine 1085-to-adenosine mutation in μS mRNA also inhibits its cleavage by IRE1 (20). RNA for wild-type or G444C mutant BLOC1S1 was transcribed in vitro and then subjected to an in vitro cleavage assay using purified IRE1 cytosolic domain. XBP1u(266–602) was used as a control for IRE1 activity. As expected, XBP1u was efficiently cleaved by IRE1, yielding fragments corresponding to 5′ and 3′ fragments (Fig. 5C, left). Wild-type BLOC1S1 was also cleaved by IRE1, yielding 5′ and 3′ fragments corresponding to cleavage at guanine 444. G444C BLOC1S1 was not cleaved by IRE1 but could bind to IRE1, as a high IRE1 concentration led to smearing on the gel (Fig. 5C, left). To investigate the selectivity of IRE1 for substrates in vitro, we carried out in vitro cleavage assays of XBP1u(266–602) and BLOC1S1 in combination, or on their own, over a time course. The resulting RNA fragments were observed by urea gel electrophoresis. On its own, XBP1u(266–602) was fully cleaved by IRE1 by 5 min of incubation, and BLOC1S1 was fully cleaved by 30 min (Fig. 5C, right). Combination of XBP1(266–602) and BLOC1S1 in the same reaction showed that they compete for cleavage by IRE1, observed as the appearance of uncleaved XBP1u(266–602) at 5 and 15 min of incubation (Fig. 5C, right). A band corresponding to the 5′ fragment of singly cleaved XBP1u(266–602) was also observed under these conditions (Fig. 5C, right). These results suggest that purified human IRE1 cleaves purified XBP1u more rapidly than purified BLOC1S1 in vitro, which matches our in vivo data (Fig. 4). The data also show that when incubated in combination, XBP1u and RIDD substrates compete for cleavage by IRE1 in vitro.
FIG 5.
BLOC1S1 is cleaved by IRE1 at guanine 444 in vitro. (A) Sequence of BLOC1S1 surrounding guanine 444 that was identified as a consensus IRE1 target sequence bioinformatically. (B) Predicted secondary structure of full-length BLOC1S1 mRNA folded in silico using the RNAfold Web server. The stem-loop surrounding G444 is magnified and annotated in panel A. The colors represent the probability of base pairing. (C) (Left) Urea gel electrophoresis of XBP1u(266–602), WT BLOC1S1, and G444C BLOC1S1 RNA after in vitro cleavage by the indicated concentrations of IRE1 for 15 min. (Right) Urea gel electrophoresis of the indicated RNAs after in vitro cleavage by IRE1 (0.5 μM) for the indicated times. The identities of RNA fragments are shown between the two gels; size markers are shown on the right. (D) Sequence of human BLOC1S1 from the start codon to the poly(A) tail. All occurrences of the sequence identical to the first 6 bases of the loop sequence around G444 are indicated in boldface.
Previous studies have suggested that IRE1 is less stringent when carrying out RIDD, in some cases cleaving RNA without a stem-loop present (17, 21). Closer inspection of the BLOC1S1 sequence revealed that the first 6 bases of the loop sequence surrounding G444 occur twice more in BLOC1S1, surrounding G141 and G172 (Fig. 5D). Neither of these additional sites was cleaved by IRE1 in vitro (Fig. 5C), suggesting that cleavage of BLOC1S1 is highly sequence specific.
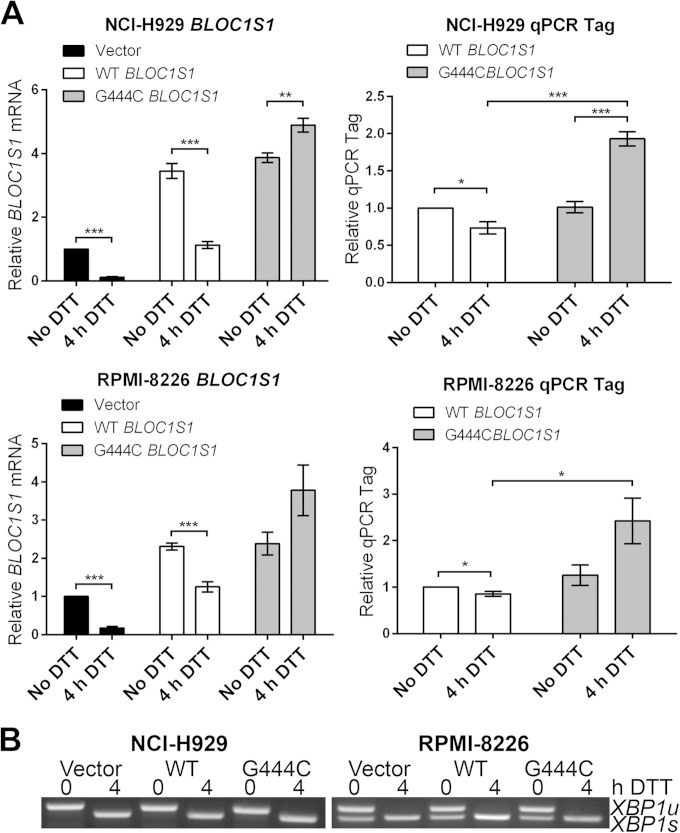
To investigate whether BLOC1S1 is cleaved at guanine 444 in vivo, wild-type or G444C mutant BLOC1S1 was expressed in myeloma cell lines, and degradation was measured by qPCR. G444 is the third base of its associated codon, and G444C mutation does not affect the amino acid sequence of BLOC1S1. WT and G444C BLOC1S1 were cloned into the pRRLsin.Neo lentiviral expression vector. A qTag with low homology to mRNA transcripts was inserted at the 3′ end, followed by the genomic polyadenylation signal from the BLOC1S1 gene, to allow specific detection of exogenous BLOC1S1 by qPCR (see Fig. S4 in the supplemental material). WT or G444C BLOC1S1-qTag was stably expressed in NCI-H929 and RPMI-8226 myeloma cells by lentiviral transduction. The cells were then treated with DTT for 4 h to induce RIDD, and BLOC1S1 or qTag was measured by SYBR green qPCR. Lentiviral transduction of myeloma cells with BLOC1S1 constructs was successful, as observed by an increase in total BLOC1S1 transcript to at least double that for untransduced cells and a measurable expression of qTag (Fig. 6A). DTT treatment induced degradation of WT BLOC1S1 but not G444C BLOC1S1 in myeloma cells, as observed by a decrease in total BLOC1S1 and qTag expression in WT-transduced cells and an increase in BLOC1S1 and qTag expression relative to GAPDH in G444C-transduced cells (Fig. 6A). XBP1 splicing was not affected by either WT or G444C BLOC1S1 expression (Fig. 6B). Interestingly, qTag.BLOC1S1 did not appear to be degraded as effectively as endogenous BLOC1S1, indicating that a further regulatory region may be required for RIDD to occur. However, the relative increase in qTag.BLOC1S1 under stress, as observed in G444C transfected cells, partially obscured the degradation of exogenous BLOC1S1 (Fig. 6A, right, compare WT BLOC1S1 and 4 h DTT with G444C BLOC1S1 and 4 h DTT). These data show that BLOC1S1 is specifically cleaved by IRE1 at guanine 444 in cells.
FIG 6.
G444C mutation inhibits cleavage of BLOC1S1 by IRE1 in vivo. (A) SYBR green qPCR quantification of relative expression of total BLOC1S1 and qTag (exogenous BLOC1S1) mRNA in myeloma cell lines transduced with the indicated virus and treated with 2 mM DTT for 4 h. Measurements were normalized to GAPDH and are shown relative to untreated control cells. The data show means ± SEM from five independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; unpaired t test. (B) Representative agarose gel electrophoresis of RT-PCR products surrounding the XBP1 splice site from the samples used in panel A.
BLOC1S1 cleavage and RIDD as a whole do not influence myeloma cell recovery from acute ER stress.
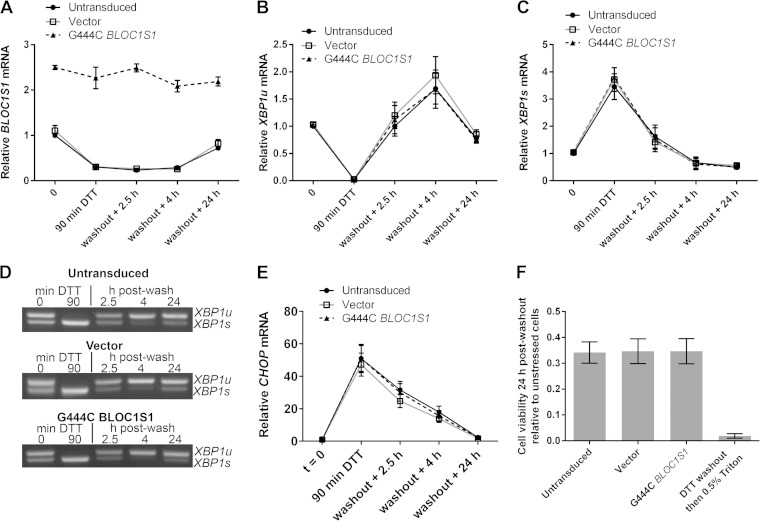
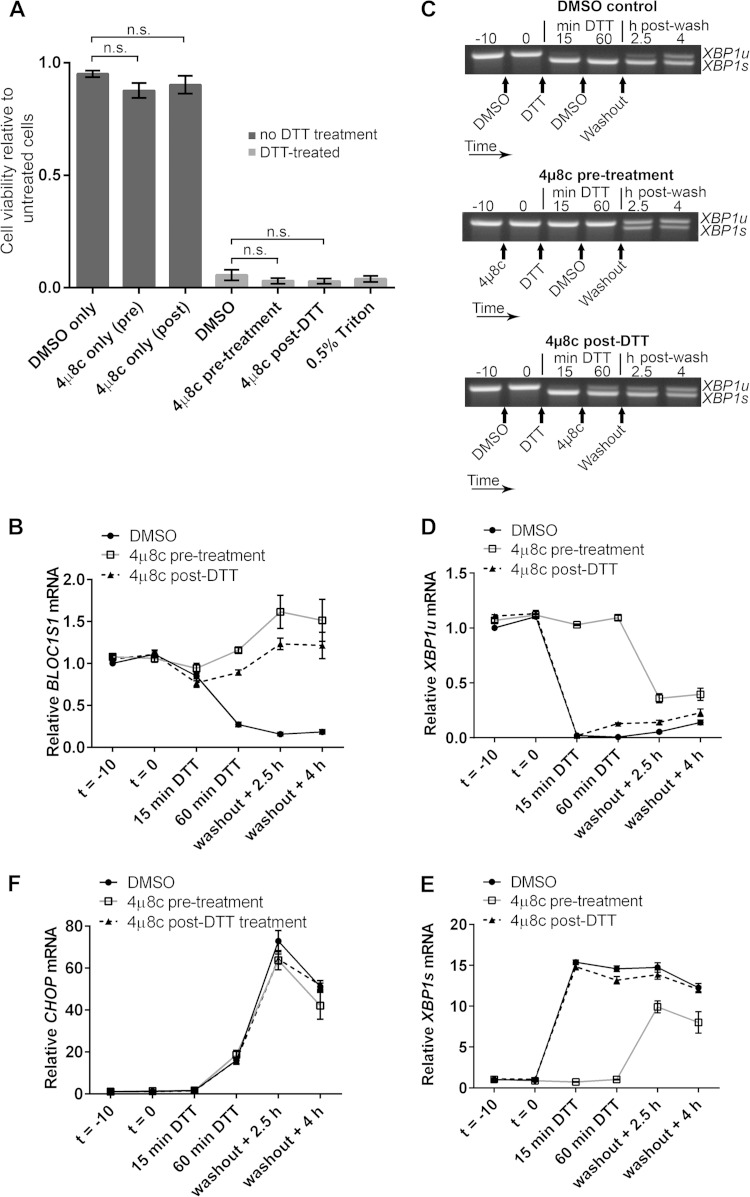
RIDD has been reported to have roles in cell survival and apoptosis under ER stress (5, 9–11, 14, 15, 17, 19–21). As BLOC1S1 is a specific and consistent RIDD target, we tested whether the degradation of BLOC1S1 has functional relevance to cell survival under stress. Myeloma cell lines lose viability rapidly under DTT treatment, so DTT washout experiments were performed. RPMI-8226 cells were chosen because they carry out RIDD more readily and recover viability more robustly after acute DTT treatment than NCI-H929 cells (Fig. 3A and data not shown). RPMI-8226 cells expressing G444C BLOC1S1 were treated with DTT for 90 min and then washed and allowed to recover for 24 h before assessment of cell viability by WST-1 assay. Vector-transduced cells and untransduced cells were used as controls. RIDD of BLOC1S1 was confirmed by SYBR green qPCR for total BLOC1S1, and XBP1 splicing was assessed by agarose gel electrophoresis of the PCR product surrounding the XBP1u splice site and by TaqMan qPCR of XBP1s and XBP1u. In untransduced and vector-transduced cells, 90 min of DTT treatment led to degradation of BLOC1S1, as expected. G444C BLOC1S1 was not degraded (Fig. 7A). XBP1 was fully spliced by 90 min of DTT treatment, observed as a reduction in XBP1u and increase in XBP1s (Fig. 7B to D). After DTT washout, XBP1 splicing recovered to less than basal levels by 4 h (Fig. 7B to D) and BLOC1S1 expression recovered by 24 h (Fig. 7A). XBP1 splicing and recovery were not affected by expression of G444C BLOC1S1 (Fig. 7B to D). To further confirm that recovery of protein homeostasis was not affected by G444C BLOC1S1 expression, induction and recovery of the transcript for C/EBP-homologous protein (CHOP) were measured by SYBR green qPCR. CHOP mRNA expression is induced by the UPR downstream of PRKR-like endoplasmic reticulum kinase (PERK) activation (1). The relative instability of CHOP mRNA means that it should be rapidly reduced after PERK has been switched off and so can be used as an indicator of the recovery of protein homoeostasis (36, 37). CHOP was induced by DTT and recovered in a similar time frame in each cell type, indicating that PERK was activated and inactivated with similar kinetics (Fig. 7E). Recovery of cell viability under these conditions was measured by WST-1 assay. To take potential differences in seeding density and growth rate into account, readings were normalized to matched untreated control cells for each transduced or untransduced sample. The WST-1 assay was not affected by residual DTT, as Triton X-100 treatment led to a reduction in signal close to baseline (Fig. 7F). Cell viability after acute ER stress was not affected by G444C BLOC1S1 expression, as it was reduced to around 35% by DTT treatment in each case (Fig. 7F).
FIG 7.
BLOC1S1 degradation is dispensable for recovery from acute stress. (A) SYBR green qPCR quantification of relative expression of BLOC1S1 in RPMI-8226 cells transduced as indicated and then treated with 2 mM DTT for 90 min, followed by washout and recovery for the indicated times. (B and C) TaqMan qPCR quantification of XBP1u (B) and XBP1s (C) in the samples used for panel A. (D) Representative agarose gel electrophoresis of the RT-PCR product surrounding the XBP1 splice site in the samples used for panel A. (E) SYBR green qPCR quantification of relative expression of CHOP in the samples used for panel A. (F) Relative cell viability measured by WST-1 assay of cells transduced as indicated and then treated with 2 mM DTT for 90 min, followed by washout and recovery for 24 h. Triton X-100 (0.5%) treatment after DTT washout in untransduced cells is shown as a control for maximal cell death. The measurements are background-subtracted absorbances relative to the absorbance of unstressed cells for each transduced sample. All qPCR measurements were normalized to GAPDH and are presented relative to 0 min of DTT treatment in DMSO-treated cells. All the graphs show means ± SEM of three (A to C and E) or four (F) independent experiments.
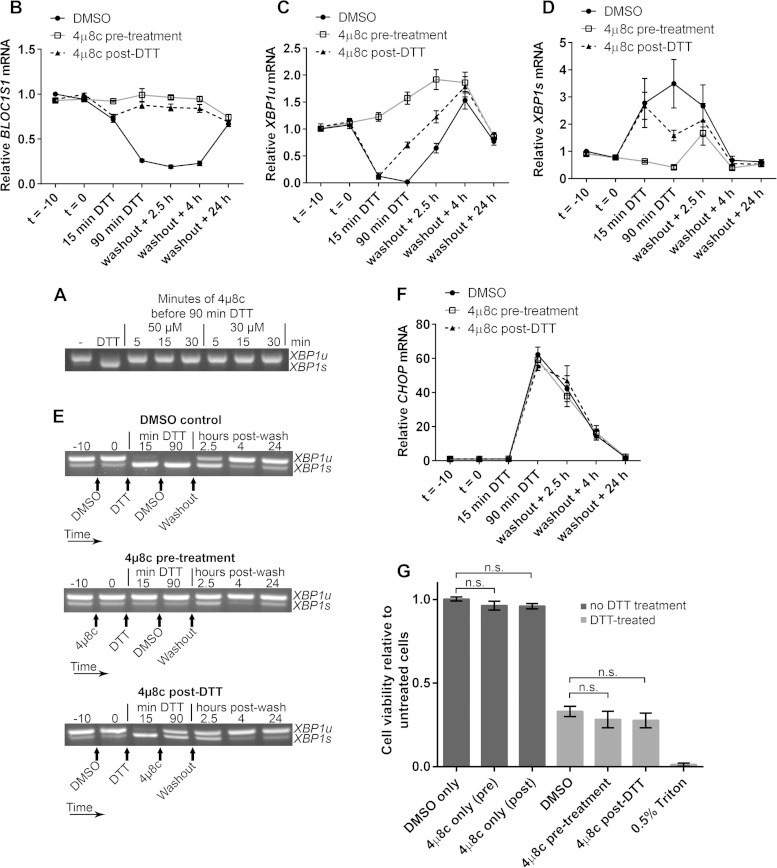
As RIDD is known to affect many transcripts, it could be that collective mRNA degradation, rather than degradation of BLOC1S1 alone, would affect viability under acute ER stress. To test this, we aimed to inhibit RIDD without inhibiting XBP1 splicing by taking advantage of the observation that RIDD is temporally separate from XBP1 splicing under DTT treatment. We first confirmed that the IRE1 RNase inhibitor 4μ8c is fast acting in cells. Pretreatment of RPMI-8226 cells with 30 μM 4μ8c for only 5 min was enough to completely inhibit splicing induced by DTT (Fig. 8A). Then, to separate XBP1 splicing from RIDD, cells were either pretreated with 4μ8c for 10 min before addition of DTT (pretreatment) or posttreated with 4μ8c 15 min after the start of DTT treatment (post-DTT). DTT and 4μ8c were washed off after 90 min, and the cells were allowed to recover. RNA samples were prepared throughout the time course of treatment, and cell viability was measured by WST-1 assay 24 h after DTT washout. BLOC1S1 expression and CHOP expression were measured by SYBR green qPCR. XBP1 splicing was measured by agarose gel electrophoresis of the PCR product surrounding the splice site and by TaqMan qPCR of XBP1u and XBP1s. Pretreatment of cells with 4μ8c inhibited both RIDD (as measured by BLOC1S1 expression) (Fig. 8B) and XBP1 splicing (Fig. 8C to E). There was a small increase in XBP1s after washout in the 4μ8c-pretreated cells (Fig. 8D); however, this was probably due to the matched increase in XBP1u (Fig. 8C), with the proportion of XBP1s compared to XBP1u returning close to basal level (Fig. 8E). Posttreatment with 4μ8c inhibited RIDD (Fig. 8B) but allowed XBP1 splicing to take place (Fig. 8C to E). Neither pre- nor posttreatment with 4μ8c significantly affected the recovery of CHOP to basal levels after DTT washout (Fig. 8F) or cell viability after DTT washout (Fig. 8G), indicating that RIDD is dispensable for both processes. We did observe a small and insignificant drop in cell viability after 4μ8c treatment, but this was also observed without DTT treatment and was therefore not related to inhibition of RIDD (Fig. 8G).
FIG 8.
Inhibition of RIDD does not affect RPMI-8226 cell viability under acute ER stress. (A) Agarose gel electrophoresis of the RT-PCR product surrounding the XBP1 splice site in samples from RPMI-8226 cells pretreated for the indicated times with the indicated concentration of 4μ8c and then treated with 2 mM DTT for 90 min. −, untreated cells; DTT, DTT alone. (B) SYBR green qPCR showing relative expression of BLOC1S1 mRNA in RPMI-8226 cells treated with 30 μM 4μ8c at −10 min (t = −10) (pretreatment) or at 15 min of DTT (post-DTT) and treated with DTT at time zero (t = 0) for 90 min before washout and recovery for the indicated times. DMSO, vehicle control-treated cells. (C and D) TaqMan qPCR quantification of XBP1u (C) and XBP1s (D) in the samples used for panel B. (E) Representative agarose gel electrophoresis of the RT-PCR product surrounding the XBP1 splice site in the samples from panel B. The arrows indicate the times of addition of the indicated treatments or washout. (F) SYBR green qPCR of relative CHOP expression in the samples used for panel B. (G) WST-1 cell viability assays of RPMI-8226 cells treated as for panel B or treated with 4μ8c or DMSO without DTT treatment. The data are relative to untreated cells; 0.5% Triton X-100 after DTT washout is shown as a control for maximal cell death. All the graphs show means ± SEM from four (B, F, and G) or three (C and D) independent experiments. n.s., P > 0.05 (unpaired t test). All qPCR measurements were normalized to GAPDH and are presented relative to time −10 for DMSO-treated cell samples.
To ensure that the lack of effect of inhibiting RIDD on the cell viability response was not cell type specific, we carried out DTT washout experiments using NCI-H929 cells. DTT treatment for 60 min was used because NCI-H929 cells were particularly sensitive to the stressor. Cells were treated with 30 μM 4μ8c (or DMSO vehicle) either 10 min before addition of 2 mM DTT (pretreatment) or 15 min after (post-DTT). Unstressed cells with 4μ8c treatment alone were included as a control. After DTT had been present for 60 min, 4μ8c and DTT were washed off, and the cells were resuspended in fresh medium to recover for 24 h. Cell viability was then measured by WST-1 assay. NCI-H929 cells were unable to recover from 60-min DTT treatment regardless of 4μ8c treatment, and 4μ8c treatment alone had no significant effect on cell viability (Fig. 9A). To observe RIDD and XBP1 splicing in these cells, BLOC1S1 expression was measured by SYBR green qPCR and XBP1 splicing was measured by agarose gel electrophoresis of the PCR product surrounding the splice site and TaqMan qPCR of XBP1s and XBP1u. In DMSO vehicle-treated cells, DTT induced RIDD of BLOC1S1 (Fig. 9B) and XBP1 splicing (Fig. 9C to E), as expected. XBP1 splicing did not fully recover by 4 h after washout (Fig. 9C to E), and a 24-h time point was not included because NCI-H929 cells did not recover viability (Fig. 9A). Treatment with 4μ8c 15 min after addition of DTT (4μ8c post-DTT) inhibited RIDD (Fig. 9B) and allowed XBP1 splicing to take place (Fig. 9C to E). 4μ8c pretreatment also inhibited RIDD (Fig. 9B) and inhibited XBP1 splicing before washout (Fig. 9C to E). However, XBP1 was spliced in 4μ8c-pretreated cells after washout (Fig. 9C to E), possibly due to the presence of misfolded proteins that built up during DTT treatment. Interestingly, XBP1 splicing at 2.5 h and 4 h postwashout in 4μ8c-pretreated cells was not accompanied by RIDD of BLOC1S1. Instead BLOC1S1 expression was slightly increased (Fig. 9B), further highlighting the mechanistic separation of XBP1 splicing and RIDD.
FIG 9.
Inhibition of RIDD does not affect NCI-H929 cell viability under acute ER stress. (A) WST-1 cell viability assays of NCI-H929 cells treated as indicated with 2 mM DTT for 60 min (from time zero) and either pretreated (at time −10 min) or posttreated (at time 15 min) with 30 μM 4μ8c and then washed and allowed to recover for 24 h. Cells treated with 0.5% Triton X-100 were included as a dead-cell control. (B) SYBR green qPCR quantification of BLOC1S1 in the DTT-treated samples from panel A. (C) Representative agarose gel electrophoresis of the RT-PCR product surrounding the XBP1 splice site in the DTT-treated samples from panel A. The timing of treatments is indicated below the gel images. (D and E) TaqMan qPCR quantification of XBP1u (D) or XBP1s (E) in the DTT-treated samples from panel A. (F) SYBR green qPCR of relative CHOP expression in the DTT-treated samples from panel A. All qPCR data are normalized to GAPDH and are presented relative to the DMSO control at time −10 min of DTT. All the graphs show means ± SEM from three independent experiments. n.s., P > 0.05 (unpaired t test).
To assess the recovery of protein homeostasis after acute DTT treatment, CHOP expression was measured by SYBR green qPCR as before as a readout for PERK activity. Regardless of 4μ8c treatment, CHOP expression increased by 60 min of DTT treatment and continued to increase after washout, dropping slightly between 2.5 h and 4 h after washout (Fig. 9F). This shows that the cells continued to increase the proapoptotic CHOP signal that contributes to cell death regardless of the presence or absence of RIDD. These results from NCI-H929 cells indicate that RIDD does not contribute to cell death under acute ER stress.
RIDD is dispensable for cell viability under ER stress in nonmyeloma cells.
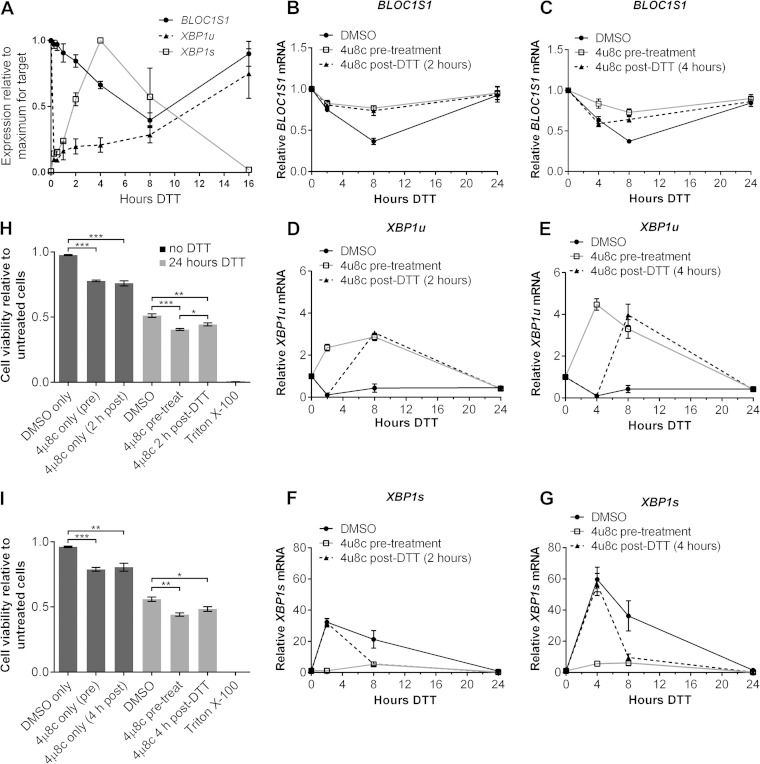
In order to test whether RIDD influences cell viability under a longer period of stress, or in nonmyeloma cells, we performed differential 4μ8c treatments in HT-1080 fibrosarcoma cells. In order to select suitable times for DTT and 4μ8c treatment, we analyzed BLOC1S1 expression and XBP1 splicing by qPCR over a time course of DTT treatment (2 mM DTT). The data were normalized to the maximum expression for each target so that each could be represented on the same graph. As expected, XBP1u was rapidly reduced in HT-1080 cells by 15 min of DTT treatment (Fig. 10A). The decrease in BLOC1S1 expression was slower, reaching a minimum at 8 h, and it then returned to basal level by 16 h (Fig. 10A). XBP1s expression increased rapidly and then continued to increase, peaking at 4 h after DTT addition (Fig. 10A). This continued increase in XBP1s was probably due to de novo transcription and subsequent splicing of XBP1u mRNA.
FIG 10.
RIDD is dispensable for cell viability under DTT treatment in HT-1080 fibrosarcoma cells. (A) TaqMan qPCR measuring BLOC1S1, XBP1u, or XBP1s in HT-1080 cells treated with 2 mM DTT for the indicated times. The data are normalized to GAPDH and are presented relative to the maximum for each transcript. (B to G) SYBR green (BLOC1S1) or TaqMan (XBP1u and XBP1s) qPCR measurements of the indicated transcripts over a time course of DTT (2 mM) treatment in HT-1080 cells that were treated with 4μ8c (30 μM) 10 min before (pretreatment) or 2 h or 4 h after (post-DTT) addition of DTT. The data are normalized to GAPDH and are presented relative to untreated cells. (H and I) WST-1 measurement of the viability of HT-1080 cells treated with 2 mM DTT or 30 μM 4μ8c, as indicated. Triton X-100 treatment is shown as a dead-cell control. The data show means ± SEM from three (A) or four (B to I) independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; unpaired t test.
In order to experimentally separate XBP1 splicing from RIDD, 4μ8c (30 μM) was added either before or after the start of DTT treatment. Because the continued increase in XBP1s expression partially overlapped RIDD, two time points for 4μ8c treatment after DTT addition were chosen (2 h or 4 h post-DTT addition). Treatment was then continued until DTT had been present for 24 h. DMSO was used as a vehicle control for 4μ8c treatments.
To confirm the separation of XBP1 splicing and RIDD, BLOC1S1, XBP1u, and XBP1s were measured over the time course by qPCR. Pretreatment with 4μ8c inhibited RIDD, measured as BLOC1S1 expression (Fig. 10B and C), and inhibited splicing of XBP1u to XBP1s (Fig. 10D to G). Pretreatment with 4μ8c also caused an increase in XBP1u upon ER stress, confirming that new XBP1 transcription had taken place (Fig. 10D and E). Treatment with 4μ8c 2 h post-DTT addition inhibited RIDD to the same extent as pretreatment (Fig. 10B) but allowed XBP1 splicing until 2 h, which decreased after 4μ8c treatment (Fig. 10D and F). Treatment with 4μ8c 4 h post-DTT addition allowed maximal XBP1s expression at 4 h (Fig. 10G) but also allowed some RIDD to take place until 4 h, after which it was inhibited (Fig. 10C).
Cell viability was measured by WST-1 assay after 24 h of DTT treatment with or without differential 4μ8c treatment. DTT was washed off prior to addition of the WST-1 reagent to prevent interference with the assay. Triton X-100 was used as a dead-cell control and abolished viability, as expected (Fig. 10H and I). DTT treatment for 24 h caused a reduction in cell viability close to 50%, and pre- or posttreatment with 4μ8c caused an additional significant decrease in viability (Fig. 10H and I). This decrease is likely to be due to the effect of 4μ8c treatment alone rather than inhibition of RIDD, as 4μ8c treatment in the absence of stress also significantly reduced cell viability relative to untreated cells and DMSO vehicle control (Fig. 10H and I). There was a slight difference in viability between pretreated (inhibition of both XBP1 splicing and RIDD) and posttreated (inhibition of only RIDD) cells after DTT treatment (Fig. 10H and I), which was significant in the case of 2-h posttreatment (Fig. 10H). This may be due to a protective effect of XBP1s that we would expect to be present in posttreated cells. These data suggest that the effect of inhibiting RIDD or XBP1 splicing on the viability of HT-1080 cells under DTT treatment is negligible, indicating that other factors are primarily responsible for cell viability and apoptosis under these conditions.
DISCUSSION
Hyperactivated IRE1 cleaves a wide array of mRNA targets, leading to their degradation via RIDD (8, 9, 11). Previous studies have suggested roles for some of these targets, including pro-cell survival and proapoptotic roles (5, 9–11, 14, 15, 17, 19–21). However, the physiological role of RIDD is not clear, as these studies used conditions of chemically induced stress, expression of IRE1 mutants, or genetic deletion of XBP1, which lead to IRE1 hyperactivation. Also, many reported RIDD targets have cleavage sites with poor structural similarity to the XBP1 stem-loops, suggesting that IRE1 is less stringent when hyperactivated (9–18, 21). We hypothesized that RIDD targets with stem-loop structures that were highly similar to XBP1 stem-loops may be more readily cleaved by IRE1 in a stringent manner and may therefore have a functional role under physiological circumstances. We found that BLOC1S1 was the only RIDD target identified in all systems tested and also contains a stem-loop structure with high similarity to XBP1. One caveat to this finding is that we analyzed only microarray data, which would be prone to false negatives. For this reason, we cannot rule out the existence of other consistent RIDD targets that may affect cell viability. However, we measured the expression of five candidate RIDD targets containing XBP1-like stem-loops that were identified in more than one microarray and found that only BLOC1S1 was degraded in both cell lines. This shows that specific substrates are selectively cleaved by IRE1, even though the consensus sequence is present in many other mRNAs that are not cleaved. In vitro and in cells, BLOC1S1 was specifically cleaved by IRE1 only at guanine 444 and not at two other sites with sequence similarity, showing that this cleavage event is highly selective, even when IRE1 is hyperactivated. This appears contrary to reports that IRE1 can cleave certain transcripts at multiple sites with less stringency (9, 13, 17). The differences may be due to the length, epitope tag, or phosphorylation status of the IRE1 used for in vitro cleavage or to the reaction conditions. In our assay, we used more stringent cleavage conditions than those used in previously published work. We used His-tagged IRE1 cytosolic domain purified from insect cells without any additional phosphatase treatment or in vitro autophosphorylation reaction. This may have different RNase selectivity than IRE1 that has been allowed to autophosphorylate in vitro (9) or glutathione S-transferase (GST)-tagged IRE1 (13), because GST is predominantly dimeric (38). Our in vitro cleavage reactions were carried out at 37°C for 15 min, which should be more stringent than 37°C for 6 h, as used by Upton et al. (17). Interestingly, these previous studies do suggest that the IRE1 RNase can be less stringent in vitro under some circumstances. However, we found that the G444C point mutation blocked degradation of BLOC1S1 in cells, even under extreme reducing conditions, showing that this cleavage event is highly sequence specific in cells.
It has previously been suggested that IRE1 kinase activity controls RIDD, as I642G kinase-dead IRE1 did not carry out RIDD, even when activated to cleave XBP1 with 1NM-PP1, nor could it cleave Ins2 mRNA in vitro (9). However, conflicting evidence has recently been published showing that I642G mutant IRE1 can cleave pre-miR-17 in vitro (17). Again, the length and temperature of the cleavage reaction may be responsible for this difference. It has also been shown that I642G IRE1 can induce RIDD of Blos1 in mouse cells when DTT is added with 1NM-PP1 (9). In addition, RIDD in Ire1-null C. glabrata can be rescued by reexpressing a kinase-dead mutant (6). It may therefore be the case that another stress-induced kinase phosphorylates IRE1 to induce RIDD. In our study, we assessed IRE1 kinase activity by measuring autophosphorylation at serine 724. We found that RIDD could take place both under conditions when S724 was highly phosphorylated (RPMI-8226 cells under Tm treatment) or when S724 was phosphorylated at or close to baseline levels (RPMI-8226 and NCI-H929 cells under DTT treatment), suggesting that IRE1 kinase activity may not affect RIDD of BLOC1S1. However, we acknowledge that there are multiple other phosphorylation sites in IRE1 (29), and they may contribute to RIDD.
In this study, we found that RIDD of BLOC1S1 occurred after XBP1 splicing and took place more slowly. In the example of DTT treatment, XBP1 was fully spliced after 15 min of treatment but BLOC1S1 degradation took place gradually over 2 h (Fig. 3). The observation that we could inhibit RIDD with 4μ8c after XBP1 splicing had taken place (Fig. 8B and 9B) shows that it is the cleavage of BLOC1S1 that is slower rather than the subsequent degradation. In vitro, we also observed that complete XBP1u cleavage was quicker than BLOC1S1 cleavage (Fig. 5C), suggesting that IRE1 cleaves XBP1 more effectively than RIDD substrates, although BLOC1S1 could compete with XBP1 for cleavage by IRE1 in vitro (Fig. 5C). In cells, we observed that XBP1 splicing can take place in the absence of RIDD, for example, in NCI-H929 cells treated with tunicamycin (Fig. 4F and J). Therefore, it seems that there is an additional mechanism for the selectivity of IRE1 for XBP1 as a substrate in vivo. We speculate that the specific cotranslational targeting of XBP1 to IRE1 at the ER membrane may be responsible for this. It has been shown that effective XBP1 splicing requires its targeting to the ER membrane via a translated 3′ region (31). Some RIDD targets may also be cotranslationally targeted, for example, SPARC in Drosophila (5). BLOC1S1 may lack this targeting, which could lead to slower cleavage by IRE1. It may also be the case that the specific targeting of XBP1u to IRE1 could inhibit RIDD of other substrates when XBP1u is present. In agreement with this hypothesis, we observed RIDD of BLOC1S1 only under conditions that led to the nearly complete conversion of XBP1u to XBP1s (Fig. 3 and 9). There may be a threshold level of XBP1u below which other mRNA substrates are able to access active IRE1. This idea is supported by previous studies showing that genetic deletion of XBP1 leads to RIDD (10, 11, 15, 17, 19, 20). After submission of the manuscript, work was published suggesting that IRE1 has distinct mechanisms for the cleavage of XBP1u or RIDD substrates (22). The authors show that the cleavage of HAC1 (the yeast orthologue of XBP1u) by ADP-activated yeast Ire1 is not inhibited by RIDD substrates under “single-turnover” conditions (high enzyme concentration) and is inhibited in a noncompetitive manner under steady-state conditions. The findings under steady-state conditions support our own data, as we found that human BLOC1S1 can compete with XBP1 for cleavage by IRE1 in vitro (Fig. 5C). Tam et al. go on to show that HAC1 or XBP1u cleavage exhibits cooperativity, whereas RIDD substrate cleavage does not. Structural analysis suggests that binding sites for HAC1 and RIDD substrates to yeast Ire1 may be different (22). More recently, it has been shown that a positively charged motif within the cytosolic “linker” domain of yeast Ire1 is responsible for mRNA recruitment (39). Interestingly, this motif is not present in human IRE1, suggesting that the mechanism for mRNA recruitment to IRE1 in humans is different from that in yeast. This highlights a key difference between yeast and human IRE1 proteins and shows that further work is needed to specifically address the binding mechanisms of XBP1 and RIDD substrates to human IRE1 in vivo.
The physiological function of RIDD in mammals remains to be proven, and several hypotheses have been proposed. It was suggested that RIDD may be a mechanism to degrade ER-localized mRNA under stress, thereby reducing the burden on the ER and aiding recovery from stress, as is the case in certain yeast species (6–8). Lu et al. later proposed that IRE1 transiently degrades DR5 mRNA to reduce caspase 8 activation under early ER stress, thereby promoting adaptation (21). In contrast to this, we did not observe significantly more cell death when RIDD was inhibited during the early phase of ER stress (Fig. 8G and 9A). This difference may be due to the duration of stress used. Lu et al. treated cells with thapsigargin for 24 h before measurement of cell viability or caspase activation, whereas we used 90 min or 60 min of DTT treatment. However, we observed no protective function for RIDD under longer DTT treatment in HT-1080 cells (Fig. 10H and I). It has also been suggested that RIDD contributes to apoptosis (9), and more recently, a specific mechanism was suggested where IRE1 cleaves a subset of microRNAs (miRNAs), leading to apoptosis via upregulation of caspase 2 (17). However, this has since been challenged by the finding that caspase 2 is not involved in apoptosis triggered by ER stress (40). Tam et al. also proposed that RIDD leads to cell death (22). In cells expressing a human-yeast hybrid Ire1 that is activatable by quercetin, they found that the IRE1 RNase inhibitor STF-083010 inhibits XBP1 splicing but not RIDD under quercetin treatment (22). In this system, they show that the addition of STF-083010 with quercetin leads to increased cell death and PARP cleavage (22). However, a control treatment of STF-083010 without quercetin was not included, so it is not clear how much the toxicity of STF-083010 alone contributes to the effect. STF-083010 or its active, hydrolyzed product 2-hydroxy-1-napthadehyde (HNA) is indeed cytotoxic at the relevant concentration (41–43), and HNA induces PARP cleavage (44). In our study, we observed no significant change in recovery of cell viability after acute ER stress when RIDD was inhibited in myeloma cells (Fig. 8G and 9A). In addition, under longer DTT treatment in HT-1080 cells, the effect of inhibiting RIDD was negligible (Fig. 10H and I). Importantly, we used temporal inhibition of endogenous IRE1 to separate XBP1 splicing and RIDD activities under the level of stress where we observed RIDD to occur. This is in contrast to previous studies in other cell types, where exogenously expressed IRE1 mutants were used (9, 22), and may be the reason for the discrepancies. Our results raise questions about the function of RIDD in mammalian cells. In certain yeast species, RIDD promotes cell survival under stress by indirectly reducing protein translation through mRNA degradation. This is particularly important in C. glabrata and S. pombe, which lack Hac1 signaling (6, 7). However, the yeast UPR is controlled solely by Ire1 (45), whereas mammalian cells have a much more complex response consisting of IRE1, ATF6, and PERK (1). It may be the case that the RIDD function of IRE1 is not required for the mammalian UPR because it is superseded by PERK, which provides a more sophisticated mechanism to promote adaptation or apoptosis under stress. PERK phosphorylates EIF2α, attenuating protein translation and alleviating ER stress but promoting translation of the proapoptotic transcription factor ATF4 (which increases CHOP expression) and the prosurvival transcription factor ATF6 (46, 47). In this way, the duration and amplitude of PERK activation can control both adaptation to stress and cell death, potentially without the need for RIDD to occur. In agreement with this hypothesis, we measured no difference in CHOP expression in the presence or absence of RIDD (Fig. 8F and 9F).
Another question raised by our work, and previous publications, is whether the conditions that activate RIDD would occur under physiological conditions. The conditions required to induce RIDD in myeloma cells were intense stress induced by 2 mM DTT, or more lengthy Tm treatment in RPMI-8226 cells. Other studies observing RIDD have also used chemically induced ER stress or genetic deletion of XBP1. It is conceivable that the gut epithelium could be exposed to chemical stressors, such as tunicamycin, a toxin produced by Streptomyces bacteria (48), or thapsigargin, a poisonous compound found in plants of the genus Thapsia (49). It could therefore be that RIDD is a mechanism to deal with extreme proteotoxic stress induced by these agents in nature. Due to the longer incubation required to induce RIDD with tunicamycin, we would be unable to separate RIDD and XBP1 splicing using the temporal 4μ8c inhibition we used for DTT treatment. It therefore remains possible that RIDD has a protective or apoptotic role under long-term tunicamycin treatment. However, our data suggest that RIDD does not have a role in cell survival under acute stress or under the extreme ER stress induced by 2 mM DTT treatment.
In summary, in this study, we found that BLOC1S1 is the most consistently identified RIDD target and contains an XBP1-like stem-loop. BLOC1S1 is specifically cleaved by IRE1 at guanine 444 in vitro and in cells, and the specificity of IRE1 for cleaving this site remains stringent even under strong ER stress. Cleavage of BLOC1S1 occurred more slowly than XBP1 splicing, and the cleavage of BLOC1S1, or RIDD as a whole, was dispensable for the control of cancer cell viability under acute ER stress.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lauren Aronson and Jade Strover for technical assistance with qPCR and XBP1 splicing assays.
We are all employees of the Institute of Cancer Research, which has a commercial interest in the development of inhibitors of the ER stress response.
M.D.B. designed research, performed experiments, analyzed data, and wrote the manuscript. D.N.I. assisted with experiment design, performed experiments, and analyzed data. C.P.W. performed bioinformatics analysis and assisted in writing the manuscript. G.J.M. assisted in writing the manuscript. F.E.D. designed research, analyzed data, and assisted in writing the manuscript.
This work was supported by Cancer Research United Kingdom (grant number C20826/A1210) and the Kay Kendall Leukaemia Fund (grant number KKL833). F.E.D. is a Cancer Research United Kingdom Senior Cancer Research Fellow. We acknowledge NHS funding to the NIHR Biomedical Research Centre at the Royal Marsden Hospital and the ICR.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00013-15.
REFERENCES
- 1.Ron D, Walter P. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Korennykh AV, Behrman SL, Walter P. 2010. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc Natl Acad Sci U S A 107:16113–16118. doi: 10.1073/pnas.1010580107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. 2001. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891. doi: 10.1016/S0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 4.Lee AH, Iwakoshi NN, Glimcher LH. 2003. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollien J, Weissman JS. 2006. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki T, Nakayama H, Nagayoshi Y, Kakeya H, Kohno S. 2013. Dissection of Ire1 functions reveals stress response mechanisms uniquely evolved in Candida glabrata. PLoS Pathog 9:e1003160. doi: 10.1371/journal.ppat.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimmig P, Diaz M, Zheng J, Williams CC, Lang A, Aragon T, Li H, Walter P. 2012. The unfolded protein response in fission yeast modulates stability of select mRNAs to maintain protein homeostasis. eLife 1:e00048. doi: 10.7554/eLife.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. 2009. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. 2009. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hur KY, So JS, Ruda V, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Iwawaki T, Glimcher LH, Lee AH. 2012. IRE1alpha activation protects mice against acetaminophen-induced hepatotoxicity. J Exp Med 209:307–318. doi: 10.1084/jem.20111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.So JS, Hur KY, Tarrio M, Ruda V, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Lichtman AH, Iwawaki T, Glimcher LH, Lee AH. 2012. Silencing of lipid metabolism genes through IRE1alpha-mediated mRNA decay lowers plasma lipids in mice. Cell Metab 16:487–499. doi: 10.1016/j.cmet.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oikawa D, Tokuda M, Hosoda A, Iwawaki T. 2010. Identification of a consensus element recognized and cleaved by IRE1 alpha. Nucleic Acids Res 38:6265–6273. doi: 10.1093/nar/gkq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pluquet O, Dejeans N, Bouchecareilh M, Lhomond S, Pineau R, Higa A, Delugin M, Combe C, Loriot S, Cubel G, Dugot-Senant N, Vital A, Loiseau H, Gosline SJ, Taouji S, Hallett M, Sarkaria JN, Anderson K, Wu W, Rodriguez FJ, Rosenbaum J, Saltel F, Fernandez-Zapico ME, Chevet E. 2013. Posttranscriptional regulation of PER1 underlies the oncogenic function of IREalpha. Cancer Res 73:4732–4743. doi: 10.1158/0008-5472.CAN-12-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oikawa D, Tokuda M, Iwawaki T. 2007. Site-specific cleavage of CD59 mRNA by endoplasmic reticulum-localized ribonuclease, IRE1. Biochem Biophys Res Commun 360:122–127. doi: 10.1016/j.bbrc.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Coelho DS, Cairrao F, Zeng X, Pires E, Coelho AV, Ron D, Ryoo HD, Domingos PM. 2013. Xbp1-independent Ire1 signaling is required for photoreceptor differentiation and rhabdomere morphogenesis in Drosophila. Cell Rep 5:791–801. doi: 10.1016/j.celrep.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tirasophon W, Lee K, Callaghan B, Welihinda A, Kaufman RJ. 2000. The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev 14:2725–2736. doi: 10.1101/gad.839400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, Papa FR, Oakes SA. 2012. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science 338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejeans N, Pluquet O, Lhomond S, Grise F, Bouchecareilh M, Juin A, Meynard-Cadars M, Bidaud-Meynard A, Gentil C, Moreau V, Saltel F, Chevet E. 2012. Autocrine control of glioma cells adhesion and migration through IRE1alpha-mediated cleavage of SPARC mRNA. J Cell Sci 125:4278–4287. doi: 10.1242/jcs.099291. [DOI] [PubMed] [Google Scholar]
- 19.Osorio F, Tavernier SJ, Hoffmann E, Saeys Y, Martens L, Vetters J, Delrue I, De Rycke R, Parthoens E, Pouliot P, Iwawaki T, Janssens S, Lambrecht BN. 2014. The unfolded-protein-response sensor IRE-1alpha regulates the function of CD8alpha+ dendritic cells. Nat Immunol 15:248–257. doi: 10.1038/ni.2808. [DOI] [PubMed] [Google Scholar]
- 20.Benhamron S, Hadar R, Iwawaky T, So JS, Lee AH, Tirosh B. 2014. Regulated IRE1-dependent decay participates in curtailing immunoglobulin secretion from plasma cells. Eur J Immunol 44:867–876. doi: 10.1002/eji.201343953. [DOI] [PubMed] [Google Scholar]
- 21.Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, Paton AW, Paton JC, Walter P, Ashkenazi A. 2014. Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 345:98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam AB, Koong AC, Niwa M. 2014. Ire1 has distinct catalytic mechanisms for XBP1/HAC1 splicing and RIDD. Cell Rep 9:850–858. doi: 10.1016/j.celrep.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo B, Lee AS. 2013. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 32:805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan GJ, Walker BA, Davies FE. 2012. The genetic architecture of multiple myeloma. Nat Rev Cancer 12:335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- 25.Aronson LI, Davies FE. 2012. DangER: protein ovERload. Targeting protein degradation to treat myeloma. Haematologicaae 97:1119–1130. doi: 10.3324/haematol.2012.064923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong H, Chen L, Chen X, Gu H, Gao G, Gao Y, Dong B. 2009. Dysregulation of unfolded protein response partially underlies proapoptotic activity of bortezomib in multiple myeloma cells. Leukemia Lymphoma 50:974–984. doi: 10.1080/10428190902895780. [DOI] [PubMed] [Google Scholar]
- 27.Davenport EL, Zeisig A, Aronson LI, Moore HE, Hockley S, Gonzalez D, Smith EM, Powers MV, Sharp SY, Workman P, Morgan GJ, Davies FE. 2010. Targeting heat shock protein 72 enhances Hsp90 inhibitor-induced apoptosis in myeloma. Leukemia 24:1804–1807. doi: 10.1038/leu.2010.168. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itzhak D, Bright M, McAndrew P, Mirza A, Newbatt Y, Strover J, Widya M, Thompson A, Morgan G, Collins I, Davies F. 2014. Multiple autophosphorylations significantly enhance the endoribonuclease activity of human inositol requiring enzyme 1alpha. BMC Biochem 15:3. doi: 10.1186/1471-2091-15-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rio DC, Ares M Jr, Hannon GJ, Nilsen TW. 2010. Polyacrylamide gel electrophoresis of RNA. Cold Spring Harb Protoc 2010:pdb.prot5444. doi: 10.1101/pdb.prot5444. [DOI] [PubMed] [Google Scholar]
- 31.Yanagitani K, Imagawa Y, Iwawaki T, Hosoda A, Saito M, Kimata Y, Kohno K. 2009. Cotranslational targeting of XBP1 protein to the membrane promotes cytoplasmic splicing of its own mRNA. Mol Cell 34:191–200. doi: 10.1016/j.molcel.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 32.Moore KA, Plant JJ, Gaddam D, Craft J, Hollien J. 2013. Regulation of sumo mRNA during endoplasmic reticulum stress. PLoS One 8:e75723. doi: 10.1371/journal.pone.0075723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, Harding HP. 2012. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci U S A 109:E869–E878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Yi P, Zhang B, Xu C, Liu Q, Pi Z, Xu X, Chevet E, Liu J. 2011. Differences in endoplasmic reticulum stress signalling kinetics determine cell survival outcome through activation of MKP-1. Cell Signal 23:35–45. doi: 10.1016/j.cellsig.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Gruber AR, Lorenz R, Bernhart SH, Neubock R, Hofacker IL. 2008. The Vienna RNA websuite. Nucleic Acids Res 36:W70–W74. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. 2007. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. 2006. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabrini R, De Luca A, Stella L, Mei G, Orioni B, Ciccone S, Federici G, Lo Bello M, Ricci G. 2009. Monomer-dimer equilibrium in glutathione transferases: a critical re-examination. Biochemistry 48:10473–10482. doi: 10.1021/bi901238t. [DOI] [PubMed] [Google Scholar]
- 39.van Anken E, Pincus D, Coyle S, Aragon T, Osman C, Lari F, Gomez Puerta S, Korennykh AV, Walter P. 2014. Specificity in endoplasmic reticulum-stress signaling in yeast entails a step-wise engagement of HAC1 mRNA to clusters of the stress sensor Ire1. eLife 3:e05031. doi: 10.7554/eLife.05031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandow JJ, Dorstyn L, O'Reilly LA, Tailler M, Kumar S, Strasser A, Ekert PG. 2014. ER stress does not cause upregulation and activation of caspase-2 to initiate apoptosis. Cell Death Differ 21:475–480. doi: 10.1038/cdd.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, Koong AC. 2011. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood 117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kharabi Masouleh B, Geng H, Hurtz C, Chan LN, Logan AC, Chang MS, Huang C, Swaminathan S, Sun H, Paietta E, Melnick AM, Koeffler P, Muschen M. 2014. Mechanistic rationale for targeting the unfolded protein response in pre-B acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 111:E2219–E2228. doi: 10.1073/pnas.1400958111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kriss CL, Pinilla-Ibarz JA, Mailloux AW, Powers JJ, Tang CH, Kang CW, Zanesi N, Epling-Burnette PK, Sotomayor EM, Croce CM, Del Valle JR, Hu CC. 2012. Overexpression of TCL1 activates the endoplasmic reticulum stress response: a novel mechanism of leukemic progression in mice. Blood 120:1027–1038. doi: 10.1182/blood-2011-11-394346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chien W, Ding LW, Sun QY, Torres-Fernandez LA, Tan SZ, Xiao J, Lim SL, Garg M, Lee KL, Kitajima S, Takao S, Leong WZ, Sun H, Tokatly I, Poellinger L, Gery S, Koeffler PH. 2014. Selective inhibition of unfolded protein response induces apoptosis in pancreatic cancer cells. Oncotarget 5:4881–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mori K, Ma W, Gething MJ, Sambrook J. 1993. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74:743–756. doi: 10.1016/0092-8674(93)90521-Q. [DOI] [PubMed] [Google Scholar]
- 46.Teske BF, Wek SA, Bunpo P, Cundiff JK, McClintick JN, Anthony TG, Wek RC. 2011. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell 22:4390–4405. doi: 10.1091/mbc.E11-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walter P, Ron D. 2011. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 48.Takatsuki A, Arima K, Tamura G. 1971. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J Antibiot 24:215–223. [DOI] [PubMed] [Google Scholar]
- 49.Patkar SA, Rasmussen U, Diamant B. 1979. On the mechanism of histamine release induced by thapsigargin from Thapsia garganica L. Agents Actions 9:53–57. doi: 10.1007/BF02024109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.