Abstract
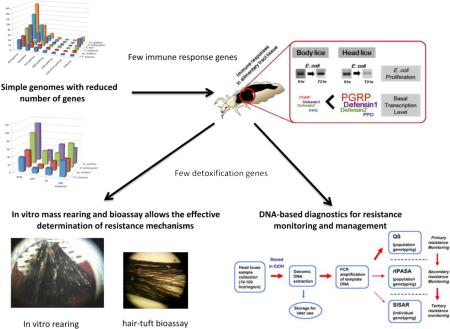
Since sequencing the human body louse genome, substantial advances have occurred in the utilization of the information gathered from louse genomes and transcriptomes. Comparatively, the body louse genome contains far fewer genes involved in environmental response, such as xenobiotic detoxification and innate immune response. Additionally, the body louse maintains a primary bacterial endosymbiont, Candidatus Riesia pediculicola, and a number of bacterial pathogens that it vectors, which have genomes that are also reduced in size. Thus, human louse genomes offer unique information and tools for use in advancing our understanding of coevolution among vectors, endosymbionts and pathogens.
In this review, we summarize the current literature on the extent of pediculicide resistance, the availability of new pediculicides and information establishing this organism as an efficient model to study how xenobiotic metabolism, which is involved in insecticide resistance, is induced and how insects modify their innate immune response upon bacterial challenge resulting in enhanced vector competence.
Keywords: Pediculus, Immune response, Body and head lice, Resistance, kdr monitoring, Bacterial challenge, Phagocytosis, Vector competence, Carboxylesterase, Pediculicides
1. Pediculicides and Resistance
The infestation of humans by lice is called pediculosis and the chemicals used to treat such infestations are called pediculicides. Before the availability of effective antibiotics, infestation by the human body louse, Pediculus humanus humanus, was common and the ability of the body louse to vector a number of bacterial diseases lead to the death of millions and in some cases changed the course of our history. Today, the infestation of the human scalp and hair by the human head louse, Pediculus humanus capitis, is more common. Although they are not vectors of disease, head louse infestations are a major economic and social concern worldwide because infestations are often associated with school-aged children, who often miss substantial number of school days and can suffer emotional distress not to mention the increased possibility of secondary infections due to self-inoculation upon intense itching [1]. Over the past 70 years, the control of pediculosis has been largely dependent upon the availability of natural and synthetic insecticides starting with DDT (1943), natural pyrethrins (1945), the organochlorine lindane (1960), organophosphorous insecticides (malathion, 1971), carbamates (carbaryl, 1977) and synthetic pyrethroids (permethrin, phenothrin, 1992) [2].
In the USA, the pyrethrins/pyrethroids have dominated the over-the-counter (OTC) market since their availability beginning in 1992, followed by the prescription only malathion-containing formulations, such as Ovide®, beginning in 1971. The pyrethrins/pyrethroids share a common target site in the nervous system, the voltage-sensitive sodium channel (VSSC), and act as agonistic neuroexcitants by increasing sodium current, leading to nerve depolarization and hyperexcitation, followed by neuromuscular paralysis and death. Malathion is a phosphorodithioate-type organophosphorous insecticide, which is an indirect nerve toxin that acts as a competitive irreversible inhibitor of acetylcholinesterase associated with the cholinergic nervous system. When inhibited, acetylcholinesterase cannot efficiently hydrolyze the neurotransmitter, acetylcholine, allowing overstimulation of post-synaptic effector organs, including muscle, leading to paralysis and death.
Insecticide resistance to currently-used pediculicides, including permethrin, synergized pyrethrins and malathion, has occurred worldwide, is increasing [3-5] and is certainly contributing to increased incidences of pediculosis. Insecticide resistance threatens the success of all control programs but is particularly problematic in the control of human lice for several reasons: (1) they are obligate human blood feeders that are exposed to pediculicides at all stages; (2) they have short generational time and high fecundity; and (3) there are few pediculicidal products, the majority of which share common chemistry and elicit cross-resistance. Because of these issues, louse resistance to most commercial pediculicides has occurred and is increasing [6].
Both clinical and parasitological pyrethroid resistance to d-phenothrin was first reported in France in 1994 [7] with additional reports of clinical control failures following: permethrin (2001) in the USA [8], phenothrin (2005) in the UK [9], and permethrin (2005) in the UK [10]. Also, parasitological resistance has been reported in the Czech Republic [11], the UK [9], Denmark [12], Israel [13], the USA [14], Argentina [15], Japan [16] and Australia [17].
Malathion resistance was first reported in France in 1995 [18], followed by the UK in 1999 [19], Australia in 2003 [17], and Denmark in 2006 [12]. The lack of extensive resistance in the USA is likely due to the use of the Ovide® formulation, which also includes pediculicidal terpenes likely resulting in a mixture that has redundant killing action on multiple target sites [20].
Current control and resistance problems underscore the need to understand the molecular mechanisms of insecticide resistance in lice. The identification of resistance mechanisms and novel target sites may allow the development of resistance-breaking compounds and specific non-toxic synergists useful in novel control strategies. Recently, a number of new topical pediculicidal products have been introduced to the marketplace. They possess novel modes of action, show little cross-resistance to existing commercially-available pediculicides and appear safe and effective. The following section has been recently reviewed and the following is in large part from Clark et al. [1].
1.1 Dimeticone-based formulations
There has been a trend, primarily in Europe, for the development of physical means to control head lice because of increasing instances of resistance, particularly to the neurotoxic pediculicides, and the increased scrutiny of the use of such products on children. The dimeticone-based anti-louse products (silicone oils) are of interest due to their low mammalian toxicity, novel modes of action (not neurotoxic) and the possibility that they will have a low potential for the development of resistance. Dimeticones are linear polydimethylsiloxanes (CH3SiO[SiO(CH3)2]nSi(CH3)2), where n is the number of repeating monomers [SiO(CH3)2] of varying chain length. The chain length substantially influences the viscosity of the dimeticones and thus, they can vary considerably in spreading characteristics. Of the different dimeticone-based products available, two products are better characterized scientifically in terms of their effectiveness and probable modes of action.
Hedrin® 4% lotion (Thornton & Ross Ltd, Huddersfield, UK) is a 4% dimeticone lotion in 96% (w/w) decamethylcyclopentasiloxane (cyclomethicone D5). Head lice treated with this product are rapidly immobilized but small movements in their extremities over several hours indicate that death is delayed. Scanning electron microscopy coupled with X-ray microanalysis revealed that Hedrin® 4% lotion was found in the spiracles, in some cases blocking the opening completely, and penetrated into the outer aspects of the tracheae [21]. Asphyxia is unlikely as a mode of action given the slow onset of mortality. The inability of the louse to excrete the excess water acquired during blood feeding by transpiration via the spiracles has been suggested as a mode of action with death occurring by either prolonged immobilization or by the rupture of organs such as the gut [21].
The second dimeticone-based anti-louse product (NYDA®, G. Pohl-Boskamp GmbH& Co. Hohenlockstedt, Germany) contains a mixture of two dimeticones, one of low and the other of higher viscosity, at a final total concentration of dimeticones of 92% (w/w). Medium-chain length triglycerides, jojoba wax and two fragrances make up the remaining constituents. NYDA® rapidly enters the tracheal system, filling even the smallest branches, due to its superior spreading characteristics [22]. Within one min of treatment with NYDA®, lice do not show any major vital signs. This effect appears to be due to an interruption in the oxygen supply leading to suffocation. Additionally, NYDA® has been shown to be an effective ovicide [23].
1.2 Ivermectin-based formulations
Ivermectin is a macrocyclic lactone produced fermentatively by Streptomyces avermitilis and is a widely-used oral anthelmintic agent for both humans and companion animals. It has a unique mode of action by reducing motility and feeding in treated nematodes [24]. In addition to muscles used in motility, ivermectin also acts to paralyze the muscles associated with the pharyngeal pump, inhibiting the pumping action needed for feeding and attachment [25]. The concentration of ivermectin needed to cause paralysis of the pharyngeal pump is 10- to 100-fold lower than the concentration needed to cause mortality [26].
Ivermectin increases chloride ion permeability in insect [27] and nematode [28] neurons and muscle membranes through binding to glutamate-gated chloride ion channels. These channels are highly expressed in the neuromuscular system of the pharyngeal pump in the mouthparts of the free living nematode, Caenorhabditis elegans, which has been shown to be highly sensitive to ivermectin. It is believed that during de-worming, nematode parasites are killed by ivermectin acting on glutamate-gated chloride channels in the cells of the neuromuscular system. Ivermectin increases chloride ion influx, which hyperpolarizes the cells, leading to paralysis of the mouthparts. This action causes the worms to detach from the mammalian gut and be excreted. A similar mode of action in head lice, however, has not been directly characterized.
Recently, successive oral ivermectin treatments were used to treat hard-to-control head louse infestations [29]. The need for successive treatments in the oral ivermectin studies indicates that one treatment, while effective against feeding lice in situ, needs to be supplemented by a second systemic treatment to kill nymphs that emerge from eggs present at the time of the initial treatment. This implies an absence of an ovicidal effect of oral ivermectin.
Ivermectin also has been formulated as a less invasive topically-applied pediculicide that possesses the ability to kill permethrin-resistant head lice [30]. A 0.5% ivermectin topical cream formulation (Sklice®, Sanofi Pasteur Inc., Swiftwater, PA) killed permethrin-resistant head lice [30] but was not directly ovicidal to treated eggs, as hatchability was not decreased [31]. Nevertheless, the percent of hatched lice from treated eggs that took a blood meal significantly decreased (80-95%) compared to lice that hatched from untreated eggs and all treated lice died within 48 h of hatching, including those that fed. Dilutions of ivermectin formulation of 0.15 and 0.2 μg/ml, which were topically applied to 0-8 d old eggs, were not lethal to lice at 24 h post-eclosion. However, 9 and 16% less lice fed when hatched from these treated eggs, respectively. This observation led us to hypothesize that ivermectin may be acting on the glutamate-gated chloride channels in the neuromuscular system of the louses piecing-sucking mouthparts in much the same way it acts on the pharyngeal pump of nematodes [31]. Using [3H] inulin, a plant polysaccharide that is not hydrolyzed by insects, as a tracer for blood uptake during louse feeding, total [3H] inulin ingested by untreated 1st instars significantly increased over a 48 h feeding interval but was significantly less in instars that hatched from eggs receiving the 0.15 (36% less) and 0.2 (55% less) μg/ml ivermectin treatments compared to placebo (formulation without ivermectin). The reduced feeding that occurred following the 0.15 and 0.2 μg/ml ivermectin treatments occurred in the absence of mortality and suggests a unique mode of action of ivermectin on feeding that is separate from the mode of action of ivermectin leading to mortality. Failure of hatched instars to take a blood meal following egg treatments with formulated ivermectin is likely responsible for its action as a post-eclosion nymphicide by acting on glutamate-gated chloride channels in the louse mouthparts.
1.3 Spinosad-based formulation
Spinosad is a macrocyclic lactone insecticide produced fermentatively by a soil actinomycete bacterium, Saccharopolyspora spinosa. It has two active ingredients, spinosyn A and spinosyn D, in a 5 to 1 ratio. Spinosad is a neurotoxic agonist at the nicotinic acetylcholine receptor of the cholinergic nervous system where it selectively modifies the non-desensitizing aspect of the current flowing through this ligand-gated channel, causing prolonged excitability and then paralysis [32].
Spinosad has been commercially formulated as a 0.9% viscous topical suspension for the treatment of head louse infestation (Natroba®, ParaPRO, LLC, Carmel, IN).
2. In Vitro Rearing and Hair Tuft Bioassay
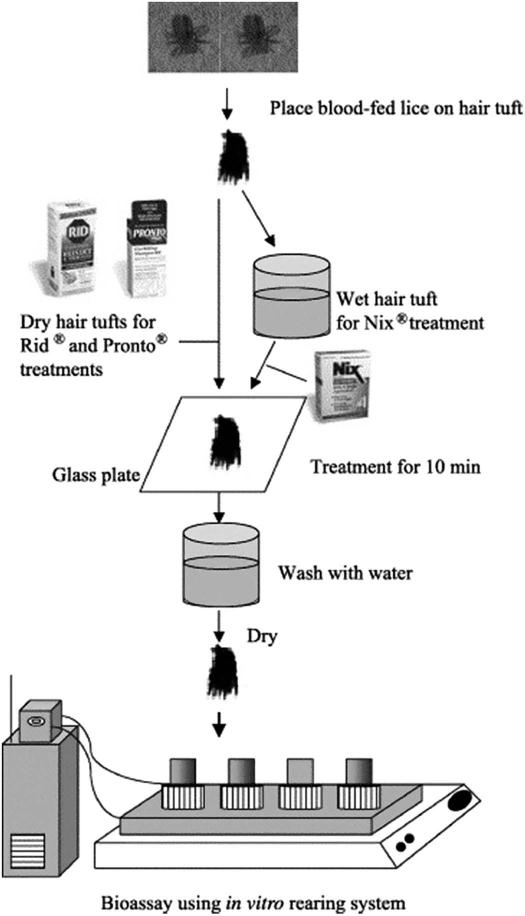
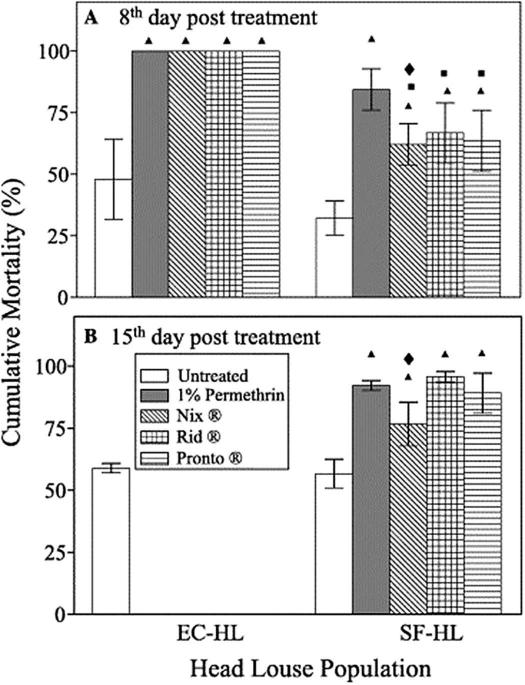
Prior to 2003, research on human lice was greatly impeded by the inability to rear lice off of their host. Without reference strains, only low numbers of lice of uncertain age and treatment history were obtained primarily from human volunteers for experiments. This scenario made detailed examination of the physiology, biochemistry and molecular biology of insecticide efficacy and resistance virtually impossible. These issues were rectified by the development of the first in vitro rearing system developed by Takano-Lee et al. [33]. This rearing system was improved upon and modified to allow the determination of resistance to formulated pediculicides using the hair-tuft bioassay (Fig. 1) [34]. This improved in vitro rearing system, based on a silicone-reinforced Parafilm® M membrane, human hair tufts and reconstituted human blood, enabled the large-scale rearing of pediculicide-susceptible (EC-HL) and resistant (SF-HL) strains of human head lice. The efficacies of three commercial pediculicide products were assessed using the hair-tuft bioassay and applying the pediculicides using the manufacturers’ instructions in a semi-clinical format (Fig. 2) [34]. Treatments of 1% permethrin in acetone, Nix®, Rid® and Pronto Plus® were highly efficacious (100% mortality) on the susceptible lice (EC-HL) but were differentially efficacious (62-84% mortality) on the resistant lice (SF-HL) when examined eight days post-treatment (Fig. 2). SF-HL that survived the first treatment received an identical second treatment. Survivors (13-30%) developed into adults and females laid viable eggs that hatched (Fig. 2). These results confirmed resistance to permethrin- and pyrethrin-based pediculicide formulations, initially reported using unformulated pediculicides and a filter paper bioassay, and the establish usefulness of the in vitro rearing system when used in conjunction with the hair-tuft bioassay as a standard protocol to test new formulated louse control agents. Furthermore, this system allowed for the inbreeding of human body and head lice strains necessary for the detailed examination of insecticide resistance mechanisms and for the sequencing of the body louse genome [35] and head louse transcriptome [36].
Fig. 1.
The hair tuft bioassay procedure performed in conjunction with the in vitro rearing system to determine the efficacy of over-the-counter (OTC) pediculicide products. Reproduced from with permission from Ref. [34]. Copyright 2006 Elsevier Inc.
Fig. 2.
Mortality of pediculicide-susceptible (EC-HL) and permethrin-resistant (SF-HL) head louse strains treated with 1% (w/v) permethrin and the OTC pediculicides (Nix®, Rid® and Pronto® Plus) using the hair tuft bioassay procedure in conjunction with the in vitro rearing system. Reproduced by permission from Ref. [34]. Copyright 2006 Elsevier Inc.
3. Permethrin Resistance Mechanisms and Monitoring
3.1 Three point mutations in the α-subunit gene of the VSSC cause knockdown resistance (kdr) and can be used for monitoring resistance
Lee et al. [37] first reported that head lice from Massachusetts and Florida were resistant to a pyrethroid, permethrin, and exhibited in vivo responses in behavioral bioassays that were consistent with kdr. kdr is a heritable trait associated with nerve insensitivity to DDT, the pyrethrins and the pyrethroids, which was first discovered in the house fly, Musca domestica [38]. Point mutations in these genes are functionally responsible for the kdr, kdr-like and super-kdr traits and nerve insensitivity to DDT, the pyrethrins and pyrethroids [39].
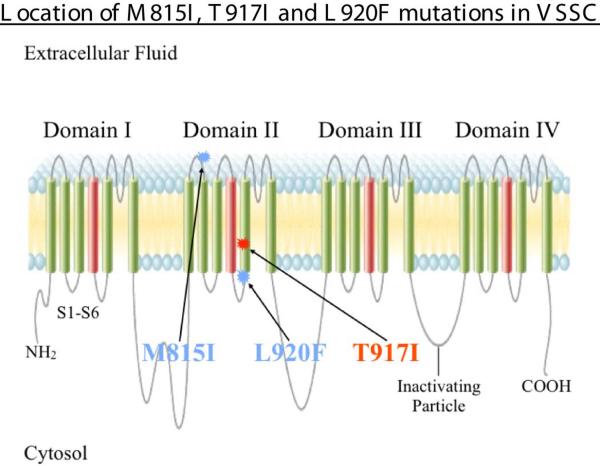
Three point mutations located in the domain IIS1-2 extracellular loop (M815I) and in the domain IIS5 transmembrane segment (T917I and L920F) of VSSC α-subunit (numbered according to the head louse amino acid sequence) were identified in permethrin-resistant head lice [38,40] (Fig. 3). T917I, corresponding to T929I in the house fly, was functionally validated as a kdr-type mutation in the diamondback moth, Plutella xylostella [41]. The other two mutations (M815I and L920F) were novel and their functional significance in pyrethroid resistance unproven.
Fig. 3.
Transmembrane topology of the voltage-sensitive sodium channel (VSSC) α-subunit showing the locations of the three amino acid substitutions, which result from point mutations in the human head louse VSSC α-subunit gene, responsible for nerve insensitivity and knockdown (kdr)-type resistance. Modified from Fig. 26A from http://www.bio.miami.edu/tom/courses/bil360/bil360goods/07_actionpot.html
Heterologous expression of insect VSSC in Xenopus laevis oocytes has been utilized to determine the functional characteristics and pharmacological significance of kdr-associated point mutations [42,43]. Using this system, Yoon et al. [44] inserted the three mutations associated with pyrethroid resistance in the head louse (M815I, T917I and L920F) in all possible combinations into the corresponding positions of the house fly Vssc1WT sequence, expressed wild type and specifically-mutated channels along with the house fly Vsscβ auxiliary subunit in Xenopus oocytes, and employed the two electrode voltage-clamp technique to electrophysiologically assess the impact of these mutations on permethrin sensitivity of the expressed channels. In the absence of the three mutations and their corresponding amino acid replacements, a dose-dependent increase in the late current seen during inactivation and a prolongation of the tail current seen during deactivation were apparent at increasing concentrations of permethrin. This finding was consistent with the action of permethrin on many wild type VSSCs expressed in Xenopus oocytes. In the presence of the three amino acid replacements, superimposed current traces obtained at increasing concentrations of permethrin were indistinguishable from DMSO control traces recorded prior to permethrin treatments. These results confirm that the MITILF haplotype results in target site insensitivity of the VSSC and contributes to permethrin resistance in the head louse [1].
3.2 Monitoring the allele frequency of kdr in North America
The extent and frequency of a knockdown-type resistance allele (kdr-type) in North American populations of human head lice has been recently determined [45]. Lice were collected from 32 locations in Canada and the USA. DNA was extracted from individual lice and used to determine their zygosity using the serial invasive signal amplification (SISAR) technique to detect the kdr-type T917I mutation (TI), which is most responsible for nerve insensitivity that results in the kdr phenotype and permethrin resistance. Previously sampled sites were re-sampled to determine if the frequency of the TI mutation was changing. Genotyping substantiated that TI occurs at high levels in North American lice (94.1%). Overall, the TI frequency in USA lice from 1999 to 2009 was 84.4%, increased to 99.6% from 2007 to 2009 and was 97.1% in Canadian lice in 2008. Thus, the frequency of TI in North American head louse populations were determined to be uniformly high, appear to be due to the high selection pressure from the intensive and widespread use of the pyrethrins/pyrethroid-based pediculicides over many years, and is likely a main cause of increased pediculosis and failure of pyrethrins/permethrin-based products in Canada and the USA [1].
4. Malathion Resistance is due to Enhanced Hydrolytic Ester Cleavage by Malathion Carboxylesterase
Enhanced malathion carboxylesterase (MCE) activity was previously reported to be involved in malathion resistance in the head louse [5]. To identify the MCE(s) involved, the transcriptional profiles of all five esterases, which were annotated to be catalytically active, were determined and compared between the malathion-resistant (BR-HL) and malathion-susceptible (KR-HL) strains of head lice [46]. An esterase gene, designated HLCbE3, exhibited approximately 5.4-fold higher transcription level, whereas remaining four esterases did not exhibit a significant increase in their transcription in BR-HL, indicating that HLCbE3 may be the putative MCE involved in malathion resistance. Comparison of the entire cDNA sequences of HLCbE3 revealed no sequence differences between the BR-HL and KR-HL strains and suggested that no single nucleotide polymorphism was associated with enhanced MCE activity [43]. Two copies of the HLCbE3 gene were found in BR-HL, implying that over-transcription of HLCbE3 is due to the combination of a gene duplication and up-regulated transcription [43]. Knockdown of HLCbE3 expression by RNA interference (RNAi) in the BR-HL strain caused increased malathion susceptibility, confirming the identity of HLCbE3 as the MCE responsible for malathion resistance in the head louse [43].
5. The Body and Head Louse Genomes and their Utility
Using flow cytometry determinations, it was discovered that body and head lice have the smallest genomes of any hemimetabolous insect reported to date, spanning 108 Mb [47]. Sequencing of the body louse genome validated this finding and revealed that despite its small size the genome retained a remarkably complete basal insect repertoire of 10,775 draft body louse gene models [35]. Reduction of the genome size was accomplished by removing intergenic DNA, reducing the size and number of introns within genes and reducing the number of genes within large gene families, particularly those involved in environmental sensing and response, including odorant and gustatory receptor, detoxification enzyme and innate immune response genes [35,48,49]. Comparison of the transcriptional profiles of body and head lice using expressed sequence tag data sets identified 10,771 body and 10,770 head louse transcripts [50]. Illumina sequence reads were mapped to the 10,775 body louse gene models and identified nine presence/absence differences. Only one gene difference between the two transcriptomes was determined using quantitative real time PCR and that gene was determined to be translated into a hypothetical protein with no function, indicating that these two organisms share virtually the same genome and are ecotypes of the same species.
With this information in hand, it became apparent that human lice could serve as an efficient model system to study how xenobiotic metabolism, which is involved in insecticide resistance, is induced [48] and how insects modify their innate immune response upon bacterial challenge resulting in enhanced vector competence [49,51].
6. Optimization of the Non-invasive Induction Assay to Identify Detoxification Genes Involved in Insecticide Tolerance as a Proactive Resistance Monitoring Approach
Identifying insect detoxification genes, based on induced transcript profiles, has been repeatedly suggested as a means of identifying the major metabolic pathways involved in insecticide resistance [52], and initial attempts using transcriptional profiling following insecticide induction in a susceptible strain of D. melanogaster did identify a number of detoxification genes [53]. However, only a limited number of the genes induced appeared to be involved in insecticide metabolism.
Because resistance monitoring is an absolute requirement for any sustainable vector control program, there is a critical need to efficiently identify detoxification genes that metabolize insecticides during the process of induced tolerance prior to resistance evolving. Some of these genes will certainly be involved in phenotypic resistance that will evolve after pesticide selection and can then be used proactively to monitor for metabolic resistance. This approach is particularly relevant with the advent of pesticides that possess “green chemistries”, such as ivermectin, and prone to rapid detoxification by xenobiotic metabolism [1].
To investigate whether detoxification genes can be selectively induced by insecticides, and thereby identify those genes involved in the actual metabolism of the insecticide, the induction scheme needs to be optimized by including: (1) an assessment of gene transcript levels at a time of peak gene induction that results in insect tolerance; (2) insecticide doses that do not result in physiological stress that can mask the identification of primary detoxification genes due to a large number of genes of secondary importance being co-induced; and (3) application of insecticides in a non-invasive manner, such as contact exposure without solvent carriers. Insecticide induction that leads to tolerance also needs to be rapid enough to protect the insect from the rapid onset of toxicity and temporary so the fitness cost associated with the long-term over-expression of detoxification gene products is minimal. For these reasons, a non-invasive induction assay (brief exposure to sub-lethal levels of insecticide administered in a low stress fashion with a rapid assessment of transcript levels that overlaps with tolerance) was envisaged to optimize the identification of inducible detoxification genes that produce tolerance via metabolism. It is likely that some of the induced genes will result in resistance once inheritable mutations causing constitutive over-expression, more sensitive induction or structural alteration occur [1].
The optimization of dose, the timing of exposure, and the assessment of transcript levels during tolerance was used to identify detoxification genes that metabolize ivermectin as a proof of principal experiment. Transcriptional profiling results, using our “optimized” non-invasive induction assay [short exposure intervals (2-5 h) to sub-lethal amounts of insecticides (<LD3 at 24 h) administered by stress reducing means (contact vs. immersion screen) and with induction assessed in a time frame when tolerance is still present (~LC90 in 2-4 h)], show that ivermectin-induced detoxification genes from body lice are identified by quantitative real-time PCR analyses (qPCR) [54].
Of the cytochrome P450 monooxygenase (CYP) and ATP binding cassette transporter (ABC) genes induced by ivermectin, CYP6CJ1, CYP9AG1, CYP9AG2 and PhABCC4 were the most significantly over-expressed, had high basal expression levels and were most closely related to genes from other organisms that metabolized insecticides, including ivermectin [54]. Injection of dsRNAs against either CYP9AG2 or PhABCC4 into non-induced female lice reduced their respective transcript level and resulted in increased sensitivity to ivermectin, indicating that these two genes are involved in the xenobiotic metabolism of ivermectin and in the production of tolerance [54].
The above transcriptional profiling results show that ivermectin-induced detoxification genes from body lice can be identified by qPCR analyses using our non-invasive induction assay. It is our contention, therefore, that resistance to ivermectin in body lice will occur, in part, by a combination of oxidative metabolism and efflux via ABC transporters driven by the over-expression of some of the genes identified above, once either constitutive over-expression or a more sensitive induction mechanism is selected for in the field. However, once identified as above, these inducible detoxification genes may be used in proactive resistance monitoring schemes (e.g., qPCR) and in the construction of metabolic maps using a variety of insecticides to establish cross- and negative cross-expression patterns during the acquisition of tolerance following induction. Such information is critical in establishing effective mixtures to be used in proactive resistance management of pediculosis [1].
7. Innate Immune Response in Human Lice
7.1 Comparison of the humoral and cellular immune responses between body and head lice following bacterial challenge
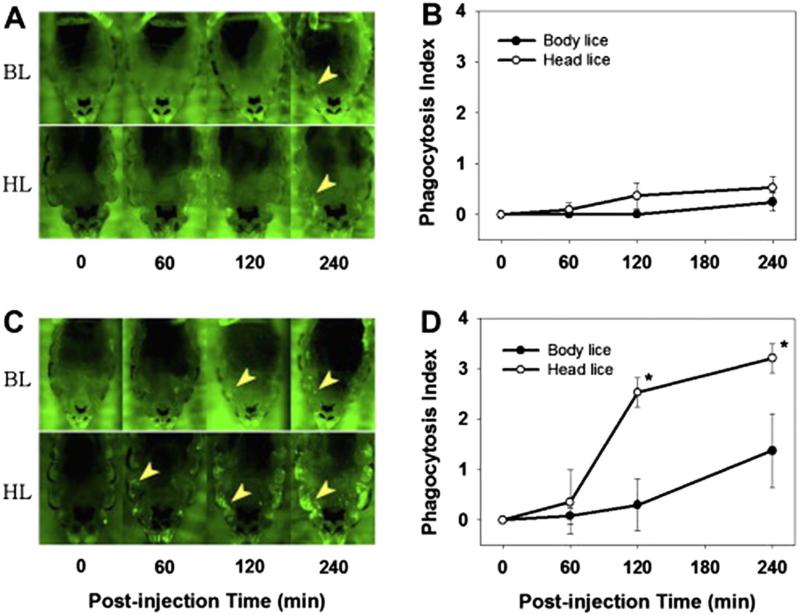
Although both body and head lice belong to a single species, P. humanus, and share virtually the same genome, only body lice are known to transmit several bacterial diseases to humans, including epidemic typhus, relapsing fever and urban trench fever. This difference in vector competence is assumed to be due, in part, to their different innate immune responses. The differences in the immune response between body and head lice were investigated initially by measuring the proliferation rates of two model bacteria, a Gram-positive Staphylococcus aureus and a Gram-negative Escherichia coli, following challenge by injection [49]. Body lice showed a significantly reduced immune response compared to head lice, particularly to E. coli at the early stage of the immune challenge. Annotation of the body louse genome identified substantially fewer immune-related genes compared with other insects [49]. Nevertheless, all required genetic components of the major immune pathways, except for the immune deficiency (Imd) pathway, were still retained in the body and head louse genomes. Transcriptional profiling of representative genes involved in the humoral immune response, following bacterial challenge, revealed that both body and head lice, regardless of their developmental stages, exhibited an increased immune response to S. aureus but little to E. coli. Head lice, however, exhibited a significantly higher phagocytotic activity against E. coli than body lice, whereas the phagocytosis against S. aureus differed only slightly between body and head lice (Fig. 4). These findings suggest that the greater immune response in head lice against E. coli is largely due to enhanced phagocytosis and not due to differences in the humoral immune response. The reduced phagocytotic activity in body lice could be responsible, in part, for their increased vector competence [49].
Fig. 4.
Fluorescence microscopic images of abdominal region of the lice injected with FITC-labeled Staphylococcus aureus (A) or Escherichia coli (C) and the time course of phagocytosis as determined by phagocytosis index (B and D). Yellow arrows in the panels A and C indicates the typical phagocytes clusters immobilized in the lateral region of abdomen. The size of body louse images was reduced 1.5 fold to make it similar to that of head lice. BL, body lice; HL, head lice.
7.2 Comparison of the immune response in alimentary tract tissue from body versus head lice following E. coli oral infection
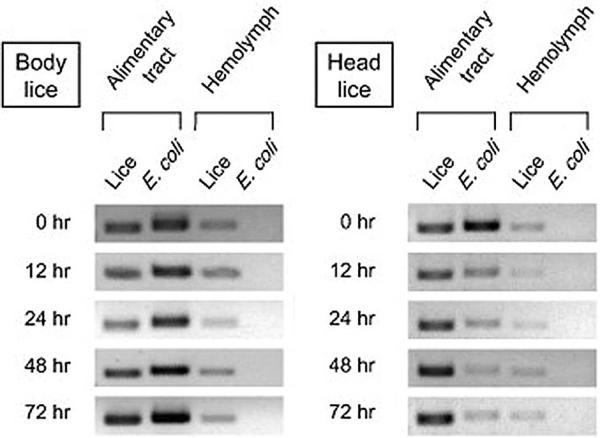
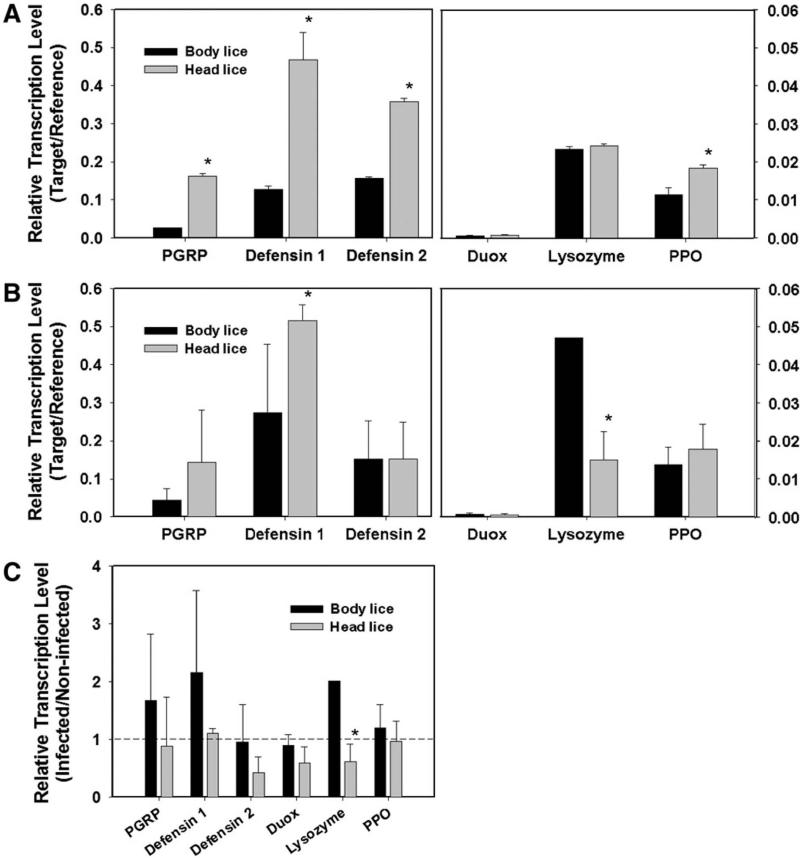
The immune reactions in the alimentary tract were investigated in both body and head lice following oral challenge of E. coli as a model Gram-negative bacterium [51]. In proliferation assays, head lice suppressed the growth of E. coli effectively at the early stage of infection, resulting in gradual reduction of E. coli number in alimentary tract tissues (Fig. 5). In contrast, the number of E. coli steadily increased in alimentary tract tissues of body lice. No apparent alteration of transcription was observed following E. coli challenge in three important genes for the humoral immune responses, peptidoglycan recognition protein as a recognition gene and defensin 1 and defensin 2 as effector genes (Fig. 6). Nevertheless, the basal transcription levels of these genes were higher in the gut tissues of head versus body lice. Considering that there is no cellular immune reaction in gut tissues, these findings suggest that the higher constitutive transcription levels of major immune genes in head lice can contribute to their rapid defense and enhanced immune capacity against intestinal bacterial infection.
Fig. 5.
Proliferation profiles of Escherichia coli in the alimentary tract tissues of orally-infected body and head lice. Louse EF1α and E. coli 16s rRNA were used as marker genes for the proper tissue preparation and the proliferation of E. coli, respectively. Reproduced with permission from Ref. [51]. Copyright 2012 Elsevier Inc.
Fig. 6.
Comparison of the relative transcription level of representative immune-related genes in the alimentary tract tissues of non-infected (A) or orally-infected (B) body and head lice and the fold-changes of transcript by infection (C). Bars with star mark (*) indicate statistical difference between body and head lice (p<0.05). Reproduced with permission from Ref. [51]. Copyright 2012 Elsevier Inc.
Highlights.
Human head and body lice offer an efficient model system to study resistance and immune response due, in part, to having small but complete genomes, reduced number of genes involved and the availability of successful mass rearing and bioassays
Kdr allele frequency, which is associated with DDT and pyrethroid resistance, is greater than 90% across the USA and Canada
Malathion resistance is due to enhanced hydrolytic ester cleavage by malathion carboxylesterase HLCbE3
Body lice appear to be more efficient vectors of pathogenic bacteria due to suppressed phagocytosis and innate immune response when compared to head lice following bacterial challenge
Abbreviations
- BR-HL
malathion- and DDT- resistant strain from Bristol, UK
- DDT
dichlorodiphenyltrichloroethane
- Duox
dual oxidase
- EC-HL
insecticide-susceptible head louse strain from Ecuador
- HLCbE3
head louse malathion carboxylesterase
- Imd
immune deficiency pathway
- kdr
knockdown resistance
- KR-HL
insecticide-susceptible head louse strain form the Republic of Korea
- MCE
malathion carboxylesterase
- PGRP
peptidoglycan recognition protein
- PPO
prophenoloxidase
- qPCR
reverse transcriptase quantitative real-time polymerase chain reaction
- RNAi
RNA interference
- SF-HL
DDT- and permethrin-resistant head louse strain from south Florida
- VSSC
voltage-sensitive sodium channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark JM, Yoon KS, Lee SH, Pittendrigh BR. Human lice: past present and future control. Pestic. Biochem. Physiol. 2013;106:162–171. [Google Scholar]
- 2.Durand R, Bouviesse S, Berdjane Z, Izri A, Chosidow O, Clark JM. Insecticide resistance in head lice: Clinical, parasitological and genetic aspects. Clin. Microbiol. Infect. 2012;18:338–344. doi: 10.1111/j.1469-0691.2012.03806.x. 2012. [DOI] [PubMed] [Google Scholar]
- 3.Hodgdon HE, Yoon KS, Previte DJ, Kim HJ, Abo El-Ghar GE, Lee SH, Clark JM. Serial invasive signal amplification reaction for the determination of kdr frequencies in global human head louse populations for efficient resistance monitoring. Pest Manag. Sci. 2010;66:1031–1040. doi: 10.1002/ps.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcoux D, Palma K, Kaul N, Hodgdon H, Van Geest A, Previte D, Abo-Elghar G, Yoon K, Clark J. Pyrethroid pediculicide resistance of head lice in Canada evaluated by serial invasive signal amplification reaction. J. Cutaneous Medicine & Surgery. 2010;14:115–118. doi: 10.2310/7750.2010.09032. [DOI] [PubMed] [Google Scholar]
- 5.Gao J-R, Yoon KS, Frisbie RK, Coles GC, Clark JM. Esterase-mediated malathion resistance in the human head louse, Pediculus capitis (Anoplura: Pediculidae). Pestic. Biochem. Physiol. 2006;85:28–37. [Google Scholar]
- 6.Durand R, Bouviesse S, Berdjane Z, Izri A, Chosidow O, Clark JM. Insecticide resistance in head lice: Clinical, parasitological and genetic aspects. Clin. Microbiol. Infect. 2012;18:338–344. doi: 10.1111/j.1469-0691.2012.03806.x. [DOI] [PubMed] [Google Scholar]
- 7.Chosidow O, Chastang C, Brue C, Bouvet E, Izri M, Monteny N, et al. Controlled study of malathion and d-phenothrin lotions for Pediculus humanus var capitis-infested schoolchildren. Lancet. 1994;344:1724–1727. doi: 10.1016/s0140-6736(94)92884-3. [DOI] [PubMed] [Google Scholar]
- 8.Hipolito RB, Mallorca FG, Zuniga-Macaraig ZO, Apolinario PC, Wheeler-Sherman J. Head lice infestation: single drug versus combination therapy with one percent permethrin and trimethoprim/sulfamethoxazole. Pediatrics. 2001;107(3):E30. doi: 10.1542/peds.107.3.e30. [DOI] [PubMed] [Google Scholar]
- 9.Burgess IF, Brown CM, Peock S, Kaufman J. Head lice resistant to pyrethroid insecticides in Britain. BMJ. 1995;311:752. doi: 10.1136/bmj.311.7007.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill N, Moor G, Cameron MM, Butlin A, Preston S, Williamson MS, et al. Single blind, randomised, comparative study of the Bug Buster kit and over the counter pediculicide treatments against head lice in the United Kingdom. BMJ. 331(2005):384–387. doi: 10.1136/bmj.38537.468623.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rupes V, Moravec J, Chmela J, Ledvinka J, Zelenkova J. A resistance of head lice (Pediculus capitis) to permethrin in Czech Republic. Cent Eur J Public Health. 1995;3:30–32. [PubMed] [Google Scholar]
- 12.Kristensen M, Knorr M, Rasmussen AM, Jespersen JB. Survey of permethrin and malathion resistance in human head lice populations from Denmark. J Med Entomol. 2006;43:533–538. doi: 10.1603/0022-2585(2006)43[533:sopamr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Mumcuoglu KY, Hemingway J, Miller J, Ioffe-Uspensky I, Klaus S, Ben-Ishai F F, et al. Permethrin resistance in the head louse Pediculus capitis from Israel. Med Vet Entomol. 1995;9:427–432. doi: 10.1111/j.1365-2915.1995.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 14.Meinking TL, Entzel P, Villar ME, Vicaria M, Lemard GA, Porcelain SL. Comparative efficacy of treatments for pediculosis capitis infestations: update 2000. Arch Dermatol. 2001;137:287–292. [PubMed] [Google Scholar]
- 15.Picollo MI, Vassena CV, Casadio AA, Massimo J, Zerba EN. Laboratory studies of susceptibility and resistance to insecticides in Pediculus capitis (Anoplura; Pediculidae). J Med Entomol. 1998;35:814–817. doi: 10.1093/jmedent/35.5.814. 1998. [DOI] [PubMed] [Google Scholar]
- 16.Tomita T, Yaguchi N, Mihara M, Takahashi M, Agui N, Kasai S. Molecular analysis of a para sodium channel gene from pyrethroid-resistant head lice, Pediculus humanus capitis (Anoplura: Pediculidae). J Med Entomol. 2003;40:468–474. doi: 10.1603/0022-2585-40.4.468. [DOI] [PubMed] [Google Scholar]
- 17.Hunter JA, Barker SC. Susceptibility of head lice (Pediculus humanus capitis) to pediculicides in Australia. Parasitol Res. 2003;90:476–478. doi: 10.1007/s00436-003-0881-y. [DOI] [PubMed] [Google Scholar]
- 18.Izri MA, Briere C. First cases of resistance of Pediculus capitis Linne 1758 to malathion in France. Presse Med. 1995;24:1444. [PubMed] [Google Scholar]
- 19.Downs AM, Stafford KA, Harvey I, Coles GC. Evidence for double resistance to permethrin and malathion in head lice. Br. J. Dermatol. 1999;141:508–511. doi: 10.1046/j.1365-2133.1999.03046.x. [DOI] [PubMed] [Google Scholar]
- 20.Yoon KS, Gao J-R, Lee SH, Clark JM, Brown L, Taplin D. Permethrin-resistant human head lice. Pediculus capitis, and their treatment. Arch. of Dermatol. 2003;139:994–1000. doi: 10.1001/archderm.139.8.994. [DOI] [PubMed] [Google Scholar]
- 21.Burgess IF. The mode of action of dimeticone 4% lotion against head lice, Pediculus capitis. BMC Pharmacology. 2009 doi: 10.1186/1471-2210-9-3. doi: 10.11186/1471-2210-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richling I, Bockele W. Lethal effects of treatment with a special dimeticone formula on head lice and house crickets (Orthoptera, Ensifera: Acheta domestica and Anoplura, Phthiraptera: Pediculus humanus)-Insights into physical mechanisms. Arzneimittelforschung. 2008;58:248–254. doi: 10.1055/s-0031-1296501. [DOI] [PubMed] [Google Scholar]
- 23.Strycharz JP, Lao AR, Alves AM, Clark JM. Ovicidal response of NYDA formulations on the human head louse (Anoplura, Pediculidae) using a hair tuft bioassay. J. Med. Entomol. 2012;49:336–342. doi: 10.1603/me11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher MH, M. H., Mrozik H. The avermectin family of macrolide-like antibiotics. In: Omura A, editor. MacrolideAntibiotics. NY. 1984. Academic Press; New York: 1984. pp. 553–606. [Google Scholar]
- 25.Brownlee DJ, Holde-Dye AL, Walker RJ. Actions of the anthelminic ivermectin on the pharyngeal muscle of the parasitic nematode, Ascaris suum. Parasitol. 1997;115:553–561. doi: 10.1017/s0031182097001601. 1997. [DOI] [PubMed] [Google Scholar]
- 26.Gill JH, Redwin JM, Van Wyk JA, Lacey E. Avermectin inhibition of larval development in Haomonchus contortus effects ivermectin resistance. Int. J. of Parasitol. 1995;25:463–470. doi: 10.1016/0020-7519(94)00087-5. [DOI] [PubMed] [Google Scholar]
- 27.Duce IC, Scott RH. Actions of dihydroavermectin B1a on insect muscle. Brit. J. Pharmacol. 1985;85:395–401. doi: 10.1111/j.1476-5381.1985.tb08874.x. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dent JA, Davis MW, Avery L. avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. The EMBO Journal. 1997;16:5867–5879. doi: 10.1093/emboj/16.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chosidow O, Giraudeau B, Cottrell J, Ozri A, Hofmann R, Mann SG, Burgess I. Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N. Engl. J. Med. 2010;362:896–905. doi: 10.1056/NEJMoa0905471. [DOI] [PubMed] [Google Scholar]
- 30.Strycharz JP, Yoon KS, Clark JM. A new ivermectin formulation topically kills permethrin-resistant human head lice (Anoplura: Pediculidae). J. Med Entomol. 2008;45:75–81. doi: 10.1603/0022-2585(2008)45[75:aniftk]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Strycharz JP, Berge NM, Alves AM, Clark JM. Ivermectin acts as a post-eclosion nymphicide by reducing blood feeding of human head lice (Anoplura, Pediculidae) that hatched from treated eggs. J. Med. Entomol. 2011;48:1174–1182. doi: 10.1603/me11051. [DOI] [PubMed] [Google Scholar]
- 32.Salgado V, Saar R. Desensitizing and non-desensitizing subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in cockroach neurons. J. Insect Physiol. 2004;50:867–879. doi: 10.1016/j.jinsphys.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Takano-Lee M, Yoon KS, Edman JD, Mullens BA, Clark JM. In vivo and in vitro rearing of Pediculus humanus capitis (Anoplura:Pediculidae). J. Med. Entomol. 2003;40:628–635. doi: 10.1603/0022-2585-40.5.628. [DOI] [PubMed] [Google Scholar]
- 34.Yoon KS, Strycharz JP, Gao JR, Takano-Lee M, Edman JD, Clark JM. An improved in vitro rearing system for the human head louse allows the determination of resistance to formulated pediculicides. Pestic. Biochem. Physiol. 2006;86:195–202. [Google Scholar]
- 35.Kirkness EF, Clark JM, et al. The Human Body Louse Genome Consortium Group Genome sequences of the human body louse and its endosymbiont provide insights into the permanent parasitic lifestyle. PNAS. 2010;107:12168–12173. doi: 10.1073/pnas.1003379107. (6th of 72) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olds BP, Coates BS, Steele LD, Sun W, Agunbiade TA, Yoon KS, Strycharz JP, Lee SH, Paige KN, Clark JM, Pittendrigh BR. Comparison of the transcriptional profiles of head and body lice. Insect. Mol. Biol. 2012;21:257–268. doi: 10.1111/j.1365-2583.2012.01132.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee SH, Yoon KS, Williamson MS, Goodson SJ, Takano-Lee M, Edman JD, Devonshire AL, Clark JM. Molecular analysis of kdr-type resistance in permethrin-resistant strains of head lice, Pediculus capitis. Pestic. Biochem. Physiol. 2000;66:130–143. 2000. [Google Scholar]
- 38.Busvine JR. Mechanism of resistance to insecticide in houseflies. Nature. 1951;168:193–195. doi: 10.1038/168193a0. 1951. [DOI] [PubMed] [Google Scholar]
- 39.Soderlund DM, Knipple DC. The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem. Mol. Biol. 2003;33:563–577. doi: 10.1016/s0965-1748(03)00023-7. 2003. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Gao J-R, Yoon KS, Mumcuoglu KY, Taplin D, Edman JD, Takano-Lee M, Clark JM. Sodium Channel Mutations Associated with Knockdown Resistance in the Human Head Louse, Pediculus capitis, (De Geer). Pestic. Biochem Physiol. 2003;75:79–91. 2003. [Google Scholar]
- 41.Schuler TH, Martinez-Torres D, Thompson AJ, Denholm I, Devonshire AL, Duce IR, Williamson MS. Toxicological, electrophysiological, and molecular characterization of knockdown resistance to pyrethroid insecticides in the diamondback moth, Plutella xylostella (L.). Pestic. Biochem. Physiol. 1998;59:169–182. [Google Scholar]
- 42.Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of TipE, a novel membrane protein that enhances Drosophila para sodium channel function. Cell. 1995;82:1001–1011. doi: 10.1016/0092-8674(95)90279-1. 1995. [DOI] [PubMed] [Google Scholar]
- 43.Smith TJ, T.J., Lee SH, Ingles S,PJ, Knipple DC, Soderlund DM. The L1014F point mutation in the house fly Vssc1 sodium channel confers knockdown resistance to pyrethroids. Insect Biochem. Mol. Biol. 1997;27:807–812. doi: 10.1016/s0965-1748(97)00065-9. [DOI] [PubMed] [Google Scholar]
- 44.Yoon KS, Symington SB, Lee S-H, Soderlund DM, Clark JM. Three mutations identified in the voltage-sensitive sodium channel α-subunit gene of permethrin-resistant human head lice abolish permethrin sensitivity of the house fly Vssc1 expressed in Xenopus oocytes. Insect. Biochem. & Mol. Biol. 2008;38:296–306. doi: 10.1016/j.ibmb.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon KS, Previte DJ, Hodgdon HE, Poole BC, Kwon DH, Abo El-Ghar GE, Lee SH, Clark JM. Knockdown resistance allele frequencies in North American head louse populations. J. Med. Entomol. 2014;51:450–457. doi: 10.1603/me13139. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwon DK, Kim JH, Kim YH, Yoon KS, Clark JM, Lee SH. Identification and characterization of the esterase involved in malathion resistance in the head louse, Pediculus humanus capitis. Pestic. Biochem. Physiol. 2014;112:13–18. doi: 10.1016/j.pestbp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Johnson JS, Yoon KS, Strycharz JP, Pittendrigh BR, Clark JM. Body and head lice (Phthiraptera: Pediculidae) have the smallest genomes of any hemimetablous insect reported to date. J. Med. Entomol. 2007;44:1009–1012. doi: 10.1603/0022-2585(2007)44[1009:blahla]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 48.Lee SH, Min JS, Yoon KS, Strycharz JP, Johnson R, Mittapalli O, Margam VM, Sun W, Berenbaum MR, Pittendrigh BR, Clark JM. Decreased detoxification genes and genome size makes the human body louse an efficient model to study xenobiotic metabolism. Insect Mol. Biology. 2010;19:599–615. doi: 10.1111/j.1365-2583.2010.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JH, Min JS, Kang JS, Kwon DH, Yoon KS, Strycharz JP, Koh YH, Pittendrigh BR, Clark JM, Lee SH. Comparison of the humoral and cellular responses between body and head lice following immune challenge. Insect Biochem. Mol. Biol. 2011;41:333–339. doi: 10.1016/j.ibmb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Olds BP, Coates BS, Steele LD, Sun W, Agunbiade TA, Yoon KS, Strycharz JP, Lee SH, Paige KN, Clark JM, Pittendrigh BR. Comparison of the transcriptional profiles of head and body lice. Insect Mol. Biol. 2012;21:257–268. doi: 10.1111/j.1365-2583.2012.01132.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim JH, Yoon KS, Previte DJ, Pittendrigh BR, Clark JM, Lee SH. Comparison of the immune response in alimentary tract tissue from body versus head lice following Escherichia coli oral infection. J. Asia-Pacific Entomol. 2012;15:409–412. [Google Scholar]
- 52.Vontas J, Blass C, Koutsos AC, David JP, Kafatos FC, Louis C, Hemingway J, Christophides GK, Ranson H. Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect Mol. Biol. 2005;14:509–521. doi: 10.1111/j.1365-2583.2005.00582.x. (2005) [DOI] [PubMed] [Google Scholar]
- 53.Willoughby L, Chung H, Lumb C, Robin C, Batterham P, Daborn PJ. A comparison of Drosophila melanogaster detoxification gene induction responses for six insecticides, caffeine and phenobarbital. Insect Biochem. and Mol. Biol. 2006;36:934–942. doi: 10.1016/j.ibmb.2006.09.004. (2006) [DOI] [PubMed] [Google Scholar]
- 54.Yoon KS, Strycharz JP, Baek JH, Sun W, Kim JH, Kang JS, Pittendrigh BR, Lee SH, Clark JM. Brief exposures of human body lice to sub-lethal amounts of ivermectin over transcribe genes involved in tolerance. Insect Mol. Biol. 2011;20:687–699. doi: 10.1111/j.1365-2583.2011.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]