Abstract
Objectives
Little is known about long-term changes in insulin-like growth Factor (IGF) proteins and glycemic status. We hypothesized that changes in IGF proteins are exaggerated in participants with type 2 diabetes or worsening glycemia versus those that remain normoglycemic over a 9-year follow-up period.
Design
Retrospective analysis of cohort study.
Setting
Participants were recruited from four States: North Carolina, California, Maryland and Pennsylvania.
Participants
897 participants enrolled in CHS All Stars, a cohort study of community dwelling adults aged ≥65 years.
Measurements
Plasma IGF-I, IGFBP-1, and IGFBP-3 levels were assessed and ADA cut-points for IGT, IFG, and diabetes were used to classify participants at baseline (1996–1997) and follow-up (2005–2006).
Results
At baseline, mean age was 76.3 years (± 3.6) and 18.5% had diabetes. Individuals with IFG alone and IGT+IFG had the highest levels of IGF-I and lowest levels of IGFBP-1, compared to individuals with normoglycemia or diabetes. The greatest percent change in IGF levels occurred in those who had diabetes at baseline (9-year changes: −9.3% for IGF-I, 59.7% for IGFBP-1, −13.4% for IGFBP-3); the smallest in individuals who remained normoglycemic at follow-up (9-year changes: −3.7% for IGF-I, 25.6% for IGFBP-1, −6.4% for IGFBP-3); and intermediate changes in those who were normoglycemic but developed IFG at follow-up.
Conclusion
Our results demonstrate that degrees of glycemic impairment are associated with varying levels of changes in IGF proteins. The exaggerated changes observed in the diabetes group have been previously shown to be associated with heart failure, cancer and non-cancer mortality.
Keywords: insulin-like growth factor, diabetes, glycemic status
INTRODUCTION
The Insulin-like growth Factor (IGF) axis is an evolutionarily conserved system with important physiologic roles during embryonic development, growth and adulthood [2]. IGF-I has structural and functional homology with insulin. Similar to insulin, IGF-I promotes glucose uptake in peripheral tissues and improves insulin resistance [3–7].
IGF-I, which is produced in the liver, circulates in the blood bound to high affinity IGF binding proteins (IGFBP-1 to IGFBP-6) and an acid-labile subunit (ALS). IGFBP-3, along with ALS, binds 90–95% of IGF-I and is considered a reservoir of circulating IGF-I [8]. IGFBP-1 has strong negative correlation with fasting insulin levels because insulin down regulates the hepatic production of IGFBP-1 [9].
The ability of IGF-I to induce physiologic responses similar to that of insulin has fueled the hypothesis that IGF-I activity can compensate for insulin in those with insulin resistance. Animal studies have shown that during the early stages of diabetes, locally expressed IGF-I increases Beta cell proliferation and induces regeneration of pancreatic islets [10, 11]. Whether there are compensatory physiologic changes in IGF proteins in humans who have developed impaired glucose tolerance (IGT), impaired fasting glucose (IFG), or type 2 diabetes is not clear.
Previous studies suggest that inflammation may also play a role in the IGF axis because inflammatory markers such as CRP, and IL-6 correlate with IGF proteins [8]. This has not been studied in a longitudinal setting and further complicating the picture are well-documented changes in IGF protein levels with aging. Among adults, IGF-I and IGFBP-3 levels decrease with age while IGFBP-1 levels increase. The association of age-related changes in levels of IGF proteins with glycemic status is not well understood. Specifically, little is known about changes in IGF-I and IGFBPs in subjects who initially had normal glucose levels but later developed IGT, IFG, both IGT and IFG, or diabetes. In addition, studies suggest that IFG and IGT likely have different pathophysiology. Peripheral insulin sensitivity is compromised more in IGT while hepatic insulin sensitivity is compromised more in IFG [12].
The current study was conducted within the Cardiovascular Health Study (CHS) All Stars, a prospective cohort of older adults [13]. The goals were to assess the association between IGF protein levels and glycemic status, and evaluate whether mean percent changes in IGF protein levels differed between participants whose glycemic status remained the same versus those who worsened over a 9-year follow-up period.
METHODS
Setting and Participants
The CHS is a population-based, prospective cohort of community-dwelling adults aged 65 years [14]. The cohort consists of 5201 participants recruited in 1989/1990 from four U.S. communities (Washington County, MD; Allegheny County, PA; Forsyth County, NC; and Sacramento County, CA). An additional 687 participants, the majority of whom were African Americans, were recruited in 1992/1993. Potential participants were recruited from a randomly generated sampling frame derived from Medicare eligibility lists of the Health Care Financing Administration (HCFA). Standardized examinations and questionnaires were administered annually through 1999. In 2005/2006, surviving participants who were at least 77 years of age were further evaluated as a part of the CHS All Stars Study [15]. This current study is a retrospective analysis of the CHS All Stars. Informed consent was provided by participants in accordance with the institutional review board guidelines at their clinic site.
Selection of visits
The 1996/1997 visit was defined as the study baseline, and the 2005/2006 visit as follow-up because IGF levels were available for those years. CHS All Stars recruited a total of 1677 individuals, but only 897 participants had at least one IGF variable, fasting glucose levels from the 1996/1997 and 2005/2006 visits, as well as a 2-hour post glucose load levels from the baseline visit.
Laboratory measurements
Fasting blood samples were collected at baseline and follow-up using standard procedures and stored at −70° C. At baseline, but not at the follow-up visit, a second blood sample was obtained 2 hours after ingestion of a 75g glucose load [16]. Measurements of IGF-I, IGFBP-1 and IGFBP-3 using the stored blood samples were performed at the Jewish General Hospital (Montreal, Canada) [17]. Assay coefficient of variation was 4–6% for IGF-I, 3–14% for IGFBP-1, and 3–5% for IGFBP-3. Standard methods were used to measure glucose, insulin and C-reactive protein (CRP) at the CHS Central Laboratory [16].
Clinical and laboratory covariates
American Diabetes Association cut-points for normal glycemia, impaired glucose tolerance (IGT), impaired fasting glucose (IFG), and type 2 diabetes were used for classification of participants [18]. Diabetes was defined as fasting plasma glucose (FPG) ≥126 mg/dl or 2-h plasma glucose ≥200 mg/dl or if the participant reported pharmacological treatment for diabetes. Normoglycemia was defined as FPG <100 mg/dl and 2-h post load plasma glucose <140 mg/dl; IGT was defined as 2-h plasma glucose measured after a 75g oral glucose tolerance test (OGTT) of 140–199 mg/dl and FPG <100 mg/dl; and IFG was defined as FPG of 100–125 mg/dl and a 2-h post load plasma glucose <140 mg/dl. Due to the fact that OGTT was not performed at follow-up, reclassification of participants at follow-up was completed using only the fasting glucose measures stated above. Among those with diabetes, treatment status was defined as use of hypoglycemic agents or insulin. Anthropometric measurements including height, weight, Body mass index (BMI(kg/m2)) and waist circumference were collected. In CHS, physical activity variable represents an estimate of weekly energy expenditure while doing leisure activities [19].
Statistical Analysis
The goals of the statistical analyses were threefold; to 1) examine baseline differences in IGF protein levels in participants with varying degrees of glycemic status; 2) assess the hypothesis that changes in IGF proteins over time would differ between those who maintained favorable glycemic status during follow-up (i.e., normal at baseline and follow-up), those whose glycemic status worsened (i.e., normal at baseline but IFG at follow-up), and those with established diabetes at baseline; and 3) examine associations between baseline IGF proteins and anthropometric factors, albumin, CRP and IL-6 within the glycemic groups.
First, participants were categorized as having normal glycemic status, IGT only, IFG only, both IGT + IFG, or diabetes based on data from the baseline (1996–1997) examinations. For IGFBP-1, non-parametric tests were used because the variable was not normally distributed. Mean or median differences in IGF proteins at baseline, adjusted for age, race, sex, BMI, insulin levels, C-reactive protein, physical activity and smoking status, were compared among groups with varying glycemic status using Student’s t-tests and bar graphs for normally distributed IGF variables and Wilcoxon rank-sum test and box and whisker plots for variables not normally distributed.
Secondly, using data from the follow-up visit (2005–2006), we defined subgroups of participants who remained normoglycemic at baseline and follow-up, advanced from normoglycemia at baseline to IFG at follow-up, or had diabetes at baseline. Other categories (i.e., those normal at baseline but had diabetes at follow-up) were not included in this analysis due to small sample sizes. Mean and median differences in absolute and percent change in IGF protein levels amongst these groups, adjusted for age, race, sex, BMI, insulin levels, C-reactive protein, physical activity and smoking status, were evaluated using Student’s t-tests and Wilcoxon rank-sum tests, respectively. Stratified analysis based on sex, median age, race, median fasting insulin and use of hormone therapy among women or diabetes medication use was undertaken to determine whether levels and changes in IGF proteins differed among subgroups. When differences were noted in the stratified analysis, figures are presented for comparison.
Additionally, to control for the effects of known confounders such as age, race and sex, we used linear regression models to compare percent change in IGF proteins among a grouped category of those who either had diabetes at baseline or were non-diabetic but progressed to a worse glycemic status (ie: NL/IFG, NL/DM or IFG/DM) to percent change in IGF proteins in a grouped category of those who either maintained stable glycemic status or reverted to a more favorable glycemic status at follow-up (ie: IFG/NL). These “reverters” were grouped with those who maintained normal glycemia because they were borderline hyperglycemics at baseline. Interactions with age, sex, race, BMI, physical activity and smoking status were formally tested (p-value < 0.15).
Finally, correlations between IGF protein levels and BMI, waist circumference, CRP, IL-6 and albumin were evaluated within each baseline glycemic status category using age-, race- and sex-adjusted Pearson partial correlation coefficients. All analyses were performed using Stata Version 12.1 (College Station, TX: StataCorp LP).
RESULTS
At baseline (1996–1997), the average age of the 897 CHS All Stars participants included in this investigation was 76.3 years (SD=3.6) (Table I), 62% of them were female and 16% were African Americans. Overall, at baseline 166 (18.5%) of the participants had diabetes, 45% of those were treated with insulin or hypoglycemic agents while 6.3% of those without diabetes at baseline developed diabetes at follow up. Of those who had prediabetes at baseline, 11% reverted back to normal glycemic status at follow up.
Table I.
Baseline Characteristics of the Cardiovascular Health Study All Stars Participants (n=897)
| Mean (±SD) | n (%) | |
|---|---|---|
| Age, years | 76.3 (3.55) | |
| Male | 340 (37.9%) | |
| African American | 144 (16.1%) | |
| Body mass index, kg/m2 | 27.3 (4.47) | |
| Waist circumference, cm | 97.1 (12.68) | |
| C-reactive protein, mg/liter | 2.28 (1.04 – 4.86)* | |
| Diabetes | 166 (18.5%) | |
| IGF-I, ng/ml | 166.8 (61.6) | |
| IGFBP-1, ng/ml | 32.66 (18.78 – 52.41)* | |
| IGFBP-3, ng/ml | 3363.1 (887.5) |
Fewer than 5% of study participants were missing data for each variable. IGFBP; Insulin-like Growth Factor Binding Protein. Only 3 participants were missing BMI values, 9 were missing waist circumference values, 7 were missing C-Reactive Protein values, 6 were missing IGFBP1 values while 2 were missing IGFBP3 values. Published units can be changed to SI units using the following conversion factors: creatinine, mg/dl × 88.4 = μmol/liter; C-reactive protein, mg/liter × 9.524 = nmol/liter; IGF-I, μg/liter × 0.131 = nmol/liter; IGFBP-1, μg/liter × 0.22 = nmol/liter; and IGFBP-3, μg/liter × 0.035 = nmol/liter.
Numbers reported are the median and the 25th–75th percentiles
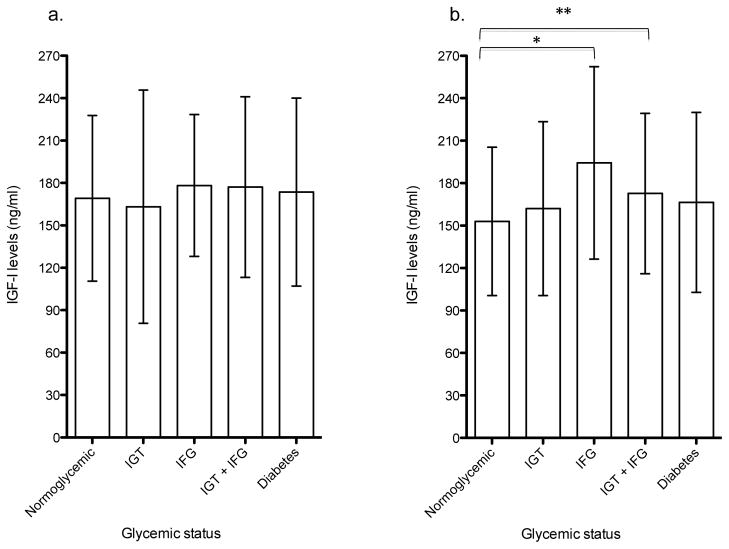
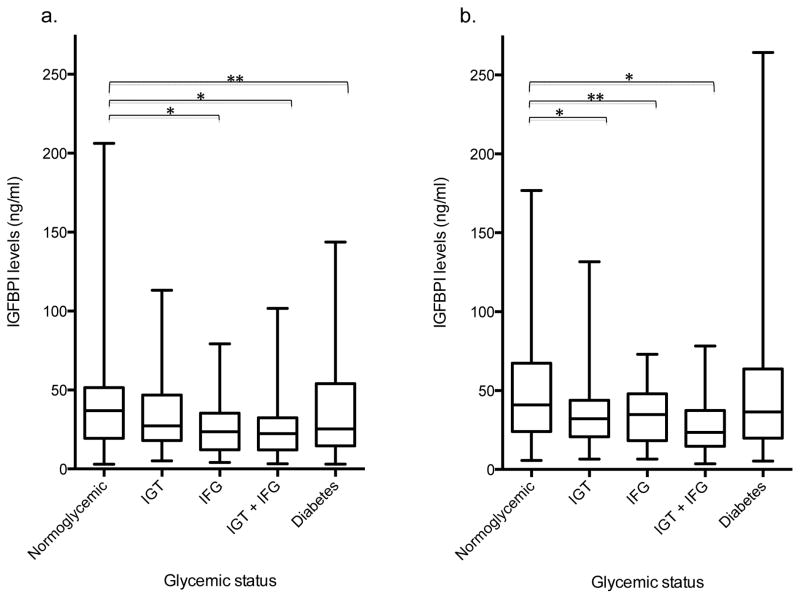
At baseline, mean IGF-I levels in the IFG alone and IGT + IFG groups were significantly higher than that of the normoglycemia group, with the highest levels in the IFG only group (Table II). Participants with diabetes had IGF-I levels that were not significantly different from those of normoglycemic participants. Mean age at baseline did not differ across glycemic groups (Table II), however, differences in IGF-I levels across groups were more prominent in those greater than or equal to the median age of 75.9 years (Figure Ia–b). Baseline median IGFBP-1 levels decreased progressively across groups of normoglycemia, IGT alone, IFG alone and IGT + IFG (Table II). The median IGFBP-1 level in each of the pre-diabetes or diabetes categories as defined by IFG or IGT was significantly different from the median IGFBP-1 level in the normoglycemia group (Table II; p<0.01 for IGT only, IFG only and IGT + IFG; p<0.05 for diabetes). Results were generally consistent within age subgroups when analyses were stratified by median age (Figure II). Baseline IGFBP-3 levels were higher in the pre-diabetes groups as compared with the normoglycemia group, and highest in the diabetes group (Table II; all p<0.05), especially in those younger than the median age of 75.9 years. In participants older than the median age, IGFBP-3 levels in the diabetes group was not significantly higher than that of the normoglycemic group (data not shown). Adjustment for age, race, sex, BMI, insulin, C-reactive protein, physical activity and smoking status did not alter associations but rather, explained some of the variability in protein levels within each glycemic category as evidenced by the decrease in standard deviations and interquartile range.
Table II.
Baseline (1996–1997) Levels of IGFs by Glycemic Status (n=897)
| Normoglycemia (n=399) | IGT only (n=148) | IFG only (n=93) | IGT + IFG (n=91) | Diabetes (n=166) | |
|---|---|---|---|---|---|
| Glucose, 2 hr post load, mg/dl | < 140 | 140–199 | < 140 | 140–199 | ≥ 200 |
| FPG, mg/dl | <100 | <100 | 100–125 | 100–125 | ≥ 126 |
|
| |||||
| Age, yearsa | 76.5 ±3.7 | 76.6 ±3.5 | 75.7 ±3.5 | 75.8 ±3.2 | 76.2 ±3.5 |
| Femaleb | 61.2 | 79.7 | 39.8 | 53.9 | 65.7 |
| African Americanb | 14.5 | 10.1 | 16.1 | 18.7 | 23.5 |
| BMI, kg/m2a | 25.9 ±3.87 | 27.4 ±4.28 | 27.6 ±4.17 | 29.2 ±4.76 | 29.3 ±4.81 |
| Insulin, μU/mla | 8.56±4.4 | 10.55±7.1 | 12.79±8.0 | 13.36±7.5 | 19.15±31.5 |
| C-reactive proteinc | 1.6 (0.9 – 3.7) | 2.8 (1.3 – 5.1) | 2.1 (1 – 4.1) | 2.8 (1.3 – 5.3) | 1.6 (3.7 – 7.1) |
| Physical Activity (kcal)c | 1102 (473 – 2275) | 956 (368 – 1913) | 1000 (304 – 2113) | 1035 (446 – 2160) | 620 (245 – 1605) |
| Smoking Statusb | 50.4 | 32.0 | 61.1 | 59.6 | 41 |
| Hormone therapyb | 24.6 | 22.0 | 5.4 | 26.5 | 21.1 |
|
| |||||
| IGF Proteins | |||||
| IGF-I, ng/mla | 160.7 ±56.0 | 162.5 ±71.6 | 185.5 ±59.1 * | 175.2 ±60.6† | 169.9 ±64.9 |
| Adjusted IGF-I, ng/mla | 161.1 ±14.6 | 162.2 ±13.4 | 184.4 ±22.8 | 172.5 ±13.9 | 168 ±17.3 |
| IGFBP-1, ng/mlc | 38.9 (22.5 – 59.1) | 30.6 (19.6 – 44.5) * | 24.9 (15.2 – 43.3) * | 23.3 (14.3 – 33.7) * | 32.7 (16.2 – 57.3) † |
| Adjusted IGFBP-1, ng/mlc | 43.9 (38.2 – 50.0) | 37.2 (30.8 – 42.5) | 29.3 (23.2 – 37.5) | 29.3 (20.7 – 38.0) | 42.2 (33.9 – 50.1) |
| IGFBP-3, ng/mla | 3246 ±805.2 | 3445 ±890.7 † | 3445 ±922.5 † | 3396 ±795.7 * | 3508 ±1059.1 † |
| Adjusted IGFBP-3, ng/mla | 3247 ±342.4 | 3441 ± 293 | 3437 ±397 | 3394 ±333 | 3481 ±342 |
Values reported are the mean (± standard deviations)
Values reported are percentages. Smoking status is percentage of those who ever smoked out of total. Hormone therapy only includes percent of females on therapy.
Values reported are the median and 25th–75th percentiles in parentheses. Physical activity is weekly energy expenditure.
Adjusted means, median, p25 and p75 are adjusted for age, sex, race, BMI, fasting insulin, C-reactive protein, physical activity and smoking status.
Versus normoglycemia, P<0.01
Versus normoglycemia, P < 0.05
IGFBP; Insulin-like Growth Factor Binding Protein; FPG, fasting plasma glucose; IGT, impaired glucose tolerance; IFG, impaired fasting glucose.
Figure I.
Mean baseline (1996–1997) IGF-I levels across glycemic groups in CHS cohort. IIa: Age < median age (75.9 years), n=445. IIb: Age ≥ median age (75.9 years), n=452. Error bars represent standard deviations. IGF-I; Insulin-like Growth Factor-I; FPG, fasting plasma glucose; IFG, impaired fasting glucose.
*P<0.01
** P<0.05
Figure II.
Median baseline (1996–1997) IGFBP-1 levels across glycemic groups in CHS cohort. a: Age < median age (75.9 years), n=440. b: Age ≥ median age (75.9 years), n=451. 6 people had missing data on IGFBP-1. IGFBP; Insulin-like Growth Factor Binding Protein-1; IFG, impaired fasting glucose; IGT, impaired glucose tolerance.
*P<0.01
** P<0.05
The proportion of participants who were African American increased with worsening glycemic status, while the proportion of female participants differed across groups defined by glycemic status (Table II). Of those who had diabetes, 37% were taking oral hypoglycemic agents while 11% were on insulin therapy at baseline while by follow-up 55% were taking oral hypoglycemic agents and 17% were on insulin therapy (data not shown). Among those with diabetes at baseline, IGF-I, IGFBP-1 and IGFBP-3 were not significantly different among those who were treated versus those who were untreated (IGF-I: p=0.10, IGFBP-1: p=0.052, IGFBP-3: p=0.09). Diabetes treatment at baseline and/or follow up had no significant effect on percent change in IGF proteins over time (IGF-I: p=0.50, IGFBP-1: p=0.89, IGFBP-3: p=0.42), after adjusting for age, race, BMI, physical activity and smoking. When analyses were restricted to whites only, or men, women, those above the median insulin, those below the median insulin, or excluding those taking diabetes medications or on hormone therapy, results were similar to the overall findings described in Tables II and III.
Table III.
Changes in IGF Protein Levels by Glycemic Status (n=559)
| Baseline Status | Normoglycemia (n=399) | Diabetes (n=166) | |
|---|---|---|---|
|
|
|
|
|
| Follow-up | Normoglycemia (n=277) | IFG (n=116) | Diabetes (n=166) |
|
|
|
|
|
| IGF-I (ng/ml) | |||
| Baseline | 158.1 ±56.5 | 166.3 ±55.5 | 169.9 ±64.9 |
| Follow-up | 146.1 ±57.6 | 152.2 ±52.3 | 144.9 ±57.8 |
| Absolute change | −12.0 ±43.6 | −14.1 ±40.9 | −25.0 ±57.4b |
| Percent change | −3.7 ±33.2 | −5.1 ±27.2 | −9.3 ±36.9 |
| Adjusted Percent change | −3.9 ±6.3 | −7.0 ±7.0 a | −8.6 ±7.8 a |
| IGFBP-1 (ng/ml)* | |||
| Baseline | 41.7 (24.0 – 61.2) | 34.4 (18.8 – 50.5) | 32.7 (16.2 – 57.3) |
| Follow-up | 52.6 (31.3 – 76.6) | 43.1 (25.6 – 66.7) | 53.4 (35.2 – 88.3) |
| Absolute change | 9.1 (−5.7 – 24.7) | 8.6 (−0.1 – 21.4) | 20.0 (−0.1 – 43.7)a |
| Percent change | 25.6 (−9.9 – 76.7) | 33.2 (−0.2 – 97.9) | 59.7 (−0.4 – 185.1)a |
| Adjusted Percent change | 51.4 (34.0 – 70.9) | 60.0 (42.3 – 77.8)b | 154.7 (134.6 – 181.3)a |
| IGFBP-3 (ng/ml) | |||
| Baseline | 3247.0 ±813.5 | 3248.4 ±793.5 | 3507.7 ±1059.1 |
| Follow-up | 3029.8 ±917.5 | 2992.3 ±777.7 | 2971.6 ±884.7 |
| Absolute change | −217.3 ±550.6 | −247.5 ±482.4 | −536.1 ±750.0a |
| Percent change | −6.4 ±17.2 | −6.9 ±14.9 | −13.4 ±19.2a |
| Adjusted Percent change | −6.3 ±2.4 | −7.2 ±2.4b | −13.3 ±2.5a |
Values reported are means (± standard deviations) unless stated otherwise.
Values reported are medians with 25th–75th percentiles in parentheses
Versus Normoglycemia, p<0.001
Versus Normoglycemia, p<0.01
Versus Normoglycemia, p<0.05
Absolute and percent change values in bold were significantly different from zero, one-sample t-test, p<0.05
Adjusted means, median, p25 and p75 are adjusted for age, sex, race, BMI, fasting insulin, C-reactive protein, physical activity and smoking status.
IGFBP; Insulin-like Growth Factor Binding Protein; FPG, fasting plasma glucose; IFG, impaired fasting glucose. Other categories (i.e., those normal at baseline but had diabetes at follow-up or those with IFG at baseline but had diabetes at follow-up) were not included in this analysis due to small sample sizes.
Table III shows the mean IGF-I and IGFBP-3 and median IGFBP-1 levels at baseline and the follow-up visit conducted 9 years subsequently. The mean and median absolute and percent changes are reported for all three IGF proteins grouped by the baseline/follow-up glycemic status. Mean IGF-I and IGFBP-3 levels decreased during follow-up, and the largest decreases were observed in those who had diabetes at baseline. The smallest changes in IGF-I and IGFBP-3 levels were observed in participants who were normoglycemic at baseline and follow-up. Among those who were normoglycemic at baseline but developed IFG, the percent decrease in IGF-I and IGFBP-3 was intermediate between those for the normoglycemic and diabetes groups. Median IGFBP-1 increased during follow-up. As with IGF-I and IGFBP-3 levels, the greatest increase in IGFBP-1 was observed in those who had diabetes, and the smallest change in IGFBP-1 was observed among those who remained normoglycemic.
After controlling for age, sex and race in the linear regression model, those who either worsened or had diabetes at baseline had a 2.6% decrease in IGF-I (p=0.30), a 60% increase in IGFBP-1 (p<0.001) and a 3.6% decrease in IGFBP-3 (p=0.007) when compared to those who either reverted or maintained normal glycemic status. The 60% change in IGFBP-1 remained statistically significant after additional adjustments for baseline BMI, total physical activity and smoking status (p<0.001). There were no significant interactions with age, sex, race, BMI, physical activity and smoking status (p-value >0.15).
IGFBP-1 had moderate-to-strong inverse correlations with BMI and waist circumference; these associations were stronger in the normoglycemia, IFG only and IGT only groups than in the IFG + IGT or diabetes groups (data not shown). CRP was inversely correlated with IGF-I and IGFBP-3, especially in the normoglycemia and IGT only groups. Serum albumin was positively correlated with IGF-I and IGFBP-3, and this association was most robust among the normoglycemia and IGT only groups.
DISCUSSION
Previous studies suggest that low total IGF-I levels are associated with increased risk of IGT and diabetes [20, 21], although a recent study in women found no association [22]. Due to a lack of longitudinal studies, it is not clear whether changes in IGF protein levels accompany worsening glycemic status. Our study addressed the hypothesis that at baseline, IGF-I levels would be elevated in older adults with prediabetes states, reflecting compensatory changes in the IGF axis in response to relatively mild glucose intolerance. We further hypothesized that stable IGF-I levels would persist among those who maintained normal glycemia over time, while groups with impaired glycemia would be associated with decline IGF-I, as is typically ascribed to the aging process.
First, in cross sectional analyses we found an inverted U-shaped relationship between IGF-I and degree of hyperglycemia. This is generally consistent with another study that reported increasing IGF-I bioactivity in the 1st to 9th deciles of HOMA-IR but a decrease in the 10th decile [23]. Maintenance of relatively high IGF-I levels in the pre-diabetes states could be a compensatory mechanism that occurs in those with mild insulin resistance in an attempt to maintain normal glucose levels. A paradoxical decrease in IGF-I in the diabetes group suggests that compensation may not occur in this group. Insulin, which is increased in the initial diabetes state, is necessary for GH stimulation of its receptor as evidenced by the GH resistance that develops in individuals with insulin-dependent diabetes [24]. However, evidence suggests that prolonged insulin exposure may have the opposite effect and could explain the lower IGF-I levels in the diabetes group. Insulin has a biphasic effect on growth hormone, where at low concentrations insulin increases the biosynthesis of GH, but at chronically high levels, insulin decreases translocation of the GH to the plasma membrane of hepatocytes [24–26]. These studies were animal/in vitro studies using human hepatoma cell lines; however, the possibility that chronically high insulin levels which occur in the later pre-diabetes and diabetes states down regulate GH receptors and in turn decrease IGF-I levels cannot be ignored. Further studies are needed to assess long-term reciprocal effects between hyperinsulinemia and IGF proteins. In light of the divergent effects of insulin on GH, it is not surprising that over nine years, those with diabetes, who undoubtedly may have exhausted their pancreatic reserve, experienced the highest percent decrease in IGF-I. Insulin levels were not measured at follow-up so assessment of the proportion of individuals with diabetes who still experience hyperinsulinemia was not feasible. While IGF-I levels decrease with age, we found that individuals who maintained normoglycemia or developed IFG had relatively small percent changes (~4% and 5% respectively) in IGF-I. This supports the hypothesis that development of prediabetes is associated with maintenance of relatively higher IGF-I levels, which can compensate for insulin action. The diabetes group who, although at baseline had IGF-I levels that were higher than the normoglycemic group, demonstrated the highest percent decrease (~ 10%) in IGF-I over time. Baseline IGF-I levels were negatively associated with CRP in the normoglycemic and IGT only groups. This suggests that inflammation is common among those with lower IGF-I levels and the previously published association of low total IGF-I with diabetes may be mediated in part or full by inflammation, though this will need to be tested formally.
Insulin down regulates the hepatic production of IGFBP-1; thus, patients developing insulin resistance have decreasing levels of IGFBP-1. Our data show that levels of IGFBP-1 decreased progressively from normoglycemia to pre-diabetes groups, but median IGFBP-1 was paradoxically high in the diabetes group. Although insulin levels were highest in the diabetes group at baseline, we still observed high levels of IGFBP-1, implying a disruption in the normal regulation of IGFBP-1 by insulin in those with type-2 diabetes [27, 28]. Because high and increasing IGFBP-1 levels are associated with increased risk of heart failure and other potentially fatal age-related conditions [17, 29, 30], it is notable that diabetics had about a 60% increase in IGFBP-1 levels in our study’s longitudinal component. Low IGFBP-1 levels are associated with high BMI and waist circumference. In our study, these inverse associations were moderate-to-strong only in the normoglycemia and pre-diabetes groups and not as apparent in the diabetes group, suggesting that those with diabetes have altered physiologic changes in the IGF axis. Inflammation may not influence the association between IGFBP-1 and glycemic status because IGFBP-1 was not associated with IL-6 or CRP.
Our study showed that IGFBP-3 levels were high in younger participants with diabetes. Although this association was based on cross-sectional analysis, a previous study suggests that high IGFBP-3 levels may be a risk factor for diabetes in middle-aged adults [22]. Overexpression of IGFBP-3 in animal studies decreases glucose-mediated insulin secretion in pancreatic islets and increases fasting hyperglycemia and glucose in tolerance [31]. In our study, IGFBP-3 decreased most markedly in those who had diabetes at baseline.
Due to the observational nature of the analysis, we cannot propose a cause and effect relationship between changes in IGF proteins and worsening glycemic status. Nonetheless, these findings are notable given that changes in IGF proteins with aging are strong independent predictors of clinical outcomes such as coronary heart disease and mortality [17, 32]. Assessment of clinical outcomes was beyond the scope of this study, although our results support the speculation that exaggerated changes in IGF proteins in individuals with diabetes may further increase the risk of other age-related diseases associated with the IGF axis and mortality [17, 29, 30].
This study has several strengths, which include a prospective design with long follow-up, and evaluation of IGF proteins in five different glycemic groups. To our knowledge, it is the first to compare changes in IGF protein levels in older individuals grouped by changes in glycemic status. Levels of IGF proteins are highly age-dependent and thus, care should be taken when generalizing these findings to other age groups. The long time interval between the baseline and follow-up measurements is possibly a weakness because other factors, apart from glycemic status, could have influenced IGF protein levels at follow-up. However, our regression models, which adjusted for several potential factors, showed similar findings.
Although we used total IGF-I levels instead of IGF-I bioactivity in our analyses, studies using IGF-I bioactivity report a statistically significant positive correlation with total IGF-I [33]. Furthermore, the cross-sectional relationship between IGF-I and glycemic status that we observed was similar to that of a study that used IGF-I bioactivity [23]. We did not have data on OGTTs for the follow-up examination. As a result, those who were normal at baseline but developed only IGT at follow-up were classified normoglycemics at follow-up since follow-up categories were based only on fasting glucose data. However, in the regression models, participants were classified using only fasting measures from baseline and follow-up blood samples and there were no substantial differences in our findings. Associations between IGF proteins and insulin resistance were stronger in the older subgroup of the cohort, which likely reflects the cumulative impact of IGF-I and IGFBP changes with age.
While age-related changes in IGF proteins are well described, our results suggest that changes in IGF proteins vary in elderly individuals with different glycemic profiles. Changes in IGF proteins seen in individuals with diabetes in our cohort are precisely the changes that identify people at high risk for cancer and non-cancer mortality, congestive heart failure, and difficulties with activities of daily living [17, 29, 30, 32]. Although treatment can potentially modify these risks [34, 35], further research is warranted on medications, diet and behavioral interventions that modify IGF protein levels in an attempt to reduce the risk of diseases associated with the IGF axis.
Acknowledgments
Funding Source: The project described was supported by the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), through CTSA grant numbers UL1TR000086, TL1RR000087, and KL2TR000088. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Additional support by contracts HHSN268201200036C, N01HC85239, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging [1]. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: The research question was formulated by Swapnil Rajpathak and Robert Kaplan. Chino Aneke, Christina Parrinello and Robert Kaplan planned all analyses and Chino Aneke completed all statistical analyses and drafted the manuscript. Christina Parrinello, Swapnil Rajpathak, Thomas Rohan, Elsa Strotmeyer, Stephen Kritchevsky, Bruce Psaty, Petra Bůžková, Jorge Kizer, Anne Newman, Howard Strickler and Robert Kaplan contributed to interpretation and discussion of data, and reviewed and edited the manuscript. The guarantor’s name is Chino S. Aneke.
Sponsor’s Role: The sponsor, Dr. Kaplan, was involved in each step of this study including formulation of the research question, data acquisition, study design and analysis.
References
- 1.Bacha F, Arslanian SA. Ghrelin suppression in overweight children: A manifestation of insulin resistance? J Clin Endocrinol Metab. 2005;90:2725–2730. doi: 10.1210/jc.2004-1582. [DOI] [PubMed] [Google Scholar]
- 2.Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633–640. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- 3.Jacob R, Barrett E, Plewe G, Fagin KD, Sherwin RS. Acute effects of insulin-like growth factor-I on glucose and amino acid metabolism in the awake fasted rat. Comparison with insulin. J Clin Invest. 1989;83:1717–1723. doi: 10.1172/JCI114072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemmons DR. Role of insulin-like growth factor in maintaining normal glucose homeostasis. Horm Res Paediatr. 2004;62 (Suppl 1):77–82. doi: 10.1159/000080763. [DOI] [PubMed] [Google Scholar]
- 5.Murphy LJ. The role of the insulin-like growth factors and their binding proteins in glucose homeostasis. J Diabetes Res. 2003;4:213–224. doi: 10.1155/EDR.2003.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulware SD, Tamborlane WV, Rennert NJ, et al. Comparison of the metabolic effects of recombinant human insulin-like growth factor-I and insulin. Dose-response relationships in healthy young and middle-aged adults. J Clin Invest. 1994;93:1131–1139. doi: 10.1172/JCI117065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moses AC, Young SC, Morrow LA, et al. Recombinant human insulin-like growth factor I increases insulin sensitivity and improves glycemic control in type II diabetes. Diabetes. 1996;45:91–100. doi: 10.2337/diab.45.1.91. [DOI] [PubMed] [Google Scholar]
- 8.Rajpathak SN, McGinn AP, Strickler HD, et al. Insulin-like growth factor-(IGF)-axis, inflammation, and glucose intolerance among older adults. Growth Horm IGF Res. 2008;18:166–173. doi: 10.1016/j.ghir.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee PD, Giudice LC, Conover CA, et al. Insulin-like growth factor binding protein-1: Recent findings and new directions. Proc Soc Exp Biol Med. 1997;216:319–357. doi: 10.3181/00379727-216-44182. [DOI] [PubMed] [Google Scholar]
- 10.George M, Ayuso E, Casellas A, et al. Beta cell expression of IGF-I leads to recovery from type 1 diabetes. J Clin Invest. 2002;109:1153–1163. doi: 10.1172/JCI12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hugl SR, White MF, Rhodes CJ. Insulin-like growth factor I (IGF-I)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. J Biol Chem. 1998;273:17771–17779. doi: 10.1074/jbc.273.28.17771. [DOI] [PubMed] [Google Scholar]
- 12.Ferrannini E, Gastaldelli A, Iozzo P. Pathophysiology of prediabetes. Med Clin North Am. 2011;95:327–339. vii–viii. doi: 10.1016/j.mcna.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Newman AB, Arnold AM, Sachs MCJ, et al. Long-term function in an older cohort--the cardiovascular health study all stars study. J Am Geriatr Soc. 2009;57:432–440. doi: 10.1111/j.1532-5415.2008.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 15.Strotmeyer ES, Arnold AM, Boudreau RM, et al. Long-term retention of older adults in the Cardiovascular Health Study: Implications for studies of the oldest old. J Am Geriatr Soc. 2010;58:696–701. doi: 10.1111/j.1532-5415.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cushman M, Cornell ES, Howard PR, et al. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 17.Kaplan RC, McGinn AP, Pollak MN, et al. Total insulin like growth factor 1 and insulin like growth factor binding protein levels, functional status, and mortality in older adults. J Am Geriatr Soc. 2008;56:652–660. doi: 10.1111/j.1532-5415.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes A. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34 (Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soares-Miranda L, Sattelmair J, Chaves P, et al. Physical activity and heart rate variability in older adults: The Cardiovascular Health Study. Circulation. 2014;129:2100–2110. doi: 10.1161/CIRCULATIONAHA.113.005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandhu MS, Heald AH, Gibson JM, et al. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: A prospective observational study. Lancet. 2002;359:1740–1745. doi: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- 21.Vaessen N, Heutink P, Janssen JA, et al. A polymorphism in the gene for IGF-I: functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes. 2001;50:637–642. doi: 10.2337/diabetes.50.3.637. [DOI] [PubMed] [Google Scholar]
- 22.Rajpathak SN, He M, Sun Q, et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes. 2012;61:2248–2254. doi: 10.2337/db11-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brugts MP, van Duijn CM, Hofland LJ, et al. Igf-I bioactivity in an elderly population: relation to insulin sensitivity, insulin levels, and the metabolic syndrome. Diabetes. 2010;59:505–508. doi: 10.2337/db09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung KC, Doyle N, Ballesteros M, et al. Insulin regulation of human hepatic growth hormone receptors: Divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000;85:4712–4720. doi: 10.1210/jcem.85.12.7017. [DOI] [PubMed] [Google Scholar]
- 25.Ji S, Guan R, Frank SJ, Messina JL. Insulin inhibits growth hormone signaling via the growth hormone receptor/JAK2/STAT5B pathway. J Biol Chem. 1999;274:13434–13442. doi: 10.1074/jbc.274.19.13434. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Ji S, Venable DY, et al. Prolonged insulin treatment inhibits GH signaling via STAT3 and STAT1. J Endocrinol. 2005;184:481–492. doi: 10.1677/joe.1.05977. [DOI] [PubMed] [Google Scholar]
- 27.Gibson JM, Westwood M, Crosby SR, et al. Choice of treatment affects plasma levels of insulin-like growth factor-binding protein-1 in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1995;80:1369–1375. doi: 10.1210/jcem.80.4.7536208. [DOI] [PubMed] [Google Scholar]
- 28.Lewitt MS, Hall K, Bang P, et al. Altered response of insulin-like growth factor-binding protein 1 to nutritional deprivation in type 2 diabetes mellitus. Metabolism. 2005;54:275–280. doi: 10.1016/j.metabol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan RC, McGinn AP, Pollak MN, et al. High insulin like growth factor binding protein 1 level predicts incident congestive heart failure in the elderly. Am Heart J. 2008;155:1006–1012. doi: 10.1016/j.ahj.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan RC, Buzkova P, Cappola AR, et al. Decline in circulating insulin-like growth factors and mortality in older adults: Cardiovascular health study all-stars study. J Clin Endocrinol Metab. 2012;97:1970–1976. doi: 10.1210/jc.2011-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen KH, Yao XH, Moulik S, et al. Human IGF binding protein-3 overexpression impairs glucose regulation in mice via an inhibition of insulin secretion. Endocrinology. 2011;152:2184–2196. doi: 10.1210/en.2010-1324. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan RC, McGinn AP, Pollak MN, et al. Association of total insulin-like growth factor-I, insulin-like growth factor binding protein-1 (IGFBP-1), and IGFBP-3 levels with incident coronary events and ischemic stroke. J Clin Endocrinol Metab. 2007;92:1319–1325. doi: 10.1210/jc.2006-1631. [DOI] [PubMed] [Google Scholar]
- 33.Vitale G, Brugts MP, Ogliari G, et al. Low circulating IGF-I bioactivity is associated with human longevity: Findings in centenarians’ offspring. Aging (Albany NY) 2012;4:580–589. doi: 10.18632/aging.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norby FL, Wold LE, Duan J, et al. IGF-I attenuates diabetes-induced cardiac contractile dysfunction in ventricular myocytes. Am J Physiol Endocrinol Metab. 2002;283:E658–666. doi: 10.1152/ajpendo.00003.2002. [DOI] [PubMed] [Google Scholar]
- 35.Seigel GM, Lupien SB, Campbell LM, et al. Systemic IGF-I treatment inhibits cell death in diabetic rat retina. J Diabetes Complications. 2006;20:196–204. doi: 10.1016/j.jdiacomp.2005.06.007. [DOI] [PubMed] [Google Scholar]