Abstract
Background/Aims
Acute pyelonephritis (APN) is the most common cause of community-onset bacteremia in hospitalized elderly patients. The objectives of this study were to investigate the differences in the clinical and microbiological data of hospitalized elderly and non-elderly women with community-onset APN.
Methods
Women with community-onset APN as a discharge diagnosis were identified from January 2004 to December 2013 using an electronic medical records system. We compared the clinical and microbiologic data in elderly and non-elderly women with community-onset APN due to Enterobacteriaceae.
Results
Of the 1,134 women with community-onset APN caused by Enterobacteriaceae, 443 were elderly and 691 were non-elderly women. The elderly group had a lower frequency of upper and lower urinary tract symptoms/signs than the non-elderly. The incidence of bacteremia, extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, patients with a C-reactive protein (CRP) level ≥ 15 mg/dL, and patients with a leukocyte count ≥ 15,000/mm3 in the blood, were significantly higher in the elderly group than in the non-elderly group. The proportion of patients requiring hospitalization for 10 days or more was significantly higher in the elderly group compared to the non-elderly group (51.5% vs. 26.2%, p < 0.001). The clinical cure rates at 4 to 14 days after the end of therapy were 98.3% (338/344) and 97.4% (519/533) in the elderly and non-elderly groups, respectively (p = 0.393).
Conclusions
Elderly women with APN exhibit higher serum CRP levels, a higher frequency of bacteremia, a higher proportion of ESBL-producing uropathogens, and require a longer hospitalization than non-elderly women, although these patients may not complain of typical urinary symptoms.
Keywords: Pyelonephritis, Aged, Non-elderly, Enterobacteriaceae
INTRODUCTION
The proportion of people age 65 and older is expected to increase in the Republic of Korea (ROK), and the increasing proportion of elderly persons will have a great impact on the healthcare system. Acute pyelonephritis (APN) is the most common cause of community-acquired bacteremia in hospitalized elderly patients [1,2]. Although most bacteriuria in the elderly is asymptomatic or accompanied by mild urinary tract infection (UTI) symptoms, a mild UTI can develop into severe and life-threatening sepsis. Many comorbid conditions and decreased immune and physiologic functions can make the elderly susceptible to UTIs. Thus, many comorbid conditions are considered to be complicating factors and include the functional or structural abnormalities of the urinary tract.
UTI symptoms or signs of infection can be atypical and vague in the elderly, even in an upper urinary tract infection such as APN. Since the clinical manifestation of community-onset APN can be different between elderly and non-elderly patients, a different approach for the diagnosis and management of the elderly may be required. The basic data necessary in order to establish diagnostic and therapeutic guidelines for elderly patients with APN include: clinical presentation, microbiology, and clinical outcomes.
Advanced age is considered to be a complicating factor of APN, without reference to the presence of other complicating factors [3]. At the initial presentation of community-onset APN, gender, menopause status, and a history of comorbid conditions can be used for the classification or stratification of APN without the need to conduct other costly examinations for the identification of complicating factors. Advanced age is one of the risk factors easily identified during the initial presentation of APN, and can be used for classifying the APN, or predicting the prognosis of patients with APN [3,4,5]. However, there have been few clinical studies comparing the clinical manifestation, microbiology, and clinical outcomes of elderly or non-elderly women with community-onset APN in the ROK. Therfore, a different approach for diagnosing and treating the elderly APN group may be required [6,7,8].
The objective of this study was to investigate differences in the clinical and microbiological data of elderly and non-elderly women with community-onset APN who were hospitalized at a university hospital in the ROK from January 2004 to December 2013.
METHODS
Study design
The current work was a retrospective study of patients with community-onset non-obstructive APN due to Enterobacteriaceae, conducted at the St. Vincent's Hospital, College of Medicine, The Catholic University of Korea in Suwon, South Korea, from January 2004 to December 2013. The study protocol was approved by the Institutional Review Board (IRB) of the St. Vincent's Hospital. The IRB waived the requirement to document informed consent for all subjects in this study.
Patient population
Women aged ≥ 18-year-old with community-onset non-obstructive APN due to Enterobacteriaceae and who were hospitalized at St. Vincent's Hospital were eligible for inclusion in this study. The clinical and demographic data were collected retrospectively using an electronic medical records system. Clinical symptoms, comorbid conditions, microbial pathogens, laboratory findings, and antimicrobial regimens were analyzed by reviewing the electronic medical records of women who were diagnosed with APN as a discharge diagnosis found in the hospital discharge database between January 2004 and December 2013. APN was defined by the presence of a fever (body temperature ≥ 38.0℃), pyuria on urinalysis (≥ 5 to 9 leukocytes/high power field), and bacteriuria with a colony count of ≥ 100,000 colony-forming units (CFU)/mL for clean voided urine [9,10]. Patients with the following conditions were excluded from the study: (1) a catheter-associated UTI; (2) an obstructive APN that demanded interventional managements, such as catheterization, percutaneous nephrostomy, or surgical treatment; or (3) APN occurring 48 hours or more after hospital admission. The following were considered as complicating factors: non-obstructive renal stone, underactive bladder, polycystic kidney disease, vesicoureteral reflux, diabetes mellitus, cerebrovascular disorder, chronic liver disease, chronic renal disease, congestive heart failure, connective tissue disorder, malignancy, and pregnancy [11,12]. Underactive bladder was diagnosed by urologists and women with underactive bladder requiring catheterization or urological interventional procedures were excluded in this study. Finally, this study solely included and analyzed urine culture-confirmed cases of community-onset non-obstructive APN caused by Enterobacteriaceae in women ≥ 18-years-old who were hospitalized and initially treated with an intravenous antimicrobial agent.
Data collection
The study collected and analyzed baseline demographic characteristics, medical history, underlying diseases, urinary tract symptoms, physical examination findings, laboratory findings, the duration of antibiotic therapy, microbiological data, defervescent time, duration of hospital stay, and mortality by reviewing the electronic medical records of the study participants. Clinical assessments including clinical symptoms, body temperature, and laboratory findings were performed at the time of admission, after 72 hours of antimicrobial treatment, and at the 4 to 14 day follow-up visit after the end of therapy (EOT).
Definitions and clinical outcome assessments
The study compared the time to defervescence after antimicrobial therapy was initiated, the duration of the hospital stay, the rate of early clinical success, and clinical cure between elderly and non-elderly women with community-onset APN. Elderly was defined as the age of 65 years or above. Women under 65 were defined as non-elderly.
Early clinical success was defined as defervescence within 72 hours after the start of the initial antimicrobial treatment. A clinical cure was defined as defervescence and the absence of UTI symptoms or signs at the 4 to 14 day follow-up visit after the EOT. Clinical failure was defined as the persistence or recurrence of pretherapy urinary tract signs and symptoms within the 4 to 14 day follow-up after completion of antimicrobial therapy [13,14].
Defervescence was defined as the afebrile state of the body temperature (tympanic) at or below 37.0℃ for 24 hours or longer [10]. The time to defervescence was defined as the time period after beginning an intravenous antimicrobial agent until defervescence.
Initial intravenous antibiotics were considered concordant if the in vitro susceptibility testing revealed that the initial antibiotics were active against the causative uropathogens.
Microbiological data and outcome assessments
Urine and blood cultures were conducted to detect the causative uropathogens and identify the in vitro susceptibility of the uropathogens in women with febrile APN at the time of admission. The etiological agents were confirmed by the presence of any micro-organisms with a colony count of ≥ 105 CFU/mL in urine cultures or the isolation of micro-organisms from blood specimens of patients with APN. Species and antibiotic susceptibility patterns of urinary tract pathogens were identified and revealed using a semiautomated microbiology system (VITEK, bioMerieux, Hazelwood, MO, USA; or Microscan, DADE Behring, West Sacramento, CA, USA) [15]. A microbiological cure was defined as the eradication of all uropathogens or a reduction of the urine pathogen population from ≥ 105 CFU/mL to < 104 CFU/mL, with no pathogenic micro-organism present in the blood.
Statistical methods
The results are presented as the number of patients (percentage of total) or median (interquartile range [IQR], 1Q to 3Q), or mean ± standard deviation. We used Fisher exact test or the Pearson chi-square test to compare categorical variables. Continuous variables were assessed and analyzed using the Mann-Whitney test or an independent t test. We conducted a multivariate analysis using logistic regression to estimate the influence of independent variables on a longer hospitalization (≥ 10 days) and early clinical failure in APN patients initially treated with intravenous antimicrobial agents. Variables with a p < 0.1 in the univariate analyses were included in the multivariate analyses. We used SPSS version 21.0 (IBM Co., Armonk, NY, USA) to analyze the resultant data. All statistical tests were two-tailed, and p values of less than 0.05 indicated a statistically significant difference.
RESULTS
Demographic and clinical characteristics
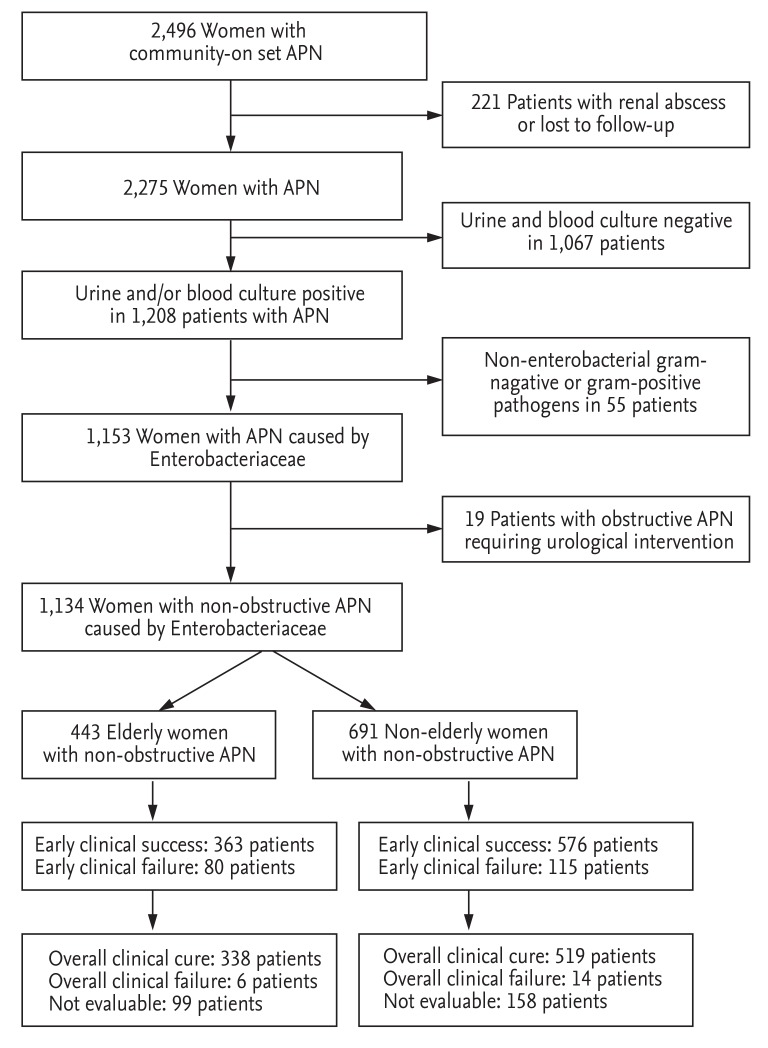
A total of 2,496 patients with community-onset APN were screened and identified from the electronic data from January 2004 to December 2013. From these, 1,134 patients with community-onset non-obstructive APN resulting from Enterobacteriaceae infection, who were hospitalized and received initial antibiotic treatment as an intravenous antimicrobial agent, were analyzed (Fig. 1). Of these 1,134 patients, 443 were classified as elderly, and 691 were considered non-elderly women. For the initial antibiotic treatment, 526 patients (46.4%) received cefuroxime, 373 patients (32.9%) gentamicin, and 235 patients (20.7%) cefotaxime. Table 1 shows a comparison of the baseline demographics, clinical characteristics, comorbid conditions, and laboratory findings in the elderly and non-elderly groups. The median ages of the elderly and non-elderly groups were 73 years (IQR, 69 to 78) and 44 years (IQR, 31 to 53), respectively (p < 0.001) (Table 1). The initial body temperature, the frequency of costovertebral angle tenderness, flank pain, and lower UTI symptoms were significantly lower in the elderly group than in the non-elderly group. The elderly group also showed a significantly higher proportion of bacteremia, extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, patients with a C-reactive protein (CRP) level ≥ 15 mg/dL, and patients with a leukocyte count ≥ 15,000/mm3 in the blood (Table 1). A history of a previous occurrence of a UTI and the frequency of antibiotic usage within 12 months prior to the hospital visit were markedly increased in the non-elderly group (Table 1).
Figure 1. A schematic diagram of the study design and subject enrollment. APN, acute pyelonephritis.
Table 1. Clinical characteristics and laboratory findings of elderly and non-elderly women with community-onset non-obstructive acute pyelonephritis caused by Enterobacteriaceae.
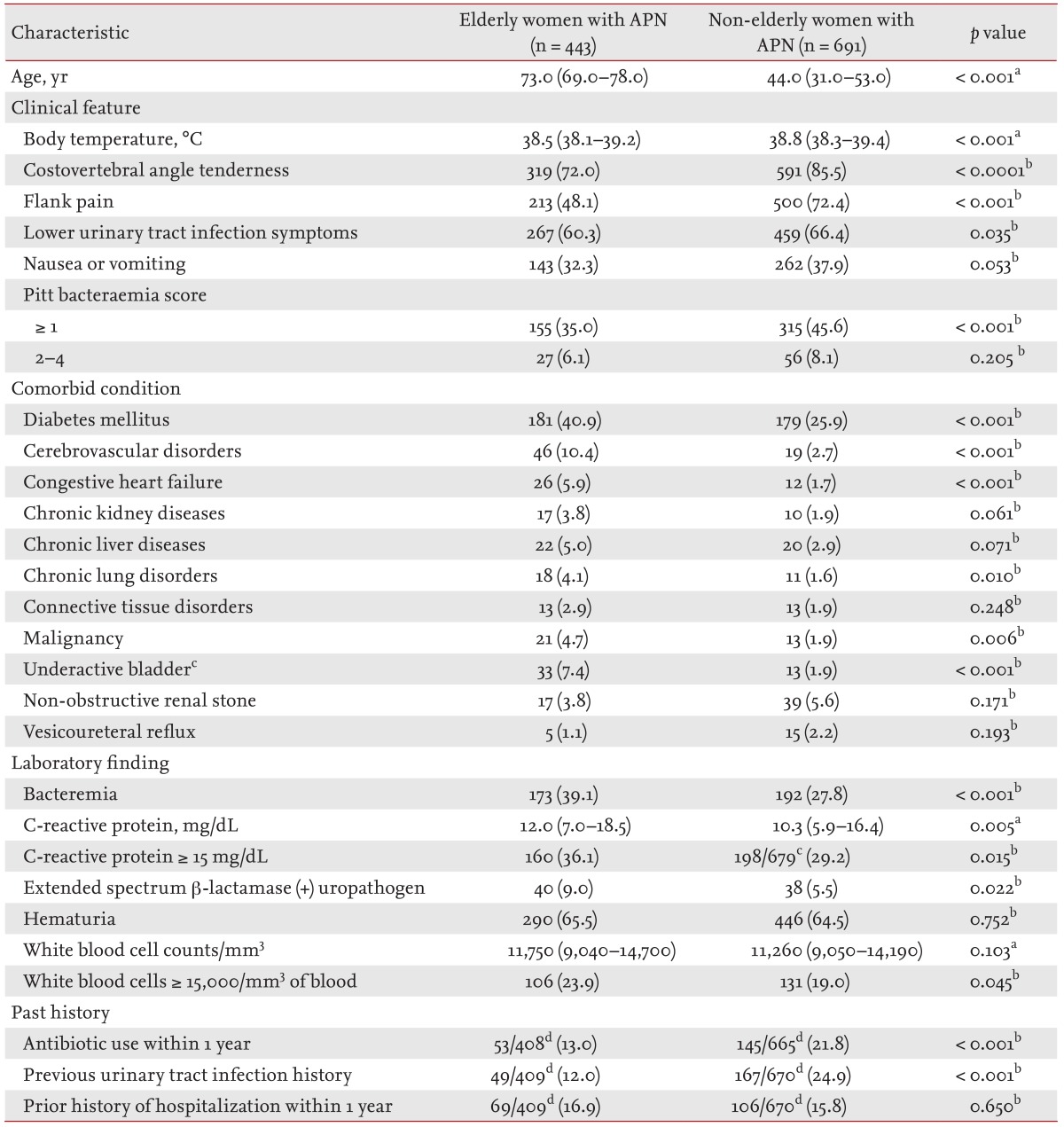
Values are presented as median (interquartile range) or number (%).
APN, acute pyelonephritis.
aMann-Whitney U test.
bPearson chi-square test or Fisher exact test.
cUnderactive bladder not requiring catheterization or urological interventional procedures.
dDenominators were the number of patients whose data were available in each group.
Microbiological data
Of the 1,134 cases enrolled in the study, Escherichia coli was the predominant uropathogen (1,069 patients, 94.3%) and non-E. coli Enterobacteriaceae were isolated from 65 patients (5.7%). The non-E. coli isolates included 31 Klebsiella pneumoniae, eight Enterobacter aerogenes, seven Enterobacter cloacae, seven Proteus mirabilis, five Citrobacter koseri, four Citrobacter freundii, two Klebsiella oxytoca, and one Klebsiella ozanae. The results of the antibacterial susceptibility testing of the 1,143 Enterobacteriaceae isolates are described in Table 2. The susceptibility of Enterobacteriaceae to amikacin, ampicillin, amoxicillin/clavulanate, cefuroxime, fluoroquinolone, gentamicin, tobramycin, piperacillin/tazobactam, and trimethoprim/sulfamethoxazole were not significantly different between the elderly and non-elderly groups.
Table 2. Antimicrobial susceptibility of enterobacteriaceae isolated from elderly or non-elderly women with community-onset non-obstructive acute pyelonephritis.
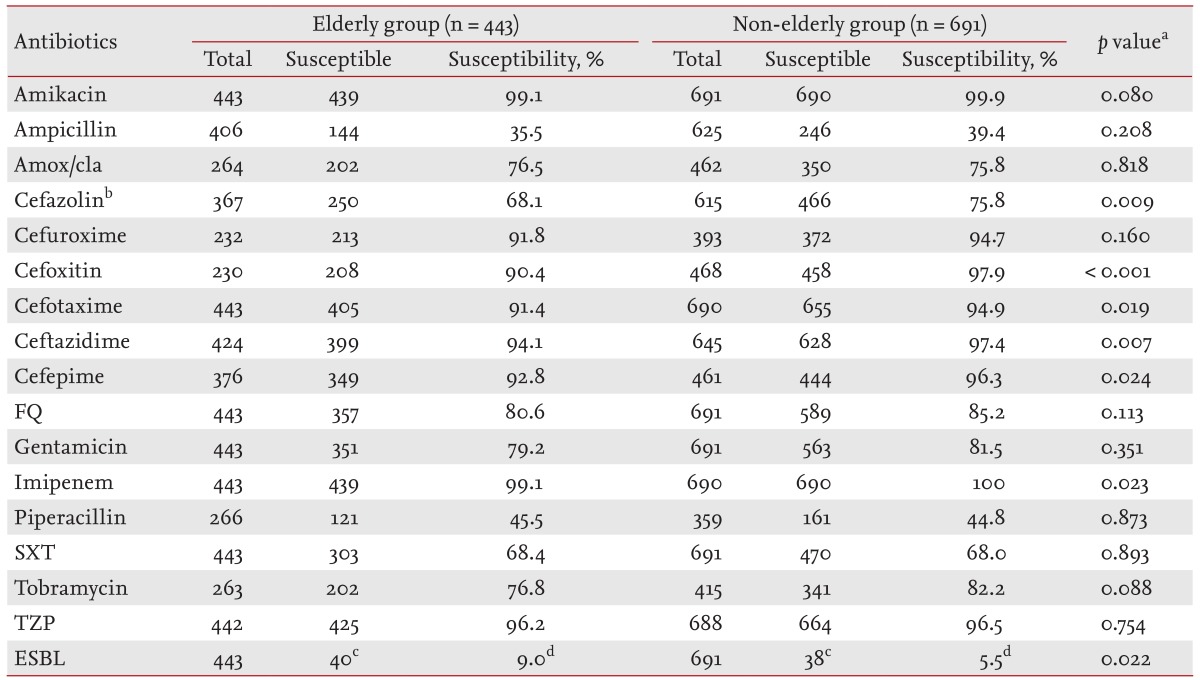
Amox/cla, amoxicillin/clavulanate; FQ, fluoroquinolone (ciprofloxacin or levofloxacin); SXT, trimethoprim/sulfamethoxazole; TZP, piperacillin/tazobactam; ESBL, extended-spectrum β-lactamase.
aPearson chi-square test or Fisher exact test.
bCefazolin, cefazolin or cephradine (first cephalosporin).
cThe number of ESBL-producing Enterobacteriaceae.
dThe percentage of ESBL-producing Enterobacteriaceae.
In the elderly versus non-elderly groups, the susceptibility of Enterobacteriaceae to cefotaxime (91.4% vs. 94.9%, p = 0.019), ceftazidime (94.1% vs. 97.4%, p = 0.007), and cefepime (92.8% vs. 96.3%, p = 0.024) was significantly higher in the non-elderly group. In addition, the proportion of ESBL-producing Enterobacteriaceae was significantly higher in the elderly group (9.0% vs. 5.5%, p = 0.022).
Comparison of clinical outcomes between the elderly and non-elderly groups
The current study compared clinical outcomes of patients in the elderly and non-elderly groups (Table 3). The duration of the initial intravenous antibiotic treatment was 7 days (IQR, 5 to 7) and 6 days (IQR, 5 to 7), respectively, in the elderly and non-elderly group (p < 0.001). The proportions of the initial concordant intravenous antimicrobial therapy were determined not to be significant (p = 0.178). Of the 1,134 patients enrolled in the study, 877 (77.3%) and 553 (48.8%), respectively, had a clinical and microbiological follow-up after 4 to 14 days after the completion of antibiotic therapy.
Table 3. Comparison of clinical outcomes of elderly or non-elderly women with community-onset non-obstructive acute pyelonephritis.
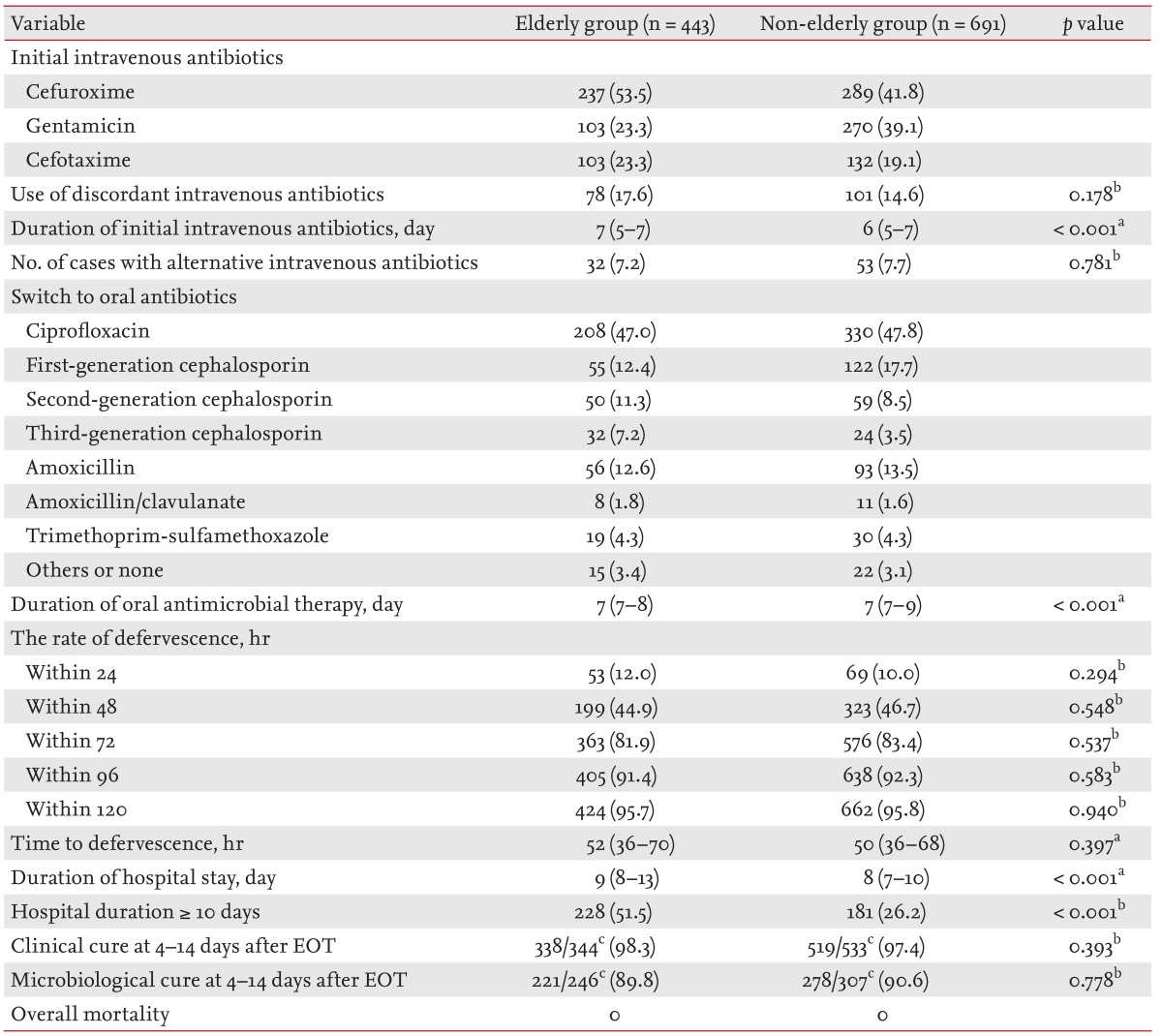
Values are presented as number (%) or median (interquartile range).
EOT, the end of therapy.
aMann-Whitney U test.
bPearson chi-square test or Fisher exact test.
cDenominators were the number of patients whose data were available in each group.
Thirty-two (7.2%) of the 443 patients in the elderly group and 53 (7.7%) of the 691 patients in the non-elderly group had their course of treatment changed to an alternative intravenous therapy. The defervescence rates were 12.0% (53/443) versus 10.0% (69/691) at 24 hours, 44.9% (199/443) versus 46.7% (323/691) at 48 hours, 81.9% (363/443) versus 83.4% (576/691) at 72 hours, 91.4% (405/443) versus 92.3% (638/691) at 96 hours, and 95.7% (424/443) versus 95.8% (662/691) at 120 hours after the initial antimicrobial agents were started in the elderly versus non-elderly groups, respectively, and these differences were not significantly different between the two groups (Table 3). The median hours to defervescence were 52 (IQR, 36 to 70) and 50 (IQR, 36 to 68) in the elderly and non-elderly groups, respectively (p = 0.397).
The clinical cure rates observed at 4 to 14 days after the EOT were 98.3% (338/344) and 97.4% (519/533) in the elderly and non-elderly groups, respectively (p = 0.393). Microbiological outcomes were available for 553 out of 1,134 women at the 4- to 14-day follow-up after the EOT. Microbiological cure rates at 4 to 14 days after the EOT were 89.8% (221/246) versus 90.6% (278/307) in the elderly versus non-elderly groups, respectively (p = 0.778).
Out of 1,134 women, 78 had APN due to ESBL-positive Enterobacteriaceae and 1,056 had APN due to ESBL-negative Enterobacteriaceae. The time to defervescence was not significantly different in the ESBL-positive versus ESBL-negative groups at 49.2 hours versus 49.3 hours (p = 0.990), respectively. Additionally, no significant differences were observed in urinary tract symptoms, comorbid conditions, frequency of previous UTI history, proportion of hematuria, and bacteremia between the ESBL-positive and ESBL-negative Enterobacteriaceae groups. However, the frequency of antibiotic use and hospitalization within 1 year prior to the hospital visit in the ESBL-positive group were significantly higher than those observed in the ESBL-negative group (p < 0.001 and p < 0.001). In contrast, the early clinical success rates at 72 hours in the ESBL-positive group were significantly lower than those in the ESBL-negative group (83.4% [881/1,056] vs. 74.4% [58/78], p = 0.041). In addition, the clinical and microbiological cure rates 4 to 14 days after the EOT in the ESBL-positive group were significantly lower than those in the ESBL-negative group (92.5% [62/67] vs. 98.1% [795/810], p = 0.005; and 77.8% [42/54] vs. 91.6% [457/499], p = 0.001).
Clinical significance and risk factors of early clinical failure in women with APN
We classified the women with APN into two groups: the early clinical success group (939 patients, 82.8%) and the early failure group (195 patients, 17.2%) according to the presence of defervescence after 72 hours of antimicrobial therapy. These two groups demonstrated no major differences in age, proportion of premenopause, urinary tract symptoms, frequency of previous UTI infection, frequency of antibiotic use, and hospitalization within 1 year prior to the hospital visit. However, the proportion of bacteremia, hematuria, and ESBL-producing Enterobacteriaceae in the early clinical success group were markedly lower. The initial leukocyte count, percentage of segmented neutrophils, and proportion of patients with a leukocyte count ≥ 15,000/mm3 in the blood were also significantly lower in the early clinical success group. Finally, a multivariate analysis using logistic regression determined that a Pitt score ≥ 1 (p = 0.022), underactive bladder (p = 0.034), bacteremia (p < 0.001), a CRP level ≥ 15 mg/dL (p < 0.001), white blood cell counts ≥ 15,000/mm3 in the blood (p < 0.001), and discordant antimicrobial therapy (p < 0.001) were closely associated with early clinical failure (Table 4).
Table 4. Factors related to early clinical failure in elderly and non-elderly women with community-onset acute pyelonephritis caused by Enterobacteriaceae in a final model of multiple logistic regression, 2004 to 2013.
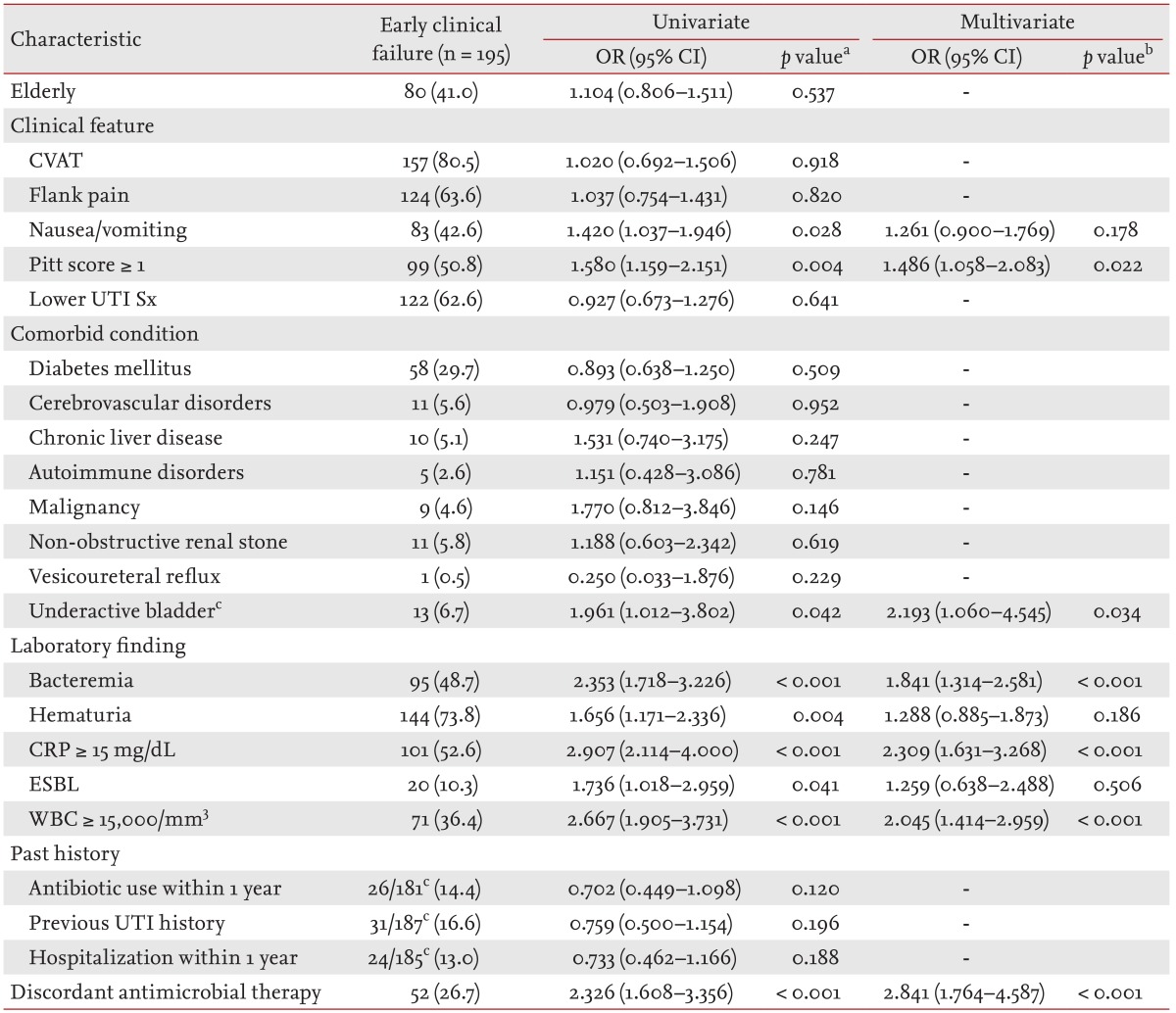
Values are presented as number (%). Final model: bacteremia, CRP ≥ 15 mg/dL in the blood, discordant antimicrobial therapy, ESBL, hematuria, nausea, or vomiting, Pitt score ≥ 1, underactive bladder, WBC counts (/mm3 in the blood) ≥ 15,000.
OR, odds ratio; CI, confidence interval; CVAT, costovertebral angle tenderness; UTI Sx, urinary tract infection symptoms; CRP, C-reactive protein; ESBL, extended-spectrum β-lactamase; WBC, white blood cell.
aUnivariate analysis by the chi-square test or Fisher exact test.
bMultivariate analysis using logistic regression.
cDenominators were the number of patients whose data were available in each group.
The clinical cure rates observed at 4 to 14 days after the EOT were not significantly different between the early clinical success and early clinical failure groups. However, the microbiological cure rate in the former was significantly higher (p < 0.001). The median times to defervescence were 45 hours (IQR, 32 to 60) and 91 hours (IQR, 83 to 103.8; p < 0.001), and the median length of the hospital stay was 8 days (IQR, 7 to 10) and 10 days (IQR, 8 to 11.8; p < 0.001) in the early clinical success and early clinical failure groups, respectively.
Factors related to a longer hospitalization in the elderly and non-elderly women with APN
The median number of hospital stays in the elderly and non-elderly groups were 9 (IQR, 8 to 13) and 8 (IQR, 7 to 10), respectively (p < 0.001) (Table 3).
The proportion of patients requiring hospitalization for 10 days or more was significantly higher in the elderly group compared to the non-elderly group (51.5% vs. 26.2%, p < 0.001). Multiple logistic regression analysis using the potential risk factors identified by univariate analysis determined that flank pain and bacteremia were significant predictors of an increased hospitalization period (p = 0.011 and p = 0.001) (Table 5).
Table 5. Factors related to a longer hospitalization (≥ 10 days) in 443 elderly women with community-onset non-obstructive acute pyelonephritis caused by Enterobacteriaceae in a final model of multiple logistic regression, 2004 to 2013.
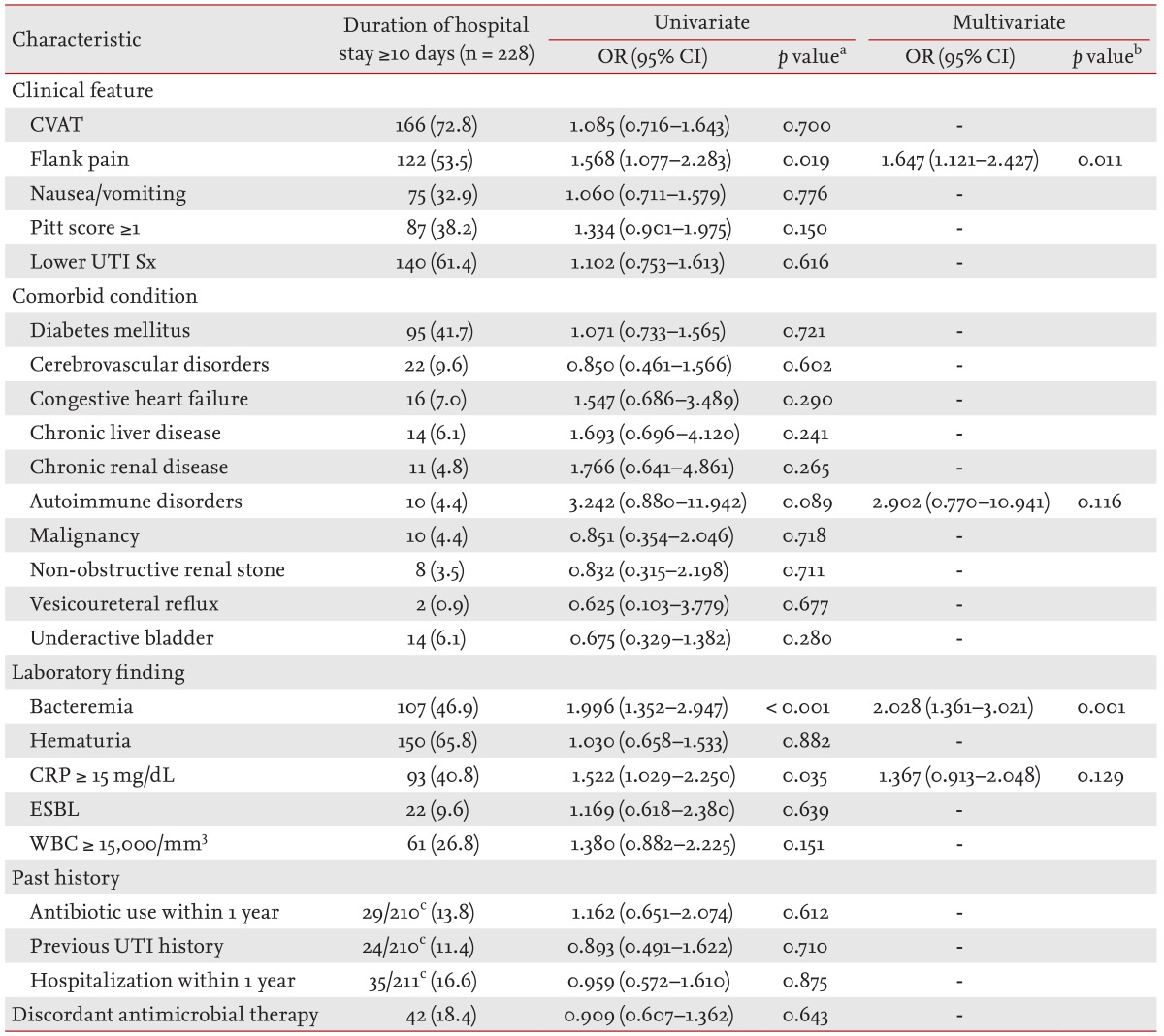
Values are presented as number (%). Final model: autoimmune disorders, bacteremia, CRP ≥ 15 mg/dL of blood, Flank pain. OR, odds ratio; CI, confidence interval; CVAT, costovertebral angle tenderness; UTI Sx, urinary tract infection symptoms; CRP, C-reactive protein; ESBL, extended-spectrum β-lactamase; WBC, white blood cell.
aUnivariate analysis by the chi-square test or Fisher exact test.
bMultivariate analysis using logistic regression.
cDenominators were the number of patients whose data were available in each group.
DISCUSSION
Advanced age is one of the complicating factors of APN. In the current study, we compared the initial clinical signs and symptoms, clinical courses, and treatment outcomes of elderly and non-elderly women with community-onset APN. The elderly group, in comparison to the non-elderly group, demonstrated fewer upper UTI symptoms/signs, such as flank pain and costovertebral angle tenderness, fewer lower UTI symptoms, and a lower frequency of nausea or vomiting. In contrast, the proportion of bacteremia, ESBL-producing Enterobacteriaceae, patients with a CRP level ≥ 15 mg/dL, and patients with a leukocyte count ≥ 15,000/mm3 in the blood were higher in the elderly group. While the median hours to defervescence were not significantly different, the length of hospitalization was notable. The proportion of patients hospitalized for 10 days or longer was significantly higher in the elderly group, even though clinical and microbiological cure rates were not significantly different.
This study determined that the proportion of bactebacteremic patients was higher in the elderly, and the frequency of upper UTI symptoms/signs, such as flank pain and costovertebral angle tenderness, were significantly lower in the elderly group. Additionally, the proportion of patients with ESBL-producing Enterobacteriaceae was higher in the elderly than in the non-elderly group. However, the proportion of patients who were treated with antibiotics within the past year, or the proportion of patients with a previous history of UTI, was lower in the elderly than in the non-elderly group. Independent risk factors contributing to APN, by ESBL-producing pathogens, were underlying comorbid conditions, antibiotic usage within the previous year, and urinary catheterization within the previous month in Korea [16]. A history of previous UTI's was determined not to be an independent risk factor of APN caused by ESBL-producing pathogens, and the occurrence of urinary catheterization within the previous month was not investigated in patients enrolled in the current study. Therefore, it is presumed that underlying comorbid conditions were a greater risk factor contributing to the development of APN caused by antibiotic-resistant pathogens than the history of antibiotic usage within the previous year.
The proportion of individuals aged 65 and over was less than 1% of the global population in 1900; however, the proportion of elderly people is expected to increase to 20% by 2050 [17,18]. Therefore, physicians should consider that aging of the immune system and underlying comorbid conditions can influence the clinical course or prognosis of APN in an elderly population [17]. Clinical research studies investigating UTIs in the elderly over the past two decades have studied catheter-associated UTI, complicated UTI, nosocomial UTI, asymptomatic bacteriuria, and antimicrobial therapy in lower UTIs. However, only a few studies have compared the clinical characteristics of APN in an elderly population, as compared to those of the non-elderly individuals with APN. These comparative studies can play a critical role in the early diagnosis and prompt treatment of the unusual presentation of APN in the elderly.
Our clinical data demonstrated that the frequency of both upper and lower urinary tract symptoms/signs were significantly lower in the elderly group compared to the non-elderly group. Therefore, the elderly may exhibit an atypical presentation of clinical symptoms/signs.
While diabetes mellitus has been shown to be an independent predictor of longer hospitalizations in a previous study [19], diabetes mellitus was not an independent risk factor contributing to longer hospitalization (≥ 10 days) in the elderly in this study. In contrast, the current study determined that flank pain and bacteremia were independent predictors of longer hospitalization among elderly patients. Analysis of 173 bacteremia cases in the current study revealed no deaths; however, a high mortality rate (33%) was observed in patients 75 years of age or older in a previous study that analyzed 191 episodes of urosepsis [20]. These contrasting results may be due to differences in age distribution, the prevalence of underlying disorders, and the exclusion of obstructive, complicated APN.
In the current study, the clinical cure rate, microbiological cure rate, and mortality rate were not significantly different between the elderly and non-elderly groups. However, we determined that the hospitalization period was much longer for the elderly group, as compared to the non-elderly group.
This study has several limitations. First, the history of prior hospitalization, antibiotic usage, and UTI may have been underestimated, since this is a retrospective study. However, we were able to demonstrate significant differences in the baseline demographics, clinical characteristics, outcomes, and laboratory findings between the elderly and non-elderly groups by performing a retrospective analysis of electronic medical records. Secondly, severe cases of APN may have been excluded from the analysis, because we did not include patients with obstructive APN who required urological interventions. Thirdly, fluoroquinolone was not administered as an initial intravenous antibiotic, although it was recommended for the treatment of hospitalized women with APN in most guidelines [21,22]. In this study, fluoroquinolone was not initially administered due to the relatively high rates of fluoroquinolone-resistant E. coli present in Korea, and was preserved for more important uses than the treatment of UTIs [3,23].
In conclusion, elderly women with APN exhibit higher serum CRP levels, an increased frequency of bacteremia, a higher proportion of ESBL-producing uropathogens, and require a longer hospitalization than non-elderly women, even though the typical signs and symptoms of urinary infections may not be observed in the elderly patients during the initial presentation.
KEY MESSAGE
Elderly women with community-onset acute pyelonephritis have a higher level of serum C-reactive protein, a higher frequency of bacteremia, a higher proportion of extended-spectrum β-lactamase-producing uropathogens, and require longer hospitalization than non-elderly women.
Elderly women with acute pyelonephritis may not exhibit typical urinary infection signs and symptoms during the initial presentation.
Acknowledgments
The authors would like to acknowledge the financial support of the St. Vincent's Hospital, Research Institute of Medical Science (SVHR-2014-08).
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Esposito AL, Gleckman RA, Cram S, Crowley M, McCabe F, Drapkin MS. Community-acquired bacteremia in the elderly: analysis of one hundred consecutive episodes. J Am Geriatr Soc. 1980;28:315–319. doi: 10.1111/j.1532-5415.1980.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 2.Gleckman R, Blagg N, Hibert D, et al. Acute pyelonephritis in the elderly. South Med J. 1982;75:551–554. doi: 10.1097/00007611-198205000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Lim SK, Park IW, Lee WG, Kim HK, Choi YH. Change of antimicrobial susceptibility among Escherichia coli strains isolated from female patients with community-onset acute pyelonephritis. Yonsei Med J. 2012;53:164–171. doi: 10.3349/ymj.2012.53.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grover ML, Bracamonte JD, Kanodia AK, Edwards FD, Weaver AL. Urinary tract infection in women over the age of 65: is age alone a marker of complication? J Am Board Fam Med. 2009;22:266–271. doi: 10.3122/jabfm.2009.03.080123. [DOI] [PubMed] [Google Scholar]
- 5.Jackson SL, Boyko EJ, Scholes D, Abraham L, Gupta K, Fihn SD. Predictors of urinary tract infection after menopause: a prospective study. Am J Med. 2004;117:903–911. doi: 10.1016/j.amjmed.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 6.Nicolle LE. A practical guide to antimicrobial management of complicated urinary tract infection. Drugs Aging. 2001;18:243–254. doi: 10.2165/00002512-200118040-00002. [DOI] [PubMed] [Google Scholar]
- 7.High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Sociey of America. J Am Geriatr Soc. 2009;57:375–394. doi: 10.1111/j.1532-5415.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavazzi G, Delerce E, Cambau E, et al. Diagnostic criteria for urinary tract infection in hospitalized elderly patients over 75 years of age: a multicenter cross-sectional study. Med Mal Infect. 2013;43:189–194. doi: 10.1016/j.medmal.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Wi YM, Kim SW, Chang HH, et al. Predictors of uropathogens other than Escherichia coli in patients with community-onset acute pyelonephritis. Int J Clin Pract. 2014;68:749–755. doi: 10.1111/ijcp.12368. [DOI] [PubMed] [Google Scholar]
- 10.Wie SH, Kim HW, Chang UI. Use of gentamicin for women with community-acquired uncomplicated acute pyelonephritis caused by gentamicin-susceptible or -resistant Escherichia coli: 10-year experience. Microb Drug Resist. 2013;19:316–322. doi: 10.1089/mdr.2012.0140. [DOI] [PubMed] [Google Scholar]
- 11.Hermanides HS, Hulscher ME, Schouten JA, Prins JM, Geerlings SE. Development of quality indicators for the antibiotic treatment of complicated urinary tract infections: a first step to measure and improve care. Clin Infect Dis. 2008;46:703–711. doi: 10.1086/527384. [DOI] [PubMed] [Google Scholar]
- 12.Naber KG, Savov O, Salmen HC. Piperacillin 2 g/tazobactam 0.5 g is as effective as imipenem 0.5 g/cilastatin 0.5 g for the treatment of acute uncomplicated pyelonephritis and complicated urinary tract infections. Int J Antimicrob Agents. 2002;19:95–103. doi: 10.1016/s0924-8579(01)00481-2. [DOI] [PubMed] [Google Scholar]
- 13.Vazquez JA, Gonzalez Patzan LD, Stricklin D, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin. 2012;28:1921–1931. doi: 10.1185/03007995.2012.748653. [DOI] [PubMed] [Google Scholar]
- 14.Park DW, Peck KR, Chung MH, et al. Comparison of ertapenem and ceftriaxone therapy for acute pyelonephritis and other complicated urinary tract infections in Korean adults: a randomized, double-blind, multicenter trial. J Korean Med Sci. 2012;27:476–483. doi: 10.3346/jkms.2012.27.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing: 15th Informational Supplement. Vol. 25. Wayne: Clinical and Laboratory Standard Institute; 2005. [Google Scholar]
- 16.Kim B, Kim J, Seo MR, et al. Clinical characteristics of community-acquired acute pyelonephritis caused by ESBL-producing pathogens in South Korea. Infection. 2013;41:603–612. doi: 10.1007/s15010-013-0441-z. [DOI] [PubMed] [Google Scholar]
- 17.Crossley KB, Peterson PK. Infections in the elderly. Clin Infect Dis. 1996;22:209–215. doi: 10.1093/clinids/22.2.209. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto T. Urinary tract infections in the elderly. Curr Urol Rep. 2001;2:330–333. doi: 10.1007/s11934-001-0073-1. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Wie SH, Chang UI, et al. Comparison of the clinical characteristics of diabetic and non-diabetic women with community-acquired acute pyelonephritis: a multicenter study. J Infect. 2014;69:244–251. doi: 10.1016/j.jinf.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Tal S, Guller V, Levi S, et al. Profile and prognosis of febrile elderly patients with bacteremic urinary tract infection. J Infect. 2005;50:296–305. doi: 10.1016/j.jinf.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 22.The Korean Society of Infectious Diseases; The Korean Society for Chemotherapy; Korean Association of Urogenital Tract Infection and Inflammation; The Korean Society of Clinical Microbiology. Clinical guideline for the diagnosis and treatment of urinary tract infections: asymptomatic bacteriuria, uncomplicated & complicated urinary tract infections, bacterial prostatitis. Infect Chemother. 2011;43:1–25. [Google Scholar]
- 23.Seo MR, Kim SJ, Kim Y, et al. Susceptibility of Escherichia coli from community-acquired urinary tract infection to fosfomycin, nitrofurantoin, and temocillin in Korea. J Korean Med Sci. 2014;29:1178–1181. doi: 10.3346/jkms.2014.29.8.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]