Abstract
Cells with certain attributes of very immature astroglial cells and their radial precursors can act as stem and/or progenitor cells during developmental and persistent neurogenesis. Neural stem/progenitor cells both express and are affected by a variety of developmentally regulated macromolecules and growth factors, and such signaling or recognition molecules are being uncovered through extensive genomic and proteomic studies, as well as tested using in vitro/in vivo cell growth bioassays. Glycosylated molecules are appreciated as distinct signaling molecules during morphogenesis in a variety of tissues and organs, with glycoconjugates (glycoproteins, glycolipids, and glycosaminoglycans) serving as mediators for the interactions of cells with each other and their substrates, to confer growth and differentiation cues to precursor cells in search of identity. Neurogenic astrocytes and associated glycoconjugates, especially extracellular matrix molecules, are discussed in the context of neurogenesis and stem/progenitor cell growth, fate choice, and differentiation.
Keywords: Astrocyte, Stem cell, Extracellular matrix, Multipotent astrocytic stem cell, Adult human neural progenitor cell
1. Introduction
In the field of neural stem cell biology and regenerative medicine, there is interest in facilitating the ex vivo expansion as well as controlling the fate and differentiation of stem/progenitor cells that reside within different neurogenic zones of the mature central nervous system (CNS), including the periventricular subependymal or subventricular zone (“SVZ” (1–4)) and hippocampus (5). With these very potent cells that are in search of fate, use of different in vitro growth conditions including the manipulation of cell–cell and cell-substrate interactions, e.g., via particular extracellular matrix (ECM) molecule exposures, it is possible to dramatically affect the growth, fate choice, and differentiation at levels that are really rather surprising (6). For the most part, ECM was not believed to affect fate choice decisions in the CNS, but instead mostly affect neuritogenesis (for review, see Ref. (7)). Glycosylated molecules, including glycoproteins, glycolipids, and glycosaminoglycans are recognition molecules during developmental neurohistogenesis (8) as well as persistent neurogenesis, or neuropoiesis, in the neurogenic niches throughout the neuraxis (e.g., see (9–13)). Cell surface glycoconjugates and their connections to extracellular signaling molecules and intracellular machinery, e.g., the cytoskeleton and the nucleus, thus provide a means for gaining molecular access to transmembrane signaling pathways that direct cell survival, proliferation, fate determination, and motility of potent precursor cells. Understanding neurogenic niches (14) and profiling developmentally regulated and neuropoietic-associated glycoconjugates thus can reveal essential macromolecules as well as bio-markers of stem/progenitor cells that can likewise elucidate targets for subsequent gene and molecular therapies in human disease. Controlling or repairing reactive neurogenesis could have positive outcomes on disease course and treatment. That is, studying biogenic factors for and from stem cells could lead to the development of new drugs that expand or deter typically quiescent stem cell populations. This in turn, could lead to their control during migration or neoplastic cell growth and invasion, as well as affect differentiation within at-risk or cell-deficient targets without the need for ex vivo manipulation and transplantation.
2. ECM Can Control Precursor Cell Fate and Specification
Early studies (15) showed that lectin-bound glycoconjugates and immunodetected ECM molecules, e.g., tenascin-C and chondroitin sulfate proteoglycans, are expressed by immature astrocytes and their precursors that we now know are neurogenic glia, i.e., radial glial cells and immature astrocyte progenitor cells ((16–19); and see Fig. 1). These cells and associated glycosylated macromolecules can act as “boundaries” around developing brain structures, and may instruct afferent fiber ingrowth through adhesive and repulsive cues, and thus play a significant role during CNS pattern formation (7). Functions of ECM might extend beyond morphogenetic effects, as loss or disarrangement of boundaries is observed in CNS diseases and malformations (8, 20–22), and as ECM is involved in regulating synaptic plasticity in the adult (23). When evaluating commonly used growth permissive substrates in an in vitro embryonic stem cell-neurogenesis assay (6), we found that laminin, fibronectin, and gelatin instruct neural fate and alter the functional specification of neurons when applied at distinct stages of development. Laminin exposure during early stages of neural differentiation generates more neurons, while gelatin induces more glial cells. Early substrate interactions did not affect functional or phenotypic profiles of protracted stages of neural maturation. However, phenotypic and electrophysiological characteristics of differentiating neurons change when substrates are modified in later developmental stages. Fibronectin leads to maturation of neurons with significantly increased sodium channel densities and typical adaptive firing behaviors, while laminin (acting through activation of the sonic hedgehog pathway) induces an upregulation of transcription factors specific for the developing medial ganglionic eminence (MGE), and subsequent specification of cholinergic and GABAergic neurons with abundant axonal branching and highly repetitive firing patterns (6). We have also tested laminin in transplants of embryonic stem cell-derived neural precursor cells, or “ESNPs” in slice cultures of the maturing nigrostriatal circuit, and found that this molecule appeared to facilitate differentiation and integration of these cells in these slices (24). Thus, although investigations of ECM-mediated control of cellular development are still in their infancy, we know now that timing and type of substrate interactions serve as specific guidelines for the control of fate choice and specification during neural development (6). Furthermore, the presence of distinct ECM expressions during brain development, and robust expression of tenascin-C, CSPGs and other ECM proteins in the rodent and human SVZ and hippocampus throughout life ((9, 22, 25); see Fig. 1) imply important roles for these molecules in neurogenesis and stem/progenitor cell growth and differentiation. We also have described ECM molecule actions on neurosphere cell motility (26), and there are certainly many more functions for these potent growth factor binders and presenters during the growth and differentiation of neurogenic astrocytes.
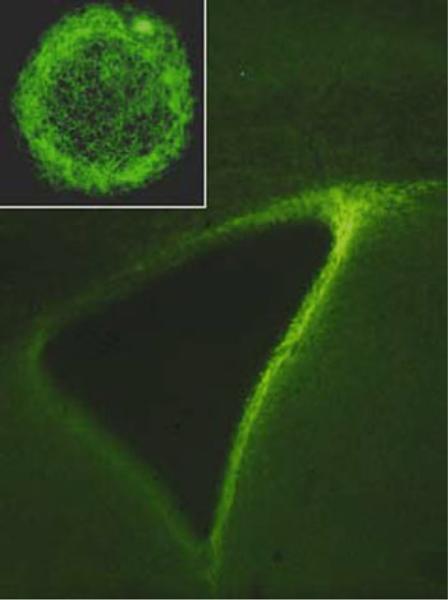
Fig. 1.
Immunofluorescence for the tenascin-C extracellular matrix (ECM) glycoprotein that is intensely expressed in both the periventricular subventricular zone (SVZ) of the lateral ventricle (main figure), as well as in neurospheres derived from a neurogenic astrocyte: the SVZ multipotent astrocytic stem cell (MASC) that is also immunostained for tenascin-C. There is a dense tenascin-C matrix surrounding cells of this cultured neurosphere (figure adapted from studies of Gates et al. (9) and Suslov et al. (62)).
3. Regulation and Potential Intrinsic Limitations for Adult Brain Neurogenesis
Astrocytes express and respond to growth factors (e.g., FGF-2, CNTF, and TGFα), neurotransmitters (i.e., 5-HT), hormones (i.e., thyroid hormone), and environmental interactions mediated through α6β1 integrin ligands are involved in neurogenesis (see the thorough review by Ref. Hagg (27)). All these factors are united in the concept of a neurogenic niche where “...(1) astrocytes serve as both stem cell and niche cell, (2) a basal lamina and concomitant vasculogenesis may be essential components of the niche, and (3) embryonic molecular morphogens and signals persist in these niches and play critical roles for adult neurogenesis...” (28) However, until now, it remains uncertain how fate choices and specification of neurogenic cells within these niches are regulated. In the postnatal brain, the subgranular zone (SGZ) of the hippocampal dentate gyrus and the SVZ lining the lateral walls of the lateral ventricles, represent the only two regions within the adult mammalian brain that support ongoing neurogenesis throughout life. Within these rare germinal niches, astrocytes, functioning as neural stem cells (17, 29), begin the cascade of events that continually renew the granule and periglomerular interneurons of the olfactory bulb (16) and granule neurons of the adult hippocampus (30, 31). In vivo, the neurogenic process within the SVZ begins with a GFAP (glial fibrillary acidic protein) expressing astrocytic stem cell, or “B-cell” (16). These unique neurogenic astrocytes, or what we refer to as multipotent astrocytic stem cells (MASCs) (17, 18, 32–34) or a putative adult human version that we refer to as adult human neural progenitor cells (or, “AHNPs,” (35)) appear to be the direct descendants of embryonic radial glia (36, 37). Unlike mature cortical astrocytes, they maintain a thin process, including a cilium, tethering them to the lateral ventricular wall and ventricular cavity (38). The relatively rare and quiescent B-cells (our “MASCs”) have been reported to give rise to a population of highly proliferative, however less-potent progenitor cells, referred to as transit-amplifiers or C-cells. Finally, from this putative intermediate progenitor pool, comes a population of young, immature neurons, or A-cells, which migrate forward over a tremendous distance through the rostral migratory stream (RMS), before a small number mature and integrate into the olfactory bulb neural circuitry as new interneurons (16, 39, 40). Interestingly, a recent publication by Danilov and colleagues has challenged the canonical B → C → A cell genesis cascade, suggesting that the multipotent B-cell astrocyte may actually give rise to its neuroblast progeny directly, without the C-cell intermediate (41). Coincidently, these results are in accord with our own in vitro model of SVZ neurogenesis, wherein cultured astrocytic stem cells are seen to robustly and directly generate a population of young neurons (42). Whether the B-cell astrocyte is directly neurogenic or requires a less-potent intermediary, its activity is limited to specific regions within the adult brain, such as the SVZ, where the expression of ECM is greatest. Under normal circumstances, the ECM molecules chondoitin sulfate proteoglycan (CSPG), heparan sulfate proteoglycan (HSPG), and tenascin-C are all intensely expressed strictly within the SVZ in the adult brain, although ECM and a distinct vascular niche ((43, 44); Fig. 1) are present within the adult hippocampus as well. Thus, the coincidental presence of an enriched ECM and persistent cell genesis, although still largely unquantified, must assuredly be of fundamental importance (28, 45). The well-accepted role of HSPG during neurogenic astrocyte expansion, as a cofactor involved in FGF-2 receptor mediated proliferation, is but one example of roles for cell surface and matrix glycoconjugates during neuropoiesis.
4. ESNPs, MASCs, and AHNPs
The differentiation of neural precursor cells during development depends on a delicate interplay between intrinsic programs of genetic expression with the myriad of extrinsic influences of local signaling cues (for review see Refs. (46, 47) and references therein). The manner in which these extrinsic environmental factors work to derive functioning neurons from a differentiating neural stem/progenitor cell is important. MASCs and AHNPs are capable of taking on different, desired (based on potential cell replacement protocols for neurological disorders) fates, and functions provided that they are exposed to the requisite environmental influences, their so-called growth niche.
There are well-described methodologies for the isolation, molecular phenotypic, electrophysiological evaluation, and transplantation of neural stem and progenitor cells (including embryonic stem cell derived neural precursor cells, or “ESNPs” that can putatively give rise to glial or neuronal restricted precursors [see Ref. (6) for review], as well as MASCs and AHNPs). Neurogenic astrocytes can be isolated from different regions within the mouse brain and expanded while adherent to plastic culture dishes using serum-containing media and mitogens (EGF + bFGF). Upon withdrawal of serum and mitogens, these cells undergo rapid glial-toneuronal phenotype transition and yield type-A neuroblasts that mature into a GABAergic interneuron phenotype (42). We have also isolated and characterized similar neurogenic astrocytes from across the entire neuraxis up until the close of the neurodevelopmental critical period – roughly the second postnatal week (17). In these studies, we found that astrocytes isolated from various regions within the late embryonic, early postnatal, and adult mammalian CNS, were capable of generating neural stem-like cells in the neurosphere assay. This demonstrates that up until the end of the second postnatal week, the cerebral cortex, cerebellum, and spinal cord harbor a population of neurogenic astrocytes which display the neural stem-cell attribute of multipotent, self-renewing neurosphere generation.
The idea of an astrocyte, or GFAP-expressing progenitor cell is best exemplified and documented in a study of the same name from the Sofroniew group (18). In that study, targeted ablation of these cells in a transgenic mouse model led to the convincing finding of GFAP-expressing cells as being “...predominant sources of constitutive adult neurogenesis...” (18) This work built on observations made from the Alvarez-Buylla, Deutsch, and van der Kooy groups (e.g., (16, 32)) describing “B cell” astrocyte-like cells as putative stem cells in the adult rodent SVZ, and our previous study showing that MASCs (e.g., see Fig. 2) can be derived from multiple brain regions including cerebral cortex, cerebellum, and SVZ. However, their ability to form neurospheres is restricted to astrocyte monolayers derived during the first two postnatal weeks, except for SVZ astrocytes, which retain this capacity throughout life (Laywell et al. (17)). Astrocyte monolayer culture approaches were later used in our laboratory to model inducible neurogenesis ex vivo (42) and further characterize the neurogenic astrocyte using cell-substrate interactions that helped to define subsequent ECM-stem cell fate control studies of Goetz et al. (6). MASCs have been shown to possess stem-like characteristics through the expression of stem cell markers such as nestin, and their ability to give rise to neurons, astrocytes, and oligodendrocytes when cultured under neurosphere-like conditions. When cultured as monolayers, MASCs are highly purified astrocyte populations that are greater than 95% immunopositive for markers including GFAP and S100β, with a few Cd11b positive microglia mixed in. From studies of induced neurogenesis within astrocyte monolayer cultures (42), we are relatively comfortable describing the so-called MASC as a nestin+/A2B5+/GFAPlight/dlx-2-/PSA-NCAM- clonogenic cell that meets all of the criteria of a neural stem cell.
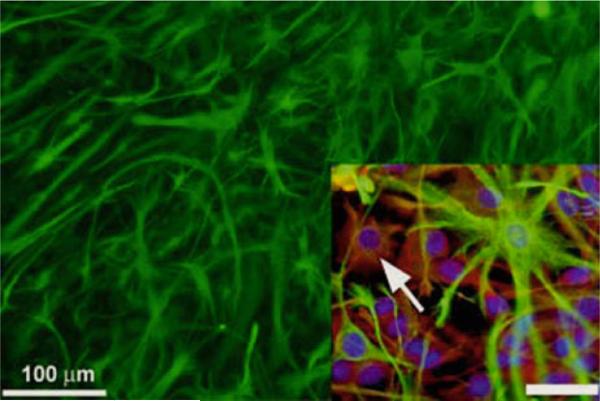
Fig. 2.
MASCs and neurogenic astrocyte-inducible neurogenesis. MASCs in monolayer cultures from transgenic mice that constitutively express green fluorescent protein, GFP (see Zheng et al. (33, 34). Monolayers of SVZ cells can be inducibly differentiated into newborn neuroblasts. Inset, lower right: low levels of GFAP (green) are found in a subpopulation of underlying nestin+/A2B5+ (red) cells. The arrow points to a GFAPlow/A2B5+ cell (inset in figure adapted from Scheffler et al. (42)). Scale bar = 30 μm.
Based upon our established methods for the derivation of rodent MASCs, we later (Walton et al. (34)) applied these techniques to isolate and expand a similar cell population from mature human brain tissue, referred to as AHNPs. These cells express a variety of astrocytic makers (e.g., see Fig. 3) and behave in culture as immature astrocytic precursor cells. Fresh tissue specimens were originally obtained from patients undergoing anterior temporal lobectomy for the treatment of intractable epilepsy. Specimens for culture were taken from three locations: the hippocampus, white matter lining the temporal horn of the lateral ventricle, and the lateral temporal neocortex. Using similar culture conditions to those previously described (42), AHNPs were successfully cultured and expanded to an average of 60 population doublings. Although the primary cell lines were not immortal, and they did upregulate their expression of telomerase, it was calculated that one cell would have the potential to generate roughly the equivalent of 4 × 107 adult brains in progeny (35). This represents a much greater capacity for expansion in vitro than had previously been reported for human progenitors (48). These proliferating cells remain dependent on externally supplied growth factors as well. Although they are clearly multipotent and have a vast proliferative potential, they appear to best meet the definition, at least for now, of progenitor cells. These cells can be encouraged to differentiate in vitro by the removal of growth factors and addition of cAMP, nerve growth factor (NGF), and 3-isobutyl-1-methylxanthine (IBMX). AHNPs are amenable to viral transduction using, e.g., lenti- vectors (e.g., see (35)), and therefore the differentiation and integration following transplantation can be easily tracked. Based on prior experience, infection efficiency has consistently been at least 95%. eGFP+ AHNPs can be FACS sorted to obtain a pure eGFP+ cell population e.g., two passages after transduction. Purified eGFP+ AHNPs are expanded for several passages before transplantation. Based on prior experience, the fluorescent marker is expressed stably during the entire culture period.
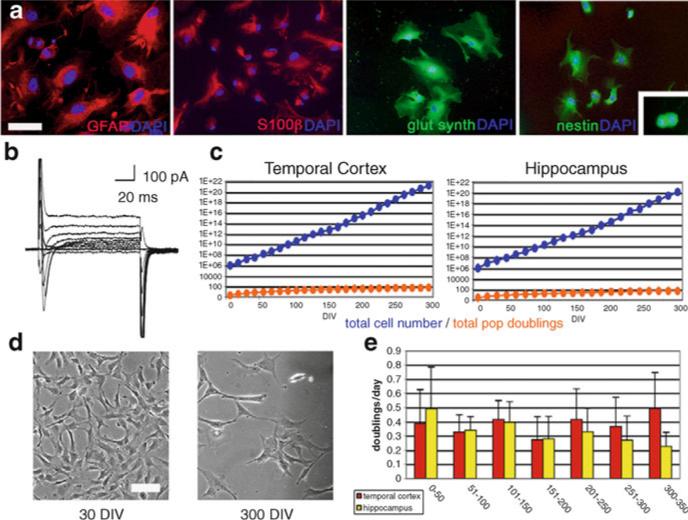
Fig. 3.
Expansion of primary neural cells as purified astroglial precursors, adult human neural progenitor cells (AHNPs) from the adult human temporal cortex. (a) High passage (>60 population doublings) cells express astrocyte markers GFAP, S100β, and glutamine synthetase. Cytoskeletal nestin (expressed in dividing cells, inset) is also present. Cells counter-stained with DAPI. (b) Voltage-clamp membrane recordings of these cells reveal prominent Na+ and minimal K+ channel activity. (c) AHNPs derived from temporal cortex and hippocampus continue logarithmic expansion throughout culture. (d) Hippocampal and temporal cortex cells maintain a stable gliotypic morphology throughout culture. (e) Both hippocampal and temporal cortex cells maintain an equivalent stable doubling rate throughout culture. Scale bars: 50 μm (GFAP), 100 μm (additional images) (a), 150 μm (d) (adapted from Walton et al. (35)).
It is also possible to distinguish intrinsic biophysical profiles of neurogenic astrocytes. When cerebellar-derived MASCs are transplanted into the lateral ventricle of normal adult mice, they migrate along the RMS into the olfactory bulb and differentiate into interneurons (34, 49). MASCs thus have the ability to respond to intrinsic environmental cues by anatomically integrating into a host brain and differentiating into neurons and astrocytes in vivo (33, 49). Further investigation by transplanting SVZ-derived MACSs into neonatal rat stroke models demonstrated that MASCs have the capability to move to an area of injury following transplantation and to differentiate into neurons and astrocytes at the site of damage. Following hypoxic injury, the neonatal brain appears receptive to MASCs and thus it appears that they may serve as potential therapeutic candidates for replacement and repair following cell loss in the CNS resulting from injury or disease (34). Transplantation of AHNPs into the adult mouse forebrain (35) yields different types of neuronal and glial cell integration. Thus, it is a key challenge to determine whether MASC- and AHNP-derived neurons can become regionally specified and integrate with appropriate functions into disparate circuitries. In vitro and in vivo studies with these two different and potent stem/progenitor cells aim to discover default cell fate determinations and interventions to modify and/or specify particular fate choice decisions.
Following injury or disease, the brain may innately attempt reactive neurogenesis since we and others (e.g., see 50) have shown that a type of astrocytic cell, the MASC, may give rise to new neurons and glia following CNS injury. Until now the consensus of the neuroregeneration field has been that the astroglial scar and its cadre of ECM-expressing astrocytes provides a neurite-growth inhibitory environment (for review, see Refs. (7, 50, 51)). However, an alternative view is that this environment may represent an attempt to recapitulate neurogenesis by deterring the differentiation of newly generated cells, e.g., their neurite growth would be limited while they are still proliferating. Because we have found that particular ECM substrate conditions can change the fate of neuronal precursor cells derived from embryonic stem cells (6), it is important to further explore the intrinsic and extrinsic determinants of neural phenotypy. It is postulated that MASCs and AHNPs have many attributes in common with neurogenic radial neuroepithelial cells (52) found throughout the neuraxis during CNS development; there are of course other astrotypic cells that exhibit stem/progenitor cell behaviors in the developing, adult, and injured CNS (see Ref. (53) for review), including a recently described pialglial cell that appears to possess stem cell attributes (54). All of these cells occupy a distinct neurogenic niche in the CNS where a dense ECM expression is almost, if not always found, but injuries and disease may induce what appear to be fully differentiated astrocytes to assume a neurogenic role whereby they upregulate their expression of developmentally regulated proteins and attempt to recapitulate neurogenic programs. In vitro conditions can be created that restore neurogenic programs of these cells, and it is necessary to resolve the growth conditions and precise molecular cascades responsible for lifelong plasticity of CNS cells. In all, unlocking multipotency of apparently differentiated mammalian CNS astrocytic cells should contribute important information toward cataloging the arsenal of molecular messengers that will help us direct reactive neurogenesis for lifelong neural repair following neurological injury or disease.
5. Glycoconjugate Signaling in Neural Stem/Progenitor Cells
ECM substrate surface coating experiments (e.g., (6)) in studies of neural stem/progenitor cells, e.g., neurogenic astrocytes, represent the tip of a very large iceberg when one has to consider all of the potential glycoconjugates that have biogenic effects on cells in search of state-dependent proliferation, fate choice (identity), differentiation, survival or death cues. There have been some recent extremely thorough reviews of roles for “glycosignaling” in neural stem cells by Yu and Yanigasawa, therefore it is not worth reiterating all aspects of the “plasma membrane glycocalyx network” (11) that are relevant to the ECM and other glycoconjugate recognition molecule events of importance to astrocytic stem/progenitor cells. However, it is worthwhile being aware of some of the most well-characterized gycoconjugates that are relevant to signal transduction and other events that underlie specific aspects of CNS pattern formation and, in particular, neurogenesis. In the spectrum of stem- and progenitor-ness, from ESNPs to neuronal- and glial-restricted precursors and adult neurogenic astrocytes (MASCs and AHNPs), such candidates include but are not restricted to: SSEA-1, prominin-1, phosphacan, GM1, A2B5, NG2, cd15 (Lewis X antigen), peanut agglutinin-binding molecules, cd44, PSA-NCAM, O4, and O1 (see Ref. (12), for review, and see Refs. (42, 55–59)). The field has paid a great deal of attention to prominin-1, or cd133 because of its apparent association with the cell surface and cilia of neural stem cells (e.g., neurogenic astrocytes), and PSA-NCAM because of its prominent expression by neuroblasts or type A cells of the SVZ and RMS (16, 42); but it is clear that the list of glycoconjugates that mediate multiple cell and substrate interactions during brain development and neuropoiesis have only begun to be elucidated.
As nicely pointed out by Yu and Yanigasawa (11), the signal transduction regulation of self-renewal and expansion, survival, fate choice, and differentiation by neural stem/progenitor cells is mediated by many different glycoconjugates including HSPG, CSPG, N-glycosylated cystatin C, galectins-1, and glycosphingolipids including GD3 that work through the PI3-kinase-Akt and Ras-MAPK pathways. In addition, ffrench-Constant, Faissner, and collaborators have reported that tenascin-C ((60); and see Fig. 1) and CSPGs including the 473 proteoglcyan (61) can affect neurosphere formation via interactions with growth factors and other glycoconjugates.
Thus, cell surface glycoconjugates on neurogenic astrocytic cells can be used as biomarkers, as well as candidates to enrich for distinct populations of these cells, using FACS and other methods, from a heterogeneous starting population (62). The biogenic actions of these glycosylated molecules can also be used, via targeting either the sugar moieties or protein cores with antibody, RNA and viral vector technologies, to facilitate neurogenesis and support migration and homing to particular neural structures in need of cell replacement, as well as discourage these cell behaviors in neoplasia where potentially transformed neurogenic astrocytes are implicated as tumor-initiating, “cancer stem cells” involved in, e.g., gliomagenesis (63, 64).
6. Conclusions
A complex set of molecular messengers help guide neurogenesis from cells that exhibit many characteristic features of immature astrocytes. Glycoconjugates on the surface of these cells, and surrounding them in the ECM, are perfectly suited for mediating many of the interactions between neurogenic astrocytes in search of molecular cues to guide their proliferation, movement, fate choice, and differentiation. In addition to an extensive inventory of surface and matrix glycoconjugates, there must also be associated receptors for these molecules, as well as coupled intracellular machinery to translate the glycosylated molecular messages to direct the different cell behaviors: motility, intracellular protein and organelle trafficking, process extension and retraction, synaptogenesis, and cell survival or death. Integrins are such receptors that can connect the ECM to actin and other components of the cytoskeleton, where this, “.... interaction can be viewed as a cyclical liaison, which develops again and again at new adhesion sites only to cease at sites of de-adhesion...” (65) The making and breaking of substrate connections by neurogenic astrocytes and their progeny is crucial for controlled migration, survival, and cell-site specific differentiation, and likewise requires distinctive cytoskeletal activities to underlie each aspect of the developmental sequalae involved in neurohistogenesis. Along with unique glycoconjugates and their receptors involved in such events, there are specific cytoskeletal proteins expressed during different stages of neurogenesis, including nestin (66), unique GFAP splice variants (67), other intermediate filament proteins and the tubulins. All of these extracellular, cell surface, and intracellular macromolecular expressions contribute to morphogenesis, reactive histogenesis following injury and disease, and neoplasia that accompanies oncogenic transformation of potent precursor cells in ways that we are only beginning to understand in this very special cell we call a “neurogenic astrocyte.” Glia, based on historic definitions, and their associated sugar-coated molecules, evoke an image of a sticky glue that binds neural elements together. Since the original discovery of the neurosphere-generating cell is in the adult mouse brain (68), it is now clear that neurogenic astroglia stem/progenitor cells, and their distinctive expressions of glycoconjugate recognition/signaling molecules, are crucial to the building and repair of the nervous system.
Acknowledgments
The author would like to thank Dr. Bjorn Scheffler for many discussions on and help with this topic, and Drs. Tong Zheng, Florian Siebzehnrubl, Oleg Suslov, Shanshan Wang, and Daniel Silver for also collaborating on all aspects of the cell and molecular biology of neural stem/progenitor cells. DAS's research is supported by NIH grant NS055165.
References
- 1.Scheffler B, Horn M, Blumcke I, Laywell ED, Coomes D, Kukekov VG, Steindler DA. Marrow-mindedness: a perspective on neuropoiesis. Trends Neurosci. 1999;22:348–357. doi: 10.1016/s0166-2236(99)01416-2. [DOI] [PubMed] [Google Scholar]
- 2.Steindler DA, Pincus D. Stem cells and neuropoiesis in the adult human brain. The Lancet. 2002;359:1047–1054. doi: 10.1016/S0140-6736(02)08096-0. [DOI] [PubMed] [Google Scholar]
- 3.Laywell E, Steindler DA, Silver DJ. Astrocytic stem cells in the adult brain. Neurosurgery Clinics of North America. 2007;18:21–30. doi: 10.1016/j.nec.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Garzón-Muvdi T, Quiñones-Hinojosa A. Neural stem cell niches and homing: recruitment and integration into functional tissues. ILAR J . 2009;51:3–23. doi: 10.1093/ilar.51.1.3. [DOI] [PubMed] [Google Scholar]
- 5.Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14:325–37. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goetz AK, Scheffler B, Chen HX, Wang S, Xiang H, Suslov O, Brustle O, Roper SN, Steindler D. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc Natl Acad Sci USA. 2006;103(29):11063–8. doi: 10.1073/pnas.0510926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faissner A, Steindler D. Boundaries and inhibitory molecules in developing neural tissues. Glia. 1995;13:233–54. doi: 10.1002/glia.440130402. [DOI] [PubMed] [Google Scholar]
- 8.Steindler DA. Glial boundaries in the developing nervous system. Annu Rev Neurosci. 1993;16:445–470. doi: 10.1146/annurev.ne.16.030193.002305. [DOI] [PubMed] [Google Scholar]
- 9.Gates MA, Thomas LB, Howard EM, Laywell ED, Sajin B, Faissner A, Götz B, Silver J, Steindler DA. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. J Comp Neurol. 1995;361:249–66. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- 10.Thomas LB, Gates MA, Steindler DA. Young neurons from the adult subependymal zone proliferate and migrate along an astrocyte, extracellular matrix-rich pathway. Glia. 1996;17:1–14. doi: 10.1002/(SICI)1098-1136(199605)17:1<1::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Yu RK, Yanagisawa M. Glycosignaling in neural stem cells: involvement of glycoconjugates in signal transduction modulating the neural stem cell fate. J. Neurochem. 2007;103(Supp 1):39–46. doi: 10.1111/j.1471-4159.2007.04710.x. [DOI] [PubMed] [Google Scholar]
- 12.Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17:57R–74R. doi: 10.1093/glycob/cwm018. [DOI] [PubMed] [Google Scholar]
- 13.Yu RK, Suzuki Y, Yanagisawa M. Membrane glycolipids in stem cells. FEBS Lett. 2010;584:1694–9. doi: 10.1016/j.febslet.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai RY, Kittappa R, McKay RD. Plasticity, niches, and the use of stem cells. Dev Cell. 2002;2:707–12. doi: 10.1016/s1534-5807(02)00195-8. [DOI] [PubMed] [Google Scholar]
- 15.Steindler DA, Cooper NGF, Faissner A, Schachner M. Boundaries defined by adhesion molecules during development of the cerebral cortex: The J1/tenascin glycoprotein in the mouse somatosensory cortical barrel field. Developmental Biology. 1989;131:243–260. doi: 10.1016/s0012-1606(89)80056-9. [DOI] [PubMed] [Google Scholar]
- 16.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 17.Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004;7:1233–41. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Bolteus AJ, Balkin DM, Henschel O, Bordey A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006;54:394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- 20.Cooper NG, Steindler DA. Critical period-dependent alterations of the transient body image in the rodent cerebral cortex. Brain Res. 1989;489:167–176. doi: 10.1016/0006-8993(89)90020-6. [DOI] [PubMed] [Google Scholar]
- 21.Steindler DA, Faissner A, Harrington KL. A unique mosaic in the visual cortex of the reeler mutant mouse. Cereb Cortex. 1994;4:129–137. doi: 10.1093/cercor/4.2.129. [DOI] [PubMed] [Google Scholar]
- 22.Scheffler B, Faissner A, Beck H, Behle K, Wolfe HK, Wiestler O, Bluemcke I. Hippocampal loss of tenascin boundaries in Ammon's horn sclerosis. Glia. 1997;19:35–46. [PubMed] [Google Scholar]
- 23.Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- 24.Kearns SM, Scheffler B, Goetz AK, Lin DD, Baker HD, Roper SN, Mandel RJ, Steindler DA. A method for a more complete in vitro Parkinson's model: slice culture bioassay for modeling maintenance and repair of the nigrostriatal circuit. J Neurosci Methods. 2006;157:1–9. doi: 10.1016/j.jneumeth.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O'Brien TF, Kusakabe M, Steindler DA. Multipotent stem/ progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–44. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 26.Kearns SM, Laywell ED, Kukekov VK, Steindler DA. Extracellular matrix effects on neuro-sphere cell motility. Exp Neurol. 2003;182:240–244. doi: 10.1016/s0014-4886(03)00124-9. [DOI] [PubMed] [Google Scholar]
- 27.Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 29.Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–32. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian fore-brain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–71. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng T, Marshall GP, 2nd, Laywell ED, Steindler DA. Neurogenic astrocytes transplanted into the adult mouse lateral ventricle contribute to olfactory neurogenesis, and reveal a novel intrinsic subependymal neuron. Neuroscience. 2006;142:175–85. doi: 10.1016/j.neuroscience.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 34.Zheng T, Rossignol C, Leibovici, Anderson KJ, Steindler DA, Weiss MD. Transplantation of multipotent astrocytic stem cells into a rat model of neonatal hypoxic-ischemic encephalopathy. Brain Res. 2006;1112:99–105. doi: 10.1016/j.brainres.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Walton NM, Sutter BM, Chen HX, Chang LJ, Roper SN, Scheffler B, Steindler DA. Derivation and large-scale expansion of multi-potent astroglial neural progenitors from adult human brain. Development. 2006;133:3671–81. doi: 10.1242/dev.02541. [DOI] [PubMed] [Google Scholar]
- 36.Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101:17528–32. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–4. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 38.Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kishi K. Golgi studies on the development of granule cells of the rat olfactory bulb with reference to migration in the subependymal layer. J Comp Neurol. 1987;258:112–24. doi: 10.1002/cne.902580109. [DOI] [PubMed] [Google Scholar]
- 40.Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–13. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danilov AI, Gomes-Leal W, Ahlenius H, Kokaia Z, Carlemalm E, Lindvall O. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia. 2009;57:136–52. doi: 10.1002/glia.20741. [DOI] [PubMed] [Google Scholar]
- 42.Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci USA. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neuro-genesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–57. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- 46.Campbell K. Cortical neuron specification: it has its time and place. Neuron. 2005;46:373–6. doi: 10.1016/j.neuron.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–10. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 48.Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–47. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 49.Zheng T, Steindler DA, Laywell ED. Transplantation of an indigenous neural stem cell population leading to hyperplasia and atypical integration. Cloning Stem Cells. 2002;4:3–8. doi: 10.1089/153623002753631995. [DOI] [PubMed] [Google Scholar]
- 50.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Götz M. Origin and progeny of reactive gliosis: A source of multi-potent cells in the injured brain. Proc Natl Acad Sci USA. 2008;105:3581–6. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brodkey JA, Gates MA, Laywell ED, Steindler DA. The complex nature of interactive neuroregeneration-related molecules. Exp Neurol. 1993;123:251–270. doi: 10.1006/exnr.1993.1158. [DOI] [PubMed] [Google Scholar]
- 52.Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steindler DA, Laywell ED. Astrocytes as stem cells: nomenclature, phenotype, and translation. Glia. 2003;43:62–9. doi: 10.1002/glia.10242. [DOI] [PubMed] [Google Scholar]
- 54.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 55.Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;16:736–9. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Han SS, Wu Y, Tuohy TM, Xue H, Cai J, Back SA, Sherman LS, Fischer I, Rao MS. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2004;276:31–46. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–75. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 58.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multi-functional cells with lineage plasticity. Nat Rev Neurosci. 2010;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 59.Siebzehnrubl FA, Jeske I, Müller D, Buslei R, Coras R, Hahnen E, Huttner HB, Corbeil D, Kaesbauer J, Appl T, von Hörsten S, Blümcke I. Spontaneous in vitro transformation of adult neural precursors into stem-like cancer cells. Brain Pathol. 2009;19:399–408. doi: 10.1111/j.1750-3639.2008.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcion E, Halilagic A, Faissner A, ffrench-Constant C. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131:3423–32. doi: 10.1242/dev.01202. [DOI] [PubMed] [Google Scholar]
- 61.von Holst A, Sirko S, Faissner A. The unique 473HD-Chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J Neurosci. 2006;26:4082–94. doi: 10.1523/JNEUROSCI.0422-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suslov ON, Kukekov VG, Ignatova TN, Steindler DA. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci U S A. 2002;99:14506–11. doi: 10.1073/pnas.212525299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ignatova T, Kukekov VG, Laywell ED, Suslov ON, Vrionis F, Steindler DA. Human cortical glial tumors contain stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 64.Singh S, Dirks PB. Brain tumor stem cells: identification and concepts. Neurosurg Clin N Am. 2007;18:31–8. doi: 10.1016/j.nec.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 65.Brakebusch C, Fässler R. The integrinactin connection, an eternal love affair. EMBO J. 2003;22:2324–33. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 67.Middeldorp J, Boer K, Slukis JA, De Filippis L, Encha-Razavi F, Vescovi AL, Swaab DF, Aronica E, Hol EM. GFAPdelta in radial glia and subventricular zone progenitors in the developing human cortex. Development. 2010;137:313–21. doi: 10.1242/dev.041632. [DOI] [PubMed] [Google Scholar]
- 68.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]