Abstract
Obese older adults with even modest functional limitations are at a disadvantage for maintaining their independence into late life. However, there is no established intervention for obesity in older individuals. The Measuring Eating, Activity and Strength: Understanding the Response --Using Protein (MEASUR-UP) trial is a randomized controlled pilot study of obese women and men aged ≥60 years with mild to moderate functional impairments. Changes in body composition (lean and fat mass) and function (Short Physical Performance Battery) in an enhanced protein weight reduction (Protein) arm will be compared to those in a traditional weight loss (Control) arm. The Protein intervention is based on evidence that older adults achieve optimal rates of muscle protein synthesis when consuming about 25-30 grams of high quality protein per meal; these participants will consume −30 g of animal protein at each meal via a combination of provided protein (beef) servings and diet counseling. This trial will provide information on the feasibility and efficacy of enhancing protein quantity and quality in the context of a weight reduction regimen and determine the impact of this intervention on body weight, functional status, and lean muscle mass. We hypothesize that the enhancement of protein quantity and quality in the Protein arm will result in better outcomes for function and/or lean muscle mass than in the Control arm. Ultimately, we hope our findings will help identify a safe weight loss approach that can delay or prevent late life disability by changing the trajectory of age-associated functional impairment associated with obesity.
Keywords: Obesity, function, sarcopenic obesity, older adults, weight loss intervention, protein
INTRODUCTION
The impact of the obesity epidemic in the older adult population on functional performance, quality of life, and psychological health is poorly recognized and under-studied. The obesity rate already exceeds 33% for U.S. adults aged 60 and older [1] and serious concerns about this trend and the associated health problems have been widely presented in the literature.[2-7] Obesity is directly linked with life-threatening chronic illnesses that also increase with aging (e.g., cardiovascular disease, type 2 diabetes), as well as a newly recognized concern, the loss of functional independence.[8, 9] As adults age, most experience a gradual loss of muscle mass in a process known as “sarcopenia.” [10] Sarcopenia is likely due to an age-associated blunting of the anabolic response to nutritional and exercise stimuli, as well as a sub-optimal protein intake; the result is a decline in physical strength, mobility, and endurance.[11] In societies with high obesity rates, sarcopenia often “co-occurs” with excess adiposity, a condition sometimes referred to as “sarcopenic obesity.”[12] A growing body of literature has established that sarcopenic obesity carries the cumulative risk of both conditions, often leading to more disability than either condition alone.[12] It is well known that having excessive stores of adipose tissue leads to increased levels of reactive oxygen species (ROS) and proinflammatory cytokines.[13] Likewise, aging is associated with increases in rates of oxidative stress and a chronic condition of low-grade inflammation, both of which lead to cellular and molecular damage to muscle tissue over time.[13] Thus, when excessive adiposity is coupled with age-related changes (reductions in the anabolic response and increased inflammation), there is an exacerbated negative effect on skeletal muscle and an increased risk for functional decline. [14] Currently, the diagnostic criteria for sarcopenic obesity are a matter of debate; at least 8 different definitions are in the published literature. [12, 15] However, the literature evidence is in agreement that having a lowered muscle mass/strength along with a heavy load of body fat contributes to functional limitations.[16, 17]
Weight reduction in obese individuals benefits physical function as well as a variety of metabolic parameters [18], but it can also have negative consequences for those with reduced lean muscle mass (LMM). With traditional weight loss approaches, 25% or more of LMM can be lost.[19] This accounts for the common recommendation to use exercise as a weight reduction intervention rather than a weight reduction diet. However, individuals with functional deficits are unlikely to achieve a level of physical training sufficient to induce a negative energy balance or to fully protect muscle mass. The Measuring Eating, Activity and Strength: Understanding the Response -Using Protein (MEASUR-UP) trial targets ways to circumvent this challenge and offer recovery of physical function for obese elders who have limited ability to exercise. Knowing that simply reducing body fat improves function [20], we sought an intervention that not only reduces body fat but also helps to protect LMM during weight reduction. We propose an intervention that favors retention of LMM by optimizing the anabolic response of muscle protein synthesis to nutritional stimuli. Increased protein intake has been shown to enhance the retention of lean mass during weight loss in younger adults [21] and higher protein intakes (exceeding the RDA level of 0.8 g/kg) are generally linked with better preservation of lean mass.[22, 23] We hypothesize that this protection may also be possible in older adults but most optimally when the protein intake is enhanced throughout the day. This is based on strong evidence from acute studies that having a generous and balanced intake of protein at each meal (> 30 grams) is essential for optimal protein synthesis in the aging muscle.[24-30] The consumption of extra protein, and the resulting hyper-aminoacidemia, stimulates a marked rise in muscle protein synthesis and a mild suppression of muscle protein breakdown. Essential amino acids, especially leucine, initiate the mTOR signaling pathway and thereby stimulate muscle protein synthesis.[22, 23] To achieve this benefit, it is important to ingest a sufficient amount of protein at each meal.[31] Additionally, there is evidence that individuals whose protein comes from animal sources have better preservation of lean muscle mass.[32] Higher protein intakes may also promote increased satiety and better adherence to weight loss diet regimens as indicated by a number of studies.[33, 34] Successful weight loss, improved function, and less problems with hunger between meals may also result in improved quality of life and psychological health.
METHODS
Hypotheses and Aims
The approach being tested in the MEASUR-UP trial builds on recent reports that higher protein intakes protect muscle mass and generous intakes of high quality protein can overcome anabolic resistance in aging muscle. The essential amino acids found in complete (animal) proteins (leucine, in particular) stimulate translation initiation of the mTOR signaling pathway and increase muscle protein synthesis.[22, 23] Unfortunately, protein intake patterns in the typical Western diet do not consistently achieve the level of intake (− 25-30 grams of high quality protein) that acute studies have shown to be needed for older adults to reach an optimal anabolic response.[24] Increasing protein intakes to spare LMM during weight loss has been previously investigated by others, but targeted enhancement of protein quantity and quality to fully achieve this threshold of optimal intake at each meal has not been studied in a long-term trial. This is important since Symons et al. [24] and others [35] have shown that 30 grams or more of high quality protein is needed for an optimal anabolic response. Mojtahedi et al.[36] evaluated the effect of providing 25 g of whey protein twice daily in an exercise and weight loss intervention. They found that higher protein intakes increased LMM relative to body mass but did not lead to improvements in function.
The MEASUR-UP trial proposes a novel intervention for weight reduction in older adults that uses meal-based threshold intakes of 30 g or more per meal of high quality (animal; 60-70% as lean beef) protein to be consumed 3 times per day to help protect lean mass (see Figure 1). While enhancing intake of high quality protein at meals has been shown to improve muscle protein synthesis in older adults in short-term experiments (< 24 hrs), this approach has never been studied in a long-term, free-living study such as our 6-month intervention. Thus, the purpose of this pilot trial is to allow us to derive effect sizes of the high protein intervention, assess its fidelity, and to assess the between group differences in the primary endpoints of function (by the Short Physical Performance Battery; SPPB) and LMM.
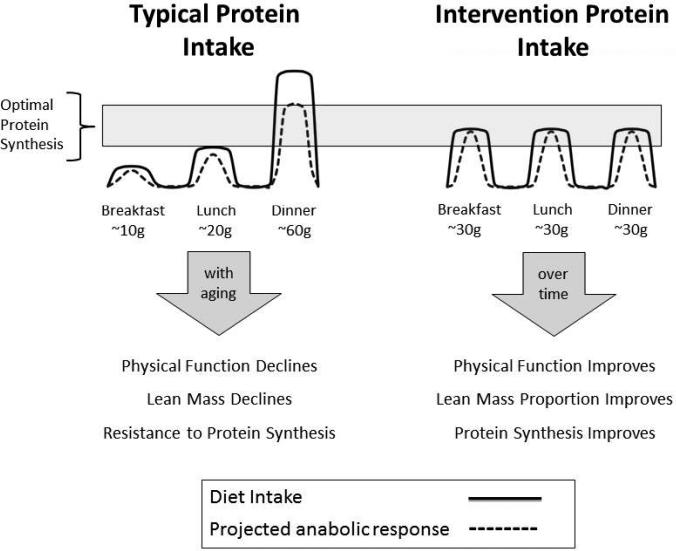
Figure 1.
In older adults, optimal protein synthesis in muscle is achieved at a target intake of 30g or more of high-quality protein. On the left is a schematic illustration of how typical protein intakes (solid line) may reach the optimal protein synthesis threshold no more than once a day resulting in an overall diminished anabolic response (dashed line). In contrast, as illustrated on the right, the MEASUR-UP high protein regimen intakes are planned to meet the threshold at each of three meals per day. The expectation, illustrated by the arrow on the right, is that the enhanced protein intervention will promote retention of lean mass and improve function
As illustrated in Figure 1, the Protein treatment group in the MEASUR-UP trial will receive counseling and provision of high-quality protein foods to achieve a goal of at least 30 grams at each of the three meals per day, keeping calories low enough to gradually lose 10% of baseline body weight. For two of the three meals, participants will be provided cooked and measured portions of lean beef. They will also receive detailed counseling on how to use these portions at the two meals and how to achieve 30 grams of protein for the third meal.
We hypothesize that following 6 months of weight loss intervention, compared to Control participants, participants in the Protein group will:
Have greater improvements in functional status as indicated by SPPB, and
Have better preservation of lean mass relative to total body mass lost.
The following sections describe key elements relevant for a comprehensive understanding of delivery of this intervention and the assessment of key study outcomes.
Study Design and Group Allocation
Obese older adults (≥60 yrs) with moderate functional impairment will be randomized to 1 of 2 study arms. Outcomes to be measured at 0, 3 and 6 months are as follows: Primary outcomes are lean muscle mass (LMM; by BodPod) and functional status (by Short Physical Performance Battery; SPPB). The Secondary outcomes include 6 minute walk, 8-foot up and go, and 30 second chair stands; total body and adipose mass, waist circumference; protein intake and overall nutritional adequacy; mood, self-rated quality of life, satiety, and sleep. The study arms are:
•Control
Participants follow a calorie-reduction diet for a weight loss of ≥10%.
•Protein
Participants follow a calorie-reduction diet for a weight loss of ≥10%, with an emphasis on allocation of high-quality protein at each meal. Intakes of ≥30 g of high quality protein will be achieved three times a day by participants in this group; all or predominantly all will come from animal sources and 60-70% of the animal protein will be lean beef. Seventy-five individuals are to be randomized in a 2:1 (protein:control) ratio.
Participant Eligibility
Women and men of all races, aged 60 years and older, who have obesity as defined by a body mass index (BMI) equal to or greater than 30 kg/m2, and who demonstrate mild to moderate impairment in function as defined by a score of 4-10 on the SPPB are eligible for enrollment. Renal function will be confirmed to be age appropriate at the time of enrollment in the study, with inclusion of those with an estimated glomerular filtration rate (GFR) of 45 ml/min/1.73 m2 or greater. For those with a GRF between 45-59 ml/min/1.73 m2, renal function will be monitored as shown in Figure 4 and discussed in detail in the safety section.
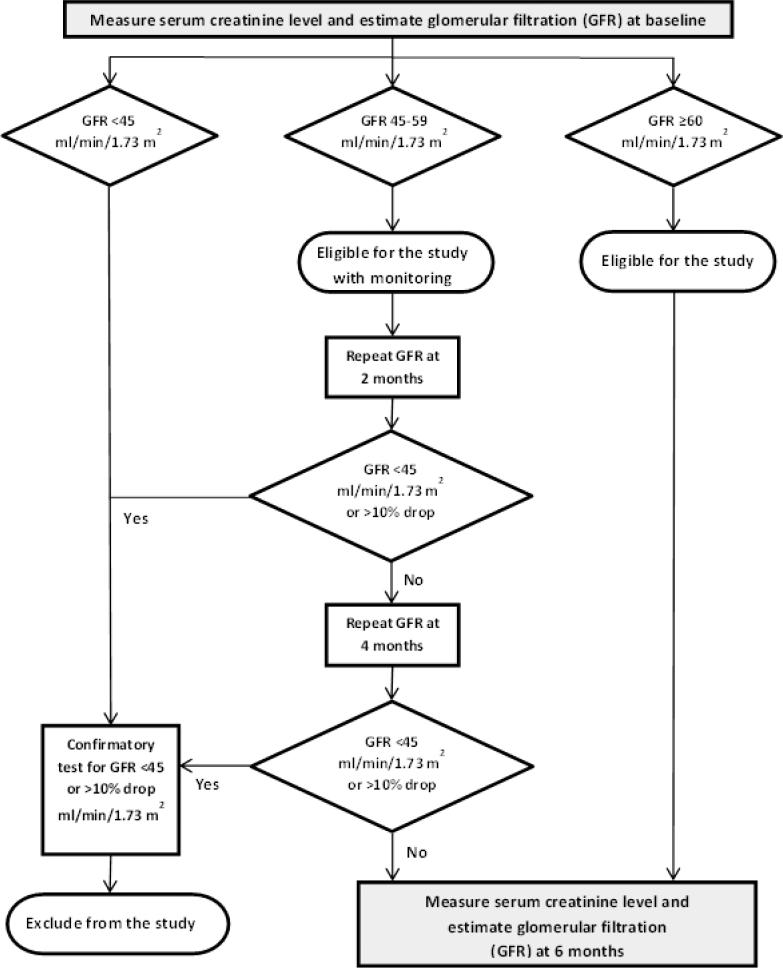
Figure 4.
The enrollment criteria for MEASUR-UP specify that eligible participants have “age-normal” renal function. We developed this algorithm using glomerular filtration rate (GFR) to guide decisions about initial enrollment and monitoring during the trial. All those with a GFR of ≥60 ml/min/1.73 m2 are study eligible. For enrollees with a GFR in the range of 45-59 ml/min/1.73 m2, GFR measurements will be repeated every two months; a drop of 10% or more or falling to a level <45 ml/min/1.73 m2 will disqualify the individual from further study participation.
Participants will be excluded for the following: body weight >495 lbs. (limits of body composition measurement instrumentation); unstable, acutely symptomatic, or life-limiting illness; positive screen for dementia (Mini-Cog) [37]; neurological conditions causing functional or cognitive impairments; significant weight instability (defined as >10 pounds weight gain or loss over 6 months); unwillingness to be randomized to either treatment, submit to study testing or participate in intervention program for six months; inability to walk independently; inability to give consent, complete written forms, including journals of eating and exercise behaviors; current use of monoamine oxidase inhibitors or prescription weight loss medications; or their primary care physician (PCP) advises against participation (PCPs will be contacted by personal letter prior to enrollment).
Recruitment Strategies
A variety of methods will be employed to recruit potential participants whose physical function is compromised, with the focus directed towards Senior Centers, retirement communities, and churches within driving distance of Duke University Medical Center. Information sessions will be held at these locations offering information about obesity, aging, and BMI. Posters, flyers, and digital billboards will be used in any receptive location frequented by older adults.
Screening and Enrollment
Interested potential participants will first be screened by telephone using a scripted list of questions to identify possible eligibility. If they pass the phone screen and are willing to be randomized to either group, each subject will then be scheduled to attend a consent presentation. After written informed consent is obtained, participants will be scheduled for 3 separate baseline screening visits in order to confirm eligibility and obtain baseline information as shown in Figure 2 (left-hand side). After successful completion of the 3 baseline visits, participants will be enrolled in the trial and randomized to one of the two treatment groups for enrollment in the study in a 1:2 ratio of Control:Protein.
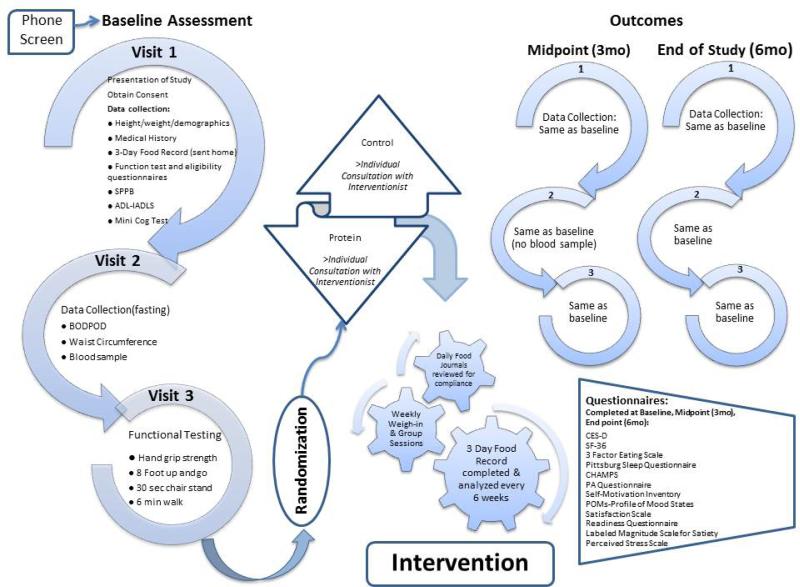
Figure 2.
This schematic flowchart explains the sequence of steps for baseline assessments, enrollment and randomization, and subsequent assessments being conducted in the MEASUR-UP trial. The assessments to be conducted are detailed under Visits 1, 2, and 3 and the questionnaires to be administered are listed in the box on far right.
Rationale for the Study Design and Randomization
The study duration was chosen to allow time for a gradual weight loss of 10% of baseline weight. The number of participants and group allocations are based on the primary outcome of LMM and our experience with similar interventions. Because considerably more is known about the results of traditional weight loss diet as compared to high protein weight loss diets, we will use a 1:2 allocation of subjects to the Control and Protein groups. This oversampling of the protein group will increase our ability to make within-group comparisons (such as by gender) in this group without substantial loss of power for the between group comparisons.
The repeated measures design assesses change over time for the Protein group relative to the Control group. Thus, we will be able to derive effects sizes (effectiveness) for this intervention. While older women significantly outnumber older men in the population, and, in general, men are much more difficult to recruit to a weight loss study than are women, we will aim to enroll equal numbers of men and women. Whatever the final enrollment, we will block randomize by gender to achieve a near equal gender ratio consistency between the study arms.
TREATMENT GROUPS
Weight Reduction Diet—Both Groups
The initial calorie prescription for weight reduction for each participant will be derived from calculations of estimated total energy expenditure (TEE) based on weight, height, gender, age and activity level using equations published by the Institute of Medicine.[38] These equations, developed using the doubly-labeled water technique, take into account metabolic rate, body composition, and other determinants of energy requirements and are currently considered the best approach for calculating total daily energy needs.[39] A TEE will be calculated for each of our participants and then reduced by about 500 kcal per day to arrive at a prescribed calorie level for a weight loss of about 1-2 pounds (average of 0.6 kg) per week with a goal of losing 10% of baseline body weight over a period of 6 months; as the intervention progresses, the prescribed TEE will be adjusted downward or upward to achieve the desired rate and amount of weight loss. Participants will meet individually with an intervention dietitian (hereafter referred to as “Interventionist”) on a weekly basis for 2 weeks to learn about their calorie prescription and how to translate it into a meal plan. Prescribed calorie intakes will be translated into daily meal patterns that are individualized on the basis of the participant's usual diet. The diet pattern specifies the number of servings from each food group allowed per day. Weekly “weigh-ins” will also occur. Tables 1 and 2 illustrate the translation of calorie prescriptions into meal plans for the Control and Protein group, respectively. For the Control group, protein intake will meet the Recommended Dietary Allowance (RDA) of 0.8 g/kg body weight, with the macronutrient distribution being 15% protein, 30% fat, 55 % carbohydrates. For the Protein group, protein intake will be approximately 1.2 g/kg body weight, with a macronutrient distribution of 30% protein, 30% fat, 40% carbohydrates.
Table 1.
Example 1-day menus for Control group (~1600-1700 kcal)
| Meal | Sample Daily Menu-Control |
|---|---|
| Breakfast | Select: |
| 1. 1 small banana, ¾ cup cereal, 1 cup skim milk, 2 slice whole wheat toast and 1 teaspoon of butter | |
| OR | |
| 2. 1 hard-boiled egg, 1 small whole wheat bagel and 1½ tsp. of nut butter, ¾ cup blueberries, 6 oz. low fat yogurt | |
| Pro (g)/Meal | Breakfast HQP ~ 15 g |
| Lunch | Select: |
| 1. Soup and Salad: 1 cup of low sodium broth based soup, 1 small roll, 2 cups lettuce greens, 1 cup assorted raw vegetables, 2 tbsp. dried fruit, 2 tbsp. low fat dressing, 1 cup whole strawberries | |
| OR | |
| 2. Deli Meat Sandwich: 1-2 oz. lean deli meat, 1 oz. slice low fat cheese, 2 slices whole wheat bread, side salad w/ 2 Tbsp. low fat dressing and a small apple | |
| Pro (g)/Meal | Lunch HQP ~ 20 g |
| Dinner | Select: |
| 5 oz. of lean protein (chicken, fish, pork or beef) | |
| 2 servings non-starchy vegetable | |
| 2 serving starch (bread, pasta, rice, legumes or starchy vegetable) | |
| Pro (g)/Meal | Dinner HQP ~ 35 g |
| Snack Options | Select: |
| 1 Fruit serving | |
| 3 cups of popcorn | |
| 6 crackers w/ 1 tsp. peanut butter | |
| Total HQ Pro (g) for Day | Total HQP ~ 70 g |
| TARGET PRO (g) | Protein ~ 0.8 g/kg body wt. |
| Sources of Calories: ~ 15% PRO; 55% CHO; 30% FAT | |
HQP = High quality protein
Table 2.
Example 1-day menus for Protein group (~1600-1700 kcal)
| Meal | Sample Daily Menu-Protein |
|---|---|
| Breakfast | Select: |
| 1. Protein Smoothie: Whey protein powder (30-40 g protein), 1 cup skim milk or 1 cup of water, and 1-2 servings of fresh or frozen fruit blended | |
| OR | |
| 2. Egg Scramble: 2 egg white omelet w/ 4 oz. lean ground beef and 1 cup of diced vegetables served with ¼ cup of salsa | |
| 3. 1 hard-boiled egg, 1 slice of whole wheat toast and 1 cup of cottage cheese, ½- 1 cup of fruit and a Splenda packet | |
| Pro (g)/Meal | Breakfast HQP ~ 30 g |
| Lunch | Select: |
| 1. Steak Wrap: 4-5 oz. fajita flank steak -study provided, 1 oz. low fat cheese, low carb tortilla wrap, lettuce, tomato, onions, ¼ cup blueberries; ½ cup sliced strawberries | |
| OR | |
| 2. Roast Beef Sandwich: 4.5 oz. Deli Roast Beef-study provided, 1 oz. slice low fat cheese, 1 whole wheat sandwich thin, side salad w/low fat dressing and an apple | |
| Pro (g)/Meal | Lunch HQP ~ 30 g |
| Dinner | Select: |
| 4.5-6 oz. of lean protein (chicken, fish, pork or beef- study provided) | |
| 2 servings non-starchy vegetable | |
| 2 serving starch (bread, pasta, rice, legumes or starchy vegetable) | |
| Pro (g)/Meal | Dinner HQP ~ 30 g |
| Snack Options | Select: |
| 1 Fruit serving | |
| Greek yogurt | |
| 3 cups of popcorn | |
| 6 crackers w/ 1 tsp. peanut butter | |
| Total HQ Pro (g) for Day | Total HQP ~ 90 g |
| TARGET PRO (g) | Protein ~ 1.2 g/kg body wt. |
| Sources of Calories: ~ 30% PRO; 40% CHO; 30% FAT | |
HQP = High quality protein
On week 3, participants begin weekly group classes with other participants in their group (Control or Protein). In addition to intensive training on diet-related topics, these classes will discuss healthy lifestyle, stress management, behavior change, and goal setting. Additional individual support will be provided by the Interventionists as needed throughout the 6 month intervention.
We recognize that physical activity helps to promote and sustain weight reduction and aids preservation of LMM. However, participants in this study will have functional limitations that hinder their exercise participation to varying degrees, so that a standardized exercise intervention would be impractical. Thus, the weekly group sessions will include discussion topics, handouts, and self-reporting of daily activities in order to provide general encouragement for regular physical activity to the extent individual functional abilities will safely allow.
Protein Quantity and Quality Enhancement—Protein Group
For participants in this group, a combination of counseling and food provision will be used to increase both the quantity and quality of protein consumed within the context of the prescribed calorie prescription. Protein quality will be enhanced by a predominant emphasis on animal sources of protein. Each week the Protein group participants will be provided cooked and chilled/frozen portions of lean beef (deli roast beef, cooked ground beef, and flank steak), as illustrated in Figure 3, and provided with instructions for safe handling practices for storing and serving the beef portions. Interventionists will provide on-going counseling on ways to include the 30 g protein portions in two meals a day as a part of the prescribed meal plan (again, see Table 2). For the third meal of the day (usually breakfast) counseling on the choice of other high quality proteins, such as yogurt, protein bars, other meats, will be used to achieve the 30 g target intake. The meal pattern will be adjusted so that the calories being consumed by the Protein participants (per gender, weight, weight loss goal) are equal to the calories prescribed to the Control group. The differences will be in the macronutrient distribution and the intentional targeting of at least 30 g high quality protein at each meal.
Figure 3.
This photograph shows the cooked lean beef products that will be provided to a Protein group participant for one week following a 1600 to 1700 kcal diet prescription. Included are 22.5 oz. ground beef, 14 oz. deli roast beef, and 36 oz. flank steak, all pre-cooked and either chilled or frozen and provided in an insulated bag for transport to home.
Nutritional Adequacy of the Diets
We have calculated the nutrient content for both the Control and Protein diets based on 5 full days of sample menus to predict the adequacy of the diets when implemented by participants. Based on these findings, low dose nutrient supplements will be provided to assure that recommended intakes for all essential nutrients are fully met. Participants will be provided a daily low dose multivitamin/mineral supplement (Teen Multivitamin for Boys 12-17, GNC Milestones), along with 400 mg calcium and 500 IU Vitamin D (Citracal, Bayer). Participants will be asked to discontinue taking any other nutritional supplements during the trial in order to avoid excessive intakes and also to remove the potential interference that might occur if participants took a variety of different supplements of their own choice.
Fidelity
We will use the Best Practices and Recommendations from the NIH Behavior Change Consortium to maximize treatment fidelity for this intervention.[40] Fidelity for study design, training of Interventionists, recruitment process and procedures, delivery of the intervention, receipt of the intervention, execution of the intervention, and retention of participants will be assessed on a continual basis to improve validity and reliability.
A Manual of Operations and Procedures (MOP) has been developed to ensure standardization of protocol implementation and data collection, and will be provided to all key personnel on the study team. The MOP will have written procedures for all assessment measurements, individual sessions, and group sessions. To ensure the weight loss intervention will be equivalent both the control and treatment arms will meet with Interventionists for equal amounts of time, both groups will be given two individual sessions, and 24-weeks of group sessions. Weekly education material will be similar and group differences will only be made to emphasize increased protein intake in the treatment arm. Additionally, the Interventionists will co-lead both the Control and the Protein arm to ensure standardization throughout the intervention. Individual taste preferences will be accommodated to ensure maximal adherence. Weight loss will be recorded via weekly in–person weigh-ins and Interventionists will collect daily food logs every week and 3-day food records every 6 weeks. Protein intake will be confirmed by review of weekly food diaries and by computerized nutrient analysis of 3-day food diaries every 2 months; corrections will be made in diet plans or implementation as needed. Attendance at weekly support group meetings will be mandatory (sessions missed will be made up at an alternate time). If a participant is not meeting their goal, individual sessions will be held to problem solve and find ways around the barriers to following the diet regimen.
ASSESSMENTS
All outcome measures will be collected at baseline, 3 and 6 months. Research staff will be cross-trained to screen eligible participants, consent participants, and to obtain body measurements and measures of functional performance. Whenever possible, staff collecting outcome measures will be blinded to the participant's treatment group. The bulk of the data will be hand entered and captured using REDCap (Research Electronic Data Capture).
Body Composition and Anthropometric Measures
Measured weight and height will be used to calculate BMI and ensure participants meet the enrollment criteria of BMI ≥30 kg/m2. . Body weight will be recorded to the nearest 0.1 kg (Salus scale, Milan, Italy), and height to the nearest 0.5 cm using a stadiometer. Body composition comprised of total fat mass (Fat, kg), lean muscle mass (LMM, kg), and percent fat mass (%Fat) will be determined using the BOD POD™ air displacement plethysmography method (Life Measurement, Inc., Concord, CA) with our previously established protocol.[41] The Bod Pod has excellent sensitivity and test-to-test reliability.[42, 43] It is relatively easy to utilize and non-invasive in nature, which is important to encourage full participation from this functionally frail population. Body proportions will be measured at the minimal waist, umbilical waist, and hip circumferences using a Gulick II tape measure (Country Technology, 1999) with the tape placed directly on the skin (not over the clothing). Measurements will be taken once at each location before completing a second measurement, and an average of the two will be recorded. A third measurement will be taken if the first two measurements are > 0.5 cm apart.
Function and Physical Activity Measures
Short Physical Performance Battery
The Short Physical Performance Battery (SPPB) [44] is a performance-based test that assesses older adults' mobility by measuring the three categories of balance, strength, and gait speed. The participant performs three different standing balance tests (side-by-side, semi-tandem, and tandem), holding each position for up to 10 seconds, along with five chair stands and a 4-meter walk. Performance in each category is scored on a scale of 0 to 4. A summary performance score will be calculated by summing the three category scores to give a final score ranging from 0 to 12. Participants with summary scores ranging from 4 to 10 will qualify to participate in the study.
Modified Senior Fitness Test
The 8-Foot Up-and-Go, 6-minute walk, and 30-second chair stands are adapted from the Senior Fitness Test[45] and will be collected as secondary measures of physical function. The 8-Foot Up-and-Go measures agility and dynamic balance and will be determined by the amount of time it takes the participant to stand from a seated position, walk 8 feet, turn, and return to seated position. The 6-minute walk will measure cardiovascular endurance, and will be administered on a 60-meter course with participants completing as many laps as possible in 6 minutes. Finally, the 30-second chair stands will be conducted to assess lower body strength. Participants will sit with arms folded across their chest and will then do as many chair stands as possible in 30 seconds.
Hand-Grip Strength
Hand grip is well established to parallel overall strength of older adults and is used often for its convenience and simplicity to administer.[46] A Jamar Hand Dynamometer (Sammons Preston Rolyan) will be used to measure isometric hand grip strength (highest of two successive scores trials for each hand).
CHAMPS Physical Activity Questionnaire
Community Healthy Activities Model Program for Seniors (CHAMPS)[47], a questionnaire designed to assess the weekly frequency and duration of activities typically undertaken by older adults at light (e.g. leisurely walking, light gardening), moderate (e.g. cycling, heavy housework) and vigorous (e.g. singles tennis, jogging) intensities, will be administered at each time point.
Protein and Overall Nutrient Intake
At baseline and every 6 weeks, food and beverage data recorded on 3-day food diaries (including 1 weekend day) will be collected, analyzed, and evaluated by an Interventionist.[48] Diet intake data will be coded and analyzed for total caloric intake, absolute amounts and distribution of macronutrients (protein, carbohydrate, fat, and type of fat) and micronutrient content using Food Processor Nutrition Analysis Software (Version 10.10, 2012, ESHA Research, Salem, OR), which provides access to information on over 50,000 food items with data for 105 nutrient components. Dietary intake will also be monitored by having participants complete daily food logs that will be turned at each weekly group session. This will ensure that participants are meeting their prescribed intake of macronutrients and that protein consumption is not being over-consumed by the control arm or under-consumed by the protein arm.
Psychological Well-being and Food-related Factors
Psychological and food-related questionnaires will be self-administered; participants will complete each questionnaire without input from the research staff at 0, 3, and 6 months.
Psychological measures will include the Center for Epidemiologic Studies Depression Scale (CES-D),[49] Profile of Mood States (POMS) 30-item version,[50, 51] Satisfaction with Life Scale (SWLS),[52]Short Form Health Survey (SF-36) to assess quality of life,[53] Pittsburgh Sleep Quality Index (PSQI),[54] Perceived Stress Scale (PSS),[55] and the Self-Motivation Inventory (SMI).[56] The CES-D measures depression symptoms and is not diagnostic for major depressive disorder. However, participants who score 16 or higher (range 0-60) are considered at risk for depression and will be informed of this potential, and they will be provided information about clinical treatment options. These participants will be given or sent a letter which informs them that they have symptoms of depression, has general information about depression (including a list of symptoms), and encourages them to follow-up with their health care provider, Duke Psychiatry, or a local behavioral health clinic (contact information is provided). In addition, participants will be evaluated and/or referred for psychiatric treatment if, at any point in the study, they endorse suicidal thoughts or behaviors.
The original POMS is an easily-administered assessment that was designed to measure mood and mood changes in psychiatric patients but has more recently been used successfully in a variety of research settings;[50] the 30-item short form further reduces respondent burden and also has the advantage of not including the less psychometrically-sound or confusing items.[51] The SWLS evaluates one component of subjective well-being, namely, global life satisfaction.[52] It has high internal consistency and reliability, and scores correlate well with other measures of subjective well-being. The SF-36 evaluates 8 domains which are roughly categorized into physical (functioning, role-physical, pain, general health) and mental health (vitality, social functioning, role-emotional, and mental health).[53] It has high reliability and validity, and is appropriate for all ages. The PSQI evaluates sleep quality and disturbances over a 1-month period, and generates seven “component” scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction.[54] It has high sensitivity and specificity for distinguishing good and poor sleepers. The PSS is a measure of the extent to which situations in one's life are appraised as stressful. Items were designed to evaluate how unpredictable, uncontrollable, and overloaded participants judge their lives, and can be used in a variety of sub-populations.[55] The SMI is a reliable and valid assessment for self-motivation, conceptualized as the behavioral trait to persevere independent of situational reinforcements.[56] These 8 psychological assessment tools will be used to evaluate predictors of weight loss as well as the impact of weight loss on psychological health.
Food-related questionnaires will include the Labeled Magnitude Scale (LMS) for satiety,[57, 58] Weight-Loss Readiness Tool (WLRT), [59] and the Three-Factor Eating Questionnaire (TFEQ).[60] The LMS includes 3 components: hunger, fullness and desire to eat.[57, 58] The WLRT is based upon the Stages of Change model[61] and has been successfully used to evaluate participants regarding chances of success to lifestyle intervention.[59] The TFEQ evaluates cognitive restraint, uncontrolled eating and emotional eating and was developed for use in an obese population.[60] These 3 food-related questionnaires will be used to examine predictors of weight loss and adherence to the dietary interventions.
SAFETY MEASURES
Surveillance
The study physician will review the self-reported medical history, medication list, and diet history forms during the screening process for any concerns or exclusions. A fasting basic metabolic panel (BMP) will be collected through peripheral venipuncture during the second visit of the baseline screening process in order to measure the serum creatinine and estimate the glomerular filtration rate. Laboratory analysis will be done by LabCorp (Laboratory Corporation of America, Burlington, NC). The study physician will review the BMP results to assess eligibility for enrollment, as well as critical lab values. Each participant will be notified of any critical lab values; i.e., any that would indicate need for prompt medical attention. As part of eligibility criteria, renal function must be found to be age-normal. [62] As illustrated by the algorithm in Figure 4, participants with an estimated glomerular filtration (GFR) rate of ≥ 60 ml/min/1.73 m2 will be eligible to enroll without surveillance monitoring. Participants with a GFR of 45 to 59 ml/min/1.73 m2 will be eligible for enrollment but must be monitored during the intervention via a repeat GFR determination every 2 months. If their GFR drops by 10% or more or falls below 45 ml/min/1.73 m2, a repeat blood draw will be done to confirm the results. If the results are confirmed, the participant will not be able to continue study participation. All participants who enroll in the study and complete the study will have their GFR estimated again at the completion with a final blood draw.
During the enrollment process, a letter will be sent to each participant's PCP informing them of the purpose of the study, enrollment criteria, GFR monitoring, and providing contact information in the event the medical provider has questions, concerns, or reasons to believe that their patient should not participate.
Data & Safety Monitoring
An internal data safety advisory group made up of physicians and other clinician scientists not involved with the project will be asked to monitor the study and assure that participant safety is being protected. This group will receive regular reports on subject enrollment and on any adverse events, and it will meet as needed and at least yearly to offer recommendations. Additionally, they will serve to advise the principle investigator and study physician concerning the interpretation of any clinical issues of concern and any needed changes in the protocol related to safety.
STATISTICAL ANALYSIS
This is a randomized clinical trial with repeated measurements, with the purpose of assessing change over time for the overall sample and the difference in the Protein group relative to the Control group with regards to primary outcomes (function by SPPB and LMM as measured by the BOD POD™) and to assess within group differences (e.g. gender) within the Protein arm. The statistical analyses will proceed chronologically in 3 phases: 1) descriptive analyses that will summarize the distribution of the covariates and dependent variables, 2) bivariate analyses of the association between group membership and the outcome measures, and 3) controlled multivariable analyses, which assess the association between experimental group and the outcomes, controlling for the important covariates.
Since this is a clinical trial, groups are randomized and balanced by gender, and should be approximately equal on other important confounders, (e.g. age, and BMI) at baseline, the analysis is particularly simple. To incorporate the full data given by the subject, to test our primary hypothesis, we will employ Mixed Models repeated measures,[63] which extends the standard repeated measures ANOVA to allow for missing values, error structures other than compound symmetry, and measurements taken non-equal intervals. The test of efficacy for the intervention will be analyzed under an 'intent to treat' criteria. We will control for important baseline variables, including baseline levels of the outcomes of interest,[64] and, to extend the 'intent to treat' mechanism, we will not employ any variables observed post-randomization[65] for the intent to treat test of group differences.
The test of the main effect of the primary outcomes will be tested by the Time effect, while the group difference will be assessed by statistical significance of the Group and the Group X Time interaction. Statistical significance will be declared at level alpha of 0.05 (two-tailed). Controlling for baseline will allow us to assess both overall and group differences (measured by the main effect of group) and for differential change over time for outcomes measured repeatedly over the course of the trial (tested by the group X time interaction).
Inclusion of covariates, including the baseline measure for the outcomes of interest, into the models will add precision to the estimates and allow us to assess the generalizability of the effects across the covariates. The list of covariates, (e.g., age, race, gender, co-morbidity) will be developed prior to any analysis. In addition, to assess a dose-response relationship between acceptance of the protocol and outcome and to provide a ‘per protocol’ estimate of the effects, a measure of adherence to the protocol can be added to the covariate list, assessing if the effects are greater among those who are more compliant with the intervention. We have increased the relative size of the Protein arm and added an interim measurement point in order to provide a more precise assessment of the functional form of the compliance X lean mass and functional status change. We hypothesize that regular intake of high quality protein achieved through this trial will provide information on the feasibility and efficacy of adding protein supplementation to standard weight loss interventions in order to (1) assess the impact of the intervention on LMM and function and (2) assess the impact of the intervention on the secondary outcomes (e.g. body fat, other functional measures).
We will measure and analyze several outcomes. We have two primary outcomes – change in LMM and change in function by SPPB. Since this is not a confirmatory intervention and the limited sample size will likely lead to Type-II errors, we will not make adjustments for the family-wise Type-I error rate. Rather, ultimately, when results are reported, we will alert the reader of the p-value issues inherent in the testing multiple of multiple outcomes. The results of these analyses will provide invaluable information regarding the process and functional form of the change in the outcomes and, potentially, the causal pathways of the intervention process.
The presence of missing data can lead to bias in the estimation of the parameters unless missing values result from a Missing at Random or Missing Completely at Random process.[66] While the dropout rate is expected to be low, we will try to assess the validity of the Missing at Random assumption and the impact of the missing observations on bias in the estimates of effect. We would expect that in a geriatric population in any intensive intervention, the bulk of dropouts will come from illness, and interference with planned activities. We will list the rates and reasons for dropout by group and overall and assess if the exits are systematic and predictable differences, using logistic regression, and assess if the weight trajectories in the dropout group differed from those in the completers.
DISCUSSION
The randomized controlled MEASUR-UP trial is designed to explore a new approach for weight reduction that could benefit obese, physically frail older adults. The use of meal-based enhancement of protein quantity and quality in the context of a reduced calorie diet has never before been studied in this population or in a long-term community-based setting. Thus the study findings will have important implications not only for this population but for the feasibility of other potential applications for long-term administration of high quality protein diets in older adults. Additionally, much will be learned about the efficacy and safety of carefully supervised weight reduction in this relatively high risk and under-studied population.
Protein Intake Modifications: More Quantity and Better Quality
There is considerable evidence supporting the health benefits of higher protein diets. With regards to quantity of protein, there are numerous reports that higher protein diets provide increased satiety and enhance compliance with calorie-restricted diets.[33, 34] The phenomenon of protein leveraging, which stipulates that protein intake is regulated more strongly than energy intake, continues to be actively explored.[26] Both of these influences have important implications for reduction of overweight and obesity in the population. Additionally, there is a growing consensus that an intake of protein of 1.0 to 1.2 g/kg body weight (as opposed to the current RDA of 0.8 g/kg body weight) favors maintenance of lean mass and function in older adults.[67, 68] Since the high protein treatment group in MEASURUP will be consuming around 1.2 g/kg body weight per day for 6 months, the results of the functional assessments will yield important answers about the optimal long-term protein intake for older adults. With regards to quality of protein, there is evidence that complete (animal-source) protein is preferential for enhancing muscle protein synthesis; the Protein arm in MEASUR-UP will have increased long-term consumption of high-quality protein relative to the Control group, allowing us to evaluate this possibility.[29, 32]
The MEASUR-UP trial will yield important information about whether a consistent and carefully monitored regimen of generous intakes of complete protein impacts LMM and/or physical function differently than a traditional weight loss control diet with mixed sources of protein. Our approach of using real food as the predominant source for enhancing the quantity and quality of protein intake is unique and likely to be appealing to this age cohort and to substantially improve their adherence to the diet regimen.
Weight Reduction Regimen for Obese and Frail Older Adults
Until recently, there has been hesitancy to initiate weight loss therapies for obese older adults because of concerns that weight loss may have untoward effects, including a reduction in lean mass; traditional approaches to weight reduction can come at a cost to lean mass.[69, 70] Reflecting these concerns, potential obesity interventions for older adults have been under-studied. To date, there is no established obesity intervention that can be recommended for older adults.[69, 71] Recently, several groups have demonstrated that successful weight loss can be achieved in obese older adults using a combination of diet and exercise. However, despite these positive findings, reductions in LMM still occur and not all studies have shown improvements in functional status.[71, 72] Moreover, many obese older adults have co-morbidities and physical limitations that hamper their ability to fully achieve the levels of exercise used in these trials.
These concerns contribute to the apprehension of health care professionals about recommending weight loss for functionally limited older adults and underscore the importance of finding ways to augment the benefits of diet and exercise in this population. Thus the MEASUR-UP trial will fill an important information void regarding weight loss in a physically and metabolically frail population. The study will include participants with a variety of co-morbidities (e.g., diabetes, hypertension) and will not have an upper age limit for enrollment. Body composition, as well as an array of functional and health- and quality of life-related measures collected at baseline, midpoint and six month endpoint will provide a rich description of the impact of study participation on many different aspects of health and independence. In particular, the issue of renal function will be important to observe. Because high protein diets are not recommended unless renal function is normal due to the potential challenge of processing protein breakdown products,[73] the assessment of renal function before and after the trial will yield important information about the applicability of higher protein diets for older adults.
In summary, the MEASUR-UP trial will indicate the feasibility and practicality of implementing an intensive weight loss intervention with enhanced protein quantity and quality in obese, community dwelling elderly adults with functional limitations; lessons learned will include findings that can be applied both with overweight/obese individuals and also with normal or underweight individuals who need to increase protein takes for other reasons, such as enhancement of LMM.
Acknowledgements
This work was funded by The Beef Checkoff and supported by the National Institutes of Health (Grant 5T32 AG00029 to KPS; BIRWCH K12 HD043446 to MEP) and the Claude Pepper Independence Center (P30 AG028716 to CFP).
References Cited
- 1.Mathus-Vliegen EM. Obesity and the elderly. J Clin Gastroenterol. 2012;46(7):533–44. doi: 10.1097/MCG.0b013e31825692ce. [DOI] [PubMed] [Google Scholar]
- 2.Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65years and older: A review of the controversy. Exp Gerontol. 2013 doi: 10.1016/j.exger.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decaria JE, Sharp C, Petrella RJ. Scoping review report: obesity in older adults. Int J Obes (Lond) 2012;36(9):1141–50. doi: 10.1038/ijo.2012.29. [DOI] [PubMed] [Google Scholar]
- 4.Darmon P. Intentional weight loss in older adults: useful or wasting disease generating strategy? Curr Opin Clin Nutr Metab Care. 2013 doi: 10.1097/MCO.0b013e32835f503f. [DOI] [PubMed] [Google Scholar]
- 5.Howel D. Waist circumference and abdominal obesity among older adults: patterns, prevalence and trends. PLoS One. 2012;7(10):e48528. doi: 10.1371/journal.pone.0048528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathus-Vliegen EM. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts. 2012;5(3):460–83. doi: 10.1159/000341193. [DOI] [PubMed] [Google Scholar]
- 7.Samper-Ternent R, Al Snih S. Obesity in Older Adults: Epidemiology and Implications for Disability and Disease. Rev Clin Gerontol. 2012;22(1):10–34. doi: 10.1017/s0959259811000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11(8):568–79. doi: 10.1111/j.1467-789X.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 9.Naugle KM, Higgins TJ, Manini TM. Obesity and use of compensatory strategies to perform common daily activities in pre-clinically disabled older adults. Arch Gerontol Geriatr. 2012;54(2):e134–8. doi: 10.1016/j.archger.2011.10.017. Epub 2011 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans WJ. Skeletal Muscle Loss: Cachexia, Sarcopenia, and Inactivity. Am J Clin Nutr. 2010;91(4):1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 11.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1(2):129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prado CM, et al. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin Nutr. 2012;31(5):583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2007;292(1):R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 14.Ershler WB. A gripping reality: oxidative stress, inflammation, and the pathway to frailty. Journal of Applied Physiology. 2007;103(1):3–5. doi: 10.1152/japplphysiol.00375.2007. [DOI] [PubMed] [Google Scholar]
- 15.Batsis JA, et al. Variation in the Prevalence of Sarcopenia and Sarcopenic Obesity in Older Adults Associated with Different Research Definitions: Dual-Energy X-Ray Absorptiometry Data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc. 2013 doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- 16.Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13(1):46–51. doi: 10.1097/MCO.0b013e32833309cf. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Guo X. Obesity and functional disability in elderly Americans. J Am Geriatr Soc. 2008;56(4):689–94. doi: 10.1111/j.1532-5415.2007.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villareal DT, et al. Weight Loss, Exercise, or Both and Physical Function in Obese Older Adults. N Engl J Med. 2011;364(13):1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinheimer EM, Sands LP, Campbell WW. A Systematic Review of the Separate and Combined Effects of Energy Restriction and Exercise on Fat-free Mass in Middle-aged and Older Adults: Implications for Sarcopenic Obesity. Nutr Rev. 2010;68(7):375–88. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 20.Beavers KM, et al. Fat mass loss predicts gain in physical function with intentional weight loss in older adults. J Gerontol A Biol Sci Med Sci. 2013;68(1):80–6. doi: 10.1093/gerona/gls092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wycherley TP, et al. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281–98. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 22.Casperson SL, et al. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clinical Nutrition. 2012;31(4):512–519. doi: 10.1016/j.clnu.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koopman R, et al. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. American Journal of Clinical Nutrition. 2006;84(3):623–632. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- 24.Symons TB, et al. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109(9):1582–6. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pennings B, et al. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012 doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- 26.Martens EA, Lemmens SG, Westerterp-Plantenga MS. Protein leverage affects energy intake of high-protein diets in humans. Am J Clin Nutr. 2013;97(1):86–93. doi: 10.3945/ajcn.112.046540. [DOI] [PubMed] [Google Scholar]
- 27.Symons TB, et al. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86(2):451–6. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 28.Dillon EL, et al. Amino Acid Supplementation Increases Lean Body Mass, Basal Muscle Protein Synthesis, and Insulin-like Growth Factor-I Expression in Older Women. J Clin Endocrinol Metab. 2009;94(5):1630–7. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips SM, Tang JE, Moore DR. The Role of Milk- and Soy-based Protein in Support of Muscle Protein Synthesis and Muscle Protein Accretion in Young and Elderly Persons. J Am Coll Nutr. 2009;28(4):343–54. doi: 10.1080/07315724.2009.10718096. [DOI] [PubMed] [Google Scholar]
- 30.Sakuma K, Yamaguchi A. Molecular Mechanisms in Aging and Current Strategies to Counteract Sarcopenia. Curr Aging Sci. 2010;3(2):90–101. doi: 10.2174/1874609811003020090. [DOI] [PubMed] [Google Scholar]
- 31.Paddon-Jones D, Rasmussen BB. Dietary Protein Recommendations and the Prevention of Sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12(1):86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips SM. The science of muscle hypertrophy: making dietary protein count. Proc Nutr Soc. 2011;70(1):100–3. doi: 10.1017/S002966511000399X. [DOI] [PubMed] [Google Scholar]
- 33.Leidy HJ, et al. The influence of higher protein intake and greater eating frequency on appetite control in overweight and obese men. Obesity (Silver Spring) 2010;18(9):1725–32. doi: 10.1038/oby.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veldhorst M, et al. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008;94(2):300–7. doi: 10.1016/j.physbeh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Pennings B, et al. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302(8):E992–9. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- 36.Mojtahedi MC, et al. The Effects of a Higher Protein Intake During Energy Restriction on Changes in Body Composition and Physical Function in Older Women. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr120. [DOI] [PubMed] [Google Scholar]
- 37.Borson S, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–4. doi: 10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- 38.Human energy requirements: report of a joint FAO/ WHO/UNU Expert Consultation. Food Nutr Bull. 2005;26(1):166. [PubMed] [Google Scholar]
- 39.Heymsfield SB, et al. How much may I eat? Calorie estimates based upon energy expenditure prediction equations. Obes Rev. 2006;7(4):361–70. doi: 10.1111/j.1467-789X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 40.Bellg AJ, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443–51. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 41.Ocampo CI,GE, Aktan SL, Hawk VH, Arendt V, Slentz C, Willis L, Gallup D, Houmard J, Samsa G, Kraus W, Bales CW. Journal of the American College of Nutrition 29: 523, . Body Composition and Nutrient Intake Responses to Aerobic and Resistance Training in Previously Sedentary Women and Men. Journal of the American College of Nutrition. 2010;29(5):523. [Google Scholar]
- 42.Fields DA, Hunter GR. Monitoring body fat in the elderly: application of air-displacement plethysmography. Curr Opin Clin Nutr Metab Care. 2004;7(1):11–4. doi: 10.1097/00075197-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Aleman-Mateo H, et al. Body composition by the four-compartment model: validity of the BOD POD for assessing body fat in Mexican elderly. Eur J Clin Nutr. 2007;61(7):830–6. doi: 10.1038/sj.ejcn.1602597. [DOI] [PubMed] [Google Scholar]
- 44.Guralnik JM, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 45.Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. Journal of aging and physical activity. 1999;7:129–161. [Google Scholar]
- 46.Stenholm S, et al. Association between Obesity History and Hand Grip Strength in Older Adults--Exploring the Roles of Inflammation and Insulin Resistance as Mediating Factors. J Gerontol A Biol Sci Med Sci. 2011;66(3):341–8. doi: 10.1093/gerona/glq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart AL, et al. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine & Science in Sports & Exercise. 2001 doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Huffman KM, et al. Dietary Carbohydrate Intake and High-sensitivity C-reactive Protein in At-risk Women and Men. Am Heart J. 2007;154(5):962–8. doi: 10.1016/j.ahj.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 50.McNair D, Lorr M, Droppleman L. Manual for the Profile of Mood States. Educational and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- 51.EDITS EDITS. Research and Developments. Educational and Industrial Testing Service; San Diego, CA: 1999. [Google Scholar]
- 52.Diener E, et al. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71–5. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 53.Stewart AL, Hays RD, Ware JE., Jr. The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26(7):724–35. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 55.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 56.Dishman RK, Ickes W. Self-motivation and adherence to therapeutic exercise. J Behav Med. 1981;4(4):421–38. doi: 10.1007/BF00846151. [DOI] [PubMed] [Google Scholar]
- 57.Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18(6):683–702. [Google Scholar]
- 58.Green BG, et al. Evaluating the 'Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses. 1996;21(3):323–34. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 59.Kong W, et al. Predictors of success to weight-loss intervention program in individuals at high risk for type 2 diabetes. Diabetes Res Clin Pract. 2010;90(2):147–53. doi: 10.1016/j.diabres.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 60.de Lauzon B, et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr. 2004;134(9):2372–80. doi: 10.1093/jn/134.9.2372. [DOI] [PubMed] [Google Scholar]
- 61.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 62.Levey AS, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 63.Laird NM, Ware JH. Random-effects Models for Longitudinal Data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 64.Products C.f.P.M. Points to consider on adjustment for baseline covariates. Stat Med. 2004:701–9. doi: 10.1002/sim.1647. [DOI] [PubMed] [Google Scholar]
- 65.Group IEEW. ICH harmonised tripartite guideline - statistical principles for clinical trials. Stat Med. 1999;18:1905–42. [PubMed] [Google Scholar]
- 66.Little RJ, Rubin DB. Statistical Analysis with. 2002 [Google Scholar]
- 67.Bauer J, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–59. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 68.Houston DK, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 69.Miller SL, Wolfe RR. The Danger of Weight Loss in the Elderly. J Nutr Health Aging. 2008;12(7):487–91. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- 70.Shah K, et al. Weight-loss therapy improves endurance capacity in obese older adults. J Am Geriatr Soc. 2008;56(6):1157–9. doi: 10.1111/j.1532-5415.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santanasto AJ, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011;2011:516576. doi: 10.1155/2011/516576. Epub 2010 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avila JJ, et al. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur J Appl Physiol. 2010;109(3):517–25. doi: 10.1007/s00421-010-1387-9. [DOI] [PubMed] [Google Scholar]
- 73.Martin WF, Armstrong LE, Rodriguez NR. Dietary protein intake and renal function. Nutr Metab (Lond) 2005;2:25. doi: 10.1186/1743-7075-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]