Abstract
Around all human activity, there are zones of pollution with pesticides, heavy metals, pharmaceuticals, personal care products and the microorganisms associated with human waste streams and agriculture. This diversity of pollutants, whose concentration varies spatially and temporally, is a major challenge for monitoring. Here, we suggest that the relative abundance of the clinical class 1 integron-integrase gene, intI1, is a good proxy for pollution because: (1) intI1 is linked to genes conferring resistance to antibiotics, disinfectants and heavy metals; (2) it is found in a wide variety of pathogenic and nonpathogenic bacteria; (3) its abundance can change rapidly because its host cells can have rapid generation times and it can move between bacteria by horizontal gene transfer; and (4) a single DNA sequence variant of intI1 is now found on a wide diversity of xenogenetic elements, these being complex mosaic DNA elements fixed through the agency of human selection. Here we review the literature examining the relationship between anthropogenic impacts and the abundance of intI1, and outline an approach by which intI1 could serve as a proxy for anthropogenic pollution.
Introduction
Humans produce and use a diverse array of compounds in domestic, industrial and agricultural settings. These compounds can contaminate ecosystems, elevating local concentrations of pollutants such as heavy metals, synthetic organic compounds and radioactive isotopes. Together with microbiological contaminants, they create a zone of impact emanating from human activities. Managing impacts requires monitoring to assess the efficacy of preventative or remedial measures, by measuring the quantities and distribution of individual pollutants. However, because some 80 000 different compounds are now traded in the marketplace, testing for all pollutants is not feasible (Rockstrom et al., 2009). Focussing on just one class of pollutant is also problematic, because the composition of pollutants varies both geographically and temporally. Furthermore, diverse classes of pollutants, such as antibiotics and endocrine disrupting compounds, have significant biological effects at extremely low concentrations (Diamanti-Kandarakis et al., 2009; Gillings, 2013).
An alternative to direct detection is to use a proxy that exhibits rapid responses to diverse environmental pressures and could thus be a generic marker for anthropogenic pollutants. We propose that the class 1 integron-integrase gene, intI1, could serve as such a marker, because:
it is commonly linked to genes conferring resistance to antibiotics, disinfectants and heavy metals (Liebert et al., 1999; Partridge et al., 2001);
it has penetrated into diverse pathogenic and commensal bacteria of humans and their domestic animals (Goldstein et al., 2001; Stokes and Gillings, 2011);
the abundance of intI1 can rapidly change in response to environmental pressures, because the class 1 integron resides in diverse bacterial species that themselves have rapid generation times, and it is often located on mobile genetic elements that can readily transfer between bacteria; and
the common ‘clinical' forms of intI1 are xenogenetic, that is, recently assembled under selection pressures imposed by human activities (Gillings et al., 2008a).
Independent studies have already begun to note remarkable correlations between intI1 and associated genetic elements with various measures of human impact (Gaze et al., 2011; Pruden et al., 2012; Jechalke et al., 2013b). Here we review the recent evolutionary origins of the clinical class 1 integron, examine a series of case studies using intI1 as an environmental marker of human pollution, and suggest methods for using this gene as a proxy for human impact.
The evolutionary history of the class 1 integron
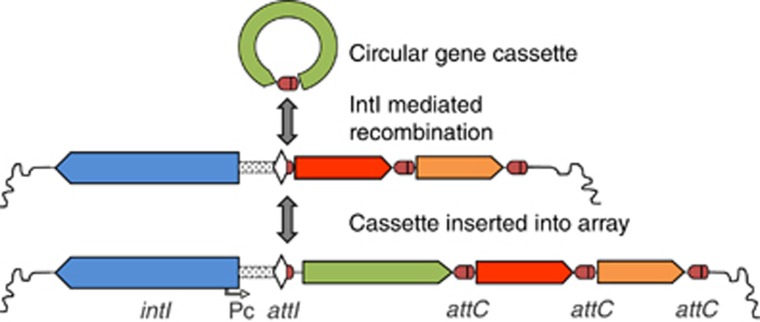
Integrons are an ancient and common feature of bacterial genomes, where they usually reside on chromosomes (Gillings, 2014a). They have three core features: an integron-integrase gene (intI), a recombination site (attI) and a promoter (PC). These features allow capture and expression of exogenous genes as part of gene cassettes that are recombined into the attI site using the integrase activity encoded by intI (Boucher et al., 2007; Cambray et al., 2010) and subsequently expressed from PC (Collis and Hall, 1995) (Figure 1). This allows genes to be acquired and expressed with minimal disturbance to the existing genome. Integrons sample cassettes from an extraordinarily diverse pool that encodes functions of potential adaptive significance. Consequently, they are a hot spot of genomic diversity in a range of genera (Gillings et al., 2005; Boucher et al., 2011; Hall, 2012; Wu et al., 2013).
Figure 1.
Integron structure and function. Integrons consist of a gene for an integron-integrase (intI) that catalyses recombination between the attC site of circular gene cassettes and the attendant integron recombination site, attI. This activity results in the sequential insertion of multiple, different cassettes to form a tandem cassette array that, in some cases, might contain hundreds of different genes. Inserted genes are expressed by an integron-encoded promoter, Pc.
Hundreds of integron classes have been described, defined on the basis of the relative homology of intI (Cambray et al., 2010; Boucher et al., 2011). Of these, the class 1 integrons, so named because they were first to be discovered, had properties that meant that they were well equipped to move by lateral DNA transfer into a wide range of commensal and pathogenic bacteria, and to accumulate diverse antibiotic resistance genes once humans tried to control bacteria with antimicrobial compounds. These fortuitous properties included: location on the chromosomes of Betaproteobacteria whose habitats intersect the human food chain; ability to move between chromosomal locations and between species (Gillings et al., 2008a); carriage by 0.002% of cells in an unaffected soil (Gaze et al., 2011) compared with as many as 5% of cells in affected soil, fresh water and biofilms (Gaze et al., 2005; Hardwick et al., 2008); ability to acquire a wide range of gene cassettes (Biskri et al., 2005); and frequent association with qac genes that encode versatile efflux pumps (Gaze et al., 2005; Gillings et al., 2009a).
When metagenomic DNA is examined from environmental sources, diverse genes belonging to intI1 can be detected. In contrast, all examples of intI1 recovered from clinical contexts have essentially identical DNA sequences, showing that there was a single common ancestor for the ‘clinical' class 1 integron that has spread antibiotic resistance among Gram-negative pathogens (Gillings et al., 2008b). Consequently, the class 1 integrons now circulating freely within human-dominated ecosystems have a conserved DNA sequence that, in the main, distinguishes them from the diverse class 1 integrons present in the more general environment.
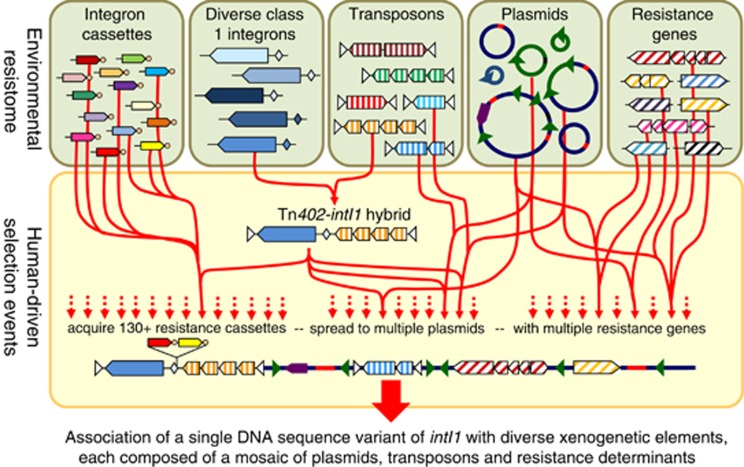
The best explanation for the origin of the clinical class 1 integron is that a chromosomal class 1 integron from an environmental betaproteobacterium was captured by a transposon of the Tn402 family (Figure 2). This integron carried a gene cassette encoding resistance to disinfectants (qacE), and subsequently captured a gene for sulphonamide resistance (sul1), deleting the terminus of the qacE cassette (Kholodii et al., 1995; Gillings et al., 2008a; Gillings, 2014a).
Figure 2.
The recent evolutionary origin of the clinical class 1 integron and its incorporation into diverse xenogenetic elements. The raw materials for the assembly of the complex mosaic DNA elements that now carry the clinical intI1 were all present in the environmental resistome. A single sequence variant from the diverse pool of class 1 integrons in natural environments was captured by a Tn402 transposon, thus forming a Tn402–intI1 hybrid, and giving the integron greater mobility. This hybrid integron, in total, has captured at least 130 different gene cassettes encoding resistance to diverse antibiotics. At the same time, the Tn402 portion of the hybrid element targeted the res sites of plasmids, transposing the whole hybrid molecule into a diverse collection of plasmids. This, in turn, promoted movement of clinical intI1 between different bacterial species by conjugation of those plasmids. Human selection events have also independently fixed the acquisition of diverse resistance genes onto the collection of plasmids invaded by the hybrid integron. These independent acquisitions resulted in the accumulation of genes for resistance to metals, antibiotics, disinfectants and other compounds, along with other genetic elements such as insertion sequences and transposons. As a result, a single molecular species (the clinical intI1 sequence variant) has become associated with an ever expanding and diverse set of plasmids, transposons and resistance genes. These mosaic elements can be thought of as xenogenetic, in the sense that they owe their current structures and abundance to human activity.
The Tn402 transposon has the unusual property of targeting the res sites of plasmids (Minakhina et al., 1999) and, consequently, the Tn402-class 1 integron hybrid was able to transpose into a wide variety of plasmids (Figure 2) that then enabled lateral transfer into an equally wide variety of bacterial species. One of the most successful of these insertion events associated the Tn402-integron with a mercury resistance operon (mer) to spawn the Tn21 element that itself went on to generate a series of complex derivatives (Liebert et al., 1999; Partridge et al., 2001). The Tn402-integron has also subsequently generated extensive internal variation by deletion of parts of qacE, sul1 and/or the Tn402 transposition machinery (Hall et al., 1994; Brown et al., 1996; Partridge et al., 2001). Variation in the cassette array has been generated by the collective acquisition of over 130 different antibiotic resistance gene cassettes (Figure 2) (Partridge et al., 2009), conferring resistance to the majority of antibiotics used to control Gram-negative pathogens (Mazel, 2006; Cambray et al., 2010; Stokes and Gillings, 2011).
Consequently, the ‘clinical' intI1 variant is now found on a range of different mobile elements that are freely transmissible between diverse commensal and pathogenic bacteria associated with humans and domestic animals (Nandi et al., 2004; Bailey et al., 2010; Djordjevic et al., 2013; Liu et al., 2013). This ‘clinical' intI1 is also closely linked to various genes that confer phenotypes of environmental significance, such as antibiotic, disinfectant and heavy metal resistances (Figure 2) (Liebert et al., 1999; Norman et al., 2009; Gillings et al., 2009b; Moura et al., 2010; Heuer et al., 2012; Domingues et al., 2013). Finally, ‘clinical' intI1 comprises a single molecular species with essentially identical DNA sequences, regardless of the diverse genetic and cellular landscapes they now inhabit (Figure 2).
Antibiotics and antibiotic resistance genes as pollutants
Between 30% and 90% of ingested antibiotic is excreted unchanged by both animals and humans (Sarmah et al., 2006). Antibiotics are only partly removed by wastewater treatment (Giger et al., 2003; Watkinson et al., 2007) and, depending on the antibiotic, can still be found at levels between 10 and 1000 ng l−1 in secondary effluent (Le-Minh et al., 2010). Antibiotics can enter soils via animal manure used for fertilization (Chee-Sanford et al., 2009), whereas other antibiotics are excreted preferentially in urine (Subbiah et al., 2012). As much as 80% of the antibiotics used in aquaculture flow into the environment (Cabello et al., 2013). Consequently, there is a zone around human activities that is enriched with antibiotics.
The use of antibiotics has vastly increased the abundance of ‘clinical' class 1 integrons, such that they are now present in up to 80% of enterobacteria in humans and farm animals (Tenaillon et al., 2010; Marchant et al., 2012; Liu et al., 2013). Consequently, large numbers of bacteria containing integrons are released into the environment, with one estimate suggesting that disposal of sewage sludge in the United Kingdom adds 1019 integron-containing bacteria to waste streams each year (Gaze et al., 2011). Wastewater treatment is not designed to remove DNAs, and the abundance of intI1 often increases during the water treatment process (LaPara et al., 2011; Ma et al., 2011b, 2013; Chen and Zhang, 2013; Cheng et al., 2013; Du et al., 2014). This might be a consequence of selection driven by the antibiotics, disinfectants and heavy metals that are also inefficiently removed during water treatment (Baker-Austin et al., 2006; Selin, 2009; Hegstad et al., 2010; Rosewarne et al., 2010). As a result, any bacteria that carry class 1 integrons associated with resistance determinants, or that are able to acquire them by lateral gene transfer, would increase in abundance during various stages of water treatment.
Resistance genes and DNA vectors are increasingly being recognized as environmental contaminants (Pruden et al., 2006; Stalder et al., 2014), and their abundance in natural environments and wild animals has been increasing since the first human use of antibiotics (Knapp et al., 2009; Gillings, 2013). The complex DNA molecules that now bear class 1 integrons often also carry genes for resistance to diverse antibiotics, disinfectants and other environmental contaminants, all embedded in a mosaic of mobile elements. These individual components often have a separate phylogenetic origin, each having been acquired in a separate event, and then fixed by human selection. Because human activities have had a direct role in the selection of sequential gene acquisitions, these complex mosaics of resistance elements can be thought of as xenogenetic. Such xenogenetic molecules have properties of both pollutants and invasive species, as they are pollutants that can replicate (Storteboom et al., 2010; Gillings and Stokes, 2012; Pruden et al., 2012). Methods to control pollution by antibiotics and their respective resistance genes have been suggested, including limiting the use of antibiotics in agriculture, and improving treatment of urban, industrial and hospital waste water (Pruden et al., 2013; Berglund et al., 2014).
IntI1 as a potential marker of anthropogenic pollution
The ‘clinical' intI1 gene has key advantages as a generic marker of anthropogenic influence. These include: universal presence and high abundance in the commensal bacteria of humans and domestic animals, a consequently high representation in waste streams, low abundance in less affected environments and a uniform and highly conserved DNA sequence. Based on these properties, a number of research groups have used quantitative analysis of intI1 to track human influence (Table 1).
Table 1. Environmental and laboratory studies examining the relationship between diverse pollutants, antibiotic resistance genes and class 1 integrons.
| System | Location | Sample, method | Comments | Reference |
|---|---|---|---|---|
| Hospital effluent | France | Water treatment, qPCR | IntI1 abundance increases because of effluent | Stalder et al. (2014) |
| Medical center effluent | France | E. coli isolation, PCR | IntI1 abundance increases because of effluent | Oberle‘X' et al. (2012) |
| Sewage treatment | USA | Aerobic digester, qPCR | IntI1 has longest half-life of genes tested | Burch et al. (2013) |
| Sewage treatment | China | Activated sludge, qPCR | IntI1 abundance increases | Ma et al. (2013) |
| Sewage treatment | China | Isolation, water, qPCR | IntI1 abundance increases in effluent | Ma et al. (2011a) |
| Sewage mesocosms | USA | Sludge, effluent, qPCR | Efficiency of intI1 removal dependent on treatment system | Ma et al. (2011b) |
| Wastewater treatment | China | Water, qPCR | Efficiency of intI1 removal dependent on treatment system | Du et al. (2014) |
| Wastewater treatment | China | Water, qPCR | Efficiency of intI1 removal dependent on treatment system | Chen and Zhang (2013) |
| Wastewater treatment | China | Water, sediment, qPCR | intI1 increases in abundance downstream from city | Zhang et al. (2009) |
| Wastewater treatment | USA | Water, sediment, qPCR | IntI1 abundance significantly increases in effluent | LaPara et al. (2011) |
| Wastewater treatment | UK | Bacterial isolation, PCR | Shows co-selection of intI1 and disinfectant resistance | Gaze et al. (2005) |
| Freshwater microcosm | USA | Bacterial isolation | Shows co-selection of antibiotic and metal resistance | Stepanauskas et al. (2006) |
| Waste streams | UK | Sludge, manure, qPCR | Shows selection of intI1 by waste antibiotics/disinfectants | Gaze et al. (2011) |
| River catchment | Cuba | Sediment, water, qPCR | Ab resistance correlates with degree of pollution | Graham et al. (2011) |
| River catchment | Pakistan | Water, qPCR | IntI1 and other gene abundance increases with human impact | Khan et al. (2013) |
| River catchment | USA | Sediment, water, qPCR | IntI1 abundance increases with industrial pollution | McArthur et al. (2011) |
| Stream catchment | Australia | Sediment, qPCR | IntI1 abundance increases with human impact | Hardwick et al. (2008) |
| Freshwater habitats | Canada | Water, floc, microarray | IntI1 cassette abundance increases with human impact | Drudge et al. (2012) |
| Estuary, catchment | France | E. coli isolation, qPCR | IntI1 and Ab resistance correlates with degree of pollution | Laroche et al. (2009) |
| Estuary | USA | Sediment, water, qPCR | IntI2 abundance increases with human impact | Uyaguari et al. (2013) |
| Estuary | Canada | Sludge, PCR | IntI1 and diverse cassettes associated with industrial waste | Koenig et al. (2009) |
| Various | Worldwide | PCR, cloning | IntI2 abundance increases with human impact | Rodríguez-Minguela et al. (2009) |
| Environ. gradient | USA | Sediment, qPCR | IntI1 abundance increases with metal/antibiotic pollution | Wright et al. (2008) |
| Environ. gradient | China | Sediment, sequencing | Integron and plasmid abundance increases with impact | Chen et al. (2013) |
| Environ. gradient | Argentina | Bacterial isolation, PCR | Trend for intI1 to increase in abundance with urbanization | Nardelli et al. (2012) |
| Environ. gradient | Australia | Sediment, qPCR | IntI1 abundance increases with heavy metal pollution | Rosewarne et al. (2010) |
| Environ. gradient | Worldwide | Soil, sediment, PCR | IncP plasmid abundance increases with pesticide impact | Dealtry et al. (2014a) |
| Swine production | Not stated | Soil and water, qPCR | IntI1 and other genes increase in abundance | Hong et al. (2013) |
| Slaughterhouse water | Portugal | Bacterial isolation, PCR | IntI1 increased in abundance during treatment | Moura et al. (2007) |
| Farm manuring | Germany | Soil, rhizosphere, qPCR | IntI1 and other genes increase in abundance | Jechalke et al. (2014) |
| Farm manuring | UK | Soil, qPCR | IntI1 increased in abundance | Byrne-Bailey et al. (2011) |
| Farm manuring | Germany | Soil, manure, PCR | IntI1 and other genes increase in abundance | Binh et al. (2009) |
| Manure, wastewater | China | Water, manure, qPCR | IntI1 and other genes increase in abundance | Cheng et al. (2013) |
| Manure treatment | China | Manure, qPCR array | Transposons and resistance genes increase in abundance | Zhu et al. (2013) |
| Animal microbiota | Various | E. coli isolation, PCR | IntI1 increases in frequency with increased human contact | Skurnik et al. (2006) |
| Archived soils | Scotland | Soil, qPCR | Correlation of resistance genes with copper pollution | Knapp et al. (2011) |
| Diverse | Various | Review | Shows co-selection of antibiotic and heavy metal resistance | Baker-Austin et al. (2006) |
| Diverse | Various | Review | Shows co-selection of antibiotic and heavy metal resistance | Seiler and Berendonk (2012) |
| Diverse | Various | Review | Shows co-selection of antibiotic and disinfectant resistance | Hegstad et al. (2010) |
Abbreviations: Ab, antibiotic; intI2, class 2 integron-integrase gene; qPCR, quantitative PCR.
Examining the relationship between pollutants, antibiotic resistance and class 1 integrons reveals a number of general trends (Table 1). IntI1 is poorly removed during water treatment, and its abundance often increases downstream from water treatment plants and human habitation. The intI1-carrying bacteria are abundant in manure, in digestates from biogas plants and in pesticide biopurification systems (Dunon et al., 2013; Jechalke et al., 2013a). Mesocosms designed to test land application of wastewater solids show that intI1 has a slow decay rate (Burch et al., 2014). In this regard, intI1-carrying bacterial populations are similar to other persistent pollutants, such as metals, antibiotics and disinfectants.
The co-occurrence of integrons, resistance genes and pollutants is probably causal, as co-selection of antibiotic resistance genes and integrons occurs in environments polluted with heavy metals and disinfectants (Baker-Austin et al., 2006; Hegstad et al., 2010; Seiler and Berendonk, 2012). This co-selection is most likely caused by the physical location of class 1 integrons on a range of transposons and plasmids that also carry genes for resistance to antibiotics, heavy metals and disinfectants (Table 1), and consequently, class 1 integrons can be selected via simple linkage. Similarly, intI1 abundance has been associated with pesticide pollution, via the co-occurrence of integrons and genes for degradative pathways on IncP-1 plasmids (Dealtry et al., 2014b).
Although the class 1 integron integrase gene does not directly confer resistance to any particular pollutant, its linkage to a diverse suite of antibiotic, metal and disinfectant resistance genes means that it is an excellent de facto measure of the general level of resistance determinants. For instance, there is a strong correlation between the abundance of intI1 in reclaimed water and the abundance of antibiotic resistance genes such as sul1 and tetG (Wang et al., 2014). Similarly, at the scale of whole watersheds, there is a strong correlation between sul1, which is commonly linked to intI1, and the upstream capacities of wastewater treatment and animal feeding operations (Pruden et al., 2012). Because resistance determinants confer selective advantages on those bacterial cells that carry them, intI1 abundance should then reflect the general response of the bacterial community to selection imposed by anthropogenic pollution. Consequently, intI1 abundance should be a good measure of general selective pressure. In contrast, targeting specific resistance determinants such as tet or sul is not a generic measure, as abundance of these genes is dependent on both their presence in a waste source and the presence of specific antibiotics to which they confer resistance.
Towards practical application of intI1 as a marker
Resistance genes and their vectors originate from environmental sources, where they form part of the resistome (D'Costa et al., 2006; Wright, 2010). This is also the case for intI1, which occurs naturally in environmental samples (Figure 2). The use of generic intI1 PCR primer pairs (Stokes et al., 2006) effectively amplifies both clinical and environmental variants of intI1, potentially contributing noise to quantification of intI1 shed from human sources. In environmental samples, intI1 exhibits considerable sequence diversity (Gillings et al., 2008b), whereas the clinical intI1 has a uniform, conserved sequence. For example, the Fungene database (http://fungene.cme.msu.edu/index.spr) (Fish et al., 2013) has over 500 sequences with >99% identity to intI1. These are mostly from clinical isolates, although a few are from environmental strains.
Sequences for environmental variants of intI1 are still in the minority in databases, and the region of intI1 for which most data are available is that amplified by primers HS464/HS463a (Gillings et al., 2008b). Examination of the sequence data (Supplementary Table S1) reveals a number of nucleotide positions where the clinical intI1 can be distinguished from most reported environmental variants. A primer pair targeting intI1 nucleotide positions 165–184 and 456–476 (intI1F165 5′-CGAACGAGTGGCGGAGGGTG-3′ and intI1R476 5′-TACCCGAGAGCTTGGCACCCA-3′) is one possibility for specifically amplifying the clinical version of intI1. Because these primers target the clinical intI1 sequence, but not the diverse intI1 variants known to be present in environmental bacteria, they should allow a more precise quantitative analysis. As more complete sequences from environmental variants of intI1 become available, better regions for discrimination could be identified.
Sample collection and processing
Environmental monitoring of human impact and the efficacy of remediation could be conducted using quantitative analysis of intI1 abundance. Careful consideration should be given to sampling strategies and data generation. Samples of sediment, soil or water should be taken in a uniform, reproducible manner. The likelihood of temporal variation should be taken into account. For instance, sewage treatment water can vary considerably over a 24-h period, and composite or flow proportionate samples should be considered. Ideally, each sampling time or point should be represented by at least triplicate samples, to be treated as triplicates in all subsequent steps such as DNA extraction and quantitative PCR (qPCR). Each sampling point for soil or sediment can be laid out in a grid to capture microvariation. At the minimum, samples should be identified by date, GPS coordinates and land use. The GSC (Genomic Standards Consortium) provides a guide to collection of environmental data under its MIMARKS environmental packages (Yilmaz et al., 2011), conveniently implemented by RDP according to habitat type with prepopulated googlesheets (http://rdp.cme.msu.edu/wiki/index.php/RDP_MIMARKS_GoogleSheets). For a detailed description of one multipurpose soil sampling procedure, see the BASE website (http://www.bioplatforms.com.au/special-initiatives/environment/soil-biodiversity/sample-collection-procedure). Soil can be stored at 4 °C, or snap-frozen immediately upon collection, and maintained frozen during transport to minimize changes to microbial populations.
IntI1 monitoring could be used for analysis of water samples, such as wastewater effluents, feedlot runoff and affected streams, rivers, lakes and oceans. Water samples can be collected by bulk grab techniques, using methods described for coliform monitoring. Water samples contain a particulate fraction, and many microbes, including microbes carrying intI1, attach to particulate matter suspended in the water. Most methods employ filtration, with 0.22 μm cutoff capturing the majority of bacteria and other particulates. The filter is then directly subject to DNA extraction. However, extracellular DNA may also be of interest, and this will pass through filters under some conditions. Recent studies have introduced techniques for analysing extracellular forms of antibiotic resistance genes (Mao et al., 2013). Further assessment and standardization of filter membrane composition and pore size employed for analysis of intI1 in water samples for different purposes (extracellular versus intracellular) would be of interest.
Sufficient sample should be taken for multiple analyses, and for archival storage. To ensure representative subsampling, the cone and quarter method can be used (Ferrari et al., 2008). The DNA extraction method employed should be suitable for diverse cell types and for removal of inhibitors present in soil, sediment, manure, sludge and other intractable substrates (Yeates and Gillings, 1998; Gillings, 2014b). The integrity of extracted DNA should be assessed using agarose electrophoresis and the concentration estimated photometrically. Alternatively, double-stranded DNA could be quantified using fluorometric methods (Singer et al., 1997).
Concentrations of intI1 can then be determined using real-time qPCR, correcting by the total bacterial abundance as measured by 16S rRNA gene PCR performed on the same sample. Ideally, three independent environmental samples should be processed in parallel to control for variation introduced during processing. PCR inhibition caused by co-extracted compounds can be overcome using bovine serum albumin (Gaze et al., 2011), an environmental master mix or template dilution. Primers for amplification of 16S rRNA genes should be specific for bacteria (Nadkarni et al., 2002). Primer sets need to be optimized across a range of concentrations and annealing temperatures. Standard curves for each target gene need to be determined, and positive control standards of known copy number prepared by PCR (Hardwick et al., 2008; McKinney and Pruden, 2012). The qPCR results could also be normalized by the total DNA in a sample, which would generate an idea of the relative abundance of intI1 in relation to the entire metagenome. If a housekeeping or other gene is used to normalize intI1 abundance, it should be established that it has a similar amplification efficiency to that of intI1.
Rapidly advancing molecular technologies will add new capabilities for understanding human impact. Highly parallel qPCR equipment from several vendors allows analysis of multiple primer sets and samples, such that hundreds of antibiotic resistance genes, mobile elements and their variants can be analysed (Looft et al., 2012; Zhu et al., 2013), allowing more comprehensive spatial and temporal studies. Furthermore, amplicons can be sequenced, providing diagnostic-level insight into the probable origin of these genes. A recent example of the latter shows clusters of intI1 and antibiotic resistance gene identities at a country scale and at an intercontinental scale for intI1 (Johnson et al., 2014).
As DNA sequencing becomes more efficient and cheaper, direct sequencing of metagenomic samples may replace qPCR approaches. In such an analysis, clinical intI1 sequences could be extracted from the sequence data and normalized to a single copy housekeeping gene. Already, such approaches are being used, based on high-throughput next-generation sequencing methods.
Conclusion
The clinical version of the intI1 gene has some unique advantages as a universal marker of selective pressures imposed by anthropogenic pollution. Its recent emergence into human-dominated ecosystems means that it has a homogenous and conserved DNA sequence, simplifying detection. It has seen a rapid increase in abundance and geographic distribution, fuelled by the extensive use of antibiotics and its insertion into diverse mobile elements, coupled with its penetration into a wide range of bacterial species associated with human-dominated ecosystems. During this expansion, it has become closely linked with genes that confer resistance to disinfectants and heavy metals, as well as the wide range of antibiotic resistance determinants for which it is well known. Consequently, versions of the clinical intI1 gene are capable of conferring diverse advantages to those cells that carry them, and these advantageous phenotypes correspond with the selective agents that are most likely to be present in human waste streams.
Acknowledgments
This paper arose from discussions held at the International Workshop on the Environmental Dimensions of Antibiotic Resistance held in Xiamen, China, December 2013. MRG is supported by the Australian Research Council, AP is supported by the Alfred P Sloan Foundation Microbiology of the Built Environment program and the National Science Foundation RAPID award no. 1402651, KS is supported by the Deutsche Forschungsgemeinschaft (DFG) funding the Research Unit FOR 566 ‘Veterinary Medicines in Soil: Basic Research for Risk Analysis' (Grant No. SM59/5-3) and by the Umweltbundesamt (3713 63 402), JMT is supported by the US National Science Foundation and Y-GZ is supported by the National Science Foundation of China.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Bailey JK, Pinyon JL, Anantham S, Hall RM. Commensal Escherichia coli of healthy humans: a reservoir for antibiotic-resistance determinants. J Med Microbiol. 2010;59:1331–1339. doi: 10.1099/jmm.0.022475-0. [DOI] [PubMed] [Google Scholar]
- Baker-Austin C, Wright MS, Stepanauskas R, McArthur JV. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006;14:176–182. doi: 10.1016/j.tim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Berglund B, Khan GA, Weisner SEB, Ehde PM, Fick J, Lindgren P-E. Efficient removal of antibiotics in surface-flow constructed wetlands, with no observed impact on antibiotic resistance genes. Sci Total Environ. 2014;476–477:29–37. doi: 10.1016/j.scitotenv.2013.12.128. [DOI] [PubMed] [Google Scholar]
- Binh CTT, Heuer H, Kaupenjohann M, Smalla K. Diverse aadA gene cassettes on class 1 integrons introduced into soil via spread manure. Res Microbiol. 2009;160:427–433. doi: 10.1016/j.resmic.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Biskri L, Bouvier M, Guérout A-M, Boisnard S, Mazel D. Comparative study of class 1 integron and Vibrio cholerae superintegron integrase activities. J Bacteriol. 2005;187:1740–1750. doi: 10.1128/JB.187.5.1740-1750.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher Y, Cordero OX, Takemura A, Hunt DE, Schliep K, Bapteste E, et al. Local mobile gene pools rapidly cross species boundaries to create endemicity within global Vibrio cholerae populations. mBio. 2011;2:e00335-10. doi: 10.1128/mBio.00335-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher Y, Labbate M, Koenig JE, Stokes HW. Integrons: mobilizable platforms that promote genetic diversity in bacteria. Trends Microbiol. 2007;15:301–309. doi: 10.1016/j.tim.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Brown HJ, Stokes H, Hall RM. The integrons In0, In2, and In5 are defective transposon derivatives. J Bacteriol. 1996;178:4429–4437. doi: 10.1128/jb.178.15.4429-4437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch TR, Sadowsky MJ, LaPara TM. Aerobic digestion reduces the quantity of antibiotic resistance genes in residual municipal wastewater solids. Front Microbiol. 2013;4:17. doi: 10.3389/fmicb.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch TR, Sadowsky MJ, LaPara TM. Fate of antibiotic resistance genes and class 1 integrons in soil microcosms following the application of treated residual municipal wastewater solids. Environ Sci Technol. 2014;48:5620–5627. doi: 10.1021/es501098g. [DOI] [PubMed] [Google Scholar]
- Byrne-Bailey K, Gaze WH, Zhang L, Kay P, Boxall A, Hawkey PM, et al. Integron prevalence and diversity in manured soil. Appl Environ Microbiol. 2011;77:684–687. doi: 10.1128/AEM.01425-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Millanao A, et al. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ Microbiol. 2013;15:1917–1942. doi: 10.1111/1462-2920.12134. [DOI] [PubMed] [Google Scholar]
- Cambray G, Guerout A-M, Mazel D. Integrons. Annu Rev Genet. 2010;44:141–166. doi: 10.1146/annurev-genet-102209-163504. [DOI] [PubMed] [Google Scholar]
- Chee-Sanford JC, Mackie RI, Koike S, Krapac IG, Lin Y-F, Yannarell AC, et al. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. Anglais. 2009;38:1086–1108. doi: 10.2134/jeq2008.0128. [DOI] [PubMed] [Google Scholar]
- Chen B, Yang Y, Liang X, Yu K, Zhang T, Li X. Metagenomic profiles of antibiotic resistance genes (ARGs) between human impacted estuary and deep ocean sediments. Environ Sci Technol. 2013;47:12753–12760. doi: 10.1021/es403818e. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang M. Occurrence and removal of antibiotic resistance genes in municipal wastewater and rural domestic sewage treatment systems in eastern China. Environ Int. 2013;55:9–14. doi: 10.1016/j.envint.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Cheng W, Chen H, Su C, Yan S. Abundance and persistence of antibiotic resistance genes in livestock farms: a comprehensive investigation in eastern China. Environ Int. 2013;61:1–7. doi: 10.1016/j.envint.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Collis CM, Hall RM. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob Agents Chemother. 1995;39:155–162. doi: 10.1128/aac.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dealtry S, Ding G-C, Weichelt V, Dunon V, Schlüter A, Martini MC, et al. Cultivation-independent screening revealed hot spots of IncP-1, IncP-7 and IncP-9 plasmid occurrence in different environmental habitats. PLoS One. 2014;9:e89922. doi: 10.1371/journal.pone.0089922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dealtry S, Holmsgaard PN, Dunon V, Jechalke S, Ding G-C, Krögerrecklenfort E, et al. Shifts in abundance and diversity of mobile genetic elements to diverse pesticides introduced into an on-farm biopurification system over a year. Appl Environ Microbiol. 2014;80:4012–4020. doi: 10.1128/AEM.04016-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocr Rev. 2009. pp. 293–342. [DOI] [PMC free article] [PubMed]
- Djordjevic SP, Stokes HW, Chowdhury PR. Mobile elements, zoonotic pathogens and commensal bacteria: conduits for the delivery of resistance genes into humans, production animals and soil microbiota. Front Microbiol. 2013;4:86. doi: 10.3389/fmicb.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues S, Toleman MA, Nielsen KM, da Silva GJ. Identical miniature inverted repeat transposable elements flank class 1 integrons in clinical isolates of Acinetobacter spp. J Clin Microbiol. 2013;51:2382–2384. doi: 10.1128/JCM.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drudge CN, Elliott AVC, Plach JM, Ejim LJ, Wright GD, Droppo IG, et al. Diversity of integron- and culture-associated antibiotic resistance genes in freshwater floc. Appl Environ Microbiol. 2012;78:4367–4372. doi: 10.1128/AEM.00405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Ren H, Geng J, Zhang Y, Xu K, Ding L. Occurrence and abundance of tetracycline, sulfonamide resistance genes, and class 1 integron in five wastewater treatment plants. Environ Sci Pollut Res. 2014;21:7276–7284. doi: 10.1007/s11356-014-2613-5. [DOI] [PubMed] [Google Scholar]
- Dunon V, Sniegowski K, Bers K, Lavigne R, Smalla K, Springael D. High prevalence of IncP-1 plasmids and IS1071 insertion sequences in on-farm biopurification systems and other pesticide-polluted environments. FEMS Microbiol Ecol. 2013;86:415–431. doi: 10.1111/1574-6941.12173. [DOI] [PubMed] [Google Scholar]
- D'Costa VM, McGrann KM, Hughes DW, Wright GD. Sampling the antibiotic resistome. Science. 2006;311:374–377. doi: 10.1126/science.1120800. [DOI] [PubMed] [Google Scholar]
- Ferrari BC, Winsley T, Gillings M, Binnerup S. Cultivating previously uncultured soil bacteria using a soil substrate membrane system. Nat Protoc. 2008;3:1261–1269. doi: 10.1038/nprot.2008.102. [DOI] [PubMed] [Google Scholar]
- Fish JA, Chai B, Wang Q, Sun Y, Brown CT, Tiedje JM, et al. FunGene: the functional gene pipeline and repository. Front Microbiol. 2013;4:291. doi: 10.3389/fmicb.2013.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze WH, Abdouslam N, Hawkey PM, Wellington EMH. Incidence of class 1 integrons in a quaternary ammonium compound-polluted environment. Antimicrob Agents Chemother. 2005;49:1802–1807. doi: 10.1128/AAC.49.5.1802-1807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze WH, Zhang L, Abdouslam NA, Hawkey PM, Calvo-Bado L, Royle J, et al. Impacts of anthropogenic activity on the ecology of class 1 integrons and integron-associated genes in the environment. ISME J. 2011;5:1253–1261. doi: 10.1038/ismej.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger W, Alder AC, Golet EM, Kohler H-PE, McArdell CS, Molnar E, et al. Occurrence and fate of antibiotics as trace contaminants in wastewaters, sewage sludges, and surface waters. CHIMIA. 2003;57:485–491. [Google Scholar]
- Gillings M, Boucher Y, Labbate M, Holmes A, Krishnan S, Holley M, et al. The evolution of class 1 integrons and the rise of antibiotic resistance. J Bacteriol. 2008;190:5095–5100. doi: 10.1128/JB.00152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillings MR. Evolutionary consequences of antibiotic use for the resistome, mobilome and microbial pangenome. Front Microbiol. 2013;4:4. doi: 10.3389/fmicb.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillings MR. Integrons: Past, present and future. Microbiol Mol Biol Rev. 2014;78:257–277. doi: 10.1128/MMBR.00056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillings MR. Rapid extraction of PCR-competent DNA from recalcitrant environmental samples. Methods Mol Biol. 2014;1096:17–23. doi: 10.1007/978-1-62703-712-9_2. [DOI] [PubMed] [Google Scholar]
- Gillings MR, Holley MP, Stokes H, Holmes AJ. Integrons in Xanthomonas: a source of species genome diversity. Proc Natl Acad Sci USA. 2005;102:4419–4424. doi: 10.1073/pnas.0406620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillings MR, Holley MP, Stokes HW. Evidence for dynamic exchange of qac gene cassettes between class 1 integrons and other integrons in freshwater biofilms. FEMS Microbiol Lett. 2009;296:282–288. doi: 10.1111/j.1574-6968.2009.01646.x. [DOI] [PubMed] [Google Scholar]
- Gillings MR, Krishnan S, Worden PJ, Hardwick SA. Recovery of diverse genes for class 1 integron-integrases from environmental DNA samples. FEMS Microbiol Lett. 2008;287:56–62. doi: 10.1111/j.1574-6968.2008.01291.x. [DOI] [PubMed] [Google Scholar]
- Gillings MR, Labbate M, Sajjad A, Giguere NJ, Holley MP, Stokes HW. Mobilization of a Tn402-like class 1 integron with a novel cassette array via flanking miniature inverted-repeat transposable element-like structures. Appl Environ Microbiol. 2009;75:6002–6004. doi: 10.1128/AEM.01033-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillings MR, Stokes HW. Are humans increasing bacterial evolvability. Trends Ecol Evol. 2012;27:346–352. doi: 10.1016/j.tree.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Goldstein C, Lee MD, Sanchez S, Hudson C, Phillips B, Register B, et al. Incidence of class 1 and 2 integrases in clinical and commensal bacteria from livestock, companion animals, and exotics. Antimicrob Agents Chemother. 2001;45:723–726. doi: 10.1128/AAC.45.3.723-726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DW, Olivares-Rieumont S, Knapp CW, Lima L, Werner D, Bowen E. Antibiotic resistance gene abundances associated with waste discharges to the Almendares River near Havana, Cuba. Environ Sci Technol. 2011;45:418–424. doi: 10.1021/es102473z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RM. Integrons and gene cassettes: hotspots of diversity in bacterial genomes. Ann NY Acad Sci. 2012;1267:71–78. doi: 10.1111/j.1749-6632.2012.06588.x. [DOI] [PubMed] [Google Scholar]
- Hall RM, Brown HJ, Brookes DE, Stokes H. Integrons found in different locations have identical 5'ends but variable 3'ends. J Bacteriol. 1994;176:6286–6294. doi: 10.1128/jb.176.20.6286-6294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick SA, Stokes HW, Findlay S, Taylor M, Gillings MR. Quantification of class 1 integron abundance in natural environments using real-time quantitative PCR. FEMS Microbiol Lett. 2008;278:207–212. doi: 10.1111/j.1574-6968.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- Hegstad K, Langsrud S, Lunestad BT, Scheie AA, Sunde M, Yazdankhah SP. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health. Microb Drug Resist. 2010;16:91–104. doi: 10.1089/mdr.2009.0120. [DOI] [PubMed] [Google Scholar]
- Heuer H, Binh CT, Jechalke S, Kopmann C, Zimmerling U, Krögerrecklenfort E, et al. IncP-1ɛ plasmids are important vectors of antibiotic resistance genes in agricultural systems: diversification driven by class 1 integron gene cassettes. Front Microbiol. 2012;3:2. doi: 10.3389/fmicb.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong P-Y, Yannarell AC, Dai Q, Ekizoglu M, Mackie RI. Monitoring the perturbation of soil and groundwater microbial communities due to pig production activities. Appl Environ Microbiol. 2013;79:2620–2629. doi: 10.1128/AEM.03760-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechalke S, Dealtry S, Smalla K, Heuer H. Quantification of IncP-1 plasmid prevalence in environmental samples. Appl Environ Microbiol. 2013;79:1410–1413. doi: 10.1128/AEM.03728-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechalke S, Focks A, Rosendahl I, Groeneweg J, Siemens J, Heuer H, et al. Structural and functional response of the soil bacterial community to application of manure from difloxacin-treated pigs. FEMS Microbiol Ecol. 2014;87:78–88. doi: 10.1111/1574-6941.12191. [DOI] [PubMed] [Google Scholar]
- Jechalke S, Schreiter S, Wolters B, Dealtry S, Heuer H, Smalla K. Widespread dissemination of class 1 integron components in soils and related ecosystems as revealed by cultivation-independent analysis. Front Microbiol. 2013;4:420. doi: 10.3389/fmicb.2013.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TA, Stedtfeld RD, Wang Q, Cole JR, Hashsham SA, Zhu YG, et al. 2014Correlation of the abundance and sequence identity of antibiotic resistance genes and mobile genetic elements in swine agriculture ISME ConferencePS19 272B; August 2014; Seoul, South Korea.
- Khan GA, Berglund B, Khan KM, Lindgren P-E, Fick J. Occurrence and abundance of antibiotics and resistance genes in rivers, canal and near drug formulation facilities—a study in Pakistan. PLoS One. 2013;8:e62712. doi: 10.1371/journal.pone.0062712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodii GY, Mindlin S, Bass I, Yurieva O, Minakhina S, Nikiforov V. Four genes, two ends, and a res region are involved in transposition of Tn5053: a paradigm for a novel family of transposons carrying either a mer operon or an integron. Mol Microbiol. 1995;17:1189–1200. doi: 10.1111/j.1365-2958.1995.mmi_17061189.x. [DOI] [PubMed] [Google Scholar]
- Knapp CW, Dolfing J, Ehlert PAI, Graham DW. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ Sci Technol. 2009;44:580–587. doi: 10.1021/es901221x. [DOI] [PubMed] [Google Scholar]
- Knapp CW, McCluskey SM, Singh BK, Campbell CD, Hudson G, Graham DW. Antibiotic resistance gene abundances correlate with metal and geochemical conditions in archived Scottish soils. PLoS One. 2011;6:e27300. doi: 10.1371/journal.pone.0027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JE, Sharp C, Dlutek M, Curtis B, Joss M, Boucher Y, et al. Integron gene cassettes and degradation of compounds associated with industrial waste: the case of the Sydney Tar Ponds. PLoS One. 2009;4:e5276. doi: 10.1371/journal.pone.0005276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPara TM, Burch TR, McNamara PJ, Tan DT, Yan M, Eichmiller JJ. Tertiary-treated municipal wastewater is a significant point source of antibiotic resistance genes into Duluth-Superior Harbor. Environ Sci Technol. 2011;45:9543–9549. doi: 10.1021/es202775r. [DOI] [PubMed] [Google Scholar]
- Laroche E, Pawlak B, Berthe T, Skurnik D, Petit F. Occurrence of antibiotic resistance and class 1, 2 and 3 integrons in Escherichia coli isolated from a densely populated estuary (Seine, France) FEMS Microbiol Ecol. 2009;68:118–130. doi: 10.1111/j.1574-6941.2009.00655.x. [DOI] [PubMed] [Google Scholar]
- Le-Minh N, Khan SJ, Drewes JE, Stuetz RM. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010;44:4295–4323. doi: 10.1016/j.watres.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Liebert CA, Hall RM, Summers AO. Transposon Tn21, flagship of the floating genome. Microbiol Mol Biol Rev. 1999;63:507–522. doi: 10.1128/mmbr.63.3.507-522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang H, Huang M, Mei Y, Gu B, Wu R, et al. Analysis of antimicrobial resistance and class 1 integrons among strains from upper respiratory tract of healthy adults. J Thorac Dis. 2013;5:149. doi: 10.3978/j.issn.2072-1439.2013.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looft T, Johnson TA, Allen HK, Bayles DO, Alt DP, Stedtfeld RD, et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc Natl Acad Sci USA. 2012;109:1691–1696. doi: 10.1073/pnas.1120238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang X-X, Cheng S, Zhang Z, Shi P, Liu B, et al. Occurrence, abundance and elimination of class 1 integrons in one municipal sewage treatment plant. Ecotoxicology. 2011;20:968–973. doi: 10.1007/s10646-011-0652-y. [DOI] [PubMed] [Google Scholar]
- Ma L, Zhang X-X, Zhao F, Wu B, Cheng S, Yang L. Sewage treatment plant serves as a hot-spot reservoir of integrons and gene cassettes. J Environ Biol. 2013;34:391–399. [PubMed] [Google Scholar]
- Ma Y, Wilson CA, Novak JT, Riffat R, Aynur S, Murthy S, et al. Effect of various sludge digestion conditions on sulfonamide, macrolide, and tetracycline resistance genes and class 1 integrons. Environ Sci Technol. 2011;45:7855–7861. doi: 10.1021/es200827t. [DOI] [PubMed] [Google Scholar]
- Mao D, Luo Y, Mathieu J, Wang Q, Feng L, Mu Q, et al. Persistence of extracellular DNA in river sediment facilitates antibiotic resistance gene propagation. Environ Sci Technol. 2013;48:71–78. doi: 10.1021/es404280v. [DOI] [PubMed] [Google Scholar]
- Marchant M, Vinué L, Torres C, Moreno MA. Change of integrons over time in Escherichia coli isolates recovered from healthy pigs and chickens. Vet Microbiol. 2012;163:124–132. doi: 10.1016/j.vetmic.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Mazel D. Integrons: agents of bacterial evolution. Nat Rev Microbiol. 2006;4:608–620. doi: 10.1038/nrmicro1462. [DOI] [PubMed] [Google Scholar]
- McArthur JV, Tuckfield RC, Lindell AH, Austin BC.2011. When rivers become reservoirs of antibiotic resistance: industrial effluents and gene nurseries. Proceedings of the 2011 Georgia Water Resources Conference.
- McKinney CW, Pruden A. Ultraviolet disinfection of antibiotic resistant bacteria and their antibiotic resistance genes in water and wastewater. Environ Sci Technol. 2012;46:13393–13400. doi: 10.1021/es303652q. [DOI] [PubMed] [Google Scholar]
- Minakhina S, Kholodii G, Mindlin S, Yurieva O, Nikiforov V. Tn5053 family transposons are res site hunters sensing plasmidal res sites occupied by cognate resolvases. Mol Microbiol. 1999;33:1059–1068. doi: 10.1046/j.1365-2958.1999.01548.x. [DOI] [PubMed] [Google Scholar]
- Moura A, Henriques I, Ribeiro R, Correia A. Prevalence and characterization of integrons from bacteria isolated from a slaughterhouse wastewater treatment plant. J Antimicrob Chemother. 2007;60:1243–1250. doi: 10.1093/jac/dkm340. [DOI] [PubMed] [Google Scholar]
- Moura A, Henriques I, Smalla K, Correia A. Wastewater bacterial communities bring together broad-host range plasmids, integrons and a wide diversity of uncharacterized gene cassettes. Res Microbiol. 2010;161:58–66. doi: 10.1016/j.resmic.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- Nandi S, Maurer JJ, Hofacre C, Summers AO. Gram-positive bacteria are a major reservoir of class 1 antibiotic resistance integrons in poultry litter. Proc Natl Acad Sci USA. 2004;101:7118–7122. doi: 10.1073/pnas.0306466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli M, Scalzo PM, Ramírez MS, Quiroga MP, Cassini MH, Centrón D. Class 1 integrons in environments with different degrees of urbanization. PLoS One. 2012;7:e39223. doi: 10.1371/journal.pone.0039223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A, Hansen LH, Sorensen SJ. Conjugative plasmids: vessels of the communal gene pool. Philos Transac R Soc B Biol Sci. 2009;364:2275–2289. doi: 10.1098/rstb.2009.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle‘X' K, Capdeville M-J, Berthe T, Budzinski Hln, Petit F. Evidence for a complex relationship between antibiotics and antibiotic-resistant Escherichia coli: from medical center patients to a receiving environment. Environ Sci Technol. 2012;46:1859–1868. doi: 10.1021/es203399h. [DOI] [PubMed] [Google Scholar]
- Partridge SR, Brown HJ, Stokes H, Hall RM. Transposons Tn1696 and Tn21 and their integrons In4 and In2 have independent origins. Antimicrob Agents Chemother. 2001;45:1263–1270. doi: 10.1128/AAC.45.4.1263-1270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge SR, Tsafnat G, Coiera E, Iredell JR. Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol Rev. 2009;33:757–784. doi: 10.1111/j.1574-6976.2009.00175.x. [DOI] [PubMed] [Google Scholar]
- Pruden A, Arabi M, Storteboom HN. Correlation between upstream human activities and riverine antibiotic resistance genes. Environ Sci Technol. 2012;46:11541–11549. doi: 10.1021/es302657r. [DOI] [PubMed] [Google Scholar]
- Pruden A, Larsson DJ, Amézquita A, Collignon P, Brandt KK, Graham DW, et al. Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ Health Perspect. 2013;121:878–885. doi: 10.1289/ehp.1206446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruden A, Pei R, Storteboom H, Carlson KH. Antibiotic resistance genes as emerging contaminants: studies in Northern Colorado. Environ Sci Technol. 2006;40:7445–7450. doi: 10.1021/es060413l. [DOI] [PubMed] [Google Scholar]
- Rockstrom J, Steffen W, Noone K, Persson Å, Chapin FS, III, Lambin E, et al. Planetary boundaries: exploring the safe operating space for humanity. Ecol Soc. 2009;14:32. [Google Scholar]
- Rodríguez-Minguela CM, Apajalahti JH, Chai B, Cole JR, Tiedje JM. Worldwide prevalence of class 2 integrases outside the clinical setting is associated with human impact. Appl Environ Microbiol. 2009;75:5100–5110. doi: 10.1128/AEM.00133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosewarne CP, Pettigrove V, Stokes HW, Parsons YM. Class 1 integrons in benthic bacterial communities: abundance, association with Tn402-like transposition modules and evidence for coselection with heavy-metal resistance. FEMS Microbiol Ecol. 2010;72:35–46. doi: 10.1111/j.1574-6941.2009.00823.x. [DOI] [PubMed] [Google Scholar]
- Sarmah AK, Meyer MT, Boxall ABA. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. 2006;65:725–759. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Seiler C, Berendonk TU. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front Microbiol. 2012;3:399. doi: 10.3389/fmicb.2012.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin NE. Global biogeochemical cycling of mercury: a review. Annu Rev Environ Resour. 2009;34:43–63. [Google Scholar]
- Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal Biochem. 1997;249:228–238. doi: 10.1006/abio.1997.2177. [DOI] [PubMed] [Google Scholar]
- Skurnik D, Ruimy R, Andremont A, Amorin C, Rouquet P, Picard B, et al. Effect of human vicinity on antimicrobial resistance and integrons in animal faecal Escherichia coli. J Antimicrob Chemother. 2006;57:1215–1219. doi: 10.1093/jac/dkl122. [DOI] [PubMed] [Google Scholar]
- Stalder T, Barraud O, Jove T, Casellas M, Gaschet M, Dagot C, et al. Quantitative and qualitative impact of hospital effluent on dissemination of the integron pool. ISME J. 2014;8:768–777. doi: 10.1038/ismej.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanauskas R, Glenn TC, Jagoe CH, Tuckfield RC, Lindell AH, King CJ, et al. Coselection for microbial resistance to metals and antibiotics in freshwater microcosms. Environ Microbiol. 2006;8:1510–1514. doi: 10.1111/j.1462-2920.2006.01091.x. [DOI] [PubMed] [Google Scholar]
- Stokes HW, Gillings MR. Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol Rev. 2011;35:790–819. doi: 10.1111/j.1574-6976.2011.00273.x. [DOI] [PubMed] [Google Scholar]
- Stokes HW, Nesbø CL, Holley M, Bahl MI, Gillings MR, Boucher Y. Class 1 integrons potentially predating the association with Tn402-like transposition genes are present in a sediment microbial community. J Bacteriol. 2006;188:5722–5730. doi: 10.1128/JB.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storteboom H, Arabi M, Davis JG, Crimi B, Pruden A. Identification of antibiotic-resistance-gene molecular signatures suitable as tracers of pristine river, urban, and agricultural sources. Environ Sci Technol. 2010;44:1947–1953. doi: 10.1021/es902893f. [DOI] [PubMed] [Google Scholar]
- Subbiah M, Shah DH, Besser TE, Ullman JL, Call DR. Urine from treated cattle drives selection for cephalosporin resistant Escherichia coli in soil. PLoS One. 2012;7:e48919. doi: 10.1371/journal.pone.0048919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenaillon O, Skurnik D, Picard B, Denamur E. The population genetics of commensal Escherichia coli. Nat Rev Microbiol. 2010;8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- Uyaguari MI, Scott GI, Norman RS. Abundance of class 1–3 integrons in South Carolina estuarine ecosystems under high and low levels of anthropogenic influence. Mar Pollut Bull. 2013;76:77–84. doi: 10.1016/j.marpolbul.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Wang F-H, Qiao M, Lv Z-E, Guo G-X, Jia Y, Su Y-H, et al. Impact of reclaimed water irrigation on antibiotic resistance in public parks, Beijing, China. Environ Pollut. 2014;184:247–253. doi: 10.1016/j.envpol.2013.08.038. [DOI] [PubMed] [Google Scholar]
- Watkinson AJ, Murby EJ, Costanzo SD. Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling. Water Res. 2007;41:4164–4176. doi: 10.1016/j.watres.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Wright GD. The antibiotic resistome. Expert Opin Drug Discov. 2010;5:779–788. doi: 10.1517/17460441.2010.497535. [DOI] [PubMed] [Google Scholar]
- Wright MS, Baker-Austin C, Lindell AH, Stepanauskas R, Stokes HW, McArthur JV. Influence of industrial contamination on mobile genetic elements: class 1 integron abundance and gene cassette structure in aquatic bacterial communities. ISME J. 2008;2:417–428. doi: 10.1038/ismej.2008.8. [DOI] [PubMed] [Google Scholar]
- Wu Y-W, Doak TG, Ye Y. The gain and loss of chromosomal integron systems in the Treponema species. BMC Evol Biol. 2013;13:16. doi: 10.1186/1471-2148-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates C, Gillings M. Rapid purification of DNA from soil for molecular biodiversity analysis. Lett Appl Microbiol. 1998;27:49–53. [Google Scholar]
- Yilmaz P, Kottmann R, Field D, Knight R, Cole JR, Amaral-Zettler L, et al. Minimum information about a marker gene sequence (MIMARKS) and minimum information about any (x) sequence (MIxS) specifications. Nat Biotechnol. 2011;29:415–420. doi: 10.1038/nbt.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu B, Zhang Y, Zhang T, Yang L, Fang H, et al. Class 1 integronase gene and tetracycline resistance genes tetA and tetC in different water environments of Jiangsu Province, China. Ecotoxicology. 2009;18:652–660. doi: 10.1007/s10646-009-0332-3. [DOI] [PubMed] [Google Scholar]
- Zhu Y-G, Johnson TA, Su J-Q, Qiao M, Guo G-X, Stedtfeld RD, et al. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci USA. 2013;110:3435–3440. doi: 10.1073/pnas.1222743110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.