Abstract
Despite being the most common electrolyte disturbance encountered in clinical practice, the diagnosis and treatment of hyponatremia (defined as a serum sodium concentration <135 mmol/L) remains far from optimal. This is extremely troubling because not only is hyponatremia associated with increased morbidity, length of hospital stay and hospital resource use, but it has also been shown to be associated with increased mortality. The reasons for this poor management may partly lie in the heterogeneous nature of the disorder; hyponatremia presents with a variety of possible etiologies, differing symptomology and fluid volume status, thereby making its diagnosis potentially complex. In addition, a general lack of awareness of the clinical impact of the disorder, a fear of adverse outcomes through overcorrection of sodium levels, and a lack of effective targeted treatments until recent years, may all have contributed to a reticence to actively treat cases of hyponatremia. There is therefore a clear unmet need to further educate physicians on the pathophysiology, diagnosis and management of this important condition. Through the use of a variety of real-world cases of patients with hyponatremia secondary to the syndrome of inappropriate secretion of antidiuretic hormone—a condition that accounts for approximately one-third of all cases of hyponatremia—this supplement aims to provide a comprehensive overview of the challenges faced in diagnosing and managing hyponatremia. These cases will also help to illustrate how some of the limitations of traditional therapies may be overcome with the use of vasopressin receptor antagonists.
Keywords: AVP, hyponatremia, SIADH, syndrome of inappropriate secretion of antidiuretic hormone, vasopressin
Introduction
M. Laville
Hyponatremia, defined as a serum sodium concentration ([Na+]) <135 mmol/L, is the most common electrolyte disturbance encountered in clinical practice, affecting up to 15–28% of hospitalized patients [1, 2]. Its incidence varies between hospitals and departments; for example, a study in the Netherlands observed an increased incidence in the departments of surgery (32%), internal medicine (36%) and intensive care (38%) compared with other departments (P < 0.05) [3]. Patients with hyponatremia also have varied clinical presentations including differing symptomology, underlying etiology and fluid volume status.
Despite frequently being observed in both outpatients and hospitalized patients, the diagnosis and management of hyponatremia remains far from optimal [4, 5]. This may be attributable to the diversity of underlying disease states associated with the condition and, until the last few years, a lack of targeted treatments. In some cases, the failure to order sufficient tests for diagnosis may also compound this issue [4].
Rationale for the treatment of hyponatremia
Hyponatremia at admission has been shown to be associated with increased length of stay (LOS) and cost of care for hospitalized patients [6]. Consequently, the proactive treatment of hyponatremia has the potential to reduce the utilization of healthcare resources.
There is some debate regarding the relationship of hyponatremia with mortality. A prospective study in 98 411 patients followed up for up to 5 years after hospital discharge found that even mild hyponatremia (serum [Na+] <135 mmol/L) independently predicted mortality [7]. Moreover, the resolution of the hyponatremia during hospitalization attenuated the increased mortality risk. The presence of hyponatremia has also been found to be an independent risk factor for increased mortality in a wide range of underlying diseases, including congestive heart failure, tuberculosis and liver failure [2]. Despite these data, causality has been difficult to prove [8, 9]. For example, in an analysis of hospitalized patients with hyponatremia, Chawla et al. [9] hypothesized that the nature of the underlying disease rather than hyponatremia itself was responsible for mortality. However, another plausible explanation is that hyponatremia may contribute to organ dysfunction and, therefore, indirectly contribute to mortality (Figure 1) [8].
Figure 1:
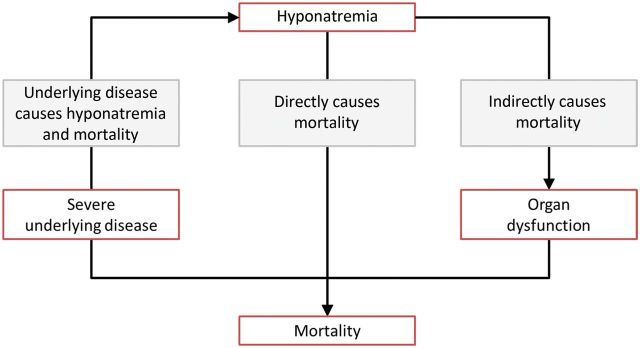
Possible relationships between hyponatremia and mortality [8]. The figure illustrates possible scenarios to explain the relationship between hyponatremia and mortality. In one scenario, hyponatremia and mortality are both caused by severe underlying disease. In another scenario, hyponatremia directly results in mortality (e.g. hyponatremia leading to cerebral edema in acute hyponatremia and the osmotic demyelination syndrome when chronic hyponatremia is corrected too rapidly). In the final scenario, hyponatremia indirectly contributes to mortality by causing organ dysfunction. Adapted from Hoorn and Zietse [8].
Support for this latter hypothesis is increasing with emerging data revealing the presence of significant morbidity in patients with hyponatremia, even in those traditionally presumed to be asymptomatic. In the past, treatment for the condition was only considered in patients with severe hyponatremia (serum [Na+] <125 mmol/L); however, even mild hyponatremia has been shown to be associated with attention deficits, increased falls and unsteadiness, which improved with the correction of hyponatremia [10]. Moreover, hyponatremia has also been shown to place elderly patients at increased risk of osteoporosis and bone fractures [11, 12]. Such symptoms are suggestive of a potential effect of hyponatremia on the nervous system and bone [8].
The syndrome of inappropriate secretion of antidiuretic hormone
Approximately one-third of all cases of hyponatremia are accounted for by the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) [13], a condition first described by Bartter and Schwartz [14]. Hyponatremia secondary to SIADH is the focus of our patient case reviews in this supplement. In a healthy body, vasopressin [also known as arginine vasopressin (AVP) or antidiuretic hormone (ADH)] acts in response to an increase in serum osmolality to retain water at the kidney nephron. As its name implies, patients with SIADH have unregulated secretion of vasopressin despite hypotonicity of the serum. Consequently, water intake combined with a high concentration of vasopressin leads to antidiuresis eventually resulting in hyponatremia.
Research over the past 40 years has uncovered a long list of potential causes of SIADH; these causes can be categorized as related to malignant diseases, pulmonary diseases and disorders of the central nervous system, as well as idiopathic forms [15]. Criteria now exist to definitively diagnose the condition, with the clinical assessment of the patient's fluid status forming a key part (Table 1).
Table 1.
Essential and supporting diagnostic criteria for hyponatremia secondary to SIADH [15]
| Essential diagnostic criteria | Case 3 values | Case 4 values |
|---|---|---|
| Serum sodium <135 mmol/L | 121 | 141 |
| Decreased measured plasma osmolality (<275 mOsm/kg) | 259 | 267 |
| Urinary osmolality >100 mOsm/kg during hypotonicity | 307 | 646 |
| Clinical euvolemia | ✓ | ✓ |
|
||
| Increased urinary sodium excretion >30 mmol/L with normal dietary salt and water intakeb | 89 | 130 |
| Normal thyroid and adrenal function determined by both clinical and laboratory assessment | ✓ | ✓d |
| No recent use of diuretic agents | ✓ | ✓ |
| Supporting diagnostic criteria | ||
| Plasma uric acid <4 mg/dL (<0.24 mmol/L) | ✓ | |
| Blood urea nitrogen <10 mg/dL (<3.6 mmol/L) | ||
| Fractional sodium excretion >1%; fractional urea excretion >55%c | ||
| Failure to improve hyponatremia after 0.9% saline infusion | ||
| Improvement of hyponatremia with fluid restriction | ||
aOrthostatic changes in blood pressure and pulse rate are defined as a ≥20 mm decrease in systolic blood pressure and/or a ≥20 b.p.m. increase upon going from a supine to a standing position.
bAlthough high urine sodium excretion generally occurs in patients with SIADH, its presence does not confirm the diagnosis, nor does its absence rule out the diagnosis; urine [Na+] can also be high in patients with Addison's disease. Conversely, some patients with SIADH can have low urinary [Na+] if they become hypovolemic or solute depleted, which are conditions sometimes produced by imposed sodium and water restriction.
cFractional sodium excretion = (urinary sodium excretion/serum sodium)/(urinary creatinine/serum creatinine) × 100; fractional urea excretion = (urinary urea/serum urea)/(urinary creatinine/serum creatinine) × 100.
dThe patient was taking corticosteroids.
Adapted from Ellison and Berl [15].
The management of SIADH
Owing to a relative lack of randomized controlled trials, the treatment of SIADH is largely based on expert opinion and uses agents commonly approved for indications other than for hyponatremia [16]. Fortunately, this situation has recently improved with the advent of vasopressin receptor antagonists (also known as ‘vaptans’; see below).
Proper evaluation of the underlying cause of SIADH is essential for its appropriate management. Once a diagnosis has been made, several conventional strategies exist for the correction of sodium concentration, many of which involve agents approved for indications other than for hyponatremia. These treatment strategies include fluid restriction, demeclocycline, lithium, loop diuretics in combination with salt tablets, urea tablets and hypertonic saline (3% NaCl). Owing to the absence of randomized controlled trials, the treatment of SIADH is largely based on expert opinion using agents commonly approved for indications other than for hyponatremia. However, these strategies can often prove challenging (Table 2), and there is little consensus as to the most effective and safe strategy in any given patient [16]. Although fluid restriction has traditionally been the mainstay of treatment in the management of SIADH despite the absence of an evidence base for its use, correct implementation is often difficult and patients can find it unpleasant, thus leading to poor compliance [17]. In fact, fluid restriction is often prescribed because of a lack of access to alternatives or simply because it is considered to be cheap. This is despite the fact that the majority of nephrologists and endocrinologists in Europe are dissatisfied with its effectiveness as a therapy for SIADH, as well as its poor tolerance by patients and feasibility even in trained staff [18].
Table 2.
Challenges of conventional treatments for SIADH [16]
| Strategy | Licensed in EU for SIADH | Drawback |
|||
|---|---|---|---|---|---|
| Slow and of low efficiency | Unreliable | Cumbersome | Invasive | ||
| Fluid restriction | ⨯ | ✓ | ✓ | ||
| 3% NaCl | ⨯ | ✓ | |||
| Loop diuretic | ⨯ | ✓ | |||
| Urea | ⨯ | ✓ | |||
| Demeclocycline | ⨯ | ✓ | ✓ | ||
| Lithium | ⨯ | ✓ | ✓ | ||
| CVVH | ⨯ | ✓ | ✓ | ||
| SLEDD | ⨯ | ✓ | ✓ | ||
CVVH, continuous veno-venous hemofiltration; SLEDD, slow low-efficiency daily dialysis.
Adapted from Gross [16].
The advent of vasopressin receptor antagonists
The recent introduction of orally available (tolvaptan) and parenteral (conivaptan) antagonists to the renal vasopressin receptor represents a new era in the management of SIADH. For the first time, agents are available that are specifically approved for the treatment of SIADH.
Vaptans work by preventing the antidiuresis caused by circulating vasopressin, by competitively blocking the binding of vasopressin to V2 receptors in the distal nephron of the kidney [19]. This decreases cAMP production, in turn reducing the number of aquaporin-2 water channels in the cells of the collecting tubules, which leads to a decrease in water reabsorption. The end result is electrolyte-free water excretion, or aquaresis, without any significant effects on renal sodium and potassium excretion. Thus, the removal of water from the body without loss of electrolytes results in an increase in serum [Na+].
Tolvaptan
In the European Union, tolvaptan—the only orally administered vaptan—is approved for use in adults with hyponatremia secondary to SIADH [20]. However, in the USA it is indicated for patients with clinically significant euvolemic or hypervolemic hyponatremia (serum [Na+] <125 mmol/L) and also for mild hyponatremia (serum [Na+] <125–135 mmol/L) in symptomatic patients, including patients with heart failure and SIADH that has resisted conventional therapy [21].
Its efficacy was evaluated in two of the largest studies ever conducted in hyponatremia [22]. These two identical multicenter, randomized, double-blind, placebo-controlled studies—known as the Study of Ascending Levels of Tolvaptan in Hyponatremia (SALT-1 and -2)—enroled 448 patients with euvolemic or hypervolemic hyponatremia (serum [Na+] <135 mmol/L) of different etiologies. Patients were randomized to receive tolvaptan (15–60 mg/day) or placebo for 30 days.
Tolvaptan significantly raised serum [Na+] within 8 h after its first administration in both the total patient population and subgroups categorized by degree of hyponatremia at baseline (all P < 0.01) [22]. Moreover, significantly more patients in the tolvaptan-treated group had normal serum [Na+] values at 30 days compared with placebo (P < 0.001). The most common adverse events were thirst and dry mouth; other adverse events included dizziness, hypotension, acute renal failure, sepsis and ascites. Furthermore, no patients exhibited any neurological symptoms suggestive of osmotic demyelination.
A subgroup analysis confirmed that the efficacy and safety profile of tolvaptan demonstrated in the heterogeneous hyponatremic population enroled in the SALT trials was applicable to patients with a diagnosis of SIADH [17]. According to the randomized design of the study, 58 patients with SIADH were assigned to receive placebo and 52 were assigned to receive tolvaptan at 15–60 mg/day. This was the largest number of patients with SIADH studied chronically using an orally active, vasopressin V2 receptor antagonist to correct hyponatremia. The authors noted that, ‘the superiority of tolvaptan over placebo was apparent for all of the endpoints related to improvement in serum [Na+] in the SIADH subgroup (average daily AUC of change from baseline in serum [Na+] at Days 4 and 30, mean serum [Na+] at each visit, time to serum [Na+] normalization, percentage of subjects with serum [Na+] normalization at Days 4 and 30, and categorical change in serum [Na+] at Days 4 and 30)’. Regarding safety issues, the profiles of potentially drug-related adverse events were relatively similar between the two treatment groups. Thirst and dry mouth occurred in nine (18%) and eight (16%) patients, respectively, on tolvaptan, and five (9%) and six (10%) patients, respectively, on placebo in this SIADH subgroup analysis. Of the 51 patients treated with tolvaptan, three (5.9%) exceeded an increase in serum [Na+] >12 mmol/L over the first 24 h and >18 mmol/L over the first 48 h; however, none of these patients experienced any neurological symptoms suggestive of osmotic demyelination.
The long-term efficacy and safety of tolvaptan was confirmed in an open-label extension trial, SALTWATER, which enroled 111 patients who completed the SALT-1 and SALT-2 studies for a mean of 701 days [23].
Upcoming guidelines for the management of hyponatremia
With the development of the vaptans, it has been suggested that these agents may become the mainstay of treatment for SIADH. However, there is a clear need for further randomized, controlled trials assessing the relative benefits of these new pharmacological agents compared with traditional standards of care. While we await the completion of these trials, the role of these newer agents in current practice has been considered in recently published evidence-based and expert panel reviewed guidelines in the USA (Verbalis JG, Goldsmith SR, Greenberg A et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 2013; 126 (10 Suppl 1): S1–S4). European guidelines are expected to follow later this year.
A review of real-life cases
For a condition that is frequently encountered in clinical practice and has the clinical consequences that hyponatremia does, its suboptimal diagnosis and management are a serious cause for concern. Furthermore, even when the condition is diagnosed, traditional treatment options may exhibit limited efficacy and can be challenging to use.
There is, therefore, an urgent need for improved education regarding the diagnosis and management of this important condition. To address these issues, a series of case studies have been brought together in this supplement to facilitate the exchange of knowledge regarding the management of hyponatremia. Discussion of these case studies will provide detailed information on the diagnosis and management of patients with hyponatremia secondary to SIADH. In addition, these cases illustrate the potential advantages that vaptans may offer over traditional therapies in certain patients.
Case 1: 47-year-old woman with a medulloblastoma
V. Burst
A 47-year-old woman with a known medulloblastoma (diagnosed 2 years previously) went to see her oncologist for a scheduled check-up. Her existing medications included temsirolimus for her tumor; she was also receiving treatment with the anticonvulsant valproate because she had experienced a number of seizures over the past year. At her check-up, she reported that she had not been feeling well for the previous few days with symptoms including drowsiness, lethargy and headaches.
A clinical assessment revealed a fever (38.5°C) but otherwise no other apparent issues; she had no signs of edema, her jugular venous pressure was normal and her heart and lungs were normal during auscultation (Table 3). Her blood analysis reported that her serum [Na+] was low at 123 mmol/L with a correspondingly low serum osmolality of 253 mOsm/kg, low creatinine (44.2 µmol/L [0.5 mg/dL]), low blood urea nitrogen (BUN; 3.9 mmol/L [11 mg/dL]) and low uric acid (125 µmol/L [2.1 mg/dL]; Table 3). Correspondingly, her urinary osmolality was high at 599 mOsm/kg and urinary [Na+] was high at 99 mmol/L.
Table 3.
Case 1: physical and laboratory data at scheduled check-up
| Investigation | Reading | Investigation | Reading |
|---|---|---|---|
| Physical examination | |||
| Weight (kg) | 58 | Blood pressure (mmHg) | 115/75 |
| Heart rate (b.p.m.) | 93 | Temperature (°C) | 38.4 |
| SpO2 (%) | 98 | ||
| Laboratory data | |||
| Serum [Na+] (mmol/L) | 123 | Uric acid (µmol/L) | 125 |
| Serum osmolality (mOsm/kg) | 253 | Urinary osmolality (mOsm/kg) | 599 |
| Serum [K+] (mmol/L) | 3.2 | Urinary [Na+] (mmol/L) | 99 |
| Creatinine (µmol/L) | 44.2 | Urinary [K+] (mmol/L) | 39 |
| Urea (mg/dL) | 24 | ||
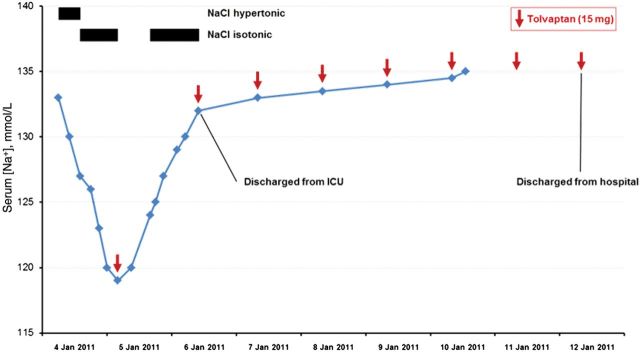
Her physician determined that her hyponatremia was most likely being caused by SIADH. To normalize her serum [Na+], she was admitted to the ward and fluid restriction (1.2 L/day) was initiated. The following day, the patient experienced ongoing motor convulsions, which were believed to be due to a focal status epilepticus so had to be transferred to the intensive care unit (ICU). After she arrived at the ICU, it was discovered that her serum [Na+] had not increased as expected but had in fact dropped further to 116 mmol/L (Figure 2)—this drop was therefore very likely to be the reason for her clinical deterioration.
Figure 2:
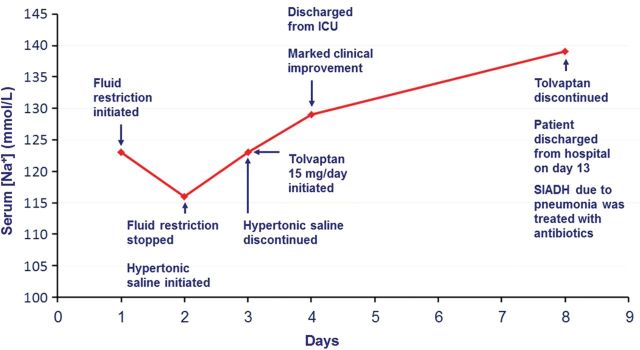
Case 1: treatment response and patient progress. SIADH, syndrome of inappropriate secretion of antidiuretic hormone.
Given the severity of her symptoms, treatment with hypertonic (3% NaCl) saline was commenced immediately. Within <24 h, sodium levels rose to 123 mmol/L. Hence, treatment with hypertonic saline was stopped and replaced with the oral vasopressin V2 receptor antagonist tolvaptan 15 mg/day (Figure 2). This resulted in a further increase in serum [Na+] with the patient exhibiting a rapid and marked clinical improvement, which allowed her to be discharged from the ICU 1 day later. Further work-up of the patient's initial feverish presentation revealed a pneumonia, which was treated successfully with antibiotics. It subsequently was suspected that the patient's SIADH had, in fact, been caused by the underlying pneumonia rather than her medulloblastoma as was initially assumed. Hence, tolvaptan administration was discontinued 5 days later when the patient's serum [Na+] had normalized at 137 mmol/L. The patient was discharged from hospital by Day 13 and her serum [Na+] has remained normal since the resolution of the pneumonia, without the need for further tolvaptan administration.
Discussion
When a patient presents with hyponatremia, there is a need to make a careful assessment of the volume status of the patient. Although it is relatively simple to detect hypervolemic patients through clinical signs, as they will show signs of edema etc., it is less straightforward to make a distinction between hypovolemic and euvolemic patients, especially in the elderly (Table 4) [24]. It is therefore important to perform a thorough clinical assessment. In addition, further diagnostic tests are key to making the right diagnosis. The latter point is important because, although SIADH was first described over 50 years ago [25], the condition continues to be under-diagnosed by physicians, often as a result of a failure to order sufficient diagnostic tests [4].
Table 4.
Matrix for the differential diagnosis of the underlying etiology of hyponatremia
| Urine [Na+] (<20 mmol/L) | Urine [Na+] (>40 mmol/L) | |
|---|---|---|
| Hypovolemia (dry tongue, decreased CVP, decreased urea, decreased pulse, decreased BP) | Vomiting, diarrhea, skin losses, burns | Diuretics, Addison's, cerebral salt-wasting syndrome, salt-losing nephropathy |
| Euvolemia | Hypothyroidism Any cause + hypotonic fluids |
SIADH Glucocorticoid deficiency Drugs |
| Hypervolemia (edema, ascites, LVF, increased JVP, increased CVP) | CHF, cirrhosis Nephrotic syndrome |
Renal failure, any cause + diuretics |
BP, blood pressure; CHF, congestive heart failure; CVP, central venous pressure; LVF, left ventricular failure; JVP, jugular venous pressure; SIADH, syndrome of inappropriate secretion of antidiuretic hormone.
Diagnosis of the underlying etiology of the hyponatremia using this system relies on an accurate assessment of the patient's volume status and measurement of urinary [Na+].
Adapted from Thompson et al. [24].
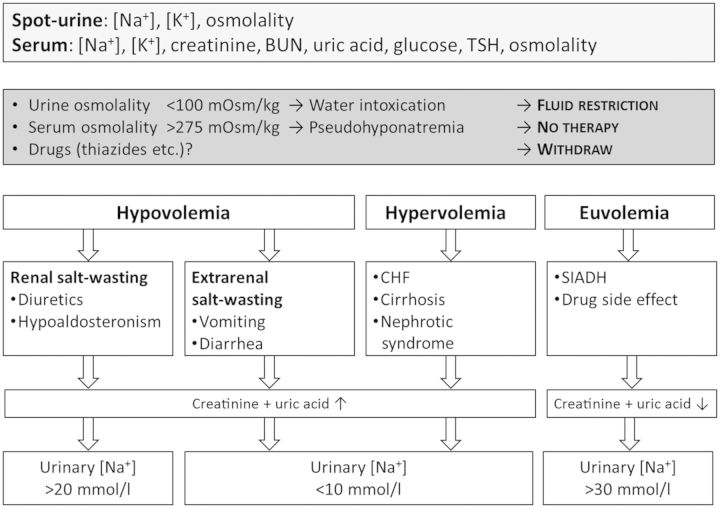
SIADH is a diagnosis of exclusion; the essential criteria for making a diagnosis of SIADH are listed in Table 1 [15, 26]. A very simple and straightforward diagnostic algorithm for hyponatremia is shown in Figure 3.
Figure 3:
Diagnostic algorithm for hyponatremia. BUN, blood urea nitrogen; CHF, chronic heart failure; SIADH, syndrome of inappropriate secretion of antidiuretic hormone; TSH, thyroid-stimulating hormone.
Moderate hyponatremia, like in our case report, is a common finding in hospitalized patients, and SIADH accounts for over a third of these cases. To treat such patients properly, it is crucial to understand that hyponatremia is always the consequence of water excess and never a matter of sodium loss. Thus, the aim of our treatment must be the elimination of water (electrolyte-free water clearance) rather than the supplementation of salt. It is only in life-threatening situations, where cerebral edema and subsequent herniation have to be suspected, when treatment with hypertonic saline is recommended. Otherwise, fluid restriction is the classical therapy for euvolemic hyponatremia. The simple logic behind that is by restricting the patient's fluid intake, the kidney is forced to excrete the highly concentrated urine it already produces anyway, due to inappropriate vasopressin action. Consequently, serum [Na+] will tend to rise. However, this increase is usually subtle, and hyponatremia needs several days to subside. It is of importance to understand that the degree of fluid restriction varies with the urinary tonicity, i.e. the concentration of osmotically active electrolytes. To excrete electrolyte-free water, urinary sodium plus urinary potassium must be less than serum [Na+]. Consequently, urinary potassium should also be regularly measured as well as urinary [Na+]; this allows electrolyte-free water clearance (CeH2O) to be calculated using the following formula:
 |
Using this formula, the calculations show that, for the next litre of urine excreted, the patient's electrolyte-free water clearance would be −122 mL, i.e. she would have an increase in free water rather than an excretion:
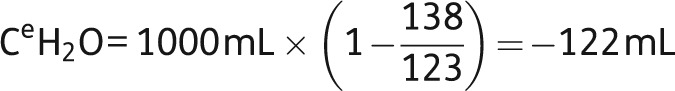 |
If you also take into account the intake (1.2 L) and the insensible losses (800 mL), the free water balance is calculated as follows:
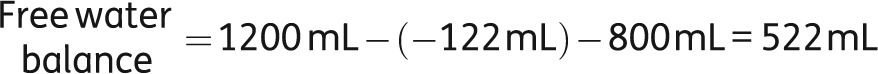 |
Hence, despite the prescribed fluid restriction, fluid accumulation continued leading to further aggravation of hyponatremia. For bedside purposes, the ratio of urinary and serum electrolytes (see below) taken from the above formula is a handy tool allowing for choosing the right therapy, as well as for making a rough estimation on the severity of the situation [27]. In this ratio, the numerator is a surrogate for the urinary tonicity, whereas the denominator is a surrogate for serum tonicity. To remove free water, the tonicity of the urine would need to be below that of the serum, i.e. the ratio should be <1.0. When the ratio is >1.0 (as was the case in this patient), serum [Na+] is being lowered because of free water being retained. Thus, the patient is at direct risk and immediate therapeutic action is mandatory. In addition, the fluid restriction in our patient is likely to be ineffective (Table 5).
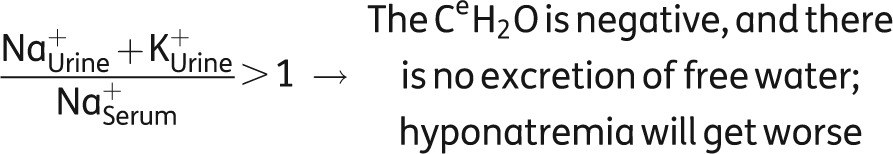 |
Table 5.
Recommendations for fluid restriction
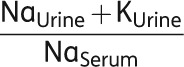 |
Fluid restriction |
|---|---|
| >1.0 | 0 mL |
| 0.5–1.0 | <500 mL |
| <0.5 | <1 L |
These recommendations assume that urinary sodium and potassium losses are replaced that a patient has an average body surface area of 1.73 m2 and eats a normal diet, and is calculated for the period during which the next 1 L of urine is excreted.
Adapted from Furst et al. [27].
Although it is possible that a more strict fluid restriction of 500 mL/day may have proved more successful, it should be borne in mind that patients with SIADH not only tend to have a high level of vasopressin but they are also thirsty, which contributes to their condition. In fact, it has been shown that patients with SIADH have a downward resetting of their osmotic threshold for thirst [28]. Therefore, putting a patient with SIADH onto 500 mL/day fluid restriction on a chronic basis is not an ideal solution and is likely to incur problems with compliance. In such cases, alternative approaches involving an increase in solute intake or a treatment that inhibits vasopressin action on the collecting duct, thereby enhancing free water excretion, should be considered.
Case 2: 47-year-old man with possible anaplastic oligoastrocytoma recurrence
V. Burst
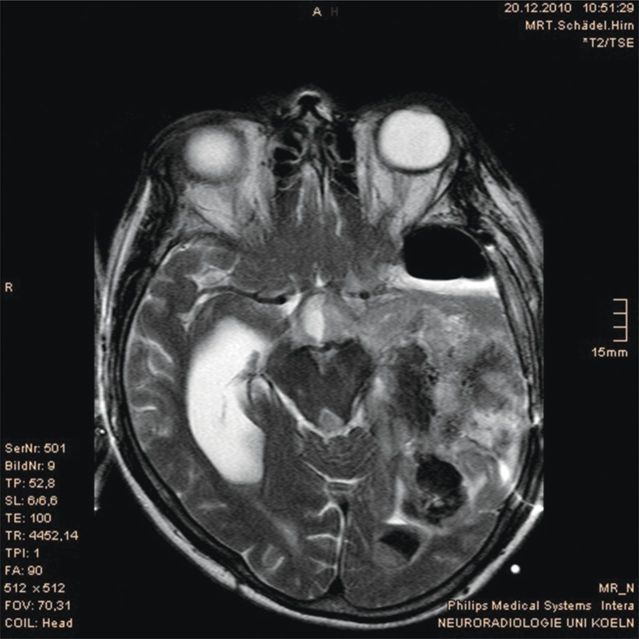
A 47-year-old man was transferred from a secondary hospital to a university hospital following suspicious magnetic resonance imaging (MRI) findings, which suggested a recurrence of his known anaplastic oligoastrocytoma and intracerebral bleeding (Figure 4). The tumor (classified as WHO grade III) had been resected 3 years previously but the patient had subsequently developed a subdural hygroma. Following several unsuccessful attempts to surgically resolve the hygroma, he had received a subdural-peritoneal shunt, which had proved successful. The patient was also coinfected with HIV and chronic hepatitis C, and had a history of alcohol abuse. He was receiving chronic treatment with antiretrovirals, carbamazepine and neuroleptics.
Figure 4:
Case 2: magnetic resonance imaging suggesting a recurrence of his known anaplastic oligoastrocytoma and intracerebral bleeding.
Following admission, the patient's clinical picture deteriorated over the course of a few days, and a steady increase in intracranial pressure mandated surgical intervention. Eight days after surgery, the patient lost consciousness; he was diagnosed with acute hyponatremia with his serum [Na+] having dropped to 116 mmol/L from a reading of 134 mmol/L just 32 h previously. The patient was therefore rushed to the ICU where hypertonic saline was administered, which led to an improvement in his clinical state.
Unfortunately, his serum [Na+] rose from 116 to 129 mmol/L within 16 h, which is considered overcorrection. Subsequently, collected urinary specimens revealed an extremely high urinary [Na+] of 222 mmol/L. This high urinary sodium output was found to have mirrored the high sodium intake caused by the administration of hypertonic saline. Several subsequent attempts to withdraw the hypertonic saline resulted in dramatic drops in serum [Na+] (e.g. falls from 133 to 119 mmol/L were observed in 24 h) thereby necessitating its continuation.
Based on the assumption that SIADH was the most likely cause of the hyponatremia, tolvaptan was initiated at a dose of 15 mg/day on Day 5 after prior discontinuation of his saline infusion (Figure 5). This resulted in an increase in serum [Na+] from 120 to 130 mmol/L in 24 h and the patient reported feeling well. He was subsequently transferred from the ICU to the neurosurgery ward 1 day later and was kept on tolvaptan without adverse effects. The patient was discharged from the hospital 8 days later. A recent attempt to withdraw the drug resulted in an aggravation of the hyponatremia; therefore, the patient is currently receiving chronic therapy with tolvaptan.
Figure 5:
Case 2: treatment response and patient progress. ICU, intensive care unit.
Discussion
When considering the management of hyponatremia, the benefits of treatment must be balanced against the potential risks. A rapid fall in serum osmolality, mirrored by a decline of serum sodium, results in a shift of water from the extracellular to the intracellular compartment. In response, many if not all cells of the body are able to undergo a process of active adaptation—accomplished by the extrusion of intracellular organic osmolytes—in order to induce water loss and restore cell volume [29]. However, if the serum [Na+] decreases more rapidly than the brain can adapt to it, then cerebral edema occurs resulting in an increase in intracranial pressure and subsequent serious complications [30]. Consequently, it is important that symptomatic acute hyponatremia is treated urgently.
Conversely, a rapid correction of serum [Na+] in cases of chronic hyponatremia has the potential to do more harm than good through possible overcorrection. Although the brain adapts in response to hyponatremia to protect itself from cerebral edema, this same adaptation also makes it vulnerable to injury if serum [Na+] is normalized too rapidly, probably due to shrinkage of solute-depleted brain cells. This shrinkage leads to demyelination of pontine and extrapontine neurons and may result in neurological dysfunction, including seizures, coma and even death [31].
The consequences of overly rapid correction (>12 mmol/L/day) were demonstrated in an analysis by Sterns et al. of post-therapeutic neurological complications in 56 patients treated for severe hyponatremia (serum [Na+] ≤105 mmol/L) [32]. Ten patients displayed permanent neurological sequelae and four had transient neurological complications that emerged after serum [Na+] was increased to >120 mmol/L. Analyses of variables determining outcome in these patients showed that neurological sequelae after the treatment of severe chronic hyponatremia were associated with increases in sodium concentration that were >12 mmol/L over the first 24 h and >18 mmol/L over the first 48 h of therapy. Furthermore, the chronicity of hyponatremia was significantly associated with complications (P < 0.01 versus acute hyponatremia).
It is now generally accepted that the rate of serum [Na+] correction in chronic hyponatremia should be limited to <10–12 mmol/L in 24 h or to <18 mmol/L in 48 h [26]. However, it should be taken into consideration that patients with alcoholism, as was the case in our patient, may be especially susceptible to osmotic demyelination syndrome (ODS) [33]. Thus, given the risk of overshooting maximal recommended increases, it is perhaps best to aim for correction by ∼8 mmol/L/day in these patients [26].
Despite overcorrection with hypertonic saline in this case (serum [Na+] increased 13 mmol/L in 16 h), the patient did not develop any neurological issues. Although there have been no reported cases of osmotic demyelination associated with the use of vaptans, to date, there is a need for careful monitoring of serum [Na+] responses during correction of hyponatremia as per the recommendations in the product licence.
Case 3: 81-year-old woman presenting with mental confusion
A. Peri
An 81-year-old Caucasian woman was brought in to the emergency department of the University Hospital in Florence suffering from mental confusion. Her past medical history showed that she had hypertension, peripheral atheromatosis, and had experienced a previous transitory ischemic attack. Over the last year, she complained of an inability to perform normal cognitive functions and had experienced frequent falls. Her existing medications included ramipril 2.5 mg/day, lansoprazole 30 mg/day and acetylsalicylic acid 100 mg/day.
The patient's blood pressure was 170/100 mmHg and heart rate 70 b.p.m.; routine laboratory testing revealed that her serum [Na+] was only 120 mmol/L (Table 6). At this point, her son recalled that the patient's serum [Na+] had been frequently documented below the normal range over the last year.
Table 6.
Case 3: laboratory values at admission and diagnostic work-up
| Laboratory measurement | Results | Normal range |
|---|---|---|
| At admission | ||
| Glucose (mmol/L) | 5.16 | 3.6–6.3 |
| Serum [Na+] (mmol/L) | 120 | 135–146 |
| Serum urea (g/L) | 0.41 | 0.1–0.5 |
| Hemoglobin (g/L) | 109 | 120–160 |
| Hematocrit (proportion of 1.0) | 0.32 | 0.36–0.46 |
| Serum creatinine (μmol/L) | 56.6 | 35.4–79.6 |
| Serum uric acid (μmol/L) | 131 | 208–387 |
| Serum [K+] (mmol/L) | 4.1 | 3.5–5.3 |
| Diagnostic work-up | ||
| TSH (miU/L) | 1.18 | 0.25–3.50 |
| Free T4 (pmol/L) | 14.8 | 10.3–19.4 |
| Serum [Na+] (mmol/L)a | 121 | 135–146 |
| Urinary [K+] (mmol/L) | 48 | 25–125 |
| Urinary [Na+] (mmol/L) | 89 | 25–250 |
| Triglycerides (mmol/L) | 0.71 | 0.57–1.92 |
| Cholesterol (mmol/L) | 4.45 | 4.14–5.70 |
| Total protein (g/L) | 0.062 | 0.06–0.086 |
| Cortisol (nmol/L) | 603 | 160–690 |
| Plasma osmolality (mOsm/kg) | 259 | 285–295 |
| Urine osmolality (mOsm/kg) | 307 | 300–1100 |
aFollowing isotonic saline infusion.
No further diagnostic tests were performed at this stage and the patient was administered intravenous isotonic saline in the emergency department in order to correct her low serum [Na+]. Unfortunately, there was no change in serum [Na+] and the patient's condition showed no improvement.
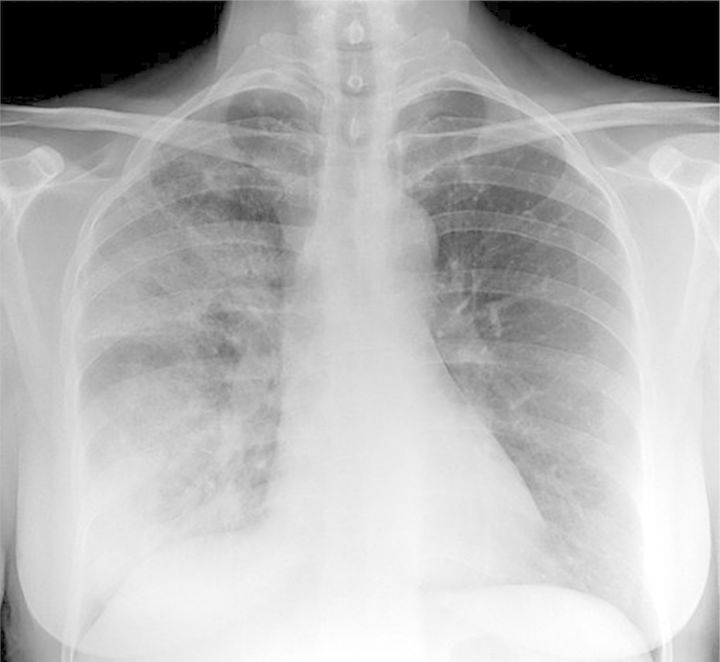
The patient was subsequently admitted to the internal medicine unit where she was given a comprehensive diagnostic work-up (Table 6). The patient was shown to be clinically euvolemic. A cranial computed tomography (CT) scan showed no signs of intracranial bleeding or subcortical ischemic encephalopathy, but a chest X-ray revealed that she had pneumonia (Figure 6). Consequently, the patient was diagnosed with hyponatremia secondary to SIADH caused by underlying pulmonary disease.
Figure 6:
Case 3: pneumonia diagnosed via chest X-ray.
The patient was treated with hypertonic saline (3% NaCl); her existing lansoprazole was stopped following a negative gastroduodenoscopy and acetylsalicylic acid replaced by ticlopidine 250 mg/day. For her pneumonia, she was prescribed antibiotics (levofloxacin 500 mg/day orally and ceftriaxone 2 g/day intramuscularly).
Following 24 h, the patient's serum [Na+] rose to 127 mmol/L, at which point her infusion was stopped. Fluid restriction was then considered. However, calculations based on the patient's ratio of electrolytes in the urine and serum (according to the formula: urinary [Na+] + urinary [K+]/serum [Na+]) [27] gave a result >1.0, which implied that the free water clearance of the patient was nil. Thus, fluid restriction was unlikely to improve serum [Na+] in this patient; therefore, therapy with tolvaptan 15 mg/day was started. This raised her serum [Na+] to 132 mmol/L after 24 h and marked clinical improvement was observed on the second day, when her serum [Na+] was 138 mmol/L. The patient was eventually discharged after 6 days on tolvaptan.
At a follow-up assessment 1 week after discharge, the patient's sodium levels remained within the normal range, her clinical condition was good and a further chest X-ray showed no evident radiological signs of pneumonia. Tolvaptan treatment was therefore stopped at a serum [Na+] of 141 mmol/L. Serum [Na+] remained within the normal range at follow-up.
Discussion
Chronic hyponatremia is associated with increased patient morbidity. A four-fold increase in the incidence of falls has been reported by Renneboog et al. [10] in patients with apparently ‘asymptomatic’ chronic hyponatremia (mean serum [Na+] 126 ± 5 mmol/L) admitted to the emergency department, compared with matched controls with normal serum [Na+] (21.3 versus 5.3%). Further analyses revealed that these falls may possibly be the result of marked gait and attention impairments in these patients [10]. Consequently, it is logical to deduce that this patient's neurological symptoms and a history of falls over the past year are linked to her chronic hyponatremia rather than to another underlying disease. Taking steps to correct her persistent hyponatremia may therefore help reverse these symptoms.
In practice, it may be a tempting diagnostic procedure to administer isotonic saline to all patients with low serum [Na+] before the fluid volume status of the patient has been determined and monitor the response to therapy. However, although isotonic saline can be used to treat hypovolemic hyponatremia, it is not an appropriate treatment in a patient with euvolemic hyponatremia and may even exacerbate problems in such patients. It is, therefore, important to perform sufficient tests in order to identify the underlying etiology of hyponatremia. Assessing the patient's serum and urinary osmolality, fluid volume status and urinary [Na+] plays a pivotal role in the differential diagnosis of hyponatremia (Table 1). In this case, the patient was found to be clinically euvolemic and diagnosed with SIADH.
The etiology of SIADH can be classified into five major categories including malignancies, pulmonary disorders, central nervous system disorders, drug-related and a fifth category that includes idiopathic SIADH and other causes (Table 7) [15, 26]. Of these, lung cancer, pulmonary infections, CNS infections, stroke, antiepileptics, antidepressants and idiopathic origins are the most common causes of SIADH [34]. Investigating the etiology of SIADH is important because if the underlying cause is not managed then the hyponatremia could return. It is also important that an underlying malignancy, particularly of the lung, is not missed.
Table 7.
Causes of SIADH [26]
| Malignancy | Pulmonary/mediastinal (bronchogenic carcinoma, mesothelioma, thymoma) Non-chest (duodenal carcinoma, pancreatic carcinoma, ureteral/prostate carcinoma, uterine carcinoma, nasopharyngeal carcinoma, leukemia) |
| CNS disorders | Mass lesions (tumors, brain abscesses, subdural hematoma) Inflammatory diseases (encephalitis, meningitis, systemic lupus, acute intermittent porphyria, multiple sclerosis) Degenerative/demyelinative diseases (Guillain–Barré syndrome, spinal cord lesions) Miscellaneous (subarachnoid hemorrhage, head trauma, acute psychosis, delirium tremens, pituitary stalk section, transsphenoidal adenomectomy, hydrocephalus) |
| Drugs | Stimulated vasopressin release Direct renal effects and/or potentiation of vasopressin antidiuretic effects Mixed or uncertain actions |
| Pulmonary diseases | Infections (tuberculosis, acute bacterial and viral pneumonia, aspergillosis, empyema) Mechanical/ventilatory (acute respiratory failure, COPD, positive pressure ventilation) |
| Other | AIDS and ARC Prolonged strenuous exercise (marathon, triathlon, ultramarathon, hot-weather hiking) Prolonged nausea and vomiting (e.g. with chemotherapy) Senile atrophy Idiopathic |
ARC, AIDS-related complex; COPD, chronic obstructive pulmonary disease.
Adapted from Verbalis et al. [26].
Investigations revealed that the patient had normal thyroid and adrenal functions, thereby ruling out glucocorticoid deficiency and hypothyroidism as possible causes. Her existing medications [an angiotensin-converting enzyme (ACE) inhibitor, a proton-pump inhibitor and acetylsalicylic acid] suggested that drug-induced hyponatremia could be a possible candidate for the underlying cause (Table 8). However, ACE inhibitors and proton-pump inhibitors are relatively rare causes of hyponatremia [35] and, as the patient had been taking her medications for a long time without ill effect, it was probable that her hyponatremia was being caused by something else.
Table 8.
Causes and underlying mechanisms of drug-induced hyponatremia [35]
| Drugs affecting sodium and water homeostasis | Diuretics |
| Drugs affecting water homeostasis | Antidepressants, antipsychotic drugs, opioids, antiepileptic drugs, anticancer agents |
| Potentiation of vasopressin effect | Antiepileptic drugs, antidiabetic drugs anticancer agents, non-steroidal anti-inflammatory drugs |
| Reset osmostat | Antidepressants, antiepileptic drugs |
| Rare causes of drug-induced hyponatremia | Antihypertensive agents, immune globulin (intravenous), 3,4-methylenedioxymethamphetamine (ecstasy), antibiotics, antiarrhythmic, theophylline, proton pump inhibitors, bromocriptine, terlipressin, duloxetine, fluorescein angiography, bupropion |
Adapted from Liamis et al. [35].
Following a chest X-ray, it was confirmed that the patient's SIADH was most likely being caused by underlying pneumonia. At this stage, she was prescribed antibiotics for her pulmonary infection. Rather than simply wait for the infection to resolve, and her hyponatremia to subsequently normalize, it is important to realize the benefits of immediately treating hyponatremia. It was the patient's hyponatremia rather than the underlying disease that was keeping her hospitalized by making her confused and prone to falls; therefore, direct treatment of her hyponatremia would hopefully result in her being discharged earlier, as well as reducing the risk of rehospitalization. She was, therefore, also treated initially with hypertonic saline (3% NaCl) 20 mL/h (according to the Adrogué and Madias formula) [31]:
Formula for the calculation of the infusion rate of saline solution
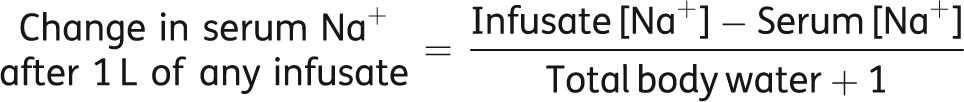 |
If potassium has been added into the infusate then the calculation should bear this in mind, i.e.
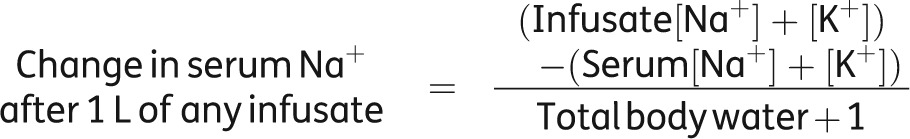 |
The infusion was stopped after 24 h with her serum [Na+] raised to 127 mmol/L. At this stage, fluid restriction—often the standard of care in patients with asymptomatic hyponatremia secondary to SIADH—was considered. However, acknowledging the difficulties patients often have in maintaining fluid restriction, its slowness in increasing serum [Na+] and the predicted absence of free water excretion, it was decided to further treat her with the vasopressin V2 receptor antagonist tolvaptan at 15 mg/day taken orally. This produced a rise in serum [Na+] to 132 mmol/L after 24 h; the patient had marked clinical improvement on the second day (serum [Na+] 138 mmol/L), and she was discharged after 6 days on tolvaptan.
Tolvaptan treatment was stopped at a serum [Na+] of 141 mmol/L after a follow-up assessment 1 week later. The patient's sodium levels remained within normal ranges, her clinical condition was good and there were no evident radiological signs of pneumonia at a control chest X-ray.
Case 4: 74-year-old man presenting with a cough and hemoptysis
A. Peri
A 74-year-old Caucasian man presented with a cough and hemoptysis, with which he had been suffering for the last 3 months. Although he had previously been prescribed antibiotics, they had proved ineffective. He was a long-term heavy smoker (smoking 40 cigarettes per day over the last 40 years); although his medical history included surgery for an inguinal hernia in 1987 and a diagnosis of benign prostatic hyperplasia in 2009, he was not receiving any chronic medications. The patient also complained of a slightly elevated temperature and asthenia. For this reason, his physician sent him to the emergency department of the University Hospital in Florence from which he was referred to the pneumology unit.
At the time of admission, the patient's laboratory findings showed a normal serum [Na+] of 141 mmol/L (Table 9). A few days following admission, the patient started complaining of chest pain for which he was prescribed an opiate (oxycodone 5 mg o.d.) together with paracetamol 325 mg t.i.d.; at this stage, he was also receiving methylprednisolone 16 mg o.d., lansoprazole 30 mg o.d. and tranexamic acid 500 mg b.i.d. for his hemoptysis.
Table 9.
Case 4: laboratory data at admission and in-patient assessments
| Investigation | Reading | Investigation | Reading |
|---|---|---|---|
| At admission | |||
| Serum [Na+] (mmol/L) | 141 | Glucose (mmol/L) | 5.6 |
| Serum [K+] (mmol/L) | 4.1 | Cholesterol (mmol/L) | 4.3 |
| Creatinine (µmol/L) | 80.4 | Triglycerides (mmol/L) | 1.2 |
| Urea (mg/dL) | 26 | Total proteins (g/L) | 0.06 |
| In-patient assessments | |||
| Plasma osmolality (mOsm/kg) | 267 | Serum [K+] (mmol/L) | 3.9 |
| Urinary osmolality (mOsm/kg) | 646 | Glucose (mmol/L) | 4.6 |
| Urinary [Na+] (mmol/L) | 130 | Urea (mg/dL) | 22 |
| TSH (miU/L) | 0.96 | Creatinine (µmol/L) | 64.5 |
| fT4 (pmol/L) | 18.9 | Uric acid (µmol/L) | 238 |
fT4, free thyroxine; TSH, thyroid-stimulating hormone.
Over the following 15 days, the patient experienced a progressive reduction in serum [Na+] down to 123 mmol/L. His plasma osmolality was low (267 mOsm/kg), urinary osmolality was 646 mOsm/kg and urinary [Na+] 130 mmol/L; other measurements such as uric acid and potassium concentration were in the normal range (Table 9). The patient was apparently asymptomatic, he was clinically euvolemic and his profile fitted that of a person with hyponatremia secondary to SIADH (Table 1)—the most common cause of euvolemic hyponatremia [36].
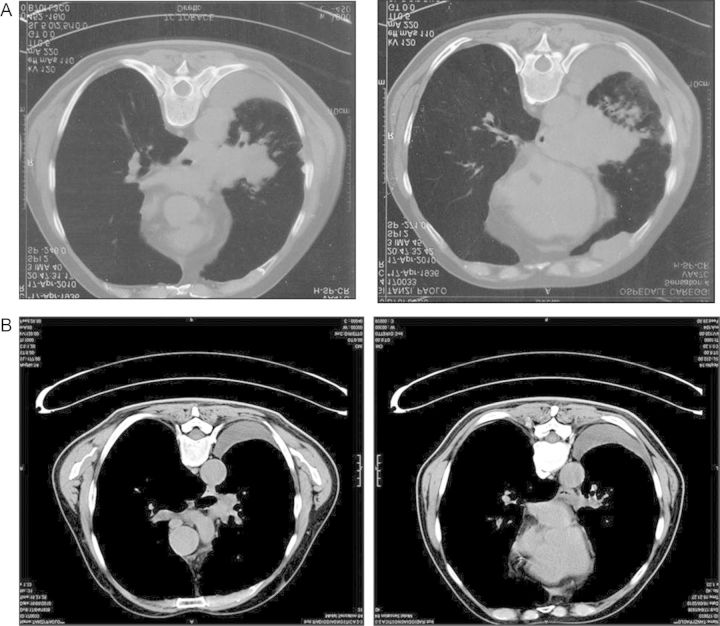
A CT scan revealed a large mediastinal mass, which turned out to be small-cell lung carcinoma (SCLC) (Figure 7A). Fluid restriction was initiated in an effort to resolve the hyponatremia; this resulted in an initial increase in serum [Na+], however, the concentration remained below the normal range of 135 mmol/L after 15 days. Because the patient required chemotherapy with etoposide and carboplatin—drugs that may also cause SIADH—it was important to fully resolve the patient's hyponatremia before chemotherapy could be initiated. Fluid restriction was therefore discontinued and the oral vasopressin antagonist tolvaptan 15 mg/day initiated, thus making this the first person to be treated with tolvaptan in Italy.
Figure 7:
Case 4: results of CT scans. CT scan taken at (A) initial presentation and (B) after 3 months’ therapy with etoposide and carboplatin.
Within 24 h, the patient's serum [Na+] had normalized allowing the prompt initiation of chemotherapy. However, over the next month the patient's serum [Na+] continued to steadily increase to 147 mmol/L at which point tolvaptan therapy was stopped (the only case in our clinic where overcorrection with tolvaptan has been observed, so far). Sodium concentrations then promptly returned to normal concentrations (Figure 8).
Figure 8:
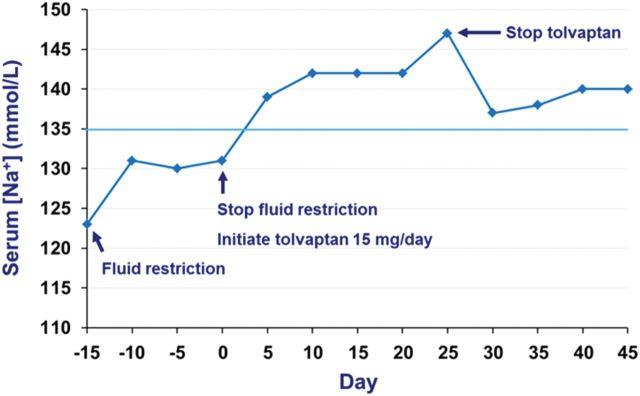
Case 4: serum [Na+] response to treatment.
After 3 months of chemotherapy, a CT scan showed a large reduction in a tumor mass (Figure 7B) and the patient's serum [Na+] remained stable. The patient was subsequently regularly monitored; serum [Na+] remained within the normal range and the patient reported a rather good quality of life at follow-up. Unfortunately, as expected, disease progression occurred after 8 months of clinical remission and the patient passed away ∼1 year after the initial diagnosis.
Discussion
Hyponatremia is a common feature in oncology practice [37], occurring most frequently in patients with SCLC [38]. A quarter of all patients with SCLC were identified as having sodium [Na+] of <136 mmol/L in a large analysis of nine consecutive clinical trials in Denmark and Sweden (n = 1684) [39]. Comparable rates (25–44%) were also observed in smaller SCLC cohort analyses using a similar cut-off [40–42].
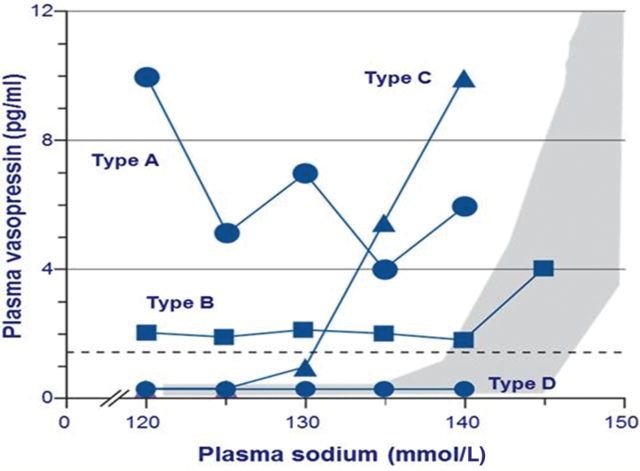
SIADH is the most common cause of hyponatremia, with higher rates found in patients with SCLC than with other malignancies [43, 44]. Four different patterns of vasopressin secretion have been identified in patients with SIADH (Figure 9):
Type A is characterized by an unregulated, erratic, secretion independent of the prevailing plasma osmolality.
Type B is characterized by an elevated basal secretion of vasopressin, despite normal regulation by osmolality.
Type C is characterized by a ‘reset osmostat’ system, whereby vasopressin is secreted at an abnormally low threshold of plasma osmolality but otherwise displays a normal response to relative changes in osmolality.
Type D is characterized by undetectable vasopressin levels (some of these patients may have gain-of-function mutations in the vasopressin V2 receptor) [15, 45, 46].
Figure 9:
Patterns of plasma vasopressin levels where compared with plasma sodium levels in patients with SIADH [15]. Type A is characterized by unregulated secretion of vasopressin, type B by elevated basal secretion of vasopressin despite normal regulation by osmolality, type C by a ‘reset osmostat’ and type D by undetectable vasopressin. The shaded area represents normal values of plasma vasopressin. Adapted from Ellison and Berl [15].
Type A vasopressin secretion tends to be usually found in paraneoplastic SIADH [47]. It has been proposed that the term ‘syndrome of inappropriate antidiuresis’ (SIAD) may be more suitable for this condition as not all patients with SIADH have elevated levels of vasopressin [15]. Many of these patients respond to the administration of vasopressin receptor antagonists with an aquaresis, suggesting that they have low levels of inappropriate vasopressin secretion despite the difficulty measuring such low levels. An exception is a patient group with gain-of-function mutations in the vasopressin V2 receptor—identified in infants with clinical and laboratory features consistent with the presence of SIADH but with undetectable vasopressin levels—who do not appear to respond to the administration of vasopressin receptor antagonists [45, 48]. Although this mutation appears to be a rare cause of hyponatremia at this time, the term SIAD would comprise these patients.
Although SCLC is a known cause of SIADH, it must also be borne in mind that the patient in this case would have had carcinoma at the time of admission, yet his serum [Na+] had been normal. However, opiates are another potential cause of SIADH and the patient's decline in serum [Na+] began with the administration of the opiate oxycodone for his chest pain. Consequently, it is possible that the patient initially had a low level of vasopressin, which then randomly increased and, together with the opiate, caused the decline in serum [Na+]. It is therefore important to continuously monitor sodium levels carefully in such patients as vasopressin levels can increase randomly.
Although this patient was treated with fluid restriction, it is nowadays likely that therapy with an oral vasopressin V2 receptor antagonist, i.e. tolvaptan, would be initiated earlier rather than after fluid restriction. The patient required urgent chemotherapy so his hyponatremia needed to be corrected as quickly as possible so that chemotherapy could be administered; however, fluid restriction usually takes several days before a significant increase in serum [Na+] is achieved, no doubt exacerbated by the fact that patient compliance with fluid restriction is often poor [38].
Tolvaptan acts by antagonizing the effects of endogenous vasopressin at the V2 receptors in the renal collecting duct. This causes an increase in urine water excretion, resulting in an increase in free water clearance (aquaresis), a decrease in urine osmolality and a resultant increase in serum [Na+]. Urine excretion of sodium and potassium is not significantly affected. The effects of tolvaptan on this patient are shown in Figure 10.
Figure 10:
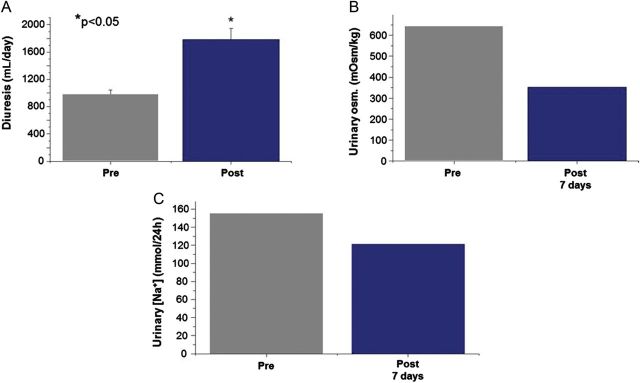
Case 4: response to tolvaptan therapy. Effect of tolvaptan before and after administration on (A) diuresis (average value between Day 1 and 7 following tolvaptan; statistical analysis conducted using Student's t-test), (B) urinary osmolality and (C) urinary [Na+].
Serum [Na+] normalized within 24 h following administration of tolvaptan, thereby allowing the prompt initiation of chemotherapy. In the only case of its kind observed in our clinic so far, the patient's sodium level continued to steadily increase above normal levels, reaching a peak of 147 mmol/L ∼25 days after initial drug administration (Figure 8). This necessitated the cessation of tolvaptan therapy upon which serum [Na+] returned to normal levels. This overcorrection was likely influenced by the patient continuing to limit his fluid intake following the initiation of tolvaptan despite feeling thirsty. Consequently, it is important to advise patients that they can, and should, drink fluid in response to thirst during tolvaptan therapy.
Case 5: 51-year-old man with subarachnoid hemorrhage
J. G. Verbalis
A 51-year-old man previously in good health except for hyperlipidemia was found unconscious in his bathroom. After awakening, he complained of a severe headache. A CT scan of the head showed a subarachnoid hemorrhage (SAH) with blood in the cisterns bilaterally. On transfer to a university hospital, he was awake and oriented with no focal neurological deficits but was noted to be drowsy and lethargic with intermittent nausea and vomiting. A physical exam was unremarkable except for a blood pressure of 149/76 mmHg, and the patient was felt to be clinically euvolemic. Admission laboratory values were all within normal limits, including electrolytes with a serum [Na+] of 139 mmol/L.
The patient was admitted to the neurosurgical ICU, where his blood pressure was controlled with labetalol and hydralazine drips. A cerebral angiogram revealed an anterior communicating artery aneurysm, which was successfully occluded by insertion of a coil by interventional neuroradiology. He was started on nimodipine for vasospasm prophylaxis and diphenylhydantoin for seizure prophylaxis. An external ventricular drain was placed to control intracranial pressure. Triple-H therapy (i.e. the induction of hypertension, hypervolemia and hemodilution to decrease the risk of vasospasm [49]) was begun for the SAH, including volume expansion via intravenous fluid administration with isotonic (0.9%) saline at rates varying between 125 and 175 mL/h. Total fluid intake ranged between 5 and 7 L/24 h over the first week of hospitalization.
The patient stabilized neurologically; but by Day 4, his serum [Na+] was noted to be intermittently low and the patient was started on NaCl tablets 8 g daily in divided doses (Figure 11). Despite this therapy, sodium levels continued to fall to the 125–127 mmol/L range and the patient became confused and lethargic. The oral NaCl was supplemented with an intravenous infusion of hypertonic (3%) NaCl, initially at 50 mL/h then increasing to 70 mL/h. By Day 8, the endocrinology service was consulted because of persistent hyponatremia and worsening neurological status despite aggressive NaCl administration, which by that time totalled as high as 62 g/day.
Figure 11:
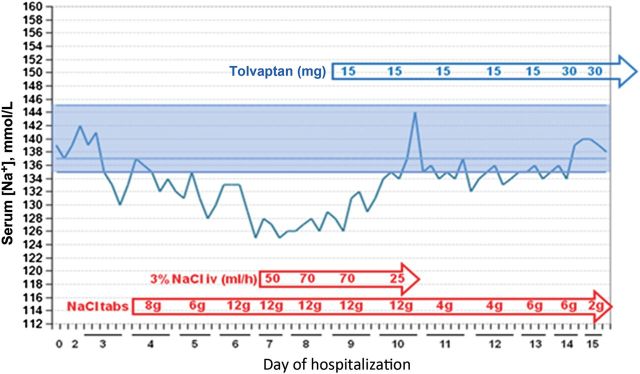
Case 5: treatment summary, serial serum [Na+] measurements, and patient progress.
On evaluation by the endocrinology department, the patient was clinically euvolemic. Serum [Na+] was in the range of 125–126 mmol/L with a plasma osmolality of 266 mOsm/kg and urine osmolalities ranging from 283 to 369 mOsm/kg. Urine [Na+] was noted to be 83 mmol/L with urine [K+] = 8.5 mmol/L prior to the start of oral NaCl tablets, and increased to 162 mmol/L with urine [K+] = 12.1 mmol/L following oral NaCl therapy. BUN was 2.1 mmol/L (6 mg/dL) and creatinine 53 µmol/L (0.6 mg/dL), with normal glucose levels. Thyroid function was normal: free T4 was 12.1 pmol/l (0.94 ng/dL) (normal range 0.58–1.64) and thyroid-stimulating hormone was 2.96 µIU/mL (normal range 0.35–5.5). A random measurement of cortisol levels showed an elevation at 792 nmol/L (28.7 µg/dL). Serum uric acid was low at 155 µmol/L (2.6 mg/dL) (normal range 208–387). Analysis of the intakes and outputs showed that the patient had been in a positive fluid balance each day over the first week of hospitalization.
Based on the patient's physical exam, laboratory values and clinical course, he was assessed to have euvolemic hyponatremia that was unresponsive to NaCl administration, which met the criteria for a diagnosis of SIADH (Table 1). Because the neurosurgeons would not consider instituting fluid restriction due to the risk of vasospasm from the SAH [49], therapy with tolvaptan was recommended at a starting dose of 15 mg/day. Although fluid restriction was relatively contraindicated in this case, it likely would not have been effective even if employed in view of the high urine to plasma electrolyte ratio that ranged from 0.73 to 1.39 (Table 5). After the first 15 mg dose, the patient developed a negative fluid balance of −2.5 L and his serum [Na+] increased from 126 to 132 mmol/L. Despite recommendations that the 3% NaCl infusion be stopped once an increase in serum [Na+] of 5 mmol/L had occurred, the 3% NaCl infusion was continued. After the second 15 mg tolvaptan dose, the patient developed a negative fluid balance of −4.2 L and his serum [Na+] increased further to a maximum of 144 mmol/L. The 3% NaCl was subsequently discontinued and serum [Na+] stabilized between 132 and 135 mmol/L. After 5 days, the tolvaptan dose was increased to 30 mg/day with normalization of serum [Na+] to 138–140 mmol/L; the patient was discharged on this dose on Day 16. His serum [Na+] remained normal at subsequent outpatient follow-up, and tolvaptan was discontinued 4 weeks after discharge without recurrence of the hyponatremia.
Discussion
Patients with acute neurological disorders, such as SAH, represent an especially challenging group of patients with hyponatremia. This is because the mild degrees of cerebral edema produced by hyponatremia that would not cause significant neurological symptoms in normal brains, can worsen both symptoms and recovery from already damaged brain tissue as a result of increased intracranial pressure [50]. Consequently, both avoidance of hyponatremia, and prompt and effective treatment of hyponatremia, when it occurs, is a crucial aspect of management in patients with acute neurological disorders.
The degree to which hyponatremia occurs primarily as a result of natriuresis has remained controversial for many years. Cerebral salt wasting (CSW) was first proposed by Peters in 1950 [51] as an explanation for the natriuresis and hyponatremia that sometimes accompanies intracranial disease, particularly SAH, in which up to one-third of patients often develop hyponatremia. Following the first clinical description of SIADH in 1957 [25], such patients were generally assumed to have hyponatremia secondary to vasopressin hypersecretion with a secondary natriuresis [52]. However, over the last decade, clinical and experimental data have suggested that some patients with SAH and other intracranial diseases may actually have a primary natriuresis leading to volume contraction rather than SIADH [53–56]; in which case, the elevated measured plasma vasopressin levels may actually be physiologically appropriate for the degree of volume contraction present. The major clinical question as to the frequency of CSW as a cause of hyponatremia is dependent on the criteria used to assess the extracellular fluid (ECF) volume status of these patients; opponents argue that there is insufficient evidence of true hypovolemia despite ongoing natriuresis [57], whereas proponents argue that the combined measures that have traditionally been used to estimate ECF volume do in fact support the presence of hypovolemia in many cases [58, 59].
With regard to the potential mechanisms underlying the natriuresis, in both plasma and cerebrospinal fluid (CSF), atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), levels are clearly elevated in many patients with SAH [56, 60–62] and have been found to correlate variably with hyponatremia in patients with intracranial diseases [56, 62, 63]. However, because SIADH is also frequently associated with elevated plasma ANP and BNP levels, this finding alone does not prove causality. Ample precedent certainly exists for hyponatremia due to sodium wasting with secondary antidiuresis in Addison’s disease, as well as diuretic-induced hyponatremia. But, characteristic of these disorders, normalization of ECF volume with isotonic NaCl infusions restores plasma tonicity to normal ranges by virtue of shutting off the secondary vasopressin secretion. If hyponatremia in patients with SAH occurred via a similar mechanism, it should also respond to this therapy. However, studies indicate that it does not. Nineteen patients with SAH were treated with large volumes of isotonic saline sufficient to maintain plasma volume at normal or slightly elevated levels, but despite removal of any volume stimulus to vasopressin secretion, 32% still developed hyponatremia in association with non-suppressed plasma vasopressin levels, an incidence equivalent to that found in previous studies of SAH [64]. In contrast, other studies have demonstrated that mineralocorticoid therapy to inhibit natriuresis can reduce the incidence of hyponatremia in patients with SAH [65]; such results are not unique to patients with intracranial diseases, since a subset of elderly patients with SIADH have also been shown to respond favorably to mineralocorticoid therapy [66]. Although seemingly disparate, these types of results support the existence of disordered vasopressin secretion as well as a coexisting stimulus to increased natriuresis in many such patients. It seems most likely that SAH and other intracranial diseases represent a mixed disorder in which some patients have both exaggerated natriuresis and inappropriate vasopressin secretion; which effect predominates in terms of the clinical presentation will depend on their relative intensities, as well as the effects of concomitant therapy.
This case graphically illustrates the important role played by SIADH in acute neurological disorders such as SAH. This patient was initially assumed to have CSW as a result of hyponatremia with an elevated urine sodium excretion. However, the patient never actually met criteria for a diagnosis of CSW, since volume depletion resulting from natriuresis was never documented. In addition, aggressive volume expansion as part of the triple-H therapy typically employed in patients with SAH failed to improve the hyponatremia; in fact, the serum [Na+] decreased in conjunction with the large volumes of fluid typically infused as a component of triple-H therapy. Subsequent therapy with the vasopressin receptor antagonist tolvaptan produced correction of the hyponatremia as a result of the induced aquaresis, thereby documenting vasopressin-induced water retention as the major etiological factor causing this patient's hyponatremia.
Treatment of hyponatremia in patients with acute neurological injury is challenging. Patients with SAH have been shown to have worse outcomes with fluid restriction, presumably as a result of vasospasm [54]. This has led to the widespread use of triple-H therapy to decrease the risk of vasospasm [49], though definitive evidence in support of this therapy is lacking [67]. Based on these findings, treatment of patients with true CSW using vaptans would be contraindicated, since such patients are by definition hypovolemic, which is a contraindication to the use of vaptans because of the risk of worsening volume depletion with resulting hemodynamic instability, hypotension and, in this group of patients, increased risk of vasospasm. However, the widespread employment of triple-H therapy effectively eliminates the possibility of CSW in most patients as a result of the volume expansion produced by NaCl administration, whether as oral, isotonic or hypertonic NaCl administration, or all three as in this case. When hyponatremia persists in the face of volume expansion, then it can only be due to vasopressin-induced water retention, which was proved by the favorable response to tolvaptan in this case. It should be remembered that the indication for therapy with a vasopressin receptor antagonist is euvolemic hyponatremia due to SIADH (EU and USA) or hypervolemic hyponatremia due to heart failure (USA only), not to the underlying cause of the SIADH. Consequently, even if this patient had a component of salt wasting due to CSW, his clinical picture was clearly one of euvolemic hyponatremia from SIADH, thereby making vaptan therapy an appropriate choice.
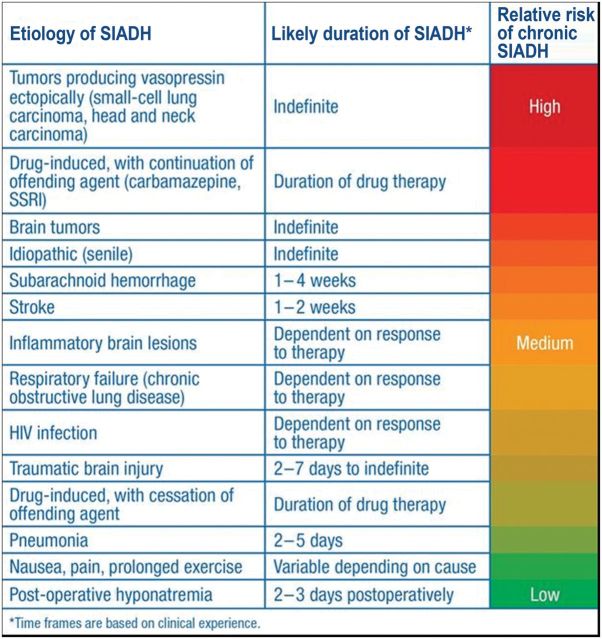
This case illustrates three additional important aspects of clinical use of vaptans. First, caution must be used when using vaptans in combination with other therapies for the treatment of hyponatremia. Extra caution should be employed if fluid restriction is used in combination with vaptan therapy to ensure that dehydration does not occur (patients should have access to drinking water), and concomitant use with hypertonic saline is not recommended in order to avoid the possibility of overly rapid correction of the hyponatremia with subsequent risk of precipitating the ODS [17]. This patient stayed within accepted guidelines for hyponatremia correction to reduce the risk of ODS [26], with a correction of 5 mmol/L in the first 24 h of therapy and 17 mmol/L in the first 48 h of therapy, but came close to exceeding these limits. If therapies are combined, in this case because of uncertainty of whether the tolvaptan would be effective at raising the serum [Na+], then the serum [Na+] must be monitored frequently in order to stop combined therapy once an acceptable response is seen. Secondly, effective therapy of hyponatremia is often a major determinant of patient discharge from ICUs and hospitals. In this case, continued use of ineffective therapies such as excessive NaCl administration would very likely have further prolonged this patient's hospital stay. Finally, many cases of hyponatremia are transient and resolve following treatment of the underlying disease process causing the hyponatremia. SAH is a classic example of this situation, in which the hyponatremia typically resolves 2–4 weeks after the clearance of blood products from the CSF. Outpatient monitoring of serum [Na+], including a planned trial of drug cessation, is required to determine whether continued therapy is necessary. Which patients should be discharged on vaptans to maintain normonatremia, and for how long, therefore depends critically upon the underlying etiology of the hyponatremia (Figure 12) [68].
Figure 12:
Estimated probability of need for long-term treatment of SIADH depending on underlying etiology [68]. Adapted from Verbalis [68].
Discussion and conclusions
J. G. Verbalis
As stated in the introduction to this article, among the many reasons why the diagnosis and treatment of hyponatremia remains far from optimal is the heterogeneous nature of the disorder, including a wide variety of possible etiologies, variable symptomology and differing fluid volume status. The five cases presented here abundantly illustrate the marked heterogeneity in the presentations of hyponatremia. As always is the case in medical practice, therapy in each case must be individualized to the patient. But despite their many differences, the cases presented here also illustrate that adherence to accepted guidelines for the diagnosis of SIADH and rational decision-making among the available therapeutic options for treating hyponatremia, importantly including the new class of vasopressin receptor antagonists, can achieve desirable outcomes in the majority of cases.
Case 1 illustrates a case of severe symptomatic hyponatremia with seizures who was appropriately treated with 3% NaCl followed by tolvaptan therapy to finish a controlled correction of the hyponatremia. The case emphasizes several important points about management of hyponatremia. First, the patient's symptoms did not deteriorate until her serum [Na+] decreased while on a fluid restriction of 1.2 L/day. As discussed by Dr Burst, employment of simple calculations such as the urine to plasma electrolyte ratio can identify patients in whom fluid restriction is likely to be ineffective, or even dangerous by virtue of negative electrolyte-free water clearance, as summarized in Table 5 [27]. Rather than assuming that all patients with SIADH should have an initial trial of fluid restriction, intelligent decision-making in 2013 should employ a careful analysis of which patients are likely to respond, and to what degree, before choosing an initial therapy. Although it is comforting that the patient responded to sequential therapy with hypertonic saline and tolvaptan with rapid discharge from the ICU and hospital, it is likely that initial therapy with tolvaptan rather than fluid restriction would have prevented the clinical deterioration that required ICU transfer and 3% NaCl rescue in the first place. This case raises the important issue of the appropriate choice of initial therapy of patients with SIADH; a careful assessment of the patient's likelihood of responding to individual therapies is clearly superior to choosing treatments simply because they are typically employed in such patients.
A second important point made by this case is that not all patients with malignancies have tumor-induced hyponatremia. There is no question that this patient met standard criteria for SIADH as shown in Table 1, but the list of potential etiologies of SIADH is long (Table 7), and one must consider many possibilities in patents with malignancies. The finding that this patient more likely had a pneumonia-induced SIADH had important implications for her long-term therapy, since it allowed discontinuation of the tolvaptan after successful therapy of the pneumonia; had this truly been tumor-associated SIADH, the patient would likely have required more chronic therapy for the hyponatremia (Figure 12).
Case 2 represents another common cause of SIADH, namely both acute and chronic neurological disorders. The case illustrates the risks of overly rapid correction using 3% NaCl. There is no question that this is the appropriate therapy for patients with severe symptomatic hyponatremia, as in this case. However, even in the best hands at advanced academic medical centers, overly rapid correction of hyponatremia remains a potential risk using hypertonic saline [69]. However, this patient did not develop symptoms of ODS despite overcorrection of his serum [Na+] by 13 mmol/L over 16 h. The most likely reason is that this was an acute hyponatremia. Studies in experimental animals have shown that the brain adapts to chronic hyponatremia by loss of solute, both as electrolytes and organic osmolytes. ODS is likely precipitated by brain dehydration that has been demonstrated to occur following correction of serum [Na+] toward normal ranges in animal models of chronic hyponatremia. Because the degree of osmotic brain shrinkage is greater in animals that are maintained chronically hyponatremic than in normonatremic animals undergoing similar increases in plasma osmolality [70–72]; by analogy, the brains of human patients adapted to hyponatremia are likely to be particularly susceptible to dehydration following subsequent increases in osmolality, which in turn can lead to pathological demyelination in some patients. MRI studies have shown that chronic hypo-osmolality predisposes rats to opening of the blood–brain barrier following rapid correction of hyponatremia [73], and that the disruption of the blood–brain barrier is highly correlated with subsequent demyelination [74]; a potential mechanism by which blood–brain barrier disruption might lead to subsequent myelinolysis via an influx of complement, which is toxic to the oligodendrocytes that manufacture and maintain the myelin sheaths of neurons into the brain [75]. Of the factors that clearly influence the susceptibility to demyelination following correction of hyponatremia, perhaps most important are the severity and duration of the pre-existing hyponatremia. Both of these risk factors likely relate to the degree of brain volume regulation that has occurred prior to the correction: the more severe the hyponatremia and the longer it has been maintained, the greater the degree of solute loss that will have occurred during the process of brain volume regulation. As larger amounts of solute are lost, the ability of the brain to buffer subsequent increases in plasma osmolality is impaired, resulting in greater degrees of brain dehydration as serum [Na+] is later raised, which in turn can lead to brain demyelination. Clinical implications of this pathophysiological mechanism are that ODS should not occur in cases of either mild or very acute hyponatremia. Both of these findings have been found to be true. ODS has only rarely been reported in patients with a starting serum [Na+] >120 mmol/L [32, 76, 77], and also does not appear to occur in most patients with psychogenic polydipsia who are well known to develop hyponatremia acutely from episodes of massive water ingestion followed by rapid correction as they diurese the excess fluid [78]. In cases in which an overcorrection has already occurred, consideration should be given to lowering serum [Na+] back to the maximally desired correction in that patient, again using water administration and/or desmopressin. Animal models have suggested that lowering serum [Na+] after overcorrection can prevent subsequent brain damage from occurring [79, 80], and this would be consistent with the occurrence of a delayed immunological demyelination as a result of complement influx into the brain following a sustained blood–brain barrier disruption [75]. A case report in which delayed lowering of serum [Na+] was associated with a reversal of symptoms suggestive of early myelinolysis also supports this as a potential therapy in similar cases [81]. However, there is no clinical evidence to support the recommendation of this for cases of acute hyponatremia, as in this case. Typical scenarios in which hyponatremia develops acutely are water intoxication from severe polydipsia in psychiatric patients, hyponatremia that occurs 24–48 h postoperatively, especially in neurosurgical patients, and exercise-associated hyponatremia following marathon and ultramarathon endurance events.
Case 3 is similar to case 1 in that it illustrates a case of pneumonia-associated SIADH. However, it occurred in a patient who represents the patient population at greatest risk of hyponatremia, namely the elderly. When hyponatremia is defined as a serum [Na+] <135 mmol/L, incidences as high as 15–30% have been observed in studies of both acutely and chronically hospitalized patients [1, 82]. However, incidences decrease to the range of 1–4% when only patients with serum [Na+] <130–131 mmol/L are included [13, 83, 84], which may represent a more appropriate level to define the occurrence of clinically significant cases of this disorder. Even when one uses these more stringent criteria to define hypo-osmolality, incidences from 7 to ∼53% have been reported in institutionalized geriatric patients [85, 86]. Many different factors are responsible for the high prevalence of hyponatremia in the elderly, including drugs, particularly thiazide diuretics and selective serotonin reuptake inhibitors antidepressants, low solute diets, underlying co-morbidities such as heart failure and lung disease, and SIADH of multiple etiologies. Unexplained or idiopathic causes of SIADH account for a relatively small proportion of all cases of SIADH, and the numbers of patients in whom an apparent cause cannot be established after consistent follow-up over time are relatively few. However, an exception to this appears to be elderly patients who sometimes develop SIADH without any apparent underlying etiology [87–89]. Coupled with the significantly increased incidence of hyponatremia in geriatric patients [1, 85, 86, 90–92], this suggests that the normal ageing process may be accompanied by abnormalities of regulation of vasopressin secretion that predispose to SIADH. Such an effect could potentially account for the fact that virtually all causes of drug-induced hyponatremia occur much more frequently in elderly patients [93–95]. In several series of elderly patients meeting criteria for SIADH, 40–60% remained idiopathic despite rigorous evaluation [96–98].
Also, similar to case 1, this case illustrates initial use of an ineffective therapy for SIADH, namely isotonic NaCl infusion. Infusion of isotonic saline was shown to be ineffective in the first patients described to have SIADH by Schwartz et al. [25]. These seminal studies documented that in response to saline infusion, the patient increased urine sodium excretion since there is no stimulus to retain sodium in a euvolemic patient; the net result was no change in serum [Na+], though in patients with low or negative electrolyte-free water excretion, serum [Na+] may actually decrease [99]. Given what has long been known about the futility of using isotonic saline in patients with SIADH, it remains surprising how often this therapy is employed as the initial therapy of such patients. Luckily, in this case, the patient was treated more efficaciously following an appropriate evaluation that documented a diagnosis of SIADH. In contrast to case 1, utilization of the urine to plasma electrolyte ratio (Table 5) prevented subsequent employment of fluid restriction as a second ineffective treatment, and therapy with tolvaptan allowed the patient to be discharged sooner than undoubtedly would have been the case had a fluid restriction been employed.
Case 4 illustrates a case of SIADH produced by small-cell carcinoma of the lung, the disease of the index cases of SIADH and still the most common tumor associated with inappropriately elevated vasopressin levels and hyponatremia. It is of interest that this patient was admitted with a normal sodium level and only became hyponatremic following admission to the hospital. Prof. Peri hypothesizes that this may have been due to the production of more severe SIADH with the institution of opiate therapy. This is certainly possible, and would fit the time course of the hyponatremia. However, it is not uncommon for patients with SIADH to present with a normal serum [Na+] as a result of limited fluid intake. This is especially true of the elderly, who are known to have a decreased thirst response [100]; in effect, they have a self-imposed fluid restriction, but when subjected to intravenous fluids as well as medications that further reduce free water excretion, they manifest the hyponatremia characteristic of SIADH. Evidence in support of a disordered thirst mechanism in this patient is the development of hypernatremia with tolvaptan therapy. Most patients would maintain a normal serum [Na+] even in the face of vaptan-induced aquaresis because they increase fluid intake commensurate with increased thirst as they become hyperosmolar.
This case, therefore, emphasizes the need to continue to follow serum [Na+] after instituting aquaretic therapies, regardless of the type, especially in elderly patients. It also illustrates the advisability of not using a fluid restriction in patients started on vaptan therapy because of the increased risk of increasing the serum [Na+] too rapidly and/or causing hyperosmolality, both of which are risk factors for the development of ODS. Finally, the case again illustrates the inability of therapies such as fluid restriction to normalize the serum [Na+] when renal electrolyte-free water excretion is low. Maintaining a serum [Na+] ∼130 mmol/L would be acceptable for most patients, but in this case, intervening therapy with chemotherapeutic agents accompanied by the increased fluids that are typically infused in conjunction with potentially nephrotoxic chemotherapeutic agents required a larger safety margin, which was far more effectively accomplished with tolvaptan therapy than continued fluid restriction.
Case 5 illustrates a case of hyponatremia accompanying SAH, one of the most common causes of hyponatremia with acute neurological injury. Although in some ways similar to case 2, this case highlights why CSW cannot account for the development of hyponatremia in most cases with neurological injury. Typical of many such cases, the patient was initially assumed to have CSW as a result of hyponatremia with an elevated urine sodium excretion, but never actually met criteria for a diagnosis of CSW since volume depletion resulting from natriuresis was never documented [101]. Aggressive volume expansion as part of the triple-H therapy employed in patients with SAH usually fails to prevent or improve the hyponatremia [64], and often is accompanied by decreases in serum [Na+] as a result of the large volumes of fluid infused as a component of triple-H therapy. When this occurs, therapy with vaptans is an appropriate consideration to correct the hyponatremia and prevent potentially detrimental increases in intracranial pressure. Concerns about vasospasm must also be considered, but this can be addressed by preventing volume depletion by infusing isotonic saline during the induced aquaresis while carefully monitoring the patient's volume status, either by hemodynamic parameters such as central venous pressure measurements, and/or by routine intake and output measurements. However, caution must always be exercised when combining therapies for hyponatremia, such as the combined use of tolvaptan and 3% NaCl in this case, to prevent overly rapid correction of hyponatremia with increased risk of ODS.
Although not specifically illustrated via a case study in this supplement, a careful drug history is essential in patients with SIADH as hundreds of different medications can potentially cause hyponatremia (Table 8) [35]. Discontinuation of treatment with these agents and avoidance of readministration is an effective management strategy in such cases (Figure 3). Despite the heterogeneous nature of these five cases, including age, disease process, acuity of hyponatremia and symptomatology, there are significant commonalities across all of the presented cases that deserve emphasis:
In all cases, the necessary clinical and laboratory evaluations were eventually, though not always initially, obtained to make a definitive diagnosis of SIADH (Table 1). Too often, treatment for hyponatremia is started without performing the analyses needed to confirm the correct diagnosis (Figure 3).
In most of the cases, initial therapies were chosen that were ineffective, or actually worsened the hyponatremia, including isotonic saline administration, NaCl tablets and fluid restriction. These errors could have been avoided, for the most part, by the evaluation of the patient's initial presentation.
More careful initial analysis of the clinical scenario, importantly including consideration of the urine and plasma laboratory values, could have led to employment of more effective therapies sooner, as was demonstrated in case 4.
Severe symptomatic hyponatremia was treated appropriately with 3% NaCl infusion, but this therapy always entails an increased risk of overly rapid correction of the hyponatremia. To guard against ODS, the serum [Na+] must be monitored at frequent intervals while the active correction is ongoing.
Treatment with vaptan therapy was successful in cases that were refractory to other therapies, usually leading to quicker hospital and ICU discharge.
Attention to the underlying etiology of the SIADH allowed cessation of vaptan therapy once the pathological process causing the SIADH had resolved (Figure 12).
Obviously, the above characteristics will not be applicable to all cases, nor will the treatment decisions made be the same. However, the cases in aggregate emphasize that paying attention to known principles of diagnosis and treatment can result in better outcomes for patients with hyponatremia from SIADH by choosing initial therapies according to a careful analysis of the patient's presenting clinical characteristics, rather than indiscriminate employment of typical therapies that are too often ineffective or deleterious.
Transparency declaration
M.L. has received honoraria from Otsuka Company for participation to clinical trials and teaching lectures. V.B. has received honoraria and consultancy fees from Otsuka. A.P. is on the Otsuka Pharmaceutical advisory board for tolvaptan and has received honoraria from Otsuka Pharmaceutical for speaking at symposia. J.G.V. serves as a paid consultant to Otsuka Pharmaceutical Co. Ltd, and has received a research grant from Otsuka America, Inc.
Acknowledgements
This supplement was commissioned by Otsuka Pharmaceutical Europe Ltd. The authors have not received any honorarium in relation to this supplement. The authors would like to thank apothecom for editorial assistance in the preparation of this supplement.
References
- 1.Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clinica Chimica Acta. 2003;337:169–172. doi: 10.1016/j.cccn.2003.08.001. doi:10.1016/j.cccn.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119:S30–S35. doi: 10.1016/j.amjmed.2006.05.005. doi:10.1016/j.amjmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21:70–76. doi: 10.1093/ndt/gfi082. doi:10.1093/ndt/gfi082. [DOI] [PubMed] [Google Scholar]
- 4.Huda MS, Boyd A, Skagen K, et al. Investigation and management of severe hyponatraemia in a hospital setting. Postgrad Med J. 2006;82:216–219. doi: 10.1136/pmj.2005.036947. doi:10.1136/pmj.2005.036947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenske W, Maier SK, Blechschmidt A, et al. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123:652–657. doi: 10.1016/j.amjmed.2010.01.013. doi:10.1016/j.amjmed.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Callahan MA, Do HT, Caplan DW, et al. Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study. Postgrad Med. 2009;121:186–191. doi: 10.3810/pgm.2009.03.1991. doi:10.3810/pgm.2009.03.1991. [DOI] [PubMed] [Google Scholar]
- 7.Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122:857–865. doi: 10.1016/j.amjmed.2009.01.027. doi:10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoorn EJ, Zietse R. Hyponatremia and mortality: how innocent is the bystander? Clin J Am Soc Nephrol. 2011;6:951–953. doi: 10.2215/CJN.01210211. doi:10.2215/CJN.01210211. [DOI] [PubMed] [Google Scholar]
- 9.Chawla A, Sterns RH, Nigwekar SU, et al. Mortality and serum sodium: do patients die from or with hyponatremia? Clin J Am Soc Nephrol. 2011;6:960–965. doi: 10.2215/CJN.10101110. doi:10.2215/CJN.10101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renneboog B, Musch W, Vandemergel X, et al. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:71.e1–71.e8. doi: 10.1016/j.amjmed.2005.09.026. doi:10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Verbalis JG, Barsony J, Sugimura Y, et al. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010;25:554–563. doi: 10.1359/jbmr.090827. doi:10.1359/jbmr.090827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinsella S, Moran S, Sullivan MO, et al. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol. 2010;5:275–280. doi: 10.2215/CJN.06120809. doi:10.2215/CJN.06120809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson RZ, Chung HM, Kluge R, et al. Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med. 1985;102:164–168. doi: 10.7326/0003-4819-102-2-164. doi:10.7326/0003-4819-102-2-164. [DOI] [PubMed] [Google Scholar]
- 14.Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42:790–806. doi: 10.1016/0002-9343(67)90096-4. doi:10.1016/0002-9343(67)90096-4. [DOI] [PubMed] [Google Scholar]
- 15.Ellison DH, Berl T. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356:2064–2072. doi: 10.1056/NEJMcp066837. doi:10.1056/NEJMcp066837. [DOI] [PubMed] [Google Scholar]
- 16.Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab. 2012;3:61–73. doi: 10.1177/2042018812437561. doi:10.1177/2042018812437561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verbalis JG, Adler S, Schrier RW, et al. Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur J Endocrinol. 2011;164:725–732. doi: 10.1530/EJE-10-1078. doi:10.1530/EJE-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoorn EJ, Bouloux PM, Burst V. Perspectives on the management of hyponatraemia secondary to SIADH across Europe. Best Pract Res Clin Endocrinol Metab. 2012;26(Suppl. 1):S27–S32. doi: 10.1016/S1521-690X(12)70005-2. doi:10.1016/S1521-690X(12)70005-2. [DOI] [PubMed] [Google Scholar]
- 19.Ohnishi A, Orita Y, Okahara R, et al. Potent aquaretic agent. A novel nonpeptide selective vasopressin 2 antagonist (OPC-31260) in men. J Clin Invest. 1993;92:2653–2659. doi: 10.1172/JCI116881. doi:10.1172/JCI116881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otsuka. 2012. Samsca (tolvaptan) Summary of Product Characteristics.
- 21.Otsuka. 2012. Samsca (tolvaptan) Prescribing Information.
- 22.Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. New Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. doi:10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 23.Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21:705–712. doi: 10.1681/ASN.2009080857. doi:10.1681/ASN.2009080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson C, Berl T, Tejedor A, et al. Differential diagnosis of hyponatraemia. Best Pract Res Clin Endocrinol Metab. 2012;26(Suppl. 1):S7–S15. doi: 10.1016/S1521-690X(12)70003-9. doi:10.1016/S1521-690X(12)70003-9. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz WB, Bennett W, Curelop S, et al. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am J Med. 1957;23:529–542. doi: 10.1016/0002-9343(57)90224-3. doi:10.1016/0002-9343(57)90224-3. [DOI] [PubMed] [Google Scholar]
- 26.Verbalis JG, Goldsmith SR, Greenberg A, et al. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120:S1–S21. doi: 10.1016/j.amjmed.2007.09.001. doi:10.1016/j.amjmed.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Furst H, Hallows KR, Post J, et al. The urine/plasma electrolyte ratio: a predictive guide to water restriction. Am J Med Sci. 2000;319:240–244. doi: 10.1097/00000441-200004000-00007. doi:10.1097/00000441-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Smith D, Moore K, Tormey W, et al. Downward resetting of the osmotic threshold for thirst in patients with SIADH. Am J Physiol Endocrinol Metab. 2004;287:E1019–E1023. doi: 10.1152/ajpendo.00033.2004. doi:10.1152/ajpendo.00033.2004. [DOI] [PubMed] [Google Scholar]
- 29.Pasantes-Morales H, Lezama RA, Ramos-Mandujano G, et al. Mechanisms of cell volume regulation in hypo-osmolality. Am J Med. 2006;119(7 Suppl. 1):S4–S11. doi: 10.1016/j.amjmed.2006.05.002. doi:10.1016/j.amjmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Arieff AI. Management of hyponatraemia. BMJ. 1993;307:305–308. doi: 10.1136/bmj.307.6899.305. doi:10.1136/bmj.307.6899.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–1589. doi: 10.1056/NEJM200005253422107. doi:10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 32.Sterns RH, Cappuccio JD, Silver SM, et al. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. J Am Soc Nephrol. 1994;4:1522–1530. doi: 10.1681/ASN.V481522. [DOI] [PubMed] [Google Scholar]
- 33.Thompson CJ, Crowley RK. Hyponatraemia. J R Coll Physicians Edinb. 2009;39:154–157. [Google Scholar]
- 34.Hoorn EJ, van der Lubbe N, Zietse R. SIADH And hyponatraemia: why does it matter? NDT Plus. 2009;2(Suppl 3):iii5–iii11. doi: 10.1093/ndtplus/sfp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis. 2008;52:144–153. doi: 10.1053/j.ajkd.2008.03.004. doi:10.1053/j.ajkd.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Baylis PH. The syndrome of inappropriate antidiuretic hormone secretion. Int J Biochem Cell Biol. 2003;35:1495–1499. doi: 10.1016/s1357-2725(03)00139-0. doi:10.1016/S1357-2725(03)00139-0. [DOI] [PubMed] [Google Scholar]
- 37.Palmer BF, Gates JR, Lader M. Causes and management of hyponatremia. Ann Pharmacother. 2003;37:1694–1702. doi: 10.1345/aph.1D105. doi:10.1345/aph.1D105. [DOI] [PubMed] [Google Scholar]
- 38.Castillo JJ, Vincent M, Justice E. Diagnosis and management of hyponatremia in cancer patients. Oncologist. 2012;17:756–765. doi: 10.1634/theoncologist.2011-0400. doi:10.1634/theoncologist.2011-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lassen U, Osterlind K, Hansen M, et al. Long-term survival in small-cell lung cancer: long-term survival in small-cell lung cancer: posttreatment characteristics in patients surviving 5 to 18+ years–an analysis of 1,714 consecutive patients. J Clin Oncol. 1995;13:1215–1220. doi: 10.1200/JCO.1995.13.5.1215. [DOI] [PubMed] [Google Scholar]
- 40.Hansen O, Sørensen P, Hansen KH. The occurrence of hyponatremia in SCLC and the influence on prognosis: A retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer. 2010;68:111–114. doi: 10.1016/j.lungcan.2009.05.015. doi:10.1016/j.lungcan.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Allan SG, Stewart ME, Love S, et al. Prognosis at presentation of small cell carcinoma of the lung. Eur J Cancer. 1990;26:703–705. doi: 10.1016/0277-5379(90)90121-9. doi:10.1016/0277-5379(90)90121-9. [DOI] [PubMed] [Google Scholar]
- 42.Osterlind K, Andersen PK. Prognostic factors in small cell lung cancer: multivariate model based on 778 patients treated with chemotherapy with or without irradiation. Cancer Res. 1986;46:4189–4194. [PubMed] [Google Scholar]
- 43.Onitilo AA, Kio E, Doi SA. Tumor-related hyponatremia. Clin Med Res. 2007;5:228–237. doi: 10.3121/cmr.2007.762. doi:10.3121/cmr.2007.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raftopoulos H. Diagnosis and management of hyponatremia in cancer patients. Support Care Cancer. 2007;15:1341–1347. doi: 10.1007/s00520-007-0309-9. doi:10.1007/s00520-007-0309-9. [DOI] [PubMed] [Google Scholar]
- 45.Feldman BJ, Rosenthal SM, Vargas GA, et al. Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med. 2005;352:1884–1890. doi: 10.1056/NEJMoa042743. doi:10.1056/NEJMoa042743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robertson GL. Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis. Am J Med. 2006;119(Suppl. 1):S36–S42. doi: 10.1016/j.amjmed.2006.05.006. doi:10.1016/j.amjmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Verbalis JG. Chapter 86: The syndrome in inappropriate antidiuretic hormone secretion and other hypoosmolar disorders. In: Schrier RW, editor. Diseases of the Kidney and Urinary Tract. 8th edn. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2214–2248. [Google Scholar]
- 48.Decaux G, Vandergheynst F, Bouko Y, et al. Nephrogenic syndrome of inappropriate antidiuresis in adults: high phenotypic variability in men and women from a large pedigree. J Am Soc Nephrol. 2007;18:606–612. doi: 10.1681/ASN.2006090987. doi:10.1681/ASN.2006090987. [DOI] [PubMed] [Google Scholar]
- 49.Kim DK, Joo KW. Hyponatremia in patients with neurologic disorders. Electrolyte Blood Press. 2009;7:51–57. doi: 10.5049/EBP.2009.7.2.51. doi:10.5049/EBP.2009.7.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters JP, Welt KG, Sims EAH, et al. A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Physicians. 1950;63:57–64. [PubMed] [Google Scholar]
- 51.Doczi T, Tarjanyi J, Huszka E, et al. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) after head injury. Neurosurgery. 1982;10:685–688. doi: 10.1227/00006123-198206010-00001. doi:10.1227/00006123-198206010-00001. [DOI] [PubMed] [Google Scholar]
- 52.Nelson PB, Seif S, Gutai J, et al. Hyponatremia and natriuresis following subarachnoid hemorrhage in a monkey model. J Neurosurg. 1984;60:233–237. doi: 10.3171/jns.1984.60.2.0233. doi:10.3171/jns.1984.60.2.0233. [DOI] [PubMed] [Google Scholar]
- 53.Wijdicks EF, Vermeulen M, Hijdra A, et al. Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysms: is fluid restriction harmful? Ann Neurol. 1985;17:137–140. doi: 10.1002/ana.410170206. doi:10.1002/ana.410170206. [DOI] [PubMed] [Google Scholar]
- 54.Wijdicks EF, Ropper AH, Hunnicutt EJ, et al. Atrial natriuretic factor and salt wasting after aneurysmal subarachnoid hemorrhage. Stroke. 1991;22:1519–1524. doi: 10.1161/01.str.22.12.1519. doi:10.1161/01.STR.22.12.1519. [DOI] [PubMed] [Google Scholar]
- 55.Diringer MN, Lim JS, Kirsch JR, et al. Suprasellar and intraventricular blood predict elevated plasma atrial natriuretic factor in subarachnoid hemorrhage. Stroke. 1991;22:577–581. doi: 10.1161/01.str.22.5.577. doi:10.1161/01.STR.22.5.577. [DOI] [PubMed] [Google Scholar]
- 56.Oh MS, Carroll HJ. Cerebral salt-wasting syndrome. We need better proof of its existence. Nephron. 1999;82:110–114. doi: 10.1159/000045385. doi:10.1159/000045385. [DOI] [PubMed] [Google Scholar]
- 57.Maesaka JK, Gupta S, Fishbane S. Cerebral salt-wasting syndrome: does it exist? Nephron. 1999;82:100–109. doi: 10.1159/000045384. doi:10.1159/000045384. [DOI] [PubMed] [Google Scholar]
- 58.Palmer BF. Hyponatremia in patients with central nervous system disease: SIADH versus CSW. Trends Endocrinol Metab. 2003;14:182–187. doi: 10.1016/s1043-2760(03)00048-1. doi:10.1016/S1043-2760(03)00048-1. [DOI] [PubMed] [Google Scholar]
- 59.Sviri GE, Shik V, Raz B, et al. Role of brain natriuretic peptide in cerebral vasospasm. Acta Neurochir (Wien) 2003;145:851–860. doi: 10.1007/s00701-003-0101-7. doi:10.1007/s00701-003-0101-7. [DOI] [PubMed] [Google Scholar]
- 60.Espiner EA, Leikis R, Ferch RD, et al. The neuro-cardio-endocrine response to acute subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2002;56:629–635. doi: 10.1046/j.1365-2265.2002.01285.x. doi:10.1046/j.1365-2265.2002.01285.x. [DOI] [PubMed] [Google Scholar]
- 61.McGirt MJ, Blessing R, Nimjee SM, et al. Correlation of serum brain natriuretic peptide with hyponatremia and delayed ischemic neurological deficits after subarachnoid hemorrhage. Neurosurgery. 2004;54:1369–1373. doi: 10.1227/01.neu.0000125016.37332.50. doi:10.1227/01.NEU.0000125016.37332.50. [DOI] [PubMed] [Google Scholar]
- 62.Weinand ME, O'Boynick PL, Goetz KL. A study of serum antidiuretic hormone and atrial natriuretic peptide levels in a series of patients with intracranial disease and hyponatremia. Neurosurgery. 1989;25:781–785. doi: 10.1097/00006123-198911000-00014. doi:10.1227/00006123-198911000-00014. [DOI] [PubMed] [Google Scholar]
- 63.Diringer MN, Wu KC, Verbalis JG, et al. Hypervolemic therapy prevents volume contraction but not hyponatremia following subarachnoid hemorrhage. Ann Neurol. 1992;31:543–550. doi: 10.1002/ana.410310513. doi:10.1002/ana.410310513. [DOI] [PubMed] [Google Scholar]
- 64.Mori T, Katayama Y, Kawamata T, et al. Improved efficiency of hypervolemic therapy with inhibition of natriuresis by fludrocortisone in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 1999;91:947–952. doi: 10.3171/jns.1999.91.6.0947. doi:10.3171/jns.1999.91.6.0947. [DOI] [PubMed] [Google Scholar]
- 65.Ishikawa S, Fujita N, Fujisawa G, et al. Involvement of arginine vasopressin and renal sodium handling in pathogenesis of hyponatremia in elderly patients. Endocr J. 1996;43:101–108. doi: 10.1507/endocrj.43.101. doi:10.1507/endocrj.43.101. [DOI] [PubMed] [Google Scholar]
- 66.Meyer R, Deem S, Yanez ND, et al. Current practices of triple-H prophylaxis and therapy in patients with subarachnoid hemorrhage. Neurocrit Care. 2011;14:24–36. doi: 10.1007/s12028-010-9437-z. doi:10.1007/s12028-010-9437-z. [DOI] [PubMed] [Google Scholar]
- 67.Velat GJ, Kimball MM, Mocco JD, et al. Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analyses in the literature. World Neurosurg. 2011;76:446–454. doi: 10.1016/j.wneu.2011.02.030. doi:10.1016/j.wneu.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 68.Verbalis JG. Managing hyponatremia in patients with syndrome of inappropriate antidiuretic hormone secretion. Endocrinol Nutr. 2010;57(Suppl. 2):30–40. doi: 10.1016/S1575-0922(10)70020-6. doi:10.1016/S1575-0922(10)70020-6. [DOI] [PubMed] [Google Scholar]
- 69.Mohmand HK, Issa D, Ahmad Z, et al. Hypertonic saline for hyponatremia: risk of inadvertent overcorrection. Clin J Am Soc Nephrol. 2007;2:1110–1117. doi: 10.2215/CJN.00910207. doi:10.2215/CJN.00910207. [DOI] [PubMed] [Google Scholar]
- 70.Verbalis JG, Baldwin EF, Robinson AG. Osmotic regulation of plasma vasopressin and oxytocin after sustained hyponatremia. Am J Physiol. 1986;250:R444–R451. doi: 10.1152/ajpregu.1986.250.3.R444. [DOI] [PubMed] [Google Scholar]
- 71.Sterns RH, Thomas DJ, Herndon RM. Brain dehydration and neurologic deterioration after rapid correction of hyponatremia. Kidney Int. 1989;35:69–75. doi: 10.1038/ki.1989.9. doi:10.1038/ki.1989.9. [DOI] [PubMed] [Google Scholar]
- 72.Cserr H, DePasquale M, Patlak CS. Regulation of brain water and electrolytes during acute hyperosmolality. Am J Physiol. 1987;253:F522–F529. doi: 10.1152/ajprenal.1987.253.3.F522. [DOI] [PubMed] [Google Scholar]
- 73.Adler S, Verbalis JG, Williams D. Effect of rapid correction of hyponatremia on the blood brain barrier of rats. Brain Res. 1995;679:135–143. doi: 10.1016/0006-8993(95)00245-l. doi:10.1016/0006-8993(95)00245-L. [DOI] [PubMed] [Google Scholar]
- 74.Adler S, Martinez J, Williams DS, et al. Positive association between blood brain barrier disruption and osmotically-induced demyelination. Mult Scler. 2000;6:24–31. doi: 10.1177/135245850000600106. [DOI] [PubMed] [Google Scholar]
- 75.Baker EA, Tian Y, Adler S, et al. Blood-brain barrier disruption and complement activation in the brain following rapid correction of chronic hyponatremia. Exp Neurol. 2000;165:221–230. doi: 10.1006/exnr.2000.7474. doi:10.1006/exnr.2000.7474. [DOI] [PubMed] [Google Scholar]
- 76.Sterns RH, Riggs JE, Schochet SS., Jr Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med. 1986;314:1535–1542. doi: 10.1056/NEJM198606123142402. doi:10.1056/NEJM198606123142402. [DOI] [PubMed] [Google Scholar]
- 77.Lohr JW. Osmotic demyelination syndrome following correction of hyponatremia: association with hypokalemia. Am J Med. 1994;96:408–413. doi: 10.1016/0002-9343(94)90166-x. doi:10.1016/0002-9343(94)90166-X. [DOI] [PubMed] [Google Scholar]
- 78.Cheng JC, Zikos D, Skopicki HA, et al. Long-term neurologic outcome in psychogenic water drinkers with severe symptomatic hyponatremia: the effect of rapid correction. Am J Med. 1990;88:561–566. doi: 10.1016/0002-9343(90)90518-i. doi:10.1016/0002-9343(90)90518-I. [DOI] [PubMed] [Google Scholar]
- 79.Soupart A, Penninckx R, Crenier L, et al. Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int. 1994;45:193–200. doi: 10.1038/ki.1994.23. doi:10.1038/ki.1994.23. [DOI] [PubMed] [Google Scholar]
- 80.Soupart A, Penninckx R, Stenuit A, et al. Reinduction of hyponatremia improves survival in rats with myelinolysis-related neurologic symptoms. J Neuropathol Exp Neurol. 1996;55:594–601. doi: 10.1097/00005072-199605000-00011. doi:10.1097/00005072-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 81.Soupart A, Ngassa M, Decaux G. Therapeutic relowering of the serum sodium in a patient after excessive correction of hyponatremia. Clin Nephrol. 1999;51:383–386. [PubMed] [Google Scholar]
- 82.Upadhyay A, Jaber BL, Madias NE. Epidemiology of hyponatremia. Semin Nephrol. 2009;29:227–238. doi: 10.1016/j.semnephrol.2009.03.004. doi:10.1016/j.semnephrol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 83.Flear CT, Gill GV, Burn J. Hyponatraemia: mechanisms and management. Lancet. 1981;2:26–31. doi: 10.1016/s0140-6736(81)90261-0. doi:10.1016/S0140-6736(81)90261-0. [DOI] [PubMed] [Google Scholar]
- 84.Brunsvig PF, Os I, Frederichsen P. Hyponatremia. A retrospective study of occurrence, etiology and mortality. Tidsskrift for Den Norske Laegeforening. 1990;110:2367–2369. [PubMed] [Google Scholar]
- 85.Sorensen IJ, Matzen LE. Serum electrolytes and drug therapy of patients admitted to a geriatric department. Ugeskrift for Laeger. 1993;155:3921–3924. [PubMed] [Google Scholar]
- 86.Miller M, Morley JE, Rubenstein LZ. Hyponatremia in a nursing home population. J Am Geriatr Soc. 1995;43:1410–1413. doi: 10.1111/j.1532-5415.1995.tb06623.x. [DOI] [PubMed] [Google Scholar]
- 87.Goldstein CS, Braunstein S, Goldfarb S. Idiopathic syndrome of inappropriate antidiuretic hormone secretion possibly related to advanced age. Ann Intern Med. 1983;99:185–188. doi: 10.7326/0003-4819-99-2-185. doi:10.7326/0003-4819-99-2-185. [DOI] [PubMed] [Google Scholar]
- 88.Hamilton DV. Inappropriate secretion of antidiuretic hormone associated with cerebellar and cerebral atrophy. Postgrad Med J. 1978;54:427–428. doi: 10.1136/pgmj.54.632.427. doi:10.1136/pgmj.54.632.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller M. Hyponatremia: age-related risk factors and therapy decisions. Geriatrics. 1998;53 32–3, 37–8, 41–2 passim. [PubMed] [Google Scholar]
- 90.Kleinfeld M, Casimir M, Borra S. Hyponatremia as observed in a chronic disease facility. J Am Geriatr Soc. 1979;27:156–161. doi: 10.1111/j.1532-5415.1979.tb06439.x. [DOI] [PubMed] [Google Scholar]
- 91.Misra SC, Mansharamani GG. Hyponatremia in elderly hospital in-patients. Brit J Clin Prac. 1989;43:295–296. [PubMed] [Google Scholar]
- 92.Miller M, Hecker MS, Friedlander DA, et al. Apparent idiopathic hyponatremia in an ambulatory geriatric population. J Am Geriatr Soc. 1996;44:404–408. doi: 10.1111/j.1532-5415.1996.tb06410.x. [DOI] [PubMed] [Google Scholar]
- 93.Pillans PI, Coulter DM. Fluoxetine and hyponatraemia—a potential hazard in the elderly. N Zealand Med J. 1994;107:85–86. [PubMed] [Google Scholar]
- 94.Rault RM. Case report: hyponatremia associated with nonsteroidal antiinflammatory drugs. Am J Med Sci. 1993;305:318–320. doi: 10.1097/00000441-199305000-00011. doi:10.1097/00000441-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 95.Sharabi Y, Illan R, Kamari Y, et al. Diuretic induced hyponatraemia in elderly hypertensive women. J Hum Hypertens. 2002;16:631–635. doi: 10.1038/sj.jhh.1001458. doi:10.1038/sj.jhh.1001458. [DOI] [PubMed] [Google Scholar]
- 96.Hirshberg B, Ben-Yehuda A. The syndrome of inappropriate antidiuretic hormone secretion in the elderly. Am J Med. 1997;103:270–273. doi: 10.1016/s0002-9343(97)00250-7. doi:10.1016/S0002-9343(97)00250-7. [DOI] [PubMed] [Google Scholar]
- 97.Arinzon Z, Feldman J, Jarchowsky J, et al. A comparative study of the syndrome of inappropriate antidiuretic hormone secretion in community-dwelling patients and nursing home residents. Aging Clin Exp Res. 2003;15:6–11. doi: 10.1007/BF03324473. doi:10.1007/BF03324473. [DOI] [PubMed] [Google Scholar]
- 98.Anpalahan M. Chronic idiopathic hyponatremia in older people due to syndrome of inappropriate antidiuretic hormone secretion (SIADH) possibly related to aging. J Am Geriatr Soc. 2001;49:788–792. doi: 10.1046/j.1532-5415.2001.49157.x. doi:10.1046/j.1532-5415.2001.49157.x. [DOI] [PubMed] [Google Scholar]
- 99.Steele A, Gowrishankar M, Abrahamson S, et al. Postoperative hyponatremia despite near-isotonic saline infusion: a phenomenon of desalination. Ann Intern Med. 1997;126:20–25. doi: 10.7326/0003-4819-126-1-199701010-00003. doi:10.7326/0003-4819-126-1-199701010-00003. [DOI] [PubMed] [Google Scholar]
- 100.Phillips PA, Rolls BJ, Ledingham JG, et al. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med. 1984;311:753–759. doi: 10.1056/NEJM198409203111202. doi:10.1056/NEJM198409203111202. [DOI] [PubMed] [Google Scholar]
- 101.Sterns RH, Silver SM. Cerebral salt wasting versus SIADH: what difference? J Am Soc Nephrol. 2008;19:194–196. doi: 10.1681/ASN.2007101118. doi:10.1681/ASN.2007101118. [DOI] [PubMed] [Google Scholar]