Abstract
Introduction:
Use of e-cigarettes has been increasing exponentially, with the primary motivation reported as smoking cessation. To understand why smokers choose e-cigarettes as an alternative to cigarettes, as well as to US Food and Drug Administration (FDA)–approved nicotine replacement therapies (NRT), we compared outcome expectancies (beliefs about the results of drug use) for the three nicotine delivery systems among vapers, i.e., e-cigarette users, who were former smokers.
Methods:
Vapers (N = 1,434) completed an online survey assessing 14 expectancy domains as well as perceived cost and convenience. We focused on comparisons between e-cigarettes and cigarettes to determine the attraction of e-cigarettes as a smoking alternative and between e-cigarettes and NRT to determine perceived advantages of e-cigarettes over FDA-approved pharmacotherapy.
Results:
Participants believed that e-cigarettes, in comparison to conventional cigarettes, had fewer health risks; caused less craving, withdrawal, addiction, and negative physical feelings; tasted better; and were more satisfying. In contrast, conventional cigarettes were perceived as better than e-cigarettes for reducing negative affect, controlling weight, providing stimulation, and reducing stress. E-cigarettes, compared to NRT, were perceived to be less risky, cost less, cause fewer negative physical feelings, taste better, provide more satisfaction, and be better at reducing craving, negative affect, and stress. Moderator analyses indicated history with ad libitum forms of NRT was associated with less positive NRT expectancies.
Conclusions:
The degree to which expectancies for e-cigarettes differed from expectancies for either tobacco cigarettes or NRT offers insight into the motivation of e-cigarette users and provides guidance for public health and clinical interventions to encourage smoking-related behavior change.
Introduction
Cigarette smoking remains the leading preventable cause of death in the United States, despite the fact that most smokers report wanting to quit smoking.1 Although there have been notable advancements in pharmacotherapies, cessation rates continue to be very low. Nicotine replacement therapies (NRT) approved by the FDA (patch, gum, lozenge, inhaler, nasal spray) have demonstrated quit rates between 18% and 31% in clinical trials.2 However, in the general population, most smokers do not use NRT,3,4 and among those who do, only about 5%–16% quit smoking.5,6
Perhaps partially in response to dissatisfaction with NRT, other forms of nicotine delivery are on the rise. Most prominently, rapid growth has occurred in the marketing, sale, and use of electronic nicotine delivery systems (“e-cigarettes”). Most e-cigarette users report smoking cessation as the primary reason for use.7–10 Given the well-known harms associated with cigarette smoking, switching to e-cigarettes is likely a form of harm reduction.11–14 However, although there is evidence that e-cigarettes may help some people reduce or even quit smoking, available prospective studies suggest that e-cigarettes are similar to NRT in that most users do not quit smoking.15–21 Despite these concerns, a recent cross-sectional, retrospective study found that e-cigarette users in the general population were more likely than NRT users to have quit cigarette smoking.22
In comparison to cigarettes, available evidence suggests that e-cigarettes are a safer nicotine delivery system. For example, levels of toxicants in e-cigarette vapor have been reported to be 9–450 times lower than in cigarette smoke12 and particle emissions have been reported to be 5–10 times lower.13 However, the relative health risks of e-cigarettes compared to NRT are less clear. E-cigarettes often contain more toxicants than approved pharmacotherapy, such as the nicotine inhaler.12 This potential for greater health risk is increased as e-cigarettes are not currently regulated and vary in content, with actual content sometimes differing from labeled content.23–25 Forthcoming regulations from the US Food and Drug Administration (FDA) are likely to reduce these concerns, but it should also be acknowledged that nicotine itself may be harmful. The 2014 Surgeon General’s Report notes the need for quantifying level of risk from long-term use of nicotine, especially if such use becomes more prevalent,1 as appears to occur commonly with e-cigarettes,26 despite being relatively rare with NRT.27
Drug outcome expectancies, that is beliefs about the results of drug use, offer a key tool in predicting substance use initiation and continued use. Drug expectancies can be understood as informational structures in long-term memory.28–30 These fundamental elements of memory are theorized to both organize input to the central nervous system and guide management of behavior, acting as a “final common pathway” that is implicated in connections between a variety of prior conditions (for example, genetic predisposition, social influence, emotional state, personality) and drug use decisions31,32.
Expectancies have been found to be robust predictors of drug use, including the initiation of cigarette smoking,33,34 dependence35, and relapse after a period of abstinence.36,37 Expectancies likely play roles in driving both the increased use of e-cigarettes and the lack of NRT usage. Prior research shows that expectancies for nicotine and NRT are generally less positive than for cigarette smoking38,39; but see40). However, no study has examined and contrasted cigarette, e-cigarette, and NRT expectancies among e-cigarette users (“vapers”). Understanding these expectancies, thought to be key drivers of behavioral choices, should help elucidate why smokers switch from cigarettes to e-cigarettes, and why they choose e-cigarettes over NRT. This information, in turn, should be useful in developing interventions or messages designed to encourage smoking-related behavior change. We expected to find that, among this sample of ex-smoking vapers, e-cigarettes would generally be rated more positively than both NRT and cigarettes on domains previously found to be important for predicting smoking.
Methods
Participants
Individuals were eligible to participate in the online survey if they were at least 18 years of age, reported a history of daily smoking, had smoked cigarettes for at least 1 year, and had used e-cigarettes in the past 30 days. Participants were not compensated.
There were 2,271 survey responses. We deleted 91 cases that were repeats from the same IP address, 130 entries that were blank, 50 where respondents indicated they had not smoked 100 or more cigarettes in their lifetime, and 187 that did not complete all the expectancy questions. Of the remaining 1,813, the majority reported no smoking in the past month (n = 1,434, 79.0%) and they constitute the sample for the current analysis. Table 1 summarizes sample demographics as well as tobacco, e-cigarette, NRT, and other medication use.
Table 1.
Sample Characteristics (N = 1,434).
| M (SD) or n (%) | |
|---|---|
| Source of survey referral | |
| Internet (e-cigarette forums, Reddit, Facebook) | 969 (67.6%) |
| News source (local news, TV, newspaper) | 172 (12.0%) |
| Other | 293 (20.4%) |
| Age (years) | 41.1 (18.1) |
| Female gender | 488 (34.0%) |
| Caucasian/White race | 1326 (92.5%) |
| Education | |
| High school diploma/G.E.D. | 202 (14.1%) |
| Some college or technical school | 776 (54.1%) |
| Four-year college degree | 412 (28.7%) |
| Total household income | |
| Under $40,000 | 391 (27.2%) |
| $40,000–$89,999 | 584 (40.7%) |
| Over $90,000 | 291 (28.7%) |
| Time since last cigarette | |
| 1–6 months ago | 622 (43.4%) |
| 6–12 months ago | 366 (25.5%) |
| More than 1 year ago | 446 (31.1%) |
| Cigarettes per day before quitting | |
| 1–9 | 107 (7.5%) |
| 10–20 | 535 (37.3%) |
| More than 20 | 788 (55.0%) |
| Time since started using e-cigarettes | |
| Less than 1 month ago | 7 (0.5%) |
| 1–6 months ago | 567 (39.5%) |
| 6–12 months ago | 360 (25.1%) |
| 12–24 months ago | 260 (18.1%) |
| More than 2 years ago | 240 (16.7%) |
| Type of e-cigarettea | |
| First generation (cigarette-like) | 112 (7.8%) |
| Second generation (refillable) | 1050 (73.2%) |
| Other | 272 (19.0%) |
| Frequency of use of e-cigarettesb | |
| 1–9 times a day | 409 (28.5%) |
| 10–20 times a day | 589 (41.1%) |
| More than 20 times a day | 436 (30.4%) |
| Ever use of NRT | |
| Patch | 908 (63.3%) |
| Gum | 950 (66.2%) |
| Lozenge | 542 (37.8%) |
| Inhaler | 198 (13.8%) |
| Nasal spray | 70 (4.9%) |
| None—never used NRT | 366 (25.5%) |
| Past 30 days use of NRT | |
| None reported | 1399 (97.6%) |
| Other medication experience | |
| Varenicline | 361 (25.2%) |
| Bupropion | 380 (26.5%) |
aThese are three exclusive categories describing e-cigarette usually used. The most common brands reported in the first generation category were V2Cigs (n = 21), Blu (n = 20), White Cloud (n = 18), Smokeless Image (n = 18), Vapor for Life (n = 16), Halo (n = 10), and NJOY (n = 9). The most common brands/categories reported in the second generation category were eGo (n = 700), Innokin (n = 71), ProVape (n = 71), “Mods” (n = 70), Vamo (n = 55), and Kanger (n = 21). Other included a variety of items including no response and brands endorsed by fewer than 10 respondents.
bOne time of e-cigarette use = 15 puffs, or around 10min.
Procedure
The online survey was publicized via local press releases capitalizing on media interest in the emerging phenomenon. These press releases resulted in television, print newspaper, and online newspaper features, which included links to the survey. We also discovered that links to the survey were reposted by others on various internet sites, including social media and e-cigarette forums. Table 1 provides information on survey referral source. Data were collected from August through November, 2013.
Measures
The online survey, developed by the authors, included the following sections.
Demographics
Information was collected on age, gender, race, ethnicity, education, income, and marital status.
Smoking History
Participants answered questions about their past use of cigarettes.
E-Cigarette History
Next, participants answered questions about their history with e-cigarettes. Participants were asked what brands of e-cigarettes they usually used and were given 15 popular options and “other” with the opportunity to write in the brand. Table 1 describes some common responses.
Pharmacotherapy History
Participants were asked if they had ever used any NRT in their lifetime and in the past 30 days. Options included all five FDA-approved NRT modalities (patch, gum, lozenge, inhaler, and nasal spray). Participants additionally indicated whether they had ever used varenicline or bupropion. Table 1 provides a summary of participant characteristics.
Expectancies
To compare the expectancies of e-cigarettes with expectancies for cigarettes and NRT, we included comparable questions for each of these products. One item from nine of the factors in Smoking Consequences Questionnaire-Adult (SCQ-A)41 was used in each version of the expectancy survey: Negative affect reduction, stimulation/state enhancement, health risk, taste/sensorimotor manipulation, social facilitation, weight control, craving/addiction (specifically craving reduction), negative physical feelings (focused on mouth and throat), and negative social impression. Items were chosen based on factor loadings and their ability to be adapted for different types of nicotine delivery. In all cases, the factor loadings were greater than 0.60. Participants also rated the degree to which they experienced craving for the type of product and withdrawal effects if going without the product for too long, and the degree to which they felt the product helped with stress reduction, was satisfying, or was addictive. An additional two questions assessed convenience and cost. As this study focuses on ex-smokers, questions about cigarette smoking were worded in the past tense, for example, “Cigarettes were satisfying.” Participants who had no prior use of NRTs were asked to report their beliefs about these products. All expectancy items were rated on a seven-point scale from 1 “Strongly Disagree” to 7 “Strongly Agree.”
Data Analysis
Using two sets of paired t-tests, we compared expectancies for cigarettes versus e-cigarettes and for e-cigarettes versus NRT. Cohen’s d was calculated for each comparison. Based on published guidelines, Cohen’s d ranges were labeled as small: 0.2–0.4; medium: 0.5–0.7; and large: 0.8 and above.42,43 Additional analyses using repeated measures analysis of variance (ANOVA) and examining partial eta-squared led to similar findings with similar categorie.44
Results
Negative Effects of Product Use
Table 2 summarizes the results of expectancy analyses. Figure 1 displays the expectancies that can broadly be conceived of as negative effects. For health risks, ex-smoking vapers rated e-cigarettes as less risky than both cigarettes and NRT. Similarly, vapers agreed less that e-cigarettes give rise to negative physical feelings than both cigarettes and NRT. They also reported e-cigarettes were less likely to lead to cravings than cigarettes, but more likely than NRT. E-cigarette users reported that e-cigarettes were less addictive, caused less withdrawal, and were more socially acceptable than cigarettes, but there were not even small effects when comparing e-cigarettes with NRT for these three expectancies. E-cigarettes were also rated as less expensive than both cigarettes and NRT.
Table 2.
Results of Expectancy Comparisons: Cigarette Versus E-Cigarette and E-Cigarette Versus Nicotine Replacement Therapy
| Domain | Cigarette versus E-cigarette | E-cigarette versus NRT | Cigarette | E-cigarette | NRT | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohen’s d | Label | Cohen’s d | Label | M | SD | M | SD | M | SD | |
| Negative effects | ||||||||||
| Health risks “ ___ is/was hazardous to my health” | 2.53* | Large | 0.83* | Large | 6.66 | 1.08 | 2.17 | 1.36 | 3.72 | 1.47 |
| Negative physical feelings “ ___ irritate(d) my mouth and throat” | 1.14* | Large | 0.80* | Large | 4.61 | 1.84 | 2.08 | 1.47 | 3.93 | 1.83 |
| Craving “I experience(d) cravings for ___” | 1.10* | Large | 0.49* | Medium | 6.52 | 1.21 | 4.37 | 1.69 | 3.10 | 1.98 |
| Addiction “ ___ is/were addictive” | 1.25* | Large | 0.14* | 6.62 | 1.80 | 4.33 | 1.65 | 4.02 | 1.74 | |
| Withdrawal “If I go/went too long without ___, I...feel bad” | 1.30* | Large | 0.11* | 6.13 | 1.45 | 3.55 | 1.81 | 3.84 | 1.93 | |
| Negative Social Impression “ People think less of me if they see me ___” | 1.13* | Large | 0.17* | 5.08 | 1.54 | 2.75 | 1.55 | 3.08 | 1.44 | |
| Cost “___ are expensive” | 1.91* | Large | 1.36* | Large | 6.67 | 1.06 | 2.85 | 1.64 | 5.98 | 1.50 |
| Positive effects | ||||||||||
| Craving reduction “ ___ satisfy/ed my nicotine cravings” | 0.16* | 1.52* | Large | 6.55 | 1.10 | 6.35 | 1.13 | 3.08 | 1.77 | |
| Negative affect reduction “ ___ help(ed) me deal with anxiety or worry” | 0.67* | Medium | 0.87* | Large | 6.15 | 1.29 | 4.97 | 1.76 | 2.93 | 1.64 |
| Weight control “ ___ control(led) my appetite” | 0.34* | Small | 0.54* | Medium | 4.69 | 1.68 | 4.02 | 1.76 | 2.82 | 1.52 |
| Social facilitation “ ___ help(ed) me enjoy people more” | 0.05 | 0.61* | Medium | 4.22 | 1.84 | 4.12 | 1.86 | 2.71 | 1.54 | |
| Stimulation “ ___ energize(d) me.” | 0.38* | Small | 0.54* | Medium | 4.61 | 1.78 | 3.92 | 1.63 | 2.83 | 1.46 |
| Stress reduction “ ___ are/were good for dealing with stress.” | 0.54* | Medium | 0.97* | Large | 5.96 | 1.33 | 5.10 | 1.61 | 2.92 | 1.57 |
| Taste “ ___ taste/tasted good.” | 0.95* | Large | 2.37* | Large | 4.54 | 1.87 | 6.52 | 0.95 | 2.20 | 1.47 |
| Satisfaction “ ___ are/were satisfying.” | 0.40* | Small | 2.57* | Large | 6.13 | 1.29 | 6.65 | 0.78 | 2.17 | 1.48 |
| Convenience “ ___ are/were convenient to use.” | 0.16* | 0.79* | Medium | 5.52 | 1.76 | 5.89 | 1.37 | 4.07 | 1.79 | |
*p < .001
Figure 1.
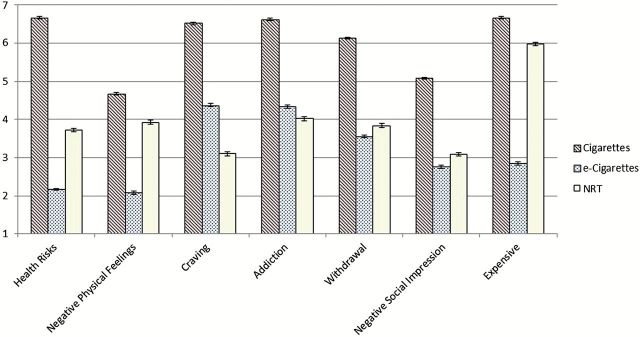
“Negative” expectancies and convenience for cigarettes, e-cigarettes and NRT among former smoking e-cigarette users (N = 1,434). Error bars represent SE.
Because it was somewhat surprising that e-cigarettes were rated as less risky to health than NRT, we examined correlations between NRT health risks and negative physical feelings expectancies, finding a significant correlation (r = 0.24, p < .001). This suggests that the harsher perceptions of NRT’s health risks may in part be driven by greater physical irritation perceived to be caused by NRTs.
Positive Effects of Product Use
In terms of positive effects, cigarettes were rated as superior to e-cigarettes in four domains, although none of the effect sizes were large (Table 2; Figure 2). Specifically, there were medium effect sizes for cigarettes to be rated as more effective in negative affect reduction and stress reduction and small effect sizes indicating perceived benefits in weight control and stimulation. However, a large effect was found for e-cigarettes to be rated as superior to cigarettes in terms of Taste, and a small effect for Satisfaction. NRT was rated as worse than e-cigarettes in all positive domains. Specifically, there were large effects for NRT to be less appealing in terms of craving reduction, negative affect reduction, stress reduction, taste, and satisfaction, and medium effects in terms of weight control, social facilitation, stimulation, and convenience.
Figure 2.
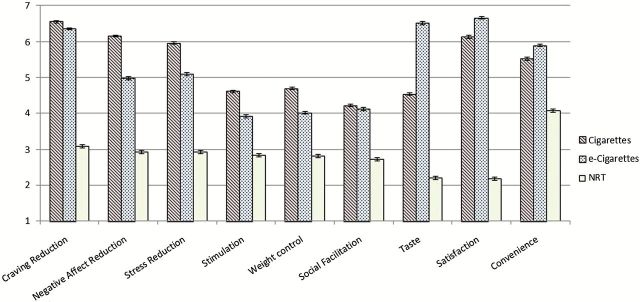
“Positive” expectancies and convenience for cigarettes, e-cigarettes and NRT among former smoking e-cigarette users (N = 1,434). Error bars represent SE.
Moderator Analyses
To examine how actual experience with NRT may have impacted the ratings, we tested previous NRT use as a moderator variable. As e-cigarettes deliver an acute dose of nicotine, we were particularly interested in understanding how expectancies differ in relation to use of NRTs that similarly deliver acute doses of nicotine (gum, lozenge, inhaler, and nasal spray), as opposed to the patch’s steady nicotine delivery system. Like e-cigarettes, acute NRT can be taken ad lib in response to acute cravings that may occur due to stress or other triggers. To address this issue, prior history with acute NRT was included as a between-subjects factor in subsequent repeated measures ANOVAs (with product comparison as a within-subjects factor), to allow for testing for moderation effects. To adjust for multiple comparisons, alpha was set at 0.001.
The majority of participants (n = 981, 68.4%) used at least one type of short-acting NRT in their lifetime. Of these, nearly all (n = 950, 96.8%) had used nicotine gum, a majority had used lozenges (n = 542, 55.2%), about a fifth had used the inhaler (n = 198, 20.2%), and less than a tenth had tried the nasal spray (n = 70, 7.1%). Moderator analyses indicated no significant effects when comparing cigarettes to e-cigarettes (all p > .01). However, when comparing e-cigarettes to NRT, there were small interaction effects for cravings, d = 0.23, cost, d = 0.22, craving reduction, d = 0.28, negative affect reduction, d = 0.24, weight control, d = 0.27, stimulation, d = 0.29, taste, d = 0.33, stress reduction, d = 0.27, and satisfaction, d = 0.37.
Of the effects moderated by NRT experience, only two were negative effects: craving and cost. Participants who had used acute NRT rated NRT cravings as less likely (M = 2.76, SD = 2.08 vs. M = 3.86, SD = 1.47), and they rated NRT as more costly (M = 6.28, SD = 1.38 vs M = 5.37, SD = 1.56). The remaining practically significant differences appeared with positive expectancies. For all of these positive expectancies, those with prior acute NRT experience reported less positive NRT expectancies. For example, those with NRT experience rated NRT lower in providing stimulation (M = 2.54, SD = 1.50 vs. M = 3.46, SD = 1.17) and satisfaction (M = 1.77, SD = 1.32 vs. M = 3.04, SD = 1.46). All other practically significant effects for positive expectancies showed the same pattern, with individuals with prior experience with acute NRT rating NRT as worse than those with no prior experience with acute NRT. By comparison, prior NRT experience was generally unassociated with ratings of e-cigarettes, with the only significant difference being that experienced NRT users rated e-cigarettes slightly better at relieving nicotine withdrawal compared to those without NRT experience (M = 3.71, SD = 1.81 vs. M = 3.22, SD = 1.77).
Some of the former smokers in this sample only recently quit smoking in the past few months. As such, their recovery may still be in a fragile state, where relapse could be likely.45 Although empirical evidence does not indicate a clear demarcation, 6 months has been used to differentiate active cessation from maintenance.46 Thus, we conducted moderator analyses using similar procedures as above, but with time since last cigarette—dichotomized for past 6 months—as a between-subjects factor. We found a significant interaction effect for cost, with those who had quit smoking for more than 6 months reporting less agreement that e-cigarettes are expensive, in comparison to those who had quit less than 6 months ago (M = 2.68, SD = 1.57 vs. M = 3.06, SD = 1.69). There were no other significant differences (all p > .001).
Discussion
The e-cigarette users in our study were similar to other samples of vapers from prior studies, representing an older, majority White, majority male, relatively well-educated, and relatively affluent sample.7–9 Unlike these other studies, our data were collected from the United States during fall of 2013, after the advent of major e-cigarette advertising and media attention. It is noteworthy that the majority of respondents (at least 73%) reported using “second generation” e-cigarettes that use a refillable reservoir, as opposed to the “first generation” products that resemble cigarettes.
E-Cigarettes Versus Cigarettes
In comparison to cigarettes, e-cigarettes were rated as leading to less craving, addiction, and withdrawal. Current evidence on nicotine delivery in e-cigarettes commercially available at the time of the survey suggests a much more delayed nicotine onset than with cigarettes.47,48 These data are consistent with a large body of evidence suggesting that rapid delivery of drugs leads to increased addictive symptomatology than slower delivery.49–51 However, e-cigarettes are likely to evolve into more rapid nicotine delivery systems as product design is improved, as we have already seen in comparisons between first and second generation e-cigarettes.47,52 E-cigarettes were further rated as less likely to cause a negative social impression and as more similar to cigarettes than NRT in enhancing social experiences. This social aspect of use is reported as a major source of appeal for ex-smokers.53
In addition, e-cigarettes were rated as superior to cigarettes in terms of both taste and satisfaction, perhaps reflecting the flavorings, which—with the exception of menthol—are no longer permitted for cigarettes. It is notable that e-cigarettes were rated higher in terms of satisfaction, given the previously mentioned slower onset of nicotine delivery. Satisfaction is generally one of the stronger effects found when nicotine is compared to placebo.54,55 Given that nicotine is increasingly recognized as a secondary, rather than a primary reinforcer,56–59 it may be that the unique flavorings associated with e-cigarettes are leading to the increased reports of satisfaction.
These former smokers did rate cigarettes as superior to e-cigarettes on a few expectancy dimensions: negative affect reduction, stress reduction, weight control, and stimulation. This is striking, given that this sample of exsmoking vapers primarily came from e-cigarette forums and have used e-cigarettes for more than 6 months. Given that these enthusiastic users of e-cigarettes still considered their product inferior to cigarettes in these areas suggests that they may be at risk of smoking relapse. In particular, affect regulation may be a prepotent motivator of tobacco use60, 61, whereas weight gain has been cited as a primary reason for putting off quit attempts, especially in women.62–64 Thus, these expectancies may represent targets for relapse-prevention efforts directed at e-cigarette users, as may be the development of relevant coping skills to prevent cigarette smoking relapse.
E-Cigarettes Versus Nicotine Replacement Therapies
As expected, e-cigarettes were rated as less risky to health than cigarettes. Prior research has shown that currently most use of e-cigarettes is driven by a desire to quit cigarettes or to have a healthier alternative to cigarettes.7,9 However, e-cigarettes, which are currently unregulated with very minimal safety or efficacy data,
65,66 were also rated as less risky than tested and regulated NRT. This may be related to perceived side effects from NRT, as suggested by reports here that NRT leads to more negative physical effects than e-cigarettes and findings that these two expectancies were correlated. Indeed, side effects are a common reason reported for discontinuation of NRT5,27.
NRT was also rated as much more expensive than e-cigarettes. In comparison to NRT, vapers reported no practically significant differences in addiction or withdrawal, but higher expectations of experiencing craving for e-cigarettes. This is consistent with findings that ex-smokers appear to use e-cigarettes for longer periods of time than ex-smokers who use NRT.26 Among these vapers, e-cigarettes were rated as superior to NRT for every positive expectancy we examined, particularly in relation to craving reduction, negative affect reduction, stress reduction, taste, and satisfaction.
Moderator analyses found that individuals with prior experience with acute NRT rated NRT less favorably than those who had only used the patch or had no prior experience with NRT. This prior experience was primarily with nicotine gum, although a majority also had used nicotine lozenges. The fact that NRT usage is associated with poorer expectancies supports the idea that NRT is perceived as an inadequate solution by a substantial proportion of the population of e-cigarette users. For NRT to compete with e-cigarettes, these factors may need to be addressed either by improving upon expectancies for NRT or by improving the products themselves. Nevertheless we cannot rule out the possibility that e-cigarettes themselves may represent a better alternative for some smokers, provided they are adequately regulated and supported by future research on their efficacy and safety.
Limitations
This research is limited by its cross-sectional nature and the sample used. The sample was self-selected, and most appeared to be dedicated vapers. They may not be generalizable to novice e-cigarette users. The association between past NRT usage and worse expectancies for NRT may similarly be related to selection bias. Specifically, the subsample that had previous NRT experience may have been particularly negative about NRT because it presumably was ineffective for them—otherwise they would have not needed to try e-cigarettes. More generally, the current sample chose e-cigarettes over both cigarettes and NRT. A more complete picture of motivational influences would require sampling of those who chose NRT over e-cigarettes, as well as those who chose to continue smoking rather than using either product. Nonetheless, the fact that the majority of this sample reported using both short-acting and long-term forms of NRT and found them lacking suggests there is a substantial proportion of vapers who are not simply avoiding NRT due to ignorance or poor motivation. Instead, they apparently tried some of the currently available evidence-based and FDA-approved treatments and were unsuccessful, but subsequently found cessation success with e-cigarettes.
Implications
Future research using longitudinal and experimental designs should investigate to what degree these cigarette, e-cigarette, and NRT expectancies are driving usage among vapers, and whether these expectancies could be altered via the development of more effective products or messaging. Such research could also attempt to understand the degree to which the expectancies precede or follow usage patterns, thus helping to determine the degree to which expectancies are causal determinants of product choice. It is known that expectancies can be altered by instructions or media campaigns54,67 and are associated with different aspects of desire to change substance use behaviors.68 As regulations for e-cigarettes are debated, this information can help guide labeling requirements, advertising restrictions, and the development of counter-messaging to discourage unhealthy behaviors. Expectancies may be targets for public health efforts to discourage de novo e-cigarette use among youth and adults, to encourage switching from cigarettes to e-cigarettes, or to encourage e-cigarette use after (or potentially even before) trying NRT and other approved medications, depending on the outcomes of future safety and efficacy studies.
Funding
This research was funded by the National Cancer Institute Behavioral Oncology Training Grant (R25CA090314) at Moffitt Cancer Center in Tampa, FL, awarded to Paul Jacobsen, and by grants R01CA134347 and R01CA154596, awarded to Thomas Brandon and Vani Simmons, respectively.
Declaration of Interests
Thomas Brandon receives research support from Pfizer, Inc. The authors alone are responsible for the content and writing of this article.
Acknowledgments
The authors wish to acknowledge the help and support of Wendy Malagon, Yohana Botero, and other staff at the Tobacco Research and Intervention Program.
References
- 1. USDHHS. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [PubMed] [Google Scholar]
- 2. Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 3. Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med. 2005;28:119–122. [DOI] [PubMed] [Google Scholar]
- 4. Cummings KM, Hyland A. Impact of nicotine replacement therapy on smoking behavior. Annu Rev Public Health. 2005;26:583–599. [DOI] [PubMed] [Google Scholar]
- 5. Balmford J, Borland R, Hammond D, Cummings KM. Adherence to and reasons for premature discontinuation from stop-smoking medications: data from the ITC Four-Country Survey. Nicotine Tob Res. 2011;13:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curry SJ, Ludman EJ, McClure J. Self-administered treatment for smoking cessation. J Clin Psychol. 2003;59:305–319. [DOI] [PubMed] [Google Scholar]
- 7. Dawkins L, Turner J, Roberts A, Soar K. ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction (Abingdon, England). 2013;108:1115–1125. [DOI] [PubMed] [Google Scholar]
- 8. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction (Abingdon, England) 2011;106:2017–2028. [DOI] [PubMed] [Google Scholar]
- 10. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an Internet survey. Drug Alcohol Rev. 2013;32:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J. Public Health Policy. 2011;32:16–31. [DOI] [PubMed] [Google Scholar]
- 12. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control. 2014;23:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pellegrino RM, Tinghino B, Mangiaracina G, et al. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Annali di Igiene: medicina preventiva e di comunità. 2012;24:279–288. [PubMed] [Google Scholar]
- 14. Wagener TL, Siegel M, Borrelli B. Electronic cigarettes: achieving a balanced perspective. Addiction (Abingdon, England). 2012;107:1545–1548. [DOI] [PubMed] [Google Scholar]
- 15. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. [DOI] [PubMed] [Google Scholar]
- 16. Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10:446–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caponnetto P, Campagna D, Cibella F, et al. (2013). EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 8:e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polosa R, Morjaria JB, Caponnetto P, et al. Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med. 2014;9:537–546. [DOI] [PubMed] [Google Scholar]
- 20. Vickerman KA, Carpenter KM, Altman T, Nash CM, Zbikowski SM. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787–1791. [DOI] [PubMed] [Google Scholar]
- 21. Wagener TL, Meier E, Hale JJ, et al. Pilot investigation of changes in readiness and confidence to quit smoking after E-cigarette experimentation and 1 week of use. Nicotine Tob Res. 2014;16:108–114. [DOI] [PubMed] [Google Scholar]
- 22. Brown J, Beard E, Kotz D, Michie S, West R. Real‐world effectiveness of e‐cigarettes when used to aid smoking cessation: a cross‐sectional population study. Addiction. 2014;109:1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cameron JM, Howell DN, White JR, Andrenyak DM, Layton ME, Roll JM. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tobacco Control. 2014;23:77–78. [DOI] [PubMed] [Google Scholar]
- 24. Cheah NP, Chong NW, Tan J, Morsed FA, Yee SK. Electronic nicotine delivery systems: regulatory and safety challenges: Singapore perspective. Tobacco Control. 2014;23:119–125. [DOI] [PubMed] [Google Scholar]
- 25. Trehy ML, Ye W, Hadwiger ME, et al. Analysis of electronic cigarette cartridges, refill solutions, and smoke for nicotine and nicotine related impurities. J Liquid Chromatogr Relat Technol. 2011;34:1442–1458. [Google Scholar]
- 26. Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39:491–494. [DOI] [PubMed] [Google Scholar]
- 27. Burns EK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking-cessation attempters. Am J Prev Med. 2008;34:212–215. [DOI] [PubMed] [Google Scholar]
- 28. Goldman MS, Darkes J. Alcohol expectancy multiaxial assessment: a memory network-based approach. Psychol Assess. 2004;16:4–15. [DOI] [PubMed] [Google Scholar]
- 29. Leventhal AM, Schmitz JM. The role of drug use outcome expectancies in substance abuse risk: an interactional-transformational model. Addict Behav. 2006;31:2038–2062. [DOI] [PubMed] [Google Scholar]
- 30. Reich RR, Ariel I, Darkes J, Goldman MS. What do you mean “drunk”? Convergent validation of multiple methods of mapping alcohol expectancy memory networks. Psychol Addict Behav. 2012;26:406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brandon TH, Herzog TA, Irvin JE, Gwaltney CJ. Cognitive and social learning models of drug dependence: implications for the assessment of tobacco dependence in adolescents. Addiction (Abingdon, England). 2004;99(Suppl. 1):51–77. [DOI] [PubMed] [Google Scholar]
- 32. Goldman MS, Del Boca FK, Darkes J. Alcohol expectancy theory: The application of cognitive neuroscience. In Psychological Theories of Drinking and Alcoholism. Vol 2; 1999;203–246. [Google Scholar]
- 33. Chassin L, Presson CC, Sherman SJ, Edwards DA. Four pathways to young-adult smoking status: adolescent social-psychological antecedents in a midwestern community sample. Health Psychol. 1991;10:409–418. [DOI] [PubMed] [Google Scholar]
- 34. Doran N, Schweizer CA, Myers MG. Do expectancies for reinforcement from smoking change after smoking initiation? Psychol Addict Behav. 2011;25:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeffries SK, Catley D, Okuyemi KS, et al. Use of a brief Smoking Consequences Questionnaire for Adults (SCQ-A) in African American smokers. Psychol Addict Behav. 2004;18:74–77. [DOI] [PubMed] [Google Scholar]
- 36. Gwaltney CJ, Shiffman S, Balabanis MH, Paty JA. Dynamic self-efficacy and outcome expectancies: prediction of smoking lapse and relapse. J Abnormal Psychol. 2005;114:661–675. [DOI] [PubMed] [Google Scholar]
- 37. Wahl SK, Turner LR, Mermelstein RJ, Flay BR. Adolescents’ smoking expectancies: psychometric properties and prediction of behavior change. Nicotine Tob Res. 2005;7:613–623. [DOI] [PubMed] [Google Scholar]
- 38. Juliano LM, Brandon TH. Smokers’ expectancies for nicotine replacement therapy vs. cigarettes. Nicotine Tob Res. 2004;6:569–574. [DOI] [PubMed] [Google Scholar]
- 39. Mooney ME, Leventhal AM, Hatsukami DK. Attitudes and knowledge about nicotine and nicotine replacement therapy. Nicotine Tob Res 2006;8:435–446. [DOI] [PubMed] [Google Scholar]
- 40. Hendricks PS, Brandon TH. Smokers’ expectancies for smoking versus nicotine. Psychol Addict Behav. 2008;22:135–140. [DOI] [PubMed] [Google Scholar]
- 41. Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire-Adult: Measurement of smoking outcome expectancies of experienced smokers. Psychol Assess. 1995;7:484. [Google Scholar]
- 42. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 43. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2–18. [DOI] [PubMed] [Google Scholar]
- 44. Ferguson CJ. An effect size primer: A guide for clinicians and researchers. Prof Psychol Res Pract. 2009;40:532. [Google Scholar]
- 45. Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–284. [DOI] [PubMed] [Google Scholar]
- 46. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47:1102–1114. [DOI] [PubMed] [Google Scholar]
- 47. Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine de livery after acute administration. Nicotine Tob Res. 2013;15:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Berridge MS, Apana SM, Nagano KK, Berridge CE, Leisure GP, Boswell MV. Smoking produces rapid rise of [11C]nicotine in human brain. Psychopharmacology. 2010;209:383–394. [DOI] [PubMed] [Google Scholar]
- 50. Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26:82–87. [DOI] [PubMed] [Google Scholar]
- 51. Samaha AN, Yau WY, Yang P, Robinson TE. Rapid delivery of nicotine promotes behavioral sensitization and alters its neurobiological impact. Biol Psychiatry. 2005;57:351–360. [DOI] [PubMed] [Google Scholar]
- 52. Rose JE, Turner JE, Murugesan T, Behm FM, Laugesen M. Pulmonary delivery of nicotine pyruvate: sensory and pharmacokinetic characteristics. Exp Clin Psychopharmacol. 2010;18:385–394. [DOI] [PubMed] [Google Scholar]
- 53. Barbeau AM, Burda J, Siegel M. Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: a qualitative approach. Addict Sci Clin Practice. 2013;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harrell PT, Juliano LM. A direct test of the influence of nicotine response expectancies on the subjective and cognitive effects of smoking. Exp Clin Psychopharmacol. 2012;20:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Juliano LM, Fucito LM, Harrell PT. The influence of nicotine dose and nicotine dose expectancy on the cognitive and subjective effects of cigarette smoking. Exp Clin Psychopharmacol. 2011;19:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. [DOI] [PubMed] [Google Scholar]
- 57. Caggiula AR, Donny EC, White AR, et al. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:230–237. [DOI] [PubMed] [Google Scholar]
- 58. Perkins KA, Karelitz JL. Influence of reinforcer magnitude and nicotine amount on smoking’s acute reinforcement enhancing effects. Drug Alcohol Depend. 2013;133:167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology. 2013;228:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. [DOI] [PubMed] [Google Scholar]
- 61. Piper ME, Schlam TR, Cook JW.et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology. 2011;216:569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Clark MM, Hurt RD, Croghan IT, et al. The prevalence of weight concerns in a smoking abstinence clinical trial. Addict Behav. 2006;31:1144–1152. [DOI] [PubMed] [Google Scholar]
- 63. Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. The Cochrane Database Syst Rev. 2012;1:CD006219. [DOI] [PubMed] [Google Scholar]
- 64. Klesges RC, Shumaker SA. Understanding the relations between smoking and body weight and their importance to smoking cessation and relapse. Health Psychol. 1992;11(Suppl):1–3. [DOI] [PubMed] [Google Scholar]
- 65. Benowitz NL, Goniewicz ML. The regulatory challenge of electronic cigarettes. JAMA. 2013;310:685–686. [DOI] [PubMed] [Google Scholar]
- 66. Harrell PT, Simmons VN, Correa JB, Padhya TA, Brandon TH. Electronic nicotine delivery systems (“e-cigarettes”): Review of safety and smoking cessation efficacy. Otolaryngol Head Neck Surg. In press. 10.1177/0194599814536847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Copeland AL, Brandon TH. Testing the causal role of expectancies in smoking motivation and behavior. Addict Behav. 2000;25:445–449. [DOI] [PubMed] [Google Scholar]
- 68. Harrell PT, Trenz RC, Scherer M, Martins SS, Latimer WW. A latent class approach to treatment readiness corresponds to a transtheoretical (“Stages of Change”) model. J Subst Abuse Treat. 2013;45:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]


