Abstract
Background
Contrast-induced nephropathy (CIN) is a common cause of hospital-acquired acute kidney injury (AKI). Neutrophil gelatinase-associated lipocalin (NGAL) represents a promising biomarker for AKI. Its role in the early diagnosis of CIN has already been examined in adults and children undergoing coronary angiography. This study was designed to prospectively evaluate plasma NGAL compared with serum creatinine (SCr) for early CIN detection among hospitalized patients undergoing contrast-enhanced computed tomography (CT).
Methods
We prospectively enrolled consecutive hospitalized patients undergoing elective CT with intravenous (IV), low-osmolar contrast administration. Patients with pre-procedure SCr >150 μmol/L (1.7 mg/dL), congestive heart failure, haemodynamic instability, sepsis, or urinary tract infection were excluded. Plasma NGAL was measured using the standardized Triage® NGAL test (Biosite Incorporated, San Diego, CA, USA) at baseline and 6 h post-procedure. SCr, blood urea nitrogen (BUN), albumin and sodium (Na) were measured and eGFR MDRD4 was calculated at the same intervals, as well as at 24 and 48 h post-procedure. CIN was defined as an increase in SCr of >25% or >44 μmol/L (0.5 mg/dL) from baseline within 48 h post-procedure, in the absence of other obvious causes.
Results
Forty-seven patients, male/female 27/20, median age 68 (31–88) years, 16/47 diabetics, with baseline SCr 91.94 ± 20.33 μmol/L (1.04 ± 0.23 mg/dL) and eGFR MDRD4 68.40 ± 18.22 mL/min/1.73 m2 were enrolled. A contrast volume of 120 mL (range 100–150 mL) was administered. CIN was found in four subjects (8.51%), but detection by SCr was only possible 24 h in 1 and 48 h post-procedure in three. In contrast, significant elevation of plasma NGAL was found at 6 h post-procedure in those with versus those without CIN (779.25 ± 361.49 versus 82.30 ± 40.64 ng/mL, P < 0.001). Using a cutoff value of 200 ng/mL, sensitivity, specificity and area under the receiver-operating characteristic (ROC) curve of 6-h plasma NGAL for CIN prediction were excellent (100, 100 and 1.00%, respectively). Subjects with CIN did not differ in baseline demographics, renal function and diabetes status compared with those without CIN. No differences in any variable were noted between diabetics and non-diabetics. Plasma NGAL at 6 h (R2 = 0.24, P < 0.001) was found to be an independent predictor of CIN.
Conclusions
Plasma NGAL 6 h after contrast administration measured by the rapid, point-of-care Triage® NGAL test appears to be a useful biomarker in the early prediction of CIN among hospitalized patients undergoing elective contrast-enhanced CT. However, the small sample size and the very small number of CIN events are important limitations. In any case, according to our evaluation, CIN incidence in this well-controlled population underlines the importance of early detection by an adequate and simple procedure such as the 6-h plasma NGAL test.
Keywords: acute kidney injury, biomarkers, computed tomography, contrast-induced nephropathy, neutrophil gelatinase-associated lipocalin
Introduction
Contrast-induced nephropathy (CIN) is a common cause of hospital-acquired acute kidney injury (AKI), accounting for up to 12% of cases, and is associated with an average in-hospital mortality of 6% [1]. Studies of large cohorts have revealed that more than half of these cases are in subjects undergoing cardiac catheterization and intra-arterial coronary angiography, and nearly a third follow computed tomography (CT) scans [2]. Technological advances in diagnostic and interventional imaging techniques have contributed to a continuously increasing number of individuals being exposed to iodinated contrast media [3]. Serum creatinine (SCr) is undoubtedly a delayed and unreliable indicator of AKI [4]. Therefore, the pursuit of biomarkers with improved performance in the early and accurate diagnosis of AKI is an area of intense contemporary research with the ultimate goal to implement currently available therapeutic interventions in a timely manner and reduce the unacceptably high morbidity and mortality rates associated with AKI [5].
Neutrophil gelatinase-associated lipocalin (NGAL) was originally identified as a 25-kDa protein covalently bound to gelatinase from neutrophils. The major ligands for NGAL are siderophores, small iron-binding molecules. Siderophores are synthesized by bacteria, and NGAL exerts a bacteriostatic effect by depleting siderophores. On the other hand, siderophores produced by eukaryotes participate in NGAL-mediated iron shuttling that is critical to various cellular responses, such as proliferation and differentiation [4–6]. Although NGAL is expressed only at very low levels in several human tissues, it is markedly induced in injured epithelial cells, including the kidney. This is likely mediated via NF-kB, which is known to be rapidly activated in kidney tubule cells after acute injuries, and plays a central role in controlling cell survival and proliferation [4–6]. NGAL, both in plasma and urine, represents a promising, non-invasive, troponin-like biomarker for the early prediction of AKI in various clinical settings [5, 6]. Several investigators have examined the role of plasma and urine NGAL as a predictive biomarker of CIN in adults and children undergoing coronary angiography with intra-arterial contrast administration and revealed good diagnostic and prognostic performance [7–11]. Much of these data have been arbitrarily extrapolated to patients receiving intravenous (IV) contrast for CT scans despite major differences in patient populations, contrast volume administered and intra-procedural complications between the two settings [12]. In addition, 90% of contrast media are used for CT imaging [13, 14]. Furthermore, although the number of coronary and conventional angiograms has been relatively stable, the number of contrast-enhanced CT scans has been increasing by ∼2 million per year in the USA over the last decade [13]. Therefore, the widespread use of contrast-enhanced CT necessitates studies focusing on NGAL performance in this setting [14].
The present study was designed to prospectively evaluate plasma NGAL measured by using a standardized, point-of-care Triage® NGAL device [15] compared with SCr for early CIN detection among hospitalized patients undergoing elective contrast-enhanced CT.
Subjects and methods
Consecutive hospitalized patients undergoing elective CT with IV contrast administration at our institution between December 2010 and December 2011 were prospectively enrolled. The study was performed in accordance with the Declaration of Helsinki and with the approval of the local ethics committee. All of the patients gave informed written consent before enrollment. Patients with pre-procedure SCr >150 μmol/L (1.7 mg/dL), overt congestive heart failure (stages III–IV according to New York Heart Association-NYHA functional classification system), haemodynamic instability of any cause, sepsis or systemic infectious disease were excluded. All of the subjects were maintained in an euvolaemic state prior to the procedure. Non-ionic, low-osmolar iopromide injection (Ultravist® 370) was used by all study participants. According to the Radiology Department protocol, a contrast volume of not >100 mL was administered in subjects with eGFR MDRD4 <60 mL/min/1.73 m2.
Plasma NGAL was measured by using the standardized Triage® NGAL test (Biosite Incorporated, San Diego, CA, USA) at baseline (within 1 h prior to contrast administration) and 6 h after contrast administration. The Triage® NGAL test is a point-of-care, fluorescence-based immunoassay used in conjunction with the Triage Meter, a portable fluorescence spectrometer, for the rapid quantitative determination of NGAL concentration (measurable range 60–1300 ng/mL) in EDTA-anticoagulated whole blood or plasma specimens [15]. The whole procedure was performed according to the instructions provided by the manufacturer (Biosite Incorporated, San Diego, CA, USA).
SCr, BUN, serum albumin (SAlb) and sodium (Na) were measured, and estimated GFR (eGFR) MDRD4 [16] was calculated at the same intervals, as well as at 24 and 48 h post-procedure. SCr levels were determined using an isotope dilution mass spectrometry-traceable kinetic Jaffe method (Thermo Scientific Konelab Prime 60i Clinical Chemistry Analyser) and all other biochemistry measurements were performed using standard laboratory methods.
CIN development was the primary outcome, defined by an increase in SCr of >25% or >44 μmol/L (0.5 mg/dL) from baseline within 48 h after contrast exposure, in the absence of other obvious causes [17].
Values are presented as means ± standard deviation. The paired two-sample t test or Mann–Whitney rank-sum test was used to compare continuous variables, whereas the Chi-square test or Fisher's exact test was used for categorical variables. Univariate and multivariate stepwise multiple logistic regression analysis was undertaken to assess predictors of CIN. To calculate the sensitivity and specificity for plasma NGAL measurements for the prediction of CIN at varying cut-off values, a receiver operating characteristic (ROC) curve was generated and the area under the curve (AUC) was calculated to quantify the accuracy of plasma NGAL as a biomarker (Medcalc, version 12.2.1). An AUC of 0.5 is no better than expected by chance, whereas a value of 1.0 signifies a perfect biomarker. P ≤ 0.05 was considered statistically significant.
Results
Forty-seven patients, male/female 27/20, median age 68 (31–88) years, 16/47 (34%) diabetics, with baseline SCr 91.94 ± 20.33 μmol/L (1.04 ± 0.23 mg/dL) and eGFR MDRD4 68.40 ± 18.22 mL/min/1.73 m2 were enrolled in the study. Thirty-five out of 47 subjects (74.47%) had a baseline eGFR MDRD4 of ≥60 mL/min/1.73 m2, whereas no participant had baseline eGFR MDRD4 <30 mL/min/1.73 m2. Contrast volume of 120 mL (range 100–150 mL) was administered.
CIN was found in four subjects (8.51%), but detection by SCr was only possible at 24 h in 1 and 48 h post-procedure in three.
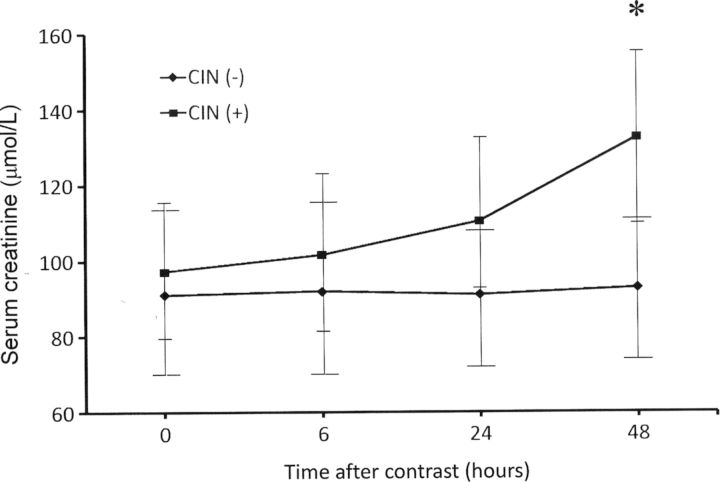
Based on the primary outcome, subjects were classified into those with and without CIN (Table 1). No significant differences were noted between CIN and non-CIN groups in baseline patient demographics, renal function, SAlb and diabetes status (Table 1). Furthermore, no differences in any variable were found between diabetics and non-diabetics. SCr did not significantly change at any time point in the non-CIN group, whereas subjects in the CIN group showed a statistically significant increase in SCr from baseline at 48 h post-procedure: 132.6 ± 22.1 μmol/L (1.50 ± 0.25 mg/dL) versus 97.24 ± 15.91 μmol/L (1.10 ± 0.18 mg/dL), P = 0.002. Between the two groups, a statistically significant difference in SCr was noted only at 48 h post-procedure (Table 1 and Figure 1). No significant differences were observed in SAlb and serum Na at any time point both within and between the two groups (data not shown).
Table 1.
Patient demographics and study results in CIN and non-CIN groups
| CIN− (n = 43) | CIN+ (n = 4) | P-value | |
|---|---|---|---|
| Age (years) | 65.65 ± 16.20 | 66.75 ± 11.35 | 0.62 |
| Weight (kg) | 71.98 ± 9.39 | 76.25 ± 7.50 | 0.8 |
| Males (n) | 25 | 2 | 0.87 |
| Diabetics (n) | 14 | 2 | 0.64 |
| Hospitalization cause | |||
| Diseases of digestive system | 15 | 2 | 0.69 |
| Diseases of circulatory system | 11 | 2 | 0.46 |
| Diseases of genitourinary system | 9 | 0 | 0.36 |
| Neoplasms | 8 | 0 | 0.39 |
| Contrast volume (mL) | 114.65 ± 10.77 | 115.00 ± 10.00 | 0.95 |
| BUN baseline (mmol/L) | 5.85 ± 2.78 | 5.58 ± 1.65 | 0.85 |
| (mg/dL) | 16.39 ± 7.79 | 15.64 ± 4.64 | |
| SCr baseline (μmol/L) | 91.05 ± 21.21 | 97.24 ± 15.91 | 0.59 |
| (mg/dL) | 1.03 ± 0.24 | 1.10 ± 0.18 | |
| eGFR MDRD4 (mL/min/1.73 m2) | 68.72 ± 18.33 | 65.00 ± 19.15 | 0.7 |
| Serum Na baseline (mmol/L) | 136.51 ± 3.50 | 136.00 ± 2.45 | 0.77 |
| SAlb baseline (g/L) | 35.10 ± 5.20 | 36.00 ± 3.40 | 0.74 |
| (g/dL) | 3.51 ± 0.52 | 3.60 ± 0.34 | |
| Plasma NGAL baseline (ng/mL) | 73.37 ± 30.48 | 98.25 ± 38.33 | 0.13 |
| SCr (μmol/L) 6 h | 91.93 ± 22.10 | 101.66 ± 21.21 | 0.42 |
| (mg/dL) | 1.04 ± 0.25 | 1.15 ± 0.24 | |
| 24 h | 91.05 ± 18.56 | 110.50 ± 18.56 | 0.06 |
| 1.03 ± 0.21 | 1.25 ± 0.21 | ||
| 48 h | 92.82 ± 19.44 | 132.60 ± 22.10 | <0.001 |
| 1.05 ± 0.22 | 1.50 ± 0.25 | ||
| Plasma NGAL 6 h (ng/mL) | 82.30 ± 40.64 | 779.25 ± 361.49 | <0.001 |
CIN, contrast-induced nephropathy; SCr, serum creatinine; Na, sodium; SAlb, serum albumin.
Fig. 1.
Serum creatinine (means ± SD) in CIN and non-CIN groups at various time points after contrast administration. *P < 0.001 when the two groups were compared at 48 h. CIN, contrast-induced nephropathy, SD, standard deviation.
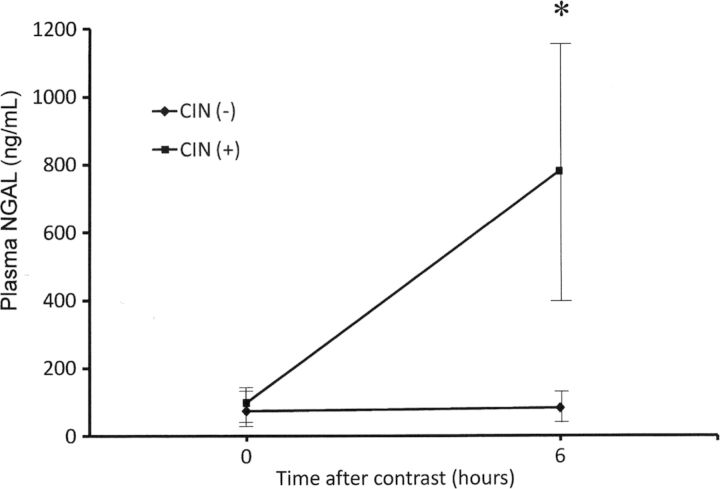
Baseline plasma NGAL levels were consistently low and similar in both groups. Subjects in the CIN group displayed a substantial increase by nearly 10-fold in plasma NGAL at 6 h after contrast administration, whereas in the non-CIN group no significant change was found in plasma NGAL levels (Table 1 and Figure 2)
Fig. 2.
Plasma NGAL (means ± SD) in CIN and non-CIN groups at baseline and at 6 h after contrast administration. * P < 0.001 when the two groups were compared at 6 h. NGAL, neutrophil gelatinase-associated lipocalin, CIN, contrast-induced nephropathy, SD, standard deviation.
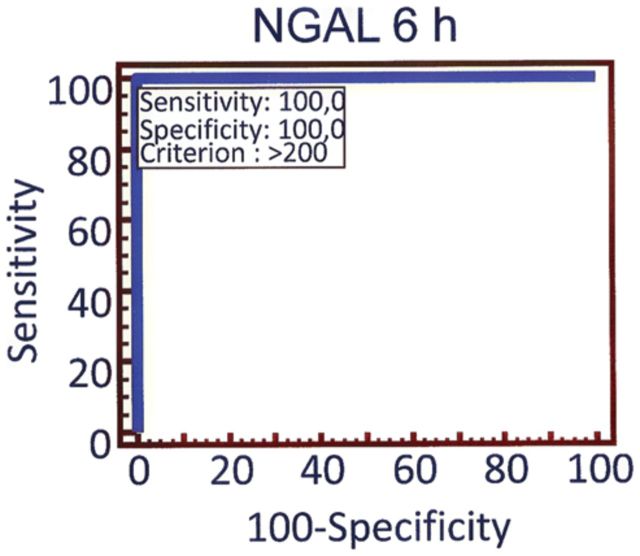
Plasma NGAL biomarker characteristics for CIN prediction at various cut-off values are shown in Table 2. For plasma NGAL at 6 h after contrast administration, sensitivity and specificity were optimal at the cut-off value of 200 ng/mL, with an area under the ROC curve of 1.00 (95% CI: 0.92–1.00) for CIN prediction (Figure 3), whereas for SCr at 6 h the area under the ROC curve was 0.59 (95% CI: 0.45–0.74), P = 0.002 for pairwise comparison between NGAL 6 h and SCr 6 h ROC curves. By multiple regression analysis, plasma NGAL at 6 h (R2 = 0.24, P < 0.001) was found to be an independent predictor of CIN.
Table 2.
Six-hour plasma NGAL test characteristics for CIN prediction at various cut-off values
| Cut-off values (ng/mL) |
|||
|---|---|---|---|
| >200 | >150 | >100 | |
| Sensitivity (%) (95% CI) | 100 (39.8–100) | 100 (39.8–100) | 100 (39.8–100) |
| Specificity (%) (95% CI) | 100 (91.8–100) | 90.7 (77.9–97.4) | 79.1 (64–90) |
| Positive predictive value (%) (95% CI) | 100 (39.8–100) | 50 (15.7–84.3) | 30.8 (9.1–61.4) |
| Negative predictive value (%) (95% CI) | 100 (91.8–100) | 100 (91–100) | 100 (89.7–100) |
CI, confidence intervals.
Fig. 3.
ROC curve analysis of 6 h plasma NGAL at the cut-off value of 200 ng/mL. The AUC was 1.00 (95% CI: 0.92–1.00). ROC, receiver operating characteristic, AUC, area under the curve, CI, confidence intervals.
Discussion
This prospective study in hospitalized patients undergoing elective conventional contrast-enhanced CT demonstrated that plasma NGAL 6 h after contrast administration measured by the rapid, point-of-care Triage® NGAL test appeared to be a clinically useful biomarker in the early prediction of CIN, defined as an increase in SCr by > 25% or 44 μmol/L (0.5 mg/dL) from baseline within 48 h post-procedure. Therefore, plasma NGAL appears to be a powerful early biomarker of CIN that precedes the increase in SCr by several hours. Furthermore, the relatively high CIN incidence detected in this well-controlled population underlines the importance of early diagnosis by an adequate and simple procedure such as the 6-h plasma Triage® NGAL test that was evaluated in our study.
Despite the widespread use of newer, less nephrotoxic contrast agents and the implementation of preventive strategies over the last decades, the risk of CIN continues to be considerable, especially in the inpatient setting. Affected patients experience a significant increase in short- and long-term morbidity and mortality [18]. A major limitation of the current literature is that most studies on CIN have been conducted in the setting of intra-arterial contrast administration, particularly coronary angiography and percutaneous coronary interventions [14]. It is currently unclear how far the conclusions of such studies can be extrapolated to patients receiving IV contrast for CT scans [13]. To the best of our knowledge, this is the first study that evaluates plasma NGAL performance for early CIN detection specifically after IV contrast administration.
A limited number of studies have evaluated the performance of plasma and urine NGAL as a predictive biomarker of CIN among children and adults with normal renal function undergoing coronary angiography [7–11]. In a prospective study of children undergoing elective cardiac catheterization and angiography with contrast administration, both urine and plasma NGAL measured by using a research-based assay at 2 h post-procedure predicted CIN, defined as a 50% increase in SCr from baseline, with an AUC-ROC of 0.91–0.92 [10]. Subjects in the CIN group of the above-mentioned study displayed a 5-fold increase in plasma NGAL at the 6-h time point, whereas a 10-fold increase was observed in our study, possibly reflecting differences in patient populations or in the assays used for plasma NGAL measurement. Furthermore, pilot studies in adults with normal SCr receiving contrast for coronary angiography and intervention detected a significant rise in both urine and plasma NGAL 2–4 h post-procedure, whereas plasma cystatin C levels increased significantly only 24 h after contrast exposure [7, 8]. However, none of these subjects developed CIN, as SCr remained unchanged 48 h post-procedure. Not surprisingly, the maximal plasma NGAL increase from baseline was only 31% at the 4-h time point, in marked contrast to our findings. A study from China detected CIN in 8.7% of adults with normal renal function undergoing coronary angiography and found that urinary NGAL measured by a research-based assay at 24 h post-procedure increased significantly in the CIN group, but not in the non-CIN group [9]. Finally, a meta-analysis revealed an overall AUC-ROC of 0.894 for CIN prediction, when NGAL was measured within 6 h after contrast administration for coronary procedures and CIN was defined as an increase in SCr of over 25% [11]. In agreement with these findings, our study demonstrated excellent performance of plasma NGAL, measured at 6 h post-procedure, as an early biomarker of CIN in the special and understudied population of subjects receiving IV contrast for elective CT scans.
The predictive performance of NGAL depends on the specific characteristics of the population studied. In particular, it varies with baseline renal function, with optimal discriminatory performance in subjects with normal pre-procedure renal function [19]. Our study population consisted of subjects at low-to-medium risk of CIN. No participant had a baseline SCr >150 μmol/L (1.7 mg/dL), whereas SCr did not exceed 115 μmol/L (1.3 mg/dL) in the subgroup of diabetics. This relatively homogenous cohort of subjects with well-preserved renal function is a noteworthy strength of our study. In addition, subjects with major confounding variables such as haemodynamic instability of any cause were excluded. Furthermore, all subjects received iopromide, a non-ionic, low-osmolar contrast agent, thereby eliminating any variability due to type of agent used. The contrast volume administered was kept at low levels, especially in participants with renal impairment. Furthermore, our study involved only elective CT scans, excluding emergent ones that are usually performed in subjects with major comorbidities and potentially at high risk of CIN [20, 21].
An additional strength of our study is that all subjects started with low levels of plasma NGAL. Furthermore, there was no significant difference in the baseline SCr between the two groups (CIN/non-CIN). The study design allowed a direct comparison of altered NGAL concentration with changes in SCr, the current reference standard test for the definition of CIN. The magnitude of rise by nearly 10-fold from baseline found in our study supports previous literature data that NGAL is a highly discriminatory biomarker with a wide dynamic range and cut-off values that allow for risk assessment and stratification [6, 11].
The reported incidence of CIN varies widely depending on the presence or absence of risk factors, in particular pre-existing chronic kidney disease (CKD), the amount and type of agent administered and the exact radiological procedure [20]. Current data coming mainly from patients undergoing coronary angiography with intra-arterial contrast administration indicated that CIN occurs in 4–20% of cases [20, 21]. In contrast to that associated with angiography, the risk of CIN associated with contrast-enhanced CT scans is quite low, even among patients with CKD [14, 22]. Therefore, in a study of in- and outpatients with eGFR <60 mL/min/1.73 m2 undergoing elective contrast-enhanced CT, only 3.5% had an increase in SCr of >0.5 mg/dL (44 μmol/L) within 48–96 h post-procedure [22]. In addition, three recent prospective trials involving contrast-enhanced CT in patients with eGFR<60 mL/min/1.73 m2 found an overall incidence of CIN, defined as a ≥25% increase in SCr, of ∼5% [23–25]. In view of these data, a CIN incidence of 8.5% as reported in our study appears to be relatively high. However, differences in study populations (participants' renal function and diabetes status, in- or outpatients, elective or non-elective CT) should be taken into account in the interpretation of the results.
Previous studies on NGAL performance as a predictive biomarker of CIN used mainly research-based enzyme-linked immunosorbent assay for plasma NGAL measurement [7–11]. Our study determined plasma NGAL by using the standardized, point-of-care Triage® NGAL test. This assay is simple and easy to perform, with quantitative results available within 15 min, requires only microlitre quantities of whole blood or plasma. A potential limitation is the suboptimal assay performance in the lower-range values. However, a meta-analysis on the accuracy of NGAL in the diagnosis of AKI revealed better performance of standardized assays compared with individually developed research-based ones [11]. However, the Triage® NGAL device, although validated in several clinical settings with favourable results [15], has not been evaluated in subjects undergoing contrast-enhanced CT scans. Thus, our study is the first example of how a standardized, point-of-care platform using plasma samples may be useful for predicting CIN.
Plasma NGAL was measured in our study, although the majority of biomarkers for AKI described to date have been determined in the urine [5, 6]. Urinary diagnostics do have several advantages, including the non-invasive nature of sample collection, the reduced number of interfering proteins, and the potential for the development of self-testing kits. However, several limitations also exist, including the lack of samples from patients with oligoanuria and potential changes in urinary biomarker concentration induced by hydration status and diuretic therapy or by co-existing urinary tract infection. Plasma-based diagnostics have revolutionized many aspects of clinical medicine, as exemplified by the use of troponins for the early diagnosis of acute myocardial infarction and the value of B-type natriuretic peptide for prognostication in acute coronary syndromes [5, 6, 15, 26].
Our study has a few limitations. An important limitation is the small sample size and the very small number of CIN events (n = 4). This is reflected by the wide confidence intervals in sensitivity and specificity (Table 2). Therefore, our results will certainly need to be validated in a larger population, including subjects at high risk of CIN such as those with advanced CKD, volume depletion and hemodynamic instability. Furthermore, it is a single-centre uncontrolled study of adult hospitalized patients undergoing elective CT scan. Finally, despite strict exclusion criteria, some confounding variables such as diabetes and pre-existing, mainly mild, renal insufficiency are present in our study population. However, their influence in study results is unlikely to be clinically significant. Interestingly, no difference in any variable was found between participants with and without diabetes. In any case, our study population is largely representative of hospitalized patients undergoing elective contrast-enhanced CT.
In conclusion, our study results showed that plasma NGAL measured by rapid, standardized assay appears to be a powerful early predictive biomarker for CIN among hospitalized patients undergoing elective contrast-enhanced CT. Although a growing body of evidence suggests that the incidence of CIN may be less with IV contrast administration than that reported in coronary procedures, the widespread use of contrast-enhanced CT examinations still leaves a sizeable population vulnerable to this event [3, 14]. A considerable incidence of CIN was confirmed in the well-controlled population of our study making early diagnosis even more desirable. Indeed, an increase in plasma NGAL would potentially trigger clinicians to monitor patients more closely, avoid additional nephrotoxins and optimize hydration and renal perfusion to prevent further injury. In any case, prevention would be the cornerstone of management for all patients at risk. However, our ability to prevent CIN is limited by the lack of an early, appropriately sensitive and specific marker of kidney injury [3, 4]. Therefore, timely detection of imminent CIN by an accurate and easy-to-perform procedure as the 6-h plasma Triage® NGAL test we evaluated might enable early initiation of interventions or, at least, promote increased scientific vigilance to improve the long-term adverse outcomes associated with this rather common clinical complication.
Conflict of interest statement. None declared.
References
- 1.Pannu N, Wiebe N, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006;295:2765–2779. doi: 10.1001/jama.295.23.2765. [DOI] [PubMed] [Google Scholar]
- 2.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 3.Solomon R. Contrast media nephropathy: how to diagnose and how to prevent? Nephrol Dial Transplant. 2007;22:1812–1815. doi: 10.1093/ndt/gfm207. [DOI] [PubMed] [Google Scholar]
- 4.Nickolas TL, Barasch J, Devarajan P. Biomarkers in acute and chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:127–132. doi: 10.1097/MNH.0b013e3282f4e525. [DOI] [PubMed] [Google Scholar]
- 5.Devarajan P. Neutrophil gelatinase-associated lipocalin: an emerging troponin for kidney injury. Nephrol Dial Transplant. 2008;23:3737–3743. doi: 10.1093/ndt/gfn531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4:265–280. doi: 10.2217/bmm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, et al. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol. 2006;26:287–292. doi: 10.1159/000093961. [DOI] [PubMed] [Google Scholar]
- 8.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, et al. Neutrophil gelatinase-associated lipocalin (NGAL) correlations with cystatin C, serum creatinine and eGFR in patients with normal serum creatinine undergoing coronary angiography. Nephrol Dial Transplant. 2007;22:295–296. doi: 10.1093/ndt/gfl408. [DOI] [PubMed] [Google Scholar]
- 9.Ling W, Zhaohui N, Ben H, et al. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108:c176–c181. doi: 10.1159/000117814. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch R, Dent C, Pfriem H, et al. NGAL Is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–2095. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- 11.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Rao QA, Newhouse JH. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysis. Radiology. 2006;239:392–397. doi: 10.1148/radiol.2392050413. [DOI] [PubMed] [Google Scholar]
- 13.Rudnick MR, Goldfarb S, Tumlin J. Contrast-induced nephropathy: is the picture any clearer? Clin J Am Soc Nephrol. 2008;3:261–262. doi: 10.2215/CJN.04951107. [DOI] [PubMed] [Google Scholar]
- 14.Solomon R. Contrast-induced acute kidney injury: is there a risk after intravenous contrast? Clin J Am Soc Nephrol. 2008;3:1242–1243. doi: 10.2215/CJN.03470708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dent CL, Ma Q, Dastrala S, et al. Plasma NGAL predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Breyer Lewis J, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541. doi: 10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 18.Finn WF. The clinical and renal consequences of contrast-induced nephropathy. Nephrol Dial Transplant. 2006;21(Suppl. 1):i2–i10. doi: 10.1093/ndt/gfl213. [DOI] [PubMed] [Google Scholar]
- 19.McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5:211–219. doi: 10.2215/CJN.04240609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon RJ, Mehran R, Natarajan MK, et al. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol. 2009;4:1162–1169. doi: 10.2215/CJN.00550109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell AM, Jones AE, Tumlin JA, et al. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol. 2010;5:4–9. doi: 10.2215/CJN.05200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisbord SD, Mor MK, Resnick AL, et al. Incidence and outcomes of contrast-induced AKI following computed tomography. Clin J Am Soc Nephrol. 2008;3:1274–1281. doi: 10.2215/CJN.01260308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett B, Thomsen H, Katzberg R. Nephrotoxicity of low-osmolar iopamidol vs iso-osmolar iodixanol in renally impaired patients: The IMPACT study. Invest Radiol. 2006;41:815–821. doi: 10.1097/01.rli.0000242807.01818.24. [DOI] [PubMed] [Google Scholar]
- 24.Thomsen HS, Morcos SK, Erley CM, et al. Investigators in the Abdominal Computed Tomography: The ACTIVE trial: comparison of the effects on renal function of iomeprol-400 and iodixanol-320 in patients with chronic kidney disease undergoing abdominal computed tomography. Invest Radiol. 2008;43:170–178. doi: 10.1097/RLI.0b013e31815f3172. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn M, Chen N, Sahani DV, et al. The PREDICT study: A randomized double-blind comparison of contrast-induced nephropathy after low- or isoosmolar contrast agent exposure. Am J Radiol. 2008;191:151–157. doi: 10.2214/AJR.07.3370. [DOI] [PubMed] [Google Scholar]
- 26.Fassett RG, Venuthurupalli SK, Gobe GC, et al. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]