See Aizenstein and Klunk for a scientific commentary on this article (doi:10.1093/brain/awv001).
In a 3-year longitudinal study, Huijbers et al. observe increased hippocampal activation, faster rates of hippocampal atrophy, and clinical progression in patients with mild cognitive impairment with elevated levels of amyloid-β. Amyloid-β accumulation likely contributes to abnormal neuronal activity on the trajectory towards Alzheimer’s disease dementia.
Keywords: amyloid deposition, MCI, hippocampal activation, functional MRI, clinical progression
Abstract
Cross-sectional functional magnetic resonance imaging studies using a memory task in patients with mild cognitive impairment have produced discordant results, with some studies reporting increased hippocampal activity—consistent with findings in genetic at-risk populations—and other studies reporting decreased hippocampal activity, relative to normal controls. However, previous studies in mild cognitive impairment have not included markers of amyloid-β, which may be particularly important in prediction of progression along the Alzheimer’s disease continuum. Here, we examine the contribution of amyloid-β deposition to cross-sectional and longitudinal measures of hippocampal functional magnetic resonance imaging activity, hippocampal volume, global cognition and clinical progression over 36 months in 33 patients with mild cognitive impairment. Amyloid-β status was examined with positron emission tomography imaging using Pittsburg compound-B, hippocampal functional magnetic resonance imaging activity was assessed using an associative face-name memory encoding task, and hippocampal volume was quantified with structural magnetic resonance imaging. Finally global cognition was assessed using the Mini-Mental State Examination and clinical progression was assessed using the Clinical Dementia Rating (Sum of Boxes). At baseline, amyloid-β positive patients with mild cognitive impairment showed increased hippocampal activation, smaller hippocampal volumes, and a trend towards lower Mini-Mental State Examination scores and higher Clinical Dementia Ratings compared to amyloid-β negative patients with mild cognitive impairment. Longitudinally, amyloid-β positive patients with mild cognitive impairment continued to show high levels of hippocampal activity, despite increasing rates of hippocampal atrophy, decline on the Mini-Mental State Examination and faster progression on the Clinical Dementia Ratings. When entered simultaneously into the same linear mixed model, amyloid-β status, hippocampal activation, and hippocampal volume independently predicted clinical progression. These results indicate that amyloid-β positive patients with mild cognitive impairment are more likely on a path towards Alzheimer’s disease dementia than amyloid-β negative patients. Increased hippocampal activity is discussed in relation to neuronal compensation and/or amyloid-β induced excitoxicity.
Introduction
Alzheimer’s disease is a progressive neurodegenerative disorder characterized by amyloid-β plaques and neurofibrillary tangles on neuropathology (Braak and Braak, 1991). Mild cognitive impairment (MCI) is often considered a prodromal stage of Alzheimer’s disease, but patients with MCI are heterogeneous in their rates of progression towards Alzheimer’s disease dementia. Similarly, both autopsy and biomarker studies using PET amyloid-β imaging or CSF assays suggest that a substantial proportion of patients with MCI do not have evidence of Alzheimer’s disease pathology as the aetiology of their cognitive impairment (Jack et al., 2008; Albert et al., 2011). Interestingly, previous studies investigating the pattern of neural activity using task-related functional MRI in MCI have yielded discordant findings (Ewers et al., 2011; Sperling, 2011), ranging from increased hippocampal activity concordant with findings in genetic-at-risk asymptomatic individuals, to decreased hippocampal activity, similar to patients with Alzheimer’s disease dementia.
Several functional MRI studies have associated increased hippocampal activity with increased risk for Alzheimer’s disease (Ewers et al., 2011; Sperling, 2011). Cognitively normal older adults who carry the apolipoprotein E ε4 (APOE4) allele are more likely to harbour amyloid-β and demonstrate increased hippocampal activity during an associative memory task (Bookheimer et al., 2000; Bondi et al., 2005; Johnson, 2006; Trivedi et al., 2008b; Dennis et al., 2010). Also, autosomal dominant mutation carriers, who develop Alzheimer’s disease with almost 100% certainty, show increased hippocampal activity during an associative memory task when compared to non-carrier family members who lack the mutation (Quiroz et al., 2010; Reiman et al., 2012). In MCI, increased hippocampal activity has also been linked to cortical thinning (Putcha et al., 2011a) and clinical progression (O'Brien et al., 2010). In animal models of Alzheimer’s disease, release of soluble amyloid-β can trigger aberrant synaptic activity, resulting in hyperactivity (Palop and Mucke, 2010). Treatment of hyperactivity in human patients with MCI, or in mice with pathological levels of amyloid-β, using the anti-epileptic drug levetiracetam reduces hippocampal activity and improves memory (Bakker et al., 2012; Sanchez et al., 2012). In cognitively normal older adults with high levels of amyloid-β deposition, increased functional MRI activity has often been linked to neuronal compensation for Alzheimer’s disease pathology (Sperling et al., 2009; Mormino et al., 2012; Elman et al., 2014). Together, these studies provide compelling evidence that increased hippocampal activity occurs transiently early in the course of Alzheimer’s disease, but it remains unclear whether this aberrant activity represents a marker of neuronal compensation, and/or evidence of neuronal excitotoxicity. Here we investigate amyloid-β deposition, and longitudinal measures of hippocampal activity, hippocampal atrophy, cognition and clinical progression in MCI to elucidate these relationships.
Cross-sectional functional MRI studies have reported mixed results in MCI. Several studies have found increased hippocampal activity during an associative memory task (Dickerson et al., 2004; Celone et al., 2006; Hämäläinen et al., 2007; Kircher et al., 2007; Lenzi et al., 2011), while several other studies have not (Small et al., 1999; Machulda et al., 2003; Petrella et al., 2006; Trivedi et al., 2008a; Hanseeuw et al., 2011). Previously, increased hippocampal activity has been linked to clinical progression in MCI in the absence of known levels of amyloid-β deposition (O'Brien et al., 2010). Interestingly, PET studies indicate that only half to two-thirds of patients with amnestic MCI show elevated amyloid-β deposition. Furthermore, patients with MCI with low levels of amyloid-β deposition are less likely to progress to Alzheimer’s disease dementia when compared to patients with MCI with elevated levels of amyloid-β (Jack et al., 2009; Wolk et al., 2009; Petersen et al., 2013). Therefore, we hypothesized that increased hippocampal activity contributes to clinical progression in MCI primarily in the presence of elevated levels of amyloid-β deposition.
Here, we examined the contribution of amyloid-β deposition, assessed with PET amyloid imaging, to cross-sectional and longitudinal measures of hippocampal functional MRI activity, hippocampal atrophy and clinical progression over 36 months. We hypothesized that patients with MCI with elevated amyloid-β deposition (Aβ+ MCI) would show increased hippocampal activity during an associative memory encoding task (Sperling et al., 2003) when compared to patients with MCI without evidence of amyloid-β deposition (Aβ− MCI). Additionally, we hypothesized that Aβ+ MCIs would show faster rates of hippocampal atrophy (Reuter et al., 2012), decline in global cognition (Folstein et al., 1975) and clinical progression (Petersen, 2004) over time than Aβ− MCIs.
Materials and methods
Thirty-three older adults with MCI with baseline amyloid PET and longitudinal clinical and structural and functional MRI data from an ongoing study on ageing and Alzheimer’s disease were included in the current study (Fig. 1 and Table 1). We utilized criteria from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) to recruit a range of early and late patients with MCI. All patients with MCI met ADNI criteria for amnestic MCI, single or multiple domain. The Wechsler Memory Scale-Revised Logical Memory Test delayed recall (LMIIa) was used to assess objective memory, using an education adjusted cut-off, as previously described (Marshall et al., 2013). The patient, and/or study partner, reported a memory complaint, but had essentially intact activities of daily living, as assessed by the Functional Activities Questionnaire and Clinical Dementia Rating (CDR), and no evidence of dementia. Criteria included a Mini-Mental State Examination (MMSE) score between 24 and 30 (Folstein et al., 1975), CDR global score of 0.5 (Petersen, 2004), and a Memory Box score 0.5. The LMIIa, MMSE and CDR scores are reported at baseline and final assessment (Table 1). Patients with MCI were medically stable, and did not have apparent cofounding neurological conditions, substance or alcohol abuse within the past 2 years, or primary psychiatric diagnoses (e.g. major depressive disorder) within the past 2 years. All MCI patients had a Modified Hachinski Ischaemic Score ≤ 4 and a Geriatric Depression Scale (long form: 30 items) ≤ 10 (Rosen et al., 1980; Yesavage et al., 1982). Written informed consent was obtained prior to experimental procedures and the study was approved, and conducted, in accordance with the Partners Human Research Committee at the Massachusetts General Hospital and Brigham and Women Hospital (Boston, MA). The patients with MCI were followed longitudinally for up to 3 years (36 months). MMSE was used to quantify global cognition and CDR Sum of Boxes score (CDRSB) to quantify clinical progression. Additionally, to assess clinical diagnosis, two experienced clinicians (R.A. and G.M.) held a consensus meeting to evaluate if a patient at a given time met criteria for Alzheimer’s disease dementia, by reviewing performance on CDR, neuropsychological testing (LMIIa and MMSE) and the Functional Activities Questionnaire (instrumental activities of daily living). Clinicians were blinded to the neuroimaging data.
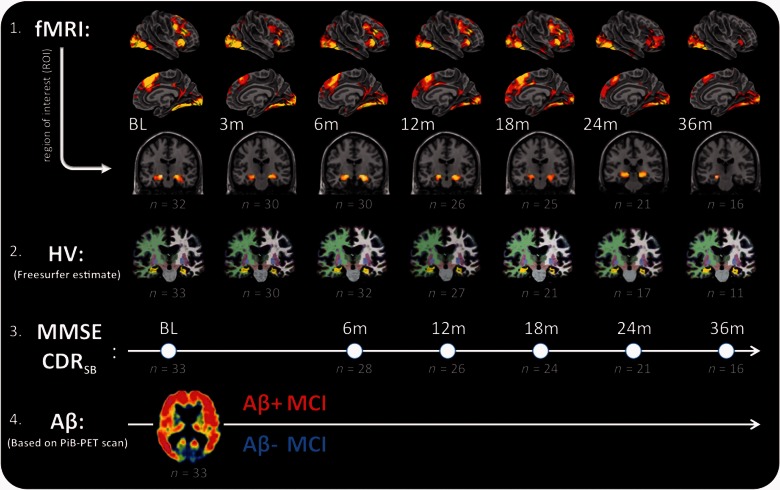
Figure 1.
Schematic representation of data collected in patients with mild cognitive impairment. From top to bottom: (1) Functional MRI (fMRI) collected during an associative face-name encoding task; (2) hippocampal volume (HV), (3) Mini-Mental State Examination (MMSE) and Clinical Dementia Rating Sum of Boxes score (CDRSB); and (4) amyloid-β PET imaging (Aβ). Functional MRI: shows brain activity depicted in yellow/red projected on an inflated brain for right hemisphere, midline and a sagittal slice with the hippocampus, shown at P < 0.001 (uncorrected) from the contrast novel-repeated at baseline (BL), 3, 6, 12, 18, 24 and 36 months (m). Hippocampal volume: shows the anatomical T1-weighted scans of one MCI patient, analysed with the longitudinal FreeSurfer pipeline and used to estimate grey matter volume of the hippocampus (shown in yellow). MMSE/CDRSB: shows time points of neuropsychological examination matched to the closest MRI visit. Amyloid-β: shows an example of an Aβ+ PET scan, using PiB. PET data acquisition was collected near baseline (4 months) and used to identify Aβ− and Aβ+ patients with MCI. Below each visit number of observations (n) are shown in grey for at each visit separately for functional MRI, hippocampal volume, CDRSB and the amyloid-β PET scans.
Table 1.
Demographics, baseline and final assessment
| Aβ− MCI | Aβ+ MCI | |||
|---|---|---|---|---|
| Baseline assessment | n | 16 | 17 | |
| gender (% female) | 19 | 24 | chi = 0.57 | |
| age | 72 ± 1.91 | 74 ± 2.03 | P = 0.57 | |
| years of education | 16 ± 0.64 | 17 ± 0.51 | P = 0.21 | |
| APOE (% ε4 carriers) | 38 | 50 | chi = 0.58a | |
| Amyloid-β (PiB/FLR) | 1.1 ± 0.02 | 1.6 ± 0.05 | P < 0.001b | |
| CDR | 0.5 | 0.5 | – | |
| LMIIa | 7.6 ± 1.32 | 5.0 ± 0.89 | P = 0.12 | |
| MMSE | 27.9 ± 0.33 | 26.9 ± 0.47 | P = 0.11 | |
| Hippocampal fMRI | 0.15 ± 0.037 | 0.31 ± 0.058 | P = 0.024 | |
| Hippocampal volume | 7197 ± 281 | 6287 ± 238 | P = 0.019 | |
| CDRSB | 1.3 ± 0.16 | 1.9 ± 0.25 | P = 0.055 | |
| Final assessment | LMIIa | 10.25 ± 1.48 | 6.1 ± 1.6 | P = 0.064 |
| MMSE | 27.9 ± 0.51 | 26.2 ± 0.54 | P = 0.037 | |
| Hippocampal fMRI | 0.10 ± 0.038 | 0.27 ± 0.068 | P = 0.039 | |
| Hippocampal volume | 7045 ± 285 | 6009 ± 273 | P = 0.013 | |
| CDRSB | 1.5 ± 0.22 | 3.2 ± 0.41 | P = 0.001 |
APOE = carriers of apolipoprotein E (APOE) ε4 allele; LMIIa = the Wechsler Memory Scale-Revised Logical Memory Test delayed recall; MMSE = Mini-Mental State Examination; CDRSB = Clinical Dementia Rating Sum of Boxes score; PiB = Pittsburg Compound-B (PiB) retention in neocortical regions of interest comprised of frontal, lateral temporal and retrosplenial (FLR) cortex.
P-values denote t-test or chi-squares test.
aAPOE genotype available in 23 MCIs.
bUsed to catogorize Aβ−/Aβ+ MCI groups.
MRI acquisition
Thirty-three patients with MCI were included in our analyses. Figure 1 lists the number of anatomical MRIs (MPRAGE), functional MRI sessions and MMSE/CDR assessments included after quality control. MRI data were acquired at the Athinoula A. Martinos Centre for Biomedical Imaging at baseline, 3, 6, 12, 18, 24 and 36 months. At each visit, we acquired anatomical and functional MRI data using a Siemens Trio 3 T system with a 12-channel phased-array head coil. Foam pads restricted motion of the head. The anatomical MRI consisted of a T1-weighted MPRAGE (isotropic 1 mm, repetition time = 2300 ms, echo time = 2.98 ms, inversion time = 900 ms, trigger delay = 600 ms, 9° flip angle, 160 sagittal contiguous slices, right to left). Functional MRI images were acquired using gradient T2*-weighted echo-planar imaging (EPI) sequence with a time of repetition of 2000 ms, echo time of 30 ms, and a 90° flip angle. EPI scans included 30 oblique coronal slices, interleaved, 5 mm thick, with 1 mm gap between slices; slices oriented perpendicular to the AC–PC line, providing whole brain coverage extending from occipital to frontal poles. The in-plane resolution was 3.125 mm, resulting in an effective voxel size of 3.125 × 3.125 × 6.0 mm. Each functional run consisted of 127 repetition times. Prior to each EPI run, five images were acquired and discarded to allow longitudinal magnetization to reach equilibrium. Each functional MRI visit consisted of a maximum of six functional runs (of 4:40 min each). Occasionally, due to time constrains or patient discomfort, we collected fewer runs. During preprocessing of the functional MRI data, we screened each individual run for head-motion and signal-to-noise and removed runs that did not meet quality criteria. Runs with translation >5 mm, or rotation >5°, where not included. Within a run, bad volumes were identified by a global signal difference >2.5 standard deviations for the run, translational movement exceeding 0.75 mm or rotational movement exceeding 1.5° per repetition time. Runs with >20 bad volumes were not included. In addition, we correlated the motion regressors with the SPM task regressors and removed runs with R2 > 0.25. Previously, we demonstrated that functional MRI data using this exact paradigm are relatively reliable with only two runs (Putcha et al., 2011b). No functional MRI visits with fewer than two functional runs of high quality data were included in the analysis. The quality control resulted in the complete exclusion of data from four patients with MCI and was performed prior to examination of neuropsychological and PET data.
Associative memory encoding task
Inside the MRI scanner, patients with MCI performed an associative memory task to probe associative encoding of face–name pairs, as previously described (Celone et al., 2006; Sperling et al., 2009; O'Brien et al., 2010; Putcha et al., 2011b). In brief, subjects completed up to six functional runs within each imaging visit. Each run consisted of two encoding blocks with seven novel face-name pairs (84 pairs in total) and two blocks with repeated, alternating, face–name pairs and blocks of passive fixation. Faces were presented for 4500 ms on a black background with a fictional first name printed in white letters underneath. During the presentation of each face–name pair, subjects were asked to indicate, via a fibre-optic button box, whether the name was a ‘good’ name for the face or not, a subjective task designed to enhance associative memory encoding (Sperling et al., 2003). Before each run, subjects were explicitly instructed to try to remember the name that was associated with each face. The intertrial interval consisted of visual fixation, jittered using OptSeq2 (Dale, 1999), varying in duration from 300–2200 ms. Between each block, a fixation cross was presented for 25 s. Stimuli were presented using MacStim 2.5 (WhiteAnt Occasional Publishing, Melbourne, Australia). Visual images were projected onto a screen at the end of the scanner bore and viewed in a mirror attached to the head coil.
Functional MRI analysis
The functional MRI images were preprocessed and analysed using SPM8 (UCL, http://www.fil.ion.ucl.ac.uk/spm). The functional images for each visit were slice time-corrected, realigned and resized to 3 × 3 × 3 mm isotropic voxels. A within-subject average was created for each subject using the realigned scans across all visits. This within-subject average was normalized to the standard SPM MNI template, and the resulting normalization parameters were subsequently applied to all scans from all visits, in the same manner as the FreeSurfer longitudinal pipeline for anatomical data (Reuter et al., 2012) to avoid a normalization bias towards a single visit. Next, the normalized time-series were smoothed with an 8 mm full-width half-maximum Gaussian kernel and high-pass filtered (1/260 Hz).
Using the general linear model (GLM), as implemented in SPM8, we modelled novel and repeated blocks by convolving the onset and block duration with the canonical haemodynamic response function. Individual beta-maps were calculated for novel versus repeated blocks. Next, we used SPM8 in combination with in-house MATLAB scripts to create group-level maps (GLM-Flex, http://mrtools.mgh.harvard.edu/, Harvard Aging Brain Study, Martinos Centre, MGH, USA). Regions of interest were defined in a manner consistent with our previous work (O'Brien et al., 2010). First, we used a one-sample t-test across baseline functional MRI data to identify activity peaks within the medial temporal lobe that showed the greatest difference between novel and repeated items (left hippocampus: Tmax = 6.27, MNIx,y,z = −18, −13, −13 and right hippocampus: Tmax = 5.66, MNIx,y,z = 21, −16, −13). At these locations, we extracted the beta-estimates for each subject’s visit using 5-mm radius spheres. Next, we calculated a single hippocampal estimate, by averaging across hemispheres. These hippocampal estimates were used to quantify baseline functional MRI activity and change in longitudinal functional MRI activity.
Anatomical MRI analysis
The anatomical MRI data were analysed using FreeSurfer v5.1 longitudinal pipeline (Dale et al., 1999; Reuter et al., 2012). Briefly, each anatomical scan was normalized to an individual within-subject template based on all data and therefore not biased to a single visit (Reuter et al., 2012). Anatomic regions of interest were then defined in template space, and warped to subject space at each visit. The white matter and pial surface segmentation was examined visually for quality assessment using FreeSurfer tools. In cases where dura or skull influenced the segmentation, voxels were either manually edited or corrected by adjusting the watershed threshold. The preprocessing steps were subsequently re-run on the edited files and re-evaluated. This cumulative quality assessment was iterated until the segmentation results were deemed either sufficient or irreparable for cases with poor T1-weighted MPRAGE images. From each of these FreeSurfer parcellations, we extracted hippocampal volume and normalized it by estimated total intracranial volume using simple regression (Mathalon et al., 1993; Buckner et al., 2004).
Amyloid PET imaging
Amyloid PET imaging data were acquired on average 4.2 ± 1.4 months after the baseline clinical visit. Deposition of amyloid-β was measured by PET using Pittsburg compound-B (N-methyl-[11C]-2(4-methylaminophenyl)-6-hydroxybenzothiazole) according to previously described methods (Johnson et al., 2007). Briefly, we acquired 60 min of dynamic PET data following intravenous administration of 11C-PiB (Pittsburg compound-B) using an HR+ PET camera (Siemens) operating in 3D mode (63 image planes; 15.2-cm axial field of view; 5.6-mm transaxial resolution; 2.4-mm slice interval; 69 frames: 12 × 15 s, 57 × 60 s). PET data were reconstructed and corrected for attenuation using standard Siemens software. Each frame was evaluated for head motion and adequate count statistics. Using Logan’s graphical analysis method, we calculated PiB retention expressed as a distribution volume ratio (DVR) using a grey matter cerebellum reference region (Price et al., 2005). Neocortical amyloid-β deposition was quantified using an aggregate DVR from a set of regions that comprised most of the association cortex, including frontal, lateral parietal, lateral temporal and retrosplenial cortex. MCIs were classified as amyloid positive (Aβ+) or amyloid negative (Aβ−) using a DVR threshold of 1.20. This threshold was determined by a Gaussian mixture modelling approach (Mormino et al., 2014) on an independent data sample with an identical amyloid PiB-PET protocol (Johnson et al., 2007) collected in the Harvard Aging Brain study.
Statistical analysis
The cross-sectional baseline results were modelled using two-sample t-tests (two-sided). Longitudinal effects were estimated using linear mixed models, as implemented in R v3.0.1 and the companion to Applied Regression Toolbox (Fox and Weisberg, 2011). For each dependent variable (functional MRI, hippocampal volume, MMSE, CDRSB), we conducted three separate models (Table 2). The first model included only time. The second model included time, amyloid-β and the interaction Time × Amyloid-β. The third model included time, amyloid-β, age, sex and the interactions Time × Amyloid-β, Time × Age and Time × Sex. Amyloid-β and sex were modelled dichotomously. Age, functional MRI, hippocampal volume, CDRSB and MMSE were modelled continuously. Amyloid-β and sex are modelled dichotomously. Aβ−MCIs were used as the reference group for amyloid-β. Females are used as the reference group for sex. Age, as a control variable, was centred at the group mean. All models included a random intercept. To visualize the slopes (Fig. 3), we interpolated missing time points in the longitudinal data using a linear estimate as implemented in the R package zoo (Zeileis and Grothendieck, 2005). The interpolated values were not included in the linear mixed models or any of the statistical analyses.
Table 2.
Predictive models of longitudinal change in functional MRI, hippocampal volume, MMSE and CDR
| Dependent variable: |
Functional MRI |
Hippocampal volume |
MMSE |
CDRSB |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Number of observations: |
180 |
171 |
147 |
148 |
|||||
| Estimate | P-value | Estimate | P-value | Estimate | P-value | Estimate | P-value | ||
| I | LM models with only Time: | −0.029 | 0.062# | −115.440 | <0.001*** | −0.267 | 0.028* | 0.303 | <0.001*** |
| II | LM models with amyloid-β: | ||||||||
| Time | −0.016 | 0.539 | −190.980 | <0.001*** | −0.604 | 0.001** | 0.62 | <0.001*** | |
| Amyloid-β | 0.144 | 0.044* | −885.696 | 0.024* | −1.198 | 0.079# | 0.60 | 0.012* | |
| Amyloid-β × Time | 0.019 | 0.543 | −125.880 | <0.001*** | −0.565 | 0.019* | 0.56 | <0.001*** | |
| III | LM models with amyloid-β, age, sex: | ||||||||
| Time | −0.013 | 0.572 | −224.995 | <0.001*** | −0.384 | 0.755 | 0.654 | 0.085# | |
| Amyloid-β | 0.153 | 0.042* | −744.046 | 0.029* | −1.244 | 0.079# | 0.648 | 0.092# | |
| Age | −0.003 | 0.482 | −50.623 | 0.023* | 0.027 | 0.549 | −0.034 | 0.177 | |
| Sex | 0.035 | 0.698 | 1242.721 | 0.004** | −0.279 | 0.745 | −0.082 | 0.860 | |
| Amyloid-β × Time | 0.014 | 0.689 | −127.572 | <0.001*** | −0.613 | 0.017* | 0.575 | <0.001*** | |
| Age × Time | 0.001 | 0.616 | 1.271 | 0.473 | 0.000 | 0.997 | −0.004 | 0.571 | |
| Sex × Time | −0.007 | 0.850 | 41.070 | 0.202 | −0.314 | 0.299 | −0.035 | 0.802 | |
The top row shows estimates from four linear mixed-effects (LM) models (Model I) with only time across all patients with MCI. The middle row shows estimates from four linear mixed models (Model II) with time (in years) and amyloid-β group as predictors without covariates. The bottom row shows the estimate from four linear mixed models (Model III) with additional covariates for age, gender and their effect by time. The first column shows models with hippocampal functional MRI activity as the dependent variable. The second column shows models with adjusted hippocampal volume, the third column shows models with Mini-Mental State Exam (MMSE) scores and the fourth column with Clinical Dementia Ratings Sum of Boxes scores (CDRSB).
Amyloid-β and sex are a dichotomous variable and age, functional MRI and hippocampal volume are continuous. Aβ− MCIs were used as the reference group for amyloid-β. Females are used as the reference group for sex. Age, as a control variable, was centred at the group mean. Significant at #P < 0.10 (trending), *P < 0.05, **P < 0.01 and ***P < 0.001.
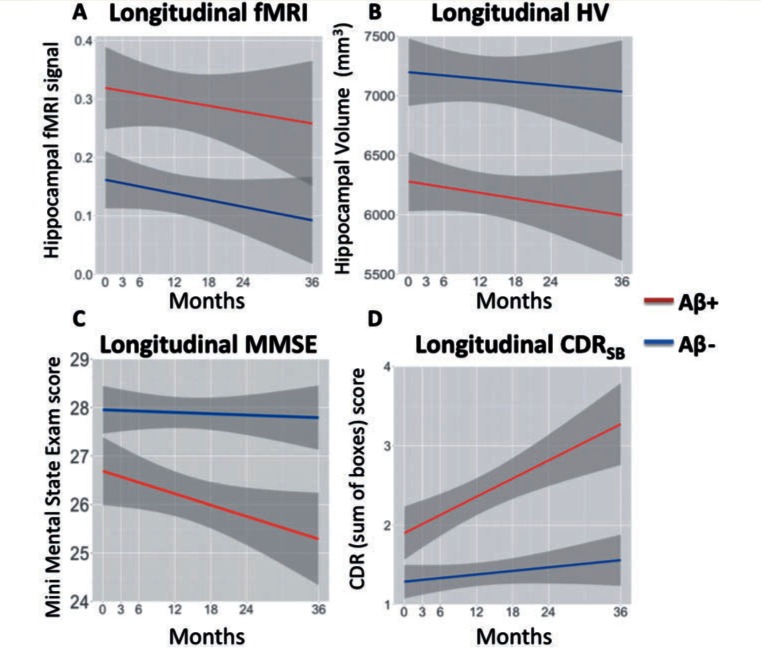
Figure 3.
Longitudinal results. (A) Estimates from linear mixed models in functional MRI (fMRI) activity. The y-axis contains the average beta-estimates from both left and right hippocampus. (B) Estimates in hippocampal volume (HV). The y-axis contains total hippocampal volume in mm3 of both left and right hippocampus. (C) Estimates in Mini-Mental State Examination (MMSE), with lower scores representing lower cognition. (D) Estimates in Clinical Dementia Rating Sum of Boxes score (CDRSB), with higher score representing greater clinical deficits. The x-axis shows time in months. The Aβ− MCI group is shown in blue and Aβ+ MCI in red. Grey area denotes the standard error of the mean.
Results
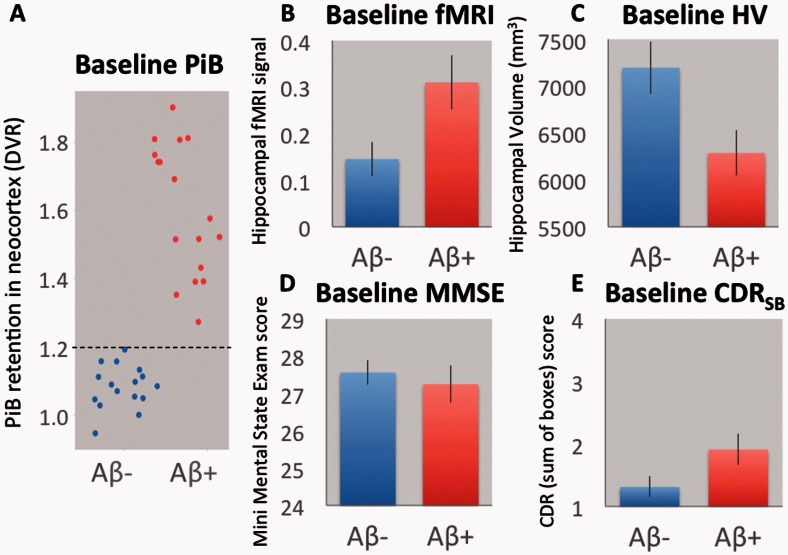
The Aβ− and Aβ+ MCI groups did not differ in terms of age, sex and years of education (Table 2). Note that a global CDR score of 0.5 was a study inclusion criterion to define MCI (Petersen, 2004). At baseline (Fig. 2), Aβ+ MCIs, showed greater hippocampal functional MRI activity (P = 0.024), smaller hippocampal volume (P = 0.019), and a trend towards lower MMSE scores (P = 0.11) and worse CDRSB (P = 0.055), as indicated by two-sample t-tests. Consistent with the MMSE/CDRSB findings, Aβ + MCIs also trended towards worse performance on LMIIa (P = 0.12).
Figure 2.
Baseline results. (A) A scatter plot of PiB retention values at baseline. The y-axis contains the neocortical PiB retention values, normalized by a DVR. The black line indicates the 1.20 cut-off used to classify Aβ+ and Aβ− MCIs. (B) Baseline functional MRI (fMRI) activity. The y-axis contains the average beta-estimate from both left and right hippocampus. (C) Average baseline hippocampal volume (HV). The y-axis contains the total hippocampal volume in mm3 of both left and right hippocampus. (D) Baseline Mini-Mental State Examination scores (MMSE). (E) Baseline Clinical Dementia Rating Sum of Boxes scores (CDRSB). The Aβ− MCI group is shown in blue and Aβ+ MCI in red. Error bars denote the standard error of the mean.
Next, we examined longitudinal change in hippocampal functional MRI activity, hippocampal volume, MMSE and CDRSB over 36 months using linear mixed models (Fig. 3). We examined whether amyloid-β status was associated with different rates of change for hippocampal functional MRI, hippocampal volume, MMSE and CDRSB (Table 2). Models were conducted without covariates (Model I), models with amyloid-β group (Model II) and models with amyloid-β group including covariates controlling for age and sex (Model III). There was a trend towards longitudinal hippocampal functional MRI activity to decrease over time across all subjects (P = 0.062), similar to a previous report that did not include amyloid-β PET imaging (O'Brien et al., 2010). We found a main effect of baseline amyloid-β, consistent with the cross-sectional baseline results, such that Aβ+ MCIs had increased activity in the hippocampus (P = 0.044) compared to Aβ−. We did not observe an interaction between time and amyloid-β (P = 0.543), suggesting that the change in functional MRI activity over time was not different across amyloid-β groups. Longitudinal measures of hippocampal volume also significantly decreased over time across all subjects (P < 0.001). We observed an interaction between time and baseline amyloid-β (P < 0.001), such that Aβ+ MCIs showed a higher rate of hippocampal atrophy over time. Longitudinal measures of MMSE decreased over time across all subjects (P = 0.028). We observed an interaction between time and baseline amyloid-β (P = 0.019), such that Aβ+ MCIs showed greater cognitive decline on the MMSE over time. A model that included covariates for age and sex showed a similar pattern (Table 2). Longitudinal measures of CDRSB increased over time across all subjects (P < 0.001). We observed an interaction between time and baseline amyloid-β (P < 0.001), such that Aβ+ MCIs showed a greater progression on CDRSB over time. Similar patterns were observed across all models when age and sex were included as covariates (Table 2).
Finally, to determine the relative contributions of these markers to prediction of longitudinal clinical progression, baseline amyloid-β group, baseline hippocampal functional MRI, baseline hippocampal volume, baseline age and baseline sex, as well as their interactions with time, were all modelled as simultaneous predictors of change in CDRSB over time. We found main effects of baseline hippocampal volume (P = 0.025) and baseline age (P = 0.012), such that a smaller hippocampus and higher age where associated with worse CDRSB (Table 3). Importantly, we observed an interaction between time and amyloid-β group (P < 0.001), such that Aβ+ MCIs showed a greater progression on CDRSB over time. These results demonstrate that amyloid-β group was the strongest independent predictor of clinical progression (Table 3). In addition, we also observed an interaction between time and baseline functional MRI (P = 0.027), such that baseline hippocampal activation was associated with greater clinical progression on CDRSB. These latter results demonstrate that functional MRI showed an independent contribution to clinical progression, even with amyloid-β in the model.
Table 3.
Predictive model of clinical progression
| Dependent variable: | CDRSB |
|
|---|---|---|
| Number of observations: | 143 |
|
| t-value | P-value | |
| Time | 0.408 | 0.684 |
| Amyloid-β | 0.590 | 0.560 |
| Functional MRI | 0.818 | 0.421 |
| Hippocampal volume | 2.399 | 0.024* |
| Age | 2.712 | 0.012* |
| Sex | 0.923 | 0.365 |
| Amyloid-β × Time | 4.740 | <0.001*** |
| Functional MRI × Time | 2.249 | 0.027* |
| Hippocampal volume × Time | 0.831 | 0.408 |
| Age × Time | 0.254 | 0.800 |
| Sex × Time | 0.011 | 0.991 |
Table shows estimates from a linear mixed model with Clinical Dementia Ratings Sum of Boxes score (CDRSB) as the dependent variable. Predictors include time (in years), amyloid-β group, hippocampal functional MRI activity, hippocampal volume, age, gender and their effects by time.
Amyloid-β and sex are a dichotomous variable and age, functional MRI and hippocampal volume are continuous.
Significant at *P < 0.05 and ***P < 0.001.
During the 3-year longitudinal study, 9 of 33 patients with MCI progressed to meet clinical criteria for the diagnosis of Alzheimer’s disease dementia. From enrolment, the average time of clinical progression to dementia was 19.3 ± 3.9 months. Seven of 9 of the MCI patients who progressed were Aβ+ at baseline. The demographics for stable MCI and progressive MCIs, baseline data and data obtained at final assessment are included in Supplementary Table 1.
Discussion
In this study, we first examined the cross-sectional relationship of amyloid-β deposition with hippocampal functional MRI activity in patients with MCI. Secondly, we examined the influence of amyloid-β deposition on 3-year longitudinal measures of hippocampal functional MRI activity, hippocampal atrophy, cognition, and functional clinical progression. At baseline, we found that patients with MCI with high amyloid-β deposition showed increased hippocampal activity, smaller hippocampal volumes and slightly greater functional impairment compared to MCI with low amyloid-β deposition. Longitudinally, we found that Aβ+ MCIs showed persistent greater functional MRI activity, higher rates of hippocampal atrophy, and greater cognitive decline on the MMSE and faster clinical progression on CDRSB. Finally, we investigated the contribution of all of these imaging markers to the prediction of clinical progression. We found that amyloid-β deposition was the strongest predictor. However, we found an independent contribution of functional MRI, such that greater hippocampal activity was associated with faster clinical progression, even when accounting for amyloid and hippocampal volume. Below we discuss our findings in the context of other cross-sectional and longitudinal studies.
Increased functional MRI activity in mild cognitive impairment
At baseline, we found increased hippocampal activity in Aβ+ MCIs compared to Aβ− MCIs. It is possible that amyloid status may at least partially explain the discrepancies between several cross-sectional functional MRI studies that found hyperactivity in MCI (Dickerson et al., 2004; Johnson et al., 2004; Celone et al., 2006; Hämäläinen et al., 2007; Kircher et al., 2007; Lenzi et al., 2011), and those studies that did not (Small et al., 1999; Machulda et al., 2003; Johnson et al., 2006; Petrella et al., 2006; Trivedi et al., 2008b; Hanseeuw et al., 2011). These previous studies did not measure amyloid-β deposition, and therefore might differ in their relative number of Aβ+ and Aβ− MCIs included in their cohorts. Previous studies have found that APOE Ɛ4 carriers show relative hyperactivity in the hippocampus (Johnson, 2006; Bookheimer et al., 2000; Bondi et al., 2005; Filippini et al., 2009; Dennis et al., 2010) and APOE Ɛ4 is known to increase the likelihood of amyloid positivity (Johnson et al., 2013). The association between high levels of amyloid-β deposition and increased hippocampal activity is consistent with reports from autosomal dominant genetic at-risk populations. Asymptomatic carriers of the PSEN1 mutation also demonstrate relative hyperactivity in the hippocampus and adjacent cortices during an associative memory task (Quiroz et al., 2010; Braskie et al., 2012; Reiman et al., 2012). Nonetheless, our observational data only provide an associative link between amyloid-β deposition and increased hippocampal activity, since other pathological processes, for example tau accumulation in the medial temporal lobe, also start before the stage of MCI (Nelson et al., 2012) and might be more closely linked to either amyloid-β or increased hippocampal activity. Taken together, our cross-sectional and longitudinal data suggest that amyloid-β deposition may be an important factor that explains at least some of the discrepant findings reported in functional MRI studies in MCI.
In addition to the influence of amyloid-β, the degree of cognitive impairment may influence the presence of increased hippocampal activity. Our cohort includes some MCIs with very mild impairment (early MCI by ADNI criteria), and we have previously speculated that early MCIs may demonstrate increased activity that diminishes with disease progression and loss of hippocampal neurons (Sperling et al., 2010; Ewers et al., 2011). Consistent with a previous longitudinal functional MRI study in a different set of MCI subjects (O'Brien et al., 2010), we reported that greater hippocampal activity at baseline was associated with decreases activity and faster cognitive decline over time. In the present study, we did not observe that rate of decline in functional MRI activity was a predictor of clinical progression. Instead we found that amyloid-β deposition is a key factor and is linked to both increased hippocampal activity and clinical progression.
At baseline, we found that Aβ+ MCIs had smaller hippocampal volumes compared to Aβ− MCIs, a finding consistent with cross-sectional studies (Jack et al., 2008; Mormino et al., 2009; Wolk et al., 2009) and the link between increased risk for Alzheimer’s disease progression in MCIs with smaller hippocampal volumes (Visser et al., 2002; Landau et al., 2010). Consistent with other longitudinal studies, we also found faster rates of hippocampal atrophy in Aβ+ MCIs (Jack et al., 2010; Chetelat et al., 2012; Villemagne et al., 2013; Ossenkoppele et al., 2014). These anatomical results support the view that Aβ+ MCIs are more likely to develop dementia due to Alzheimer’s disease.
The cross-sectional association of smaller hippocampal volume with increased functional MRI activity suggests that grey matter loss alone does not account for the functional MRI results. Both hippocampal activity and volume are influenced by amyloid-β deposition, but in opposite directions. In addition, it is likely that hippocampal volume has an independent influence on hippocampal activity. At baseline across all subjects, smaller hippocampal volume is associated with decreased activity. Longitudinal atrophy of the hippocampus, present in both amyloid-β groups, may partially explain the trend towards decreased hippocampal activity also present in both amyloid-β groups. Thus, we hypothesize that while amyloid-β deposition contributes to increased hippocampal activity, likely beginning prior to the clinical stage of MCI, hippocampal atrophy, increasing over the course of MCI, contributes to decreasing hippocampal activity.
Increased hippocampal activity predicts cognitive decline in the setting of hippocampal atrophy and amyloid-β
The increased hippocampal activity in Aβ+ MCIs support the view that increased neuronal activity and amyloid-β are associated (Palop and Mucke, 2010; Jagust, 2013). Yet, our longitudinal data do not provide evidence of whether initial amyloid-β deposition induces increased hippocampal activity (Palop and Mucke, 2010; Jack et al., 2013) or whether initial hyperactive neurons drive deposition of amyloid-β (Bero et al., 2011; Jagust and Mormino, 2011). In the context of recent longitudinal studies that estimated the rate of amyloid-β accumulation, it is likely that amyloid-β continues to accumulate in Aβ+ MCIs (Vlassenko et al., 2011; Jack et al., 2013; Villemagne et al., 2013). As we only acquired amyloid PET data at baseline, it remains unclear if the rate of amyloid-β accumulation is directly linked to the level of aberrant functional MRI activity. Based on longitudinal studies, it is also likely that Aβ+ MCI began to accumulate amyloid-β years before the start of our study (Morris et al., 2009; Vlassenko et al., 2011; Jack et al., 2013; Roe et al., 2013; Villemagne et al., 2013) and showed increased hippocampal activity, possibly before the clinical stage of MCI. Studies in Aβ+ cognitively normal older adults typically found increased functional MRI activity in the default network (manifesting as failure of deactivation), including the entorhinal cortex, while demonstrating normal levels of activity in the hippocampus (Sperling et al., 2009; Kennedy et al., 2012; Oh and Jagust, 2013; Elman et al., 2014; Huijbers et al., 2014). In early stages of amyloid-β deposition, the default network, including the functionally connected entorhinal cortices, might demonstrate the earliest signs of relative hyperactivity, prior to change in the hippocampus, consistent with our recent cross-sectional observation using a different functional MRI task in a group of clinically normal older individuals (Huijbers et al., 2014). In a previous cross-sectional study (Sperling et al. 2009), we only observed evidence of amyloid-β associated aberrant activity in the default network of clinically normal older individuals (CDR 0) but increases in hippocampal activity were only seen in a small group of individuals with subtle memory impairment (CDR 0.5). Thus, we postulate that increased hippocampal activity, in the setting of amyloid-β deposition, may mark the onset of memory impairment and predict more rapid cognitive decline. Future longitudinal studies in preclinical Alzheimer’s disease (Sperling et al., 2011) will test this hypothesized anatomic progression of aberrant activity, with initial aberrant activity (decreased deactivation) in the default network followed by increased activity in the hippocampus.
Several studies have interpreted increased functional MRI activity in Aβ+ adults as a marker for neuronal compensation (Sperling et al., 2009; Mormino et al., 2012; Oh and Jagust, 2013). A recent study demonstrated compelling evidence for compensation by increased functional MRI activity in Aβ+ cognitively normal older adults (Elman et al., 2014). Elman and colleagues (2014) found increased functional MRI activity in task-positive brain regions and this coincided with more vivid memory encoding, suggesting increased functional MRI activity is linked to maintaining performance in the presence of Alzheimer’s disease pathology. At the stage of MCI, increased hippocampal activity might reflect compensatory activity that occurs relatively late in the Alzheimer’s disease trajectory and could coincide with increased activity in the default network and task-positive regions. Wide-spread co-activation in the hippocampus, default-network and task-positive brain regions is also consistent with de-differentiation theories of ageing (Cabeza, 2002; Park and Reuter-Lorenz, 2009), as brain regions become less specialized. Previous functional MRI studies in MCI have suggested that increased hippocampal activity is associated with increased rates of cortical atrophy and more rapid cognitive decline (O'Brien et al., 2010; Putcha et al., 2011a). Whether age-related changes in activity reflect aberrant or compensatory processes remain a topic of debate (Stern, 2012; Jagust, 2013). The causal association of hyperactivity and amyloid accumulation in the evolution of sporadic Alzheimer’s disease remains to be elucidated. The interpretation of increased hippocampal activity, as compensatory neuronal activity, versus evidence of excitotoxicity is not mutually exclusive. Early in the trajectory of Alzheimer’s disease, as pathology starts to accumulate, increased neuronal activity might be compensatory and benefit cognition. However, these overactive neurons may produce greater amounts of soluble amyloid-β (Cirrito et al., 2005), which might further perpetuate the cycle and contribute to disease progression.
Limitations
A foremost limitation of this study is the modest cohort size; this study was designed for analysis of longitudinal data obtained at multiple visits, which limited the total number of patients who could be included. Also, we have a limited number of observations at 36 months (Table 1), as several MCIs did not complete their 36-month visit due to increased impairment or enrolment into clinical trials. In this cohort, males were over-represented (Table 2), consistent with the higher number of males diagnosed with MCI (Petersen et al., 2010), which has been suggested to reflect a faster rate of Alzheimer’s disease progression in females with MCI. Yet, we did not detect interactions with sex and merely observed a main effect of baseline hippocampal volume. In addition, we found that longitudinal changes in hippocampal functional MRI activity were not different between Aβ+ and Aβ− MCI groups and did not predict clinical progression. A second potential limitation is the PET acquisition times (>60 min). Longer acquisition or qualitative assessment of the PET scans (Ossenkoppele et al., 2013), might help discriminate between MCIs with low and intermediate levels of amyloid-β deposition (Villemagne et al., 2013). Thus, the acquisition method and the DVR threshold applied here (1.2) might slightly underestimate the number of MCIs with clinically relevant amyloid-β deposition. However, this likely would have decreased our ability to detect amyloid-β related differences between the MCI groups. Finally, we did not find evidence that learning or habituation of the associative memory task could account for the longitudinal changes in hippocampal functional MRI activity. Nevertheless, we hypothesize that as the Aβ+ MCI group continues to progress towards dementia, memory performance and increased hippocampal activity will diminish, in accordance with cross-sectional observations in patients with Alzheimer’s disease (Sperling et al., 2003; Trivedi et al., 2008a; Ewers et al., 2011).
Implications for future clinical research
The longitudinal models demonstrate that these neuroimaging markers provide information above and beyond clinical status at baseline. About half of the early MCIs (17 of 33) in this study, as defined in accordance with ADNI criteria (Aisen et al., 2010), showed high levels of amyloid-β deposition and slightly more impaired cognition. This percentage of Aβ+ MCIs is roughly consistent with previous studies (Jack et al., 2009; Wolk et al., 2009; Petersen et al., 2013), and is markedly higher than the 30% Aβ+ typically observed in clinically normal older adults (Sperling et al., 2011). Our findings provide further support for the role of biomarkers in patients with a clinical diagnosis of MCI to define a prodromal stage of Alzheimer’s disease (Albert et al., 2011; Hinrichs et al., 2011; Dubois et al., 2014). The MMSE scores and CDRSB, in combination with the neuroimaging data, demonstrate that Aβ− MCIs are more stable, consistent with a trajectory of suspected non-Alzheimer’s disease pathology (Petersen et al., 2013). In contrast, the Aβ+ MCI group in our study progressed clinically and are more likely on the path to Alzheimer’s disease dementia, consistent with other recent longitudinal studies in MCI (Jack et al., 2010; Nordberg et al., 2012; Hatashita and Yamasaki, 2013).
Currently, there is no effective treatment for Alzheimer’s disease (Selkoe, 2012). The contributions of both amyloid-β deposition and increased hippocampal activity to clinical progression suggest two potential targets for clinical intervention: amyloid-β and excitotoxicity. The current findings provide support for an initial study that used anti-epileptic drugs to reduce hippocampal activity and demonstrated some cognitive benefit in MCI (Bakker et al., 2012). In individuals who are already experiencing symptoms due to Alzheimer’s disease with manifest evidence of neuronal dysfunction and neurodegeneration, we will likely need to pursue other mechanisms beyond amyloid-β (Sperling et al., 2014), and consider combination therapies of anti-amyloid agents with drugs to mitigate excitotoxicity.
Funding
This research was supported by the European Molecular Biology Organization: ALTF 318-2011 (W.H.), the National Institutes of Health: F32 AG044054 (E.M.), K23 AG033634 (G.M.), K24 AG035007 (R.S.), K23 AG033634 (G.M.), R01 AG027435 (R.S.), P01AG036694 (R.S.), P50AG00513421 (R.S.) and the Alzheimer’s Association: IIRG-06-27374 (R.S.) and a philanthropic gift by Foundation for Neurologic Diseases (W.H./R.S.). This research was carried out in part at the Athinoula A. Martinos Centre for Biomedical Imaging, using resources provided by the Centre for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- DVR
distribution volume ratio
- MCI
mild cognitive impairment
- PiB
Pittsburg compound-B
References
- Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, et al. Clinical core of the Alzheimer's disease neuroimaging initiative: progress and plans. Alzheimers Dement. 2010;6:239–46. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–74. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Pub Group. 2011;14:750–6. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–8. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–6. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Medina LD, Rodriguez-Agudelo Y, Geschwind DH, Macias-Islas MA, Cummings JL, et al. Increased fMRI signal with age in familial Alzheimer’s disease mutation carriers. NBA. 2012;33:424. doi: 10.1016/j.neurobiolaging.2010.09.028. e11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, et al. Alterations in memory networks in mild cognitive impairment and alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–31. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Villemagne VL, Villain N, Jones G, Ellis KA, Ames D, et al. Accelerated cortical atrophy in cognitively normal elderly with high -amyloid deposition. Neurology. 2012;78:477–84. doi: 10.1212/WNL.0b013e318246d67a. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–22. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–14. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, et al. Temporal lobe functional activity and connectivity in young adult. Alzheimers Dement. 2010;6:303–11. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Elman JA, Oh H, Madison CM, Baker SL, Vogel JW, Marks SM, et al. Neural compensation in older people with brain amyloid-β deposition. Nat Neurosci. 2014;17:1316–8. doi: 10.1038/nn.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia. Trends Neurosci. 2011;34:430–42. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc Natl Acad Sci USA. 2009;106:7209–14. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S. An R companion to applied regression. 2nd edn., University of Minnesota, Sage Publications; 2011. [Google Scholar]
- Hanseeuw B, Dricot L, Kavec M, Grandin C, Seron X, Ivanoiu A. Associative encoding deficits in amnestic mild cognitive impairment: a volumetric and functional MRI study. Neuroimage. 2011;56:1743–8. doi: 10.1016/j.neuroimage.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Hatashita S, Yamasaki H. Diagnosed mild cognitive impairment due to alzheimer’s disease with pet biomarkers of beta amyloid and neuronal dysfunction. PLoS One. 2013;8:e66877. doi: 10.1371/journal.pone.0066877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen A, Pihlajamaki M, Tanila H, Hänninen T, Niskanen E, Tervo S, et al. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2007;28:1889–903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Hinrichs C, Singh V, Xu G, Johnson SC, Initiative TADN. Predictive markers for AD in a multi-modality framework: an analysis of MCI progression in the ADNI population. Neuroimage. 2011;55:574–89. doi: 10.1016/j.neuroimage.2010.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Mormino EC, Wigman SE, Ward AM, Vannini P, McLaren DG, et al. Amyloid deposition is linked to aberrant entorhinal activity among cognitively normal older adults. J Neurosci. 2014;34:5200–10. doi: 10.1523/JNEUROSCI.3579-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer‘s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–65. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010;133:3336–48. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Knopman DS, Lowe V, Vemuri P, et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology. 2013;81:1732–40. doi: 10.1212/01.wnl.0000435556.21319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77:219–34. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Mormino EC. Lifespan brain activity. Trends Cogn Sci. 2011;15:520–6. doi: 10.1016/j.tics.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–34. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9:S72–83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Susskind-Wilder L, Connor DJ, Sabbagh MN, Caselli RJ. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42:980–9. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, et al. Activation of brain regions vulnerable to Alzheimer's disease: the effect of mild cognitive impairment. Neurobiol Aging. 2006;27:1604–12. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC. The influence of alzheimer disease family history and apolipoprotein e 4 on mesial temporal lobe activation. J Neurosci. 2006;26:6069–76. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Devous MD, Sr, Hebrank AC, Bischof GN, Park DC. Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. Neuroimage. 2012;62:1–8. doi: 10.1016/j.neuroimage.2012.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher TT, Weis S, Freymann K, Erb M, Jessen F, Grodd W, et al. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatr. 2007;78:812–8. doi: 10.1136/jnnp.2006.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–8. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Serra L, Perri R, Pantano P, Lenzi GL, Paulesu E, et al. Single domain amnestic MCI: a multiple cognitive domains fMRI investigation. NBA. 2011;32:1542–57. doi: 10.1016/j.neurobiolaging.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O'Brien PC, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology. 2003;61:500–6. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GA, Donovan NJ, Lorius N, Gidicsin CM, Maye J, Pepin LC, et al. Apathy is associated with increased amyloid burden in mild cognitive impairment. J Neuropsychiatry Clin Neurosci. 2013;25:302–7. doi: 10.1176/appi.neuropsych.12060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Res. 1993;50:121–39. doi: 10.1016/0925-4927(93)90016-b. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, et al. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–7. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Brandel MG, Madison CM, Marks S, Baker SL, Jagust WJ. A deposition in aging is associated with increases in brain activation during successful memory encoding. Cereb Cortex. 2012;22:1813–23. doi: 10.1093/cercor/bhr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated -amyloid deposition in elderly subjects. Brain. 2009;132:1310–23. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic. Alzheimer Dis Arch Neurol. 2009;66:1469–75. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status. J Neuropathol Exp Neurol. 2012;71:362–81. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2012;40:104–14. doi: 10.1007/s00259-012-2237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JL, O'Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, et al. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74:1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Jagust WJ. Frontotemporal network connectivity during memory encoding is increased with aging and disrupted by beta-amyloid. J Neurosci. 2013;33:18425–37. doi: 10.1523/JNEUROSCI.2775-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Prins ND, van Berckel BN. Amyloid imaging in clinical trials. Alzheimers Res Ther. 2013;5:36. doi: 10.1186/alzrt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, van der Flier WM, Verfaillie SCJ, Vrenken H, Versteeg A, van Schijndel RA, et al. Long-term effects of amyloid, hypometabolism, and atrophy on neuropsychological functions. Neurology. 2014;82:1768–75. doi: 10.1212/WNL.0000000000000432. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-–induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–8. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Aisen P, Boeve BF, Geda YE, Ivnik RJ, Knopman DS, et al. Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol. 2013;74:199–208. doi: 10.1002/ana.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men the mayo clinic study of aging. Neurology. 2010;75:889–97. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Krishnan S, Slavin MJ, Tran T-TT, Murty L, Doraiswamy PM. Mild cognitive impairment: evaluation with 4-T functional MR imaging 1. Radiology. 2006;240:177–86. doi: 10.1148/radiol.2401050739. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–47. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Putcha D, Brickhouse M, O'Keefe K, Sullivan C, Rentz D, Marshall G, et al. Hippocampal hyperactivation associated with cortical thinning in alzheimer's disease signature regions in non-demented elderly adults. J Neurosci. 2011a;31:17680–8. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha D, O’Keefe K, LaViolette P, O’Brien J, Greve D, Rentz DM, et al. Reliability of functional magnetic resonance imaging associative encoding memory paradigms in non-demented elderly adults. Hum Brain Mapp. 2011b;32:2027–44. doi: 10.1002/hbm.21166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz YT, Budson AE, Celone K, Ruiz A, Newmark R, Castrillón G, et al. Hippocampal hyperactivation in presymptomatic familial Alzheimer's disease. Ann Neurol. 2010;68:865–75. doi: 10.1002/ana.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11:1048–56. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–18. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–91. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–8. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci USA. 2012;109:E2895–903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Preventing Alzheimer's disease. Science. 2012;337:1488–92. doi: 10.1126/science.1228541. [DOI] [PubMed] [Google Scholar]
- Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol. 1999;45:466–72. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sperling R. The potential of functional MRI as a biomarker in early Alzheimer's disease. Neurobiol Aging. 2011;32:S37–43. doi: 10.1016/j.neurobiolaging.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer‘s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatr. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, et al. Functional alterations in memory networks in early alzheimer’s disease. Neuromol Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O’Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–88. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. The A4 study: stopping AD before symptoms begin? Sci Trans Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11:1006–12. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MA, Murphy CM, Goetz C, Shah RC, Gabrieli JDE, Whitfield-Gabrieli S, et al. fMRI activation changes during successful episodic memory encoding and recognition in amnestic mild cognitive impairment relative to cognitively healthy older adults. Dement Geriatr Cogn Disord. 2008a;26:123–37. doi: 10.1159/000148190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Hess TM, Fitzgerald ME, Atwood CS, et al. fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: risk factors for Alzheimer's disease. Neuropsychologia. 2008b;46:1667–78. doi: 10.1016/j.neuropsychologia.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Verhey FRJ, Hofman PAM, Scheltens P, Jolles J. Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatr. 2002;72:491–7. doi: 10.1136/jnnp.72.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko AG, Mintun MA, Xiong C, Sheline YI, Goate AM, Benzinger TLS, et al. Amyloid-beta plaque growth in cognitively normal adults: longitudinal [11C]Pittsburgh compound B data. Ann Neurol. 2011;70:857–61. doi: 10.1002/ana.22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–68. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zeileis A, Grothendieck G. zoo: S3 infrastructure for regular and irregular time series. J Stat Softw. 2005;14:1–27. [Google Scholar]