Abstract
Background
Epidemiology of minimal-change disease (MCD) in adults differs from that in children and is not studied well in Indian population.
Methods
We retrospectively studied the records of 61 adult patients with MCD to assess clinical, laboratory and histopathological features, and to evaluate the response to treatment, course and complications of the disease and therapy.
Results
The male to female ratio was 1.17:1. Mean age was 30.46 years. Of the total, 6.55% had hypertension; 13.11% had microhaematuria. After initial treatment with steroids, 68.85% had complete remission (CR) and 13.1% had partial remission (PR). Twelve of 14 (85.71%) steroid-resistant cases had CR or PR after alternative immunosuppression with cyclophosphamide, or mycophenolate mofetil. Of all patients, 44.2% had at least one relapse; 8.19% were frequently relapsing and 26.22% were steroid dependent. After a mean follow-up of 149.9 weeks, 38 (61.29%) patients were in CR and 16 (26.22%) in PR with a mean proteinuria of 1.28 g/day, 3 being treated for relapse. Mean serum creatinine was 89.28 μmol/L (1.01 mg/dL). Fourteen (22.95%) had acute kidney injury (AKI). All but two recovered completely.
Conclusions
This single-centre study with a medium-term follow-up shows that majority of patients respond to steroids or alternative immunosuppressants. AKI is common and may not be completely reversible in some cases.
Keywords: minimal-change disease in adults, nephrotic syndrome, treatment outcomes
Introduction
Minimal-change disease (MCD) refers to the occurrence of nephrotic syndrome (NS) with no glomerular lesions by light microscopy (or only minimal mesangial prominence), no staining on immunofluorescence microscopy (or low-intensity staining for C3 and IgM) and foot process effacement but no electron-dense deposits on electron microscopy [1].
MCD, the most frequent cause of primary NS in children, is the third most common cause of primary NS in adults contributing to 10–15% of the cases [2–4]. Cross-sectional studies from various parts of India have identified MCD as a more frequent cause of primary NS than that observed in Western studies. It was the commonest cause (33–44%) of primary NS in adults in the studies from the North [5–7] while accounting for 11–22% of the cases from the Southern Indian studies [8, 9].
Epidemiology and course of MCD in adults differ from that in children. Adult-onset MCD is associated with kidney failure in 33% of patients, hypertension in 35% and microscopic haematuria in 47% [10].
In view of paucity of data from India, we took up this study to examine the epidemiology and course of MCD in adults. In this retrospective observational study, we aimed to analyse the clinical, biochemical and histological profile of adult patients with primary NS due to MCD and to study the course of the disease, response to therapy and outcome.
Subjects and methods
The Institutional Ethics Committee approved the study. The study included patients >12 years of age with the diagnosis of MCD during the period from January 2005 to June 2012. All of the patients had at least one outpatient department visit in the preceding 6 months. Patients with secondary glomerular disorders were excluded. Clinical and laboratory features, kidney biopsy findings, treatment details and outcome data were noted in a pre-defined format.
The following definitions were used while collecting the data:
MCD: NS with no glomerular lesions by light microscopy (or only minimal mesangial proliferation), no staining on immunofluorescence microscopy (or low-intensity staining for C3 and/or IgM) and foot process effacement but no electron-dense deposits on electron microscopy;
Complete remission (CR): reduction of proteinuria to 0.3 g/day or 300 mg/g urine creatinine, serum albumin >35 g/L (3.5 g/dL);
Partial remission (PR): reduction of proteinuria to 0.3–3.5 g/day (300–3500 mg/g urine creatinine);
Relapse: proteinuria >3.5 g/day or >3500 mg/g (4350 mg/mmol) urine creatinine after CR has been obtained;
Frequently relapsing NS (FRNS): four or more relapses within 1 year. Steroid-dependent NS (SDNS): two relapses during or within 2 weeks of completing steroid therapy;
Steroid resistant NS (SRNS): failure to achieve remission despite at least 16 weeks of prednisolone;
Acute kidney injury (AKI): rise in serum creatinine by >26.52 μmol/L (0.3 mg/dL) over the baseline or urine output <0.5 mL/kg/h for 6 h.
A single pathologist reported all of the histopathology. General principles of the treatment remained the same over the study duration. Initial treatment given was daily prednisolone 1 mg/kg for 4–16 weeks followed by tapering over the next 6 months. Relapses were treated in a similar manner. Oral cyclophosphamide (CYP) 1.5–2 mg/kg was used in FRNS, SDNS with steroid intolerance and in SRNS. Calcineurin inhibitors (CNIs) were used if CYP was contraindicated or refused by the patient. Mycophenolate mofetil (MMF) was used when CYP or CNIs did not achieve remission or were contraindicated, declined or not tolerated.
Statistical analysis was performed using the software SPSS version 16.0. Continuous variables were expressed as mean and standard deviation (SD). Difference between the two groups was tested by Student's t-test. Categorical data were evaluated using chi square test. Variables that were evaluated for possible association with the remission included serum albumin, cholesterol, 24-h urine proteins, glomerulomegaly, mesangial proliferation and interstitial fibrosis. For all statistical tests, a P-value of <0.05 was considered significant.
Results
Between 2005 and 2011, 61 patients >12 years of age presenting with NS were diagnosed with MCD on the basis of kidney biopsy. Clinical and laboratory features of these patients are shown in Table 1. Thirty-three (54.09%) were males. Mean age at presentation was 30.46 years (SD 13.43, range 13–65). All presented with oedema. Eight had ascites. Four (6.55%) had hypertension on presentation. Two patients had fever with joint pain and one had varicella zoster on presentation. Two had a history of hypertension before presentation. Two had type 2 diabetes mellitus. One was operated for schwannoma in the past. One of the adolescent girls was treated before referral with Ayurvedic medicines, a form of traditional alternative medicine in India, for phrynoderma (follicular keratosis). These drugs, whose chemical nature was unknown, were discontinued just before presentation. No patient had family history of NS. Eight (13.11%) had microscopic haematuria. Fourteen had AKI. Mean serum creatinine was 102.54 μmol/L (1.16 mg/dL) (SD 62.76, range 44.2–388.96). Mean follow-up was 149.9 weeks (16–390).
Table 1.
Clinical and laboratory features of patients with MCD
| n = 61 | |
|---|---|
| Age (years) | 30.46 ± 13.43 |
| Male/female | 33/ 28 |
| Hypertension (n) | 4 (6.55%) |
| Microhaematuria (n) | 8 (13.11%) |
| Serum albumin (g/L) | 20 ± 7.1 |
| Serum cholesterol (mmol/L) | 9.86 ± 3.51 |
| 24-h urine proteins (g/day) | 5.33 ± 3.75 |
On histopathological examination, 16 (26.22%) patients had glomerulomegaly judged subjectively by the pathologist. Twenty-eight (45.9%) had a mild increase in mesangial cellularity. Four had foci of tubular atrophy and interstitial fibrosis. Twelve had lymphocytic infiltrates in the interstitium. One had hyaline arteriosclerosis. Six had IgM deposition in the mesangium, one being associated with C3 deposition.
One patient achieved spontaneous remission. All others were treated with prednisolone 1 mg/kg/day for a mean duration of 9.46 weeks (SD 3.89, range 5–16). Forty-two (68.85%) and eight (13.1%) achieved CR and PR, respectively.
Patients who had CR received prednisolone for an average duration of 8.14 weeks (SD 3.27, range 5–16) followed by tapering of the dose over a mean duration of 16.38 weeks (SD 8.02, range 8–22). Mean duration to achieve remission was 5.33 weeks (SD 3.81, range 2–16).
Eight patients achieved PR after initial treatment with prednisolone; five were treated with the lowest possible dose of prednisolone and three were treated with oral CYP in view of clinical features of steroid toxicity or steroid dependence. Two of them could discontinue the steroid therapy.
Serum cholesterol and 24-h urine protein on presentation were the significant predictors of the initial response, complete or partial, to prednisolone (P = 0.04 and 0.02, respectively), while histological features like glomerulomegaly, mesangial proliferation or interstitial fibrosis were not.
Twenty-seven (44.2%) patients had at least one relapse with twelve (19.67%) having more than one relapse. Mean duration to first relapse was 12.2 months (SD 11.51). Five of these were FRNS. Three of these were treated with CYP and one with tacrolimus while remission could be maintained in one with low-dose prednisolone. All of the patients treated with CYP achieved CR while the patient treated with tacrolimus was being treated for relapse at the time of data collection after having achieved CR.
Sixteen (26.22%) were steroid dependent. Nine were treated with low-dose steroids, of whom six achieved CR (including one being treated for relapse at the time of data collection) while three were in PR. Seven were treated with CYP, of whom, at the time of data collection, four were in CR, and two achieved PR. One was treated with MMF after remaining steroid dependent after CYP achieved CR.
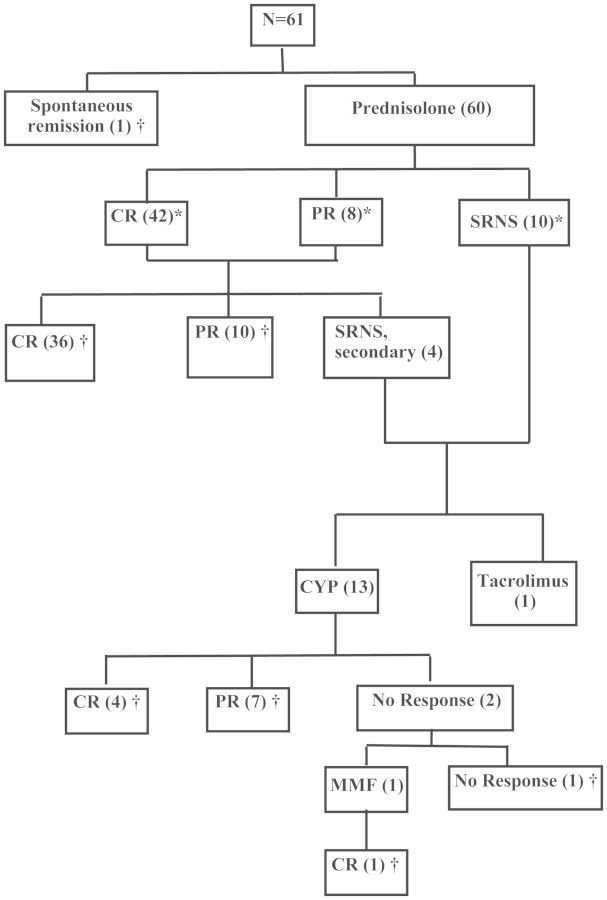
Fourteen were SRNS (including four having been steroid responsive on presentation). Of these, 13 were treated with CYP and 1 with tacrolimus. Of the 13 patients treated with CYP, 4 achieved CR, 7 achieved PR and 2 did not respond to CYP. One such patient was treated with MMF achieved CR over 14 weeks and had completed 11 months on MMF. The one patient treated with tacrolimus had not achieved remission at the 10th week at the time of data collection. Figure 1 shows the treatment outcome of the patients.
Fig. 1.
Minimal-change disease: treatment outcomes. *Initial response, †outcome at the time of data collection.
Twenty-eight (45.9%) patients were treated with angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARBs). Eleven (18.03%) were treated with statins.
The cumulative dose for oral CYP was 6577 ± 1660 mg (range 4480–10752 mg) over a mean duration of 10 weeks. No patient received a repeat course of CYP. One patient treated with CYP had microhaematuria at Week 8 of therapy, one was diagnosed to have sputum-positive pulmonary tuberculosis and none had leukopenia.
Mooning of face, striae, acne, weight gain, hyperglycaemia, cataract and proximal muscle weakness were found in patients treated with prednisolone. MMF and tacrolimus were tolerated well.
Fourteen patients had AKI. Mean serum creatinine was 167.08 ± 95.47 mole/μL. Three episodes were associated with lower respiratory tract infection. One had bacterial peritonitis. Two were on ARBs when AKI was diagnosed. All but two recovered completely. More patients with ascites than without ascites developed AKI, although the difference did not reach statistical significance. Serum albumin (P = 0.76), serum cholesterol (P = 0.77) and urine protein (P = 0.26) levels were not significantly different between the patients with or without AKI. Table 2 shows features of patients with and without AKI.
Table 2.
Clinical and laboratory features of patients with and without AKI
| AKI (n = 14) | No AKI (n = 47) | P-value | |
|---|---|---|---|
| Ascites | 4 | 4 | 0.0509 |
| Serum creatinine (μmol/L) | 167.08 ± 95.47 | 83.1 ± 30.06 | 0.0061 |
| Serum albumin (g/L) | 21 ± 10.4 | 20 ± 5.9 | 0.76 751 |
| Serum cholesterol (mmol/L) | 9.61 ± 3.91 | 9.95 ± 3.43 | 0.77 816 |
| Serum triglyceride (mmol/L) | 31.64 ± 14.81 | 29.04 ± 12.86 | 0.55 295 |
| Proteinuria (g/day) | 6.24 ± 3.33 | 5.05 ± 3.85 | 0.26 879 |
| Hypertension | 2 | 2 | 0.81 |
After a mean follow-up of 149.9 weeks, 38 (61.29%) patients were in CR and 16 (26.22%) in PR with mean proteinuria 1.28 g/day (SD 0.92, range 0.4–2.8 g/day), three being treated for relapse. Mean serum creatinine was 89.28 μmol/L (1.01 mg/dL) (SD 38.01, range 44.2–353.6), not different from serum creatinine at presentation (P = 0.13).
In patients with steroid-resistant NS, mean serum creatinine at presentation and at last follow-up were 76.9 ± 29.17 and 94.58 ± 76.02 μmol/L (0.87 ± 0.33 mg/dL and 1.07 ± 0.86 mg/dL), the difference being statistically insignificant (P = 0.36). However, one patient, who had AKI which recovered only partially, was in CKD stage 4 (eGFR = 20.4 mL/min) at the end of 29 weeks of follow-up.
Twelve (four with CR, four with PR and three without remission) had hypertension requiring antihypertensive medications.
Discussion
This retrospective audit found that MCD was diagnosed in adults in the third and fourth decade of life with almost equal gender prevalence. Of the total, 75.4% patients achieved CR or PR after initial prednisolone therapy. Of the 14 patients with SRNS (primary or secondary), 11 could achieve CR or PR after treatment with immunosuppressive medications. AKI occurred in 22.95% patients, which did not recover completely in two.
Epidemiology of MCD in adults is not studied well in the Indian population. We could find cross-sectional studies, but no study examined the course and response to treatment. We could not find any study examining epidemiology of AKI in MCD from this part of the world.
Although children with MCD tend to have hypertension or microhaematuria infrequently, these features are described more frequently in adult patients in Western series. We found that 6.55% of adolescents and adults in our study had hypertension. This proportion is lower than that seen in other case series from the West and Asia [11–13] which report 22–43% of patients presenting with hypertension. Although classically MCD does not involve disruption of the basement membrane or inflammation, microhaematuria is well known, with Waldman et al. [11] reporting this feature in about 29% of patients with MCD. Microhaematuria was seen in 13.11% of our patients which is similar to that reported by Dias et al. [12] in a Brazilian population and by Huang et al. [14] in a Taiwanese population.
No patient in our series had reported a history of allergies in contrast to 33% reported in the British population studied by Mak et al. [15].
Treatment of adult MCD is challenging, as response to steroid is late and steroid resistance is more common than seen in children [11, 16]. Treatment options for adults with MCD are based on randomized controlled trials (RCTs) in children. Efficacy and safety of immunosuppressive agents, including inexpensive drugs like CYP, have not been subjected to rigorous RCTs. Observational studies have supported the use of CYP in SDNs, FRNS as well as SRNS [11, 15]. We observed the use of immunosuppressive drugs in 4 FRNS, 7 SDNS and 14 SRNS patients. The treatment protocol was the same throughout the study period.
One patient achieved spontaneous remission in our study. Eighty-two per cent of the patients achieved PR or CR. This is less than that seen in other studies in a Taiwanese population (94%) [14] or in a British (92%) [15] and Brazilian study (97%) [12]. Mean duration to achieve remission was 5.3 weeks, in contrast to 13 weeks seen by Waldman et al. [11] but similar to that reported by Mak et al. [15] in a British population.
Relapses were seen in 44.26% of the patients, five of whom were frequently relapsing. Four of the frequent relapsers could achieve remission with CYP or low-dose prednisolone. FRNS is more common in children than adults. There appears to be a wide variation in proportion of patients having FRNS in various studies in adults. While the average age was similar in the British and American group, the former did not report any case of FRNS, the latter reporting FRNS in 28.6% patients [11, 15]. Tse et al. [13] reported an 8% incidence of FRNS, with a similar incidence in younger and older Asian patients.
All 17 patients with SDNS achieved remission. All nine treated with low-dose prednisolone and six of seven treated with CYP-achieved remission with one achieving CR after treatment with MMF. This proportion is higher compared with 82% reported by Waldman et al. [11].
Twelve of the 14 steroid-resistant patients achieved remission with CYP or MMF. Waldman et al. [11] reported a much lower response (43%) to treatment with various immunosuppressive drugs including CYP. Mak et al. [15] observed that two of the three steroid-resistant patients could achieve remission. Only steroid-resistant patients in the study by Tse et al. [13] responded to CYP followed by cyclosporine. In the British study, two of three patients with SRNS were treated with CYP, both achieving remission [15]. There are no RCTs examining efficacy of CYP in adults with steroid-resistant MCD. Although long-term safety has to be determined, given the efficacy and acceptable short-term safety of this inexpensive drug, as seen by us, it should be considered for RCTs.
The entity of PR in MCD has been described even when patients with subsequent renal biopsies showing another glomerular histology were excluded [15]. Although we did not perform repeat biopsies in any of the patients with PR, this entity needs to be followed up to examine the long-term outcome.
AKI was seen in 14 patients and none required renal replacement therapy. Two such cases recovered only partially, depicting the importance of AKI associated with the otherwise benign entity of MCD where end-stage renal disease due to progression of the disease is not reported. Nephrotoxic drugs and infections contribute further to the renal impairment.
We did not see thromboembolism in any of the patients. But varicella zoster, spontaneous bacterial peritonitis and pulmonary tuberculosis were seen. These assume importance as therapy contributes to the immunosuppression and to the adverse outcomes associated with this disease with otherwise good long-term outcome.
The major limitations of our study were the retrospective nature of data collection and single-centre analysis in a referral hospital. Although histopathological features were recorded subjectively, they were reported by a single pathologist in a standard format. Repeat biopsies were not done in resistant cases or cases with PR, as in individual cases they were not considered to be helpful to decide or change the treatment.
To conclude, we found that features of adults with MCD in our study differ from Western population with less frequency of hypertension, microhaematuria and less incidence of FRNS. Apart from SDNS and FRNS, steroid-resistant NS may be treated with oral CYP with acceptable results.
Conflict of interest statement
The results presented in this paper have not been published previously in whole or in part, except in abstract format.
Acknowledgements
We acknowledge Dr Sunil Jawale and Dr Dinesh Mahajan for the intellectual inputs and Mr Kishor Vardhan and Mr Ramesh More for their help in retrieving the records.
References
- 1.Jefferson JA, Nelson PJ, Najafian B, et al. Podocyte disorders: Core Curriculum 2011. Am J Kidney Dis. 2011;58:666–677. doi: 10.1053/j.ajkd.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haas M, Spargo BH, Coventry S. Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: a 20-year renal biopsy study. Am J Kidney Dis. 1995;26:740–750. doi: 10.1016/0272-6386(95)90437-9. [DOI] [PubMed] [Google Scholar]
- 3.Korbet SM, Genchi RM, Borok RZ, et al. The racial prevalence of glomerular lesions in nephrotic adults. Am J Kidney Dis. 1996;27:647–651. doi: 10.1016/s0272-6386(96)90098-0. [DOI] [PubMed] [Google Scholar]
- 4.Cameron JS. Nephrotic syndrome in the elderly. Semin Nephrol. 1996;16:319–329. [PubMed] [Google Scholar]
- 5.Agarwal SK, Dash SC. Spectrum of renal diseases in Indian adults. J Assoc Physicians India. 2000;48:594–600. [PubMed] [Google Scholar]
- 6.Aggarwal HK, Yashodara BM, Nand N, et al. Spectrum of renal disorders in a tertiary care hospital in Haryana. J Assoc Physicians India. 2007;55:198–202. [PubMed] [Google Scholar]
- 7.Reshi AR, Bhat MA, Najar MS, et al. Etiological profile of nephrotic syndrome in Kashmir. Indian J Nephrol. 2008;18:9–12. doi: 10.4103/0971-4065.41281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grace M, Thomas V. Clinicopathological correlation of primary nephrotic syndrome in adults. Saudi J Kidney Dis Transpl. 2012;23:1292–1293. doi: 10.4103/1319-2442.103580. [DOI] [PubMed] [Google Scholar]
- 9.Narasimhan B, Chacko B, John GT, et al. Characterization of kidney lesions in Indian adults: towards a renal biopsy registry. J Nephrol. 2006;19:205–210. [PubMed] [Google Scholar]
- 10.Nakayama M, Katafuchi R, Yanase T, et al. Steroid responsiveness and frequency of relapse in adult-onset minimal change nephrotic syndrome. Am J Kidney Dis. 2002;39:503–512. doi: 10.1053/ajkd.2002.31400. [DOI] [PubMed] [Google Scholar]
- 11.Waldman M, Crew RJ, Valeri A, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2:445–453. doi: 10.2215/CJN.03531006. [DOI] [PubMed] [Google Scholar]
- 12.Dias CB, Pinheiro CC, Silva Vdos S, et al. Proteinuria predicts relapse in adolescent and adult minimal change disease. Clinics (Sao Paulo) 2012;67:1271–1274. doi: 10.6061/clinics/2012(11)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tse KC, Lam MF, Yip PS, et al. Idiopathic minimal change nephrotic syndrome in older adults: steroid responsiveness and pattern of relapses. Nephrol Dial Transplant. 2003;18:1316–1320. doi: 10.1093/ndt/gfg134. [DOI] [PubMed] [Google Scholar]
- 14.Huang JJ, Hsu SC, Chen FF, et al. Adult-onset minimal change disease among Taiwanese: clinical features, therapeutic response, and prognosis. Am J Nephrol. 2001;21:28–34. doi: 10.1159/000046215. [DOI] [PubMed] [Google Scholar]
- 15.Mak SK, Short CD, Mallick NP. Long-term outcome of adult-onset minimal-change nephropathy. Nephrol Dial Transplant. 1996;11:2192–2201. doi: 10.1093/oxfordjournals.ndt.a027136. [DOI] [PubMed] [Google Scholar]
- 16.Takei T, Koike M, Suzuki K, et al. The characteristics of relapse in adult-onset minimal-change nephrotic syndrome. Clin Exp Nephrol. 2007;11:214–217. doi: 10.1007/s10157-007-0484-5. [DOI] [PubMed] [Google Scholar]