Abstract
Haemophagocytic syndrome (HPS) is a rare and potentially lethal condition characterized by pancytopoenia, fever, organomegaly and widespread proliferation of macrophages phagocytosing blood elements. Among the triggers of this syndrome, excessive immunosuppression in a context of acute rejection has been rarely reported, although it might be underdiagnosed. Here, we report the case of a kidney transplant recipient with allograft dysfunction due to chronic antibody-mediated rejection treated with antithymocyte globulin and plasmapheresis. The patient developed high fever, pancytopoenia, diarrhoea and respiratory symptoms with no apparent infectious or neoplastic cause, despite an extensive work-up. Haemophagocytosis was found in bone marrow examination, along with hyperferritinaemia and hypertriglyceridaemia. The clinical profile improved after treatment with intravenous immunoglobulin and reduction of the basal immunosuppression.
Keywords: allograft rejection, haemophagocytic syndrome, intravenous immunoglobulin, kidney transplantation
Background
Haemophagocytic syndrome (HPS), also known as macrophage activation syndrome, is associated with uncontrolled and ineffective immune activation, leading to cell damage and multiple organ dysfunction as well as excessive activation of macrophages. The activated macrophages then transform into histiocytes that phagocytose erythrocytes, leukocytes, platelets and their precursor cells, leading to various degrees of cytopoenia, hepatosplenomegaly and lymphadenopathy [1]. Cases of HPS can occur in patients with hereditary immune dysfunction, infections, malignancies or autoimmune diseases. There is also a familial form of HPS [2–7]. A diagnosis of HPS can be made if at least five of the following criteria are met: fever; hepatosplenomegaly; haemophagocytosis; cytopoenia in at least two cell lines; a markedly elevated ferritin level; high triglycerides or a low fibrinogen level; high levels of soluble interleukin 2 receptor/CD25 and little or no natural killer (NK) cell activity. In most suspected cases, the diagnosis can be confirmed through a bone marrow biopsy showing haemophagocytosis of red blood cells and other precursor cells [1, 8].
Although there have been reports of HPS associated with excessive immunosuppression, few of those cases were directly associated with acute rejection [4, 9, 10]. Herein, we report a case of a renal transplant recipient with allograft dysfunction due to chronic antibody-mediated rejection, which developed HPS after treatment with antithymocyte globulin (ATG) and plasmapheresis 8 years following transplantation.
Case report
A 34-year-old white male with a primary diagnosis of focal segmental glomerulosclerosis had undergone kidney transplantation from a one-haplotype-matched living-related donor 8 years previously and was on triple immunosuppression consisting of prednisone, cyclosporine and mycophenolate mofetil (MMF), with stable graft function. His baseline serum creatinine (SCr) was 123.76 µmol/L (normal range 61.88–106.8). During the 6-month follow-up period, he presented with an increase in SCr (from 123.76 to 371.28 µmol/L). He was then subjected to a renal biopsy, which showed chronic antibody-mediated rejection (diffuse C4d staining in peritubular capillaries), probably due to non-compliance with immunosuppression. Class I and II panel reactive antibody levels were 0 and 97%, respectively, and human leukocyte antigen class-II donor-specific antibody was detected by single-antigen bead assay (Luminex; One Lambda Inc., Canoga Park, CA). After hospital admission, ATG was started (cumulative dose 6 mg/kg) with plasmapheresis (six sessions). He was discharged 7 days later after a reduction in SCr (to 334.76 µmol/L). One month later, he sought treatment with a 1-week history of fever, diarrhoea, anorexia, odynophagia, cough and dyspnoea. On physical examination, his blood pressure was 170/100 mmHg and the body temperature 38°C. The patient was alert and oriented. No rash or jaundice was noted, nor were there any signs of hepatosplenomegaly or lymphadenopathy. A chest X-ray and abdominal ultrasound revealed no abnormalities. The patient was readmitted for diagnostic evaluation. Laboratory tests at admission showed pancytopoenia, high serum ferritin, elevated fasting triglyceride and worsening of graft function (Table 1).
Table 1.
Laboratory test results and normal ranges (for males)
| Variable | Admission | After IVIg | Normal range |
|---|---|---|---|
| Haemoglobin, g/L | 62 | 89 | 130–180 |
| White blood cell count, 109/L | 0.970 | 5450 | 4000–11 000 |
| Platelets, 109/L | 84 000 | 290 000 | 140 000–450 000 |
| Serum ferritin, pmol/L | 7781 | 3127 | 67–899 |
| Fasting triglyceride, mmol/L | 4.9 | 0.8 | <1.7 |
| Lactate dehydrogenase, IU/L | 223 | 298 | 240–480 |
| SCr, µmol/L | 609.96 | 291.72 | 61.88–106.8 |
IVIg, intravenous human immunoglobulin.
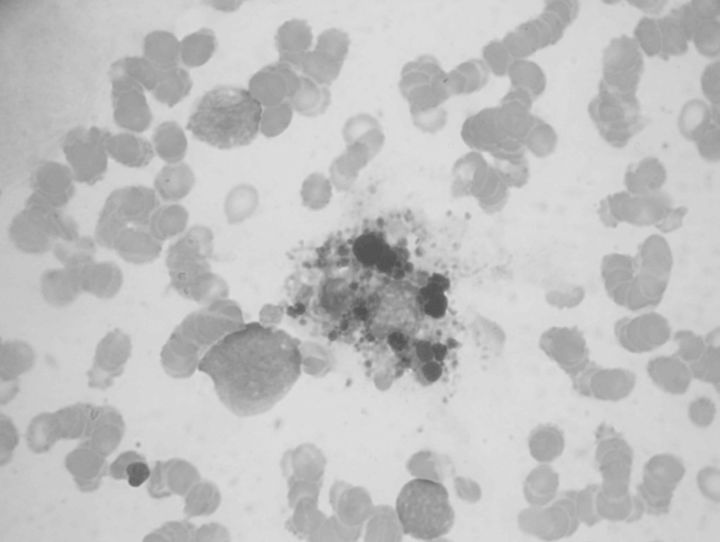
Because the patient presented with febrile neutropaenia, MMF was discontinued and he was started on broad-spectrum antibiotics and recombinant human granulocyte colony-stimulating factor (Granulokine®; Hoffmann-La Roche, Basel, Switzerland). Multiple blood and urine cultures were negative for bacteria and fungi. Further aetiological inquiry was negative for numerous pathogens: he had tested negative on serologic tests for human immunodeficiency virus, hepatitis B and C, Chagas disease, syphilis, human T-lymphotropic virus; polymerase chain reaction was negative for cytomegalovirus, herpes simplex virus, varicella-zoster virus, Epstein-Barr virus, parvovirus, adenovirus and BK polyomavirus; screenings for mycobacteria, Pneumocystis spp. and helminths were negative. Analysis of bone marrow aspirate revealed activated macrophages phagocytosing immature red blood cells, suggestive of haemophagocytosis (Figure 1).
Fig. 1.
Bone marrow aspirate showing an activated macrophage phagocytosing immature red blood cells. Note the abundant cellular debris in the inner cytoplasm of the macrophage.
Subsequently, we administered high-dose intravenous human immunoglobulin (IVIg, 400 mg/kg daily for five days) and increased the dose of prednisone (from 7.5 to 20 mg/day). The overall health status of the patient gradually improved and fever disappeared, even without antibiotics. Laboratory tests after 2 weeks of treatment with IVIg showed recovery from the cytopoenia, decreased serum ferritin, lower triglyceride levels and improve allograft function (Table 1). The immunosuppressive regimen was restarted with tacrolimus instead of cyclosporine.
Discussion
Potentially life-threatening multiple organ failure is common in HPS. Kidney transplant recipients are at risk of developing HPS as a result of their immunocompromised state [4, 11]. In the first description of reactive, infection-associated HPS (by Risdall et al. in 1979), 13 of the 19 affected individuals had undergone kidney transplantation [12].
The clinical presentation of HPS is non-specific, making it difficult to differentiate between HPS and severe sepsis. High-grade fever and constitutional symptoms are constant; lymphadenopathy, lung infiltration and skin rash might also be present. Central nervous system involvement, including encephalopathy, seizures and meningitis, is seen in approximately half of HPS patients. The most consistent laboratory finding is pancytopoenia. Hyperferritinaemia, typically associated with HPS, is also pronounced in other inflammatory states [1, 4, 8, 13]. Our patient presented fever, pancytopoenia, hypertriglyceridaemia, hyperferritinaemia and haemophagocytosis, which collectively allowed us to establish the diagnosis.
The pathogenesis of post-transplant HPS is comparable with that hypothesized for non-transplant patients. The high production of tumour necrosis factor-α and interferon-γ associated with impairment of CD8+ T lymphocyte and NK cell cytotoxicity can render the immune system incapable of controlling a trigger infectious agent. Lack of cytotoxicity might explain the excessive activity of Th1 and increased cytokine production, which explains the overwhelming inflammatory state observed during HPS. The unbalanced macrophage proliferation causing organ infiltration explains the hepatosplenomegaly, lymphadenopathy and bone marrow suppression. Cytokine inhibition of lipoprotein lipase is considered to be the cause of elevated fasting triglyceride levels [1, 14, 15].
In most cases, post-transplant HPS is triggered by an opportunistic infection following intensive immunosuppression. Many patients who develop HPS have received lymphocyte-depleting antibodies as induction therapy or have had their immunosuppression therapy ramped up because of rejection [9, 12]. In the largest sample evaluated to date, Karras et al. described 17 cases among 4230 kidney transplant recipients, an estimated prevalence of 0.4% [9]. The HPS tended to occur during the initial post-transplant period in highly immunosuppressed patients. Fourteen of those patients had received lymphocyte-depleting antibodies and seven had been treated for acute rejection in the previous 2 months. Infectious or neoplastic disease was identified in 15 patients: most cases (9 of the 17) were associated with viral infections (cytomegalovirus; adenovirus; Epstein-Barr virus; human herpes viruses 6 and 8; parvovirus, hepatitis C; and BK polyomavirus). Bacterial infections (tuberculosis and Bartonella henselae), other opportunistic infections (toxoplasmosis and Pneumocystis jirovecii) or lymphoproliferative diseases were encountered in seven patients [9]. However, in a number of cases reported in the literature (∼20%) the cause of HPS was not identified [1–3, 11].
Despite the extensive work-up performed in the case reported here, we were unable to identify any triggering infectious agent or malignancy. In addition, empirical treatment against infection was ineffective. However, the overall health status of the patient gradually improved after the administration of high-dose IVIg and increase in steroid dosage. We hypothesize that HPS could be triggered directly by allograft rejection and the associated ramping up of immunosuppression therapy, especially when lymphocyte-depleting antibodies are employed.
Haemophagocytosis is sometimes observed in association with autoimmune diseases in the absence of any triggering factor, suggesting that the disease itself or its immunosuppression therapy can directly cause HPS. Raffray et al. [10] proposed that MMF is a cause of HPS after kidney transplantation. In the case described no infectious agent or malignancy was identified: MMF was discontinued and a dramatic improvement was observed. Another such case was described in a recipient of an ABO-incompatible kidney transplant with acute rejection [16].
The prognosis of post-transplant HPS is poor. In the cohort evaluated by Karras et al., 8 (47%) of the 17 patients died and 4 of the 9 survivors lost the allograft. The authors found that aetiology of HPS was not predictive of the outcome. The risk of death was significantly associated with organomegaly, elevated aminotransferase levels, abnormal prothrombin time and thrombocytopaenia [9].
There is as yet no consensus regarding the preferred treatment of post-transplant HPS. Efforts should be made to promptly recognize and treat the aetiological agent. The use of IVIg might be beneficial. Asci et al. [17] evaluated 13 patients with post-transplant HPS and reported that the 6 who recovered had all been treated with IVIg. A reasonable management strategy might be to minimize the administration of immunosuppressive drugs while giving high-dose steroids. Corticosteroids at high doses can protect against allograft rejection, reducing macrophage activation, as well as inhibiting cytokine production [18]. Karras et al. [9] found that steroid doses at diagnosis of HPS were significantly higher in survivors than in non-survivors. On the other hand, cyclosporine and thymoglobulin have been recommended in the treatment of HPS in non-transplant patients, like in hereditary forms of HPS [19].
In conclusion, reactive HPS is a rare complication after kidney transplantation and should be suspected when fever and organomegaly are accompanied by pancytopoenia in highly immunosuppressed patients. Bone marrow analysis is the most conclusive test for a positive diagnosis. Due to its rarity, it is difficult to determine whether HPS is caused by immunosuppression alone or if therapy favours the emergence of infectious or neoplastic processes that lead to HPS. Although other underlying causes cannot be ruled out, our findings indicate that the intensity of the immunosuppression therapy induces HPS directly.
Conflict of interest statement
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. We also declared that the results presented in this paper have not been published previously in whole or part.
References
- 1.Ponticelli C, Alberighi OD. Haemophagocytic syndrome—a life-threatening complication of renal transplantation. Nephrol Dial Transplant. 2009;24:2623–2627. doi: 10.1093/ndt/gfp282. [DOI] [PubMed] [Google Scholar]
- 2.Rouphael NG, Talati NJ, Vaughan C, et al. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7:814–822. doi: 10.1016/S1473-3099(07)70290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21:245–253. doi: 10.1016/j.blre.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Karras A. What nephrologists need to know about hemophagocytic syndrome. Nat Rev Nephrol. 2009;5:329–336. doi: 10.1038/nrneph.2009.73. [DOI] [PubMed] [Google Scholar]
- 5.Silva DAF, Anunciação FA, Arcoverde JAC, et al. Hemophagocytic Syndrome: a clinical presentation of systemic lupus erythematosus. Acta Reumatol Port. 2008;33:91–97. [PubMed] [Google Scholar]
- 6.Bea Granell S, Beneyto Castello I, Ramos Escorihuela D, et al. Cytomegalovirus-associated haemophagocytic syndrome in a kidney transplant patient. Nefrologia. 2011;31:236–238. doi: 10.3265/Nefrologia.pre2010.Nov.10639. [DOI] [PubMed] [Google Scholar]
- 7.Esposito L, Hirsch H, Basse G, et al. BK virus-related hemophagocytic syndrome in a renal transplant patient. Transplantation. 2007;365 doi: 10.1097/01.tp.0000248807.63325.cc. United States. [DOI] [PubMed] [Google Scholar]
- 8.Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 9.Karras A, Thervet E, Legendre C, et al. Hemophagocytic syndrome in renal transplant recipients: report of 17 cases and review of literature. Transplantation. 2004;77:238–243. doi: 10.1097/01.TP.0000107285.86939.37. [DOI] [PubMed] [Google Scholar]
- 10.Raffray L, Couzi L, Viallard JF, et al. Mycophenolate mofetil: a possible cause of hemophagocytic syndrome following renal transplantation? Am J Transplant. 2010;10:2378–2379. doi: 10.1111/j.1600-6143.2010.03254.x. [DOI] [PubMed] [Google Scholar]
- 11.Rostaing L, Fillola G, Baron E, et al. Course of hemophagocytic histiocytic syndrome in renal transplant patients. Transplantation. 1995;60:506–509. doi: 10.1097/00007890-199509000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Risdall RJ, McKenna RW, Nesbit ME, et al. Virus-associated hemophagocytic syndrome: a benign histiocytic proliferation distinct from malignant histiocytosis. Cancer. 1979;44:993–1002. doi: 10.1002/1097-0142(197909)44:3<993::aid-cncr2820440329>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology Am Soc Hematol Educ Program. 2009;2009:127–131. doi: 10.1182/asheducation-2009.1.127. [DOI] [PubMed] [Google Scholar]
- 14.Kürşat S, Cağirgan S, Ok E, et al. Haemophagocytic-histiocytic syndrome in renal transplantation. Nephrol Dial Transplant. 1997;12:1058–1060. doi: 10.1093/ndt/12.5.1058. [DOI] [PubMed] [Google Scholar]
- 15.Filipovich A, McClain K, Grom A. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant. 2010;16:S82–S89. doi: 10.1016/j.bbmt.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Yoshiaki K, Jiro M, Masaki Y, et al. Hemophagocytic syndrome in an ABO-incompatible living renal transplant recipient with acute rejection during the chronic phase. Nishinihon J Urol. 1999;61:341–343. [Google Scholar]
- 17.Asci G, Toz H, Ozkahya M, et al. High-dose immunoglobulin therapy in renal transplant recipients with hemophagocytic histiocytic syndrome. J Nephrol. 2006;19:322–326. [PubMed] [Google Scholar]
- 18.Wong PK, Cuello C, Bertouch JV, et al. Effects of pulse methylprednisolone on macrophage chemotactic protein-1 and macrophage inflammatory protein-1alpha in rheumatoid synovium. J Rheumatol. 2001;28:2634–2636. [PubMed] [Google Scholar]
- 19.Kaito K, Otsubo H, Takei Y, et al. Immunosuppressive therapy with antithymocyte globulin and cyclosporine for prolonged marrow failure after hemophagocytic syndrome. Ann Hematol. 2003;82:699–701. doi: 10.1007/s00277-003-0714-1. [DOI] [PubMed] [Google Scholar]