Abstract
The dialysis disequilibrium syndrome (DDS) is characterized by progressive neurological symptoms and signs attributable to cerebral edema that occurs due to fluid shifts into the brain following a relatively rapid decrease in serum osmolality during hemodialysis (HD). Since continuous renal replacement therapy (CRRT) is less efficient at solute clearance than intermittent HD, it seems logical that this mode of therapy is less likely to cause DDS. This entity has not been previously reported to occur with this modality. Here, we report two cases of DDS associated with CRRT that provide insights into its pathophysiological mechanisms and suggest strategies for its prevention.
Keywords: CRRT, dialysis, disequilibrium, osmolality, renal failure
Background
The dialysis disequilibrium syndrome (DDS), first reported by Kennedy et al. [1] in 1962, is a clinical syndrome of acute neurological decompensation of varying severity, occurring during or after hemodialysis (HD), following the rapid removal of urea. While the final common pathway leading to DDS is the development of cerebral edema, controversy prevails as to whether the pathogenesis is due to the slower removal of urea from the brain relative to plasma, i.e. reverse urea effect [2, 3] versus the presence of idiogenic osmoles and intracerebral acidosis [4, 5].
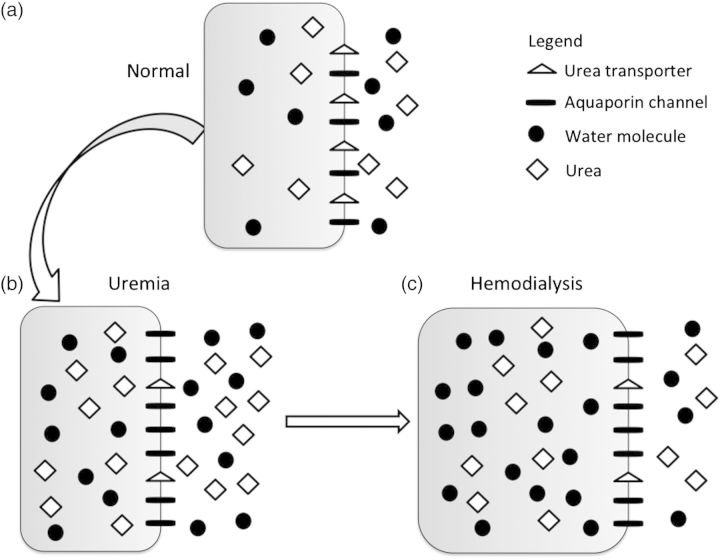
The rapid removal of urea during HD results in a concomitant reduction in serum osmolality, leading to an osmotic gradient between the intracellular and extracellular compartments that promotes water movement into cells and subsequent cerebral edema. Even though urea diffuses freely across cell membranes, it lags behind the rapidity of urea removal from the serum during HD [2]. Studies in rat models of DDS confirmed that the osmolal content of the brain does not change with HD, but the brain-to-plasma urea ratio increases significantly after dialysis and was associated with a 6% increase in brain water content [2]. Furthermore, altered brain aquaporin and urea transporter expression in advanced chronic kidney disease (CKD) may result in reduced urea exit from cells while allowing more rapid water entry along an osmotic gradient, resulting in cerebral edema [6] (Figure 1).
Fig. 1.
Changes in brain urea transporter (UT) and aquaporin channel (AQP) expression. (a) Normal, non-uremic milieu. (b) During chronic uremia, UT expression decreases by ∼50%, while that of AQP increases by ∼165% [6]. (c) During rapid urea removal, as occurs during HD, the reduced number of brain UT results in slower movement of urea from the intracellular to extracellular compartment, than is removed from the extracellular compartment by dialysis. The resulting osmotic gradient, coupled with increased brain AQP expression, results in water movement into cells, and subsequent cerebral edema.
Animal models revealed that DDS occurred in the context of acute intracerebral acidosis and the formation of idiogenic osmoles in response to plasma hyperosmolality [5]. Both these processes increase intracellular osmolality and, thus, cause cerebral edema. However, studies by other investigators did not show an increase in brain organic osmolytes after rapid HD [7].
Continuous renal replacement therapy (CRRT) is an inpatient dialysis and hemofiltration modality used in critically ill patients who may not tolerate treatment with conventional HD due to hemodynamic instability. Under standard conditions, CRRT has significantly lower solute clearance than conventional HD, resulting in slower removal of uremic toxins, and thus postulated to decrease the risk of DDS [8]. DDS has not been previously reported to occur with CRRT. Here, we report two cases of DDS associated with CRRT.
Case reports
Patient 1
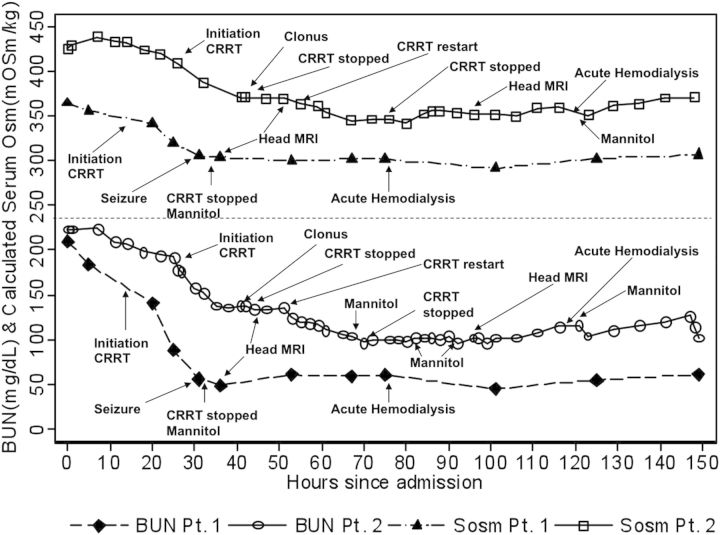
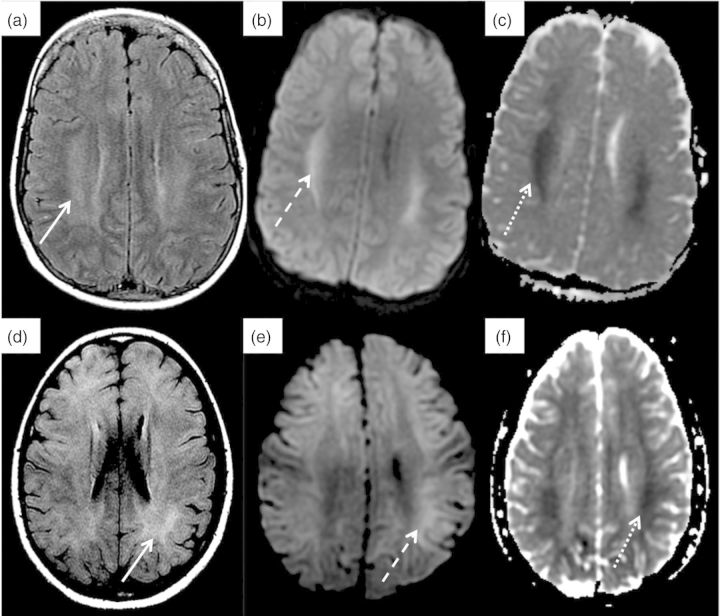
A 14-year-old male with known developmental delay presented with 7 days of emesis, decreased fluid intake and lethargy. He had weight loss and had suffered from anorexia for ∼1 year. Intravenous fluid resuscitation led to hemodynamic and mental status improvement. Initial laboratory evaluation was significant for normal serum sodium, blood urea nitrogen (BUN) of 75 mmol/L (210 mg/dL), creatinine 1520 µmol/L (17.2 mg/dL), bicarbonate 7 mmol/L and calcium 1.4 mmol/L (5.6 mg/dL). Based on renal ultrasound findings of small echogenic kidneys, he was presumed to have advanced CKD. Continuous veno-venous HD (CVVHD) was initiated 14 h after admission with a blood flow rate of 150 mL/min and dialysate flow rate of 1000 mL/h. Fourteen hours later, he became increasingly agitated and had a 30 s generalized seizure. CRRT was discontinued and intravenous mannitol administered. As shown in Figure 2, the urea reduction over the 14 h of CRRT prior to seizure activity was 65%. Brain magnetic resonance imaging (MRI) following the seizure revealed findings consistent with DDS (Figure 3a–c), including restricted diffusion and T2 hyperintensity of the deep white matter involving the posterior frontal, parietal and mesial temporal lobes, and posterior limb of the internal capsule. Six hours after CRRT discontinuation, his mental status returned to near baseline. He made a complete neurological recovery.
Fig. 2.
Changes in BUN and calculated serum osmolality (Sosm) associated with CRRT in Patients 1 and 2. Multiply BUN (mg/dL) by 0.357 to convert to mmol/L. 1 mOsm/kg is equal to 1 mmol/kg.
Fig. 3.
Head MRI findings in two patients with DDS. Patients 1 (a–c) and 2 (d–f): (a and d) axial fluid attenuated inversion recovery shows abnormal signal (vasogenic edema) within the white matter (solid arrow). (b and e) T2-weighted trace showing cytotoxic edema within the white matter (dashed arrow). (c and f) apparent diffusion coefficient mapping showing cytotoxic edema within the white matter (dotted arrow).
Patient 2
An 11-year-old previously healthy female presented with lethargy and confusion after 1 week of left ear pain, sore throat and decreased urine output. At presentation, she was dehydrated and had a Glasgow Coma Scale score of 9. She was volume resuscitated and electively intubated. An initial head computed tomography scan revealed inflammation and fluid in the left ear canal but no brain abnormalities. Laboratory evaluation was significant for serum sodium of 168 mmol/L, potassium 6.3 mmol/L, bicarbonate 13 mmol/L, BUN 79.6 mmol/L (223 mg/dL) and creatinine 831 µmol/L (9.4 mg/dL). Renal ultrasound revealed normal sized kidneys with increased cortical echogenicity. Intravenous fluids and free water replacement led to a reduction in serum sodium from 174 to 166 mmol/L. CVV hemofiltration (CVVH) was initiated with a blood flow rate of 100 mL/min and replacement flow rate of 500 mL/h. Sodium chloride was added to increase the replacement fluid sodium concentration to165 mmol/L. After 10 h on CVVH, she developed clonus and decreased spontaneous movements of the lower extremities. Urea reduction at that time was 23% (Figure 2) and serum sodium concentration unchanged. Brain MRI revealed diffuse symmetric, high T2 signal abnormality involving the basal ganglia, corpus callosum and supratentorial subcortical white matter, consistent with DDS (Figure 3d–f). CVVH was restarted to manage hypervolemia, with a reduction in replacement fluid sodium concentration to 160 mmol/L and flow rate to 300 mL/h. Mannitol was given every 12 h. Repeat head MRI 48 h later revealed significant improvement. She subsequently made a complete neurological and renal recovery.
Discussion
These two cases are the first reported of DDS occurring in the context of CRRT. They highlight several important findings providing insights into the pathogenesis of DDS.
First, despite a slower rate of urea reduction during CRRT, DDS can develop if the decreases in BUN and serum osmolality (Figure 2) are of sufficient magnitude to induce fluid shifts in the brain. This can occur even in severe acute kidney injury (AKI), as occurred in Patient 2. Unless the dose of CRRT is significantly reduced, even inefficient continuous urea reduction may be too rapid to allow sufficient time for re-equilibration of urea and/or metabolism of idiogenic osmoles to occur. Furthermore, as it is a continuous modality, the urea gradient between intra- and extracellular compartments is maintained, in contrast to HD where the patient is given time in between dialysis sessions for equilibration to occur without ongoing reductions in BUN.
Secondly, DDS can occur even when the reduction in BUN is modest if there is another electrolyte (e.g. sodium) contributing to the hyperosmolar state. Patient 2 developed DDS despite a urea reduction rate (URR) of 23%, due to the likely combined effects on osmolality of a small reduction in serum sodium coupled with modest urea clearance. Compared with recommendations for the correction of chronic hypernatremia, which is not to exceed 0.5 mmol/L/h, equivalent to no more than a 24-mmol/kg/day (24 mOsm/kg/day) change in serum osmolality, Patient 2 decreased her serum osmolality by 38 mmol/kg (38 mOsm/kg) in 10 h, which may have precipitated the DDS. In fact, the recommendation is to correct hypernatremia even more slowly when it is chronic to prevent cerebral edema. Therefore, in a patient with coexisting hypernatremia, correction of BUN and serum sodium must be gradual, limiting reduction in serum osmolality to <24 mmol/kg (24 mOsm/kg) in 24 h. In Patient 1 (Figure 2), the decrease in serum osmolality with CVVHD was 42 mmol/kg (42 mOsm/kg) in 14 h. In a patient with normal serum sodium, BUN should, therefore, not be decreased by >24 mmol/L (67 mg/dL) in 24 h. In Patient 1, who initiated CVVHD at a BUN of 80 mmol/L (224 mg/dL), this equates to a URR of 30% in 24 h. However, the higher the BUN, the lower the goal URR should be, to limit the absolute drop in osmolality to <24 mmol/kg (24 mOsm/kg) in 24 h.
Thirdly, DDS can occur in patients with AKI. Patient 2 had full recovery of renal function, but developed DDS in the context of hypernatremia and severe AKI. Animal models have confirmed that DDS can occur in AKI with rapid HD [5].
The MRI findings in our two patients revealing demyelinating white matter injury are consistent with previously reported abnormalities, which can involve both pontine and extra-pontine sites [9]. The explanation for this injury may be rooted in the observation that brain white matter has a lesser blood flow and hence, delayed efflux of urea [5], potentially making it more susceptible to the effects of a reduction in plasma urea with dialysis. A neuropathology study in a patient who developed severe DDS confirmed the presence of cerebral edema in the white matter [10]. In cerebral gray matter, the reverse urea effect does not seem to play a prominent role in the development of cerebral edema as cerebral cortical urea concentrations after rapid HD differ from plasma concentrations by only ∼7 mmol/kg H2O [5]. In contrast, there is a paradoxical drop in both cerebral gray matter and cerebrospinal fluid pH during dialysis [4]. This decrease in pH may occur due to either the production of increased organic acids or the displacement of intracellular bound sodium and potassium ions from proteins, leading to an increase in intracellular osmolality [11].
It is clear that both the magnitude and rate of urea or osmolality decrease play important roles in the occurrence of DDS. As such, CRRT prescriptions must necessarily be reduced to prevent a significant drop in BUN and osmolality, even if this occurs gradually over 24 h.
Conflict of interest statement
None declared.
References
- 1.Kennedy AC, Linton AL, Eaton JC. Urea levels in cerebrospinal fluid after haemodialysis. Lancet. 1962;1:410–411. doi: 10.1016/s0140-6736(62)91365-x. doi:10.1016/S0140-6736(62)91365-X. [DOI] [PubMed] [Google Scholar]
- 2.Silver SM, DeSimone JA, Jr, Smith DA, et al. Dialysis disequilibrium syndrome (DDS) in the rat: role of the ‘reverse urea effect. Kidney Int. 1992;42:161–166. doi: 10.1038/ki.1992.273. doi:10.1038/ki.1992.273. [DOI] [PubMed] [Google Scholar]
- 3.Silver SM, Sterns RH, Halperin ML. Brain swelling after dialysis: old urea or new osmoles? Am J Kidney Dis. 1996;28:1–13. doi: 10.1016/s0272-6386(96)90124-9. doi:10.1016/S0272-6386(96)90124-9. [DOI] [PubMed] [Google Scholar]
- 4.Arieff AI, Guisado R, Massry SG, et al. Central nervous system pH in uremia and the effects of hemodialysis. J Clin Invest. 1976;58:306–311. doi: 10.1172/JCI108473. doi:10.1172/JCI108473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arieff AI, Massry SG, Barrientos A, et al. Brain water and electrolyte metabolism in uremia: effects of slow and rapid hemodialysis. Kidney Int. 1973;4:177–187. doi: 10.1038/ki.1973.100. doi:10.1038/ki.1973.100. [DOI] [PubMed] [Google Scholar]
- 6.Trinh-Trang-Tan MM, Cartron JP, Bankir L. Molecular basis for the dialysis disequilibrium syndrome: altered aquaporin and urea transporter expression in the brain. Nephrol Dial Transplant. 2005;20:1984–1988. doi: 10.1093/ndt/gfh877. doi:10.1093/ndt/gfh877. [DOI] [PubMed] [Google Scholar]
- 7.Silver SM. Cerebral edema after rapid dialysis is not caused by an increase in brain organic osmolytes. J Am Soc Nephrol. 1995;6:1600–1606. doi: 10.1681/ASN.V661600. [DOI] [PubMed] [Google Scholar]
- 8.Bagshaw SM, Peets AD, Hameed M, et al. Dialysis disequilibrium syndrome: brain death following hemodialysis for metabolic acidosis and acute renal failure—a case report. BMC Nephrol. 2004;5:9. doi: 10.1186/1471-2369-5-9. doi:10.1186/1471-2369-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarhan NC, Agildere AM, Benli US, et al. Osmotic demyelination syndrome in end-stage renal disease after recent hemodialysis: MRI of the brain. AJR Am J Roentgenol. 2004;182:809–816. doi: 10.2214/ajr.182.3.1820809. doi:10.2214/ajr.182.3.1820809. [DOI] [PubMed] [Google Scholar]
- 10.Harris CP, Townsend JJ. Dialysis disequilibrium syndrome. West J Med. 1989;151:52–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Arieff AI. Dialysis disequilibrium syndrome: current concepts on pathogenesis and prevention. Kidney Int. 1994;45:629–635. doi: 10.1038/ki.1994.84. doi:10.1038/ki.1994.84. [DOI] [PubMed] [Google Scholar]