Abstract
Inherited and acquired metabolic disorders are responsible for renal intracellular accumulation of phospholipids. Ultrastructural analysis revealing typical myeloid or zebra bodies was previously thought to be exclusive to Fabry disease. However, chloroquine/hydroxychloroquine toxicity can cause similar abnormalities. Recent studies have mentioned curvilinear bodies (CLB) in renal cells in such cases, never described in Fabry nephropathy. We report a 31-year-old patient with systemic lupus erythematosus who was on long-term hydroxychloroquine treatment. The presence of zebra bodies on electron microscopy lead to initial interpretation of Fabry disease, but subsequent genetic analysis did not show a relevant mutation. Further evaluation revealed CLB in renal cells, supporting the diagnosis of hydroxycholoroquine-induced renal phospholipidosis.
Keywords: Fabry disease, hydroxychroloquine, toxicity, zebra body, curvilinear body
Introduction
Fabry disease is a rare X-linked metabolic disorder caused by deficient activity of the lysosomal hydrolase, alpha-galactidosidase A (AGL), resulting in intracellular accumulation of globotriasylceramide in numerous cells (vascular endothelium, cardiac, skeletal and smooth muscle and renal cells) [1]. These glycosphingolipid inclusions can be found within a variety of renal cells. Light microscopic examination usually shows enlarged podocytes with abundant, finely vacuolated cytoplasm due to lipid inclusions. Although podocytes are affected predominantly, accumulation of globotriasylceramide can also occur in glomerular or vascular endothelium, mesangial cells, tubular epithelium, interstitial foam cells or in vascular myocytes. The ultrastructural appearance of the inclusions is of whorled layers of alternating dense and pale material (‘zebra bodies’ or myelin figures) that are highly characteristic of Fabry disease [2] Although heterozygous females were previously thought to be clinically unaffected, subclinical or fully symptomatic forms can occur due to differential X-chromosomal inactivation [3].
Various drugs including amiodarone, chloroquine and hydroxychloroquine may mimic phospholipidosis of Fabry disease. In previous years, case reports have been published that have drawn attention to chloroquine, causing histomorphologic changes similar to those of Fabry nephropathy. In all cases, the almost identical inclusions led to misdiagnosis until a complete history was available, followed by confirmation of normal enzymatic activity or absence of α-galactosidase A gene mutation [2, 4–6].
Recent reports [5, 6] with ultrastructural analyses of chloroquine-induced phospholipidosis found a unique feature never reported in Fabry disease. Curvilinear inclusion bodies were first described by Müller-Höcker et al. [4] as an intermingled twisted microtubular substructure found in vascular smooth muscle cells and podocytes. More recently, Ferluga [7] demonstrated similar curvilinear bodies (CLB) in renal biopsies in a small case series of chloroquine-induced iatrogenic phospholipidosis.
We report a 31-year-old systemic lupus erythematosus (SLE) patient on hydroxychloroquine therapy who underwent a kidney biopsy that revealed findings typical of Fabry disease.
Case report
A 31-year-old female was diagnosed with SLE in 1998 when she presented with arthritis, anaemia and skin lesions (lupus discoid). Blood analysis showed hypocomplementaemia and positive antinuclear antibody and anti-double-stranded DNA antibodies. Therapy was commenced with steroids and methotrexate. She had been on hydroxychloroquine since 2009 (150 mg/day, cumulative dose, 219 g) and developed proteinuria (600 mg/day) over the 3-year observation period. The serum creatinine was normal at 81 µmol/L (0.9 mg/dL). As the skin lesions worsened in 2010, steroids were increased (0.5 mg/kg/day with subsequent tapering to 7.5 mg/day after a 6-month period), and azathioprine was commenced (later switched to mycophenolate mofetil). This treatment regimen brought about only partial improvement. A kidney biopsy was performed in February 2012 for evaluation of renal histology to facilitate the decision of giving belimumab therapy.
Kidney biopsy
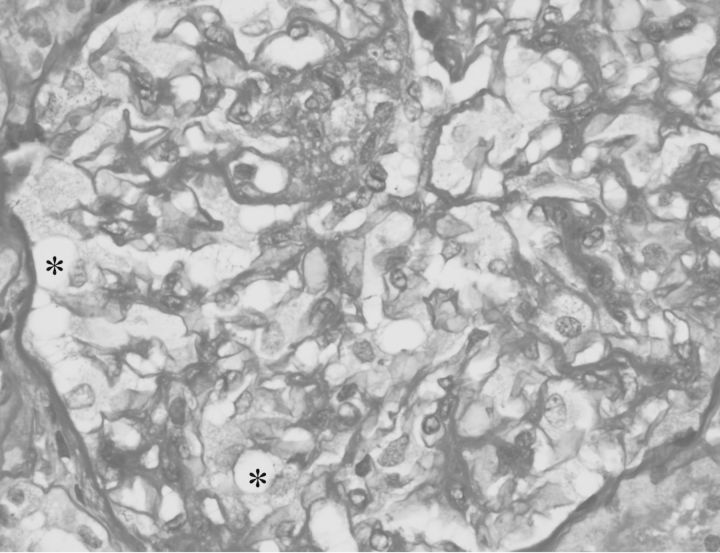
Light microscopic examination (Figure 1) of paraffin-embedded sections stained with haematoxylin and eosin, and periodic acid–Schiff stain showed renal cortex containing 23 glomeruli, 2 of which were sclerosed. Glomerular visceral epithelial cells were diffusely enlarged with fine uniform vacuolations. Mesangial matrix and cellularity were slightly increased throughout. There was no inflammation, segmental sclerosis, adhesions or crescents. Minimal interstitial fibrosis and tubular atrophy were observed. Arterioles showed only mild sclerosis.
Fig. 1.
Visceral epithelial cells showing finely vacuolated cytoplasm (*). Diffuse increase in mesangial matrix and cellularity (Periodic acid–schiff stain, ×400).
Immunofluorescence showed mild mesangial staining for IgA, IgG, IgM and complement factor C3. Immunostaining for complement factors C4 and C1q, κ and λ light chains, and fibrinogen was negative.
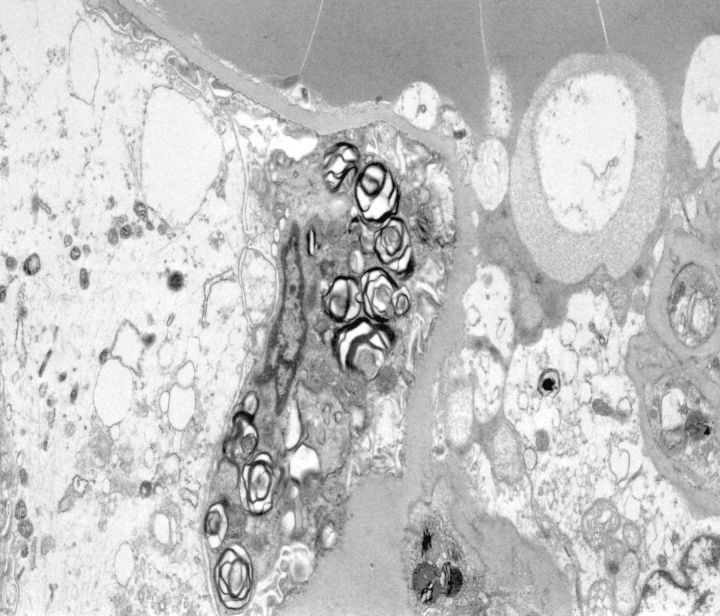
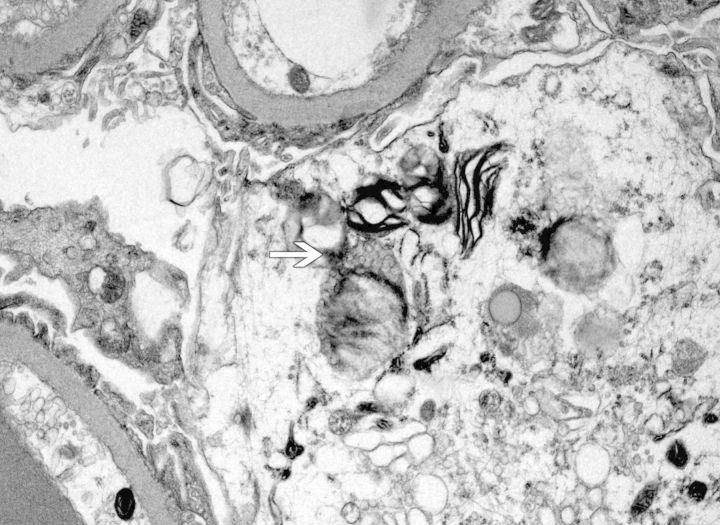
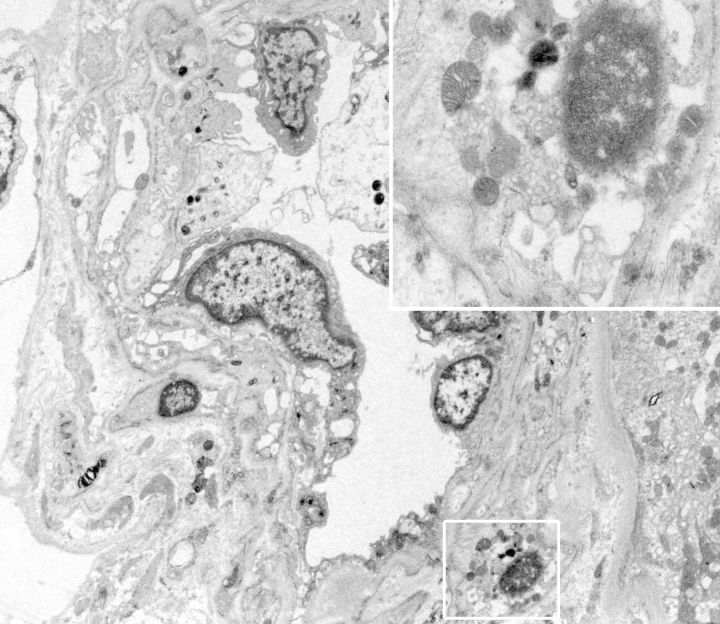
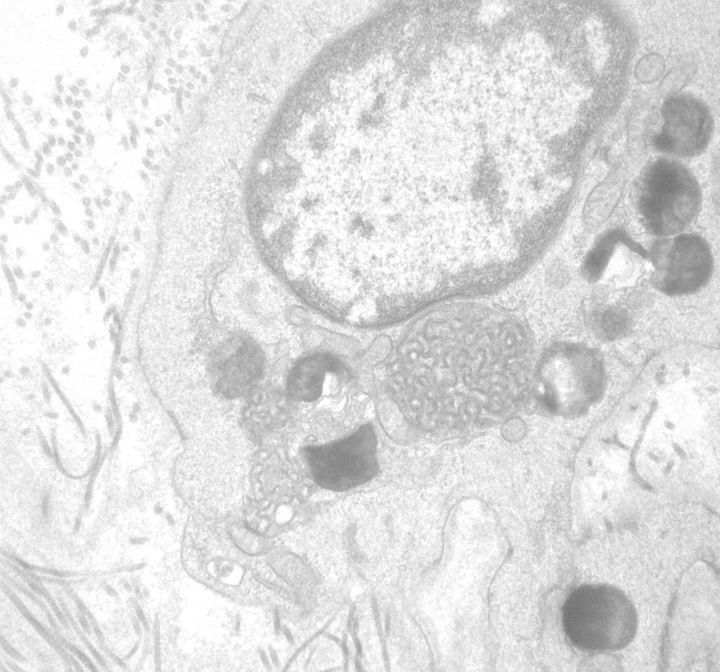
Methylene blue-stained thin sections cut at 1 µm were prepared for electron microscopy and contained one glomerulus and arteriole. Ultrastructural examination of glomerular visceral epithelial cells demonstrated prominent membrane-bound electron-dense bodies with a multilamellar appearance, with changes characteristic of myelin figures or zebra bodies (Figure 2). Similar structures were also present, although to a far lesser degree, within glomerular endothelium and vascular smooth muscle cells. Few lamellated bodies were present in tubules, with predominance of proteinaceous material in the tubular lysosomes. CLB, expressing twisted microtubular structure, were found in glomerular visceral epithelial and arteriolar endothelial cells (Figures 3 and 4). Foot processes of overlying epithelial cells showed patchy effacement. Expansion of mesangial matrix was observed and immune-type electron-dense deposits were present within the mesangium and focally in subendothelium.
Fig. 2.
Visceral epithelial cells containing myeloid/zebra body inclusions (electron micrograph; original magnification ×5200).
Fig. 3.
Curvilinear (arrow) with myeloid/zebra bodies in the cytoplasm of a podocyte (electron micrograph; original magnification ×7500).
Fig. 4.
Curvilinear and myeloid cytoplasmic inclusions in an arteriolar smooth muscle cell (electron micrograph, original magnification ×3500–15 000).
The biopsy specimen showed features of lupus nephritis Class II (ISN/RPS 2004), with superimposed intracytoplasmatic inclusions characteristic of Fabry disease.
Clinical follow-up
The patient had no clinical signs or familiar history suggestive of Fabry disease. Leucocyte α-galactosidase A enzyme activity was normal at 8.18 nmol/mL/h (normal 2.1–13.6 nmol/mL/h) and mutation analysis showed no abnormalities in the patient’s α-galactosidase A gene.
Ophthalmologic examination revealed periocular eczema (related to SLE) and discrete bilateral corneal subepithelial opacities with normal campimetry. Skin biopsy ultrastructural analysis (Figure 5) showed difuse intralysosomal electron-dense deposits and larger inclusions with typical CLB. Since Fabry disease was excluded, these findings were attributed to hydroxychloroquine toxicity and it was discontinued.
Fig. 5.
Skin biopsy (electron microscopy, ×12 000), showing intracellular CLB and electron-dense intralysosomal inclusions.
Improvement in skin lesions was observed 6 months after stopping hydroxychloroquine and bilateral corneal subepithelial opacities disappeared. Renal function remained stable (serum creatinine 81 µmol/L (0.9 mg/dL) with proteinuria 400 mg/24 h) while the patient remained on azathioprine and low-dose steroids.
Discussion
It was a surprise to find features of classic Fabry disease on kidney biopsy of this patient. However, lack of symptoms, a negative family history of Fabry disease, combined with normal enzyme activity, and absence of genetic mutations excluded this diagnosis.
The unique findings on kidney biopsy used to establish diagnosis of Fabry disease have been questioned in recent years. There are few case reports to support this, and they describe inclusions similar to Fabry nephropathy in patients with long-term therapy with chloroquine and hydroxychloroquine [2, 4–6]. These drugs have been shown to become sequestered in tissues (the liver, spleen, lungs and kidneys). This extensive distribution has been attributed to its amphiphilic nature, which allows for lysosomal trapping and inhibition of key lysosomal enzymes, including α-galactosidase [8]. Accordingly, renal phospholipidosis, similar to that observed in Fabry nephropathy, has been associated with chloroquine and hydroxychloroquine toxicity. In these cases, ultrastructural analysis has variously shown small dense cytoplasmic bodies, larger concentrically lamellated myeloid bodies and straight parallel-arranged lamellated zebra bodies, resembling Fabry disease. Even the distribution within renal cells is similar, with a dominant involvement of podocytes and endothelial cells and, to a lesser extent, smooth muscle cells of the vessel walls. Therefore, in such cases, exclusion of Fabry disease is only possible by demonstrating normal α-galactosidase activity and/or absence of genetic mutations.
Ferluga [7] reported a small series of 25 patients (21 males and 4 females) who were on long-term treatment with chloroquine, most with a diagnosis of SLE with a clinically low activity or lupus-like mixed connective tissue disease. The presence of proteinuria or renal failure was not referred to in this study. In six female patients (24%), histopathologic and ultrastructural intracytoplasmatic inclusions similar to Fabry disease were found, with quantity and type of distribution resembling a female heterozygous mild form of Fabry nephropathy. In these patients, kidney biopsy also showed milder forms of lupus nephritis and occasional focal sclerosing lesions. They had been on chloroquine for a period ranging from 11 days to 5 years. One year after stopping chloroquine, total remission of myeloid inclusion bodies in the kidney biopsy was seen in one patient and partial resolution in two patients.
In the six cases declared as chloroquine-induced phospholipidosis, four expressed CLB (three in vascular smooth muscle cells and two within podocytes and cytoplasm of distal tubular epithelial cells). They were described as inclusion bodies surrounded by single membrane and in lamellated and twisted microtubular substructure. Typically found in ceroid lipofuscinosis, CLB are also an ultrastructural feature of chloroquine toxicity found in myocytes and cardiomyocytes [9]. Despite the fact that the first description of CLB in chloroquine-induced phospholipidosis in the kidney biopsy was described in a case report by Müller-Höcker et al. [4], this finding was not observed in other published cases. The presence of CLB does not seem to be a uniform finding in renal biopsy specimens, but this small series published by Ferluga [7] suggests that it may be frequently found. Therefore, the presence of CLB may be an ultrastructural clue that allows differentiation between Fabry and chloroquine-induced nephropathy.
Conflict of interest statement
None declared.
Acknowledgements
The authors thank M.J. Mihatsch (Institute of Pathology – University Hospital Basel) for the important contribution in this case evaluation and Carmen Navarro (Institute of Pathology, University Hospital Vigo) for providing electron microscopy images of the skin biopsy.
References
- 1.Alroy J, Sabnis S, Kopp JB. Renal pathology in Fabry’s disease. J Am Soc Nephrol. 2002;13:134–138. [PubMed] [Google Scholar]
- 2.Albay D, Adler S, Philipose J, et al. Chloroquine-induced lipidosis mimicking Fabry disease. Mod Pathol. 2005;18:733–738. doi: 10.1038/modpathol.3800344. [DOI] [PubMed] [Google Scholar]
- 3.Gubler MC, Lenoir G, Grünfeld JP, et al. Early renal changes in hemizygous and heterozygous patients with Fabry’s disease. Kidney Int. 1978;13:223–235. doi: 10.1038/ki.1978.32. [DOI] [PubMed] [Google Scholar]
- 4.Müller-Höcker J, Schmid H, Weiss M, et al. Chloroquine-induced phospholipidosis of the kidney mimicking Fabry’s disease: case report and review of the literature. Hum Pathol. 2003;34:285–289. doi: 10.1053/hupa.2003.36. [DOI] [PubMed] [Google Scholar]
- 5.Bracamonte ER, Kowalewska J, Starr J, et al. Iatrogenic phospholipidosis mimicking Fabry disease. Am J Kidney Dis. 2006;48:844–850. doi: 10.1053/j.ajkd.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Woywodt A, Hellweg S, Schwarz A, et al. A wild zebra chase. Nephrol Dial Transplant. 2007;22:3074–3077. doi: 10.1093/ndt/gfm462. [DOI] [PubMed] [Google Scholar]
- 7.Ferluga D. Nephropathy in Fabry disease and iatrogenic phospholipidosis mimicking Fabry disease; Presented at the First International Renal Pathology Conference (Coruña) in La Coruña, Spain, in 2010. Data available at www.nephropathology-esp.org/meetings/first-international-renal-pathology-conference. (21 July 2013, date last accessed) [Google Scholar]
- 8.Fredman P, Klinghardt GW, Svennerholm L. Effect of chloroquine on the activity of some lysosomal enzymes involved in ganglioside degradation. Biochem Biophys Acta. 1987;917:1–8. doi: 10.1016/0005-2760(87)90276-1. [DOI] [PubMed] [Google Scholar]
- 9.Neville HE, Maunder-Sewry CA, McDougall J, et al. Chloroquine-induced cytosomes with curvilinear profiles in muscle. Muscle Nerve. 1979;2:376–381. doi: 10.1002/mus.880020509. [DOI] [PubMed] [Google Scholar]