Abstract
Myosin heavy chain-9-related disorders (MYH9-RDs) are a group of autosomal-dominant disorders caused by mutations in the MYH9 gene. The features include congenital macrothrombocytopaenia, inclusion bodies in neutrophils and a variable risk of developing sensorineural deafness, progressive renal impairment and presenile cataracts. A 44-year-old Caucasian man was initially thought to have Alport's syndrome and thrombocytopaenia secondary to idiopathic thrombocytopaenic purpura (ITP). A detailed family history and genetic analysis revealed a diagnosis of MYH9-RD. This case highlights the implications of a delayed diagnosis and the ongoing challenges encountered during management of individuals with this condition.
Keywords: MYH9-related disorders, end-stage renal disease, MYH9 gene, thrombocytopaenia
Background
Myosin heavy chain-9-related disorders (MYH9-RDs) are a group of autosomal-dominant disorders caused by mutations in the MYH9 gene. We present a case of a delayed diagnosis of MYH9-RD and highlight the challenges encountered during management of individuals with this condition.
Case report
A 44-year-old Caucasian man with a diagnosis of Alport's syndrome, established at another centre, and two previous kidney transplants was referred to our renal department in June 2009. Graft function was failing and he was approaching the need to start renal replacement therapy.
In 1973, he was investigated for hereditary nephritis and megathrombocytopaenia (platelet count of 7 × 109/L, size not available). He was also diagnosed with bilateral sensorineural deafness. A kidney biopsy was performed at this stage; however, the patient's early clinical reports were not available; hence, there was no record of the biopsy result. It could not be ascertained whether he was diagnosed with any ocular abnormalities. He eventually started haemodialysis via a tunnelled line in 1987 and later that year he received a kidney transplant from his father. This failed in 1999, when he went on to peritoneal dialysis for 16 months. He then received a second kidney transplant from his brother.
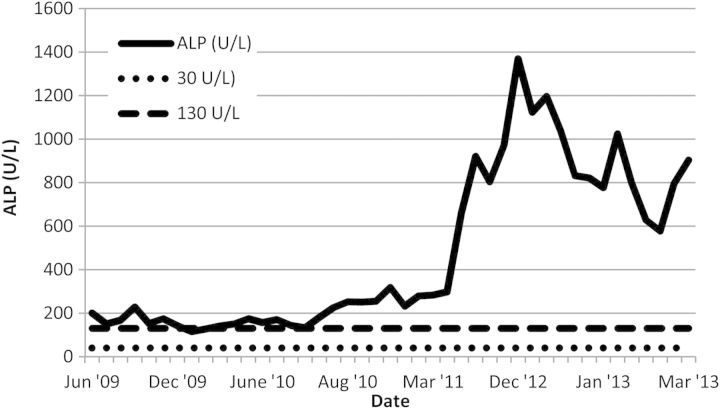
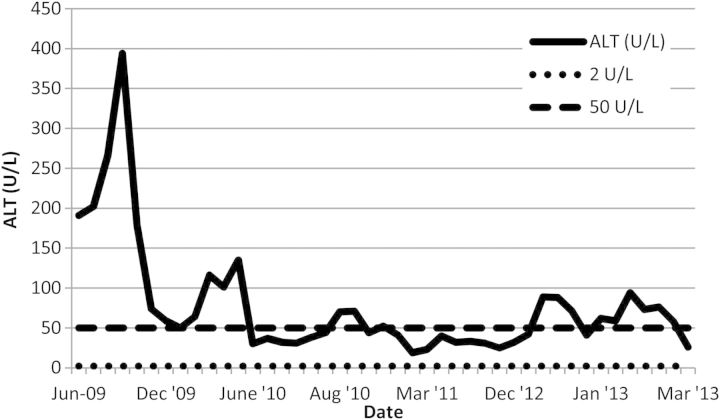
On presentation to our low-clearance clinic in 2009, liver impairment was noted (Figures 1 and 2). This was longstanding as records from 1985 revealed an alkaline phosphatase (ALP) of 420 U/L and an alanine transaminase (ALT) of around 68–92 U/L. There was a moderate improvement in the transaminase levels on withdrawing azathioprine and simvastatin. His platelet count was 5 × 109/L, and a blood film showed large platelets. Platelet size was not documented as this is not routinely measured in our hospital. A bone marrow examination showed active haematopoiesis and abundant megakaryocytes. Despite several courses of steroids, the thrombocytopaenia did not improve, and a possible diagnosis of steroid-refractory idiopathic thrombocytopaenic purpura (ITP) was considered.
Fig. 1.
ALP values between June 2009 and March 2013.
Fig. 2.
ALT levels between June 2009 and March 2013.
A year later, he started on peritoneal dialysis; however, this had to be stopped due to recurrent episodes of staphylococcal peritonitis. Haemodialysis was initiated via a tunnelled line. A left radiocephalic arteriovenous fistula was created a few months later, but this was never used due to persistent thrombocytopaenia.
During this period of time, it was revealed that this man's son was similarly affected with deafness, renal impairment and thrombocytopaenia. It was also confirmed that his brother (not the brother who had donated a kidney) and his two sons had a similar phenotype. The brother was also noted to have deranged liver function tests; however, we had no access to this patient's records; hence, we have no further details. This clear male-to-male hereditary transmission and the close association with megathrombocytopaenia made the initial diagnosis of X-linked Alport's syndrome less likely and genetic studies were performed. Sequential analysis revealed that he was heterozygous for a missense mutation in exon 17 of the myosin heavy chain-9 (MYH9) gene on chromosome 22, in the arginine residue at amino acid 702 of the motor domain. Steroid treatment for the presumed ITP was stopped and his family was offered genetic testing. After interrogating other members of the family, it was revealed that our patient's brother and his family were diagnosed with the same genetic mutation in 2005.
In the meantime, a deterioration in the man's liver function tests was observed (Figures 1 and 2), together with the development of ascites and encephalopathy. Both the patient and his family denied alcohol intake. A computed tomography scan of his abdomen revealed mild smooth peritoneal thickening in the lower abdomen and posterior pelvis. He is currently being investigated for a possible diagnosis of encapsulating peritoneal sclerosis (EPS).
Discussion
MYH9-RD is a form of inherited thrombocytopaenia that is now thought to be more prevalent than originally thought. It is caused by mutations in the MYH9 on chromosome 22 coding for the non-muscle myosin heavy chain IIa and is inherited in an autosomal-dominant fashion. The hallmark of MYH9-RD is congenital macrothrombocytopaenia characterized by large platelets (>4 μm in diameter) and thrombocytopaenia (<150 × 109/L) together with Dohle-like bodies in the cytoplasm of neutrophils [1]. These are best characterized by immunofluorescence; however, this was not performed in our index case. Affected individuals develop other features with variable degrees of severity and combinations, namely high-frequency sensorineural hearing loss, progressive nephropathy and presenile cataracts. Individuals with a mutation in the motor domain of the myosin-9 protein as in our index case exhibit a more severe phenotype than those with a mutation in the tail domain [2, 3] and present with severe thrombocytopaenia, nephritis and deafness at a juvenile age.
Alport's syndrome is similarly characterized by a hereditary glomerular disease which progresses to end-stage renal disease (ESRD) and bilateral sensorineural deafness. Presenile cataracts can also occur; however, anterior lenticonus is pathognomonic of the disease [4]. Platelet abnormalities are not associated with Alport's syndrome. Inheritance is X-linked in around 80% of the cases. In 5%, transmission is autosomal dominant but there is usually a slow progression to ESRD [5].
This case highlights the importance of obtaining a detailed family history, especially when a rare genetic disorder is suspected, or when an individual with a presumed inherited condition exhibits atypical features. In this particular case, macrothrombocytopaenia is not a recognized feature of Alport's syndrome. It is not yet clear why our index case was not aware of the diagnosis established in his brother's family in 2005. Lack of an accurate diagnosis can have an adverse impact on both the management of affected individuals and that of potentially affected family members. The longstanding thrombocytopaenia in our index case was attributed to ITP, and he received multiple courses of steroids without a satisfactory response, increasing the risk of serious steroid-related side-effects [3]. A splenectomy was also considered but this was declined by the patient. It is, however, difficult to say whether early diagnosis in affected individuals will alter the course of the disease. There have been reports that treatment of affected individuals with renal involvement with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers has a positive impact on the degree of proteinuria [6].
Interestingly, this man underwent two uncomplicated kidney transplants despite the chronic thrombocytopaenia. However, on creation of an arteriovenous fistula, concerns were raised regarding the safety of puncturing the fistula for haemodialysis. Haemodialysis via a tunnelled line was continued despite the availability of a functioning fistula, thus increasing the risk of intravascular catheter-related infections. The safety of puncturing fistulas to enable haemodialysis in the context of MYH9-RD is still not clear, and more research is required in order to assess individual bleeding risks [7].
The cause for the grossly deranged liver function tests in our index case is not clear. Deranged liver profile is a known feature of MYH9-RD; however, this is not known to cause progressive liver disease or impairment of liver function [8]. Our patient is currently being investigated for the possibility of EPS.
In conclusion, our report highlights the diagnostic and management challenges encountered when dealing with individuals affected by rare genetic disorders. Increased awareness of MYH9-RD is essential both to ensure early diagnosis and to cultivate more research to help improve management of affected individuals.
Conflict of interest statement
None declared.
Acknowledgements
The authors thank the Department of Medical Genetics at Central Manchester University Hospital NHS Foundation Trust.
References
- 1.Sun XH, Wang ZY, Yang HY. Clinical, pathological, and genetic analysis of ten patients with MYH9-related disease. Acta Haematol. 2013;129:106–113. doi: 10.1159/000342123. [DOI] [PubMed] [Google Scholar]
- 2.Pecci A, Panza E, Pujol-Moix N, et al. Position of nonmuscle myosin heavy chain IIA (NMMHC-IIA) mutations predicts the natural history of MYH9-related disease. Hum Mutat. 2008;29:409–417. doi: 10.1002/humu.20661. [DOI] [PubMed] [Google Scholar]
- 3.De Rocco D, Zieger B, Platokouki H, et al. MYH9-related disease: five novel mutations expanding the spectrum of causative mutations and confirming genotype/phenotype correlations. Eur J Med Genet. 2013;56:7–12. doi: 10.1016/j.ejmg.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flinter F. Alport's syndrome. J Med Genet. 1997;34:326–330. doi: 10.1136/jmg.34.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcocci E, Uliana V, Bruttini M, et al. Autosomal dominant Alport syndrome: molecular analysis of the COL4A4 gene and clinical outcome. Nephrol Dial Transplant. 2009;24:1464–1471. doi: 10.1093/ndt/gfn681. [DOI] [PubMed] [Google Scholar]
- 6.Pecci A, Granata A, Fiore CE, et al. Renin–angiotensin system blockade is effective in reducing proteinuria of individuals with progressive nephropathy caused by MYH9 mutations (Fechtner-Epstein syndrome) Nephrol Dial Transplant. 2008a;23:2690–2692. doi: 10.1093/ndt/gfn277. [DOI] [PubMed] [Google Scholar]
- 7.Balduini CL, Pecci A, Savoia A. Recent advances in the understanding and management of MYH9-related inherited thrombocytopenias. Br J Haematol. 2011;154:161–174. doi: 10.1111/j.1365-2141.2011.08716.x. [DOI] [PubMed] [Google Scholar]
- 8.Pecci A, Biino G, Fierro T, et al. Alteration of liver enzymes is a feature of the MYH9-related disease syndrome. PLoS One. 2012;7:e35986. doi: 10.1371/journal.pone.0035986. [DOI] [PMC free article] [PubMed] [Google Scholar]